Abstract
Electrodiagnostic studies, may help orthopaedic surgeons to identify and confirm nerve pathology, determine severity of disease, localize the lesion, identify concomitant or alternative pathology, and prognosticate potential outcomes with non-operative or operative treatment. Surgeons should recognize the indications for electrodiagnostic studies, principles of their performance, and how to assess the primary data generated by the examination and how it can inform their treatment plans.
Keywords: electrodiagnostic, nerve conduction studies, electromyography, nerve injury, carpal tunnel syndrome, cubital tunnel syndrome, cervical radiculopathy, peripheral nerve injury
Introduction
Electrodiagnostic studies (EDX) can be used to assess the function of the peripheral nervous system. Outpatient EDX are typically comprised of both nerve conduction studies (NCS) and needle electromyography (EMG). NCS examine the integrity of the nerve fiber itself and its constitutive components (axon and myelin), while EMG interrogates the resting membrane electrical activity of muscle. While EDX are frequently used to diagnose and guide treatment for patients with compressive neuropathy and nerve injury, they can be costly, uncomfortable and anxiety provoking for patients.
As with all diagnostic tests, the sensitivity and specificity of EDX for specific conditions are related to the cut-off values used11 and there is no consensus reference standard for the diagnosis of compression neuropathies at the carpal or cubital tunnel. In the absence of a consensus reference standard, the difficulty lies in quoting sensitivity or specificity for objective pathology based on subjective symptoms and interpretation of the physical examination. The clinical usefulness of EDX also heavily depends on the pre-test probability of disease.1 When disease is likely, EDX can be used to measure severity for prognosis, or location when it is in question. When disease is unlikely, and especially for non-specific symptoms, the low pre-test odds of disease increase the probability that EDX may be inconclusive or even misleading. In low prevalence testing circumstances, a normal test makes disease very unlikely and indicates normal or near normal nerve physiology.
Surgeons familiar with the diagnosis and treatment of peripheral nerve issues may use the test as confirmatory, while others who are less familiar with the conditions may use the test as a screening tool. Nevertheless, when compared to other diagnostic tests such as patient questionnaires, the sensitivity of EDX in the diagnosis of CTS ranges from 82 to 85%, with some studies showing false negative rates as high as 10–20%, and a specificity reported ranging between 95–99%.2–3 The diagnostic performance of EDX in cubital tunnel syndrome (CuTS) is reported as lower, with sensitivity reported between 37–86% and specificity estimated at >95%.4–6 Nerve conduction studies are more sensitive than EMG in detecting both CTS and CuTS.2,7
Most common conditions affecting peripheral nerves, such as CTS, CuTS, cervical radiculopathy, and traumatic nerve injury are diagnosed and treated based on history and clinical examination. Normal EDX may indicate a very mild neuropathy, which typically would be treated non-operatively. EDX are most useful to orthopaedic surgeons to determine severity of disease, localize a neurologic lesion, exclude concomitant pathology, and prognosticate potential outcomes with non-operative or operative treatment. EDX should not be perceived as the sine qua non of assessing peripheral nerve pathology. Rather, EDX should be seen as an extension of the clinical assessment, and while limited, may be particularly helpful in certain situations, such as monitoring changes in nerve pathology over time, or clarifying exam findings. EDX may also be helpful in settings where symptoms are not clearly described by the patient, or the physical examination is equivocal or difficult to obtain.
Basics of electrodiagnostic studies
In this review, we provide an overview and direct readers to the excellent description by Lee et al in a prior JAAOS article for additional details on the anatomic and physiologic basis of EDX.8
Nerve conduction studies
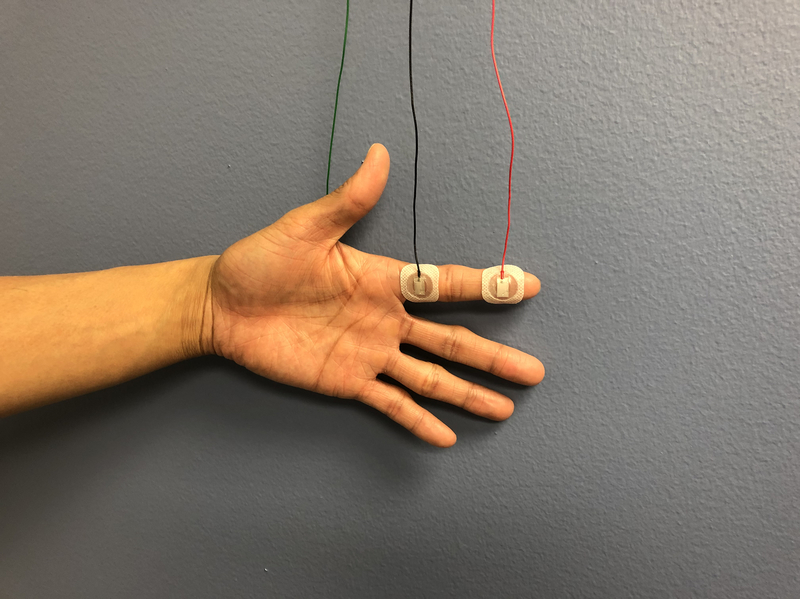
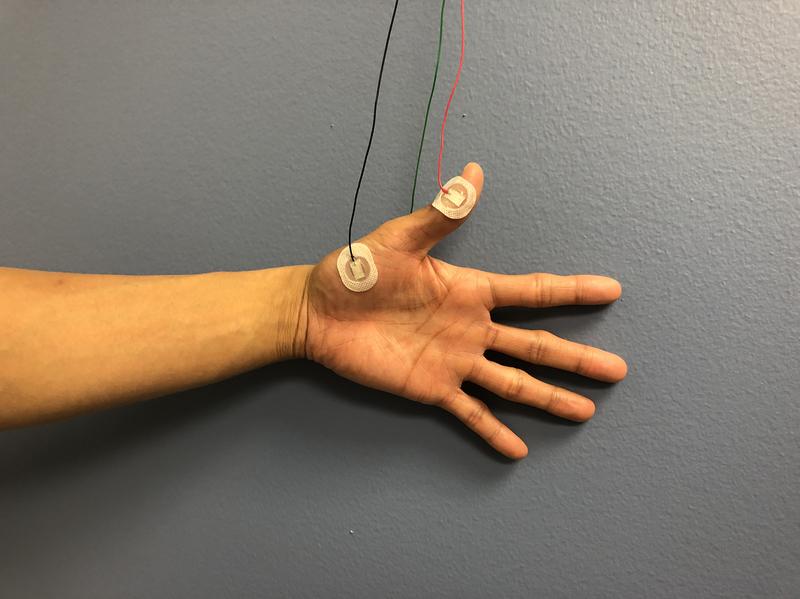
In a nerve conduction study, a stimulus is applied along the course of the nerve and recorded over a muscle. An example of a set up for median nerve sensory and motor NCS, often used in CTS, is shown in Figure 1.9 [FIGURE 1] The performance of NCS is reliant on technical factors, including a thorough knowledge of surface anatomy and appropriate measurement of distances between the recording electrode and the location of stimulus. Physiologic variables, such as room temperature, skin temperature, patient age, and patient height, can also affect the reliability of NCS measurements.9 Accordingly, the normal values for each NCS laboratory should be noted.
Figure 1.
A. Median nerve sensory nerve conduction study setup.
B. Median nerve motor nerve conduction study setup.
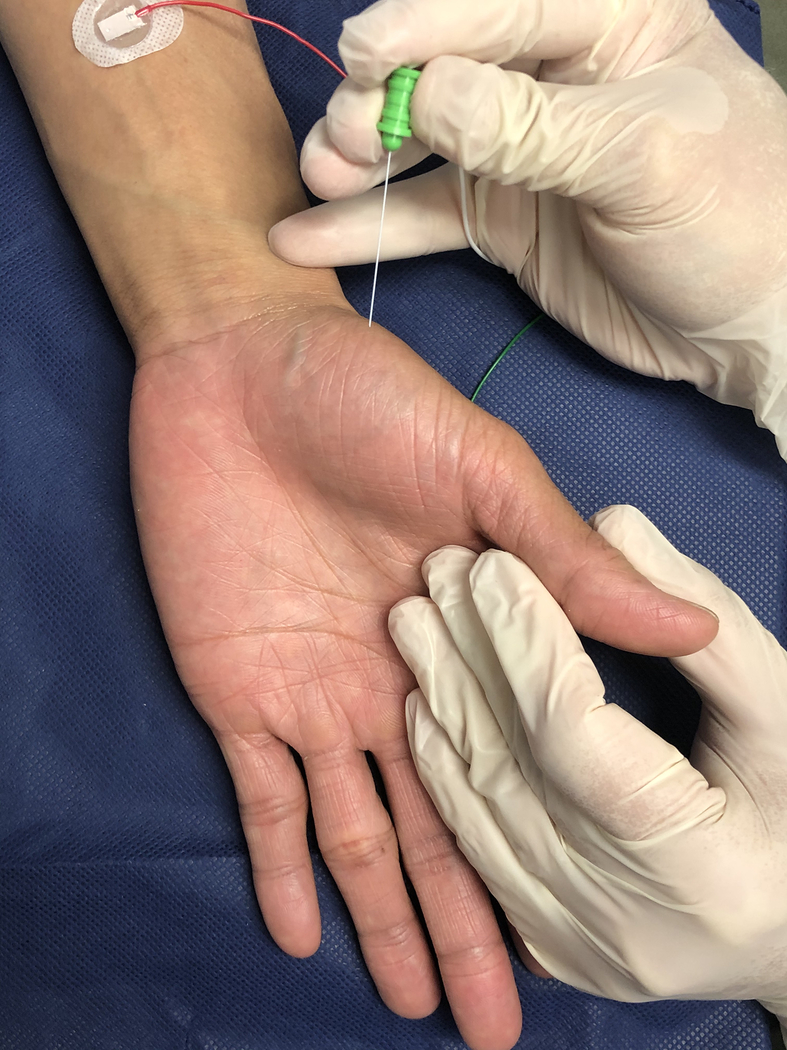
C. Electromyography needle insertion into abductor pollicis brevis.
An understanding of neural anatomy is paramount when interpreting NCS, as each component of the NCS reflects the health or function of a particular part of the nerve. Peripheral nerves have both sensory and motor fibers and therefore NCS are comprised of sensory nerve action potentials (SNAP) and compound motor action potentials (CMAP). The three main measures assessed on NCS are: (1) latency (peak latency for SNAPs, onset latency for CMAPs; measured in milliseconds), (2) nerve conduction velocity (NCV; measured in meters/second), and (3) amplitude (measured in microvolts for SNAPs, millivolts for CMAPs). Both latency and NCV reflect the speed of conduction along a nerve, which is a direct reflection of the integrity of the myelin around the axon. Healthy myelin is essential to rapid conduction of nerve signals, through the process of saltatory conduction.
Latency is a measurement of how long it takes for nerve transmission from the two points of stimulus to recording: this increases if the myelin is injured. The NCV is calculated by dividing the conduction time by the distance between the points of stimulus to recording, and conversely this decreases with myelin injury. It should be noted that proximal nerve segments generally conduct faster than distal segments10, a property intrinsic to the architecture of the nerve, as explained below.
The fastest conducting portions of the nerve are the large myelinated fibers responsible for motor, light touch, and vibration.9 The smaller diameter and unmyelinated fibers within a nerve detect pain and temperature10. During assessment with latency and NCV, the smaller and slower fibers may be “overshadowed” by the faster and larger fibers driving the first recorded impulse10. This distinction marks a potential limitation of using latency and NCV in assessment of peripheral nerve function – a relatively small number of large myelinated fibers can make latency and NCV values appear “normal”, even if other portions of the nerve are affected by compressive neuropathy.
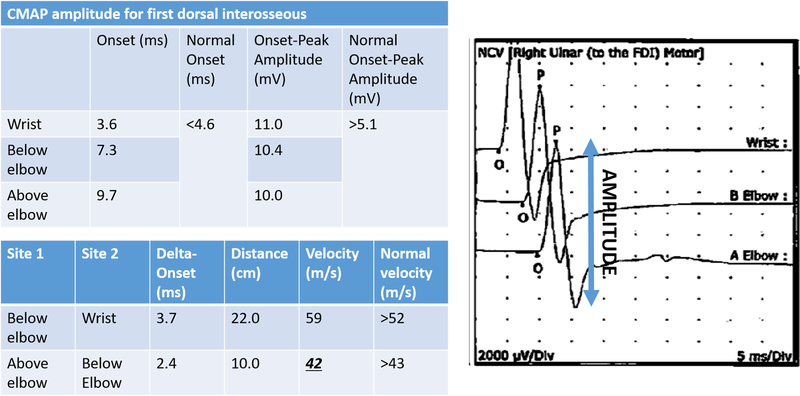
Amplitude is the other main parameter assessed on NCS. It reflects the number of functioning fibers within the nerve and is not reliant on the speed of nerve conduction. Abnormalities in the amplitude are best seen in the waveform of the nerve conduction study (FIGURE 2). In general, more axons firing in concert give a tall, but narrow peak as their voltages are summed together over a relatively short time period. Fewer axons firing, or the same axons firing over a longer period of time, gives a lower amplitude.
Figure 2.
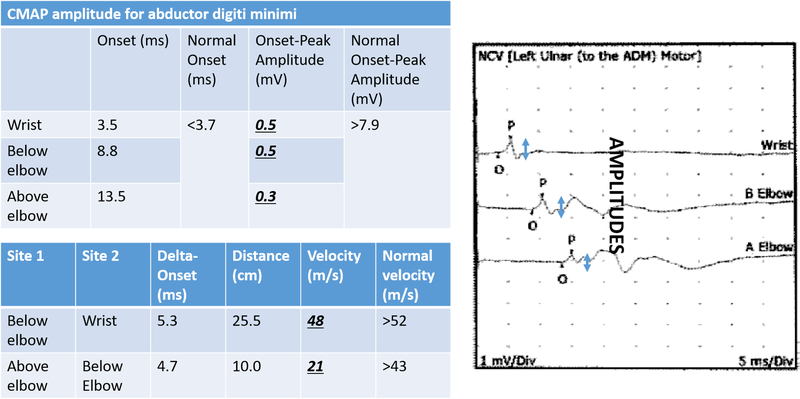
A. Motor nerve conduction study from the first dorsal interosseous muscle in a patient with mild cubital tunnel syndrome. Note the normal compound motor action potential (CMAP) amplitude levels are normal, but there is some slight slowing in the nerve conduction velocity across the elbow.
B. Motor nerve conduction study from the abductor digiti minimi muscle in a patient with severe cubital tunnel syndrome. There is muscle wasting and loss of two-point discrimination on this patient’s clinical exam. Note the drastically decreased compound motor action potential (CMAP) amplitude levels in addition to marked slowing in the nerve conduction velocity across the elbow.
Chronic compressive neuropathy can lead to axonal loss due to intraneural fibrosis and subsequent loss of amplitudes on NCS.9 However, a more proximal site of neuropathology that leads to axonal loss, such as concomitant cervical radiculopathy, may also manifest as a loss of amplitude on NCS. Power et al. demonstrated the association between CMAP amplitude and motor function (grip and pinch strength) in patients with CuTS.11 Their findings suggest that loss of CMAP amplitude is a sensitive indicator of advanced ulnar neuropathy and a possible predictor of outcomes after surgical treatment. CMAP amplitudes are generally considered more reliable than SNAP amplitudes, as the former are more easily detected due to motor neurons activating multiple muscle fibers.
Abnormalities in the components of the NCS will reflect the pathophysiologic processes of individual diseases and their various stages of severity. For example, early CTS is a focal demyelinating process and is reflected by abnormalities in NCS latencies. Later stage CTS has axonal loss from chronic ischemia, which is demonstrated by the decreases in NCS motor amplitudes. Purely neurapraxic peripheral nerve injuries will demonstrate slowing of latency and NCV but normal motor NCS amplitudes, while axonotmetic injuries will show decreased motor NCS amplitudes.
Electromyography
In a needle EMG study, a needle is inserted into a muscle, which is then interrogated both at rest and with voluntary muscle contraction. Thorough knowledge of surface anatomy is necessary to ensure accurate needle placement, and ultrasound-guidance of needle placement can improve accuracy of insertion into deep muscles. It is also important to note that an individual needle EMG assessment only reflects a single neuromuscular unit. Repeating the study in different portions of the same muscle can decrease variability and increase diagnostic sensitivity of the assessment12, as it is possible that the injured fascicles within one nerve may be associated with partially denervated portions of the muscle.
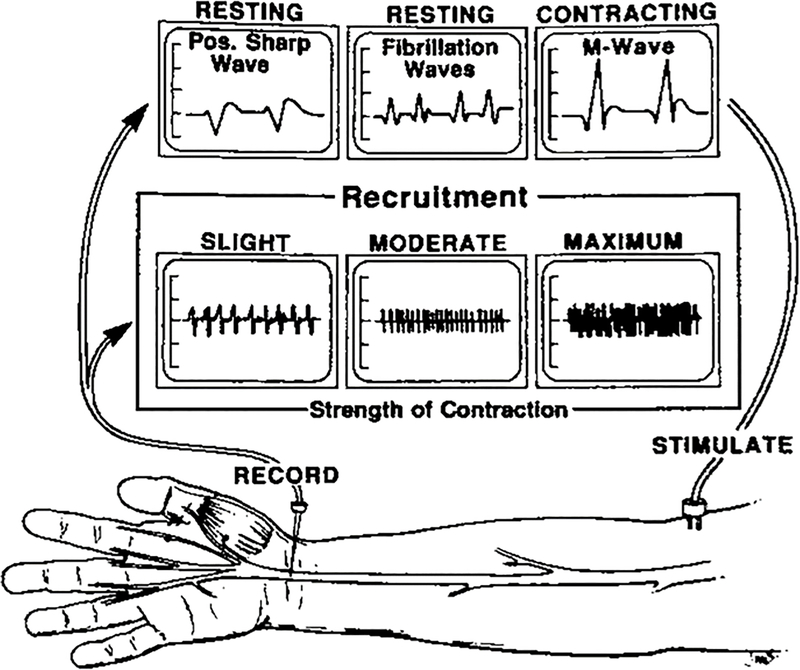
An EMG study has three phases: insertional activity (when the needle is inserted); resting phase (when the muscle is not contracting); and activation phase (when the muscle is contracting) (FIGURE 3).9 Insertional activity is noted as being increased or decreased. In the setting of a hyperexcitable muscle membrane, which can occur with Wallerian degeneration after peripheral nerve injury, the insertional activity will be increased. In chronic muscle atrophy with fibrosis and/or fatty infiltration, the insertional activity will be decreased. The resting phase activity on an EMG may include spontaneous potentials occurring within the muscle even when it is not contracting. When individual muscle fibers are deprived of their innervation, there is spontaneous depolarization due to muscle fiber hypersensitivity. This hypersensitivity is reflected in the generation of fibrillation potentials and positive sharp waves, ranging from persistent single runs in two areas (1+) to continuous discharges in all areas (4+). These changes will be present with both partial and complete nerve injuries, will occur as early as 10 days from the nerve injury and be present for months. In the activation phase of an EMG, characteristics of the motor unit action potential (MUAP) are analyzed (referred to as the M-wave in Figure 3). MUAP analysis helps determine the presence of a disorder, whether it is neuropathic or myopathic, the time course of the disorder, and its severity. MUAPs will be absent after neurotmetic (complete) injuries and decreased or absent after high-grade axonotmetic injuries. The rate and pattern of MUAP recruitment provide qualitative assessments of activity within a muscle. Following nerve injury, there is a decrease in the number of muscle fibers contracting. This leads to a reduced recruitment pattern, which can be visualized in the waveform and audibly discerned during waveform capture. Electromyographers will typically provide a characterization of muscle recruitment by describing the interference pattern, grading them as full or normal, reduced, discrete, a single MUAP, or absent MUAP. In a full or normal pattern, individual MUAPs cannot be detected due to the density of contracting motor units. In a reduced pattern, some individual MUAPs are detected but many MUAPs overlap. In a discrete pattern, each individual MUAP is detectable, reflecting a very low density of contracting motor units and a more severe level of denervation. Single MUAPs and absent MUAPs portend an even poorer prognosis.13
Figure 3.
Waveforms seen during insertion, resting, and activation phases of EMG. (Reproduced with permission from Gelberman RH: Operative Nerve Repair and Reconstruction [Fig 10–2]; Ed: Gelberman RH, 1991. Lippincott)
The amplitude of the MUAP reflects the number of motor fibers recorded nearest to the needle, while the duration of the MUAP indicates the number of muscle fibers within a motor unit. The amplitude and duration of the MUAP may be altered in subacute and chronic denervation and reinnervation settings. Following partial nerve injury, any surviving motor neurons expand the number and density of muscle fibers they innervate (collateral sprouting of injured neurons into denervated muscle).14 This is reflected with high amplitude and long duration MUAPs. Increased amplitude is typically associated with chronic denervation/reinnervation changes, while increased duration is typically seen in the subacute setting. With true nerve regeneration (nerve regrowth down an endoneural tube into denervated muscle), the MUAP will be low in amplitude with variable (low, normal, or possibly long) duration.13 While detecting nascent MUAPs can be helpful in establishing axonal continuity and successful regeneration, it is subject to inter- and intra-rater variability due to the technical difficulty of picking up the low amplitude signals.
How to counsel your patients about electrodiagnostic studies
Electrodiagnostic testing can take anywhere from 30 minutes to over 90 minutes, depending on the condition(s) being tested and the findings of each portion. Although well tolerated in general, patients may experience discomfort with electrical stimulations during the nerve conductions and with insertion of the needle electrode during the needle EMG. The most common adverse effect of EDX is pain, which can be attributed to several patient, physician, and study-related factors. Pain has been shown to negatively impact EDX results by preventing completion of the tests, therefore judicious selection of muscles to be tested may improve accuracy and patient compliance.15–16 We advise our surgical trainees to observe the performance of these studies whenever possible, to better understand the perspectives of both the patient and the electromyographer.
It is also important to consider the additional cost associated with obtaining EDX. In their analysis of commercially insured patients undergoing treatment for carpal tunnel syndrome (CTS), Sears et al demonstrated that preoperative EDX added nearly $1000 in additional cost (and $112 of additional out-of-pocket cost) compared to clinical diagnosis alone.17
Collaboration with the physician performing the electrodiagnostic studies
In order to guide the electrodiagnostic testing, the physiatrist or neurologist performing the testing should understand the differential diagnoses and treatment options being considered by the referring team. This referral should convey the symptoms being evaluated and the disorder(s) being ruled in or out. This enables the electromyographer to perform the appropriate examination to address the clinical question(s). It is also important for the electromyographer to understand the potential surgical interventions being considered. Surgeries, such as nerve transfers, may prompt the electromyographer to perform additional testing to evaluate the function of potential donor nerves.
What will electrodiagnostic studies tell you? What will they not tell you?
EDX can be used to confirm the diagnosis of nerve pathology, determine concomitant pathology such as a more proximal lesion or demyelinating disease, and help in localizing the level(s) of neurologic lesions. EDX can aid in staging severity of chronic compressive neuropathy. Associations between EDX-graded severity of nerve compression and response to surgical release are less well defined and beyond the scope of this manuscript.
In the setting of peripheral nerve injury, EMG studies can help in determining the likelihood of spontaneous nerve recovery. Although this assertion is based on expert opinion rather than higher levels of evidence, the absence of MUAPs by 3 months after nerve injury is commonly used as a predictor of a nerve that is unlikely to recover on its own, particularly for suspected stretch injuries to a nerve.18–19 Based on our clinical experience it is our opinion that prognosis after ballistic injuries to nerves is harder to predict, as recovery can occur as early as 3 months and as late as 9 months.20 This variable course may make early EMG less useful following ballistic injuries.
EMG studies are also helpful to determine whether denervated muscle is still receptive to reinnervation, as irreversible fibrosis may occur within 9–12 months of nerve injury. Typically, muscles with remaining fibrillations and/or sharp waves in the resting phase are still “salvageable”. However, the absence of these findings likely reflect muscles with fibrosis related to denervation and lack of capacity for reinnervation.21 Similarly, muscles severely damaged from trauma may be unable to fire motor units in a coordinated fashion, and demonstrate poor CMAP response even in the setting of normal nerve conduction.
EMG studies can be used as a supplement to the physical examination to determine the health of donor neuromuscular units for potential nerve transfer. Ideally, completely healthy and uninjured nerves are used as donors for nerve transfer. In some settings, partially injured nerves can recover adequately and be used as donors for nerve transfer.22–23 Tzou et al. and Chang et al. have demonstrated in animal models that motor recovery is possible after using partially-injured donor nerves, but that greater recovery is seen with healthier donors.22–23 Schreiber et al. demonstrated that donor nerve units that were normal or had reduced recruitment patterns were associated with superior outcomes compared to those donor neuromuscular units with discrete recruitment patterns.24
Common conditions
Carpal tunnel syndrome
Compressive neuropathy of the median nerve at the wrist can manifest as CTS. Classic clinical findings are paresthesias and decreased sensation in the median nerve distribution. While clinical assessment remains the foundation for diagnosis, electrophysiologic studies can help correlate this with physiologic changes in median nerve function at the wrist, although the ultimate diagnosis relies on the clinician’s summation of all findings.25
CTS results from compression of the median nerve within the carpal tunnel beneath the transverse carpal ligament. As myelin is the first component of the nerve to be affected in CTS, early changes found on NCS are attributable to deficits in myelin, leading to increased latency. Progressive axonal loss indicates more severe disease and increased damage to the nerve, manifesting as decreasing SNAP and CMAP amplitudes and EMG abnormalities in the median-innervated thenar muscles.26 While a prior version of the American Academy of Orthopaedic Surgeons clinical practice guidelines for the diagnosis of carpal tunnel syndrome recommended electrodiagnostic studies prior to surgery27, the most recent version does not mandate them and instead suggests that EDX be ordered based on clinical judgment.28
The natural history of resolution of these pathologic electrophysiologic changes after carpal tunnel release is not completely documented nor understood. Electrophysiologic recovery does not seem to correlate well with patient reported outcomes, with symptomatic and functional improvement occurring much earlier in the post-operative period, within the first few weeks to months.29–30 Long-term follow up studies of electrophysiologic tests after surgery have shown that the most significant changes occur in the first 3 months before reaching a plateau, though there is some degree of continued improvement of all NCS parameters for up to 2 years as the nerve continues to heal and regenerate.31–32 In particular, distal motor latency and NCV may continue to advance towards physiologic values, though the results seldom reach normal limits even years after clinical resolution of symptoms.31–32 Merolli et al. demonstrate that, among other parameters, there was persistence of a double peak shift, which presents as two distinct SNAP on NCS representing the latency difference between radial and median nerves, in 84% of patients who were 2–20 years post-surgical treatment.24,32
Cubital tunnel syndrome
Compressive neuropathy of the ulnar nerve at the elbow is referred to as CuTS. Paresthesias, numbness, and tingling in the ring and small fingers are classically associated with CuTS. The pathophysiology of CuTS differs from that of CTS. While compression of the median nerve occurs due to increased pressure within the carpal tunnel, compression of the ulnar nerve at the cubital tunnel is theorized to be due to a combination of both compression and traction. Flexion of the elbow causes narrowing of the space beneath the arcuate ligament, leading to compression of the ulnar nerve from increased extraneural pressure.33–34 Flexion of the elbow also lengthens the ulnar nerve as it stretches across the medial epicondyle, adding a traction neuropathy.35
In early stages of CuTS, electrodiagnostic testing may be normal despite persistent and bothersome clinical symptoms (sensitivity ranging from 37–86%)4–6, although changes in nerve morphology such as increased cross sectional area may be evident.36–37 The patient’s clinical symptoms may correspond to demyelination of smaller diameter fibers and compression of unmyelinated fibers, and the presence of functioning large myelinated fibers may produce false negative results in electrophysiologic testing38, reflecting relatively mild compressive. The benefit of surgery in these EDX-normal patients who fail non-operative management is unclear.39 Additional diagnostic testing, such as peripheral nerve blocks, may be helpful in these settings. The wide range of sensitivity of EDX has led to an interest in using ultrasound for diagnosis of CuTS.3,40
Cervical radiculopathy
Cervical radiculopathy is attributable to symptomatic compression of one or more cervical nerve roots at or near the neural foramen as they exit the spinal cord. Proximal compression of the nerve root can manifest as pain in a defined dermatomal pattern that is not well explained by peripheral nerve innervation patterns. Severe compression can result in weakness and EMG changes within multiple muscles innervated by different peripheral nerves. Atypical presentations of weakness or sensory disturbances that do not match those described above for common peripheral nerve compression should alert the astute clinician to the potential for cervical radiculopathy. Changes in NCS when interrogating individual nerves are not distinct in cervical radiculopathy compared to peripheral neuropathy, but the pattern of neuromuscular involvement should increase clinician suspicion for cervical radiculopathy being an alternative or concurrent diagnosis.
Paraspinal muscles are innervated solely by cervical nerve roots, and not peripheral nerves, thus they provide an objective measure to assess the proximal extent of the nerve pathology. If a paraspinal muscle to an isolated cervical nerve root is affected (on EMG or motor NCS), it is suggestive of cervical radiculopathy. A thorough radiculopathy screen involving five or more muscles should be performed to best identify the presence of a radiculopathy.41 EDX in the cervical spine also suffer from the same diagnostic testing limitations noted in the limbs. A recent study evaluating inter- and intra-rater reliability in diagnosing cervical radiculopathy found a 77% sensitivity, 71% specificity and relatively poor inter-rater reliability, but good intra-rater reliability43, suggesting that diagnostic uncertainty may persist even with the use of EDX, and that the clinical usefulness of EDX will depend on the pre-test probability of disease. EDX of the cervical nerves is sometimes ordered to evaluate for double crush syndrome, where a peripheral nerve is compressed at two or more locations along its course. Double crush syndrome is a controversial diagnosis, as some feel it is used to create an objective explanation for persistent subjective symptoms and/or dissatisfaction.42 However, peripheral and compressive neuropathy can coexist, which may contribute to suboptimal outcomes following nerve decompression.
Peripheral nerve injury
Early diagnosis of peripheral nerve injuries can aid in establishing prognosis and guide treatment, with early intervention potentially leading to improved recovery in both sensory and motor outcomes.44 Because abnormalities in EDX are unlikely to appear until Wallerian degeneration has occurred, initial post-injury EDX are not typically obtained until 3–4 weeks after injury, if clinical recovery is not already apparent. In these cases, EDX can help guide treatment depending on the nerve injured and distance to the target end organs.
When the degree of nerve injury (neurapraxic, axonotmetic, or neurotmetic) is not clear, NCS and EMG can help make the distinction. Neurapraxia is caused by a focal injury to myelin, resulting in a conduction block across the injury site. Stimulation proximal to the nerve site will demonstrate an increase in latency and decreased velocity compared to stimulation distal to the injury site on both motor and sensory NCS. Stimulation and measurement of segments distal to the injury site will show a normal waveform due to the absence of Wallerian degeneration.46 EMG stimulation distal to the conduction block will show normal waveforms without any evidence of spontaneous activity, but stimulation proximal to the conduction block may show reduced or absent recruitment. Therefore, if at the 4 week post-injury EMG there is nerve conduction distal to the lesion, the neurons are intact (neurapraxia) and recovery prognosis is good.
Axonotmetic injuries represent a distinctly different pathology due to the presence of Wallerian degeneration. Wallerian degeneration occurs after axonal loss and results in reduction of the amplitude of the sensory or motor action potential. In a partial axonotmetic injury, preserved fibers may demonstrate near normal conduction velocity and latency, but the overall lower number of intact axons signaling to motor fibers will result in lower conduction waveform amplitude and lower amplitude EMG signals. The presence of spontaneous activity (such as fibrillations and positive sharp waves) during the rest phase of an EMG differentiates axonotmetic injuries from neurapraxic injuries, as these findings will not be present in the latter due to a lack of Wallerian degeneration.
In complete neurotmetic lesions, there will be no response during NCS. In the months following an acute injury, there will be spontaneous activity during the rest phase of the EMG. There will be no motor unit recruitment on EMG. Distinguishing a high grade axonotmetic injury from a neurotmetic injury can be challenging on EMG and nerve studies, as the objective findings can be identical. Serial electrophysiologic studies may provide the best clue as to the extent of injury – axonotmetic injuries have potential for recovery if the endoneural tubes are intact, while complete neurotmetic injuries do not recover without surgical intervention, but the decision to obtain EDX and the frequency of studies while awaiting nerve recovery should be a shared decision between surgeon and patient.
Summary
EDX are an important extension of the clinical assessment of patients with peripheral nerve pathology. Surgeons should be aware of how EDX are performed, how to assess the primary data generated by the examination, and how to use EDX to guide their management. Detailed understanding of EDX can aid surgeons in localizing neurologic lesions, determining presence of concurrent or alternative diagnoses, and guiding the selection of nonsurgical and surgical treatments.
Acknowledgments
Sources of support: Author CJD is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR073928. None of the other authors have and sources of support or conflicts of interest to disclose.
References
- 1.Nodera H, Herrmann DN, Holloway RG, Logigian EL. A Bayesian argument against rigid cut-offs in electrodiagnosis of median neuropathy at the wrist. Neurology. 2003. January;60(3):458–464. [DOI] [PubMed] [Google Scholar]
- 2.Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002. June;58(11):1589–92. [DOI] [PubMed] [Google Scholar]
- 3.Pastare D, Therimadasamy AK, Lee E, Wilder-Smith EP. Sonography versus nerve conduction studies in patients referred with a clinical diagnosis of carpal tunnel syndrome. J Clin Ultrasound. 2009. September;37(7):389–93. [DOI] [PubMed] [Google Scholar]
- 4.Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005. June;28(5):345. [DOI] [PubMed] [Google Scholar]
- 5.Rayegani SM, Raeissadat SA, Kargozar E, Rahimi-Dehgolan S, Loni E. Diagnostic value of ultrasonography versus electrodiagnosis in ulnar neuropathy. Med Devices (Auckl). 2019;12:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medicine AAoE. Guidelines in electrodiagnostic medicine. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome. Muscle Nerve Suppl. 1999;8:S141–67. [PubMed] [Google Scholar]
- 7.Raynor EM, Shefner JM, Preston DC, Logigian EL. Sensory and mixed nerve conduction studies in the evaluation of ulnar neuropathy at the elbow. Muscle Nerve. 1994. July;17(7):785–92. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Claussen GC, Oh S. Clinical nerve conduction and needle electromyography studies. J Am Acad Orthop Surg. 2004. 2004 July-August;12(4):276–87. [DOI] [PubMed] [Google Scholar]
- 9.Van Beek AL, Heyman P. Electrophysiologic Testing, in Gelberman RH, ed: Operative Nerve Repair and Reconstruction. Philadelphia, PA, J.B. Lippincott, 1991, pp 171–184. [Google Scholar]
- 10.Mallik A, Weir AI. Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry. 2005. June;76 Suppl 2:ii23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power HA, Sharma K, El-Haj M, Moore AM, Patterson MM, Mackinnon SE. Compound Muscle Action Potential Amplitude Predicts the Severity of Cubital Tunnel Syndrome. J Bone Joint Surg Am. 2019. April;101(8):730–8. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom JW, Olney RK. Quantitative motor unit analysis: the effect of sample size. Muscle Nerve. 1992. March;15(3):277–281. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg J EMG: myths and facts. HSS J. 2006. February;2(1):19–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlfart G Collateral regeneration in partially denervated muscles. Neurology. 1958. March;8(3):175–80. [DOI] [PubMed] [Google Scholar]
- 15.Richardson JK, Evans JE, Warner JH. Information effect on the perception of pain during electromyography. Arch Phys Med Rehabil. 1994. June;75(6):671–5. [DOI] [PubMed] [Google Scholar]
- 16.London ZN, Burke JF, Hazan R, Hastings MM, Callaghan BC. Electromyography-related pain: muscle selection is the key modifiable study characteristic. Muscle Nerve. 2014. April;49(4):570–4. [DOI] [PubMed] [Google Scholar]
- 17.Sears ED, Swiatek PR, Hou H, Chung KC. Utilization of Preoperative Electrodiagnostic Studies for Carpal Tunnel Syndrome: An Analysis of National Practice Patterns. J Hand Surg Am. 2016. June;41(6):665–72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008. September;119(9):1951–1965. [DOI] [PubMed] [Google Scholar]
- 19.Dumitru D, Zwarts MJ, Amato AA. Peripheral nervous system’s reaction to injury, in Dumitru D, Amato AA, Zwarts MJ, eds: Electrodiagnostic medicine, ed 2. Philadelphia, PA, Hanley and Belfus, 2001, pp 115–156. [Google Scholar]
- 20.Omer GE Jr. Injuries to nerves of the upper extremity. J Bone Joint Surg Am. 1974. December;56(8):1615–1624. [PubMed] [Google Scholar]
- 21.Kraft GH. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990. September;13(9):814–821. [DOI] [PubMed] [Google Scholar]
- 22.Chang TN, Shafarenko M, Dadouch R, et al. Can a partially injured donor nerve restore elbow flexion in an acute brachial plexus injury in rats?. Plast Reconstr Surg. 2019. November;144(5):1105–1114. [DOI] [PubMed] [Google Scholar]
- 23.Tzou CH, Lu CJ, Chang TN, Chuang DC. Can an injured nerve be used as a donor nerve for distal nerve transfer?-An experimental study in rats. Microsurgery. 2017. September;37(6):647–654. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber JJ, Feinberg JH, Byun DJ, Lee SK, Wolfe SW. Preoperative donor nerve electromyography as a predictor of nerve transfer outcomes. J Hand Surg Am. 2014. January;39(1):42–9. [DOI] [PubMed] [Google Scholar]
- 25.Graham B The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am. 2008. December;90(12):2587–93. [DOI] [PubMed] [Google Scholar]
- 26.Chang CW, Wang YC, Chang KF. A practical electrophysiological guide for nonsurgical and surgical treatment of carpal tunnel syndrome. J Hand Surg Eur Vol. 2008. February;33(1):32–7. [DOI] [PubMed] [Google Scholar]
- 27.Keith MW, Masear V, Chung K, et al. Diagnosis of carpal tunnel syndrome. J Am Acad Orthop Surg. 2009. December;17(6):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Academy of Orthopaedic Surgeons: Management of carpal tunnel syndrome evidence-based clinical practice guideline. www.aaos.org/ctsguideline. Published February 29, 2016. [DOI] [PubMed]
- 29.Heybeli N, Kutluhan S, Demirci S, Kerman M, Mumcu EF. Assessment of outcome of carpal tunnel syndrome: a comparison of electrophysiological findings and a self-administered Boston questionnaire. J Hand Surg Br. 2002. June;27(3):259–64. [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Yoon JS, Kim SJ, Won SJ, Jeong JS. Carpal tunnel syndrome: Clinical, electrophysiological, and ultrasonographic ratio after surgery. Muscle Nerve. 2012. February;45(2):183–188. [DOI] [PubMed] [Google Scholar]
- 31.Kanatani T, Fujioka H, Kurosaka M, Nagura I, Sumi M. Delayed electrophysiological recovery after carpal tunnel release for advanced carpal tunnel syndrome: a two-year follow-up study. J Clin Neurophysiol. 2013. February;30(1):95–97. [DOI] [PubMed] [Google Scholar]
- 32.Merolli A, Luigetti M, Modoni A, Masciullo M, Lucia Mereu M, Lo Monaco M. Persistence of abnormal electrophysiological findings after carpal tunnel release. J Reconstr Microsurg. 2013. October;29(8):511–516. [DOI] [PubMed] [Google Scholar]
- 33.Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. An experimental study in human cadavera. J Bone Joint Surg Am. 1998. April;80(4):492–501. [DOI] [PubMed] [Google Scholar]
- 34.Bozentka DJ. Cubital tunnel syndrome pathophysiology. Clin Orthop Relat Res. 1998. June(351):90–4. [PubMed] [Google Scholar]
- 35.Apfelberg DB, Larson SJ. Dynamic anatomy of the ulnar nerve at the elbow. Plast Reconstr Surg. 1973. January;51(1):76–81. [PubMed] [Google Scholar]
- 36.Chang KV, Wu WT, Han DS, Özçakar L. Ulnar Nerve Cross-Sectional Area for the Diagnosis of Cubital Tunnel Syndrome: A Meta-Analysis of Ultrasonographic Measurements. Arch Phys Med Rehabil. 2018. April;99(4):743–757. [DOI] [PubMed] [Google Scholar]
- 37.Yoon JS, Walker FO, Cartwright MS. Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil. 2010. February;91(2):318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. J Hand Surg Am. 2010. April;35(4):668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomaino MM, Brach PJ, Vansickle DP. The rationale for and efficacy of surgical intervention for electrodiagnostic-negative cubital tunnel syndrome. J Hand Surg Am. 2001. November;26(6):1077–1081. [DOI] [PubMed] [Google Scholar]
- 40.Pelosi L, Mulroy E. Diagnostic sensitivity of electrophysiology and ultrasonography in ulnar neuropathies of different severity. Clin Neurophysiol. 2019. 02;130(2):297–302. [DOI] [PubMed] [Google Scholar]
- 41.Dillingham TR, Lauder TD, Andary M, et al. Identification of cervical radiculopathies: optimizing the electromyographic screen. Am J Phys Med Rehabil. 2001. February;80(2):84–91. [DOI] [PubMed] [Google Scholar]
- 42.Kane PM, Daniels AH, Akelman E. Double crush syndrome. J Am Acad Orthop Surg. 2015. September;23(9):558–62. [DOI] [PubMed] [Google Scholar]
- 43.Narayanaswami P, Geisbush T, Jones L, et al. Critically re-evaluating a common technique: Accuracy, reliability, and confirmation bias of EMG. Neurology. 2016. January;86(3):218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kömürcü F, Zwolak P, Benditte-Klepetko H, Deutinger M. Management strategies for peripheral iatrogenic nerve lesions. Ann Plast Surg. 2005. February;54(2):135–9; discussion 40–2. [DOI] [PubMed] [Google Scholar]
- 45.Wang E, Inaba K, Byerly S, et al. Optimal timing for repair of peripheral nerve injuries. J Trauma Acute Care Surg. 2017. 11;83(5):875–81. [DOI] [PubMed] [Google Scholar]
- 46.Holland NR. Electrodiagnostic testing for nerve injuries and repairs, in Elkwood AI, Kaufman M, Schneider LF, eds: Rehabilitative surgery, ed 1. Springer, 2017. p. 89–94. [Google Scholar]