Human (iatrogenic) transmission of amyloid-β (Aβ) pathology has been shown in brain biopsy or autopsy tissues in patients with and without iatrogenic Creutzfeldt–Jakob disease (iCJD) [1, 5–18, 20–22] and these Aβ seeds have been detected in the archival vials containing human cadaver-derived growth hormone (hcGH) [7, 19]. Whilst tau seeds were also found in these hcGH vials [7, 19], to date, no substantial tau pathology has been observed in patients with iCJD, iatrogenically transmitted Aβ pathology or both.
Here we show that a significant tau pathology, similar to that seen in patients with Alzheimer’s disease, can develop in patients with iatrogenic Aβ pathology after incubation periods exceeding 3 decades.
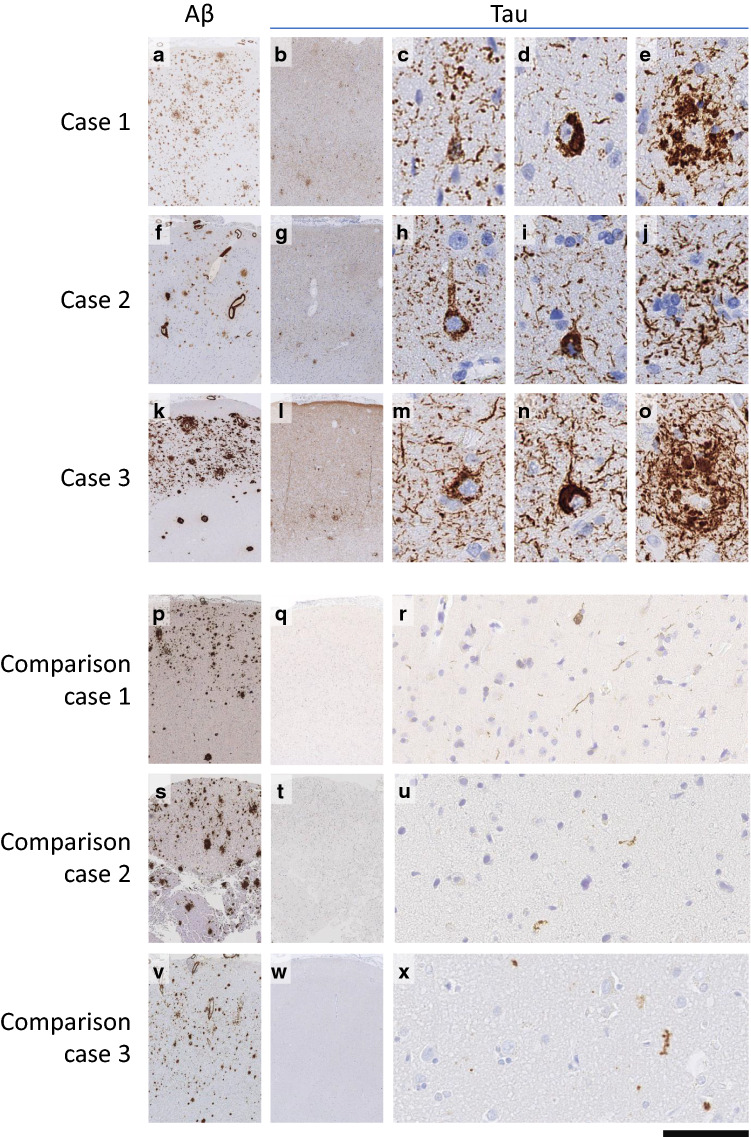
Case 1: a 46-year-old male presented with a 12-month history of cognitive decline, progressive ataxia and myoclonus. He had a medulloblastoma resected at the age of 4 years, but it is not known if a dura patch was used. The patient had learning difficulties since the radio-chemotherapy of his tumour but several months after a caudate nucleus haemorrhage at age 44, he developed gradual cognitive decline. A right frontal brain biopsy showed leptomeningeal and cortical Aβ angiopathy (CAA), parenchymal amyloid-β with diffuse deposits and plaques with central amyloid cores (Fig. 1a), and a tauopathy forming a meshwork of neuropil threads, pre-tangles, tangles and neuritic plaques (Fig. 1b–e). The patient died at the age of 47. APOE testing was not performed, but genetic testing did not identify any pathogenic mutations in 17 genes associated with neurodegenerative diseases [2], notably including the amyloid precursor protein gene (APP) (including duplication of APP), Presenilin 1 (PSEN1), Presenilin 2 (PSEN2) and microtubule-associated protein tau (MAPT).
Fig. 1.
Amyloid-β and tau pathology in cases 1–3 with an overview of Aβ pathology (a, f, k), tau pathology (b, g, l) and high power details of pre-tangles (c, h, m), tangles (d, i, n) and neuritic plaques (e, j, o), detected with antibodies against Amyloid-β (Clone 6F3D, DAKO M0872) and Tau (Clone AT8, Thermo MN1020). In three comparison cases with comparable incubation periods and similarly widespread Aβ load (p, s, v), there is minimal tau pathology: comparison case 1, incubation 37 years (case 1 in [1]; q, r, comparison case 2, incubation 35 years (case 3 in [16]) t, u, and comparison case 3, incubation 36 years (case 4 in [16]) w, x. Comparison cases are also highlighted with an asterisk (*) in Fig. 2
Case 2: a 39-year-old male presented with intracerebral haematoma and underwent emergency blood-clot evacuation. As a child, he had multiple haemangiomas, embolised at the age of 3 years (retro-auricular, embolisation agent unknown), 4 years [facial, embolised with lyophilised cadaveric dura (hcDM)], 8 years (re-embolisation of the facial lesion, with Ivalon (polyvinyl alcohol particles) and at the age of 9 (re-embolisation of the retro-auricular lesion with Ivalon). A parietal lobe biopsy from the perihaematoma region (Fig. 1f–j) showed leptomeningeal and cortical Aβ angiopathy, parenchymal Aβ with diffuse deposits and plaques with central amyloid cores, and tau pathology with a loose meshwork of neuropil threads, occasional pre-tangles and rare tangles and neuritic plaques. At follow-up, the patient had no cognitive impairment. APOE genotype was ε2/ε3, and no genetic risks or pathogenic mutations associated with early Aβ pathology were identified [2].
Case 3: a 45-year-old female presented with a convexity subarachnoid haemorrhage. As a child, she underwent multiple embolisations of facial haemangiomas including lyophilised cadaveric dura at age of 6 years. A right frontal brain biopsy (Fig. 1k–o) showed leptomeningeal and cortical Aβ angiopathy, parenchymal Aβ with diffuse deposits and plaques with central amyloid cores, and a widespread cortical tauopathy with neuropil treads, pre-tangles, tangles, and neuritic plaques. At the time of the biopsy, the patient had no cognitive impairment. Genetic testing showed an APOE ε3/ε3 genotype, and a NOTCH3 c.2183G>A (p.(Arg728His)) variant of unknown significance but no genetic risks associated with early Aβ pathology [2].
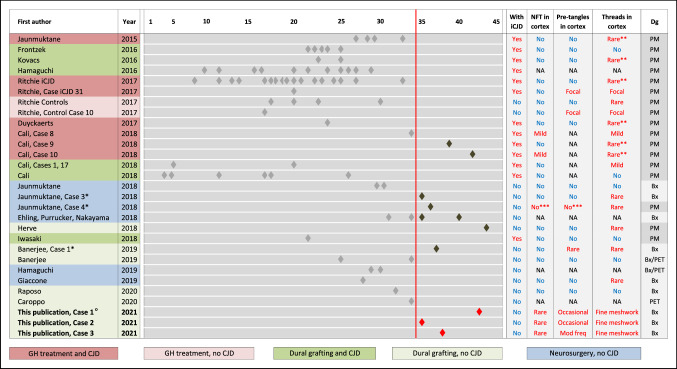
All three cases reported here have in common particularly long incubation times, exceeding 35 years for iatrogenically transmitted Aβ. No patient had a history of brain trauma, and neocortical tau pathology is extremely rare in young adults [3]. The few cases with long incubation periods reported to date (Fig. 2) do not show an obvious correlation between the extent of parenchymal Aβ pathology or the type of Aβ plaques (diffuse or plaques with central cores). Notably, plaques with central cores but without associated tau positive neurites are not uncommon in patients with iatrogenically transmitted Aβ [15].
Fig. 2.
Visualisation of iatrogenic Aβ pathology incubation times in the current and in published studies. Left columns, first author and publication year, and case ID in the respective publication, where indicated. Centre, timeline of reported incubation times. Each diamond indicates a published case. Cases with incubation times of 35 and more years are highlighted in dark grey (published) and red (this study). The reported presence of tau pathology is indicated in the four columns on the right. The column on the far right indicates the sample type (Bx—diagnosed on biopsy; PM—diagnosed on post-mortem material; PET—diagnosed on in vivo PET imaging). * (leftmost column) indicate three comparison cases shown in Fig. 1; ** (column “threads in cortex”) highlight cases, where rare neocortical threads or granular tau pathology were reported in the context of abnormal prion protein pathology; *** (columns “NFT (neurofibrillary tangles) in cortex” and “pre-tangles in cortex”) corresponds to a case in which tau pathology is seen in the medial temporal lobe but not in the neocortex. °For case 1 it is unknown if a cadaver-derived dural graft was used during neurosurgery
Whilst this study cannot answer if the tauopathy was transmitted or is a consequence of Aβ pathology, the observations described here are important as they show that tau pathology can develop in patients with iatrogenically transmitted Aβ.
Our observations give some insight into the temporal development of tau pathology. Given that substantial tau pathology in non-iCJD patients has not been seen previously (Fig. 2), at least 35 years appears to be necessary for the development of neocortical neurofibrillary tangle and widespread thread tau pathology. However, tau pathology of similar severity is not present in all patients with iatrogenic Aβ pathology with an incubation period exceeding 35 years. The three cases reported by us previously [1, 16], with equally long incubation, and similarly widespread parenchymal Aβ pathology did not show such severe tau pathology in the neocortical biopsies, although one of these cases for which whole brain tissue was available for analysis, did show tau pathology in the medial temporal lobe corresponding to Braak & Braak stage II [16].
This study highlights the importance of enquiring about previous potential iatrogenic exposure and searching medical records for treatments with hcGH or interventions using hcDM in patients with early-onset intracranial (intracerebral or non-aneurysmal subarachnoid) haemorrhages as hcDM was used not only for neurosurgical repairs but also for interventional embolisation and other surgeries [4]. The severe, often fatal, haemorrhagic consequences of iatrogenic vascular Aβ pathology have been documented [1, 6, 8, 10, 11, 13, 14, 16, 18, 21] (Fig. 2). The cases reported here indicate that in addition to CAA and parenchymal Aβ pathology, tau pathology, indistinguishable from Alzheimer’s type changes, can develop after particularly long incubation periods.
Acknowledgements
ZJ, AKT, SFF, and SB are supported by the Department of Health’s NIHR Biomedical Research Centre’s funding scheme to UCLH. GB receives funding from the Stroke Association, NIHR and Alzheimer’s Research UK. APJ is supported by the Stroke Association Margaret Giffen Reader Award. SM is National Institute of Health Research Senior Investigator. Human tissues were obtained from University College London NHS Foundation Trust as part of the UK Brain Archive Information Network (BRAIN UK, Ref: 16/006) which is funded by the Medical Research Council and Brain Tumour Research UK.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zane Jaunmuktane, Email: z.jaunmuktane@ucl.ac.uk.
Sebastian Brandner, Email: s.brandner@ucl.ac.uk.
References
- 1.Banerjee G, Adams ME, Jaunmuktane Z, Alistair Lammie G, Turner B, Wani M, et al. Early onset cerebral amyloid angiopathy following childhood exposure to cadaveric dura. Ann Neurol. 2019;85:284–290. doi: 10.1002/ana.25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J, Pittman A, Adamson G, Campbell T, Kenny J, Houlden H, et al. Validation of next-generation sequencing technologies in genetic diagnosis of dementia. Neurobiol Aging. 2014;35:261–265. doi: 10.1016/j.neurobiolaging.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 4.Brooke FJ, Boyd A, Klug GM, Masters CL, Collins SJ. Lyodura use and the risk of iatrogenic Creutzfeldt-Jakob disease in Australia. Med J Aust. 2004;180:177–181. doi: 10.5694/j.1326-5377.2004.tb05863.x. [DOI] [PubMed] [Google Scholar]
- 5.Cali I, Cohen ML, Hasmallyi US, Parchi P, Giaccone G, Collins SJ, et al. Iatrogenic Creutzfeldt–Jakob disease with amyloid-beta pathology: an international study. Acta Neuropathol Commun. 2018;6:5. doi: 10.1186/s40478-017-0503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caroppo P, Marucci G, Maccagnano E, Gobbo CL, Bizzozero I, Tiraboschi P, Redaelli V, Catania M, Di Fede G, Caputi L et al (2021) Cerebral amyloid angiopathy in a 51-year-old patient with embolization by dura mater extract and surgery for nasopharyngeal angiofibroma at age 17. Amyloid 28:142–143. 10.1080/13506129.2020.1854715 [DOI] [PubMed]
- 7.Duyckaerts C, Sazdovitch V, Ando K, Seilhean D, Privat N, Yilmaz Z, et al. Neuropathology of iatrogenic Creutzfeldt–Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol. 2018;135:201–212. doi: 10.1007/s00401-017-1791-x. [DOI] [PubMed] [Google Scholar]
- 8.Ehling R, Helbok R, Beer R, Lackner P, Broessner G, Pfausler B, et al. Recurrent intracerebral haemorrhage after coitus: a case report of sporadic cerebral amyloid angiopathy in a younger patient. Eur J Neurol. 2012;19:e29–31. doi: 10.1111/j.1468-1331.2011.03624.x. [DOI] [PubMed] [Google Scholar]
- 9.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG, Budka H. Amyloid-beta pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt–Jakob disease after dural grafting. Swiss Med Wkly. 2016;146:w14287. doi: 10.4414/smw.2016.14287. [DOI] [PubMed] [Google Scholar]
- 10.Giaccone G, Maderna E, Marucci G, Catania M, Erbetta A, Chiapparini L, et al. Iatrogenic early onset cerebral amyloid angiopathy 30 years after cerebral trauma with neurosurgery: vascular amyloid deposits are made up of both Abeta40 and Abeta42. Acta Neuropathol Commun. 2019;7:70. doi: 10.1186/s40478-019-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamaguchi T, Komatsu J, Sakai K, Noguchi-Shinohara M, Aoki S, Ikeuchi T, et al. Cerebral hemorrhagic stroke associated with cerebral amyloid angiopathy in young adults about 3 decades after neurosurgeries in their infancy. J Neurol Sci. 2019;399:3–5. doi: 10.1016/j.jns.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi T, Taniguchi Y, Sakai K, Kitamoto T, Takao M, Murayama S, et al. Significant association of cadaveric dura mater grafting with subpial Abeta deposition and meningeal amyloid angiopathy. Acta Neuropathol. 2016;132:313–315. doi: 10.1007/s00401-016-1588-3. [DOI] [PubMed] [Google Scholar]
- 13.Herve D, Porche M, Cabrejo L, Guidoux C, Tournier-Lasserve E, Nicolas G, et al. Fatal Abeta cerebral amyloid angiopathy 4 decades after a dural graft at the age of 2 years. Acta Neuropathol. 2018;135:801–803. doi: 10.1007/s00401-018-1828-9. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki Y, Imamura K, Iwai K, Kobayashi Y, Akagi A, Mimuro M, et al. Autopsied case of non-plaque-type dura mater graft-associated Creutzfeldt–Jakob disease presenting with extensive amyloid-beta deposition. Neuropathology. 2018;38:549–556. doi: 10.1111/neup.12503. [DOI] [PubMed] [Google Scholar]
- 15.Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature. 2015;525:247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- 16.Jaunmuktane Z, Quaegebeur A, Taipa R, Viana-Baptista M, Barbosa R, Koriath C, et al. Evidence of amyloid-beta cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol. 2018;135:671–679. doi: 10.1007/s00401-018-1822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs GG, Lutz MI, Ricken G, Strobel T, Hoftberger R, Preusser M, et al. Dura mater is a potential source of Abeta seeds. Acta Neuropathol. 2016;131:911–923. doi: 10.1007/s00401-016-1565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama Y, Mineharu Y, Arawaka Y, Nishida S, Tsuji H, Miyake H, et al. Cerebral amyloid angiopathy in a young man with a history of traumatic brain injury: a case report and review of the literature. Acta Neurochir (Wien) 2017;159:15–18. doi: 10.1007/s00701-016-3004-0. [DOI] [PubMed] [Google Scholar]
- 19.Purro SA, Farrow MA, Linehan J, Nazari T, Thomas DX, Chen Z, et al. Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature. 2018;564:415–419. doi: 10.1038/s41586-018-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purrucker JC, Hund E, Ringleb PA, Hartmann C, Rohde S, Schonland S, et al. Cerebral amyloid angiopathy—an underdiagnosed entity in younger adults with lobar intracerebral hemorrhage? Amyloid. 2013;20:45–47. doi: 10.3109/13506129.2012.746937. [DOI] [PubMed] [Google Scholar]
- 21.Raposo N, Planton M, Siegfried A, Calviere L, Payoux P, Albucher JF, et al. Amyloid-beta transmission through cardiac surgery using cadaveric dura mater patch. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2019-321927. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie DL, Adlard P, Peden AH, Lowrie S, Le Grice M, Burns K, et al. Amyloid-beta accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 2017;134:221–240. doi: 10.1007/s00401-017-1703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]