Abstract
Objectives
CACNA1A variants underlie three neurological disorders: familial hemiplegic migraine type 1 (FHM1), episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6). EEG is applied to study their episodic manifestations, but findings in the intervals did not gain attention up to date.
Methods
We analyzed repeated EEG recordings performed between 1994 and 2019 in a large cohort of genetically confirmed CACNA1A patients. EEG findings were compared with those of CACNA1A-negative phenocopies. A review of the related literature was performed.
Results
85 EEG recordings from 38 patients (19 EA2, 14 FHM1, 5 SCA6) were analyzed. Baseline EEG was abnormal in 55% of cases (12 EA2, 9 FHM1). The most common finding was a lateralized intermittent slowing, mainly affecting the temporal region. Slowing was more pronounced after a recent attack but was consistently detected in the majority of patients also during the follow-up. Interictal epileptic discharges (IEDs) were detected in eight patients (7 EA2,1 FHM1). EEG abnormalities and especially IEDs were significantly associated with younger age at examination (16 ± 9 vs 43 ± 21 years in those without epileptic changes, p = 0.003) and with earlier onset of disease (1 (1–2) vs 12 (5–45) years, p = 0.0009). EEG findings in CACNA1A-negative phenocopies (n = 15) were largely unremarkable (p = 0.03 in the comparison with CACNA1A patients).
Conclusions
EEG abnormalities between attacks are highly prevalent in episodic CACNA1A disorders and especially associated with younger age at examination and earlier disease onset. Our findings underpin an age-dependent effect of CACNA1A variants, with a more severe impairment when P/Q channel dysfunction manifests early in life.
Keywords: Voltage-gated calcium channels, Familial hemiplegic migraine type 1, Episodic ataxia type 2, Spinocerebellar ataxia type 6, Epilepsy, Intermittent epileptic discharges
Introduction
The gene CACNA1A encodes the pore-forming α1A subunit of the neuronal channel P/Q, which belongs to the superfamily of voltage-gated calcium channels [1]. P/Q channels are ubiquitous in the central nervous system and particularly abundant in cerebellar granules and Purkinje cells. P/Q channels play a pivotal role in neurotransmission due to their localization at the presynaptic terminals where they ensure the calcium-induced vesicular exocytosis of neurotransmitter. The selective biological functions of the voltage-gated calcium channels are determined by their specific α1 subunits. Indeed, the α1 subunit defines the channel-gating property and thus represents the main modulator of calcium currents. In this light, it is not surprising that CACNA1A gene is especially nontolerant to functional variants [2].
Ion channel variants typically underlie disorders with episodic manifestations. Accordingly, pathogenic CACNA1A variants have been initially identified in the setting of familial hemiplegic migraine type 1 (FHM1) and episodic ataxia type 2 (EA2) [3]. FHM1 is a monogenic migraine form characterized by motor deficits during the aura and is typically associated with gain-of-function CACNA1A variants [3]. EA2 manifests with self-limiting attacks of ataxia with dizziness, ocular disturbances, and dysarthria, and is generally caused by nonsense mutations or large rearrangements resulting in a loss-of-function [3]. Both disorders usually present in the first 2 decades and may display various degrees of phenotypic overlap. Beyond paroxysmal manifestations, mild to moderate chronic cerebellar signs can develop in the course of the disease in up to 75% of patients with FHM1 and EA2 [4, 5]. Interestingly, a late-onset chronic disorder, the spinocerebellar ataxia type 6 (SCA6), has been also linked to CACNA1A variants. SCA6 is underlined by an expanded polyglutamine tract in the C-terminal of the gene, a modification which does not primarily affect calcium currents [6, 7]. SCA6 shares with other spinocerebellar ataxias a similar phenotype with chronic progressive cerebellar dysfunction. Nevertheless, paroxysmal symptoms often occur at disease onset in SCA6 [8], resembling those of its allelic episodic disorders.
In the clinical approach to paroxysmal neurological disorders, electroencephalogram (EEG) is an essential part of the initial diagnostic workup. In the present study, we analyzed routine scalp EEGs (rsEEG) of a large cohort of genetically confirmed CACNA1A patients referring to the Center for Rare Movement Disorders at the Medical University of Innsbruck. Furthermore, we performed a comparison with EEG findings from CACNA1A phenocopies referring to our center and reviewed the current related literature on the topic. We aimed at defining the EEG pattern of CACNA1A-related disorders to support clinical evaluation and shed further light on the phenomenology resulting from P/Q channel dysfunction.
Methods
Patients
Patients with genetically confirmed CACNA1A disorders were retrieved from the database of the Center for Rare Movement Disorders at the Department of Neurology of the Medical University of Innsbruck. Additionally, CACNA1A-negative phenocopies were considered as the control group. Across the manuscript, families are labeled with Arabic numbers and family members with Roman numbers. Basic clinical and demographic data as well as findings from neurological examination were collected. Brain MR imaging was performed within the routine workup either at a 1.5 or 3 T scanner.
EEG evaluation
CACNA1A patients underwent rsEEG examinations within their regular clinical follow-up. We retrieved 85 rsEEG recordings which were performed between 1994 and 2019 either at the Department of Neurology or at the Department of Pediatrics of our university hospital, including the recordings from video EEG monitoring with scalp electrodes. All rsEEGs were recorded during wakefulness. EEG terminology report and interpretation were classified according to the definition by Kane et al. [9]. The following EEG findings were defined as abnormal EEG patterns: (a) abnormal changes in normal rhythm, (b) abnormal slow wave activity including theta- and/or delta-frequencies, and (c) interictal epileptiform discharges (IEDs). Each of these findings may be altered alone or in combination, may be localized (i.e., in one or two lobes), lateralized, bilateral or generalized and may occur continuously or intermittently.
EEG findings were analyzed by EI and reviewed by a senior neurologist with long-time experience in EEG evaluation (IU, MK, FZ or EH). Senior physicians were blinded to clinical diagnosis and medical history at the time of EEG.
Statistical analysis
We performed statistical analyses using SPSS version 25. Data are reported as mean ± SD or median (interquartile range) according to their distribution, which was tested by means of Kolmogorov–Smirnov test. Intergroup comparisons were performed by means of t test, Mann–Whitney-U and chi-squared test depending on the variable category. Statistical significance was set at p < 0.05.
Review of the literature
A Medline search was conducted applying the following keywords: CACNA1A, hemiplegic migraine, episodic ataxia, epilepsy, and electroencephalogram/EEG. Relevant references from the retrieved papers were also selected. We considered only the report of genetically confirmed CACNA1A cases.
Results
Innsbruck CACNA1A cohort: clinical history and neurological findings
Fifty-three genetically confirmed CACNA1A patients were registered in our database. We considered for the present study 38 patients (13 women, 25 men) who underwent rsEEG examination at least once. Twenty-five subjects have already been described in the previous publications of our group [10, 11]; 13 patients and 4 new CACNA1A variants are described herein for the first time. Clinical and demographic data are summarized in Table 1.
Table 1.
Clinical features of Innsbruck CACNA1A patients who underwent EEG examination
| Family | Genotype | Phenotype | Pt. ID | Age at onset | Age at first EEG | Chronic cerebellar signs | Cerebellar atrophy at MRI | Pathologic findings at first EEG | IEDsa |
|---|---|---|---|---|---|---|---|---|---|
| 1 | R1667W | FHM1 | 1-I* | 12 | 56 | x | x | x | |
| 1-II | 34 | 42 | x | x | x | ||||
| 1-III | 58 | 58 | x | x | x | ||||
| 1-IV | 1 | 11 | x | x | x | x | |||
| 2 | c.3102 + 2 T > C | EA2 | 2-I | 1 | 28 | x | x | x | x |
| 2-II* | 27 | 37 | x | x | x | ||||
| 3 | T666M | FHM1 | 3-I | 40 | 71 | x | x | ||
| 3-II | 5 | 47 | x | x | x | ||||
| 3-III | 13 | 42 | x | x | |||||
| 3-IV | 4 | 18 | x | x | |||||
| 3-V | 7 | 10 | x | x | x | ||||
| 3-VI | 1,5 | 2 | na | ||||||
| 3-VII* | 11 | 11 | x | x | |||||
| 3-VIII* | 1,5 | 3 | x | na | |||||
| 4 | c.3089 + 2 T > C | EA2 | 4-I | 10 | 54 | x | x | ||
| 4-II | 45 | 54 | x | x | x | ||||
| 4-III | 2 | 15 | x | x | x | ||||
| 4-IV | 10 | 20 | x | X | x | ||||
| 4-V | 1 | 26 | x | x | x | ||||
| 4-VI* | 1 | 3 | na | x | x | ||||
| 5 | R198Q | EA2 | 5-I | 1 | 22 | x | x | x | x |
| 6 | S218L | FHM1 | 6-I | 1 | 6 | x | x | x | |
| 7 | c.959G > A | EA2 | 7-I | 8 | 48 | x | x | ||
| 8 | c.3603dup | EA2 | 8-I | 7 | 45 | x | x | ||
| 9 | G540R | EA2 | 9-I | 1,5 | 18 | x | x | ||
| 10 | C1869R | EA2 | 10-I* | 36 | 41 | x | x | x | |
| 11 | I239T** | EA2 | 11-I* | 61 | 68 | x | x | ||
| 11-II* | 55 | 73 | x | x | |||||
| 12 | R2248H** | EA2 | 12-I* | 45 | 41 | x | x | x | |
| 13 | D2173Y** | EA2 | 13-I* | 50 | 52 | x | x | x | |
| 14 | T666M | FHM1 | 14-I* | 2 | 41 | x | x | x | |
| 15 | A754V** | EA2 | 15-I* | 1 | 1 | x | |||
| 16 | Q1154X | EA2 | 16-I* | 3 | 64 | x | x | x | |
| 17 | 12/23 | SCA6 | 17-I | 55 | 67 | x | x | ||
| 13/23 | 17-II | 40 | 62 | x | x | ||||
| 14/23 | 17-III | 47 | 48 | x | x | ||||
| 18 | SCA6 | 18-I | 63 | 67 | x | x | |||
| 19 | 8/23 | SCA6 | 19-I | 36 | 39 | x | x |
IEDs: interictal epileptic discharges
*These patients have been described herein for the first time
**Newly reported CACNA1A variants
aBoth first EEG and follow-up EEGs are considered
Our CACNA1A cohort comprehended 14 patients from 4 FHM1 families, 19 patients from 12 EA2 families, and 5 patients from 3 SCA6 families. Mean age at examination was 37 ± 22 years (range 1–73 years). Episodic symptoms were reported by 25 patients at the time of referral (66%) and 7 other patients (18%) had experienced episodic symptoms at the beginning of the disease. Thirty-three patients (87%) showed chronic cerebellar signs at the neurological examination.
Brain MR imaging was available for all patients but two. In 35 cases (92%), cerebellar atrophy was evident. The degree of cerebellar atrophy was rather mild in the majority of FHM1 and EA2 patients as well as mostly limited to the vermis.
First EEG findings
A majority of patients were drug-naive at the time of the first rsEEG (92%, n = 34). Of the remaining 4, n = 3 were on acetazolamide and n = 1 on flunarizine. Seven patients (18%) had experienced attacks in the week preceding the examination.
Overall, pathological EEG findings were detected in 21 patients (55%; 11 FHM1, 10 EA2). Clue pathologic findings consisted of an intermittent slowing in delta–theta-frequency range (n = 19, 90%). In one further patient, EEG displayed a continuous left lateralized hemispheric slowing indicative of a recent attack. Delta–theta activity was lateralized in five cases (n = 3 left hemisphere, n = 2 right hemisphere), bilateral in nine cases (of which four with more pronounced slowing in the left hemisphere) and generalized in seven cases. In the majority of the cases, delta–theta activity involved more markedly or selectively the temporal region (n = 13, 62%). Three patients (6%) had IEDs in their first EEG (see Tables 2 and 3 for details).
Table 2.
Synopsis of pathological findings at first rsEEG
| Pt. ID | Phenotype | Age | Background rhythm | Intermittent slowing (frequency range) | Continuous slowing (frequency range) | Bilateral or generalized | Lateralized (R/L) | Regional (F/C/T/P/O) | IEDs |
|---|---|---|---|---|---|---|---|---|---|
| 1-I | FHM1 | 56 | A | T/D | Bilateral | FT | |||
| 1-II | FHM1 | 42 | A | D/T | Non lateralized/bilateral | L > R | T | ||
| 1-III | FHM1 | 58 | A | D/T | L | ||||
| 1-IV | FHM1 | 11 | A | T | Bilateral | FT | |||
| 2-I | EA2 | 28 | A | T/D | Generalized | ||||
| 2-II | EA2 | 37 | A | D/T | Generalized | ||||
| 3-II | FHM1 | 47 | A | T/D | Bilateral | T | |||
| 3-V | FHM1 | 10 | A | T/D | L | TP | |||
| 3-VII | FHM1 | 11 | A | T/D | L | T | |||
| 4-II | EA2 | 54 | A | T | Generalized | ||||
| 4-III | EA2 | 15 | A | T/D | Generalized | yes | |||
| 4-V | EA2 | 26 | A | D/T | Non lateralized/bilateral | L > R | T | yes | |
| 4-VI | EA2 | 3 | T | Generalized | yes | ||||
| 5-I | EA2 | 22 | A | T | Bilateral | T | |||
| 6-I | FHM1 | 6 | D | D | R | FT | |||
| 9-I | EA2 | 18 | A | T | Bilateral | T | |||
| 10-I | EA2 | 41 | A | D/T | Generalized | ||||
| 12-I | EA2 | 41 | A | T | Generalized | ||||
| 13-I | EA2 | 52 | A | D/T | Non lateralized/bilateral | L > R | T | ||
| 14-I | FHM1 | 41 | A | T | R | T | |||
| 16-I | EA2 | 64 | A | T/D | Non lateralized/bilateral | L > R | T |
Concerning frequencies: A alpha, T theta, D delta; concerning localization: L and R = left and right hemispheres respectively, F = frontal, C = central, T = temporal, P = parietal, and O = occipital regions. IEDs: intermittent epileptic discharges. When both T and D frequencies were detected, the one that recurred most in EEG is reported after the slash. The slowing can occur continuously or intermittently; it can be generalized, bilateral or lateralized. In case of bilateral slowing, the presence of a side difference is also reported (for example L > R). Bilateral or lateralized slowing can also affect specifically one or two lobes (regional slowing)
Table 3.
Interictal epileptic discharges (IEDs) in CACNA1A patients
| Pt. ID | Phenotype | Age at onset | Age at EEG | IEDs |
|---|---|---|---|---|
| 1-IV | FHM1 | 1 | 11 | Bilateral frontal sharp waves |
| 2-I | EA2 | 1 | Childhood* | Generalized spike waves |
| 4-III | EA2 | 2 | 15 | Generalized spike wave complexes |
| 4-IV | EA2 | 10 | 20 | Superimposed spikes in generalized rhythmic delta |
| 4-V | EA2 | 1 | 26 | Left temporal sharp waves |
| 4-VI | EA2 | 1 | 3 | Superimposed spikes in generalized rhythmic delta |
| 5-I | EA2 | 1 | 30 | Left temporal sharp waves |
| 15-I | EA2 | 1 | 6 | Superimposed spikes in generalized rhythmic delta |
*The first rsEEG at our department was performed at 28 years of age. IEDs were documented in previous EEGs
Analyzing CACNA1A subgroups, all five SCA6 patients had normal EEG, while FHM1 and EA2 patients had a similar frequency of unspecific slowing. IEDs at the first EEG were detected only in EA2 patients. Although EEG findings were more severe in patients with recent attacks as compared to patients without recent attacks, this difference was not statistically significant (p = 0.2). Interestingly, age at examination was lower in patients with pathological EEG findings (32 ± 19 vs 43 ± 25 years in those with normal EEG, p = 0.1, non-significant). Moreover, patients with pathological EEG findings had an earlier onset of symptoms as compared to those with normal EEGs (16 ± 19 years of age at onset vs 26 ± 25, p = 0.1, non-significant).
EEG findings during an attack
Three EA2 patients (Pt. 4-V, 9-I, 13-I) were admitted to the hospital during an attack because of severe neurological impairment and underwent in-patient prolonged video-EEG monitoring. In this setting, several habitual attacks were documented. During clinically observed attacks EEG was unremarkable. Four FHM1 patients underwent EEG recordings during migraine attacks (Pt. 3-I, 3-IV, 3-V, 6-I). In these cases, a continuous delta activity was documented over the hemisphere contralateral to their aura symptoms.
Follow-up EEG findings
Eighteen patients underwent repeated rsEEG examinations during the disease course.
Short-term follow-up in two patients (Pt. 3-VII and 9), 1–2 months after the first rsEEG, which in both cases was performed after an attack, showed unchanged slowing. Another short-time rsEEG follow-up after starting acetazolamide remained without improvement as compared to the first rsEEG (Pt. 2-II).
In the remaining patients repeated rsEEG examinations were performed during a mean follow-up of 9 ± 5 years. In five patients (36%) the findings remained unchanged. Other five patients (36%) displayed a worsening consisting of appearing of slowing, whereas the first rsEEG findings were unremarkable. In the four remaining patients (28%) findings normalized in follow-up EEGs. Notably, these latter cases reported a marked reduction or even ceasing of attacks.
IEDs were documented for the first time during the follow-up in four further patients (see Table 3).
Epilepsy suspicion and IEDs
Five patients received a diagnosis of epilepsy prior to admission at our center. Three of them were on antiepileptic therapy (Pt. 3-I valproate, 4-III phenytoin, 6-I phenobarbital) at the time of referral. Eventually, none of the five patients had a convincing history of seizures and diagnosis of epilepsy was not confirmed during the work-up. Antiepileptic drugs were stopped after the diagnosis of FHM1/EA2.
Conversely, IEDs were detected at different time points in eight patients (21%, 7 EA2 and 1 FHM1, see also Table 3 and Fig. 1). None of them met the diagnostic criteria for epilepsy [12]. In one case (Pt. 15-I), clinical history was suggestive of absences, though no correlating EEG changes were detected in repeated in-patient stays and video-EEG monitoring. Patients with IEDs were significantly younger (16 ± 9 years of age at examination vs 43 ± 21 in those without IEDs, p = 0.003) and had a significantly lower median age at onset (1(1–2) years vs 12(5–45) in those without IEDs, p = 0.0009) (Figs. 2, 3, 4, 5).
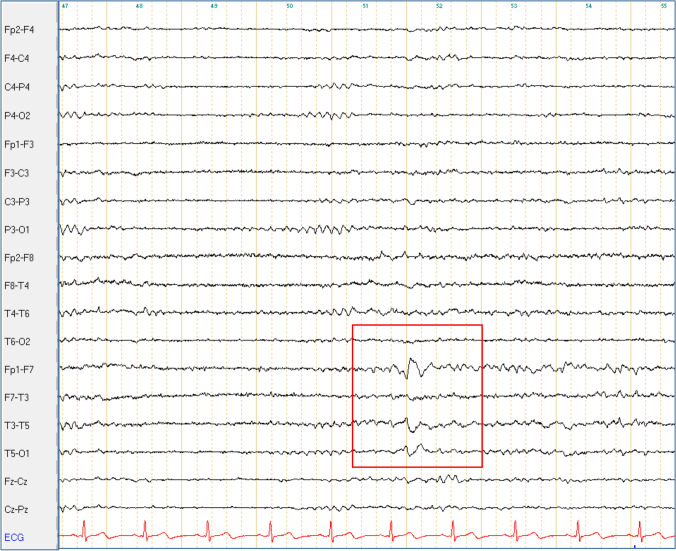
Fig. 1.
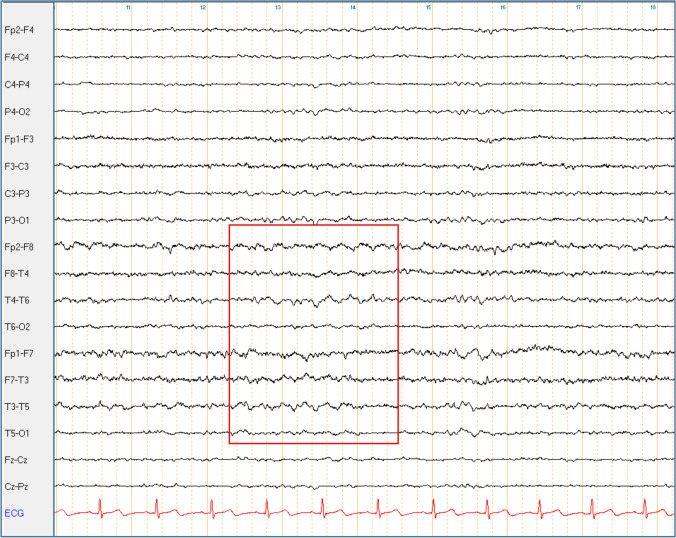
Bitemporal delta activity in Pt. 5-I. Bipolar longitudinal montage with 70 Hz filter and time constant of 0.3 s; sensitivity 7 μV
Fig. 2.
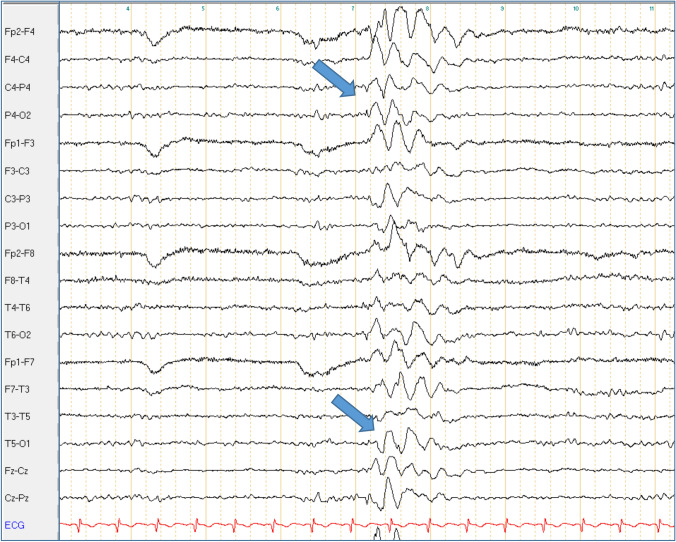
Superimposed bilateral occipital spikes in generalized rhythmic delta activity in Pt. 4-IV. Bipolar longitudinal montage with 70 Hz filter and time constant of 0.3 s; sensitivity 7 μV
Fig. 3.
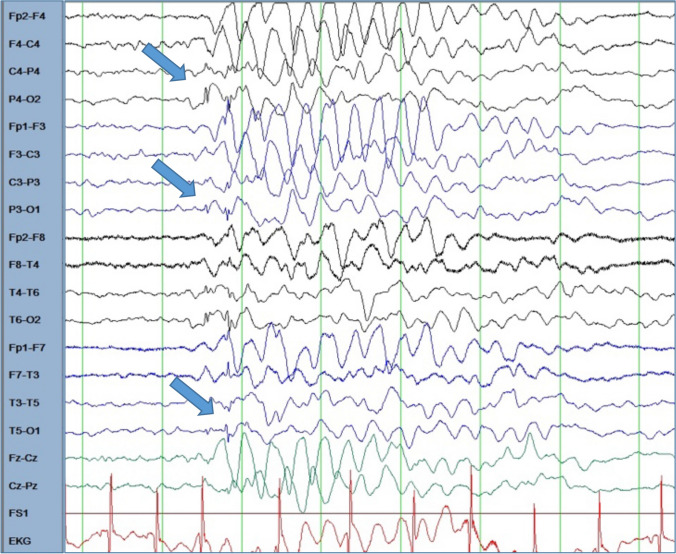
Superimposed bilateral occipital spikes in generalized rhythmic delta activity in Pt. 4-VI. Bipolar longitudinal montage with 70 Hz filter and time constant of 0.3 s; sensitivity 10 μV
Fig. 4.
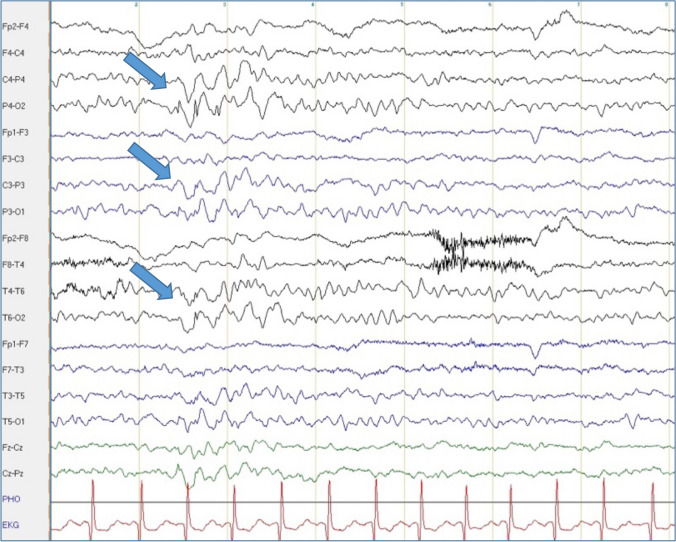
Superimposed bilateral occipital spikes in generalized rhythmic delta activity in Pt. 15-I. Bipolar longitudinal montage with 70 Hz filter and time constant of 0.3 s; sensitivity 10 μV
Fig. 5.
Left temporal sharp waves in Pt. 5-I. Bipolar longitudinal montage with 70 Hz filter and time constant of 0.3 s; sensitivity 7 μV
During the follow-up, a single generalized tonic–clonic seizure was observed during a severe hemiplegic migraine attack in a patient with the S218L mutation (Pt. 6-I). He never displayed epileptic changes in EEG nor had seizures independently from hemiplegic attacks.
Comparison with CACNA1A phenocopies
We retrieved from our database 18 patients with episodic ataxia or sporadic hemiplegic migraine which had been tested negative for CACNA1A variants. Fifteen of them underwent EEG examinations at least once and were thus considered for this study (n = 10 with EA phenotype, n = 3 with sporadic HM, n = 2 overlapping phenotype). CACNA1A-negative phenocopies were significantly older than CACNA1A patients (median age at EEG 59 (29; 67) years in phenocopies vs 39 (12; 51) in CACNA1A patients, p = 0.008).
In all but four cases, who displayed intermittent bilateral temporal slowing (3 cases theta, 1 case delta), EEG findings were unremarkable. Hence, in the comparison with the CACNA1A cohort, CACNA1A-negative phenocopies had a significantly lower frequency of abnormal findings (p = 0.03).
Review of the literature
The literature search yielded 57 pertinent reports. Notably, available EEG findings were mostly limited to the description of changes occurring during attacks or documentation of IEDs.
Concerning FHM1, several reports described EEG findings during attacks consisting mostly of severe slowing contralateral to hemiparesis [13–22]. Fewer descriptions of EEG findings beyond the attacks are available. These were reported to be normal in several cases [21, 23–27]. In single case, a residual unspecific focal slowing in the hemisphere contralateral to aura symptoms has been reported [13, 14]. Seizures concomitant to severe hemiplegic attacks have been described in at least 16 families [5, 13–17, 19, 20, 22, 24, 27–34]. Seven of these families (44%) harbored the genotype S218L, which is associated with severe attacks with coma—or even death—induced by minor head trauma [14, 16, 19, 29, 31, 33, 34]. Other genotypes recurrently associated with a seizure during attacks were R1349Q (n = 3, 19%) [20, 27, 32] and Y1385C (n = 2, 12%) [5, 22]. Epilepsy or seizures occurring independently from hemiplegic migraine have been described in at least eight pedigrees [15, 24, 28, 30, 35]. In only two of these cases IEDs were documented [15, 35].
Concerning EA2, numerous reports described IEDs [36–42]. Very few cases reported additional information on EEG findings, which were reported to be normal [43] or with interposed slowing[36, 40]. Descriptions of EEG findings during an attack are lacking. In a report by Labrum et al. EEG during an attack did not differ from baseline EEG in a patient with EA2[40]. In at least 21 EA2 families epilepsy/seizures occurred independently from episodic ataxia [4, 20, 36–41, 44–51]. In virtually all cases, epilepsy manifested in the childhood and preceded episodic ataxia. Absences represented the most common manifestations (n = 12, 57%) [20, 36–41, 48, 50, 51]. In 10 out of these 21 families, IEDs could be detected [36–41]. Notably, IEDs were also detected in several other EA2 families with no history of seizures [41, 42, 52, 53]. Generalized 3 Hz spike-wave discharges were a recurrent EEG pattern [37, 39, 41, 54, 55].
Discussion
In the present work, we systematically analyzed repeated rsEEG recordings of a large cohort of genetically confirmed CACNA1A patients, which were collected during a long-term clinical follow-up. In our CACNA1A cohort, 55% of the baseline EEGs showed abnormal findings. The key pathological findings consisted of a variable slowing, preferentially affecting the temporal lobe. Generally, slowing pattern was more pronounced following recent attacks. A normalization of EEG findings was mostly observed in association with clinical remission of episodic symptoms. However, during follow-up intermittent lateralized slowing was present in a majority of patients. IEDs were recorded in 21% of patients. Overall, earlier onset of disease was associated with EEG abnormalities, especially IEDs. None of our patients met the diagnostic criteria of epilepsy. Looking at the genotype, all SCA6 patients had normal EEG findings, while EA2 and FHM1 patients had a similar frequency of abnormal findings.
Historically, EEG has been applied in the differential diagnosis of “acetazolamide-responsive periodic ataxia” from epilepsy before a genetic allocation was possible [56]. After the identification of CACNA1A mutations, a growing body of literature focused on the co-occurrence of epileptic disorders in FHM1 and EA2 patients. Though, most of the evidence of an association between CACNA1A disorders and epilepsy originated from small case series. Systematic electrophysiological studies in larger collectives have been not carried out to date. By analyzing a genetically assigned large single-center cohort, we were able to detect an EEG pattern across two CACNA1A genotypes, consisting of a recurrent lateralized EEG slowing in the interval between the attacks. Even if non-specific, this EEG pattern discriminated CACNA1A patients from CACNA1A-negative phenocopies in our study. We hypothesize that the chronic EEG abnormalities in CACNA1A patients primarily reflect a functional disruption of cerebro-cerebellar connections. The occurrence of such network disturbances has been theorized upon observation that FHM1 and EA2 patients frequently display cognitive impairment, developmental delay and behavioral disorders (6). Indeed, cerebellar tuning extends beyond motor control to cognitive and emotional processing through multiple connections with associative areas, especially prefrontal and temporal (58). To date, powerful structural correlates of functional disruption of cerebro-cerebellar pathways are lacking. Indeed, supratentorial structures appear to be spared at conventional brain imaging [57]. Therefore, our findings strengthen the pivotal role of electrophysiology in the clinical workup of CACNA1A disorders, even in the interval between attacks.
IEDs were detected in seven EA2 and one FHM1 patients at different time points. All but one were children or young adults with EA2. Interestingly, IEDs in EA2 were recorded without the evidence of a clinical attack during video-monitoring in our cohort and were not recorded in the patients that underwent long-term follow-up and were beyond their mid-20s. These findings are similar to evidence collected in the literature, which recurrently reported IEDs and or seizures in EA2 during childhood, sometimes before the development of episodic ataxia and chronic cerebellar signs [40, 44–46, 50, 51]. Generalized epileptic seizures may also occur in FHM1, as in one of our patients. A recurrent association between severe migraine attacks with prolonged aura accompanied by hemiplegia, aphasia or coma was associated with specific genotypes such as the S218L mutation (see also patient 6-I) [14, 16, 19, 34]. Concomitant and even occasionally fatal brain edema seen in these so-called “malignant” FHM1 mutations imply that these seizures are symptomatic [29]. Interestingly, de novo CACNA1A mutations have been recently associated with a sporadic early infantile epileptic encephalopathy (the so-called EIEE42) resulting in a severe clinical phenotype with developmental delay and epilepsy (2). One of our patients (6-I) displayed as child such a severe phenotype that could be classified as EIEE42 within the spectrum of CACNA1A mutations. If EIEE42 has to be considered a separate “epileptic” entity or rather an age-dependent manifestation of CACNA1A channelopathy will be part of the future studies.
It is widely established that P/Q channels are ubiquitary but unequivocally distributed in the brain. In supratentorial regions, there is especially evidence of their expression in the hippocampus, where their alteration in human specimens and in animal models has been associated to epileptogenesis [58–60], as well as in thalamic neurons [61]. Pivotal studies in murine models showed that thalamic mediated arousal upon stimuli is coupled by the activation of P/Q channels [61]. The depolarization of P/Q channels in the thalamic neurons promotes high-frequency membrane oscillations which facilitate synaptic inputs and the transition in that low-voltage fast activity, the so-called gamma waves, typical of the attentive state and engagement in cognitive tasks [62]. In mice lacking P/Q channel, a residual excitatory transmission between thalamus and cortex is detected, but it is insufficient to maintain high-frequency oscillations [61]. Furthermore, the P/Q-channel deficient mice display bursts of spike and wave activity, resembling an absence status. This finding supports a selective susceptibility for IEDs in the presence of loss-of-function of P/Q channels. Although a propagation of epileptic activity from subcortical structures to the cortex in humans is unusual, impaired P/Q function in CACNA1A disorders may result in such a phenomenon. Both, the presence of such propagation and remission of IEDs with aging and maturation of the brain as pathophysiological substrate for EEG abnormalities in our cohort remain speculative.
Cumulatively, our findings show that in patients with episodic ataxia and hemiplegic migraine EEG slowing between the attacks and intermittent IEDs during childhood and adolescence are indicative—together with a positive family history—of a CACNA1A mutation. While in the presence of a positive family history diagnosis is straightforward, in sporadic or late onset cases with non-informative family history, detection of EEG abnormalities may support the choice of a genetic screening. Also, persistence of slowing in the weeks after attacks appears useful to discriminate CACNA1A-related hemiplegic migraine.
The pathophysiological background of the EEG abnormalities in the setting of P/Q dysfunction in humans remains elusive. Anyhow, the present findings support the increasing evidence that functional pathologic changes expand beyond the cerebellar circuitry in non-polyglutamine CACNA1A diseases and strongly suggest an age-dependent effect of P/Q dysfunction.
Future studies are advocated to gain further knowledge on the functional changes related to CACNA1A variants in vivo as well as to shed light on the clinical course of early-onset phenotypes.
Author contributions
EI and SB conceived the study; EI, SB, WN, AE, and MA collected the clinical data; EI, IU, MK, FZ, and EH analyzed EEG data; EI performed statistical analysis; EI and SB wrote the first draft which was reviewed and approved by all authors.
Funding
Open Access funding provided by University of Innsbruck and Medical University of Innsbruck. No funding to report related to the present research.
Availability of data
The dataset analyzed in the current study is available upon request. All newly reported CACNA1A mutations have been submitted to ClinVar at the time of their detection.
Compliance with ethical standards
Conflict of interest
There are no competing interests related to the present work.
Ethical approval
All investigations were conducted in accordance with the Declaration of Helsinki on ethical principles for medical research involving human subjects. The present study consists of a retrospective data analysis and was approved by the ethics committee of the Medical University of Innsbruck (Nr. 1022/2020). Written informed consent was given by the patients and their legal guardians prior to performing genetic testing.
Consent to participate
Not applicable since the present study consists of a retrospective data analysis.
Consent for publication
Traceable patients gave their written consent to publication.
Footnotes
Elisabetta Indelicato, Wolfgang Nachbauer, Andreas Eigentler, Matthias Amprosi, Sylvia Boesch are members of the European Reference Network for Rare Neurological Diseases—Project ID No 739510.
References
- 1.Rajakulendran S, Kaski D, Hanna MG. Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS. Nat Rev Neurol. 2012;8(2):86–96. doi: 10.1038/nrneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- 2.Epi KC. De Novo Mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet. 2016;99(2):287–298. doi: 10.1016/j.ajhg.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 4.Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology. 2004;62(1):17–22. doi: 10.1212/01.wnl.0000101675.61074.50. [DOI] [PubMed] [Google Scholar]
- 5.Ducros A, Denier C, Joutel A, Cecillon M, Lescoat C, Vahedi K, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345(1):17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 6.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15(1):62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 7.Bavassano C, Eigentler A, Stanika R, Obermair GJ, Boesch S, Dechant G, et al. Bicistronic CACNA1A gene expression in neurons derived from spinocerebellar ataxia type 6 patient-induced pluripotent stem cells. Stem Cells Dev. 2017;26(22):1612–1625. doi: 10.1089/scd.2017.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jen JC, Yue Q, Karrim J, Nelson SF, Baloh RW. Spinocerebellar ataxia type 6 with positional vertigo and acetazolamide responsive episodic ataxia. J Neurol Neurosurg Psychiatry. 1998;65(4):565–568. doi: 10.1136/jnnp.65.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane N, Acharya J, Benickzy S, Caboclo L, Finnigan S, Kaplan PW, et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract. 2017;2:170–185. doi: 10.1016/j.cnp.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indelicato E, Nachbauer W, Karner E, Eigentler A, Wagner M, Unterberger I, et al. The neuropsychiatric phenotype in CACNA1A mutations: a retrospective single center study and review of the literature. Eur J Neurol. 2019;26(1):66–e7. doi: 10.1111/ene.13765. [DOI] [PubMed] [Google Scholar]
- 11.Nachbauer W, Nocker M, Karner E, Stankovic I, Unterberger I, Eigentler A, et al. Episodic ataxia type 2: phenotype characteristics of a novel CACNA1A mutation and review of the literature. J Neurol. 2014;261(5):983–91. doi: 10.1007/s00415-014-7310-2. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 13.Beauvais K, Cave-Riant F, De Barace C, Tardieu M, Tournier-Lasserve E, Furby A. New CACNA1A gene mutation in a case of familial hemiplegic migraine with status epilepticus. Eur Neurol. 2004;52(1):58–61. doi: 10.1159/000079546. [DOI] [PubMed] [Google Scholar]
- 14.Chan YC, Burgunder JM, Wilder-Smith E, Chew SE, Lam-Mok-Sing KM, Sharma V, et al. Electroencephalographic changes and seizures in familial hemiplegic migraine patients with the CACNA1A gene S218L mutation. J Clin Neurosci. 2008;15(8):891–4. doi: 10.1016/j.jocn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.de Vries B, Stam AH, Beker F, van den Maagdenberg AM, Vanmolkot KR, Laan L, et al. CACNA1A mutation linking hemiplegic migraine and alternating hemiplegia of childhood. Cephalalgia. 2008;28(8):887–91. doi: 10.1111/j.1468-2982.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- 16.Debiais S, Hommet C, Bonnaud I, Barthez MA, Rimbaux S, Riant F, et al. The FHM1 mutation S218L: a severe clinical phenotype? A case report and review of the literature. Cephalalgia. 2009;29(12):1337–9. doi: 10.1111/j.1468-2982.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia Segarra N, Gautschi I, Mittaz-Crettol L, Kallay Zetchi C, Al-Qusairi L, Van Bemmelen MX, et al. Congenital ataxia and hemiplegic migraine with cerebral edema associated with a novel gain of function mutation in the calcium channel CACNA1A. J Neurol Sci. 2014;342(1–2):69–78. doi: 10.1016/j.jns.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Ohmura K, Suzuki Y, Saito Y, Wada T, Goto M, Seto S. Sporadic hemiplegic migraine presenting as acute encephalopathy. Brain Dev. 2012;34(8):691–5. doi: 10.1016/j.braindev.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Stam AH, Luijckx GJ, Poll-The BT, Ginjaar IB, Frants RR, Haan J, et al. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. J Neurol Neurosurg Psychiatry. 2009;80(10):1125–9. doi: 10.1136/jnnp.2009.177279. [DOI] [PubMed] [Google Scholar]
- 20.Tantsis EM, Gill D, Griffiths L, Gupta S, Lawson J, Maksemous N, et al. Eye movement disorders are an early manifestation of CACNA1A mutations in children. Dev Med Child Neurol. 2016;58(6):639–44. doi: 10.1111/dmcn.13033. [DOI] [PubMed] [Google Scholar]
- 21.Topakian R, Pischinger B, Stieglbauer K, Pichler R. Rare clinical findings in a patient with sporadic hemiplegic migraine: FDG-PET provides diminished brain metabolism at 10-year follow-up. Cephalalgia. 2014;34(5):392–6. doi: 10.1177/0333102413513182. [DOI] [PubMed] [Google Scholar]
- 22.Vahedi K, Denier C, Ducros A, Bousson V, Levy C, Chabriat H, et al. CACNA1A gene de novo mutation causing hemiplegic migraine, coma, and cerebellar atrophy. Neurology. 2000;55(7):1040–2. doi: 10.1212/wnl.55.7.1040. [DOI] [PubMed] [Google Scholar]
- 23.Kors EE, Haan J, Giffin NJ, Pazdera L, Schnittger C, Lennox GG, et al. Expanding the phenotypic spectrum of the CACNA1A gene T666M mutation: a description of 5 families with familial hemiplegic migraine. Arch Neurol. 2003;60(5):684–8. doi: 10.1001/archneur.60.5.684. [DOI] [PubMed] [Google Scholar]
- 24.Kors EE, Melberg A, Vanmolkot KR, Kumlien E, Haan J, Raininko R, et al. Childhood epilepsy, familial hemiplegic migraine, cerebellar ataxia, and a new CACNA1A mutation. Neurology. 2004;63(6):1136–7. doi: 10.1212/01.wnl.0000138571.48593.fc. [DOI] [PubMed] [Google Scholar]
- 25.Blumkin L, Michelson M, Leshinsky-Silver E, Kivity S, Lev D, Lerman-Sagie T. Congenital ataxia, mental retardation, and dyskinesia associated with a novel CACNA1A mutation. J Child Neurol. 2010;25(7):892–7. doi: 10.1177/0883073809351316. [DOI] [PubMed] [Google Scholar]
- 26.Liguori C, Albanese M, Sancesario G, Stefani A, Marciani MG, Pierantozzi M. May a suspicious psychiatric disorder hide sporadic hemiplegic migraine? Genetic test as prompting factor for diagnosis. Neurol Sci. 2013;34(10):1845–6. doi: 10.1007/s10072-013-1325-9. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Albisua I, Schoning M, Jurkat-Rott K, Lerche H. Possible effect of corticoids on hemiplegic attacks in severe hemiplegic migraine. Pediatr Neurol. 2013;49(4):286–8. doi: 10.1016/j.pediatrneurol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Riant F, Ducros A, Ploton C, Barbance C, Depienne C, Tournier-Lasserve E. De novo mutations in ATP1A2 and CACNA1A are frequent in early-onset sporadic hemiplegic migraine. Neurology. 2010;75(11):967–72. doi: 10.1212/WNL.0b013e3181f25e8f. [DOI] [PubMed] [Google Scholar]
- 29.Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, et al. Delayed cerebral edema and fatal coma after minor head trauma: role of the CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49(6):753–60. doi: 10.1002/ana.1031. [DOI] [PubMed] [Google Scholar]
- 30.Stam AH, Vanmolkot KR, Kremer HP, Gartner J, Brown J, Leshinsky-Silver E, et al. CACNA1A R1347Q: a frequent recurrent mutation in hemiplegic migraine. Clin Genet. 2008;74(5):481–5. doi: 10.1111/j.1399-0004.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 31.Carreno O, Garcia-Silva MT, Garcia-Campos O, Martinez-de Aragon A, Cormand B, Macaya A. Acute striatal necrosis in hemiplegic migraine with de novo CACNA1A mutation. Headache. 2011;51(10):1542–6. doi: 10.1111/j.1526-4610.2011.02014.x. [DOI] [PubMed] [Google Scholar]
- 32.Malpas TJ, Riant F, Tournier-Lasserve E, Vahedi K, Neville BG. Sporadic hemiplegic migraine and delayed cerebral oedema after minor head trauma: a novel de novo CACNA1A gene mutation. Dev Med Child Neurol. 2010;52(1):103–4. doi: 10.1111/j.1469-8749.2009.03493.x. [DOI] [PubMed] [Google Scholar]
- 33.Zangaladze A, Asadi-Pooya AA, Ashkenazi A, Sperling MR. Sporadic hemiplegic migraine and epilepsy associated with CACNA1A gene mutation. Epilepsy Behav. 2010;17(2):293–5. doi: 10.1016/j.yebeh.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki S, Ikeno K, Abe T, Tohyama J, Adachi Y. Hemiconvulsion-hemiplegia-epilepsy syndrome associated with CACNA1A S218L mutation. Pediatr Neurol. 2011;45(3):193–6. doi: 10.1016/j.pediatrneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Romaniello R, Zucca C, Tonelli A, Bonato S, Baschirotto C, Zanotta N, et al. A wide spectrum of clinical, neurophysiological and neuroradiological abnormalities in a family with a novel CACNA1A mutation. J Neurol Neurosurg Psychiatry. 2010;81(8):840–3. doi: 10.1136/jnnp.2008.163402. [DOI] [PubMed] [Google Scholar]
- 36.Damaj L, Lupien-Meilleur A, Lortie A, Riou E, Ospina LH, Gagnon L, et al. CACNA1A haploinsufficiency causes cognitive impairment, autism and epileptic encephalopathy with mild cerebellar symptoms. Eur J Hum Genet. 2015;23(11):1505–12. doi: 10.1038/ejhg.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imbrici P, Jaffe SL, Eunson LH, Davies NP, Herd C, Robertson R, et al. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127(Pt 12):2682–92. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- 38.Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, Kullmann DM, et al. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358(9284):801–7. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- 39.Jung J, Testard H, Tournier-Lasserve E, Riant F, Vallet AE, Berroir S, et al. Phenotypic variability of episodic ataxia type 2 mutations: a family study. Eur Neurol. 2010;64(2):114–6. doi: 10.1159/000315145. [DOI] [PubMed] [Google Scholar]
- 40.Labrum RW, Rajakulendran S, Graves TD, Eunson LH, Bevan R, Sweeney MG, et al. Large scale calcium channel gene rearrangements in episodic ataxia and hemiplegic migraine: implications for diagnostic testing. J Med Genet. 2009;46(11):786–91. doi: 10.1136/jmg.2009.067967. [DOI] [PubMed] [Google Scholar]
- 41.Rajakulendran S, Graves TD, Labrum RW, Kotzadimitriou D, Eunson L, Davis MB, et al. Genetic and functional characterisation of the P/Q calcium channel in episodic ataxia with epilepsy. J Physiol. 2010;588(Pt 11):1905–13. doi: 10.1113/jphysiol.2009.186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaido M, Furuta M, Nakamori M, Yuasa Y, Takahashi MP. Episodic ataxia type 2 manifests as epileptiform electroencephalographic activity with no epileptic attacks in two family members. Rinsho Shinkeigaku. 2016;56(4):260–4. doi: 10.5692/clinicalneurol.cn-000854. [DOI] [PubMed] [Google Scholar]
- 43.Yugrakh MS, Levy OA. Clinical reasoning: a middle-aged man with episodes of gait imbalance and a newly found genetic mutation. Neurology. 2012;79(16):e135–9. doi: 10.1212/WNL.0b013e31826e9b40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi JH, Seo JD, Choi YR, Kim MJ, Shin JH, Kim JS, et al. Exercise-induced downbeat nystagmus in a Korean family with a nonsense mutation in CACNA1A. Neurol Sci. 2015;36(8):1393–6. doi: 10.1007/s10072-015-2157-6. [DOI] [PubMed] [Google Scholar]
- 45.Choi KD, Yook JW, Kim MJ, Kim HS, Park YE, Kim JS, et al. Possible anticipation associated with a novel splice site mutation in episodic ataxia type 2. Neurol Sci. 2013;34(9):1629–32. doi: 10.1007/s10072-013-1298-8. [DOI] [PubMed] [Google Scholar]
- 46.Imbrici P, Eunson LH, Graves TD, Bhatia KP, Wadia NH, Kullmann DM, et al. Late-onset episodic ataxia type 2 due to an in-frame insertion in CACNA1A. Neurology. 2005;65(6):944–6. doi: 10.1212/01.wnl.0000176069.64200.28. [DOI] [PubMed] [Google Scholar]
- 47.Kaunisto MA, Harno H, Kallela M, Somer H, Sallinen R, Hamalainen E, et al. Novel splice site CACNA1A mutation causing episodic ataxia type 2. Neurogenetics. 2004;5(1):69–73. doi: 10.1007/s10048-003-0161-0. [DOI] [PubMed] [Google Scholar]
- 48.Mantuano E, Romano S, Veneziano L, Gellera C, Castellotti B, Caimi S, et al. Identification of novel and recurrent CACNA1A gene mutations in fifteen patients with episodic ataxia type 2. J Neurol Sci. 2010;291(1–2):30–6. doi: 10.1016/j.jns.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Riant F, Lescoat C, Vahedi K, Kaphan E, Toutain A, Soisson T, et al. Identification of CACNA1A large deletions in four patients with episodic ataxia. Neurogenetics. 2010;11(1):101–6. doi: 10.1007/s10048-009-0208-y. [DOI] [PubMed] [Google Scholar]
- 50.Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T. Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology. 2004;62(9):1623–5. doi: 10.1212/01.wnl.0000125691.74109.53. [DOI] [PubMed] [Google Scholar]
- 51.Stendel C, D'Adamo MC, Wiessner M, Dusl M, Cenciarini M, Belia S, et al. Association of A Novel Splice Site Mutation in P/Q-Type Calcium Channels with Childhood Epilepsy and Late-Onset Slowly Progressive Non-Episodic Cerebellar Ataxia. Int J Mol Sci. 2020;21(11). [DOI] [PMC free article] [PubMed]
- 52.Baloh RW, Yue Q, Furman JM, Nelson SF. Familial episodic ataxia: clinical heterogeneity in four families linked to chromosome 19p. Ann Neurol. 1997;41(1):8–16. doi: 10.1002/ana.410410105. [DOI] [PubMed] [Google Scholar]
- 53.Cuenca-Leon E, Banchs I, Serra SA, Latorre P, Fernandez-Castillo N, Corominas R, et al. Late-onset episodic ataxia type 2 associated with a novel loss-of-function mutation in the CACNA1A gene. J Neurol Sci. 2009;280(1–2):10–4. doi: 10.1016/j.jns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Du X, Chen Y, Zhao Y, Luo W, Cen Z, Hao W. Dramatic response to pyridoxine in a girl with absence epilepsy with ataxia caused by a de novo CACNA1A mutation. Seizure. 2017;45:189–91. doi: 10.1016/j.seizure.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Nardello R, Plicato G, Mangano GD, Gennaro E, Mangano S, Brighina F, et al. Two distinct phenotypes, hemiplegic migraine and episodic Ataxia type 2, caused by a novel common CACNA1A variant. BMC Neurol. 2020;20(1):155. doi: 10.1186/s12883-020-01704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neufeld MY, Nisipeanu P, Chistik V, Korczyn AD. The electroencephalogram in acetazolamide-responsive periodic ataxia. Mov Disord. 1996;11(3):283–8. doi: 10.1002/mds.870110312. [DOI] [PubMed] [Google Scholar]
- 57.Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011;10(5):457–70. doi: 10.1016/S1474-4422(11)70048-5. [DOI] [PubMed] [Google Scholar]
- 58.Djamshidian A, Grassl R, Seltenhammer M, Czech T, Baumgartner C, Schmidbauer M, et al. Altered expression of voltage-dependent calcium channel alpha(1) subunits in temporal lobe epilepsy with Ammon's horn sclerosis. Neuroscience. 2002;111(1):57–69. doi: 10.1016/s0306-4522(01)00528-0. [DOI] [PubMed] [Google Scholar]
- 59.Xu JH, Tang FR. Voltage-dependent calcium channels, calcium binding proteins, and their interaction in the pathological process of epilepsy. Int J Mol Sci. 2018;19(9). [DOI] [PMC free article] [PubMed]
- 60.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123(4):949–62. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llinas RR, Choi S, Urbano FJ, Shin HS. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci USA. 2007;104(45):17819–24. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedroarena C, Llinas R. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94(2):724–8. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed in the current study is available upon request. All newly reported CACNA1A mutations have been submitted to ClinVar at the time of their detection.