Abstract
Background:
Small bowel neuroendocrine tumors (SB-NET) frequently metastasize to regional lymphatic or distant sites. While most prognostication of SB-NET focuses on lymph node involvement, findings from studies of NETs from other primary sites have suggested that preoperative serum chromogranin-A (CgA) levels may provide a more accurate metric.
Study Design:
Using the National Cancer Database (2004–2016), we analyzed patients with locoregional SB-NET who underwent curative resection including an adequate lymphadenectomy (n = 1,274). A statistically optimized cut-point was used to dichotomize CgA cohort based on preoperative serum CgA levels.
Results:
We determined that a CgA ≥139ng/mL identified patients with significantly shorter estimated mean overall survival (6.6 years vs. 7.6 years, log-rank p = 0.00001). These patients were also older (63 vs. 57 years, p < 0.001) and more likely to have poorly-differentiated tumors (2.1% vs. 0.7%, p = 0.04) or primary tumors >1cm (88.2% vs. 79.2%, p = 0.001). Clinical features associated with shorter overall survival included preoperative CgA ≥139ng/mL (HR = 2.19, 95% CI 1.22 – 3.92; p = 0.009), age at diagnosis (HR = 1.06, 95% CI 1.03 – 1.09; p < 0.001), Charlson-Deyo score ≥2 (HR = 3.93, 95% CI 1.71 – 9.01; p = 0.001), and poorly-differentiated tumors (HR = 11.22, 95% CI 4.16 – 30.24; p < 0.001). Neither lymph node metastasis nor T-stage were independently associated with shorter overall survival in patients with locoregional SB-NET.
Conclusions:
Elevated preoperative serum CgA is an adverse prognostic marker associated with shorter overall survival in patients with locoregional SB-NET.
Keywords: Neuroendocrine Tumor, Cancer Staging, Tumor Biomarkers, Chromogranin-A, Small Bowel Tumors, Lymphadenectomy
Two Sentence Summary:
“Elevated preoperative serum chromogranin-A is an adverse prognostic marker independently associated with shorter overall survival in patients with locoregional SB-NET. Neither lymph node metastasis nor T-stage is independently associated with shorter overall survival.”
Introduction
Small bowel neuroendocrine tumors (SB-NETs) arise from enterochromaffin cells and account for over one-third of all small bowel tumors.1 Their incidence has risen 6-fold over the last 40 years, and the presence of associated regional metastases at presentation has more than doubled.2 Characteristic patterns of SB-NET growth and spread parallel those of NETs from other visceral organs through regional lymphatic and distant hepatic metastases, but SB-NETs also seem to be biochemically unique, as they are more commonly associated with carcinoid syndrome and less often with mutational driver events.3–6 Despite their propensity for metastasis, NETs originating from most primary sites often exhibit an indolent course that allows for survival-prolonging cytoreductive procedures even in the presence of distant disease. Furthermore, the advancement of systemic treatment options for NETs is promising and metastatic SB-NETs have the highest median overall survival of NETs arising from any visceral organ.2 To provide appropriate expectations with respect to treatment and surveillance for patients with NET, it is imperative to better understand the disease’s biological behavior and response to available therapy. Thus, advancements in staging accuracy and prognostication, longstanding areas of intense clinical investigation, remain of the utmost importance.7, 8
While there are limits to radiographic metrics to detect disease, the use of biomarkers has shown promise in the prognostication of select tumors, including NETs. Much like NETs of other primary sites, SB-NETs can secrete one or more active peptides with potential functional and prognostic relevance. One such peptide is Chromogranin A (CgA). CgA is a hydrophilic glycoprotein that is cleaved into bioactive peptides upon release in chromaffin granules of neuroendocrine cells.9 Serum CgA levels accurately reflect the clinical status of patients with advanced disease, and have demonstrated utility in both tumor surveillance and assessment of treatment response.10 An elevated serum CgA is frequently used as both a diagnostic and prognostic adjunct for NETs, given its correlation with metastatic disease burden and association with disease-specific-survival in several NET types; however, its significance in locoregional disease remains poorly defined.11–15 Elevated preoperative serum CgA levels in localized pancreatic NETs has been used to identify patients with tumors who may benefit from resection, though a similar prognostic role for preoperative CgA levels in SB-NET has yet to be established.13
In recent years, most investigations aiming to improve prognostication in SB-NET have centered on assaying lymphatic metastasis through histopathologic examination. While a regional lymphadenectomy remains standard surgical practice, the prognostic significance of lymph node metastases and the staging system used to classify them remains heavily contested.16–18 A recent evaluation of the American Joint Committee on Cancer (AJCC) 8th edition staging classification using 1,925 patients with locoregional SB-NET found no association between lymph node stage and overall survival.19 Additionally, patients with low-volume lymph node metastases (<4 nodes involved) have demonstrated similar recurrence-free survival to those without lymphatic spread following resection with adequate lymphadenectomy (≥8 nodes resected).16 Furthermore, a novel classification system utilizing lymph-node-ratio more accurately stratified patients with locoregional SB-NETs by overall survival than the present AJCC classification.17 Given the unvalidated prognostic value of lymph node metastasis and the lack of data on the utility of preoperative CgA in patients with locoregional SB-NET, we aimed to further elucidate the prognostic capacity of clinical features in SB-NET by using a large, national cancer registry database. Specifically, we sought to evaluate the prognostic relevance of lymph node spread and the utility of elevated preoperative serum CgA in predicting outcomes for locoregional SB-NET. We hypothesized that preoperative serum chromogranin-A would be more prognostically useful than nodal metastasis in predicting overall survival in patients with locoregional SB-NET.
Materials & Methods
Data Source
The National Cancer Database participant user files (NCDB PUFs) were the source of all data in our study. The NCDB is a nationwide repository of de-identified patient data related to cancer care metrics and outcomes in the United States derived from the submissions of over 1,500 Commission on Cancer (CoC)-accredited programs.20 The NCDB captures over 70% of new cancer diagnoses in the United States per year. The CoC is a multidisciplinary association maintained by the American College of Surgeons and the American Cancer Society that accredits US hospitals based on various aspects of cancer care. Due to our study’s inclusion of only de-identified data, it was exempt from institutional review board approval at the National Institutes of Health.
Patient Selection
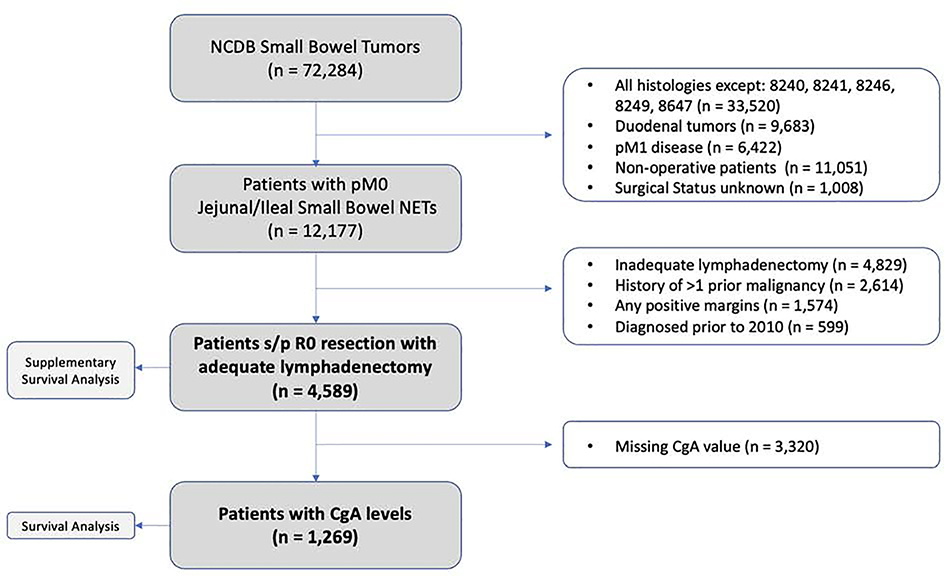
Patient selection criteria are outlined in Figure 1. The NCDB was queried for patients from 2010 to 2016 with neuroendocrine tumors of the jejunum and ileum using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes (C171, C172, C178, and C179) and histology codes (8240, 8241, 8246, 8249, and 8467). Preoperative serum chromogranin-A values are largely missing from the NCDB prior to 2010, and thus, only 5 patients were excluded by this cut-off. Of note, we excluded patients with duodenal tumors due to the high incidence of gastrinoma (and thus, proton-pump inhibitor usage) from the proximal small bowel due to potential false elevation of serum CgA levels. Additionally, we excluded all patients with evidence of distant metastatic disease to control for the effect of tumor burden on CgA levels. Patients who had a prior history of more than one prior malignancy, or those who did not undergo resection, underwent a margin-positive resection (R1 or R2), those with missing nodal (or other pertinent) information, and those who underwent an inadequate lymphadenectomy (<8 lymph nodes examined) were excluded.21 Staging was determined as per the American Joint Committee on Cancer (AJCC), 8th Edition clinical staging system. Among the 4,589 patients who met our inclusion criteria, 1,269 (27.6%) had preoperative serum CgA levels recorded. CgA levels reported in the NCDB represent the highest pre-treatment level in the medical record and are recorded to the nearest nanogram per milliliter (ng/mL). We collected demographic, clinicopathologic, and outcome-related data for all patients. Survival analysis for patients without reported preoperative CgA levels was performed separately.
Figure 1.
Consort diagram of patient inclusion and exclusion criteria.
Statistical Methods
The primary endpoint of the study was overall survival. Cut-point determination for optimal preoperative serum CgA level was performed using Contal & O’Quigley methods.22 Briefly, this model calculates the largest difference in patient mortality at each of the available preoperative serum CgA cut-points using the absolute log-rank statistic and tests the statistical significance at each point using a Q (maximization) statistic. The preoperative serum CgA level with the highest absolute log-rank and Q-statistic represents the optimal cut-off point, which, in this case, was 139ng/mL. Patients were divided into two groups based on their serum CgA levels, either ≥139ng/mL (high) or <139ng/mL (low).
Descriptive statistics were calculated for clinicopathologic and treatment variables, and associations between variables were analyzed by Pearson’s chi-square test and Mann-Whitney U test for normally and non-normally distributed data, respectively. This statistical method was also used to evaluate associations between patient and tumor characteristics and either high or low preoperative serum CgA levels. Univariable and multivariable Cox proportional hazards regression models were used to test for associations between clinicopathologic characteristics and overall survival. A two-tailed P <0.05 was considered statistically significant. The statistically significant clinical features associated with overall survival with a P <0.1 on the univariable analysis were then included in the multivariable analysis. Survival was further estimated using the Kaplan-Meier method and groups were compared using the log-rank test. Statistical analyses were performed using R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 26.0 (IBM Corp., Armonk, N.Y., USA).
Results
A total of 4,589 patients met the inclusion criteria. Sociodemographic, clinicopathologic, and treatment characteristics of these patients are presented in Table 1. Most patients were Caucasian with a well-balanced sex distribution and had a median age of 60 years. Most tumors were well-differentiated, ileal lesions of at least 1cm in maximal diameter, and had a high rate of lymph node metastasis (pN1). Patients with higher N-stages had correspondingly increasing median numbers of regional lymph nodes examined (14 nodes [pN0] vs. 16 nodes [pN1] vs. 26 nodes [pN2], p < 0.001).
Table 1.
Demographics and clinical characteristics of patients with locoregional small bowel neuroendocrine tumors.
| Variable | All Patients |
Patients with CgA “CgA Cohort” |
Patients without CgA | p-value | |||
|---|---|---|---|---|---|---|---|
| n = 4589 | % | n = 1269 | % | n = 3320 | % | ||
| Age at Diagnosis | 0.0001 | ||||||
| Mean (Range) | 60 (18 – 90) | 59 (22 – 90) | 60 (18 – 90) | ||||
| Median (IQR) | 60 (51 – 69) | 59 (51 – 67) | 60 (52 – 69) | ||||
| Sex | 0.508 | ||||||
| Male | 2242 | 48.9% | 630 | 49.6% | 1612 | 48.6% | |
| Female | 2347 | 51.1% | 639 | 50.4% | 1708 | 51.4% | |
| Race | 0.029 | ||||||
| White | 3915 | 85.3% | 1106 | 87.2% | 2809 | 84.6% | |
| Non-white | 674 | 14.7% | 163 | 12.8% | 511 | 15.4% | |
| Charlson-Deyo Score | 0.57 | ||||||
| 0 | 3507 | 76.4% | 976 | 76.9% | 2531 | 76.2% | |
| 1 | 820 | 17.9% | 228 | 18.0% | 592 | 17.8% | |
| ≥2 | 262 | 5.7% | 65 | 5.1% | 197 | 5.9% | |
| Number of Prior Malignancies | 0.284 | ||||||
| 0 | 4361 | 95.0% | 1213 | 95.6% | 3148 | 94.8% | |
| 1 | 228 | 5.0% | 56 | 4.4% | 172 | 5.2% | |
| Primary Site | 0.0001 | ||||||
| Jejunum | 166 | 3.6% | 52 | 4.1% | 114 | 3.4% | |
| Ileum | 3383 | 73.7% | 985 | 77.6% | 2398 | 72.2% | |
| Overlapping Lesions of SB | 39 | 0.8% | 13 | 1.0% | 26 | 0.8% | |
| SB NOS | 1001 | 21.8% | 219 | 17.3% | 782 | 23.6% | |
| Surgical Procedure | 0.04 | ||||||
| Simple / Partial Resection | 2961 | 64.5% | 790 | 62.3% | 2171 | 65.4% | |
| Total Resection | 1307 | 28.5% | 371 | 29.2% | 936 | 28.2% | |
| Debulking Surgery | 11 | 0.2% | 5 | 0.4% | 6 | 0.2% | |
| Radical Resection | 310 | 6.8% | 103 | 8.1% | 207 | 6.2% | |
| Tumor Size | 0.143 | ||||||
| ≤1cm | 931 | 20.7% | 235 | 18.9% | 696 | 21.3% | |
| >1cm | 3572 | 79.3% | 1008 | 81.1% | 2564 | 78.7% | |
| Differentiation | 0.452 | ||||||
| Well Differentiated | 3300 | 80.7% | 918 | 79.8% | 2382 | 81.0% | |
| Moderately Differentiated | 744 | 18.2% | 221 | 19.2% | 523 | 17.8% | |
| Poorly Differentiated | 47 | 1.1% | 11 | 1.0% | 36 | 1.2% | |
| Median Pre-op CgA (IQR) | 44 (8 – 117) | 44 (8 – 117) | n/a | n/a | |||
| Mitotic Count | 0.176 | ||||||
| <2 / 10HPFs | 2528 | 84.1% | 751 | 82.3% | 1777 | 84.9% | |
| 2–20 / 10HPFs | 462 | 15.4% | 157 | 17.2% | 305 | 14.6% | |
| >20 / 10HPFS | 15 | 0.5% | 4 | 0.4% | 11 | 0.5% | |
| pT Stage | 0.069 | ||||||
| T1 | 581 | 12.8% | 145 | 11.5% | 436 | 13.2% | |
| T2 | 1582 | 34.7% | 472 | 37.4% | 1110 | 33.7% | |
| T3 | 1750 | 38.4% | 464 | 36.8% | 1286 | 39.1% | |
| T4 | 641 | 14.1% | 180 | 14.3% | 461 | 14.0% | |
| pN Stage | 0.0001 | ||||||
| N0 | 599 | 13.1% | 117 | 9.2% | 482 | 14.5% | |
| N1 | 3809 | 83.0% | 1090 | 86.0% | 2719 | 81.9% | |
| N2 | 179 | 3.9% | 61 | 4.8% | 118 | 3.6% | |
| Regional Nodes Examined | 0.0001 | ||||||
| Mean (Range) | 19 | (8–90) | 20 | (8–90) | 18 | (8–90) | |
| Median (IQR) | 16 | (12–22) | 17 | (13–24) | 16 | (12–22) | |
The clinical and demographic characteristics of patients with preoperative serum CgA levels (the “CgA cohort”) were similar to those of the patients without CgA values available, except a lower rate of non-white patients (12.8% vs. 15.4%, p = 0.029) and a higher rate of pN1–2 disease (90.8% vs. 85.5%, p = 0.0001), likely attributable to an increased mean number of regional lymph nodes examined (20 vs. 18, p = 0.0001) (Table 1). The median CgA level was 44ng/mL, and the statistically optimized cut-point was 139ng/mL (Supplementary Figure 1). Overall, 1,269 patients were included in the preoperative serum CgA analysis, and most patients fell into the CgA-Low subgroup (n = 998, 78.6%). Compared to patients in the CgA-Low subgroup, patients in the CgA-High subgroup had a statistically significantly higher median age (63 vs. 57, p < 0.001), had higher rates of tumor size >1cm (88.2% vs. 79.2%, p = 0.001). A pairwise chi-square analysis showed no significant difference in the rate of well-differentiated NET by CgA groups. However, we observed a significantly higher rate of poorly differentiated (2.1% vs. 0.7%, p = 0.04) in the CgA-High group. Inversely, the CgA-Low group had a significantly higher rate of moderately-differentiated NETs (20.6% vs. 13.9%, p = 0.02). We found no statistically significant differences in T-stage, pathologic nodal stage, number of regional nodes examined, mitotic count, surgical procedure undergone, or comorbidity score by CgA subgroup (Table 2).
Table 2.
Demographics and clinical characteristics of patients with recorded preoperative serum chromogranin-A (CgA).
| Variables | CgA Cohort | CgA Level | |||||
|---|---|---|---|---|---|---|---|
| <139 | ≥139 | P | |||||
| n = 1269 | % | n = 998 | % | n = 271 | % | ||
| Age at Diagnosis | <0.001 | ||||||
| Mean (Range) | 59 (22 – 90) | 58 (22 – 89) | 63 (30 – 90) | ||||
| Median (IQR) | 59 (51 – 67) | 57 (50 – 65) | 63 (53 – 72) | ||||
| Sex | 0.534 | ||||||
| Male | 630 | 49.6% | 500 | 50.1% | 130 | 47.8% | |
| Female | 639 | 50.4% | 498 | 49.9% | 142 | 52.2% | |
| Race | 0.391 | ||||||
| White | 1106 | 87.2% | 874 | 87.6% | 232 | 85.6% | |
| Non-white | 163 | 12.8% | 124 | 12.4% | 39 | 14.4% | |
| Charlson-Deyo Score | 0.12 | ||||||
| 0 | 976 | 76.9% | 779 | 78.1% | 197 | 72.7% | |
| 1 | 228 | 18.0% | 173 | 17.3% | 55 | 20.3% | |
| ≥2 | 65 | 5.1% | 46 | 4.6% | 19 | 7.0% | |
| Number of Prior Malignancies | 0.728 | ||||||
| 0 | 1213 | 95.6% | 955 | 95.7% | 258 | 95.2% | |
| 1 | 56 | 4.4% | 43 | 4.3% | 13 | 4.8% | |
| Primary Site | 0.001 | ||||||
| Jejunum | 52 | 4.1% | 42 | 4.2% | 10 | 3.7% | |
| Ileum | 985 | 77.6% | 792 | 79.4% | 193 | 71.2% | |
| Overlapping Lesions of SB | 13 | 1.0% | 6 | 0.6% | 7 | 2.6% | |
| SB NOS | 219 | 17.3% | 158 | 15.8% | 61 | 22.5% | |
| Surgical Procedure | 0.37 | ||||||
| Simple / Partial Resection | 790 | 62.3% | 618 | 61.9% | 172 | 63.5% | |
| Total Resection | 371 | 29.2% | 288 | 28.9% | 83 | 30.6% | |
| Debulking Surgery | 5 | 0.4% | 4 | 0.4% | 1 | 0.4% | |
| Radical Resection | 103 | 8.1% | 88 | 8.8% | 15 | 5.5% | |
| Tumor Size | 0.001 | ||||||
| ≤1cm | 235 | 18.9% | 204 | 20.8% | 31 | 11.8% | |
| >1cm | 1008 | 81.1% | 777 | 79.2% | 231 | 88.2% | |
| Adequate Lymphadenectomy (Y/N) | |||||||
| No | 0 | 0.0 | 0 | - | 0 | 0.0% | |
| Yes | 1269 | 100.0 | 1002 | 100.0% | 272 | 100.0% | |
| Differentiation | 0.01 | ||||||
| Well Differentiated | 918 | 79.8% | 719 | 78.8% | 199 | 84.0% | |
| Moderately Differentiated | 221 | 19.2% | 188 | 20.6% | 33 | 13.9% | |
| Poorly Differentiated | 11 | 1.0% | 6 | 0.7% | 5 | 2.1% | |
| Median Pre-op Serum CgA (IQR) | 44 (8 – 117) | 27 (5 – 62) | 314 (198 – 602) | <0.001 | |||
| Preoperative Serum CgA | |||||||
| <139 | 998 | 78.6% | 998 | 100.0% | 0 | - | |
| ≥139 | 271 | 21.4% | 0 | - | 271 | 100% | |
| Mitotic Count | 0.14 | ||||||
| <2 / 10HPFs | 751 | 82.3% | 608 | 83.3% | 143 | 78.6% | |
| 2–20 / 10HPFs | 157 | 17.2% | 120 | 16.4% | 37 | 20.3% | |
| >20 / 10HPFS | 4 | 0.4% | 2 | 0.3% | 2 | 1.1% | |
| pT Stage | 0.055 | ||||||
| T1 | 145 | 11.5% | 126 | 12.7% | 19 | 7.1% | |
| T2 | 472 | 37.4% | 371 | 37.4% | 101 | 37.5% | |
| T3 | 464 | 36.8% | 360 | 36.3% | 104 | 38.7% | |
| T4 | 180 | 14.3% | 135 | 13.6% | 45 | 16.7% | |
| pN Stage | 0.493 | ||||||
| N0 | 117 | 9.2% | 97 | 9.7% | 20 | 7.4% | |
| N1 | 1090 | 86.0% | 854 | 85.6% | 236 | 87.4% | |
| N2 | 61 | 4.8% | 47 | 4.7% | 14 | 5.2% | |
| Regional Nodes Examined | 0.58 | ||||||
| Mean (Range) | 20 | (8–90) | 20 | (8–90) | 20 | (8–65) | |
| Median (IQR) | 17 | (13–24) | 17 | (13–24) | 16 | (13–24) | |
Of the patients in the CgA cohort (n = 1,269) 1,010 were included in the survival analysis with others excluded due to lack of follow-up information. With a median follow-up for living patients of 44 months (interquartile range: 24–67 months), the actual median overall survival was not reached in either subgroup (Figure 2). However, patients in the CgA-High group had a statistically significantly shorter estimated mean overall survival compared to those in the CgA-Low group (6.6 years [95% CI 6.27 – 6.91] vs. 7.6 years [95% CI 7.35 – 7.61], log-rank p = 0.00001).
Figure 2.
Kaplan-Meier analysis of patients in CgA-High and CgA-Low subgroups (n = 1010).
The clinical variables significantly associated with overall survival in univariate analysis included age at diagnosis, Charlson-Deyo score ≥2, prior malignancy, primary tumor in ileum or overlapping lesions in small bowel, tumor size >1cm, preoperative serum CgA ≥139ng/mL, pT3 and pT4 tumors (Table 3). Multivariate Cox proportional hazards analysis for the CgA cohort is summarized in Table 3. Age at diagnosis (HR = 1.06, 95% CI 1.03 – 1.09; p < 0.001), Charlson-Deyo score ≥2 (HR = 3.93, 95% CI 1.71 – 9.01; p = 0.001), poorly differentiated tumors (HR = 11.22, 95% CI 4.16 – 30.24; p < 0.001) and preoperative serum CgA ≥139ng/mL (HR = 2.19, 95% CI 1.22 – 3.92; p = 0.009) were independently associated with shorter overall survival on multivariable Cox analysis. Of note, pathologic T-stage and N-stage were not independently associated with overall survival (p > 0.05). When a survival analysis was performed on all patients, without the preoperative CgA variable (Supplementary Table 1), pN1 was independently associated with an improved overall survival compared to pN0 (HR = 0.47, 95% CI 0.33 – 0.65, p < 0.001). Kaplan Meier analysis of all patients (n = 3,793) for pathologic N-stage demonstrated longer mean overall survival in patients with pN1 compared to pN0 (7.07 years vs. 6.86 years, p = 0.003) and pT1 compared to pT3 (7.14 years vs. 6.93 years, p = 0.01), but not pT2 (7.13 years, p = 0.23) or pT4 (7.03 years, p = 0.053) (Supplementary Figure 2).
Table 3.
Cox proportional hazards survival analysis of patients in the CgA cohort.
| HR |
Univariate 95.0% CI |
P | HR |
Multivariable 95.0% CI |
P | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Age at Diagnosis | 1.09 | 1.06 | 1.11 | <0.001 | 1.06 | 1.03 | 1.09 | <0.001 |
| Sex | ||||||||
| Male | Ref | |||||||
| Female | 0.83 | 0.50 | 1.37 | 0.457 | ||||
| Race | ||||||||
| White | Ref | |||||||
| Non-white | 1.70 | 0.88 | 3.27 | 0.112 | ||||
| Charlson-Deyo Score | ||||||||
| 0 | Ref | Ref | ||||||
| 1 | 1.47 | 0.79 | 2.76 | 0.227 | 1.45 | 0.68 | 3.07 | 0.335 |
| ≥2 | 4.35 | 2.11 | 8.99 | <0.001 | 3.93 | 1.71 | 9.01 | 0.001 |
| Number of Prior Malignancies | ||||||||
| 0 | Ref | Ref | ||||||
| 1 | 2.51 | 1.14 | 5.52 | 0.022 | 1.11 | 0.41 | 3.00 | 0.844 |
| Primary Site | ||||||||
| Jejunum | Ref | Ref | ||||||
| Ileum | 0.44 | 0.17 | 1.13 | 0.089 | 0.55 | 0.15 | 1.98 | 0.362 |
| Overlapping Lesions of SB | 4.62 | 1.10 | 19.48 | 0.037 | 3.38 | 0.61 | 18.75 | 0.163 |
| SB NOS | 0.79 | 0.29 | 2.15 | 0.638 | 0.55 | 0.14 | 2.06 | 0.373 |
| Surgical Procedure | ||||||||
| Simple / Partial Resection | Ref | |||||||
| Total Resection | 0.93 | 0.52 | 1.67 | 0.803 | ||||
| Debulking Surgery | 3.33 | 0.46 | 24.31 | 0.236 | ||||
| Radical Resection | 1.27 | 0.54 | 3.00 | 0.590 | ||||
| Tumor Size | ||||||||
| ≤1cm | Ref | Ref | ||||||
| >1cm | 3.53 | 1.28 | 9.74 | 0.015 | 1.41 | 0.39 | 5.08 | 0.596 |
| Differentiation | ||||||||
| Well Differentiated | Ref | Ref | ||||||
| Moderately Differentiated | 1.18 | 0.59 | 2.39 | 0.640 | 1.69 | 0.82 | 3.48 | 0.157 |
| Poorly Differentiated | 17.68 | 7.81 | 40.02 | <0.001 | 11.22 | 4.16 | 30.24 | <0.001 |
| Preoperative Serum CgA | ||||||||
| <139ng/mL | Ref | Ref | ||||||
| ≥139ng/mL | 3.09 | 1.87 | 5.12 | <0.001 | 2.19 | 1.22 | 3.92 | 0.009 |
| Mitotic Count | ||||||||
| <2 / 10HPFs | Ref | |||||||
| 2–20 / 10HPFs | 1.42 | 0.62 | 3.25 | 0.409 | ||||
| >20 / 10HPFS | 0.00 | 0.00 | n/a | 0.980 | ||||
| pT Stage | ||||||||
| T1 | Ref | Ref | ||||||
| T2 | 3.54 | 0.45 | 27.68 | 0.228 | 1.81 | 0.18 | 18.60 | 0.617 |
| T3 | 7.60 | 1.04 | 55.47 | 0.046 | 2.91 | 0.27 | 30.96 | 0.376 |
| T4 | 8.64 | 1.14 | 65.45 | 0.037 | 3.09 | 0.28 | 34.15 | 0.358 |
| pN Stage | ||||||||
| N0 | Ref | |||||||
| N1 | 1.41 | 0.51 | 3.89 | 0.509 | ||||
| N2 | 0.82 | 0.15 | 4.45 | 0.814 | ||||
Discussion
Our study demonstrates the prognostic utility of preoperative serum CgA in patients with locoregional SB-NETs who are surgical candidates. We highlighted that, unlike preoperative serum CgA, common pathological features such as T-stage and lymph node metastasis poorly predicted overall survival in patients with locoregional SB-NETs. Neither Cox proportional hazards nor Kaplan-Meier analyses demonstrated shorter overall survival in patients with lymph node metastasis (pN1–2) in patients with locoregional SB-NET. These findings call into question the prognostic relevance of lymph node metastases as defined by the current staging system and suggest that preoperative serum CgA may provide both a more accurate and accessible prognosticator for SB-NET.
Although the utility of CgA is well-established in the diagnosis and surveillance of patients with neuroendocrine tumors, its prognostic significance in the non-metastatic setting remains ill-defined.11 The significant elevations in CgA levels have been associated with shorter overall survival in small pancreatic NETs while, conversely, it has been proposed that patients whose values are below this threshold may safely defer resection of small, well-differentiated tumors.13, 23 Our study demonstrates the independent association between the elevated preoperative CgA levels and shorter overall survival in patients with locoregional SB-NET which can be useful in preoperative counseling. Our findings may help identify a subset of patients with more aggressive tumor biology despite a relatively benign clinical presentation, such as those with possible subclinical or occult metastatic disease. Furthermore, using an accepted statistical method of dichotomization, we determined 139ng/mL to be the optimal cut-off above which patients in this cohort experienced the largest disparities in overall survival. Even when compared to potentially more accurate, ratio-based nodal staging systems, the advantages of preoperative serum CgA are 1) it informs a preoperative discussion of prognosis and 2) unlike histopathology, it is not subject to interpretation or adequacy of the surgical specimen. Additionally, our CgA cut-off exposed a difference in overall survival within 5 years of follow-up, delineating a subgroup of patients with unfavorable biology despite reported 5-year survival rates surpassing 94%.17 Thus, patients with locoregional SB-NET with an elevated preoperative CgA may benefit from a sensitive metastatic workup such as 68-Ga DOTATATE PET/CT (FDA-approved in 2016) or 18F-FDOPA PET/CT (currently investigational only), which were not used clinically in this study. Additional detection of metastatic sites can alter the extent of the operation and treatment strategy. The clinical utility of preoperative serum CgA levels, beyond preoperative discussion of prognosis, warrants further investigation. To date, ours is the largest study evaluating the prognostic implications of serum CgA in locoregional SB-NET and the only one to focus on its use in the preoperative setting. Others have examined the prognostic utility of CgA in SB-NET as part of a nomogram, thereby failing to examine the variable’s independent association with survival.7,24 Furthermore, these studies utilized arbitrary cut-offs for CgA lacking clinical significance or statistical derivation. While our cut-off of 139ng/mL was identified from a proper statistical analysis, the CgA levels in this cohort may be from various assays. Thus, the cut-off of 139ng/mL may not be clinically applicable to other cohorts and requires prospective validation using a common assay. Our approach to dichotomization of the variable emphasizes the prognostic value of elevated preoperative CgA in patients with locoregional SB-NET.
While a regional lymphadenectomy that includes at least 8 lymph nodes represents the standard of care advocated by the National Comprehensive Cancer Network guidelines, the implications of lymph node metastases themselves among patients with SB-NET remain incompletely understood.21, 25 The first study to establish eight harvested nodes as the minimal number required for an adequate lymphadenectomy also attributed its associated survival benefit to the minimization of the lymph node ratio, and not the resection of nodal tissue itself.25 This finding may suggest that the resection of nodal metastases likely does not improve long-term outcomes in these patients, but rather that these patients were spared from the inevitable understaging that would ensue if few or no lymph nodes were harvested during resection of the primary tumor. Similarly, a 2018 Surveillance, Epidemiology, and End Results (SEER) database analysis of patients with non-metastatic SB-NETs demonstrated the superior prognostic value of positive-lymph-node ratio to absolute nodal involvement and validated a novel lymph node staging system.17 Finally, in a 2019 study, patients with SB-NETs who received an adequate lymphadenectomy only exhibited shortened recurrence-free survival if ≥4 nodes were positive. Of note, recurrence-free survival was not associated with nodal involvement in patients who received sub-optimal lymphadenectomies. Adequate lymphadenectomy allows the more accurate prognostication as the extent of the tumor progression can be more precisely assessed.16 These data argue for further discrimination amongst, and a more objective assessment of, the overwhelming majority of SB-NETs staged as pN1. Our study, the largest appraisal of the current pathologic nodal staging system to date, utilizes a nationwide database to evaluate the importance of nodal involvement in SB-NETs. While our analysis found no evidence of association between pathologic node stage and overall survival, it is possible that our study was underpowered to assess this variable, given that only 13.1% of all patients had no lymph node metastases. Given the size of our study, however, it is more likely that this finding indicates a lack of granularity in the current staging system that may hinder its prognostic ability in patients with locoregional disease. This is supported by the fact that 83% of patients in our study had pN1 disease. Other well-known negative prognostic indicators, such as advanced age, Charlson-Deyo comorbidity score, and poorly-differentiated tumors, retained their established independence with overall survival, lending confidence to our model as an assessment tool. Furthermore, when chromogranin-A level was removed from the multivariable analysis to facilitate the assessment of pN in all patients, pN1 was paradoxically associated with improved overall survival; though this may be explained by more complete surgery and an upstaging due to a more extensive lymphadenectomy, it might also highlight a lack of power due to the small number of pN0 patients. This supplementary analysis also failed to demonstrate an independent association between pathologic T-stage and overall survival, despite finding such association with tumor size >1cm. While this study was not designed specifically to assess the accuracy to the existing T- and N-staging systems, these data further add to the uncertainty surrounding the current AJCC classifications and highlight the need for adjuvant metrics to guide discussions of prognosis with patients who present with locoregional SB-NETs, a role that we believe serum CgA levels can help to fulfill.
In addition to demonstrating the value of serum CgA levels, our study has a number of pertinent ancillary findings. Unsurprisingly, patients with poorly-differentiated tumors had a significantly shorter overall survival than their better-differentiated counterparts. Mitotic count, a variable used in contemporary staging systems of other neuroendocrine tumors, did not demonstrate an association with overall survival, which may be due to type II statistical error. These data, coupled with the unconventional relationships between T-, N-stage, and overall survival, reflect the unique biology underlying SB-NETs and indicate the need for a tailored staging system. Alternative staging systems, such as the 2010 WHO classification of pancreatic neuroendocrine tumors, prioritize measures of biologic aggressiveness over conventional appraisals, such as the ENETS and AJCC ‘TNM-based’ systems. Similarly, our findings demonstrate a stronger association of overall survival with histopathologic and biochemical markers of aggression, like poor differentiation and elevated CgA, thereby advocating for the prospective reassessment of prognostic variables in SB-NETs.
Despite our use of a large national database, our study is limited by its relatively small yield of patients with node-negative (pN0) or advanced nodal disease (pN2), and thus, may be underpowered to detect an overall survival difference. The retrospective nature of our analysis can be particularly problematic due to missing data, selection bias, non-standardized follow-up, absence of disease-specific survival, and the variability in laboratory assessment of serum CgA levels, which may contribute to differences in measured values. The NCDB also obscures information about non-neuroendocrine etiologies of CgA elevation, such as false elevations secondary to gastric acid suppression drugs, chronic atrophic gastritis, chronic kidney disease, systemic inflammatory diseases (i.e. rheumatoid arthritis, systemic lupus erythematosus), and cardiovascular diseases.26 The lack of postoperative CgA in the NCDB limited our ability to assess the tumor-derived CgA and the effect of cytoreduction on CgA levels. Furthermore, because the current T-staging system is more discriminatory on the depth of invasion, our attempts to control for tumor burden by eliminating patients with metastatic disease leaves room for undetected variability in primary tumor size. Based on our findings, we support the call for a reassessment of the current pathologic nodal staging system in SB-NETs and suggest prospective evaluation of preoperative serum chromogranin-A, a standardized, non-invasive, and objective biochemical measure, as a superior prognosticator.
Conclusion
Elevated preoperative serum CgA is an adverse prognostic marker associated with shorter overall survival in patients with locoregional SB-NET. Thus, it can be useful in preoperative counseling. Neither lymph node metastasis nor T-stage was independently associated with shorter overall survival. We believe that our findings are cause for consideration of serum CgA as an established prognosticator of SB-NET, and indicate the need for dedicated prospective reassessment of SB-NET staging in a comprehensive, multicenter study to establish clinicopathologic variables with the strongest association with long-term survival.
Supplementary Material
Supplementary Figure 1. Graphical representation of Contal O’Quigley method of dichotomization.
Supplementary Figure 2. (A) Kaplan Meier analysis of all patients (n = 3793) based on pathologic nodal staging (AJCC 8th Edition). P-values reflect log-rank comparison to pN0. (B) Kaplan Meier analysis of all patients (n = 3778) based on pathologic T-staging (AJCC 8th Edition). P-values reflect log-rank comparison to pT1.
Supplementary Table 1. Cox proportional hazards survival analysis (excluding the CgA variable) of all patients (n = 3778).
Acknowledgments
Funding/Support
This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Conflict of Interest/Disclosure
The authors declare that the research detailed here was conducted in the absence of any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis A, Raoof M, Ituarte PHG, Williams J, Melstrom L, Li D, et al. Resection of the Primary Gastrointestinal Neuroendocrine Tumor Improves Survival With or Without Liver Treatment. Ann Surg 2019;270:1131–7. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–94. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. [DOI] [PubMed] [Google Scholar]
- 6.Scarpa A. The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann Endocrinol (Paris). 2019;80:153–8. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Gustafsson BI, Pavel M, Svejda B, Lawrence B, Kidd M. A nomogram to assess small-intestinal neuroendocrine tumor (‘carcinoid’) survival. Neuroendocrinology. 2010;92:143–57. [DOI] [PubMed] [Google Scholar]
- 8.Kelly S, Aalberg J, Kim MK, Divino CM. A Predictive Nomogram for Small Intestine Neuroendocrine Tumors. Pancreas. 2020;49:524–8. [DOI] [PubMed] [Google Scholar]
- 9.Braga F, Ferraro S, Mozzi R, Dolci A, Panteghini M. Biological variation of neuroendocrine tumor markers chromogranin A and neuron-specific enolase. Clin Biochem 2013;46:148–51. [DOI] [PubMed] [Google Scholar]
- 10.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol 2001;12 Suppl 2:S69–72. [DOI] [PubMed] [Google Scholar]
- 11.Lyubimova NV, Churikova TK, Kushlinskii NE. Chromogranin As a Biochemical Marker of Neuroendocrine Tumors. Bull Exp Biol Med 2016;160:702–4. [DOI] [PubMed] [Google Scholar]
- 12.Khan TM, Garg M, Warner RR, Uhr JH, Divino CM. Elevated Serum Pancreastatin Is an Indicator of Hepatic Metastasis in Patients With Small Bowel Neuroendocrine Tumors. Pancreas. 2016;45:1032–5. [DOI] [PubMed] [Google Scholar]
- 13.Raoof M, Jutric Z, Melstrom LG, Lee B, Li D, Warner SG, et al. Prognostic significance of Chromogranin A in small pancreatic neuroendocrine tumors. Surgery. 2019;165:760–6. [DOI] [PubMed] [Google Scholar]
- 14.Ter-Minassian M, Chan JA, Hooshmand SM, Brais LK, Daskalova A, Heafield R, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer. 2013;20:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose KJ, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol 2008;6:820–7. [DOI] [PubMed] [Google Scholar]
- 16.Zaidi MY, Lopez-Aguiar AG, Dillhoff M, Beal E, Poultsides G, Makris E, et al. Prognostic Role of Lymph Node Positivity and Number of Lymph Nodes Needed for Accurately Staging Small-Bowel Neuroendocrine Tumors. JAMA Surg 2019;154:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Chen F, Chen S, Wang L. The Lymph Node Ratio Optimizes Staging in Patients with Small Intestinal Neuroendocrine Tumors. Neuroendocrinology. 2018;107:209–17. [DOI] [PubMed] [Google Scholar]
- 18.Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol 2013;31:420–5. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Song Y, Zhang Y, Chen M, Chen J. Exploration of the Exact Prognostic Significance of Lymphatic Metastasis in Jejunoileal Neuroendocrine Tumors. Ann Surg Oncol 2018;25:2067–74. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693–702. [DOI] [PubMed] [Google Scholar]
- 22.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis. 1999;30:253–70. [Google Scholar]
- 23.Mirkin KA, Hollenbeak CS, Wong J. Impact of chromogranin A, differentiation, and mitoses in nonfunctional pancreatic neuroendocrine tumors </= 2 cm. J Surg Res 2017;211:206–14. [DOI] [PubMed] [Google Scholar]
- 24.Kelly S, Aalberg J, Agathis A, Phillips K, Haile S, Haines K, et al. Predicting Survival of Small Intestine Neuroendocrine Tumors: Experience From a Major Referral Center. Pancreas. 2019;48:514–8. [DOI] [PubMed] [Google Scholar]
- 25.Motz BM, Lorimer PD, Boselli D, Hill JS, Salo JC. Optimal Lymphadenectomy in Small Bowel Neuroendocrine Tumors: Analysis of the NCDB. J Gastrointest Surg 2018;22:117–23. [DOI] [PubMed] [Google Scholar]
- 26.Gut P, Czarnywojtek A, Fischbach J, Baczyk M, Ziemnicka K, Wrotkowska E, et al. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci 2016;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Graphical representation of Contal O’Quigley method of dichotomization.
Supplementary Figure 2. (A) Kaplan Meier analysis of all patients (n = 3793) based on pathologic nodal staging (AJCC 8th Edition). P-values reflect log-rank comparison to pN0. (B) Kaplan Meier analysis of all patients (n = 3778) based on pathologic T-staging (AJCC 8th Edition). P-values reflect log-rank comparison to pT1.
Supplementary Table 1. Cox proportional hazards survival analysis (excluding the CgA variable) of all patients (n = 3778).