Abstract
Background:
For the developing kidney, the prenatal period may represent a critical window of vulnerability to environmental insults resulting in permanent nephron loss. Given that the majority of nephron formation is complete in the 3rd trimester, we set out to test whether 1) prenatal lead exposure is associated with decreased preadolescent kidney function and 2) whether preadolescent obesity acts synergistically with early life lead exposure to reduce kidney function.
Methods:
Our study included 453 mother-child pairs participating in the PROGRESS birth cohort. We assessed prenatal blood lead levels (BLLs) in samples collected in the 2nd and 3rd trimesters and at delivery, as well as tibial and patellar bone lead measures assessed one-month postpartum. Preadolescent estimated glomerular filtration rate (eGFR) was derived from serum levels of creatinine and/or cystatin C measured at age 8–12 years. We applied linear regression to assess the relationship between prenatal bone and BLL with preadolescent eGFR, and adjusted for covariates including age, sex, BMI z-score, indoor tobacco smoke exposure, and socioeconomic status. We also examined sex-specific associations and tested for effect modification by BMI status.
Results:
We observed null associations between prenatal lead exposure and eGFR. However, in interaction analyses we found that among overweight children, there was an inverse association between BLL (assessed at 2nd and 3rd trimester and at delivery) and preadolescent eGFR. For example, among overweight participants, a one ln-unit increase in 2nd trimester BLL was associated with a 10.5 unit decrease in cystatin C-based eGFR (95% CI: −18.1, −2.8; p = 0.008). Regardless of lead exposure, we also observed null relationships between BMI z-score and eGFR overall, as well as among overweight participants. However, among participants with preadolescent obesity, we observed a significant 5.9-unit decrease in eGFR. We observed no evidence of sex-specific effects.
Conclusions:
Our findings, if confirmed in other studies, suggest a complex interplay between the combined adverse effects of adiposity and perinatal lead exposure as they relate to adolescent kidney function. Future studies will assess kidney function and adiposity trajectories through adolescence to better understand environmental risk factors for kidney function decline.
Keywords: Lead, prenatal, kidney function, eGFR, obesity
Background:
Decreased kidney function is a driver of hypertension, which is a major risk factor for cardiovascular disease, the leading cause of death in the developed world. Furthermore, in the last 30 years, the average weight of a U.S. child has risen by >5 kg.1 Obesity is a well-known risk factor for hypertension and chronic kidney disease (CKD), and to our knowledge, the convergence of nephrotoxic environmental chemicals and child obesity has not been previously studied. Nephron number at birth varies across individuals2 and nephrogenesis is largely complete at 36 weeks’ gestation.3 Beyond 36 weeks, the kidneys continue to grow, but this is typically due to the expansion and maturation of existing nephrons rather than the formation of new nephrons. Low nephron number is an established risk factor for CKD4,5 and it has been suggested that the prenatal milieu may permanently alter adult organ function.5
Lead is an established tubular and glomerular nephrotoxicant.6–8 Lead can also cross the placenta9, and thus maternal exposure results in fetal exposure in utero.10 Prenatal lead exposure is widespread in the U.S. and Mexico. In Mexico, more than 20% of Mexican mothers have blood lead levels (BLLs) greater than 5 μg/dL during pregnancy which is the CDC guideline level of concern.11,12 In the systemic circulation, lead has an approximately 28-day half-life, which refers to transfer out of the blood.13 While the blood levels are a small percentage of the total body burden, the bloodstream is responsible for distributing lead to other tissues.
In contrast, bone lead levels have a half-life of approximately 25–30 years14 and can be measured noninvasively by X-ray fluorescence. Bone lead levels are therefore more reflective of cumulative exposure to lead due to the longer half-life.15 In the context of nephron development, BLLs measured in pregnancy may be particularly informative, as BLLs peak in the late 2nd and 3rd trimester secondary to physiologic mobilization of bone calcium stores to meet fetal requirements. Because lead can replace calcium in the bony matrix, the same physiologic processes will remobilize bone lead resulting in increased BLLs later in pregnancy.16 Given that the majority of nephron formation is completed in the 3rd trimester, the concomitant increase in maternal BLL coupled with active kidney development may represent a critical window of susceptibility for the developing kidney.17,18
In addition to lead, overweight and obesity are independent risk factors for kidney dysfunction. Increased adiposity is one of the strongest risk factors for CKD and preclinical kidney disease.19,20 Obesity leads to kidney injury through glomerular hyperperfusion and hyperfiltration, which can lead to further kidney decline due to insulin resistance, inflammation, and hypertension.21 The major increase in childhood obesity in recent decades further underscores the potential importance of the early life environment on kidney function in adulthood.22,23 Furthermore, our group has shown that lead may act synergistically with established risk factors (such as preterm birth), to negatively influence kidney outcomes.12
In this study, we set out to assess whether 1) prenatal lead exposure (assessed in maternal blood and bone matrices) is associated with decreased preadolescent kidney function and 2) whether preadolescent obesity acts synergistically with early life lead exposure to reduce kidney function.
Methods:
PROGRESS Cohort Description:
We conducted an analysis of 453 mother-child pairs participating in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study. PROGRESS is a prospective, ongoing, NIH-funded birth cohort study in Mexico City founded in 2007. Pregnant women receiving care through the Mexican Social Security System (IMSS) were enrolled between July 2007 and February 2011. All participants provided informed and written consent. The study protocols were approved by the institutional review boards of the Brigham and Women’s Hospital, the Icahn School of Medicine at Mount Sinai and the National Institute of Public Health in Mexico.
Women were eligible for enrollment if they were 18 years or older, pregnant at fewer than 20 weeks’ gestation, had access to a telephone, and planned to reside in Mexico City for the following three years. Women were excluded if they had heart or kidney disease, used steroids or anti-epilepsy drugs, or consumed alcohol on a daily basis. All measures of interest were gathered during visits at the 2nd and 3rd trimester, delivery, one-month postpartum, and 8–12 years of age.
Of 948 mother-infant pairs assessed, a total of 581 preadolescents attended the 8–12 year-old visit. The study participants for this analysis were restricted to the 453 dyads who had complete measures of serum cystatin C, 2nd trimester blood lead concentration, and covariates of interest.
Blood Lead Measurements:
Maternal blood was collected at the 2nd and 3rd trimester visit. Cord blood lead was also collected after parturition. All blood specimens were drawn in trace metal free tubes and refrigerated at 2–6°C until analysis. Lead concentration was measured by external calibration using the Agilent 8800 ICP Triple Quad (Agilent, Santa Clara, CA) in MS/MS mode in the trace metals laboratory at the Icahn School of Medicine at Mount Sinai. The limit of detection was <0.2μg/dL and the instrument precision (given as %RSD) was approximately 5%. Blinded quality control samples obtained from the Maternal and Child Health Bureau and the Wisconsin State Laboratory of Hygiene Cooperative Blood Lead Proficiency Testing Program showed good precision and accuracy.
Bone Lead Measurements:
One month postpartum, tibia (cortical bone) and patella (trabecular bone) lead concentrations were measured in mothers using a K-shell X-ray fluorescence instrument.24 Lead concentrations were estimated for 30 minutes for each leg, and the measures were averaged by the inverse of the proportion of the measurement error corresponding to each determination. With this instrument, negative values can be obtained when the true bone lead concentration value is close to 0: the instrument produces a continuous unbiased point estimate that fluctuates around the true bone lead value.25 Téllez-Rojo et al. found similar results26 between measurements that included the negative values and those that used simulated estimates randomly generated from a normal distribution. In this analysis, bone lead measurements were treated as a continuous variable in linear regression models.
Cystatin C and Creatinine Measurements:
Cystatin C (previously stored at −70 C) was measured in serum sample using Quantikine ® ELISA Human Cystatin C Immunoassay (R&D Systems, Minneapolis, MN). Optical density was measured by a microplate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT) set to 450 nm. The limit of detection was 31 pg/ml. Creatinine was measured in serum/plasma samples (previously stored at −70 C) using Creatinine FS reagent and Respons 910 (both by DiaSys, Holzheim, Germany), based on Jaffe’s kinetic method without deproteinization, with the detection limit of 0.2 mg/dL.
eGFR Calculations:
Estimated glomerular filtration rate (eGFR) was calculated using cystatin C and/or creatinine according to multiple previously validated formulas. These included the Schwartz equation27, wherein (k=0.7 for adolescent boys, and 0.55 for adolescent girls, and children (male or female) <13 years old), the 2012 cystatin C equation28, wherein eGFRCysC2012 = 70.69 ∗ (Cystatin C) −0.931 and cystatin C is in mg/L.
Covariates:
Child weight and standing height were measured using a professional digital scale (InBody230, InBody, Seoul, Korea) and stadiometer with the head in the Frankfort plane at the 8–12 year visit. Weight and height were used to calculate BMI categories according to the WHO z-scores for age and sex, defined as obesity (z-score greater than 2), overweight (z-score between 1 and 2), and normal weight (z-score between −2 and 1). Participants in the underweight category (z-score below −2) were excluded (n=2). Information on maternal age, socioeconomic status, and environmental tobacco exposure were collected at the 2nd trimester visit using a standardized questionnaire. Household environmental tobacco smoke exposure was dichotomized as yes/no based on the mother’s report that at least one household member smoked during the pregnancy. The SES index during pregnancy was calculated based on the 1994 Mexican Association of Intelligence Agencies Market and Opinion (AMAI) rule 13 * 6. The index classifies families into 6 levels based on 13 questions related to the characteristics of the household. The majority of families were middle or low SES. Thus, the 6 resultant levels were further collapsed into 3 SES categories: lower, medium, and higher. Gestational age at delivery was calculated using maternal report of last menstrual period (LMP) and confirmed with Capurro physical examination at birth.29 When the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam was used in lieu of the LMP-determined gestational age.
Statistical Analyses:
BLLs were transformed via natural logarithm to approximate a normal distribution. Bone lead concentration was used on its original scale. Covariates in adjusted models (age, sex, socioeconomic status, BMI z-score, secondhand tobacco exposure) were selected a priori. Potential influential data points were assessed using Cook’s distance. We used multiple linear regression models to assess the association between each of the lead exposure biomarkers and the outcome variables. The outcome variables included BMI z-score (to assess direct effects with obesity) as well as eGFRSchwartz and eGFRCysC2012. For simplicity, we present results with eGFRCysC2012 in the main tables. Preadolescent BMI categorization thresholds were defined according to WHO recommendations. We performed two sensitivity analyses that excluded participants 1) born preterm (less than 37 weeks) or 2) low birth weight (less than 2.5 kg). When there was evidence that the variance in eGFR increased with the mean, quasi-likelihood estimation was used resulting in a scaled Poisson objective function, and results agreed with linear regression models (data not shown).30 All analyses were performed using R Studio version 3.6.2.
Results:
Table 1 shows the demographic characteristics of the 453 mother-child pairs with complete data. Participants were 8 to 12 years of age, and 50% were girls. A majority of participants (53%) were lower SES, and 29% of mothers reported exposure to indoor secondhand tobacco smoke in the home. Approximately half were normal weight, and 24% and 23% were considered overweight or obese respectively. The average serum creatinine was 0.43 mg/dL, which is within the expected range for this age.31 The eGFRCysC2012 values were within the normal physiological range, with a median value of 97.1 mL/min/1.73m2, and four were below 60 mL/min, a level associated with adult CKD.32 Maternal 2nd trimester BLLs ranged between 0.7 and 17.8 μg/dL, and nearly 20% of maternal 2nd trimester BLLs were above the CDC guidance level of 5 μg/dL, consistent with prior studies in the PROGRESS cohort.11,12
Table 1:
Participant demographics for n=453 child-mother pairs in the PROGRESS cohort.
| Arithmetic mean (SD) or median [IQR] | N | |
|---|---|---|
| Age, years | 9.6 [9.2, 10.2] | 453 |
| Male, % | 50.1% | 453 |
| Socioeconomic status | 453 | |
| Lower | 52.5% | |
| Medium | 37.5% | |
| Higher | 9.9% | |
| Exposure to secondhand smoke (yes/no) | 28.7% | 453 |
| Preadolescent BMI | 453 | |
| Underweight | 0.4% | |
| Normal Weight | 52.8% | |
| Overweight | 23.8% | |
| Obese | 23.0% | |
| Cystatin C (mg/L) | 0.71 [0.6, 0.8] | 453 |
| BUN (mg/dL) | 12.0 [10.0, 13.7] | 367 |
| Creatinine (Plasma) (mg/dL) | 0.44 (0.08) | 448 |
| Creatinine (Serum) (mg/dL) | 0.43 (0.1) | 368 |
| eGFRSchwartz (Plasma) (mL/min/1.73m2) | 130.8 [115.2, 148.7] | 448 |
| eGFRSchwartz (Serum) (mL/min/1.73m2) | 173.4 [150.4, 204.4] | 368 |
| eGFRCysC2012 (mL/min/1.73m2) | 97.1 [83.4, 111.8] | 453 |
| Prenatal Pb levels: | ||
| Second trimester blood Pb (μg/dL) | 2.9 [2.0, 4.5] | 453 |
| Third trimester blood Pb (μg/dL) | 3.1 [2.0, 4.8] | 375 |
| Cord Blood Pb (μg/dL) | 3.3 [2.1, 5.3] | 362 |
| Tibia Pb | 3.3 [−1.7, 7.8] | 356 |
| Patella Pb | 3.4 [−1.2, 9.0] | 353 |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; BUN, blood urea nitrogen; eGFRCysC2012, estimated glomerular filtration rate from 2012 cystatin C equation.
We first examined relationships between eGFR and important covariates, including age and BMI. In models adjusted for sex, BMI z-score, indoor tobacco smoke exposure, and socioeconomic status, a single year increase in age was significantly associated with an 8.6-unit increase in eGFRCysC2012 (95% CI: 5.45, 11.8; p < 0.0001). As BMI is a significant risk factor for CKD, we also assessed the relationship between continuous BMI z-score and eGFR, and also stratified according to WHO-established BMI categories. We observed a marginal relationship between continuous BMI z-score and eGFRCysC2012 (βunadjusted = −1.37, 95% CI: −3.0, 0.26, p = 0.1) (Supplemental Figure 1). We also observed a null relationship between raw BMI and eGFRCysC2012 (βunadjusted = −0.39, 95% CI: −0.95, 0.18, p = 0.2). Stratified by BMI category, we observed a null relationship among normal weight and overweight participants (Supplemental Figure 2). However, among participants with obesity, we observed a significant 5.9-unit decrement in eGFRCysC2012 (β= −5.9, 95% CI: −11.0, −0.8, p = 0.02). We also observed null relationships between measures of bone and blood lead levels with preadolescent continuous BMI z-score (data not shown).
Null overall and sex-specific relationships between prenatal lead exposure and preadolescent eGFR
Overall, we observed null relationships between measures of bone and blood lead and preadolescent eGFR (Table 2). To evaluate whether there was evidence of sex-specific effects, we conducted analyses stratified by boys and girls (Table 2). Among girls (n=226), we observed null relationships between all measures of lead and eGFR. Among boys (n=227), we observed null relationships between 2nd trimester, 3rd trimester, cord blood at delivery, and tibia lead levels and eGFR. We observed a significant, inverse association between patella lead levels and eGFR among boys, wherein a one-unit increase in patella lead was associated with a 0.4-unit decrease in eGFRCysC2012 (95% CI: −0.8, −0.03, p = 0.03).
Table 2: Associations between overall prenatal lead exposure and preadolescent eGFRCysC2012 at age 8 to 12 years, as well as sex-specific associations.
Estimates were adjusted for BMI z-score, SES, tobacco exposure, age, and sex (sex excluded from sex-stratified models).
| All Participants | Male Participants | Female Participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-Value | n | Estimate (95% CI) | P-Value | n | Estimate (95% CI) | P-Value | n | |
| 2T BLL | −0.81 (−4.1, 2.5) | 0.63 | 453 | −2.25 (−7.03, 2.53) | 0.35 | 227 | 0.79 (−3.82, 5.41) | 0.73 | 226 |
| 3T BLL | 0.27 (−3.2, 3.8) | 0.88 | 375 | 2.78 (−2.49, 8.06) | 0.30 | 182 | −0.71 (−5.53, 4.11) | 0.77 | 193 |
| Cord BLL | −2.14 (−5.8, 1.5) | 0.25 | 362 | −3.27 (−8.58, 2.05) | 0.23 | 188 | −1.10 (−6.38, 4.18) | 0.68 | 174 |
| Tibia Pb | 0.07 (−0.2, 0.3) | 0.63 | 356 | 0.12 (−0.25, 0.50) | 0.52 | 171 | 0.02 (−0.39, 0.44) | 0.91 | 185 |
| Patella Pb | −0.15 (−0.4, 0.1) | 0.27 | 353 | −0.42 (−0.81, −0.03) | 0.03 | 174 | 0.06 (−0.31, 0.44) | 0.74 | 179 |
2T, second trimester; 3T, third trimester; BLL, blood lead level after ln-transformation.
Effect modification by BMI
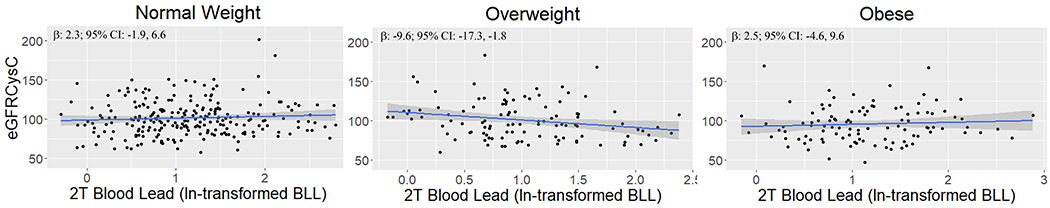
When stratified by BMI status, we observed null relationships between all measures of lead and eGFR in the normal weight and obese groups (Table 3; Figure 1). However, among overweight participants, we observed significant, inverse associations between 2nd trimester and 3rd trimester BLLs as well as cord blood at delivery and children’s eGFR (Figure 1). For example, among overweight participants, a one ln-unit increase in 2nd trimester BLL was associated with a 10.5-unit decrease in eGFRCysC2012 (95% CI: −18.1, −2.8; p = 0.008). Similarly, among overweight participants, a one ln-unit increase in 3rd trimester BLL was associated with a 10.0-unit decrease in eGFRCysC2012 (95% CI: −17.6, −2.4; p = 0.01). Among overweight participants, a one ln-unit increase in cord BLL was associated with an 8.9-unit decrease in eGFRCysC2012 (95% CI: −16.8, −0.9, p = 0.03). We observed evidence of multiplicative interaction between overweight status and second trimester BLLs among all participants (p = 0.003). We also observed no significant interaction between obesity status and second trimester BLLs (p=0.9).
Table 3: Associations between prenatal lead exposures and preadolescent eGFRCysC2012, stratified according to BMI status.
Estimates were adjusted for SES, tobacco exposure, age, and sex.
| Normal weight | Overweight | Obese | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-value | n | Estimate (95% CI) | P-value | n | Estimate (95% CI) | P-value | n | |
| 2T BLL | 1.76 (−2.4, 6.0) | 0.42 | 239 | −10.49 (−18.1, −2.8) | 0.008 | 108 | 1.66 (−5.9, 9.2) | 0.67 | 104 |
| 3T BLL | 2.94 (−1.5, 7.4) | 0.20 | 196 | −9.97 (−17.6, −2.4) | 0.01 | 95 | 4.29 (−4.7, 13.2) | 0.34 | 82 |
| Cord BLL | 1.94 (−2.9, 6.8) | 0.43 | 195 | −8.87 (−16.8, −0.9) | 0.03 | 82 | −6.96 (−15.9, 2.0) | 0.13 | 84 |
| Tibia Pb | 0.19 (−0.2, 0.6) | 0.29 | 195 | −0.13 (−0.8, 0.5) | 0.69 | 84 | −0.06 (−0.7, 0.6) | 0.84 | 75 |
| Patella Pb | −0.12 (−0.4, 0.2) | 0.47 | 194 | −0.52 (−1.3, 0.3) | 0.22 | 81 | −0.16 (−0.8, 0.5) | 0.60 | 76 |
2T, second trimester; 3T, third trimester; BLL, blood lead level after ln-transformation.
Figure 1:
Association between second trimester BLL and eGFRCysC2012 stratified by normal weight (n=239), overweight (n=108), and obese (n=104) as defined by WHO age- and sex- standardized BMI z-score.
Sensitivity Analyses
In a sensitivity analysis that excluded participants born preterm (n=45), we observed results similar to our main findings (Table 4). We found a significant inverse association between 2nd trimester and 3rd trimester BLLs and eGFR among overweight participants (Supplemental Figure 3). A one ln-unit increase in 2nd trimester BLL was associated with an 8.4-unit decrease in eGFRCysC2012 (95% CI: −16.7, −0.1, p = 0.05). A one ln-unit increase in 3rd trimester BLL was associated with an 8.9-unit decrease in eGFRCysC2012 (95% CI: −16.9, −0.8, p = 0.03). We observed a marginally significant inverse association between cord BLL and eGFR, such that a one ln-unit increase in cord BLL at delivery was associated with a 7.4-unit decrease in eGFRCysC2012 (95% CI: −15.9, 1.1, p = 0.09). We observed null relationships between all measures of lead (blood and bone) and eGFR in the normal weight and obese strata. Although not significant, one ln-unit increase in cord BLL was associated with an 8.3-unit decrease in eGFRCysC2012 (95% CI: −16.9, 0.35, p=0.06) among participants with obesity.
Table 4: Sensitivity analyses excluding children born preterm (n=45) or low birth weight (n=36): Associations between prenatal BLLs (μg/dL) and preadolescent eGFRCysC2012.
Estimates were adjusted for SES, tobacco exposure, age, and sex.
| Normal weight | Overweight | Obese | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-value | n | Estimate (95% CI) | P-value | n | Estimate (95% CI) | P-value | n | ||
| Term Participants | 2T BLL | 2.67 (−1.8, 7.2) | 0.24 | 220 | −8.43 (−16.75, −0.12) | 0.05 | 96 | −0.66 (−8.6, 7.3) | 0.87 | 91 |
| 3T BLL | 2.84 (−1.8, 7.5) | 0.23 | 183 | −8.86 (−16.90, −0.82) | 0.03 | 86 | 1.44 (−7.3, 10.1) | 0.74 | 71 | |
| Cord BLL | 2.08 (−2.9, 7.1) | 0.41 | 181 | −7.39 (−15.88, 1.11) | 0.09 | 73 | −8.26 (−16.9, 0.35) | 0.06 | 74 | |
| Normal Birth Weight Participants | 2T BLL | 1.58 (−2.7, 5.9) | 0.47 | 220 | −8.57 (−16.4, −0.7) | 0.03 | 100 | 0.40 (−7.7, 8.5) | 0.92 | 95 |
| 3T BLL | 2.50 (−2.0, 7.0) | 0.28 | 182 | −9.26 (−17.2, −1.3) | 0.02 | 88 | 2.03 (−7.6, 11.6) | 0.67 | 74 | |
| Cord BLL | 0.48 (−4.4, 5.3) | 0.84 | 179 | −8.22 (−16.2, −0.2) | 0.04 | 77 | −9.04 (−18.4, 0.3) | 0.06 | 76 | |
We performed an additional sensitivity analysis that excluded participants with low birth weight status (n=36). Some participants were both born preterm and at low birth weight and were therefore excluded from both sensitivity analyses. Excluding low birth weight participants, we similarly observed a significant, inverse association between 2nd trimester, 3rd trimester and cord BLLs and eGFR among overweight participants (Table 4; Supplemental Figure 4). A one ln-unit increase in 2nd trimester BLL was associated with an 8.6-unit decrease in eGFRCysC2012 (95% CI: −16.4, −0.7, p = 0.03). A one ln-unit increase in 3rd trimester BLL was associated with a 9.3-unit decrease in eGFRCysC2012 (95% CI: −17.2, −1.3, p = 0.02). A one ln-unit increase in cord BLL was associated with an 8.2-unit decrease in eGFRCysC2012 (95% CI: −16.2, −0.2, p = 0.04). Again, we observed null relationships between all measures of lead (blood and bone) and eGFR in the normal weight and obese strata. Although not significant, one ln-unit increase in cord BLL was associated with a 9.0-unit decrease in eGFRCysC2012 (95% CI: −18.4, 0.3, p=0.06) among participants with obesity.
Discussion:
In this study, we found that among overweight children, an increase in BLL at multiple timepoints in pregnancy and at delivery was associated with a significant decrease in eGFR, suggesting that low-level prenatal lead exposure may have important implications for kidney health in adolescence in the context of childhood adiposity. Given the near global epidemic of child overweight and obesity and the ubiquity of low-level lead exposure worldwide, if confirmed in other studies, the findings may have important public health implications for mitigating the burden of CKD and maintaining a normal weight through childhood.
There are several important observations from this work. One appropriate interpretation of this data is that preadolescent BMI modifies the effect of prenatal lead exposure on kidney function by the end of the first decade of life. Because the results depend on assessing interaction, it may explain why previous studies have demonstrated conflicting results regarding the effect of early life lead exposure on children’s kidney function: both factors must be present. For example, in a prospective study of 117 Bangladeshi children 4–5 years of age, an increase of 85 μg/kg in erythrocyte lead levels at 30 weeks’ gestation was associated with a 6.0 cm3/m2 decrease in kidney volume after adjusting for potential confounders.33 The authors found no evidence of effect modification by BMI z-score, although comparison is limited by the difference in lead biomarker, younger study population age,33 and the much lower prevalence of child obesity in Bangladesh.34 Indeed, child obesity is more prevalent in middle income and developed countries. Also, in a cross-sectional analysis of a prospective cohort of 391 Canadian and American participants aged 1–19 years of age, BLLs were associated with a decrease in eGFR, although the finding did not reach statistical significance.35 Child obesity is less common in children under five36 and lead exposure is less common in older children, which may have obscured evidence of interaction in prior studies with a wide age range. In the same North American study, subgroup analyses that stratified by anemia status or glomerular disease status found significant, inverse associations between BLLs and eGFR. Other cross-sectional studies have assessed the effects of lead exposure with other kidney parameters including proteinuria and observed mixed results.37
Potential mechanisms of lead-induced nephrotoxicity include the production of reactive oxygen species and a weakened antioxidant response.38,39 Although not well understood, manifestations of acute lead nephrotoxicity include glomerular and proximal tubule dysfunction, alterations in mitochondrial structure and energy production, and formation of nuclear and cytosolic inclusion bodies.40 Prenatal lead exposure has been shown to alter genomic DNA methylation and microRNA expression15,41 that could impact fetal nephrogenesis.42 Additionally, changes in DNA methylation patterns in the kidney cortex have been shown to influence glomerular filtration rate (GFR) and kidney fibrosis.43
The interplay between lead exposure and early life metabolic health may be in part explained by lower nephron endowment coupled with alterations in the renin-angiotensin-aldosterone system. Fetal nephrogenesis is subject to modification according to the energy made available to the fetus.44 Prenatal lead exposure can disrupt mitochondrial energy production45 potentially resulting in decreased nephron number at birth, which carries an increased risk for CKD later in life.44 Furthermore, lead can increase angiotensin-converting enzyme activity in rats as well as renin and aldosterone concentrations in both humans and animals38,46, which could compound obesity-associated kidney decline. By driving a greater increase in renin and aldosterone concentrations, lead may worsen hypertension and glomerular hyperfiltration, leading to more rapid kidney decline.47
However, effect modification by BMI does not completely explain the counterintuitive null results observed when stratified by children with obesity. There may be additional factors that are not accounted for, and there is a complex relationship between metabolic and renal health. For example, among adults, increased adiposity acts as a risk factor for de novo CKD while also conferring survival advantages in advanced CKD, a phenomenon known as the obesity paradox.48 Despite consistent data, the mechanisms of this paradox are unknown and it is unclear whether the obesity paradox may present in adolescents with obesity. It is also worth noting that in sensitivity analyses that excluded both preterm or low birth weight participants, we observed marginally significant inverse results between cord blood lead levels and eGFR among the obese stratum. Furthermore, it is possible that eGFR estimating equations that use either cystatin C or creatinine are less appropriate for children with higher BMI. This could explain the lack of association between lead and eGFR in the obese group. The greater level of uncertainty in eGFR among individuals with higher BMI is evidenced by studies in adults20,49, and in this study, we observed larger confidence intervals among overweight and obese participants (Supplemental Fig 2). Finally, it is possible to conclude that the lack of association between lead and eGFR in the obese stratum conflicts with the conclusion that BMI is an effect modifier, and there may be additional unmeasured confounding contributing to the observed associations. Overall, our findings may underscore the importance of maintaining a healthy weight through adolescence to promote kidney health.
Our study has many strengths. PROGRESS is an established prospective birth cohort with well-characterized demographic and covariate data, as well as simultaneous exposures. Maternal lead was measured years prior to eGFR, and therefore cannot be biased with respect to eGFR. We assessed lead in two biological matrices highly relevant to the prenatal period: 1) bone, more reflective of cumulative lead exposure and 2) blood level, more reflective of more recent, active lead exposure.26 The lead biomarkers have relatively strong correlation, which has been described previously.50 Our findings of consistent null relationships between bone lead and eGFR, even when stratified by BMI, suggest that blood lead is more biologically active and therefore has a more significant role in impaired kidney function. We assessed eGFR using three common equations, including the recently updated cystatin C equation for children.28 Decrements in kidney function have been historically assessed by biomarkers such as serum creatinine and urea.51 However, changes in nearly all of these biomarkers may not manifest until substantial kidney impairment is present or clinical kidney disease is apparent. For example, after accounting for risk based on serum creatinine concentration in children with CKD, there is a wide variation in CKD progression.52 We note that models using the Schwartz formula eGFR did not replicate the results of models that used cystatin C-based eGFR. The cystatin C univariate equation for children has advantages over these estimates, and eGFR calculations that are based on the metabolite cystatin C have been proposed as an improved assessment that more closely estimates the gold standard of measured GFR in pediatric populations.49,53 Cystatin C is produced by all mononucleated cells and freely filtered at the glomerulus. Further, compared to serum creatinine, cystatin C is not as subject to variation with muscle mass, BMI and delayed sensitivity to kidney damage.54 While differences between the two eGFR equations could be attributed to the differences in biomarker, the Schwartz formula would be expected to trend in the same direction. Another possible explanation for the variations is that serum creatinine was measured using the Jaffe method standardized according to the reagent provider, and was not calibrated via isotope dilution mass spectrometry.55
Our study has limitations. We assessed eGFR at a single time point between 8 and 12 years of age, which does not capture the adolescent growth spurt, although longitudinal follow-up is ongoing and will include adolescence. The PROGRESS cohort is also ethnically homogeneous, which limits the extent to which this analysis is externally generalizable but strengthens its internal generalizability by reducing confounding due to genetics or social factors. In addition, the WHO BMI Z-score reference population is American, and may not be generalizable to our cohort of Mexican children.56 As this is an observational study, we are also unable to report a specific mechanistic pathway between prenatal lead exposure, increased adiposity, and decreased preadolescent glomerular function. As increased adiposity can cause glomerular hyperperfusion and hypertrophy followed by kidney function decline, it is also possible that there is greater heterogeneity of eGFR at a single timepoint among participants with obesity. The hyper- vs. hypofiltration state may be dependent on the length of time an individual has been affected by obesity rather than BMI at a single time point. Longitudinal assessments of the relationship between obesity and kidney function trajectories through adolescences are needed.
Conclusion
In conclusion, our findings suggest a complex relationship between preadolescent BMI and prenatal lead exposure, in which the two may act together to worsen kidney function later in life. These findings underscore the public health need to prevent lead exposure in pregnancy as well as childhood obesity. To better understand the complex relationship between environmental toxic metal exposure and the origins of CKD, future longitudinal assessments of nephrotoxicant exposure and preadolescent kidney function will improve our understanding of risk factors for kidney impairment and associated comorbidities.
Supplementary Material
Highlights:
Overall, we observed null associations between perinatal lead exposure and kidney function
Perinatal BLLs were associated with reduced preadolescent eGFR among overweight participants
We describe possible effect modification by adiposity status
The complex relationship between lead exposure and adiposity warrants further study
Acknowledgments:
This work was supported in part by funding from the NIH/NIEHS: K99ES027508, R00ES027508, R24ES028522, P30ES023515, R01ES013744, R01ES026033, and R01ES021357.
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lobstein T, Jackson-Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385(9986):2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–2122. [DOI] [PubMed] [Google Scholar]
- 3.Ryan D, Sutherland MR, Flores TJ, et al. Development of the Human Fetal Kidney from Mid to Late Gestation in Male and Female Infants. EBioMedicine. 2018;27:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luyckx VA, Brenner BM. Clinical consequences of developmental programming of low nephron number. Anat Rec (Hoboken). 2019. [DOI] [PubMed] [Google Scholar]
- 5.Ingelfinger JR. Disparities in renal endowment: causes and consequences. Adv Chronic Kidney Dis. 2008;15(2):107–114. [DOI] [PubMed] [Google Scholar]
- 6.Loghman-Adham M Renal effects of environmental and occupational lead exposure. Environ Health Perspect. 1997;105(9):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basgen JM, Sobin C. Early chronic low-level lead exposure produces glomerular hypertrophy in young C57BL/6J mice. Toxicol Lett. 2014;225(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R, Xu Y, Shen J, et al. Urinary KIM-1: a novel biomarker for evaluation of occupational exposure to lead. Sci Rep. 2016;6:38930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyer RA. Transplacental transport of lead. Environ Health Perspect. 1990;89:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann Epidemiol. 2014;24(12):915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantic I, Tamayo-Ortiz M, Rosa-Parra A, et al. Children’s Blood Lead Concentrations from 1988 to 2015 in Mexico City: The Contribution of Lead in Air and Traditional Lead-Glazed Ceramics. Int J Environ Res Public Health. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders AP, Svensson K, Gennings C, et al. Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environment international. 2018;120:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin TB, Coulston F, Wills H, Russell JC. Biologic effects of airborne particulate lead on continuously exposed rats and rhesus monkeys. Environ Qual Saf Suppl. 1975;2:202–220. [PubMed] [Google Scholar]
- 14.Abadin H, Ashizawa A, Stevens YW, et al. In: Toxicological Profile for Lead. Atlanta (GA)2007. [PubMed] [Google Scholar]
- 15.Pilsner JR, Hu H, Ettinger A, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect. 2009;117(9):1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manton WI. Total contribution of airborne lead to blood lead. Br J Ind Med. 1985;42(3):168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solhaug MJ, Bolger PM, Jose PA. The developing kidney and environmental toxins. Pediatrics. 2004;113(4 Suppl):1084–1091. [PubMed] [Google Scholar]
- 18.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64(6):777–784. [PubMed] [Google Scholar]
- 19.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33. [DOI] [PubMed] [Google Scholar]
- 20.Sabanayagam C, Wong TY, Liao J, Sethi S, Teo BW. Body mass index and preclinical kidney disease in Indian adults aged 40 years and above without chronic kidney disease. Clin Exp Nephrol. 2014;18(6):919–924. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava T Nondiabetic consequences of obesity on kidney. Pediatr Nephrol. 2006;21(4):463–470. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Peterson KE, Scanlon KS, et al. Trends in overweight from 1980 through 2001 among preschool-aged children enrolled in a health maintenance organization. Obesity (Silver Spring). 2006;14(7):1107–1112. [DOI] [PubMed] [Google Scholar]
- 23.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Milder FL, Burger DE. The use of K X-ray fluorescence for measuring lead burden in epidemiological studies: high and low lead burdens and measurement uncertainty. Environ Health Perspect. 1991;94:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol. 1995;40(9):1475–1485. [DOI] [PubMed] [Google Scholar]
- 26.Tellez-Rojo MM, Hernandez-Avila M, Lamadrid-Figueroa H, et al. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol. 2004;160(7):668–678. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders AP, Burris HH, Just AC, et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics. 2015;10(3):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. London; New York: Chapman and Hall; 1989. [Google Scholar]
- 31.Chavers BM, Rheault MN, Foley RN. Kidney function reference values in US adolescents: National Health And Nutrition Examination Survey 1999–2008. Clin J Am Soc Nephrol. 2011;6(8):1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3. [DOI] [PubMed] [Google Scholar]
- 33.Skroder H, Hawkesworth S, Moore SE, Wagatsuma Y, Kippler M, Vahter M. Prenatal lead exposure and childhood blood pressure and kidney function. Environ Res. 2016;151:628–634. [DOI] [PubMed] [Google Scholar]
- 34.Saha M, Adhikary DK, Parvin I, Sharma YR, Akhter F, Majumder M. Obesity and Its Risk Factors of among School Children in Sylhet, Bangladesh. J Nepal Health Res Counc. 2018;16(2):205–208. [PubMed] [Google Scholar]
- 35.Fadrowski JJ, Abraham AG, Navas-Acien A, Guallar E, Weaver VM, Furth SL. Blood lead level and measured glomerular filtration rate in children with chronic kidney disease. Environ Health Perspect. 2013;121(8):965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hales CMC, Margaret D; Fryar Cheryl D; Ogden Cynthia L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. In. NCHS Data Brief. Atlana, GA: CDC; 2017. [PubMed] [Google Scholar]
- 37.Zheng LY, Sanders AP, Saland JM, Wright RO, Arora M. Environmental exposures and pediatric kidney function and disease: A systematic review. Environ Res. 2017;158:625–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri ND, Sica DA. Lead-induced hypertension: role of oxidative stress. Curr Hypertens Rep. 2004;6(4):314–320. [DOI] [PubMed] [Google Scholar]
- 39.Chiba M, Shinohara A, Matsushita K, Watanabe H, Inaba Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. Tohoku J Exp Med. 1996;178(1):49–62. [DOI] [PubMed] [Google Scholar]
- 40.Nolan CV, Shaikh ZA. Lead nephrotoxicity and associated disorders: biochemical mechanisms. Toxicology. 1992;73(2):127–146. [DOI] [PubMed] [Google Scholar]
- 41.Sanders AP, Burris HH, Just AC, et al. Altered miRNA expression in the cervix during pregnancy associated with lead and mercury exposure. Epigenomics. 2015;7(6):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanner N, Vornweg J, Combes A, et al. DNA Methyltransferase 1 Controls Nephron Progenitor Cell Renewal and Differentiation. J Am Soc Nephrol. 2019;30(1):63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu AY, Tin A, Schlosser P, et al. Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat Commun. 2017;8(1):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevalier RL. Bioenergetic Evolution Explains Prevalence of Low Nephron Number at Birth: Risk Factor for CKD. Kidney360. 2020;1(8):863–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Guerra M, Peng C, Trevisi L, et al. Altered cord blood mitochondrial DNA content and pregnancy lead exposure in the PROGRESS cohort. Environment international. 2019;125:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simoes MR, Ribeiro Junior RF, Vescovi MV, et al. Acute lead exposure increases arterial pressure: role of the renin-angiotensin system. PLoS One. 2011;6(4):e18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Praga M Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Transplant. 2005;20(12):2594–2597. [DOI] [PubMed] [Google Scholar]
- 48.Kalantar-Zadeh K, Rhee CM, Chou J, et al. The Obesity Paradox in Kidney Disease: How to Reconcile it with Obesity Management. Kidney Int Rep. 2017;2(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal N, Porter AC, Tang IY, Becker BN, Akkina SK. Creatinine-based estimations of kidney function are unreliable in obese kidney donors. J Transplant. 2012;2012:872894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renzetti S, Just AC, Burris HH, et al. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environ Res. 2017;152:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan E, Batuman V, Lertora JJ. Emergence of biomarkers in nephropharmacology. Biomark Med. 2010;4(6):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenberg JH, Abraham AG, Xu Y, et al. Plasma Biomarkers of Tubular Injury and Inflammation Are Associated with CKD Progression in Children. J Am Soc Nephrol. 2020;31(5):1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA. Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int. 2018;94(1):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerchman F, Tong J, Utzschneider KM, et al. Body mass index is associated with increased creatinine clearance by a mechanism independent of body fat distribution. J Clin Endocrinol Metab. 2009;94(10):3781–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shanthaveeranna G, Devanath A. Jaffe’s kinetic method comparison between isotope dilution mass spectrometry standardized versus nonstandardized method. Indian Journal of Health Sciences and Biomedical Research KLEU. 2020;13(2):137–139. [Google Scholar]
- 56.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl):S15–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.