Abstract
In a canine model of pre-sensitization using donor blood transfusions, 100% of historical control dogs receiving 9.2 Gy total body irradiation (TBI) conditioning before dog leukocyte antigen (DLA)-identical marrow grafts had graft rejection. In this pre-sensitization model, we investigated whether the addition of monoclonal antibody (mAb)- based targeted radioimmunotherapy (RIT) with astatine-211 (211At) to TBI could overcome graft rejection. 211At is an alpha-particle-emitting isotope, which has a short path length, very high energy, and a short t 1/2 of 7.2 hours, which allowed targeting radiation to the T cells responsible for graft rejection. Normal canine recipients were given three preceding transfusions of unirradiated whole blood on days -24, -17, -10 before transplant from their DLA-identical marrow donors. 211At-anti-CD45 mAb was administered on day -3, and TBI followed by marrow grafts on day 0. Six of the seven dogs (86%) achieved sustained engraftment as assessed by 100% donor chimerism in mononuclear cells, granulocytes, and CD3+ T cells. One dog receiving the lowest CD34+ cell content (0.35 × 106 cells/kg) rejected the graft. There were no late rejections in dogs followed up to one year. Graft-versus-host disease (GVHD) was seen in one dog. 211At-anti-CD45 mAb in combination with TBI as conditioning was successful in abrogating graft rejection in 86% of dogs in this pre-sensitization model. 211At-anti-CD45 mAb conditioning with TBI may serve as a novel promising strategy to overcome graft rejection in heavily transfused patients with red cell disorders.
Keywords: Astatine-211, DLA-identical marrow transplantation, transfusion-sensitized, graft rejection
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment option for patients with serious, non-malignant blood disorders including aplastic anemia and hemoglobinopathies. Rejection of the allogeneic graft has been a significant complication especially in heavily transfused patients, with rejection probabilities in the range of 35–60% in the earliest transplant series. Rejections were seen despite the use of human leukocyte antigen (HLA)-identical sibling donors and were attributed to sensitization to minor non-HLA antigens via transfusions [1].
We had predicted graft rejection in human patients based on early findings in a preclinical canine model that used total body irradiation (TBI)-based conditioning [1–3]. The model included canine donor-recipient pairs that were matched for the major histocompatibility locus, called Dog Leukocyte Antigen (DLA) region. It involved sensitizing recipient against minor histocompatibility antigens of the donor by one 50 ml whole blood transfusion each on days -24, -17 and -10 before TBI and marrow transplantation. After the three blood transfusions from their respective marrow donors, recipients uniformly rejected the subsequent marrow grafts (27 of 27 dogs) while un-transfused recipients nearly uniformly engrafted (61 of 62 dogs). Further canine studies in this model showed that both using buffycoat poor transfusions and ex vivo irradiated transfusions significantly reduced the risk of marrow graft rejection [3–5], These findings have led to changes in clinical transfusion policies. In other canine studies, use of an alkylating agent, such as procarbazine or cyclophosphamide, alternating with anti-thymocyte globulin in the conditioning regimen was successful in reducing the risk of rejection [6,7], This finding has also been translated into the clinic and has significantly reduced graft rejection rates.
However, intensification of conditioning regimens using high-dose chemotherapy or TBI has led to worse outcomes in patients with non-malignant diseases who had serious comorbidities because of regimen-related mortality. Total lymphoid irradiation (TLI), which is used in some regimens in place of TBI, uses high doses (12 Gy) of penetrating gamma rays for much of the body and increases the risk of secondary cancers. Conversely, when trying to accommodate such patients by lowering regimen intensity, the risk of graft rejection was dramatically increased [8], In order to address this quandary, we studied here whether targeted radio-immunotherapy (RIT) in the conditioning regimen could replace systemic chemotherapy, thereby retaining efficacy but reducing toxicity.
To this end, we used the DLA-identical canine model to ask whether marrow graft rejection after three donor blood transfusions could be prevented by adding targeted RIT with an alpha-emitting radionuclide, astatine 211, to the TBI conditioning regimen. Previously, we introduced targeted radiation into the clinic in the 1990s using beta-emitting radionuclides, including iodine-131 (131I) and yttrium-90 (90Y), which were coupled to an anti-CD45 monoclonal antibody (mAb) or an anti-CD20 mAb. The two beta-emitters were limited by their long half-lives of 8 and 2.5 days, respectively, their relatively low energy, and their long path lengths, which ranged from 0.8 to 11.3 mm, resulting in off-target effects. These limitations led us to investigate astatine-211 (211At), which we conjugated to the pan-hematopoietic cell surface antigen, CD45. 211At has a short half-life of 7.2 hours, and its decay results in emission of very high energy alpha particles (5.87 & 7.45 MeV) that have short path lengths of 60 80 μm in tissues [9–11]. 211At undergoes a branched chain decay process, with 41.8% decaying by alpha emission to yield the long-lived bismuth-207 (207Bi; 31.6 y) and 58.2% by electron capture to the short half-lived polonium-211 (211Po; 0.52 sec). 211Po subsequently decays rapidly through 100% alpha emission to stable lead-207 (207Pb), making the decay of 211At effectively 100% alpha emission. Decay of 211At also emits low energy X-rays (77 & 79 keV) but has very low abundance of higher energy gamma rays and no beta emissions, which makes it possible to easily administer 211At-labeled mAb in a clinical setting.
The mAb-targeted antigen CD45 is expressed on all hematopoietic cells except for platelets and erythrocytes. CD45 has a high density with about 200,000 copies per cell expressed on the surface of circulating lymphocytes [12,13] Because of its high antigen density, it permits efficient targeting of the short-lived alpha-emitting radionuclide 211At via the antibody. This way, targeted cells are destroyed without the risk of faulty DNA repair and with few off-target effects.
Our group has previously explored radioimmunotherapy (RIT) preparative regimens for DLA-identical HCT in the non-sensitized canine model, concluding that conditioning with 211At-anti-CD45-radioimmunoconjugates was efficacious, without significant nonhematopoietic toxicity [14–17],
METHODS
Dogs
The dogs were raised and housed at Fred Hutchinson Cancer Research Center (Fred Hutch) and given standard immunizations. The experimental protocol was approved by the Fred Hutch Institutional Animal Care and Use Committee, and the study was executed according to principles outlined in the Guide for the Care and Use of Laboratory Animals [18]. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care, International. The median age of the dogs was 11 (range, 7–20) months, and their median weight was 8.5 (range, 8–18) kg. DLA-identical littermates were selected on the basis of identity for highly polymorphic major histocompatibility complex class I and class II microsatellite markers, and identity for DLA-DRB1 alleles as determined by direct sequencing [19,20],
Antibodies and Antibody Conjugates
For targeted radioimmunotherapy, we used the anti-canine CD45 mAb CA12.10C12 (immunoglobulin IgGl) coupled to 211At [14–16], 211At was generated by irradiating bismuth metal with an MC-50 cyclotron (Scanditronix) at the University of Washington and isolated through a wet-chemistry method [21,22], Subsequent 211At-labeling of CA12.10C12-B10 was performed using the procedure of Chen et al. [17] Flow cytometry was performed as described by Sandmaier et al. [14]. The process of 211At production and isolation took approximately 3.5 hours, the mAb labeling/purification process took approximately 1 hour, and with transport between the two institutions, the 211At-labeled CA12.10C12 was ready for injection 6–7 hours after initiation of the target irradiation.
All radioactive materials were handled according to approved protocols at Fred Hutch and the University of Washington.
Experimental Design
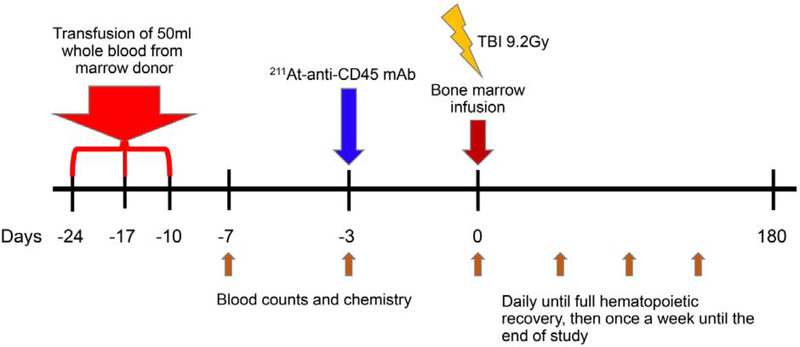
Recipient dogs received a total of three transfusions, each of 50 mL heparinized, unirradiated, whole blood from their respective DLA-identical donor dog on days -24, -17, -10 before transplant. Three days before transplant, dogs were given an intravenous injection of 211At-anti-CD45 mAb with doses ranging from 0.188 mCi/kg (7.0 MBq/kg) to 0.387 mCi/kg (14.3 MBq/kg). Dogs were given 0.05 mg/kg of unlabeled mAb to prevent nonspecific localization before injection of the 211At-anti-CD45 mAb. A total of 0.5 mg/kg of mAb was used. On day 0, dogs were given a single dose of 9.2 Gy TBI delivered at 7cGy/min from a 6 MEV Linear Accelerator (Varian CLINAC 4, Palo Alto, CA) followed by intravenous infusion of marrow grafts (mean, 6.3 × 108 total nucleated cells/kg; range, 1.3–8.3 × 108/kg) from their respective DLA-identical littermates. Proportions of CD34+ cells were determined by flow cytometry using mAb directed toward canine CD34, 1H6 (IgG1) [23], TBI dose de-escalation was performed with 7.5 Gy TBI. No post-grafting immunosuppression was administered. Dogs were monitored by regular blood counts and blood chemistry on days -7, -3, pre injection day 0, then daily for the first two months until full hematopoietic recovery, then once a week until the end of study (Figure 4). When necessary, dogs were supported with blood transfusions. Dogs were treated with ursodeoxycholic acid (7.5 mg/kg given orally twice a day), 10 days before transplantation up to day 180 [24,25],
Figure 4. Diagram of treatment plan.
Canine recipients were given three preceding transfusions of irradiated whole blood from DLA-identical marrow donors on days -24, -17, and -10. 211-At-labeled anti-CD45 mAb was given on day -3. TBI (9.2Gy) and bone marrow were given on day 0. Blood counts and chemistry were analyzed on days -7, -3, 0. They were evaluated daily until full hematopoietic recovery, then, once a week until the end of study.
Chimerism Analysis
Donor and host cell chimerism were evaluated using a polymerase chain reaction (PCR)-based assay of polymorphic (CA)n dinucleotide repeats with primers specific for informative microsatellite markers. Genomic DNA from the cells of interest was extracted, and PCR was performed under conditions described previously [26]. Engraftment was defined as greater than or equal to 5% mononuclear cell (MNC) donor chimerism, and graft rejection was defined as the first day when donor MNC chimerism was less than 5% without subsequent increase. Donor chimerism was assessed weekly until the end of study.
Mixed Leukocyte Cultures (MLCs)
Mixed leukocyte cultures (MLCs) were performed as described [27] to assess sensitization by donor blood transfusion to evaluate cellular immune function at baseline (day -24) and following donor transfusion before transplantation (day -3).
RESULTS
Hematopoietic Recovery after 9.2Gy TBI
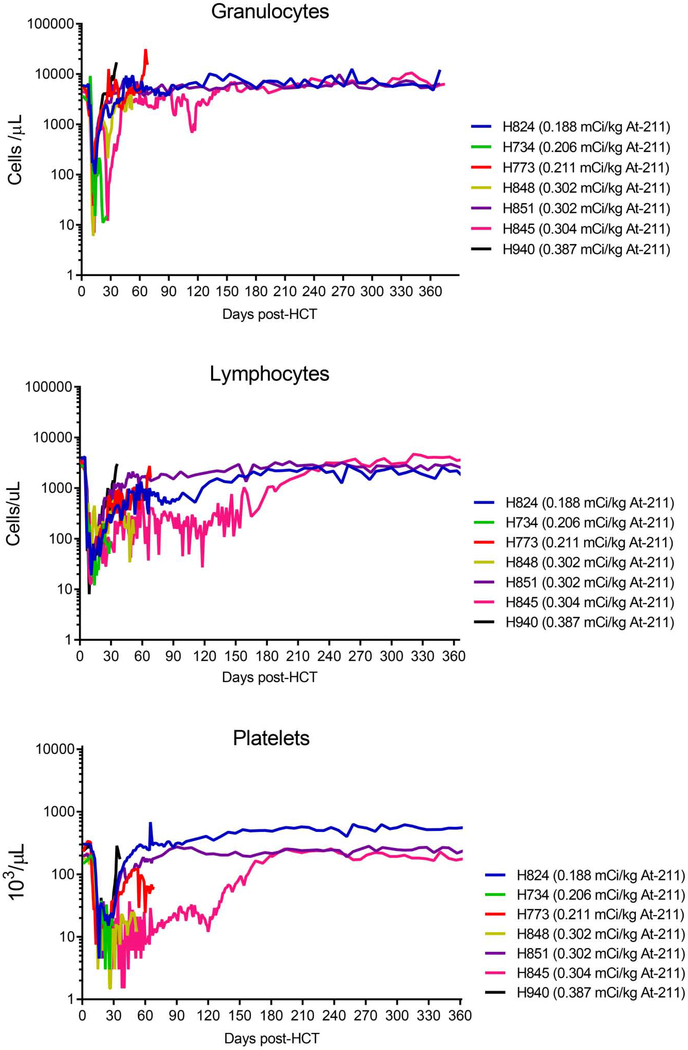
All seven dogs experienced marked cytopenia after conditioning with 211At-anti-CD45 mAb and 9.2 Gy TBI (Figure 1). The neutrophil nadir occurred at a median of 4 days post-transplant (range 4–6 days), with a mean neutrophil count of 20 (range; 6–103) cells/ μ L. The lymphocyte nadir occurred at a median of 3 days post-transplant (range 1–6 days), with a mean lymphocyte count of 18 (range; 8–35) cells/ μ L.
Figure 1. Hematology following 211At-anti-CD45 mAb conditioning with 9.2Gy TBI.
All dogs experienced neutropenia, lymphocytopenia, and thrombocytopenia with a median neutrophil nadir of 20 cells/μ L, median lymphocyte nadir of 18 cells/μ L and median platelet nadir of 4.5 × 103/μ L.
The platelet nadir occurred at a median of 8 days post-transplant (range 5–11 days), with a mean platelet count of 4.5 (range; 3–9) × 103/ μ L. Neutrophil recovery (defined as absolute neutrophil counts >1,000 cells/μ L) occurring at 11 days post-transplant (range 9–33 days), and the median platelet recovery (defined as >100,000 PLT/ μ l) occurring at 38 days post-transplant (range 25–150 days).
Chimerism
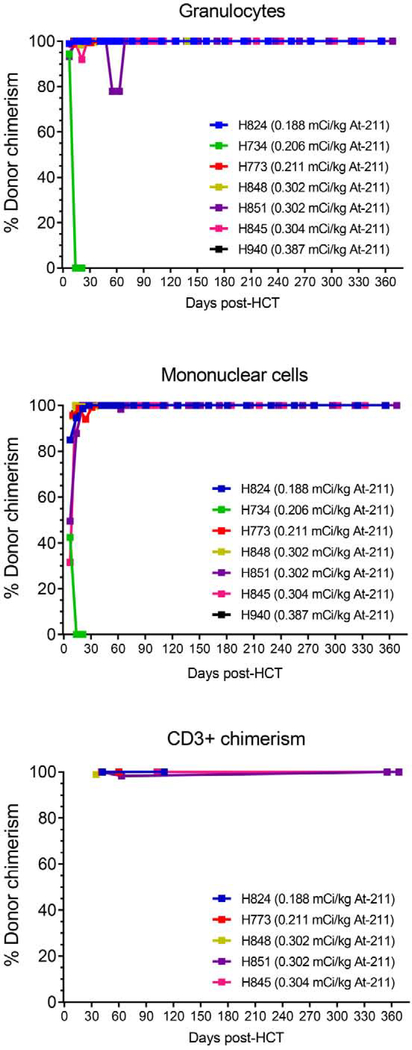
Seven dogs received 211At-labeled anti-CD45 mAb in addition to the 9.2Gy TBI (Table 1). All dogs tolerated both antibody infusion and the transplantation with no immediate side effects. Six of 7 dogs achieved 100% donor chimerism in mononuclear cells, granulocytes, and CD3+ T cells (Figure 2). Only one dog, H734, rejected the graft on day 14 after transplantation; it achieved a maximum chimerism of 42% on day 7 (in MNC); however, final chimerism declined to 0% by day 14. Of note, this dog received an inadequate number of marrow cells in its graft (1.33 × 108 total nucleated cells/kg containing 0.35 × 106 CD34+ cells/kg).
Table 1.
Transfusion sensitized DLA-identical HCT with 211At-anti-CD45 mAb and 9.2Gy TBI
| Dog ID | H824 | H734 | H773 | H848 | H851 | H845 | H940 |
|---|---|---|---|---|---|---|---|
| At-211 activity (mCi/kg) | 0.188 | 0.206 | 0.211 | 0.302 | 0.302 | 0.304 | 0.387 |
| TNC/kg (× 10s) | 5.4 | 1.33 | 8.31 | 7.45 | 4.22 | 6.27 | 6.52 |
| CD34+/kg (106) | 4.26 | 0.35 | 5.48 | 1.91 | 1.73 | 1.97 | 2.28 |
| Maximum chimerism (% MNC) | 100 | 42 | 100 | 100 | 100 | 100 | 100 |
| Final chimerism (% MNC) | 100 | 0 | 100 | 100 | 100 | 100 | 100 |
| Rejection | NO | YES | NO | NO | NO | NO | NO |
| Survival days | 367 | 21 | 60 | 44 | 368 | 367 | 28 |
| Cause of death (euthanized) | End of Study | Rejection | Liver toxicity | Pneumonia | End of Study | End of Study | Severe gastroenteritis |
A total of 7 dogs received 211At-anti-CD45 mAb. Six of seven dogs achieved 100% donor chimerism. H 734 rejected the graft.
DLA: dog leukocyte antigen; HCT: hematopoietic cell transplantation; TBI: total body irradiation; TNC: total nucleated cell count; MNC: mononuclear cell; GVHD: graft versus host disease.
Figure 2. Percentage of donor chimerism in granulocytes, mononuclear cells, and CD3+ cells in dogs treated with 211At-anti-CD45 mAb conditioning with 9.2Gy TBI DLA-identical HCT.
Six of seven dogs achieved 100% donor chimerism. H734 rejected the graft on day 14 after transplantation.
TBI Dose De-Escalation
Six dogs received 7.5 TBI as dose de-escalation and 211At-labeled anti-CD45 mAb ranging from 0.243 to 0.528 mCi/kg 211At (Table 2).
Table 2.
Transfusion sensitized DLA-identical HCT with 211At-anti-CD45 mAb and 7.5Gy TBI
| Dog ID | H911 | H920 | H923 | H935 | H933 | H929 |
|---|---|---|---|---|---|---|
| At-211 activity (mCi/kg) | 0.243 | 0.435 | 0.439 | 0.486 | 0.501 | 0.528 |
| TNC/kg (× 108) | 4.50 | 5.31 | 4.66 | 3.97 | 2.77 | 6.51 |
| CD34+/kg (106) | 1.22 | 1.32 | 2.38 | 2.91 | 0.99 | 3.50 |
| Maximum chimerism (% MNC) | 51 | 77.7 | 51.7 | 0.5 | 100 | 4.7 |
| Final Chimerism (% MNC) | 0 | 68.6 | 13.6 | 0 | 100 | 0 |
| Rejection | YES | NO | NO | YES | NO | YES |
| Survival days | 70 | 28 | 44 | 27 | 364 | 32 |
| Cause of death (euthanized) | Rejection | Sepsis | Bone marrow aplasia | Rejection | End of Study | Rejection |
A total of 6 dogs received 7.5 Gy TBI as dose de-escalation.
DLA: dog leukocyte antigen; HCT: hematopoietic cell transplantation; TBI: total body irradiation; TNC: total nucleated cell count; MNC: mononuclear cell.
Only one dog (H933) achieved 100% donor chimerism. 3 dogs rejected the graft (H911, H935, H929). A fifth dog, H923, was euthanized on day 44 due to poor condition with low counts with 13.6% donor chimerism, and the sixth dog, H920, on day 28 due to sepsis with 68.6% donor chimerism.
Cellular Immune Function Before Transplantation
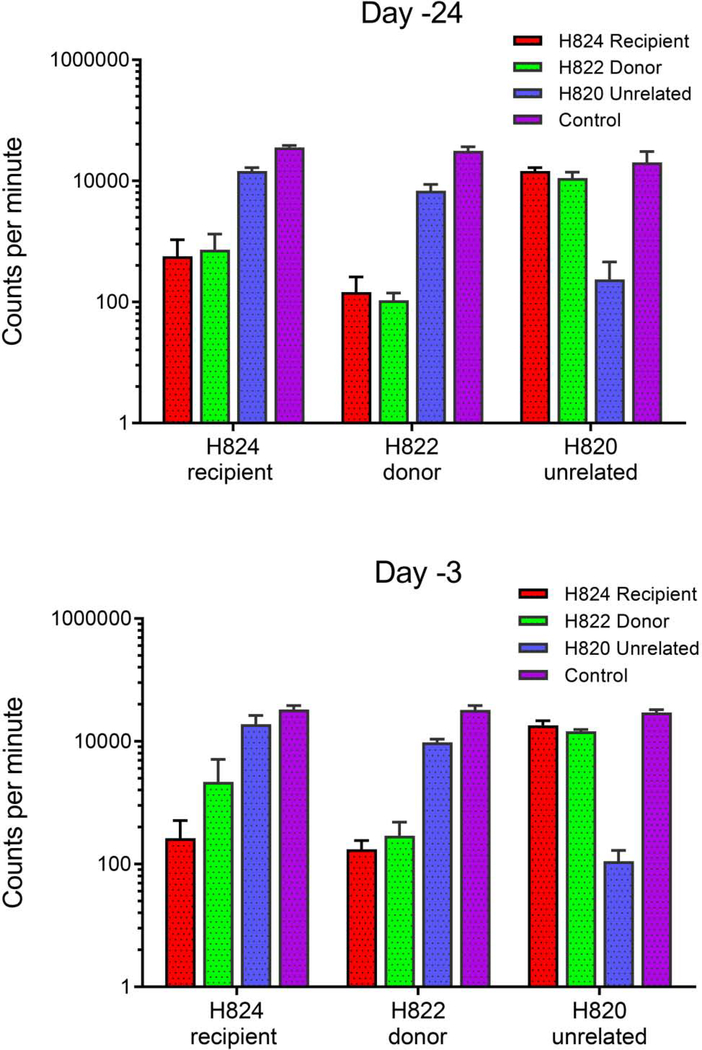
Mixed leukocyte cultures (MLC) were performed on 3 of the 12 dogs to evaluate immune function before transplantation at baseline and following sensitization. Data of one representative dog (H824) was shown (Figure 3). It was analyzed on day -24 and day -3 before transplantation. In Figure 3, data are expressed as mean counts per minute from triplicate wells of recipient response to donor cells, cells from an unrelated dog, and to concanavalin A (Con A) as positive control. 3H-thymidine uptake was higher in the recipient’s cells in response to the donor on day -3 compared to baseline (day -24). The recipient had increased thymidine uptake in response to donor cells on day -3, whereas there was no increase in the uptake in the donor cells in response to the recipient. This suggests that the recipient has been sensitized by transfusions and showed alloimmunization against donor.
Figure 3. Mixed leukocyte cultures.
Results from mixed leukocyte cultures performed on PBMC collected from a representative dog (H824) treated with 0.188 mCi/kg 211At-labled anti-CD45 mAb is shown here. Testing was performed on PBMC collected prior to sensitization (day -24) and one week after the last sensitization prior to infusion of the 211At-labled anti-CD45 mAb (day-3 before transplantation.) Data are expressed as mean counts per minute from triplicate wells of recipient response to donor cells, cells from an unrelated dog, and to concanavalin A as internal assay control.
Toxicity
All but one dog given 9.2Gy TBI with the 211At-labeled anti-CD45 mAb developed elevations of amino transferases (CTCAE grade 2–4) between day 23 and day 70. H773 died from liver toxicity on day 60. Histopathological evaluation showed collapse and regenerative hyperplasia of hepatocytes, which was not GVHD, but was suspected to be the adverse event of conditioning. H824, H851 and H845 showed transient increases of amino transferases, and their peaks came down to within normal limits after a few weeks. H940 developed severe gastroenteritis. The histologic findings showed severe mucosal necrosis in stomach, small bowel and duodenum, which were not indicative of GVHD. H848 developed grade II/III GVHD on skin without liver and intestine involvement. Four of six dogs with 7.5 Gy TBI showed elevation of amino transferases (CTCAE grade 2–4) between day 21 and day 42. Values in three dogs returned to normal within a few weeks, whereas H933 had elevated amino transferases (CTCAE grade 3) until the end of study. Histologic findings in that dog showed no significant changes in liver.
DISCUSSION
The aim of the current study was to assess whether 211 At-labeled anti-CD45 mAb combined with conditioning by 9.2 Gy TBI can overcome rejection of DLA-identical marrow grafts in transfusion--sensitized recipients. We previously demonstrated the feasibility of using a 211 At-labeled anti-CD45 mAb to promote engraftment of DLA-identical HCT in 8 non-sensitized, normal dogs [17]. Seven dogs had long-term donor mononuclear cell chimerism ranging from 19% to 58%, whereas 1 dog treated with the lowest 211At dose had 5% persisting donor mononuclear cell chimerism. Based on these results, we concluded that conditioning with 211At-labeled anti-CD45 mAb was minimally toxic and sufficiently immunosuppressive to allow stable long-term engraftment of DLA-identical marrow grafts.
Marrow graft rejection in the MHC-matched donor-recipient setting is effected by host T cells. This has been well documented in previous studies of in vitro reactivity of T-cells from patients with aplastic anemia against cells from their respective HLA-identical sibling marrow donors [28,29], A contribution of host B cells to graft rejection can be ruled out since, as a rule, it has been impossible to raise antibodies to minor histocompatibility antigens in various animal species and in humans. A growing number of human minor antigens has been identified using T lymphocytes from patients with graft rejection or GVHD [30,31],
The host anti-donor T-cell immunity induced by only three blood transfusions from the donor was powerful and could not be overcome by a conditioning regimen of high-dose (9.2 Gy) TBI, as shown by uniform rejection of marrow grafts (27 of 27 dogs in historic controls). This finding contrasted sustained engraftment in 61 of 62 dogs not given preceding transfusions (Table 3). The current study demonstrated that the powerful host T-cell responses could be overcome when targeted RIT with the 211At- anti-CD45 mAb was added to 9.2 Gy TBI conditioning since six of seven dogs showed sustained marrow engraftment.
Table 3.
Comparison with prior studies*
| 50 mL Blood transfusions, days | Conditioning | Dogs (n) | Graft rejection (%) |
|---|---|---|---|
| None[26,27] | 9.2Gy TBI | 62 | 2 |
| Marrow donor day-24, day-17, day-10 [4,26] | 9.2Gy TBI | 27 | 100 |
| Marrow donor day-24, day-17, day-10 | 9.2Gy TBI with 211At-anti-CD45 mAb | 7 | 14 |
In our well-established canine model, following 9.2Gy TBI conditioning, only 2% (1/62) of un-transfused dogs rejected the marrow grafts from their DLA-identical littermate donors. However, 100% (27/27) of the dogs pretreated with three unirradiated transfusions from their respective DLA-identical marrow donors rejected their grafts. 211At-anti-CD45 mAb in combination with 9.2Gy TBI is successful in abrogating graft. Rejection was only 14% (1/7) of dogs in the pre-sensitization model. n: number; TBI: total body irradiation.
Only one dog (H734) rejected the graft, an event that was likely due to the fact that this dog’s graft consisted of inadequate numbers of overall nucleated cells and CD34+ cells.
Two severe adverse events were seen; liver toxicity and gastroenteritis. The histologic findings with liver toxicity were collapse and regenerative hyperplasia. It was not consistent with GVHD, but likely due to radiation-induced hepatic injury. In previous studies, the biodistribution of 211At-labeled mAb showed higher accumulation in the liver than in other nontarget organs, as is typical for radioimmunotherapy because of internalization of circulating immunoglobulins by hepatic Kupffer cells and endothelial cells [32,33], In the prior dose escalation study of 211At-anti-CD45 mAb, hepatic aberrations of clinical relevance were observed in dogs treated with more than 15.0 MBq/kg [22]. Our conclusion was radiation-induced hepatic damage is dose dependent. In our previous studies at optimal dose of 211At, liver toxicity was transient and reversible [17,32,33].
In the current study hepatotoxicity and gastroenteritis were likely due to combined effects of the alpha-emitter and the high-dose gamma radiation delivered by the linear accelerator. For clinical translation, the targeted RIT is combined with a chemotherapy-based conditioning regimen using fludarabine, cyclophosphamide and low-dose TBI. The trial has recently opened to accrual (NCT04083183).
An attempted dose de-escalation of TBI to 7.5 Gy resulted in a sustained engraftment rate of only 50%, re-emphasizing the powerful responses of host T cells to minor antigens expressed on cells in the transfusion product.
In conclusion, 211At-anti-CD45 mAb in combination with 9.2 Gy TBI as conditioning is successful in establishing DLA-identical grafts. The addition of 211At-anti-CD45 mAb to conditioning may serve as a novel promising strategy to overcome graft rejection in red cell disorders and other nonmalignant conditions treated with allogenic transplantation.
HIGHLIGHTS.
Canine model of transfusion-induced sensitization and marrow graft rejection
Alpha-emitter astatine-211 is a very high energy isotope with a short path length
Astatine-211 labeled anti-CD45 antibody targets radiation to cells causing rejection
Astatine-211 anti-CD45 can prevent graft rejection in sensitized recipients
ACKNOWLEDGMENTS
We are grateful to the pathology support from George Sale, MD. We thank Michele Spector, DVM for veterinary support and the technicians at the canine facilities at Fred Hutch. We greatly appreciate Helen Crawford’s assistance with manuscript and figure preparation.
Funding: Research funding was provided by the National Institutes of Health, Bethesda, MD, grants P01 HL122173 from the National Heart, Lung, and Blood Institute and P30 CA015704 from the National Cancer Institute. DLA typing was provided by the Core Center of Excellence in Hematology (CCEH) funded by U54 DK106829 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes.
Footnotes
Disclosure
The authors declare no conflicts of interest in relation to the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105:627–633. [PubMed] [Google Scholar]
- 2.Storb R, Rudolph RH, Graham TC, Thomas ED. The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol. 1971;107:409–413. [PubMed] [Google Scholar]
- 3.Storb R, Weiden PL, Deeg HJ, et al. Rejection of marrow from DLA-identical canine littermates given transfusions before grafting: antigens involved are expressed on leukocytes and skin epithelial cells but not on platelets and red blood cells. Blood. 1979;54:477–484. [PubMed] [Google Scholar]
- 4.Bean MA, Storb R, Graham T, et al. Prevention of transfusion-induced sensitization to minor histocompatibility antigens on DLA-identical canine marrow grafts by gamma irradiation of marrow donor blood. Transplantation. 1991;52:956–960. [DOI] [PubMed] [Google Scholar]
- 5.Bean MA, Graham T, Appelbaum FR, et al. Gamma-irradiation of pretransplant blood transfusions from unrelated donors prevents sensitization to minor histocompatibility antigens on dog leukocyte antigen-identical canine marrow grafts. Transplantation. 1994;57:423–426. [DOI] [PubMed] [Google Scholar]
- 6.Storb R, Floersheim GL, Weiden PL, et al. Effect of prior blood transfusions on marrow grafts: Abrogation of sensitization by procarbazine and antithymocyte serum. Journal of Immunology. 1974;112:1508–1516. [PubMed] [Google Scholar]
- 7.Weiden PL, Storb R, Slichter S, Warren RP, Sale GE. Effect of six weekly transfusions on canine marrow grafts: Tests for sensitization and abrogation of sensitization by procarbazine and antithymocyte serum. J Immunol. 1976;117:143–150. [PubMed] [Google Scholar]
- 8.Iannone R, Casella JF, Fuchs EJ, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and β-thalassemia. Biology of Blood and Marrow Transplantation. 2003;9:519–528. [DOI] [PubMed] [Google Scholar]
- 9.McDevitt MR, Sgouros G, Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. [Review]. Eur J Nucl Med. 1998;25:1341–1351. [DOI] [PubMed] [Google Scholar]
- 10.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted {alpha}-particle therapy (Review). J Nucl Med. 2005;46 (Suppl. 1): 199S–204S. [PubMed] [Google Scholar]
- 11.Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60:1402–1406. [DOI] [PubMed] [Google Scholar]
- 12.Andres TL, Kadin ME. Immunologic markers in the differential diagnosis of small round cell tumors from lymphocytic lymphoma and leukemia. American Journal of Clinical Pathologv. 1983;79:546–552. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell CW, Patterson WP, Hakami N. Alterations of HLe-1 (T200) fluorescence intensity on acute lymphoblastic leukemia cells may relate to therapeutic outcome. Leukemia Research. 1987; 11:103–106. [DOI] [PubMed] [Google Scholar]
- 14.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100:318–326. [DOI] [PubMed] [Google Scholar]
- 15.Bethge WA, Wilbur DS, Storb R, et al. Radioimmunotherapy with Bismuth-213 as conditioning for nonmyelo ablative allogeneic hematopoietic cell transplantation in dogs: a dose deescalation study. Transplantation. 2004;78:352–359. [DOI] [PubMed] [Google Scholar]
- 16.Nakamae H, Wilbur DS, Hamlin DK, et al. Biodistributions, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the alpha-emitting radionuclides bismuth-213 or astatine-211. Cancer Res. 2009;69:2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using alpha-emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 19.Wagner JL, Burnett RC, DeRose SA, Lrancisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. [DOI] [PubMed] [Google Scholar]
- 20.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication). Tissue Antigens. 1998;52:397–401. [DOI] [PubMed] [Google Scholar]
- 21.Wilbur DS, Chyan MK, Nakamae H, et al. Reagents for astatination of biomolecules. 6. An intact antibody conjugated with a maleimido-closo-decaborate(2-) reagent via sulfhydryl groups had considerably higher kidney concentrations than the same antibody conjugated with an isothiocyanato-closo-decaborate(2-) reagent via lysine amines. Bioconjug Chem. 2012;23:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilbur DS, Vessella RL, Stray JE, Goffe DK, Blouke KA, Atcher RW. Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab')2. In vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab')2. Nucl Med Biol. 1993;20:917–927. [DOI] [PubMed] [Google Scholar]
- 23.McSweeney PA, Rouleau KA, Wallace PM, et al. Characterization of monoclonal antibodies that recognize canine CD34. Blood. 1998;91:1977–1986. [PubMed] [Google Scholar]
- 24.Storb R, Yu C, Wagner JF, et al. Stable mixed hematopoietic chimerism in DFAidentical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 25.Fadiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–15. [PubMed] [Google Scholar]
- 26.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 27.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 28.Hansen JA, Clift RA, Thomas ED, Buckner CD, Mickelson EM, Storb R. Histocompatibility and marrow transplantation. Transplantation Proceedings. 1979;11:1924–1929. [PubMed] [Google Scholar]
- 29.Livnat S, Seigneuret M, Storb R, Prentice RL. Analysis of cytotoxic effector cell function in patients with leukemia or aplastic anemia before and after marrow transplantation. Journal of Immunology. 1980;124:481–490. [PubMed] [Google Scholar]
- 30.Spierings E, Goulmy E. Human minor histocompatibility antigens: recognition and application for hematopoietic transplantation. In: Atkinson K, Champlin R, Ritz J, Fibbe WE, Ljungman P, Brenner MK, eds. Clinical Bone Marrow and Blood Stem Cell Transplantation. Third ed. Cambridge, UK: Cambridge University Press; 2014:1,968. [Google Scholar]
- 31.Warren EH. Biology of the human graft-versus-tumor repsonse and how to exploit it. In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, eds. Thomas' Hematopoietic Cell Transplantation, 5th Edition. Chichester, UK: John Wiley & Sons, Ltd; 2016:166–177. [Google Scholar]
- 32.Johansson AG, Lovdal T, Magnusson KE, Berg T, Skogh T. Liver cell uptake and degradation of soluble immunoglobulin G immune complexes in vivo and in vitro in rats. Hepatology. 1996;24:169–175. [DOI] [PubMed] [Google Scholar]
- 33.Frost SH, Miller BW, Back TA, et al. Alpha-imaging confirmed efficient targeting of CD45-positive cells after 211 At-radioimmunotherapy for hematopoietic cell transplantation. J Nucl Med. 2015;56:1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]