Abstract
Objective:
Circulating nucleosomes and their component histones have been implicated as pathogenic in sepsis and acute respiratory distress syndrome (ARDS) in adults. However, their role in pediatric ARDS is unknown.
Design:
We performed a prospective cohort study in children with ARDS, with plasma collection within 24 hours of ARDS onset. We associated nucleosome levels with severity of ARDS and with non-pulmonary organ failures and tested for association of nucleosomes with pediatric intensive care unit (PICU) mortality and ventilator-free days (VFDs) at 28 days in univariate and multivariable analysis. We also performed proteomics of DNA-bound plasma proteins in a matched case-control study of septic children with and without ARDS in order to identify specific histone proteins elevated in ARDS.
Setting:
Large academic tertiary care PICU.
Patients:
Intubated children meeting Berlin criteria for ARDS.
Interventions:
None.
Measurements and Main Results:
We enrolled 333 children with ARDS, with 69 (21%) non-survivors. Plasma nucleosomes were correlated with ARDS severity and with the number of non-pulmonary organ failures at ARDS onset. Nucleosomes were higher (p < 0.001) in non-survivors (0.40 [interquartile range 0.20, 0.71] arbitrary units) relative to survivors (0.10 [IQR 0.04, 0.25] AU). Nucleosomes were associated with PICU mortality in multivariable analysis (adjusted odds ratio 1.84 per 1 standard deviation increase, 95% confidence interval 1.38 to 2.45, p < 0.001). Nucleosomes were also associated with a lower probability of being extubated alive by day 28 after multivariable adjustment (adjusted subdistribution hazard ratio 0.74, 95% CI 0.63 to 0.88, p = 0.001). Proteomic analysis demonstrated higher levels of the core nucleosome histones H2A, H2B, H3, and H4 in septic children with ARDS, relative to septic children without ARDS.
Conclusions:
Plasma nucleosomes are associated with ARDS severity, non-pulmonary organ failures, and worse outcomes in pediatric ARDS.
Keywords: histones, damage associated molecular patterns, DAMPs, ARDS, PARDS
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a syndrome of acute non-cardiogenic pulmonary edema which has primarily been defined for and studied adults (1, 2), despite longstanding awareness of the existence of the syndrome in children (3–5). In 2015, the Pediatric Acute Lung Injury Consensus Conference (PALICC) developed a pediatric-specific definition for pediatric ARDS (6). Epidemiological studies using the PALICC definition have begun to dissect the epidemiology of pediatric ARDS (7–9). Relative to adults, ARDS occurs less frequently in children and has a lower mortality rate (7, 9, 10). However, while the epidemiology of pediatric ARDS has begun to be investigated, less attention has been devoted to its pathogenesis.
Damage associated molecular patterns (DAMPs) are upstream mediators of sepsis and ARDS. Nucleosomes are histone/DNA byproducts of chromatin degradation released after cell death, and are implicated in sepsis (11–14), aspiration (15), and trauma-related ARDS (16) in adults. Normally localized to the nucleus, nucleosomes released into the circulation are directly cytotoxic (12, 14, 16) and induce platelet aggregation (17), thereby offering a mechanism linking diverse inciting insults with lung injury. In a pilot study, we showed plasma nucleosomes were associated with mortality in pediatric ARDS (18). The small sample size and the low mortality rate in that cohort precluded a complete assessment of the relevance of nucleosomes, and the role of nucleosomes in pediatric ARDS remains largely unexplored. We therefore aimed in this current study to understand the role of nucleosomes in pediatric ARDS, and specifically tested the association of nucleosomes with the development of organ failures and the association with clinical outcomes. Additionally, we used proteomics to identify the specific histone proteins comprising the circulating nucleosomes in pediatric ARDS in order to identify potential therapeutic targets. We hypothesized that higher nucleosome levels are associated with worse outcomes in pediatric ARDS.
METHODS
Study Design and Patient Selection
This was a prospective cohort study of children with Berlin-defined (2) ARDS enrolled at the Children’s Hospital of Philadelphia (CHOP) between July 2014 and December 2019. The study was approved by the CHOP Institutional Review Board (CHOP IRB 13–010578), and informed consent was obtained prior to enrollment. Consecutive pediatric intensive care unit (PICU) patients were screened daily. Inclusion criteria were 1) acute respiratory failure requiring invasive ventilation, 2) arterial access, 3) age > 1 month and < 18 years, 4) two consecutive PaO2/FIO2 ≤ 300 ≥ 1 hour apart on positive end-expiratory pressure (PEEP) ≥ 5 cmH2O, and 5) bilateral parenchymal infiltrates. Exclusion criteria were 1) respiratory failure primarily from cardiac failure, 2) underlying chronic respiratory disease, 3) ventilator dependence, 4) mixing cyanotic heart disease, 5) ventilation for > 7 days before PaO2/FIO2 ≤ 300, 6) ARDS established outside of the CHOP PICU, and 7) inability to obtain consent. As the study was initiated prior to the PALICC definitions of pediatric ARDS, we did not screen based on oxygenation index (OI); however, all patients met PALICC criteria. The first 67 subjects have been previously reported (18).
Equations and Definitions
Metrics of oxygenation utilized were PaO2/FIO2 and OI ((mean airway pressure x FIO2 x 100)/ PaO2). The vasopressor score (19, 20) was: dopamine dose (μg/kg/min) x 1 + dobutamine (μg/kg/min) x 1 + epinephrine (μg/kg/min) x 100 + norepinephrine (μg/kg/min) x 100 + phenylephrine (μg/kg/min) x 100 + milrinone (μg/kg/min) x 10 + vasopressin (U/kg/min) x 10,000. Non-pulmonary organ failures at time of ARDS diagnosis were identified using accepted definitions (21). Severity of illness was scored using the Pediatric Risk of Mortality (PRISM) III at 12 hours. The designation “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency (22, 23).
Etiology of ARDS was determined prospectively as previously described (10). Infectious pneumonia, aspiration, drowning, pulmonary contusion, and smoke inhalation were considered direct ARDS; non-pulmonary sepsis, non-thoracic trauma, non-cardiogenic shock, transfusion-related acute lung injury, and pancreatitis were indirect. Infectious pneumonia and non-pulmonary sepsis were considered infectious ARDS; all other etiologies were non-infectious.
Exposure and Outcomes
The primary exposure was nucleosome levels, and the primary outcome PICU mortality. We also reported ventilator-free days (VFDs) at 28 days and length of ventilation in survivors. “Mechanical ventilation” denoted “invasive ventilation;” non-invasive support was not counted. For VFD and duration of ventilation, the first day was ARDS onset. Extubation for > 24 hours defined duration of ventilation. Patients requiring re-initiation of invasive ventilation had extra days counted towards total ventilator days. VFD were determined by subtracting total ventilator days from 28 in survivors. All patients with total ventilator days ≥ 28 days, and all PICU non-survivors were assigned VFD = 0.
Plasma Collection and Nucleosome Measurements
Blood was collected within 24 hours of ARDS onset (time of meeting all Berlin criteria) in citrated tubes, centrifuged within 30 minutes of collection (2000 g, 20 minutes, 20C), aliquoted to prevent freeze/thaw cycles, and stored at −80C until analysis. Platelet levels closest to blood draw were recorded. Nucleosomes were measured in duplicate using an enzyme-linked immunosorbent assay (ELISA; Cell Death detection ELISAPLUS, Roche; Basel, Switzerland). Nucleosome levels are reported as arbitrary units (AU), which are individual sample values normalized to the positive control included in the kit. Inter-assay coefficient of variation was 5.2%. Using the approximate odds ratio (OR) for the association between nucleosomes and mortality in our pilot study (18), and assuming an overall mortality rate of 20% for the cohort, 325 total subjects would provide 80% power to detect OR = 2.0 for the association between nucleosomes (per change in one standard deviation [SD]) and PICU mortality at α = 0.05. We estimated that this sample size would allow adequate adjustment of confounding.
Histone Co-Immunoprecipitation and Identification
We were interested in identifying specific histones comprising the nucleosomes of children with ARDS. Therefore, we performed a case-control study of 12 ARDS cases and 12 non-ARDS controls. We identified 12 controls meeting Sepsis-3 criteria (24), and matched these 12 septic controls without ARDS to 12 septic cases with ARDS based on age strata (≤ 2 years, 2 to 6 years, 6 to 12 years, 12 to 15 years, 15 to < 18 years), PRISM III severity of illness score (± 2), and pulmonary versus non-pulmonary source of sepsis. We ensured that the 12 cases with ARDS met Sepsis-3 criteria by confirming organ dysfunction and lactate levels. Measuring 12 samples per group provided 80% power to detect a 50% difference in histone levels at α = 0.05, similar to the difference in nucleosome levels between ARDS and non-ARDS children in our pilot study (18). We performed co-immunoprecipitation on plasma using anti-ds DNA antibody as bait to isolate DNA-bound proteins. The resulting elutions were then subject to liquid chromatography/tandem mass spectrometry for protein identification. After filtering, histone subtypes were compared between subjects with and without ARDS (Supplementary Methods).
Statistical Analysis
Clinical data were reported as median [interquartile range, IQR], and differences between groups compared using non-parametric statistics. Cuzick’s non-parametric test of trend was used to assess for monotonic trends across ordered groups (25). Categorical data were compared using Fisher exact test. Histone subtypes were compared using paired t-tests followed by Benjamini-Hochberg correction for false discovery rate. To test the association of nucleosomes with mortality, we performed multivariable logistic regression in a causal framework adjusting a priori for age, ARDS etiology, immunocompromised status, and OI at ARDS onset. Variables were chosen as plausible confounders of the relationship between nucleosomes and mortality. We purposely chose not to include metrics of severity of illness (PRISM III, vasopressor score), OI at 24 hours, or organ failures as they were potential mediators of the association between nucleosomes and outcome, although we did test whether controlling for OI at 24 hours would affect results. Because we have previously shown that different clinical subtypes of ARDS have different predictors of mortality (10), we assessed for effect modification by clinical categorization of ARDS (direct versus indirect; infectious versus non-infectious) and by immunocompromised status. We also tested the association between nucleosomes and the probability of extubation using multivariable Fine and Gray competing risk regression, treating extubation as the primary outcome and death as a competing risk (26, 27), censoring at day 28. Effect sizes are presented as ORs or as subdistribution hazard ratios (SHRs) per change in one SD (~0.25 AU) of plasma nucleosome concentration. Analyses were performed with Stata/SE 14.2 (College Station, TX).
RESULTS
Description of the Cohort
During the study period, 333 children with ARDS were enrolled (Supplementary Figure 1), with 69 (21%) non-survivors (Table 1). Non-survivors had higher severity of illness, were more likely to be immunocompromised, and more commonly had non-pulmonary sepsis as an ARDS etiology (all p < 0.001). Non-survivors had worse oxygenation and ventilator pressures 24 hours after ARDS onset.
Table 1:
Demographics of the ARDS cohort
| Variables |
Whole cohort (n = 333) |
Survivors (n = 264) |
Non-survivors (n = 69) |
p value |
| Age (years) | 5.8 [1.8, 13.1] | 5.4 [1.9, 13.1] | 7.2 [1.8, 13.3] | 0.744 |
| Female (%) | 149 (45) | 119 (45) | 30 (43) | 0.892 |
| Severity of illness PRISM III at 12 hours Non-pulmonary organ failures Vasopressor score |
11 [6, 17] 2 [1, 3] 8 [0, 18] |
10 [5, 16] 1 [1, 2] 7 [0, 15] |
16 [10, 23] 3 [2, 4] 20 [, 58] |
< 0.001 < 0.001 < 0.001 |
| Co-morbidities (%) Immunocompromised Stem cell transplant |
84 (25) 39 (12) |
46 (17) 15 (6) |
38 (55) 24 (35) |
< 0.001 < 0.001 |
| Cause of ARDS (%) Direct Indirect |
237 (71) 96 (29) |
201 (76) 63 (24) |
36 (52) 33 (48) |
< 0.001 |
| Cause of ARDS (%) Infectious Non-infectious |
245 (74) 88 (26) |
197 (75) 67 (25) |
48 (70) 21 (30) |
0.444 |
| Cause of ARDS (%) Infectious pneumonia Non-pulmonary sepsis Aspiration pneumonia Other |
162 (49) 83 (25) 50 (15) 38 (11) |
144 (55) 53 (20) 39 (15) 28 (11) |
18 (26) 30 (43) 11 (16) 10 (14) |
< 0.001 |
| ARDS onset PaO2/FIO2 OI ΔP (PIP minus PEEP) |
153 [99, 225] 11.1 [7.3, 19.3] 21 [17, 25] |
155 [105, 225] 11 [7.2, 17.4] 21 [17, 25] |
142 [77, 228] 11.8 [8.3, 29.1] 22 [18, 25] |
0.258 0.021 0.307 |
| 24 hours after onset PaO2/FIO2 OI ΔP (PIP minus PEEP) |
223 [155, 288] 7.4 [4.8, 12.9] 17 [14, 21] |
231 [165, 304] 6.7 [4.5, 11.5] 17 [13, 21] |
182 [118, 260] 11.4 [6.2, 21.6] 19 [16, 23] |
0.001 < 0.001 0.010 |
| Ancillary therapies in first 72 hours Inhaled nitric oxide Neuromuscular blockade Corticosteroids Alternative ventilator modes |
124 (37) 172 (52) 165 (50) 83 (25) |
88 (33) 131 (50) 123 (47) 60 (23) |
36 (52) 41 (59) 42 (61) 23 (33) |
0.005 0.176 0.042 0.085 |
| Nucleosomes (arbitrary units) | 0.14 [0.05, 0.34] | 0.10 [0.04, 0.25] | 0.40 [0.20, 0.71] | < 0.001 |
ARDS: acute respiratory distress syndrome; OI: oxygenation index; PRISM III: Pediatric Risk of Mortality III; ΔP: driving pressure; PIP: peak inflating pressure; PEEP: positive end-expiratory pressure
Nucleosomes Associated with ARDS Severity and Organ Failures
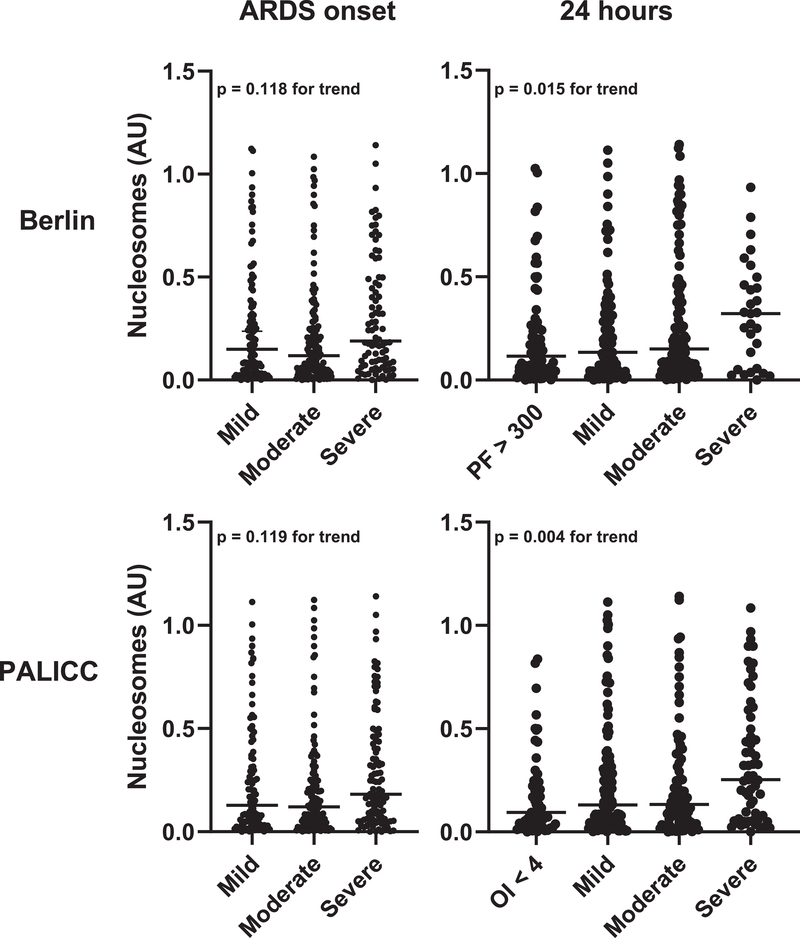
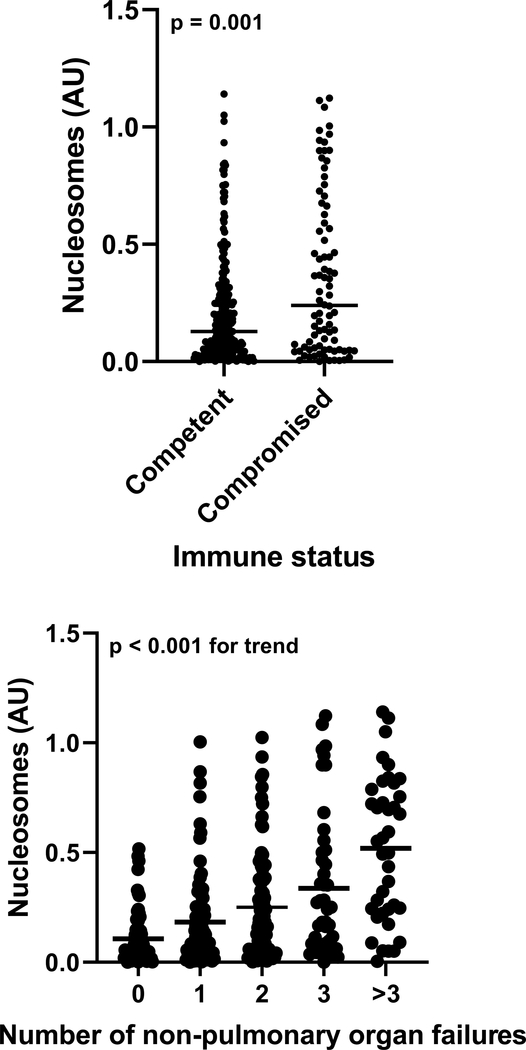
Plasma nucleosomes were correlated with both Berlin (p = 0.015) and PALICC (p = 0.004) oxygenation categories at 24 hours, but not at ARDS onset (Figure 1). Nucleosomes were higher in indirect etiologies of ARDS (Supplementary Figure 2) and in immunocompromised subjects (Figure 2). Nucleosomes were correlated with the number of non-pulmonary organ failures at ARDS onset (Figure 2), and were higher in every individual organ failure assessed (Supplementary Figure 3). When specifically examining platelet levels as a marker of hematologic failure, nucleosome levels were indirectly correlated with platelet levels in both the whole cohort and when the analysis was restricted to immunocompetent subjects (Supplementary Figure 4).
Figure 1:
Association between nucleosomes and Berlin (top) or PALICC (bottom) oxygenation categories, both at ARDS onset (left) and 24 hours later (right). P values represent results of a non-parametric test of trend.
Figure 2:
Association between nucleosomes and immunocompromised status and number of non-pulmonary organ failures. P values represent results of a rank-sum test or of a non-parametric test of trend.
Nucleosomes Associated with Worse Outcomes
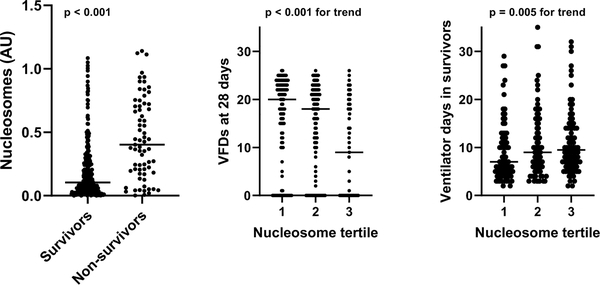
Nucleosomes were higher in non-survivors (0.40 [IQR 0.20, 0.71] AU) relative to survivors (0.10 [IQR 0.04, 0.25] AU), and higher nucleosome levels correlated with fewer VFDs and more ventilator days in survivors (Figure 3). Nucleosomes were associated with PICU mortality in multivariable analysis (adjusted OR 1.84 per 1 SD increase, 95% CI 1.38 to 2.45, p < 0.001)(Table 2). The effect size was nearly identical when adjusting for OI at 24 hours, rather than OI at ARDS onset (adjusted OR 1.87, 95% CI 1.40 to 2.49, p < 0.001). There was evidence of effect modification by direct/indirect classification (p = 0.011 for interaction), with a stronger association in direct ARDS. Nucleosomes were associated with a lower probability of being extubated alive (i.e., fewer VFDs) by day 28 (adjusted SHR 0.74, 95% CI 0.63 to 0.88, p = 0.001)(Supplementary Table 1), with no evidence for effect modification.
Figure 3:
Association of plasma nucleosomes with PICU mortality, ventilator-free days (VFDs) at 28 days, and duration of invasive mechanical ventilation in survivors. P values represent results of a rank-sum test or of a non-parametric test of trend.
Table 2:
Association of nucleosomes with PICU mortality
| Model | Odds ratio (95% confidence interval) | p value | Interaction p value |
|---|---|---|---|
| Unadjusted | 2.34 (1.80 to 3.04) | < 0.001 | - |
| Adjusted | 1.84 (1.38 to 2.45)a | < 0.001 | - |
| Stratified analyses | |||
| Direct Indirect |
2.91 (1.78 to 4.76)a 1.39 (0.96 to 2.01)a |
< 0.001 0.077 |
0.011 |
| Infectious Non-infectious |
1.87 (1.31 to 2.67)a 2.36 (1.28 to 4.34)a |
0.001 0.006 |
0.901 |
| Immunocompetent Immunocompromised |
2.06 (1.32 to 3.22)b 1.83 (1.22 to 2.75)b |
0.001 0.004 |
0.333 |
Adjusted for age, ARDS etiology, immunocompromised status, OI at onset
Adjusted for age, ARDS etiology, OI at onset
Identification of Histones
In order to identify which specific DNA-bound histones were enriched in ARDS, we compared proteins in matched subjects with and without ARDS (Supplementary Table 2). Co-immunoprecipitation and proteomics identified several differentially expressed histones. After Benjamini-Hochberg correction, two histone 2 (H2AX and H2BC12) and one each of H3 and H4 were significantly higher (at a false discovery rate < 0.05) in children with ARDS, relative to those without (Supplementary Figure 5).
DISCUSSION
Plasma nucleosomes are elevated in pediatric ARDS non-survivors, correlate with severity of ARDS and with non-pulmonary organ failures, and are independently associated with mortality and VFDs. Proteomic analysis confirmed higher levels of the nucleosome core histones H2A, H2B, H3, and H4 in children with ARDS. Our results support nucleosomes and their component histones as clinically significant DAMPs in pediatric ARDS, and a viable potential therapeutic target (28, 29).
Nucleosomes can be released from any dying cell, including inflammatory cells like neutrophils during neutrophil extracellular trap (NET) formation (30, 31). Nucleosomes are also released from tissue cells dying via either regulated or unregulated pathways. In the circulation, the component histones and cell-free nuclear DNA act as DAMPs (11, 32). The strong association of nucleosomes with organ failures in our study is consistent with nucleosomes as markers of cell stress and death. Furthermore, histones have previously been implicated in adult ARDS (15, 16, 18, 29) and in pre-clinical models (11, 16, 29, 33). Histones can propagate lung injury (15, 16, 29, 33) and worsen myocardial dysfunction and shock in animal models (13, 34, 35), consistent with their association with ARDS severity and non-pulmonary organ failures in our study. Exogenous histone administration can induce NETs (14), suggesting a feedback loop wherein NETosis and tissue death released during the initial insult release nucleosomes into the circulation, which in turn induce further NET formation.
Alternatively, it is possible that the major deficit is in nucleosome clearance, rather than excess production. Nucleosomes are normally cleared rapidly in circulation, primarily by the liver (36). However, it is possible that sepsis and ARDS have reduced nucleosome clearance due to sub-clinical or overt liver dysfunction during the acute phase of inflammation and shock. This would contribute to the persistence of nucleosomes in circulation to propagate the inflammatory insult as a DAMP. Indeed, nucleosome levels were higher in subjects with any organ failure, including hepatic.
Exogenous histones can also induce platelet aggregation and thrombocytopenia within minutes in mice (17), and elevated levels of histones are associated with thrombocytopenia in critically ill adults (37). Given the association between thrombocytopenia and poor clinical outcomes, we specifically investigated whether this was present in our cohort. Indeed, higher nucleosome levels were associated with lower concurrent platelet levels, consistent with a pathogenic role for nucleosomes in pediatric ARDS reflected, in part, by circulating platelet levels.
Recent studies have offered potential clues for mitigating histone-associated toxicity, primarily via direct scavenging. Exogenous administration of C1 esterase inhibitor has been shown to bind histones and attenuate cytotoxicity in a bleomycin-induced lung injury mouse model (29). Additionally, complexes of C1 esterase inhibitor bound to histones have been recovered from the alveolar fluid of adults with ARDS. Additional studies have also implicated heparin as a potential therapeutic agent which exerts its beneficial effects in part via direct histone binding, thereby preventing histone-induced cytotoxicity (28) and histone-induced platelet aggregation (17).
In proteomic analysis, we identified histone subtypes H2AX, H2BC12, H3, and H4 as elevated in septic children with ARDS, relative to matched septic non-ARDS. H2A, H2B, H3, and H4 comprise the nucleosome core (38). H2AX is a variant of H2A enriched in cells experiencing stress and ds DNA breaks where it localizes (39–41). H2AX is also increased during apoptosis (40), and enrichment of H2AX is suggestive of apoptotic cells as a likely source for nucleosomes. Additional proteomic analyses may be able to identify specific post-translational modification (PTMs), such as citrullination of H3, a hallmark of NETosis (42–44). While we did not find specific PTMs on histones using liquid chromatography/tandem mass spectrometry in our study, it is possible that this is a limitation of the sensitivity of the technology. This remains an area of interest, as it is possible that identification of PTMs would provide insight regarding the origin of circulating histones and nucleosomes, which is currently speculative.
Our study has limitations. Patients were from a single center, and while severity of illness and ARDS demographics are similar to other published pediatric cohorts (7, 45), generalizability cannot be assumed. We required an arterial blood gas for eligibility, and so may have missed subjects with ARDS lacking a diagnostic PaO2. It is possible that a study using PALICC criteria, screening using OI and allowing SpO2-based stratification, would come to different conclusions. However, we feel this is unlikely as the association between nucleosomes and outcome is driven by those with worse ARDS and multisystem organ failure, both of which commonly eventually receive invasive arterial access. Ventilator and ARDS management specific to CHOP could potentially impact nucleosome concentrations and outcomes, thereby giving a biased effect on the relationship between nucleosomes and outcome in other ARDS cohorts. However, our results are consistent with adult studies on the prognostic significance of histones in ARDS (15, 16), and the associations with ARDS severity and with non-pulmonary organ failure provide a plausible mechanism.
Our study also has several strengths. This is a well characterized prospective pediatric ARDS cohort using modern ventilator and fluid management. This is one of the largest pediatric biomarker studies to date, allowing for the ability to adjust for potential confounders of the association between nucleosomes and outcomes. The focus on a DAMP makes this one of the few studies in children addressing the mechanisms underlying ARDS. Plasma was collected early after ARDS onset, at a median of 10 [IQR 5, 18] hours. We tested the independent association of nucleosomes with both PICU mortality as well as VFDs at 28 days, a commonly used endpoint in pediatric ARDS trials (27). Finally, we performed a novel anti-ds DNA co-immunoprecipitation to identify the specific histone subtypes in the nucleosome, a technique which may have utility beyond this study. Our overall results are consistent with adult ARDS and suggest nucleosomes and their component histones are clinically significant DAMPs, and potential therapeutic targets, in pediatric ARDS.
Supplementary Material
Acknowledgments
Support: K23-HL136688 (NY); K24-HL115354 (JDC)
Copyright Form Disclosure: Dr. Yehya’s institution received funding from the National Institutes of Health (NIH) and Pfizer. Drs. Lawrence and Worthen’s institutions received funding from the NIH. Drs. Yehya, Fazelinia, Lawrence, and Christie received support for article research from the NIH. Dr. Mai disclosed that he has submitted a software disclosure to his institution for a software platform he developed, called TRAILS: TRainee Individualized Learning System, which is also unrelated to the research being submitted for publication. He currently receives no royalties from this software. He also received grant funding from AMA for Accelerating Change in Medical Education and from two internal institutional grants at the Children’s Hospital of Philadelphia for other projects.
REFERENCES
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149(3 Pt 1):818–824. [DOI] [PubMed] [Google Scholar]
- 2.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2(7511):319–323. [DOI] [PubMed] [Google Scholar]
- 4.Davis SL, Furman DP, Costarino AT Jr. Adult respiratory distress syndrome in children: associated disease, clinical course, and predictors of death. The Journal of pediatrics 1993;123(1):35–45. [DOI] [PubMed] [Google Scholar]
- 5.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2005;172(2):206–211. [DOI] [PubMed] [Google Scholar]
- 6.Pediatric Acute Lung Injury Consensus Conference G. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. The Lancet Respiratory medicine 2019;7(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowan CM, Klein MJ, Hsing DD, et al. Early Use of Adjunctive Therapies for Pediatric Acute Respiratory Distress Syndrome: A PARDIE Study. Am J Respir Crit Care Med 2020;201(11):1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehya N, Harhay MO, Klein MJ, et al. Predicting Mortality in Children With Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit Care Med 2020;48(6):e514–e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehya N, Keim G, Thomas NJ. Subtypes of pediatric acute respiratory distress syndrome have different predictors of mortality. Intensive Care Med 2018;44(8):1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009;15(11):1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekaney ML, Otto GP, Sossdorf M, et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care 2014;18(5):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alhamdi Y, Abrams ST, Cheng Z, et al. Circulating Histones Are Major Mediators of Cardiac Injury in Patients With Sepsis. Crit Care Med 2015;43(10):2094–2103. [DOI] [PubMed] [Google Scholar]
- 14.Raffray L, Douchet I, Augusto JF, et al. Septic shock sera containing circulating histones induce dendritic cell-regulated necrosis in fatal septic shock patients. Crit Care Med 2015;43(4):e107–116. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wen Z, Guan L, et al. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology 2015;122(1):127–139. [DOI] [PubMed] [Google Scholar]
- 16.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013;187(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011;118(13):3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehya N, Thomas NJ, Margulies SS. Circulating nucleosomes are associated with mortality in pediatric acute respiratory distress syndrome. American journal of physiology Lung cellular and molecular physiology 2016;310(11):L1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92(8):2226–2235. [DOI] [PubMed] [Google Scholar]
- 20.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11(2):234–238. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 22.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med 2014;15(4):e147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med 2015;43(5):937–946. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuzick J A Wilcoxon-type test for trend. Statistics in medicine 1985;4(1):87–90. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94(446):496–509. [Google Scholar]
- 27.Yehya N, Harhay MO, Curley MAQ, et al. Reappraisal of Ventilator-Free Days in Critical Care Research. Am J Respir Crit Care Med 2019;200(7):828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildhagen KC, Garcia de Frutos P, Reutelingsperger CP, et al. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014;123(7):1098–1101. [DOI] [PubMed] [Google Scholar]
- 29.Wygrecka M, Kosanovic D, Wujak L, et al. Antihistone Properties of C1 Esterase Inhibitor Protect against Lung Injury. Am J Respir Crit Care Med 2017;196(2):186–199. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology 2007;176(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stortz JA, Hawkins RB, Holden DC, et al. Cell-free nuclear, but not mitochondrial, DNA concentrations correlate with the early host inflammatory response after severe trauma. Scientific reports 2019;9(1):13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosmann M, Grailer JJ, Ruemmler R, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27(12):5010–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalbitz M, Grailer JJ, Fattahi F, et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2015;29(5):2185–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alhamdi Y, Zi M, Abrams ST, et al. Circulating Histone Concentrations Differentially Affect the Predominance of Left or Right Ventricular Dysfunction in Critical Illness. Crit Care Med 2016;44(5):e278–288. [DOI] [PubMed] [Google Scholar]
- 36.Gauthier VJ, Tyler LN, Mannik M. Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol 1996;156(3):1151–1156. [PubMed] [Google Scholar]
- 37.Alhamdi Y, Abrams ST, Lane S, et al. Histone-Associated Thrombocytopenia in Patients Who Are Critically Ill. JAMA 2016;315(8):817–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Huang Y, Mao P, et al. Sepsis and ARDS: The Dark Side of Histones. Mediators Inflamm 2015;2015:205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogakou EP, Nieves-Neira W, Boon C, et al. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 2000;275(13):9390–9395. [DOI] [PubMed] [Google Scholar]
- 40.Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell 2006;23(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Parry JA, Chin A, et al. Soluble histone H2AX is induced by DNA replication stress and sensitizes cells to undergo apoptosis. Mol Cancer 2008;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Seminars in thrombosis and hemostasis 2014;40(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirose T, Hamaguchi S, Matsumoto N, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PloS one 2014;9(11):e111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Liu Z, Liu B, et al. Citrullinated histone H3: a novel target for the treatment of sepsis. Surgery 2014;156(2):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012;40(12):3238–3245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.