Summary
Introduction:
Enhanced Recovery Pathways (ERPs), also known as ERAS® pathways, are standardized pathways composed of 21-24 perioperative elements designed to improve post-surgical recovery. ERP has been shown to be safe and effective in children undergoing bladder reconstruction but has not been widely utilized.
Objective:
The aim of this study was to assess utilization of ERPs in pediatric urology and identify barriers to establishing these standardized pathways.
Study Design:
Pediatric urologists who were members of the Societies for Pediatric Urology (SPU) were surveyed regarding their familiarity with standardized ERPs, current use of ERP elements, and encountered or perceived barriers to standardized ERP implementation. Willingness to implement ERP elements in a child undergoing bladder reconstruction was assessed with a 5-point Likert scale. Descriptive analysis was performed; Fisher’s exact test was performed to assess associations between respondent demographics and ERP familiarity.
Results:
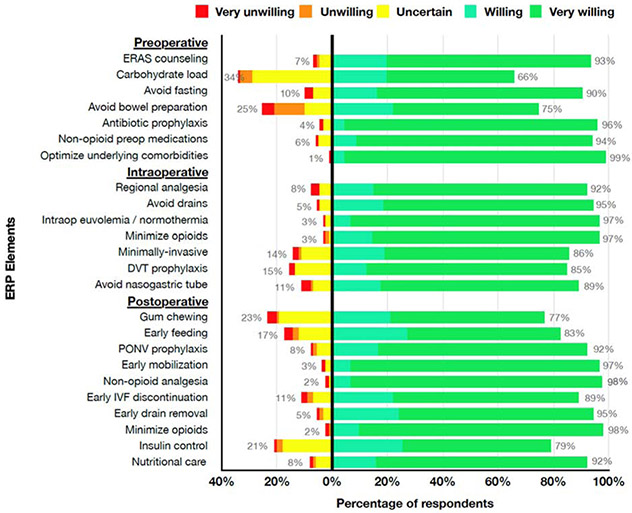
Of 714 distributed surveys, 113 (16%) valid responses were collected. 69% of respondents were male, 58% practiced at academic institutions, and 57% performed 1-5 bladder reconstructions a year. 61% were somewhat familiar or not familiar with standardized ERP. While 54% currently utilize individual ERP elements, only 20% have standardized pathways. Out of 24 possible ERP elements, a median of 15 elements (range 0-24) were implemented by the respondents whether they reported they were implementing ERP elements or had standardized pathways in place. 15 of 24 ERP elements were found to be nearly universally acceptable, with greater than 90% of respondents being somewhat or very willing to implement them in the presented case scenario (Summary Figure). 62% and 56% of those who currently implement ERP elements and experienced barriers noted lack of administrative/leadership support and inability to achieve consensus among pediatric colleagues, respectively, as common barriers in standardization. For those who have not attempted standardization, the most common perceived barrier was pathway unfamiliarity (48%).
Discussion:
Over half of respondents were not familiar with enhanced recovery pathways but were willing to implement a majority of the pathway elements, suggesting potential for ERP standardization in pediatric urology. Buy-in from colleagues and leadership would be necessary to overcome perceived barriers of standardized pathway development.
Conclusion:
Administrative support and more widespread knowledge of ERP amongst pediatric urologists are necessary to facilitate further implementation in children undergoing bladder reconstruction.
Keywords: Enhanced Recovery After Surgery, Clinical Pathways, Bladder Reconstruction
Summary Figure
Introduction
Standardized, evidence-based, peri-operative pathways focused on optimizing care of patients undergoing major surgical procedures have been promoted since the 1990s.[1, 2] These programs, commonly known as Enhanced Recovery After Surgery (ERAS®) pathways, enhanced recovery pathways (ERPs), or fast-track recovery, promote an integrated, multi-modal, multi-disciplinary approach towards incorporating evidence-based guidelines in peri-operative care.[3] Standardized pathways contain various elements that are implemented in the pre-operative, intra-operative, and post-operative phases of care. Although individual elements may vary by institution and specialty, standardized pathways contain a combination of elements focused on minimizing the physiologic stress on the body caused by surgical intervention.[3] While surgeons may implement individual ERP elements in their practices, use of a standardized, integrated pathway, in which the various elements are consistently implemented by a multidisciplinary care team, has increased in recent years. ERP implementation has shown robust and consistent results in reducing length of stay and complications in many adult surgical specialties.[4-6]
ERPs have also yielded beneficial results in pediatric surgical specialties. Pathways adapted from that of adult colorectal surgeries have been implemented in children undergoing elective colorectal surgery, resulting in decreased length of stay and opioid use.[7] Similar results were also seen in a study of ERP implementation in 13 children who underwent urologic reconstruction using a pathway adapted from adult ERAS® pathways. ERP implementation in this cohort was associated with reduced length of stay, decreased complication rates, and reduced post-operative opioid use compared to a historical cohort.[8] Despite these encouraging preliminary results, adoption of ERPs in pediatric surgical specialties has been slow. A survey of members of the American Pediatric Surgical Association in 2016 noted that only 19.2% of pediatric surgeons had an active standardized ERP at their institutions.[9] While studies of ERP implementation in pediatric surgery are increasing, an understanding of applications of ERPs and barriers to pathway implementation in pediatric urology, especially in bladder reconstructive cases where bowel anastomoses are frequently performed, remain largely unknown.[10, 11]
The objective of this study was to assess the utilization of individual ERP elements and standardized, integrated ERPs in pediatric urology in the United States, pediatric urologists’ willingness to implement specific, individual ERP elements in cases of bladder reconstruction requiring bowel anastomoses and encountered or perceived potential barriers to creation of standardized pathways. We hypothesized that utilization of standardized ERPs in pediatric urology would be low, and lack of administrative support would be a common barrier.
Materials and Methods
Ethical Approval
The study was submitted for review by the Institutional Review Board and was deemed exempt (IRB number 2019-2351).
Study Design
A survey designed iteratively by the authors, with representation from pediatric urology, pediatric anesthesiology, and pediatric surgery, was distributed to pediatric urologists who are members of the Societies for Pediatric Urology (SPU). The survey was administered through REDCap, a secure, online data collection platform.[12] The anonymous survey assessed the respondents’ familiarity with ERPs on a 4-point Likert scale (not familiar, somewhat familiar, very familiar, and extremely familiar), where they learned about ERPs, their experience with implementing standardized ERPs or individual ERP elements, and encountered or perceived barriers with establishing a standardized ERP at their respective institutions. The respondents were also surveyed regarding their willingness to implement each of 24 individual ERP elements on a 5-point Likert scale (very unwilling, somewhat unwilling, uncertain, somewhat willing, and very willing) using a case scenario of an 8-year-old boy with neurogenic bladder and bowel scheduled to undergo bladder augmentation and creation of a continent catheterizable stoma. Demographic data and details regarding the respondent’s current pediatric urology practice were also obtained.
The survey was distributed from December 28, 2018 to March 28, 2019 via electronic mail by the administrative office of the Societies for Pediatric Urology (SPU). To maximize the response rate, two reminders were delivered during this time period.
Statistical Analysis
Descriptive analysis was performed. Fisher’s exact test was performed to assess associations between respondent demographics and overall ERP familiarity. All statistical analyses were performed with Stata version 14 (StataCorp, College Station, TX) with significance set at p<0.05.
Results
The survey communication electronic mail was distributed to 714 SPU members, of whom 263 (37%) opened the message. One hundred and twenty-nine respondents (18% of the surveyed cohort and 49% of those who opened the message) started the survey. Surveys without responses (N=12) and those completed by pediatric urologists practicing outside of the United States (N=4) were excluded from analysis. A total of 113 valid surveys (16%) were included in the final analysis.
Demographics
Of the 113 survey respondents, 91 (81%) provided demographic data. Sixty-nine percent were male; 66% completed a formal pediatric urologic fellowship prior to 2010 (Table 1). One individual did not complete a formal pediatric urologic fellowship, and two were still in fellowship. Fifty-eight percent of individuals work in an academic setting. Thirty percent of respondents perform >5 bladder reconstruction cases a year.
Table 1.
Demographics
| Gender (N=91) | |
| Female | 28 (31%) |
| Male | 63 (69%) |
| Year completed fellowship (N=92) | |
| Did not complete a formal pediatric urology fellowship | 1 (1%) |
| Before 1981 | 6 (6%) |
| 1981-1990 | 11 (12%) |
| 1991-2000 | 26 (28%) |
| 2001-2010 | 18 (20%) |
| 2011-2018 | 28 (30%) |
| Still in pediatric urology fellowship | 2 (2%) |
| Practice Location (N=92) | |
| Northeast | 26 (28%) |
| Midwest | 17 (18%) |
| South | 21 (23%) |
| West | 21 (23%) |
| Canada | 1 (1%) |
| Other | 6 (7%) |
| Practice Setting (N=92)* | |
| Academic/University affiliated hospital | 65 (58%) |
| Private Practice | 14 (12%) |
| Community hospital | 10 (9%) |
| Free standing pediatric hospital | 25 (22%) |
| Other | 2 (2%) |
| Number of pediatric urologists in practice (N=92) | |
| 1 | 13 (14%) |
| 2 | 22 (24%) |
| 3 | 14 (15%) |
| 4 | 5 (5%) |
| 5 | 7 (8%) |
| >5 | 31 (34%) |
| Number of bladder reconstruction cases a year (N=91) | |
| None | 12 (13%) |
| 1-5 cases | 52 (57%) |
| 6-10 cases | 16 (18%) |
| >10 cases | 11 (12%) |
ERP Familiarity
Sixty-one percent of respondents noted that they were somewhat or not familiar with ERP (Supplemental Table 1). Respondents learned about ERP while in training (23%), in practice (38%), at conference/grand rounds (32%), through journal articles (37%), or from scientific meetings (29%) (Supplemental Table 1). No association was found between demographic factors and familiarity with ERP (Supplemental Table 2). There was also no association between practice location, number of pediatric urologists in the practice, and number of bladder reconstruction cases performed per year with ERP familiarity (Supplemental Table 2).
ERP Implementation
Twenty-six percent (29/112) of respondents have experience with implementing a standardized ERP (Supplemental Table 1). Twenty-one percent (23/110) of respondents noted that they have a standardized pediatric ERP while 37% (40/109) percent reported that their adult urologic colleagues have a standardized ERP in place. Although only 26% of respondents have a standardized pathway, 54% (60/111) reported that they are performing ERP elements with or without standardization (Supplemental Table 1). Of those performing ERP elements, 92% (55/60) reported on the types of surgeries in which they were implementing ERP elements. These included robotic surgeries (45%), laparoscopic surgeries (38%), bladder surgeries without bowel anastomoses (64%), bladder surgeries with bowel anastomoses (80%), open kidney surgeries (47%), and open ureteral surgeries (45%).
Although only 54% of respondents reported performing ERP elements, when asked specifically about individual ERP elements they are currently implementing, respondents reported implementing a median of 4 of 7 pre-operative elements, 5 of 7 intra-operative, and 6 out of 10 post-operative elements, yielding a total of 15 of 24 elements (Table 2). Elements that were not commonly implemented were pre-operative ERP counseling and carbohydrate loading.
Table 2.
Elements Currently Implemented (N=103)
| Preoperative | |
| Preoperative counseling regarding ERP | 25 (24%) |
| Carbohydrate load | 14 (14%) |
| Avoid prolonged fasting | 71 (69%) |
| Avoid additional bowel preparation | 52 (50%) |
| Provide preoperative antibiotic prophylaxis | 76 (74%) |
| Administer non-opiate preoperative analgesic medications | 64 (62%) |
| Optimize underlying medical conditions | 81 (78%) |
| None of the above | 8 (7%) |
| Median (IQR) [range] preop elements implemented (out of 7) | 4 (IQR 3-5) [range 0-7] |
| Mean (SD) preop elements implemented (out of 7) | 3.7 (2.0) |
| Intraoperative | |
| Use a standardized anesthesia protocol with use of regional anesthesia when possible | 67 (65%) |
| Avoid excess drains | 67 (65%) |
| Maintain intraoperative euvolemia and normothermia | 73 (70%) |
| Minimize intraoperative opioid use | 69 (67%) |
| Minimally invasive approach when able | 74 (72%) |
| Prophylaxis against thromboembolism | 51 (50%) |
| Avoid routine nasogastric tube use | 66 (64%) |
| None of the above | 5 (5%) |
| Median (IQR) [range] intraop elements implemented (out of 7) | 5 (IQR 3-6) [range 0-7] |
| Mean (SD) preop elements implemented (out of 7) | 4.0 (1.0) |
| Postoperative | |
| Encourage post-operative gum chewing | 19 (18%) |
| Implement early feeding (clears ad lib POD 0, regular diet POD 1) | 71 (69%) |
| Have pharmacological medications available for nausea/vomiting prophylaxis as opposed to NGT (Patients may have NGT placed post op if refractory nausea/vomiting) | 71 (69%) |
| Implement early mobilization (out of bed POD 1) | 90 (87%) |
| Use adjunctive pain medication (scheduled acetaminophen or NSAID for first 24 hours) | 86 (84%) |
| Early discontinuation of IV fluids by POD 2 if patient is tolerating oral intake | 62 (60%) |
| Early drain (not foley or other bladder catheters) removal (by POD 4) | 56 (54%) |
| Minimize opioids | 87 (84%) |
| Use insulin to control severe hyperglycemia | 14 (14%) |
| Provide perioperative nutritional optimization (screening or nutritional status evaluation) | 27 (26%) |
| None of the above | 4 (4%) |
| Median (IQR) [range] postop elements implemented (out of 10) | 6 (IQR 4-7) [range 0-10] |
| Mean (SD) postop elements implemented (out of 10) | 5.7 (2.4) |
| Median (IQR) [range] total elements implemented (out of 24) | 15 (IQR 11-18) [range 0-24] |
| Mean (SD) elements implemented (out of 24) | 14.0 (5.5) |
Barriers to Implementation
Of the 60 respondents who noted that they were currently implementing ERP elements, 15 (25%) had not attempted to institute standardized pathways, 24 (40%) noted no barriers to standardization, 7 (12%) did not respond to this question, and 16 (27%) reported experiencing barriers to standardization. The most common barriers encountered were difficulty achieving consensus among pediatric urologic colleagues (62%) and lack of administrative/leadership support (56%) (Table 3).
Table 3.
Current and Potential Barriers (n=16)
| Current Barriers for those with standardized pathways (n=16) | |
|---|---|
| Lack of administrative/leadership support | 9 (56%) |
| Lack of anesthesia support | 5 (31%) |
| Difficulty achieving consensus among my pediatric urology colleagues to implement a standardized enhanced recovery pathway | 10 (62%) |
| Difficulty achieving consensus among my pediatric urology colleagues to implement any standardized pathways | 6 (38%) |
| Resistance from patients or families | 0 |
| Difficulty initiating the enhanced recovery pathway | 6 (38%) |
| Difficulty maintaining compliance with the enhanced recovery pathway | 6 (38%) |
| Patients will not benefit | 0 |
| There is not enough familiarity with the pathway | 6 (38%) |
| Other ______________ | 2 (12%) |
| *Unable to achieve buy in from other surgical colleagues | |
| *Patients cared for by hospitalists | |
| Potential Barriers for those without standardized pathways (n=56) | |
| Lack of administrative/leadership support | 10 (18%) |
| Lack of anesthesia support | 11 (20%) |
| Difficulty achieving consensus among my pediatric urology colleagues to implement a standardized enhanced recovery pathway | 17 (30%) |
| Difficulty achieving consensus among my pediatric urology colleagues to implement any standardized pathways | 5 (9%) |
| Resistance from patients or families | 3 (5%) |
| Difficulty initiating the enhanced recovery pathway | 9 (16%) |
| Difficulty maintaining compliance with the enhanced recovery pathway | 18 (32%) |
| Patients will not benefit | 1 (2%) |
| There is not enough familiarity with the pathway | 27 (48%) |
| Other ______________ | 3 (5%) |
| *None | |
| *Just implement key elements and not a pathway | |
| *Don’t do enough cases to justify a pathway | |
Of those who were not implementing elements of ERP (N=51) or who were implementing elements of ERP without standardization (N=15), 56 (85%) individuals responded with what they would perceive to be barriers to creating a standardized ERP at their respective institutions. The most common perceived barriers were not enough familiarity with the pathway (48%), difficulty maintaining compliance with an ERP (32%), and difficulty achieving consensus among pediatric urologic colleagues to implement a standardized ERP (30%) (Table 3).
Willingness to Implement
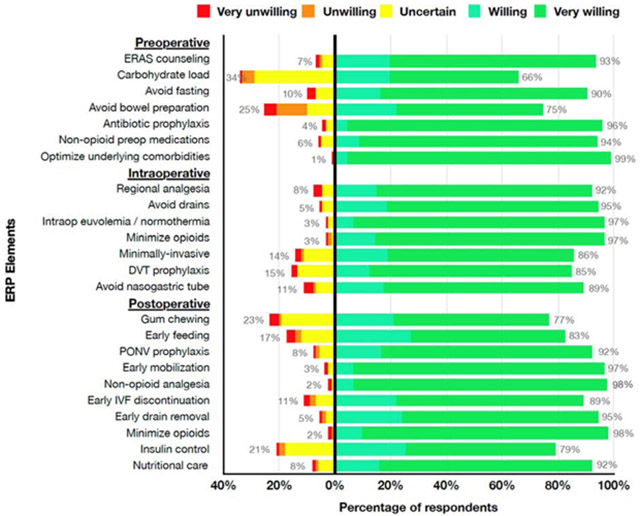
The respondents were asked to rate their willingness to perform each individual ERP element in a case of an 8-year-old boy scheduled to undergo bladder augmentation with creation of a catheterizable channel. Over 90% of respondents were very willing or somewhat willing to implement 15 of the 24 ERP elements (Figure 1). More than 10% of respondents were uncertain, somewhat unwilling, or very unwilling to perform two pre-operative elements (carbohydrate load and avoid additional bowel preparation), three intra-operative elements (minimally invasive approach to procedure, thromboembolism prophylaxis, and avoid routine nasogastric tube placement), and four post-operative elements (post-operative gum chewing, early feeding, early discontinuation of intravenous fluids, and management of hyperglycemia with insulin). Avoiding additional pre-operative bowel preparation, performing pre-operative carbohydrate loading, encouragement of gum-chewing post-operatively, and management of severe hyperglycemia with insulin were the most controversial elements with over 20% of respondents indicating that they were uncertain, somewhat unwilling, or very unwilling to implement them (Figure 1).
Figure 1.
Willingness to Implement ERP Elements
Discussion
While ERPs have become more widely utilized in adult surgical specialties,[5, 13, 14] their role in pediatric surgical specialties and specifically in pediatric urology is still slowly being defined.[10, 15] In accordance with our hypothesis and similar to findings of Short et al. in their study of standardized ERP utilization in pediatric colorectal surgery,[9] standardized ERP implementation rates are low in pediatric urology. Only 26% of respondents reported having standardized pathways at their institutions. Despite this, 54% of respondents report implementing ERP elements with or without standardized pathways. Respondents reported a median of 15 of 24 common elements implemented. Furthermore, over 90% of respondents were very or somewhat willing to implement each of 15 of the 24 presented individual ERP elements in the proposed case scenario. These results are promising and suggest interest with ERPs within the field of pediatric urology in the United States.
Results of this study indicate that lack of familiarity with ERP is a potential barrier to creating standardized pathways in pediatric urology. Sixty-one percent of survey respondents stated that they were somewhat or not familiar with ERP. In recent years, ERP has become more established in adult surgical practices, with guidelines statements released by the ERAS® society that are specific to each surgical procedure.[16-18] Similar practices are slowly rising in pediatric surgery with the recent release of guidelines for neonatal intestinal surgery.[19] However, such guidelines are currently lacking in pediatric urology, which may reflect the relative paucity of data on ERP application and unfamiliarity with ERP within the field. This knowledge gap may be slowly overcome with active participation of pediatric urologists in pediatric-specific ERP meetings, such as the World Congress in Pediatric ERAS® which first took place in 2018, and with increasing data evaluating ERP application and outcomes in pediatric urologic reconstruction.[8, 20] Increased education and discussion of the applicability of ERPs would be instrumental in facilitating more widespread implementation of standardized ERPs in pediatric urologic practices.
Inability to reach a consensus amongst pediatric colleagues to implement standardized ERPs was another commonly perceived barrier noted by respondents. The adoption of ERPs may require changes in practice, especially in cases of bladder augmentation, which may be difficult for some surgeons to accept. A controversial element noted in this study was avoiding pre-operative bowel preparation in the case scenario of a child undergoing bladder augmentation. Over 20% of respondents reported that they were uncertain, somewhat unwilling, or very unwilling to implement this element despite the presence of previous studies reporting relatively low complication rates and no additional benefit to bowel preparation for children undergoing bladder augmentation.[21, 22] Buy-in from colleagues, especially the primary reconstructive surgeons, is critical for successful ERP implementation. Future high-quality studies demonstrating the outcomes of ERPs in pediatric urology, such as among children undergoing urinary reconstruction, are needed to help facilitate ERP adaption. More importantly, these studies are needed to refine ERPs to offer the maximal benefit for children undergoing urologic procedures.
A significant barrier noted by those who have and have not implemented standardized pathways was the lack of leadership/administrative support and a perceived barrier was difficulty maintaining pathway compliance. These findings were similar to that found in Vacek et al. in their evaluation of ERP implementation in practices performing pediatric inflammatory bowel disease surgeries.[11] Successful standardized ERP implementation requires the dedication of a multidisciplinary team with stakeholders in surgery, anesthesia, nursing, physical therapy, and others.[3, 23] Pathway adherence and maintenance requires regular monitoring and audits which can often be simplified with development of electronic medical record dashboards and order sets. Communication and collaboration amongst various fields are also critical.[24] These factors require strong leadership and administrative support which may be difficult for institutions without these resources to accommodate.
These barriers may pose significant challenges for ERP implementation at centers that infrequently perform bladder reconstruction procedures. In 2006, Lendvay et al. assessed bladder augmentation rates in the United States utilizing the pediatric health information system database and noted a mean annual per institution rate of 4 cases[25]. Schlomer et al. noted a 25% decrease in bladder augmentation rates from 2000 to 2009. Based on these studies, we may infer that bladder augmentation rates may be decreasing [26], which is also reflected by our data in which 70% of our respondents reported performing fewer than 5 bladder reconstruction cases a year. The infrequency of these cases may make ERP implementation and maintenance at low volume institutions difficult as care teams may need constant reminders on pathway elements. Allocation of institutional resources to these infrequent cases may also be limited. Collaboration with other surgical specialties with established care pathways and dedicated personnel who may assist may be considered in these cases.[3]
Despite these barriers, respondents still reported implementing a median of 15 of 24 individual ERP elements with or without standardized pathways in place. As ERP elements are evidence-driven, many individual elements, such as use of pre-operative antibiotic prophylaxis, optimizing underlying medical conditions, and early mobilization, are likely already incorporated into routine medical care.[27, 28] Increased attention to opioid use in pediatric peri-operative care and adoption of opioid-sparing pain regimens may have also promoted opioid minimizing practices in current pediatric peri-operative care.[29, 30] Advances in applications of regional anesthesia may have had a similar effect.[31] As individual ERP elements are slowly incorporated into standard peri-operative care, we suspect that ERP acceptance and creation of standardized pathways would increase in the future.
Although ERP promotes practice changes, only one (2%) respondent believed that patients would not benefit, and only 3 (5%) respondents expect resistance from patients or families. These results suggest that most respondents believe that ERP may benefit patients to some degree. Further data regarding the effects of ERPs and support in creating standardized pathways, potentially as quality improvement endeavors, may promote ERP implementation.
This study has certain limitations. The survey response rate was low at 16% which was lower than the 24% response rate reported by Short et al.[9]. In part, this was due to the fact that the survey was also inadvertently sent to senior members of the SPU who were retired and nonactive despite the authors’ initial request to survey active members only. As the survey was not designed to determine who was actively practicing, we were unable to filter out responses from non-active members, even though these responses are likely low. As such, the denominator of our response group was higher and resulted in a lower response rate. Given the low response rate, however, the results may not be interpreted as a complete representation of the SPU membership at large. Our results may be more representative of sentiments of reconstructive than non-reconstructive surgeons and may reflect more readiness for ERP adoption by these surgeons. Respondents were primarily male and practiced in academic/university affiliated hospitals. The results of this study may not be applicable to non-respondents who may have different demographic profiles, different levels of familiarity with ERPs, and different degrees of willingness to perform ERP elements. As the survey was anonymous, characteristics of non-responders could not be characterized. Results are also not reflective of practices outside of the United States. As ERPs originated in Europe, ERP implementation and acceptance are likely different than that in the United States.[3] Further studies of ERP implementation in pediatric surgical practices outside the United States would be beneficial for comparison. Respondents did not report their specific institutions, meaning respondents may be clustered at certain institutions that carry the same barriers to implementation of ERPs. Only one case scenario was presented, preventing assessment of respondents’ willingness to implement each ERP element in other case scenarios. Lastly, while reports of ERP implementation in pediatric urology have mainly been in cases of bladder augmentation with bowel anastomoses, this study reflects responses of institutions with ERPs in place for other procedures, including robotic procedures or ureteral surgeries, which should be considered when interpreting our results.
Nevertheless, the results of this survey offer some perspective on the implementation rate of standardized ERPs in pediatric urology in the United States, barriers to standardization, and respondents’ willingness to implement ERP elements. Results of this study highlight the need for further education regarding ERPs to increase familiarity within the field. Ideally, there is a need for multi-center, high-quality studies to help identify key ERP elements specific to pediatric urologic procedures and bladder reconstruction that may help those uncomfortable with changes in practice to consider ERP more strongly. Identification of barriers is also helpful for institutions interested in implementing standardized ERPs seek resources and guidance in overcoming these barriers.
Conclusion
While familiarity with ERPs is low in pediatric urology, many are willing to implement a majority of the elements. Leadership/administrative support and more widespread knowledge of ERPs amongst pediatric urologists will be necessary to facilitate further ERP implementation in pediatric urology in the United States.
Supplementary Material
Acknowledgments
Funding: Dr. Chu is supported by K23 DK125670 from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIH and NIDDK had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the official view of the NIH nor NIDDK.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Engelman RM, Rousou JA, Flack JE 3rd, Deaton DW, Humphrey CB, Ellison LH, Allmendinger PD, Owen SG, Pekow PS, Fast-track recovery of the coronary bypass patient, Ann Thorac Surg 58(6) (1994) 1742–6. [DOI] [PubMed] [Google Scholar]
- [2].Kehlet H, Multimodal approach to control postoperative pathophysiology and rehabilitation, Br J Anaesth 78(5) (1997) 606–17. [DOI] [PubMed] [Google Scholar]
- [3].Ljungqvist O, Scott M, Fearon KC, Enhanced Recovery After Surgery: A Review, JAMA Surg 152(3) (2017) 292–298. [DOI] [PubMed] [Google Scholar]
- [4].Palumbo V, Giannarini G, Crestani A, Rossanese M, Calandriello M, Ficarra V, Enhanced Recovery After Surgery Pathway in Patients Undergoing Open Radical Cystectomy Is Safe and Accelerates Bowel Function Recovery, Urology 115 (2018) 125–132. [DOI] [PubMed] [Google Scholar]
- [5].Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O, Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study, World J Surg 40(7) (2016) 1741–7. [DOI] [PubMed] [Google Scholar]
- [6].Heathcote S Sr., Duggan K, Rosbrugh J, Hill B, Shaker R, Hope WW, Fillion MM, Enhanced Recovery after Surgery (ERAS) Protocols Expanded over Multiple Service Lines Improves Patient Care and Hospital Cost, Am Surg 85(9) (2019) 1044–1050. [PubMed] [Google Scholar]
- [7].Short HL, Heiss KF, Burch K, Travers C, Edney J, Venable C, Raval MV, Implementation of an enhanced recovery protocol in pediatric colorectal surgery, J Pediatr Surg 53(4) (2018) 688–692. [DOI] [PubMed] [Google Scholar]
- [8].Rove KO, Brockel MA, Saltzman AF, Donmez MI, Brodie KE, Chalmers DJ, Caldwell BT, Vemulakonda VM, Wilcox DT, Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations, J Pediatr Urol 14(3) (2018) 252 e1–252 e9. [DOI] [PubMed] [Google Scholar]
- [9].Short HL, Taylor N, Thakore M, Piper K, Baxter K, Heiss KF, Raval MV, A survey of pediatric surgeons' practices with enhanced recovery after children's surgery, J Pediatr Surg 53(3) (2018) 418–430. [DOI] [PubMed] [Google Scholar]
- [10].Cain MP, Enhanced Recovery after Surgery Protocols in Pediatric Urology-How are we Doing and What Should we be Doing?, J Urol 200(5) (2018) 952–953. [DOI] [PubMed] [Google Scholar]
- [11].Vacek J, Davis T, Many BT, Close S, Blake S, Hu YY, Holl JL, Johnson J, Strople J, Raval MV, A baseline assessment of enhanced recovery protocol implementation at pediatric surgery practices performing inflammatory bowel disease operations, J Pediatr Surg (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE, The REDCap consortium: Building an international community of software platform partners, J Biomed Inform 95 (2019) 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Auyong DB, Allen CJ, Pahang JA, Clabeaux JJ, MacDonald KM, Hanson NA, Reduced Length of Hospitalization in Primary Total Knee Arthroplasty Patients Using an Updated Enhanced Recovery After Orthopedic Surgery (ERAS) Pathway, J Arthroplasty 30(10) (2015) 1705–9. [DOI] [PubMed] [Google Scholar]
- [14].Azhar RA, Bochner B, Catto J, Goh AC, Kelly J, Patel HD, Pruthi RS, Thalmann GN, Desai M, Enhanced Recovery after Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs, Eur Urol 70(1) (2016) 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pearson KL, Hall NJ, What is the role of enhanced recovery after surgery in children? A scoping review, Pediatr Surg Int 33(1) (2017) 43–51. [DOI] [PubMed] [Google Scholar]
- [16].Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, Kassouf W, Muller S, Baldini G, Carli F, Naesheimh T, Ytrebo L, Revhaug A, Lassen K, Knutsen T, Aarsether E, Wiklund P, Patel HR, Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations, Clin Nutr 32(6) (2013) 879–87. [DOI] [PubMed] [Google Scholar]
- [17].Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O, Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations: 2018, World J Surg 43(3) (2019) 659–695. [DOI] [PubMed] [Google Scholar]
- [18].Macones GA, Caughey AB, Wood SL, Wrench IJ, Huang J, Norman M, Pettersson K, Fawcett WJ, Shalabi MM, Metcalfe A, Gramlich L, Nelson G, Wilson RD, Guidelines for postoperative care in cesarean delivery: Enhanced Recovery After Surgery (ERAS) Society recommendations (part 3), Am J Obstet Gynecol 221(3) (2019) 247 e1–247 e9. [DOI] [PubMed] [Google Scholar]
- [19].Brindle ME, McDiarmid C, Short K, Miller K, MacRobie A, Lam JYK, Brockel M, Raval MV, Howlett A, Lee KS, Offringa M, Wong K, de Beer D, Wester T, Skarsgard ED, Wales PW, Fecteau A, Haliburton B, Goobie SM, Nelson G, Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery After Surgery (ERAS((R))) Society Recommendations, World J Surg 44(8) (2020) 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brindle ME, Heiss K, Scott MJ, Herndon CA, Ljungqvist O, Koyle MA, on behalf Pediatric ES, Embracing change: the era for pediatric ERAS is here, Pediatr Surg Int 35(6) (2019) 631–634. [DOI] [PubMed] [Google Scholar]
- [21].Gundeti MS, Godbole PP, Wilcox DT, Is bowel preparation required before cystoplasty in children?, J Urol 176(4 Pt 1) (2006) 1574–6; discussion 1576-7. [DOI] [PubMed] [Google Scholar]
- [22].Victor D, Burek C, Corbetta JP, Sentagne A, Sager C, Weller S, Paz E, Bortagaray JI, Lopez JC, Augmentation cystoplasty in children without preoperative mechanical bowel preparation, J Pediatr Urol 8(2) (2012) 201–4. [DOI] [PubMed] [Google Scholar]
- [23].Kleppe KL, Greenberg JA, Enhanced Recovery After Surgery Protocols: Rationale and Components, Surg Clin North Am 98(3) (2018) 499–509. [DOI] [PubMed] [Google Scholar]
- [24].Pearsall EA, Meghji Z, Pitzul KB, Aarts MA, McKenzie M, McLeod RS, Okrainec A, A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program, Ann Surg 261(1) (2015) 92–6. [DOI] [PubMed] [Google Scholar]
- [25].Lendvay TS, Cowan CA, Mitchell MM, Joyner BD, Grady RW, Augmentation cystoplasty rates at children's hospitals in the United States: a pediatric health information system database study, J Urol 176(4 Pt 2) (2006) 1716–20. [DOI] [PubMed] [Google Scholar]
- [26].Schlomer BJ, Saperston K, Baskin L, National trends in augmentation cystoplasty in the 2000s and factors associated with patient outcomes, J Urol 190(4) (2013) 1352–7. [DOI] [PubMed] [Google Scholar]
- [27].Choong K, Canci F, Clark H, Hopkins RO, Kudchadkar SR, Lati J, Morrow B, Neu C, Wieczorek B, Zebuhr C, Practice Recommendations for Early Mobilization in Critically Ill Children, J Pediatr Intensive Care 7(1) (2018) 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lightner DJ, Wymer K, Sanchez J, Kavoussi L, Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis, J Urol 203(2) (2020) 351–356. [DOI] [PubMed] [Google Scholar]
- [29].Cravero JP, Agarwal R, Berde C, Birmingham P, Cote CJ, Galinkin J, Isaac L, Kost-Byerly S, Krodel D, Maxwell L, Voepel-Lewis T, Sethna N, Wilder R, The Society for Pediatric Anesthesia recommendations for the use of opioids in children during the perioperative period, Paediatr Anaesth 29(6) (2019) 547–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Franz AM, Dahl JP, Huang H, Verma ST, Martin LD, Martin LD, Low DK, The development of an opioid sparing anesthesia protocol for pediatric ambulatory tonsillectomy and adenotonsillectomy surgery-A quality improvement project, Paediatr Anaesth 29(7) (2019) 682–689. [DOI] [PubMed] [Google Scholar]
- [31].Vargas A, Sawardekar A, Suresh S, Updates on pediatric regional anesthesia safety data, Curr Opin Anaesthesiol 32(5) (2019) 649–652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.