Abstract
Diabetes Mellitus is a group of diseases characterized by high blood glucose levels due to patients' inability to produce sufficient insulin. Current interventions often require implants that can detect and correct high blood glucose levels with minimal patient intervention. However, these implantable technologies have not reached their full potential in vivo due to the foreign body response and subsequent development of fibrosis. Therefore, for long-term function of implants, modulating the initial immune response is crucial in preventing the activation and progression of the immune cascade. This review discusses the different molecular mechanisms and cellular interactions involved in the activation and progression of foreign body response (FBR) and fibrosis, specifically for implants used in diabetes. We also highlight the various strategies and techniques that have been used for immunomodulation and prevention of fibrosis. We investigate how these general strategies have been applied to implants used for the treatment of diabetes, offering insights on how these devices can be further modified to circumvent FBR and fibrosis.
Keywords: diabetes, type I diabetes, type II diabetes, implants, biomaterials, encapsulation, sensors, fibrosis, foreign body response, immune system
1. INTRODUCTION
Diabetes mellitus (DM) is a group of common metabolic syndromes with a typical hyperglycemic phenotype, caused by decreased insulin sensitivity, reduced insulin secretion, increased glucose production, and decreased glucose utilization [1-9]. The two broad categories of DM are type 1 diabetes mellitus (T1 DM) and type 2 diabetes mellitus (T2 DM). T1 DM is the result of insulin deficiency due to immune-mediated or idiopathic β-cell destruction. Although the precise mechanism of β-cell directed autoimmunity is still ambiguous, it has been shown that β-cells are more susceptible to cytokines, such as tumor necrosis factor-α (TNFα), interleukin 1-β (IL-1β) and interferon-γ (INFγ) [10-14]. T2 DM, on the other hand, results from varying degrees of insulin resistance, impaired insulin secretion, and relative insulin deficiency. In the early stages of T2 DM, β-cells become hyperinsulinemic to compensate for insulin resistance and maintain standard glucose tolerance. However, as the disease progresses, islets are unable to sustain the hyperinsulinemic state, leading to development of overt diabetes, which further causes a decline in insulin secretion. Over time, an increase in hepatic glucose and lipid production can lead to the failure of β-cells [5-7, 15-17].
Although the exact causes for both T1 DM and T2 DM are unknown, numerous factors have been implicated, including metabolic disorders of late pregnancy, genetic defects of β-cells, genetic defects in insulin action, diseases of exocrine and endocrine pancreas, specific drugs/chemicals, infections, and other idiopathic syndromes [11, 17-20]. DM is diagnosed with tests of fasting plasma glucose level and oral glucose tolerance, as determined by the American Diabetes Association. The 2020 National Diabetes Statistics Report, released by the Center for Disease Control and Prevention (CDC), has identified that 34.2 million (10.5% of the US population) have DM, while 88 million (34.5% of the US population) are prediabetic [21]. According to the World health organization (WHO), in 2014, the global prevalence of DM accounted for 422 million (8% of the world population), and this number is expected to rise to 592 million by 2035 [22-24]. From the given numbers, more than 85% of the patients have been diagnosed with T2 DM, while the remaining are diagnosed with T1 DM [21-25].
Despite the high prevalence of this disease, currently there is no cure for DM [3, 6, 26-29]. The cornerstone for management of diabetes is rigorous monitoring of blood glucose levels using finger pricking and administrating exogenous insulin to help regulate blood glucose levels. Current technologies used for insulin administration include syringes, injection aids such as pens or injection ports, and insulin pumps for continuous open-loop subcutaneous infusion or intraperitoneal infusion. Despite these advances, these strategies are expensive, painful, require high patient compliance, lead to insulin dependence, and are unable to provide accurate glycemic control, resulting in more frequent hypoglycemic episodes [30-35].
Many groups have been developing alternative strategies to achieve effective blood glucose homeostasis that require low patient attention. The two broad categories for these therapies are closed-loop insulin delivery systems and pancreas or β-cell replacement therapies (Table 1) [36-45].
Table 1.
Summary of the four different types of implants used to treat T1DM and T2DM that are outlined in this review [32, 34, 36-38, 75-77,79-86]].
| Treatment | Disease Type | Purpose |
|---|---|---|
| Continuous Glucose Monitors (CGMs) | T1DM, T2DM | Allows diabetes management through continuous measurement of glucose concentration in interstitial fluid. Can be connected to insulin pumps that can release insulin once high glucose levels are detected by CGM |
| Vascular Perfusion Devices | T1DM | Intravascular bioartificial pancreas (iBAP) are semi-permeable islet encapsulation devices that are anastomosed to blood vessels, allowing blood perfusion through the device. Rely on connective mass transfer of glucose and insulin across the membrane instead of passive diffusion. |
| Islet Microencapsulation | T1DM, T2DM | Provides insulin delivery without the need for patient compliance by replacing islets/ β cells. Islets or stem-cell derived β cells are encapsulated in micron-sized semi-permeable encapsulation devices, which can be implanted in various parts of the body. Provides high surface area to volume ratio for increased access to nutrients and oxygen, thereby increasing encapsulated cell viability. |
| Islet Macroencapsulation | T1DM, T2DM | Provides insulin delivery without the need for patient compliance by replacing islets/ β cells. Islets or stem-cell derived β cells are encapsulated in macro-scale sized semi-permeable encapsulation devices. Allows transfer of larger number of islets. |
Also known as artificial pancreases, closed-loop insulin delivery systems offer great promise as these systems can detect transient hyper and hypoglycemic events and project the blood glucose dynamics. Continuous blood glucose monitoring (CGM) is one such example of a closed-loop insulin delivery system that has brought a monumental change in exogenous insulin administration. It is considered to be the ideal candidate for self-management of diabetes as it can measure interstitial glucose using a subcutaneous sensor and continuously report real-time glucose levels and trends, while also detecting and predicting the hypo- and hyper-glycemic events. The close loop insulin delivery system integrates the CGM with insulin delivery pump to administer the right amount of insulin based on CGM-predicted real-time blood glucose level. This intelligent sensor-augmented insulin pump uses CGM with a feedback loop to implement timely and optimal insulin dosing maintaining long term euglycemia [32, 34, 36-38, 40, 41, 46-53].
Pancreas or β-cell replacement is another promising therapy, especially for T1 DM, that has progressed immensely in the last decade. This strategy, first established using Edmonton’s protocol, has shown great potential in achieving insulin independence in more than 50% – 85% of patients for approximately 2 – 5 years [54-59]. Unfortunately, the limited supply of donor tissue and life-long immunosuppression severely limit the application of this therapy [60-62]. To address the issue of donor tissue shortage, efforts have been directed at developing stem-cell-derived insulin-producing cells or using a xenogeneic source of islets [60, 62-70]. Other groups have been focusing on eliminating the need for immunosuppressive drugs by developing an immuno-isolation technology that will allow for successful encapsulation and transplantation of islets or insulin-producing cells [60,61,65,71-78]. The success of these devices depends primarily on the ability of a semipermeable yet immuno-isolating membrane to allow sufficient exchange of nutrients, oxygen, and insulin, while preventing immune cell infiltration. The two broad categories of immune-isolation modalities that are under investigation are extravascular devices and intravascular devices [75,79]. Extravascular micro- or macro- capsules containing islets are transplanted in extravascular spaces such as peritoneal cavity and subcutaneous cavity [75-77,79-83]. On the other hand, intravascular devices are directly anastomosed to blood vessels [79,84-86]. Regardless of the two types of devices, this immune-isolation technology, combined with a replenishable islet source, has enormous potential in the successful treatment of DM [87-90].
Despite advances, these state-of-the-art technologies remain in their experimental stage. The limitations are not inherent to the sensors or immuno-isolation devices but rather to their performance in vivo. The technologies have demonstrated excellent performance in vitro, but in the in vivo environment, there is a drastic decline in the performance of the glucose sensors and immuno-isolation devices [72-74, 79, 81-83, 88, 91-93]. The decline in function is largely attributed to the multifaceted and dynamic foreign body response (FBR) that occurs upon activation of the host immune response [79,88,94-101]. The various stages of FBR include inflammation, degradation, biofouling, loss of host microvasculature, and complete fibrous encapsulation and isolation of the implant, which leads to implant failure [97, 102-109].
CGMs and vascular perfusion devices require glucose and oxygen diffusion through a membrane for sensor function, and the formation of fibrotic capsule around these implants creates a barrier that prevents the ingress of oxygen and glucose, and seriously lowers the CGMs sensor performance. Additionally, immuno-isolation devices require glucose, oxygen, and nutrient diffusion through a membrane for survival and function of encapsulated cells [75, 79, 103, 110-113]. Fibrotic overgrowth around these devices creates a barrier that prevents diffusion of oxygen, glucose, nutrients, which ultimately leads to loss of graft due to β-cell starvation and hypoxia. Moreover, fibrous tissue around the implant can also prevent diffusion of insulin out of the implant, rendering the implant ineffective [114-119]. Therefore, it is imperative to develop efficient strategies that can target and modulate the FBR.
In this review, we will detail the host-material immune and foreign body response that occurs post-implant, particularly in the context of implants used for the management and treatment of DM. Next, we will discuss the various biomaterial properties and cellular microenvironment that are at play and dictate the progression of foreign body response. We also discuss general strategies that have traditionally been used to mitigate fibrosis. Lastly, we highlight different modification techniques that have been applied to suppress fibrotic overgrowth and enhance the subsequent function of diabetes implants.
2. Activation of Immune Cascade and Subsequent Cellular Interactions at the Implant – Tissue Interface
The immune system is comprised of the innate immune system and the adaptive immune system. The innate immune system elicits a non-specific immune response immediately after recognition of foreign material, while the adaptive immune system, typically activated by the innate immune response, elicits an antigen-specific immune response. The cross-talk between both these systems, through soluble factors, determines the host response to implants [14,107].
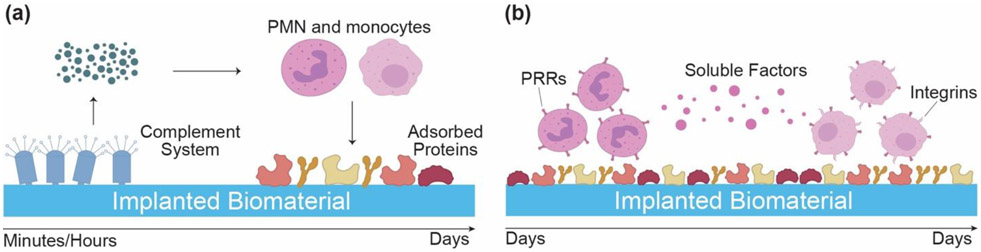
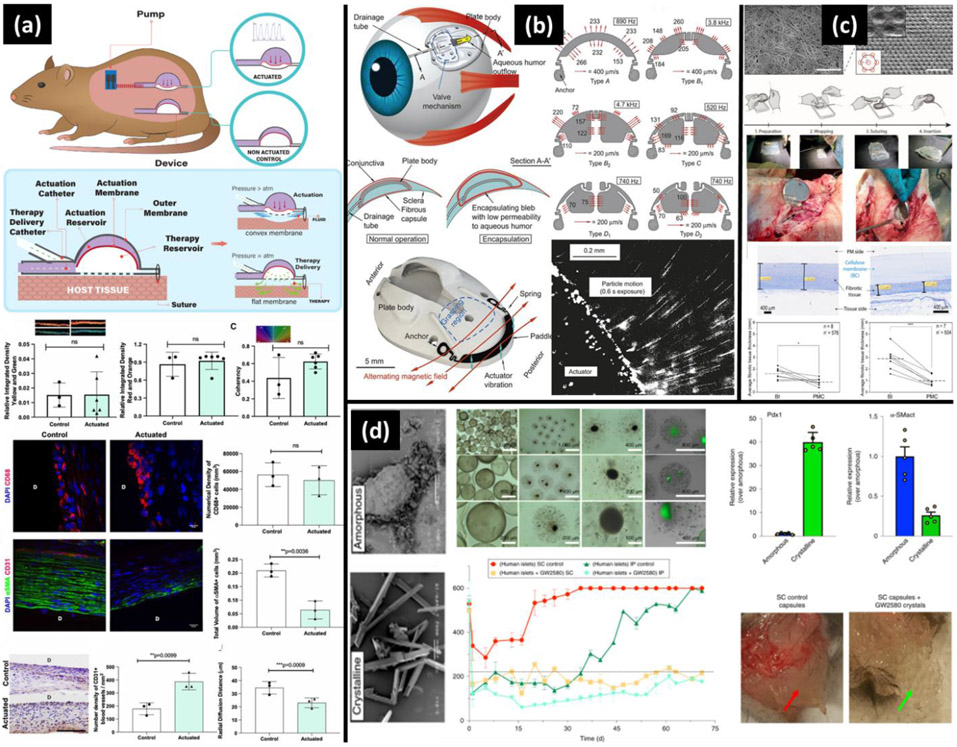
During implantation, nicked blood vessels around the implant cause accumulation of platelets and biomolecules that initiate the coagulation cascade, leading to the formation of a provisional matrix. This fibrin-dominant provisional matrix is linked with protein adsorption that occurs on the implant's surface and is considered key in subsequent leukocyte adhesion interactions [120,121]. Moreover, activation of the complement system synergistically supports matrix formation and activation of the immune system (Figure 1a). There are separate pathways in the complement system that lead to the production of anaphylatoxins, C3a and C5a. The released C3a and C5a induce the innate inflammatory response around implants by increasing vascular permeability, activating monocytes and neutrophils through the release of chemokines and chemo-attractants, and stimulating the release of reactive oxygen species (ROS) from granulocytes. Other pathways that also initiate the cellular inflammatory response include recognition and uptake of biomaterial associated pathogen-associated molecular patterns (PAMPs) or injured host tissue associated damage-associated molecular patterns (DAMPs) and alarmins. PAMPs and DAMPs are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and C-type lectins that are present on the surface of innate immune cells [102,120-127] (Figure 1b).
Figure 1.
Multiple interrelated pathways activate the immune cascade post-implantation. (a) Soluble factors released from the activation of complement system (C3a, C5a), prime polymorphonuclear leukocytes (PMN) and macrophages. (b) The primed immune cells interact with adsorbed proteins through pattern recognition receptors (PRRs) that recognize pattern associated molecular patterns (PAMPs) on biomaterial. Soluble factors released from PMNs further activate monocytes, which use both PRR and integrins to interact with the implanted biomaterial. Monocytes differentiate into macrophages and control the subsequent immune response.
2.1. Innate immune response
Nicked blood vessels lead to focal hemorrhage and edema at the implant site, causing migration and adsorption of biomolecules on to the surface of the implant and the formation of plasma protein-enriched interstitial matrix around the implant. Immune cells such as neutrophils, monocytes, and macrophages recognize proteins with damaged conformations and are activated to release a barrage of cytokines and chemokines. These cells govern the acute inflammatory response and release proteolytic enzymes that degrade the implant while clearing cellular debris. Additionally, the phagocytes (macrophages) engulf and present the antigens to the thymocytes or T cells.
2.2. Adaptive immune response
Macrophage and dendritic cells are antigen-presenting cells (APCs) that can internalize foreign antigens i.e. ions from CGMs and antigens from encapsulated islets and present them to T cells via major histocompatibility complex (MHC) molecules. The allogenic and xenogenic antigen or cell debris exacerbate the T cell response. CD4+ helper T cells get activated to display pro-inflammatory Th 1 mode and secrete pro-inflammatory cytokines and chemokines such as Interleukin-1β (IL-1β), IL-6, TNFα, iNOS. The excessive pro-inflammatory response can lead to uncontrolled damage and loss of implanted islets. Over time, inflammation resolves, and the reparative macrophages dominate the environment around the implant. These macrophages mediate the anti-inflammatory Th 2 secretory profile, including IL-4, IL-5, IL-13, and IL-10, which increases tolerance of implants and delays FBR. The reparative macrophages extenuate a pro-inflammatory state with parallel immune-regulation and positive remodeling to achieve tissue homeostasis.
Successful remodeling at the implant site happens when the innate immune response shifts from pro-inflammatory to the reparative environment and facilitates the development of site-specific functional tissue and tolerates the implant (Figure 2a) [128-131]. Due to the intricate nature of this immune cascade, slight variations could lead to the development of a foreign body response, as seen with diabetes implants (Figure 2b) [141-145]. Next, we will outline the activation of FBR and key determinants of fibrosis.
Figure 2.
The fate of the implant depends the resolution of the inflammatory immune cascade. (a) If macrophages are able to polarize from the inflammatory stage (M1) to their reparative stage (M2), they release soluble factors that promote fibroblasts to secrete collagen and promote integration of the implant. (b) If macrophages are unable to successfully transition from M1 to M2 phenotype, foreign body giant cells (FBGC) form and adhere to the implant surface. FBGC secrete more inflammatory soluble factors that activate myofibroblasts (fibrotic phenotype of fibroblasts), which secrete excessive amounts of collagen, leading to fibrous encapsulation of implant.
2.3. Dysfunctional immune system, autoimmunity crossover and implants in T1 and T2 DM
Immune system activation is a common predisposition for T1 DM and T2 DM. Independent of etiopathogenetic causes the inflammation seems to be a common mechanism among different diabetes [17-19,132-134]. The central and peripheral immune tolerance failure contribute to the autoreactive T cells. The regulatory T cell (Tregs) are shown to be defective in phenotypic autoimmune T1 DM while several islet auto-antigens and peptide epitopes are targeted by effector T cell (Teff) [10,19]. During DM progression the immune cells such as B cell, macrophages, dendritic cells, and natural killer cells mediates the inflammation. The disruption in regulation and control of local inflammatory cytokines production are also a critical factor in progression of DM [135,136]. T2 DM is mostly considered as metabolic disorder characterized by dyslipidemia, hyperinsulinemia and obesity, but with the credible hypothesis pathogenesis and progression of T2 DM is credibly linked with inflammation [137-139]. Inflammation contributes to the promotion of metabolic abnormalities such as dyslipidemia, hyperinsulinemia and obesity, which in turns regulate immune cell functions to establish systemic low-grade inflammation (LGI) [16,137]. The metabolism and immune system are bidirectionally linked. The presence of chronic LGI, infiltration of immune cells, oxidative stress intensifies metabolic impairments in insulin sensitive tissue and promotes insulin resistance. The stressed islets further stimulate the local immune inflammation and result in abnormal innate and adaptive immunity characterized by alteration in proliferations and impairment of functions of T cells, macrophages, B cells, NK cells, the release of inflammatory mediators promote systemic insulin resistance, β-cell damage and underline the considerable role of autoimmunity in T2 DM pathogenesis [138,140,141]. Thus, T1 and T2 DM has been recognized with the coexistence of insulin resistance, and auto and allo-reactivity against islet antigens lead to a vicious cycle in which initial cytokine stress surge the metabolic stress resulting in additional loss in β-cell function [18,142].
The shared etiology and pathophysiology by T1 and T2 DM present similar immunological makeup to the implants for the monitoring and treatment in DM. The active or memory autoimmune response to islet is presented to the islet implants. The fast-tracked inflammatory islet infiltrations and selective toxicity to the β-cells in transplanted implants lowers the success of allo-islet transplants in autoimmune patients as compared with non-auto immune patients. Despite the use of immunosuppressive drugs, many transplant recipients have shown marked increased in antibodies to glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2) representing indirect, or direct re-exposure to autoantibodies [143]. The relative difference in the amount of islet autoantibodies pretransplant, autoantibody titer, cytotoxic T cell response posttransplant is strongly associated with graft rejection. The relative contribution of islet autoimmunity to graft survival, however, remains unclear. Presence of HLA class I class II specific antibodies in addition to GAD, IA-2 autoantibodies also indicated the autoreactive may be independent of allo-rejection [143,144]. Growing evidence shows that regardless of the use of immunosuppression to enhance islet graft survival, the chronic islet autoimmunity may eventually lead to graft rejection and recurrent diabetes. The autoimmunity may accelerate the inflammatory response toward implants but follows similar mechanistic processes towards regeneration or fibrosis depending on the cue presented at the implant-tissue interface [143,145-148].
In parallel with the progression of DM, decline in cellular response is observed, resulting in the decline in general immunity against opportunistic infections. Low complement factors, diminished cytokine response on stimulus constituting progressively dysfunctional humoral immunity. Decreased functional efficiency of polymorphonuclear cells and macrophages are also observed in long term DM patients [132,149,150]. Though this aspect is out of the scope for this review, it is a crucial aspect of immune dysfunction leading to increased prevalence of infectious and non-infectious diseases in patients with T1 and T2 DM.
3. Development of Foreign Body Response and Fibrosis – A Multifront War
Fibrosis is defined as the formation of fibrotic capsule around the implant and occurs due to the activation of the immune cascade. If the innate immune response is not resolved (marked by the unsuccessful elimination of the foreign material and transition into the reparative environment), macrophages fuse together to form foreign body giant cells (FBGCs). FBGCs, considered as the hallmark of chronic inflammation and FBR, are multi-nucleated cells that adhere onto the surface of the implant and have increased inflammatory and phagocytic capacity, further amplifying the immune response. The inflammatory signals produced further promote proliferation of vascular endothelial cells and fibroblasts, which secrete proteoglycans and collagen for the organization of extracellular matrix. Due to excess inflammatory signals, there is superfluous secretion/production of collagen III, resulting in the formation of granulated tissue and fibrous tissue around the implant. This process eventually leads to implant isolation from host tissue, rendering it ineffective. Additionally, fibrous encapsulation formation also depends on the regenerative capacity of the tissue surrounding the implant. When composed primarily of the dormant cells, the tissue usually experiences greater inflammatory cascade and leads to fibrosis.
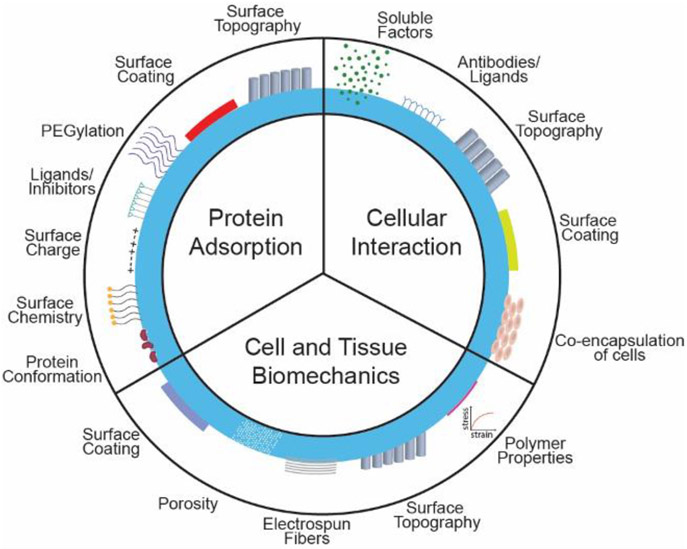
The root of the FBR lies in the first step – the nature of protein interaction with the implant’s surface. The protein-surface interaction is a complex phenomenon influenced by the protein quantity, composition, conformational changes, diffusion coefficient, size, and surface affinity. Protein characteristics are dictated by the physicochemical properties of the implant such as surface chemistry, energy, charge, geometry, porosity, topography [120,151-154]. Additionally, implant-tissue biomechanics is another crucial factor that heavily contributes to the FBR. The implants impose chronic mechanical loading and disrupt the tissue, which induces tissue remodeling and elicits FBR. Lastly, the immune cascade is an orchestra of cells, with each cell type interacting through soluble factors or direct activation. It is widely accepted that sequential transition between cell activation states and cell types is crucial in resolving the immune response. The next section will describe these three components and factors of these components that can alter the implant – tissue response.
4. Areas for Control at the Implant – Tissue Interface
4.1. Protein Adsorption and Interaction
Proteins adsorption on the implant’s surface is based on concentration gradient and surface affinity of the proteins as many proteins are competing for surface binding sites. Protein adsorption is principally driven by the accumulation of considerable noncovalent bonds, protein conformations, and the redistribution of charged groups at the interface [154,155]. However, the hydrophobic interactions, the composition of biomaterial, charge, and topography at the tissue-implant interface are also of vital importance.
4.1.1. Hydrophobic interactions
Protein adsorption is a thermodynamically driven interaction between proteins and implant surfaces. The strong interactions between a hydrophobic implant surface and neighboring polar water molecules lower the overall entropy. Unfolding of proteins compensates for the energetically unfavorable loss in entropy at the hydrophobic surface. The hydrophobic moieties on proteins form weak noncovalent interactions with the surface to exclude water molecules and favorably increase the entropy of water while driving protein adsorption. These weak noncovalent interactions collectively contribute to proteins’ total adsorption on the implants with hydrophobic and weak hydrophilic surfaces. The displacement of water on hydrophilic surfaces present a large energy barrier, making it unfavorable for protein adsorption [154-158]. For instance, fibrinogen loses its compact secondary structure and expose sequestered moieties for enhance cellular binding on the residential biomaterial surface based on its hydrophobicity.
4.1.2. Charge-charge interactions and protein conformational change
The favorable charge interactions and conformational changes in protein structure help overcome the energy barrier and displace the water molecules that drive protein adsorption on the hydrophilic and hydrophobic surfaces. The pH around implant alters the electrostatically driven charge-charge interaction between implant surface and proteins. In aqueous environment pH alters the charges on the material surface and proteins. Specially at the isoelectric point of protein small ionic interaction and formation of hydration bond can favor adsorption. Conformational changes of proteins increase the overall entropy and enhances adsorption kinetics. Proteins with favored structure overcome comprehensive charge barriers to form noncovalent bonds with the implant surface irrespective of hydrophobicity. The heterogenicity in structural conformation of proteins across implant interface varies with the quantity of protein present and the surface chemistry of implant. Depending on concentration and extent of conformational changes, these proteins may expose the integral binding motifs that are usually unavailable in their native state [151, 152, 154, 155, 158]. For example, fibrinogen at very low concentration preferential adopt the β-sheet conformation while unraveling integral platelet binding motif which favors high concentration of platelet adsorption on the hydrophobic surface. Fibrinogen forms spectrum of conformation while adsorbing at different rate based on concentration and surface chemistry. Bioactivity of exhibited motifs preferentially enhances pro-inflammatory cell phenotype, contributing to FBR.
4.1.3. Surface energy and charge
Hydrophobicity or hydrophilicity of the implant’s biomaterial defines the surface energy, which is critical in adsorption of proteins. Conformational change in proteins allows greater protein adsorption on hydrophilic implant surfaces, whereas, proteins adsorbed on hydrophobic surfaces that did not undergo structural modification preserve their native biological activity. The protein’s flexibility, reversibility, and the extent of conformational modification play a vital role in a subsequent inflammatory cascade involving immune cells and surface interactions. The overall high binding strength of noncovalent interactions at hydrophobic implant surface interface impairs the anti-inflammatory cellular interaction and reorganization. The different ratios of fibronectin and vitronectins are observed with increase in positive charge surface [151, 152, 155, 159-162].
Additionally, the polarity of implant surface also plays a role in protein adsorption and subsequent cellular interactions. Surface charge modulates the distribution and composition of adsorbed proteins, and differential and preferential protein binding to the polar region on charged implant surface interface influences downstream inflammatory cellular responses leading to FBR.
4.1.4. Surface topographies
Topographic features on ECM modulate cell behavior and imprinting these ECM patterns on surface of implants may mimic the ECM topography induced changes in cell behavior. Surface topography modulates protein adsorption, which sequentially alters macrophage adhesion, proliferation, cytokine secretion, and FBR. Changing the scale, shape, and spatial arrangement of topographical features also alters protein adsorption and subsequent cellular response. Nano-scaled topographies offer relatively higher surface area than micron-scaled topographies, thus allowing more protein adsorption. Addition of topography also alters surface energy and charge density of the material, further influencing the variable protein adsorption profile, conformational change, and cellular response.
Topographical structures induce complex physical stresses at the cellular level, generating differential cytoskeletal tensions, which activate mechanotransduction and gene expression cascades. Fibroblast and macrophages, which play a vital role in FBR, are sensitive to topographical features, and the downstream cellular response of these cells is the result of topographically induced cell behavior, which include contact guidance, cell selection, cell differentiation, and cell-mediated matrix organization. Discontinuous features and topographical roughness lead to preferential cell selection, accumulation, and interaction around the implant, while in some cases, selective cell proliferation and differentiation are also achieved [102, 103, 107, 127, 163-173]. The degree of spatial arrangement of topographies also regulates cell behavior. Ordered topographic features reduce cell adhesion compared to random arrangement of the topographic features or planar surface [103,107,168,175-180]. However, the multiple responses to topographical features makes it challenging to isolate the effectors.
4.1.5. Surface chemistry
Modulating surface properties of biomaterials to make them nonimmunogenic or hypoimmunogenic can limit macrophage adhesion, activation, and formation of FBGCs. The terminal chemistry on implant surface commands the conformation of adsorbed protein, which provides a binding site for protein-specific receptors on leukocytes and phagocytes. For example, ionic chemistry on the surface affects protein composition and conformation as counterions in the local microenvironment can stabilize the protein structure, altering protein adsorption dynamics [106, 108, 142, 154, 164, 168, 178-180].
4.1.6. Surface coating
The implant surface can be coated to generate a barrier that masks the nonspecific protein adsorption and subsequent leukocyte adhesion. A pre-adsorbed coating of known noninflammatory or less inflammatory protein can alter the receptor-ligand binding, leading to minimal fibrosis. Coating the implant surface with lower immunogenic biomaterial also masks the immune reaction leading to FBR with a similar mechanism [107, 118, 135, 155, 163].
4.2. Cellular and Tissue Level Biomechanics
Biomechanics and cell interaction with the material plays an important role in the development of fibrotic overgrowth [104, 108, 181, 182]. Cells interact with ECM and implants through proteins known as integrins that physically couple the ECM or implant surface to the cell cytoskeleton. Integrins act as mechanotransducers that transmit signals across the membrane through cytoplasmic-domain-associated focal adhesion molecules. Force-dependent focal adhesion complexes grow larger and mature as integrin clustering increases, leading to force-dependent cytoskeletal changes that ultimately lead to activation of transcription factors [187-191]. Mechanical cues provided by the ECM or implant surface, along with chemical and topographical cues, dictate cellular processes such as cell adhesion, migration, proliferation, gene expression, and apoptosis. Other mediators that function similarly to integrins and alter cellular activity are G proteins, receptor tyrosine kinase (RTK), mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinases (JNK), extracellular signal-regulated kinases (ERK), and calcium ions [121, 188-191].
Mechanical changes in the cell cytoskeleton ultimately change the biochemical molecules secreted by cells. The cytoskeleton is made up of microfilaments (α-actinin, filamin A, talin, vinculin), microtubules, and intermediate filaments, and all these components work together to provide mechanical properties that maintain cell shape and tensegrity in the presence of external stress. The external mechanical forces can lead to gene regulation and protein synthesis through pathways such as MAPK phosphorylation by activating the transcription regulatory proteins in the cytoplasm and nucleus. Moreover, mechanosensitive ion channels can also control cellular processes through intracellular calcium ion levels that are altered by mechanical force on the cells.
In terms of tissue-level biomechanics, the external mechanical forces generate motion and pressure at the tissue implant interface. The magnitude, duration, and transmission of each force varies based on the implants’ apparent relative motion and source of each force. These forces are widely classified as normal forces, transverse force, torsional force, hydrostatic pressure, stiffness, elasticity, and viscoelasticity [192,195-199]. Normal forces, i.e., tensile force, arise from pushing or pulling the implant, while compressive force is the force the implant and tissue apply to each other. Shear stress occurs due to transverse and torsional loading and determines implant sliding. Acute shear stress arises from pulling and brushing against the implant, while chronic shear stress results from repeated abuse due to walking, running, or any cyclical activity. Fluid surrounding the implant exerts nondeforming, random hydrostatic pressure, which thermodynamically affects stability of the implant. Once external force has been applied, this resistance to deformation depends on the inherent stiffness, elasticity, and viscoelastic properties of implant biomaterial or ECM [200].
Thus, biomechanics play a crucial role at the macroscale level (where implant and tissue interact), microscale level (cells are affected), and nanoscale level (protein adsorption is affected). Mechanical properties of biomaterial affects all cell types, especially immune cells and fibroblasts, and cyclic mechanical loading on these cells promotes secretion of the autocrine and paracrine soluble factors that regulate ECM protein production along with inflammatory cytokines such as IL-1β, IL-6, TNFα, and oxidative stress markers such as cyclooxygenase-2, nitric oxide, prostaglandins E2 [102, 127, 138, 183, 197-201]. The cellular response to the biomechanical forces has a self-perpetuating and deleterious response leading to FBR and fibrous capsule formation.
4.3. Cellular Interactions
Immune cells and their interaction with the environment and with each other play a key role in determining the resolution of the immune response.
4.3.1. Innate immune response
Polymorphonuclear leukocytes (PMNs) such as neutrophils, eosinophils, and basophils provide the first line of host defense as they migrate quickly to the implantation site. TGF-β, platelet-derived growth factor (PDGF), and histamine are chemoattractants that guide PMNs to activate enzymatic degradation of implanted material by stimulating release of proteolytic enzymes and ROS. These cells also secrete cytokines including TNFα, IL-1β, IFNγ along with other signals such as monocyte chemotactic protein-1, chemokines, and macrophage inflammatory protein-1β. Together these cytokines and proteins help PMNs remove cellular debris around the implant while also further amplifying the immune response by activating monocytes, tissue-resident macrophages, immature dendritic cells, and lymphocytes. Neutrophils, in particular, release neutrophil extracellular traps (NETs) to trap pathogens in place, and sustained release of these traps have been linked with fibrosis and excessive production of the dense fibrotic matrix.
Chemoattractants such as CCL2, CCL3, and CCL4 have been implicated in recruiting monocytes to the implantation site. The β2 integrin receptors of recruited monocytes bind to IgG, fibronectin, fibrinogen, and complement fragment iC3b on the implant surface, causing monocyte to differentiate into inflammatory macrophages (typically known as classically activated M1 macrophages) [122-127]. These macrophages secrete pro-inflammatory cytokines such as IL-1β, IL-6, TNFα that recruit other immune cells, along with chemokines, ROS, and proteolytic enzymes that help phagocytose apoptotic PMNs, clear debris, and attempt to degrade the implanted biomaterial. The accumulation of cytokines and chemokines also activates tissue-resident macrophages near the implant. Within 48 to 96 hours of implantation, macrophages are considered the predominant cell type that orchestrates and determines the subsequent immune response based on the chemical and physical properties of the biomaterial. Since most implants, especially for the treatment of diabetes, are larger than macrophages, adherent macrophages will be unable to phagocytose the material, at which point they enter the “frustrated phagocytosis” zone. However, specific cues or properties of the biomaterial can promote macrophages to shift to other activation states (typically known as alternatively activated M2 macrophages), in which they produce anti-inflammatory cytokines that promote tissue remodeling and angiogenesis. Together, the soluble factors released by M2 macrophages lead to recruitment of fibroblasts and endothelial cells, promoting angiogenesis and the integration of the implant [103, 128-132].
4.3.2. Adaptive immune response
The adaptive immune system can be activated through antigen presentation by macrophages and dendritic cells (DCs) (Figure 3). These antigen-presenting cells (APCs) activate T cells by presenting MHC and costimulatory molecules that activate naïve T cells. Some example of DAMPs in diabetes implants include ions from CGMs and antigens from encapsulated islets. The DAMPs are recognized by PRRs, and depending on which PRRs, DCs can mature and upregulate specific T cell activation. Local cytokines and growth factors such as IL-10, TGF-β, hepatocyte growth factor, and granulocyte colony stimulating factor produced by other immune cells around the implant have been shown to inhibit DC maturation, demonstrating the importance of biomaterial properties in directing maturation of DCs.
Figure 3.
Macrophages and dendritic cells work together to activate the adaptive immune system. Dendritic cells and sometimes, macrophages present antigens to T cells that stimulate activation of different T cell subtypes. These subtypes are influenced by the soluble factors present in the local microenvironment. If pro-inflammatory macrophages are present, the secreted soluble factors activate the inflammatory Th1 CD4 cells. Meanwhile, if reparative macrophages are present, they secrete factors that activate the reparative Th2 CD4 cells along with regulatory CD4 (Treg) cells.
Maturation of DC has been associated with activation of various T cells, including CD 4 helper Th1, Th2, Th17, and regulatory T cells (Tregs). Typically, especially in cases of chronic inflammation, Th1 and Th2 cells are primarily responsible in modulating the local inflammatory response around the implant as these cells produce large quantities of cytokines that activate local macrophages to their different phenotypes. The role of these T lymphocytes has also been linked with innate lymphoid cells (ILCs), which lack T and B cell receptors. ILC2, especially, have been associated with inhibiting Th1 and promoting Th2 polarization of CD4 helper T cells, indicating the potential role of ILCs in regulating implant induced fibrosis as well. Additionally, activation of Tregs could influence wound healing as these cells regulate the activation of CD8 cytotoxic T cells and produced IL-10, a cytokine that can activate the anti-inflammatory responses of macrophages and CD4 helper T cells. Tregs also produce growth factors that promote differentiation of local stem cells along with fibroblasts. Recently, a different T lymphocyte subset, Th17 cells, has been linked with fibrosis due to their ability to produce IL-17, a cytokine that promotes the pro-fibrotic phenotype of macrophages and fibroblasts. . Depletion of γδ T cells can also prevent wound healing, suggesting their possible involvement in implant induced fibrosis. Despite this information, the role and activation of T cells by implants has not been fully elucidated, and research on the crosstalk between T lymphocytes and macrophages could serve as a powerful and unique tool in modulating local inflammation and subsequent fibrosis of implants [102, 125, 130, 133, 135-140].
4.3.2. Other Cell Types
Apart from immune cells, other cell types, including fibroblasts and mesenchymal stem cells (MSCs), play a critical role in biomaterial-mediated fibrosis. Fibroblasts are highly dynamic, extracellular matrix depositing mesenchymal cells that are recruited by macrophages during inflammation. Once recruited, local cytokines such as PDGF, VEGF, and TGF-β, activate the fibrotic phenotype of fibroblasts. These cells are typically known as myofibroblasts, which deposit type I and III collagen around the implant until there are no local inflammatory cytokines present or until the ECM provides physical cues that promote release of the weak focal adhesions formed between myofibroblasts and the ECM. If the myofibroblasts cannot detect these local changes and continue secreting excessive collagen, fibrotic tissue can encapsulate the implant, cause a fibrotic scar formation, and prevent implant function.
MSCs are also present at the implant site and have regenerative and immunomodulatory properties that can activate the innate and adaptive system and determine the fate of the implant. MSCs activate macrophage polarization to its anti-inflammatory by secreting factors such as prostaglandin E2, which increases the production of IL-10 while reducing the secretion of TNFα and IL-12. Since anti-inflammatory macrophages are associated with Tregs, it is assumed that MSCs also lead to the induction of Tregs. Moreover, secretion of these factors leads to a decrease in dendritic cell maturation and a decrease in T lymphocyte and natural killer cell proliferation. Due to these multi-functional properties, MSCs are typically used for wound-healing purposes; however, their exact role and mechanism in preventing biomaterial induced fibrosis has not been fully explored [103,183,210,214-217].
Knowing the three critical determinants of fibrosis, we can apply various strategies to biomaterials to modulate either protein adsorption, biomechanics of material and tissue, and manipulate surrounding immune cells to control the local microenvironment post-implant. In the next section, we have outlined the general strategies that have been used to regulate FBR and fibrosis.
5. Biomaterial Strategies to Regulate the FBR and fibrosis
FBR and fibrous encapsulation are common problems associated with implantable CGMs or islet encapsulation devices. At the implant – tissue interface, the adverse host immune response leading to FBR/fibrosis can be minimized with two practical approaches: immune evasive strategies and immune-interactive strategies [186, 202, 203]. Immune evasive strategies involve use of intrinsically inert biomaterials that are recognized as foreign material but do not directly activate a specific immune response. Immune-interactive strategies, on the other hand, engage and elicit the controlled cellular responses, favorably modulating it to minimize the FBR [186, 202, 204].
5.1. Immune evasive strategies
Immune evasive biomaterials used for implants are inert and elicit minimal host response. An extensive array of biomaterials has been investigated, including natural biomaterials and synthetic biomaterials.
5.1.1. Natural biomaterials
A wide range of natural biomaterials with boundless functionalities is available. These materials are usually derived from materials present in the living system through the process of physical, chemical, or enzymatic decellularization [104, 107, 147, 205, 206]. These biomaterials are usually biocompatible and may display specific protein binding sites and biochemical signals, driving downstream cellular response towards regeneration and away from FBR and fibrosis. The downsides of natural biomaterials are premature biodegradation and unpredictable mechanical failure. Moreover, xenogeneic natural biomaterials have very high immunogenicity. Examples include gelatin, which is one of the commonly used natural biomaterials in pancreatic islet encapsulation. It has a triple helical structure with a repetitive sequence of glycine–proline/hydroxyproline–proline/hydroxyproline. Due to the structure, gelatin can immobilize water and make the implant surface hydrophilic, leading to low binding of FBR specific proteins [127, 168, 180, 205, 207-209]. Another natural polymer, chitosan, is obtained by the alkaline hydrolysis of chitin derived from the fungal cell wall, insects, and shrimp’s exoskeleton. It is a polysaccharide with repeated D-glucosamine and N-acetyl-D-glucosamine units and carries a positive charge due to the cationic amine group. It exhibits, both, pro- and anti-inflammatory responses, depending on the degree of deacetylation of chitin, molecular weight, ionic charge, and solubility. Lower molecular weight chitosan shows upregulation of pro-inflammatory cytokines such as TNFα, IL-6, IFNγ, while higher molecular weight shows downregulation of these cytokines. Moreover, downregulation of pro-inflammatory cytokines is also observed at increased polymer solubility and in its zwitterionic state [110, 137, 208, 210-212]. Hyaluronic acid is also a non-immunogenic biopolymer derived from the ECM of connective and epithelial tissues. It is a negatively charged polysaccharide with repeated units of D-glucuronic acids and N-acetyl glucosamine. At lower molecular weight, it is pro-inflammatory as it induces upregulation of TNFα and IL-1β, but at higher molecular weight, it becomes less immunogenic as it increases secretion of IL-10, an anti-inflammatory cytokine [103, 205, 208, 211, 212]. Heparin, used widely for intravascular implants in DM, is a linear glycosaminoglycan with a negative charge due to the high content of sulfonic and carboxyl groups in its D-glucuronic and D-glucosamine repeating units. Heparin and its derivative inhibit inflammatory cytokines such as TNFα, IL-6, IL-8, IL-1β at lower molecular weight in a dose-dependent manner [80, 81, 137, 163, 212]. Agarose is another natural polysaccharide composed of linear chains of D-galactose and 3,6-anhydro-L-galactopyranose. It is a thermosetting polymer with very low immunogenicity as it can locally inhibit the complement system, thus reducing the immune response leading to FBR [80, 213-215]. Alginate is a widely used biopolymer, especially for islet encapsulation for the treatment of T1 DM. It is a negatively charged biopolymer with the repeated units of mannuronic and guluronic acids and has excellent gelation property in the presence of divalent cations. Its immunogenic property depends on the ratio of mannuronic and guluronic acid. Unsaturated oligomers upregulate TNFα and induce a greater pro-inflammatory response as compared to saturated oligomers. [80, 205, 216-218]. Another polymer that has extensively improved islet transplantation outcomes is collagen. It has numerous subtypes that are present in connective tissues, with the most prominent subtypes being collagen type I, II, and III. It has a triple helical structure with a repeated unit of glycine, proline, and hydroxyproline sequence, and the immunological profile of collagen depends on this helical structure as well as the fibril’s amino acid sequence [135, 171, 182, 219-222]. Lastly, silk is a natural polymer with a core structural protein, fibroin, that is surrounded by sericin, which is responsible for the immunogenicity of silk [80, 107, 214, 223, 224]. Isolated fibroin has very low immunogenicity, controllable biodegradability, and excellent mechanical properties, making it an excellent candidate for improving islet encapsulation efficiency and long-term graft function with limited FBR.
5.1.2. Synthetic biomaterials
Synthetic biomaterials are easy to synthesize, inexpensive to produce, and have predictable and tunable functional properties. Though they have excellent physicochemical, mechanical, and degradation properties, they are more prone to induce a pro-inflammatory response, causing difficulty in integrating with host tissues [204, 206, 225]. An example of synthetic biomaterial is polycaprolactone (PCL), which is used widely with implants as it is an inert, biodegradable, linear aliphatic polyester. It is a hydrophobic, biocompatible polymer with a prolonged degradation rate that can be easily modified by changing the molecular weight [80, 106, 107, 197, 202, 226]. Polyethylene glycol (PEG) is another inert, non-immunogenic, flexible, biocompatible hydrophilic polymer of ethylene oxide. It is resistant to protein adsorption and is known to minimize the protein corona formation. It has a linear and branched structure that can be easily modified to covalently attach a variety of functional groups [81, 126, 149, 212, 215, 227, 228]. Polyvinyl alcohol (PVA) is yet another synthetic polymer that has been successfully used as a biomaterial for implants. It is derived from hydroxylation of polyvinyl acetate and has varying chemical properties based on the percentage of hydrolysis. It is highly non-immunogenic due to its hydrophilicity, low protein adsorption, and high-water solubility [110, 137, 171, 229, 230]. Polyurethane (PU) is composed of aliphatic or aromatic units derived from polyether or polyester monomers. Its immunogenicity mostly depends on the ratio of polyoxyethylene (PEO) to polytetramethylene oxide (PTMO). Water absorption and hydrophilicity of PU depends on the quantity of PEO present as PEO has low interfacial free energy with water and high surface mobility [126, 163, 180, 206, 212, 231, 232]. Polytetrafluoroethylene (PTFE), commonly known as Teflon, is a highly crystalline fluoropolymer of tetrafluoroethylene. It has a hydrophobic, electronegative, and low-friction surface that is suitable for most blood-contacting implants but does induce a mild inflammatory response [81, 144, 206, 212, 229]. Polyglycolic acid (PGA) is a polyester with a high degradation rate and a linear aliphatic structure that is synthesized using ring-opening polymerization of glycolic acid. There is no standard agreement on the immunological profile of PGA as it prevents initiation of lymphocyte DNA synthesis but also promotes the pro-inflammatory response by activating MHC-II and IL-2 receptor [135, 206, 212, 233]. Polylactic acid (PLA) is a linear, aliphatic polymer of lactic acid with slow degradation rate and excellent mechanical properties. It is used in blood-contacting implants and causes no thrombosis and minimum stenosis. However, acidic degradation products of the polymer are reported to provoke a pro-inflammatory response [80, 234-236]. Lastly, polylactic-co-glycolic acid (PLGA) is a blend of PLA to PGA, with varying ratios resulting in different immune responses in terms of immune cell infiltration and FBR [211, 230, 237-239].
5.2. Immune engaging strategies
Biomaterials can also provide structural, biochemical, and biomechanical cues that will activate the immune system at the implant-tissue interface. Some properties of biomaterials make them inherently immune engaging [186, 202, 206, 240, 241]; one such example is decellularized extracellular matrices [143, 212, 221, 242-244]. These large, structural, protein-based matrices derived from native tissue are lipid and cell-free, making them highly immune-privileged biomaterial that can modulate and downregulate a myriad of immune responses. Another example of an inherently immune engaging material is fibrin [199, 208, 234], a filament-forming soft network formed by an enzymatic reaction between fibrinogen and thrombin. Presence of fibrin ligands in several integrin receptors downregulates pro-inflammatory cellular response leading to FBR.
Other immune engaging strategies include local delivery of pro-inflammatory and anti-inflammatory molecules. Examples of these molecules include antibodies, cytokines, chemokines, prostaglandins, leukotrienes, proteolytic enzymes, free oxygen radicals, and nitric oxide [82, 107, 133, 137, 141, 147, 202, 211, 245]. Pro-inflammatory molecules such as heat shock protein 70 (HSP-70), lipopeptide-2, cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG) target the immune system through TLR pathways to help initiate acute inflammation that eventually leads to the reparative response [14, 126-128, 137, 246]. A significant acute and chronic anti-inflammatory response with inhibition of FBGC and fibrosis is obtained using glucocorticoids, superoxide dismutase, and nonsteroidal anti-inflammatory molecules. Other anti-inflammatory factors such as IL-4, IL-10, anti-TNFα also play a significant role in promoting tissue repair and regeneration [126, 133, 137, 139, 211, 247-249]. Local delivery of these pro-resolvin mediators end the acute inflammatory response by inducing macrophage polarization to its reparative M2 phenotype, which begins the process of tissue granulation and regeneration [126, 138, 250]. Promoting integrin clustering, activating immune cells, and providing growth factors can also induce tissue regeneration while suppressing FBR. Immune cells, such as macrophages and MSCs can be used as a biological source to produce immune-modulatory molecules [210,214,268]. Epidermal growth factor (EGF) [103,106,269], vascular endothelial growth factor (VEGF) [108,210,270], fibroblast growth factor (FGF) [81,210,271,272], granulocyte-macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor (PDGF), and TGF-β together form a complex signaling network, which helps guide cross-talk between the immune cells, tissue cells, and leukocytes to successfully modulate the reparative immune response [103,126,210,227,273-275]. Additionally, hydrophilic biomaterials [156,157,186] induce a lower local immune response as compared to hydrophobic polymers [172,201,276] as hydrophilic polymers have significantly lower monocyte adhesion and formation of FBGCs. Surface topographies on commonly used biomaterials for implants, such as polycaprolactone (PCL) [106, 177, 262], polylactic acid (PLA) [142, 182, 207], polydimethyl-siloxane (PDMS) [80, 107, 207], promote macrophage polarization and also reduce FBR. Lastly, surface coating reduces non-specific adhesion of proteins on the implant-tissue interface and prevents biofouling. Polymer coatings of PEG, PAA, polyethyleneglycol-block-poly l-lysine hydrochloride (PEG-b-PLL) [81,231,278], polyethylene glycol diacrylate (PEGDA) [279,280], poly N-isopropyl acrylamide, and poly 2-hydroxyethyl methacrylate (PHEMA) [80,107,210] have demonstrated minimal protein adsorption.
6. Engineering Immune Engaging Biomaterials
Immune engaging biomaterials have shown great potential in modulating FBR and fibrosis, as these materials can induce specific immune cell response that promotes implant integration and function. Essential strategies for designing such biomaterials include altering surface chemistry through biofunctionalization, changing surface topography, and emphasizing the role of biomechanics in implant design.
6.1. Surface chemistry
The bio-functionality of the material depends on how surface chemistry influences protein adsorption [155,178,281]. Immune-modulating surface chemistries are engineered by modifying the original implant surface through non-covalent deposition and adsorption of biomolecules and through covalent cross-linking of functional groups such as thiols [183], silanes [171,231], or biomolecules [110,206,210] on the material surface. Ion-beam implantation [282,283], chemical conjugation, silanization [171,231,281], self-assembly of monomers [80,81,110,284,285], and plasma-assisted techniques[169,286,287] are a few of the most critical processes used to modify surface chemistry. Ion-beam implantation injects accelerated ions (cations and anions) into a material to alter its surface charge, energy, and chemistry, directly affecting implant-protein interaction. Surface coating can also be added to materials containing functional groups, such as -OH, -COOH, -NH2, via electron beams as high energy ionizing radiations, upon exposure to the reactive groups, can react to form a functional coating on the biomaterial surface. Silanization is another technique in which silane molecule reacts with a hydroxylated substrate, which, upon polymerization, produces a covalently linked surface coating. This technique is commonly used on implant surfaces to alter chemical properties such as surface energy. Self-assembly monolayers (SAMs) [170, 252] are highly ordered surfactants that spontaneously assemble by covalently anchoring on the biomaterial surface. Alkanethiols [288] is a well-known SAM facilitating in hydrophilic, hydrophobic, non-fouling short chain, and polysaccharide terminal modification. Some of the molecules with no functional alkyl groups such as proteins, porphyrins, nucleotide bases, and hydrocarbons with aromatic rings can also form SAMs. Plasma assisted techniques, such as radiofrequency glow discharge plasma-induced surface ablation, etching, and coating using low pressure suitable ionized gas, are used to modulate the cell-material interactions by tuning the density of the functional group deposition on biomaterials [106,134, 167, 168, 170, 199, 233, 272, 274].
6.2. Biofunctionalization, coating, and patterning
Bioactive molecules can be covalently coupled to functional groups on the surface of a biomaterial. Favorable modulation of downstream immune response is proportional to the density of immobilized ligands, spatial distribution, colocalization with agonistic or synergistic ligands, and steric hindrance. The practical approach is to mimic properties of the ECM onto biomaterial surface to accelerate tissue regeneration. Material surface can be functionalized with peptides, proteins, growth factors, and endothelial cells to alter protein adhesion, improve blood compatibility, inhibit foreign body response, and increase the patency of implants. Small oligopeptide sequences such as arginine-glycine-aspartic acid (RGD) [106,254,290] and proline-histidine-serine-arginine-asparagine (PHSRN) [127,291] contain receptor binding domains for macrophage-specific adhesive proteins that can regulate macrophage phenotype. PEGylation (PEG coating or brush layers on the implant surface) prevents protein adsorption, and its non-biofouling activity depends on its molecular weight, chain length, chain density, and conformation and is directly proportional to the degree of polymerization and the density of surface brush bristles [110, 127, 281-283]. To further contain the pro-inflammatory immune response, rapamycin and other active biomolecules can be doped in the PEG coating and slowly released into the microenvironment to inhibit non-specific binding and proliferation of inflammatory macrophages [107, 284, 285]. Di block PEG copolymer, such as PEG-b-PLL and VEGF/bFGF linked PEGDA, support neovascularization with minimal fibroblast adhesion while simultaneously masking pro-inflammatory entity with an outer anti-fouling layer to ameliorate the FBR. Examples of notable anticoagulants and anti-fouling agents that successfully prevent the pro-inflammatory response are warfarin [172,231], heparin [164,210,297], hirudin [269,298], argatroban [298], chlorothalonil [299], phosphorylcholine-PDMS [300-302], PEG-fluoropolymer [171], ethisorb [228,303], zwitterionic polymers such as phosphorylcholine, sulfobetaine (SB), carboxybetaine (CB) [110,171,231,304], and many more. Materials can also be modified using biochemical patterning to spatially control cell organization, attachment, and differentiation. These well-defined and ordered colocalization of synergist, agonist, or even antagonist molecules can be achieved using microcontact printing and other Lithographie, Galvanoformung, Abformung (LIGA) processes [305-309].
6.3. Surface Topography
Topographies are precisely engineered geometric features that can be nano- or micron-scaled. The size of the features, shape, geometry, spatial arrangement, frequency, geometry, randomness, and roughness can influence protein adsorption, cell differentiation, and the overall FBR [107,135,167,179,186,202,211,298-301]. Several techniques can be used to install surface topography. For example, photolithography, limited to the sub-micron scale, uses ultraviolet exposure to transfer topographical features on photosensitive material through a patterned mask. For nanoscale features ranging above 4 nm, electron beam lithography (EBL) uses high energy focused electron beam, in the range of 15 – 30 kV, to transfer nanotopography on substrate coated with photosensitive material [178,287,310]. Additionally, soft lithography can used to replicate and transfer these features on the biomaterial substrate. Similar to EBL, high energy reactive focused ion beam (FIB) can also be used to fabricate nanoscale topographies using etch masks consisting of self-assembled nano colloids [80, 127, 143, 177, 272, 302-304]. This technique, known as colloidal lithography, is used to make nanocolumns, nanosphere, and nanocones on material surface. Polymer demixing uses spontaneous phase separation of blended polymer to fabricate random, disordered, sub-micrometric to micrometric scale, co-localized features, such as pores, pits, islands, and ribbons [166,169,183,287]. Electrospinning can also be used to fabricate nanoscale, fiber-like topography. In this method, high voltage, typically in the range of 25 – 50 kV, is used to draw charged polymer solution or eject polymer melt at a controlled rate to yield nanoscale fibers. The topographical arrangement of these fibers depends on the collection methods used; aligned fibers are obtained if collected on the rotating drum collector while random fibers are obtained if collected on the planar collector [184,279,311-314]. Similarly, electrospraying uses electrohydrodynamic process and high voltage electric field to spray a charged polymer solution at low concentration to obtain self-dispersed nanoscale particle topography on substrates [310,315,316]. Additionally, techniques such as dip coating [108,183,262], laser machining, embossing [164,178,183,288], acid etching[164,284,317], dry etching [253,284,318,319], sandblasting [169,202], grinding [284,320], etc., can also be used to successfully install surface topography.
6.4. Biomechanics at the implant level
Mechanical properties of implant that can induce FBR include several metrics such as implant location, relative motion, force intensity, shape, size, thickness, and bulk material mechanical properties of the biomaterial [104,108,205,321]. Implant location affects biomechanics of the implant as different external forces are at play, which, ultimately, limits the functionality, performance, and lifetime of the implant. For example, percutaneous implants are subjected to micromotions, exterior pressures, and forces that propagate along the implant and impact cells at the interface [108,205,321]. On the contrary, subcutaneous or intravascular devices experience less direct forces [80,205,231,322]. Moreover, implant shape significantly affects the distribution of the interfacial forces as well. Higher stress concentrates at sharp angles, curves, and edges, inducing strong FBR with thicker fibrous encapsulation [104,198,205,323]. Implant size is also another crucial factor in determining development of fibrotic overgrowth, as it has been demonstrated that smaller implants cause less tissue trauma, with reduced acute inflammatory response, and can sometimes evade the FBR completely. The induced pro-inflammatory response is less dependent on the length of the implant, as compared to the height, as there is lower disruption in collagen fibers that are parallel to the implant. On the other hand, thicker implants (higher heights) create a separation between the parallel running collagen fibers, triggering the ECM to fill the voids, resulting in a thicker fibrous capsule. For example, paper-thin polyvinyl chloride/polyacrylonitrile (PVC/PAN) [226,250,278] and silicone-coated PVC/PAN [107,127,168,262,324] implants showed lower fibrosis as compared to thicker implants. Lastly, differences in the modulus of the material and surrounding tissue can lead to accumulation of stress at the implant interface, resulting in fibrosis [102,104,186,205]. In fact, it has been demonstrated that implants with modulus similar to that of the surrounding have reduced interfacial stress, which downregulates the pro-inflammatory response leading to FBR.
In the next section, the application of these aforementioned strategies in the development of implants for DM will be evaluated.
7. Strategies Used to Modulate Fibrosis for Diabetes Implants
Treatments for DM include closed-loop insulin delivery systems, also known as artificial pancreas, along with glucose sensors that detect blood glucose levels and secrete the appropriate amount of insulin based on the detected glucose levels [38,245,325,326]. Islets or β cell replacement using intravascular or extravascular encapsulation devices can also serve as long-term treatment for DM, specifically for T1 DM [92,246,311]. Though these technologies have their own inherent challenges, the issue of FBR and fibrosis remains unresolved [80,81,92,327].
7.1. Continuous glucose monitoring systems
Implantable glucose sensor is a highly valuable, clinical technology that improves the quality of life of DM patients through real-time monitoring of glycemic variability. It notifies hypoglycemic and hyperglycemic events with early predictions and makes maintaining euglycemia an achievable goal when combined with closed-loop insulin administration technologies [49, 149, 318]. However, contemporary CGMs lose their lifespan, reliability, and accuracy approximately one week after implantation. This loss occurs due to FBR that results in avascular collagenous tissue encapsulation of the CGM and due to the metabolically active inflammatory cells around the implant that change local pH and glucose concentration [49, 52, 149, 321].
Numerous strategies for improving longevity of CGMs have been evaluated. Traditionally, platinum-iridium (Pt/Ir) [322, 323], silver/silver chloride (Ag/AgCl) [180, 207, 324] based amperometric electrode, isotonic fluid perfused microdialysis fibers, and other enzyme-based electrochemical sensors were used for glucose sensing [332-334]. However, these materials have limited biocompatibility, which is why tremendous progress has been made in developing new materials, such as carbon nanomaterials [328, 329], polymer microgels [337-339], and semiconductor quantum dots (QDs) [333, 334], that enhance the glucose-sensing capabilities and biocompatibility of the sensors. Carbon nanotubes (CNTs) [149, 328], graphene-based electrodes [245,335,342], PAA hydrogel with reduced graphene and lutetium phthalocyanine [343], and other non-enzymatic sensors [333,335,342], have shown excellent glucose sensitivity with relatively greater biocompatibility in vitro. Moreover, boronic acid-functionalized glucose-responsive polymer gels, metal nanoparticles infused with phenylboronic acid (PBA) [339] functionalized microgels, poly(amidoamine) (PAMAM) [339] functionalized microgel, photoluminescent cadmium selenide/zinc sulfide (CdS/ZnS) [339], have also shown great promise in providing excellent glucose sensitivity and higher biocompatibility.
Though the new materials have outstanding glucose sensitivity, their biocompatibility and success as implantable CGMs relies heavily on favorable immunological response at the interface. To do this, polymer coatings can be applied as they can potentially mitigate fibrosis without changing implant function. Several inorganic, organic, bio-functional polymers with anti-fouling properties have been assessed. For example, nafion coated CGM probe showed greater function for more than a week, even though it eventually failed due to mineralization [344-346]. Polyether based aliphatic PU, PU with silicone, and polyethylene oxide (PU-S-PEO) coated sensors significantly inhibit leukocyte adhesion, FBGC formation, and reduce downstream inflammatory cascade up to 2 months [347-349]. PEG-modified hydrogels [336,348,349], copolymers of 2-hydroxyethyl methacrylate (HEMA) [350-352], and ethylene dimethacrylate [353,354] coated implants have also shown lesser fibrous encapsulation compared to PU control. Zwitterionic polymer coating can also be used as an anti-fouling coating for CGMs as its net neutral charge and high hydrophilicity can resist protein adsorption and subsequent cell adhesion [107,171,348,355]. In fact, it has been shown that zwitterionic pSBAA [356-358] and pCBAA [359,360] coated surfaces suppress leukocyte attachment significantly better than PEG-coated surfaces. Moreover, inorganic composites, such as sol-gel derived silicates and silica-based material, and naturally occurring materials, such as alginate and collagen, have shown minimal inflammatory response when coated on biosensors.
Topographical and biomechanical properties of the CGMs can also influence the inflammatory response. For example, PLLA foam coated implantable glucose sensor reduced the thickness of the fibrous capsule with a better capillary density [349,361]. Glucose sensor with a porous expanded PTFE membrane demonstrated better integration of implant with the surrounding tissue for more than 5 months [180, 355]. High precision PMMA templated porous PHEMA hydrogel, silicone, and fibrin coated implant developed only a very thin fibrous capsule, increased vascularization, and reduced pro-inflammatory macrophage phenotype [362,363]. Electrospun, aligned, single and coaxial PU and gelatin fibers of different diameters have also been used to coat glucose sensors, in which monodispersed fiber diameter, permeability, and orientation influences the host response. The coaxial PU-gelatin electrospun fibers effectively prevent formation of fibrous capsule as compared to their counterpart [363-365]. Also, since mechanical properties of fibers or other coating materials play a crucial role in modulating FBR, coating materials with modulus that is similar to that of the surrounding tissue induces lower pro-inflammatory response [366,367]. An implants’ perceived modulus can also be modulated with a brush-like coating of an interpenetrating polymer network that can reduce the FBR. Additionally, polymer coating of double network N-isopropyl acrylamide and 2-methylpropane sulfonic acid membrane (NIPAAm: AMPS) with characteristic thermoresponsive cyclic swelling-deswelling inhibits protein adsorption and subsequent leukocyte attachment [366-370].
To further augment the sensor integration ability and downregulate the, active small or large molecules can be delivered. Sensors coated with dexamethasone, a glucocorticoid, have decreased vascular permeability and leukocyte extravasation. It also prevents leukocyte adhesion and recruitment by reducing the production of proteolytic enzymes and cytokines [371-374]. PLGA particles releasing tyrosine kinase inhibitor (masitinib) from CGMs modulate macrophage polarization and decrease the FBR [375-378]. Extended local delivery of VEGF from HEMA, PEG hydrogels, and PLGA microspheres can also modulate the pro-inflammatory response. PLGA particle coated on the sensor has been used for dual delivery of dexamethasone and VEGF with no significant synergistic effect. Nitric oxide (NO) has also been used to mitigate fibrotic overgrowth as it upregulates VEGF production and vascularization while downregulating pro-inflammatory cytokine secretion [379-381]. A variety of delivery systems are developed for localized long-term NO delivery from CGMs sensors. S-nitrosothiol and N-diazonium diolates are used as a source for NO generation and are incorporated in various polymer-based delivery vehicles coated on glucose sensors. S-nitrosothiol surface-functionalized silica nanoparticle and xerogels doped surface coating led to NO release from the sensor surface, resulting in decreased inflammatory cells, thin fibrous capsule formation, and long-term sensor function[347,348] .
7.2. Microencapsulation and PEGylation of Islets
Microencapsulation for islet transplantation is a widely used strategy in which islets are entrapped within a polymeric membrane that provides three-dimensional architecture to the cells while also providing a high surface area to volume ratio for increased access to nutrients and oxygen. Despite normalizing blood glucose levels in diabetic animals, microcapsules have not achieved clinical outcomes due to the formation of fibrosis around the capsules. This section of the review outlines strategies used for commonly used T1 DM microencapsulation materials to prevent fibrotic overgrowth.
7.2.1. Alginate Microcapsules
Microencapsulation of islets in alginate hydrogel is a conventional approach for treatment of T1D. Alginate is a natural, hydrophilic copolymer that exists as calcium, magnesium, or sodium salts of alginic acid in cell walls of brown seaweed. It is a non-toxic, low cost unbranched polysaccharide composed of D-mannuronic and L-guluronic acid residues joined by glycoside linkages. The monomeric composition of alginate hydrogels affects the biodegradability, porosity, and mechanical integrity, making it an easily modifiable hydrogel. Moreover, divalent cations such as calcium (Ca2+) are typically used to gel the hydrogel under mild conditions. Despite the success of these microcapsules in T1D, due to batch-to-batch variability as well as the presence of endotoxins, cellular fibrotic overgrowth is still an issue that has prevented successful clinical advances of the material. Approaches to preventing the fibrotic overgrowth for alginate hydrogel include changes in monomeric composition, increasing size of microcapsules, surface modification, co-delivery of anti-inflammatory drugs, and co-encapsulation [375-379, 381, 382]
One of the major components that can be controlled to prevent fibrosis in alginate microcapsules is material purity. Although alginate is a natural polymer, it contains endotoxins such as liposaccharides that can activate host inflammatory response through TLRs. Many studies have been conducted that show that alginate purity is a key component in reducing capsular overgrowth [375, 376]. Moreover, alginate composition as well as its molecular weight can also affect the biocompatibility of the microcapsules. However, there are many conflicting reports regarding what ratio of repeating units of guluronic (G) and mannuronic (M) acid in alginate leads to higher biocompatibility [377, 378]. Also, using a low molecular weight, lower viscosity alginate induce a greater fibrotic response as compared to intermediate and high molecular weight alginate capsules [386].
Size of the microcapsules can also determine activation of FBR. Typically, microencapsulation spheres are 0.8-1.5 mm in diameter, however, this size range creates a large diffusion barrier for encapsulated cells, leading to necrosis and accelerated fibrotic encapsulation. Smaller, 0.25-0.35 mm in diameter alginate-polylysine-alginate microcapsules, on the other hand, were more biocompatible and showed prolong graft survival [387]. However, a recent study has shown contradictory results and also demonstrated that device geometry also plays a key role in modulating FBR and fibrosis in rodents and non-human primates. The study showed that spherical materials of 1.5 mm diameter or greater caused lower fibrosis than smaller spheres or disc shaped hydrogels. This remains true across materials of different stiffnesses such as alginate, glass, polycaprolactone, polystyrene and stainless steel. Moreover, encapsulation of rat pancreatic islet cells in 1.5-mm alginate capsules were able to control blood-glucose levels for up to 180 days in a diabetic mouse. This was particularly significant as the widely accepted 0.5-mm alginate capsules have shown control of blood-glucose levels for approximately only 30 days. The increased graft survival and functionality was largely dictated by the reduced cellular deposition and fibrosis formation observed on alginate spheres of larger diameters [304].
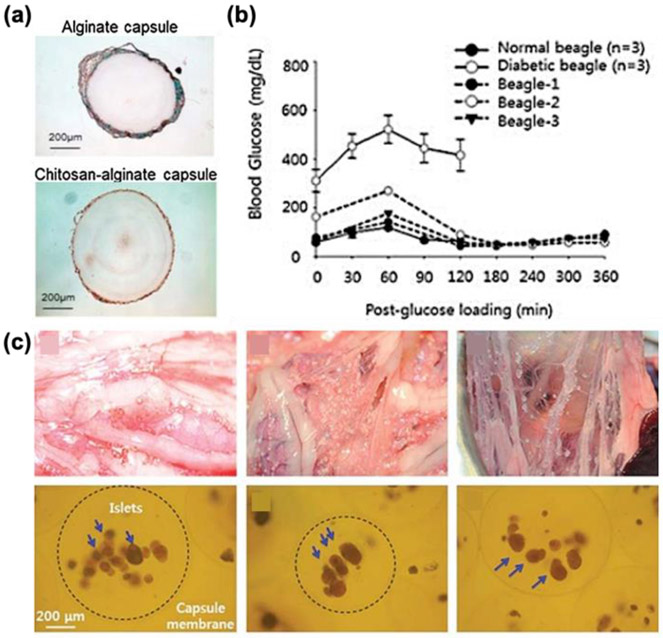
Additionally, many groups have modified the surface of alginate microcapsules in order to increase the biocompatibility. Alginate beads with alternating layers of polyethyleneimine, polyacrylacid, or carboxymethylcellulose were tested, and the use of any of these multilayer-membrane had no adverse fibrotic effects on the grafts. Results also showed high insulin secretion, indicating increased survival of islets [388]. Additionally, coating rapamycin-containing polyethylene glycol significantly reduced fibrosis around the implant by decreasing macrophage cell proliferation [102, 211, 285, 381]. However, this biocompatibility of PEG coated alginate microspheres depends greatly on the transplant site [81, 378]. Chemical conjugation of triazole-thiomorpholine dioxide to alginate demonstrated lower fibrosis around empty alginate microspheres transplanted in rodents and non-human primates [137, 383-385]. Encapsulation of islet stem cell clusters in these chemically modified microspheres also showed little evidence of fibrotic overgrowth after 6 months and excellent glucose control when transplanted into diabetic rodents [392,394-396]. Similarly, chemically modified alginate using corline heparin conjugate had no negative effects on the encapsulated islets and helped reduce fibrotic overgrowth in syngeneic and allogeneic rat transplantation model by ~65% and 43%, respectively [102, 389]. Coating alginate microcapsules with chitosan also had significantly lower fibrosis around the implant after 1 year of, both, xenogeneic and allogeneic transplant (Figure 5) [81,234,398,399].
Figure 5.
Chitosan-coated alginate capsules show demonstrate high efficacy and biocompatibility as islet microencapsulation vehicles. (a) After 1 year of xenogenic islet transplantation, Masson’s trichrome staining chitosan-alginate capsules show lower pericapsular fibrillar collagen network as compared to alginate capsules. (b) Oral blood glucose tolerance test of diabetic canines with 1 year of allogenic islet transplantation showed similar results to test conducted on non-diabetic canines. (c) Moreover, there was no gross fibrosis or cell adhesion on any of the 3 beagles after 1 year of implantation, highlighting the biocompatibility of these capsules [380].
Moreover, a large library of amines, alcohols, azides, and alkynes have been covalently conjugated to alginate to modify the latent functionalities and properties of the polymeric alginate backbone. Barium alginate microspheres of 300-350 μm size modified with Z2-Y12, Z1-Y15, and Z1-Y19 showed lower fibrotic overgrowth after 28 days of implantation in the subcutaneous space and almost no fibrous deposition after 14 days of implantation in the intraperitoneal space of C57BL/6J mice. These modified materials contained triazole functionality and showed little to no presence of macrophages, myofibroblasts, or general cellular deposition around the microcapsules. Although the mechanism of how triazole-containing materials mitigate foreign body responses is still unknown, there is strong evidence that triazole derivatives may prevent activation of macrophages and other immune cells, thus, disrupting the process of fibrosis [400]. Allogenic transplantation of islets encapsulated Z1-Y15 modified alginate microspheres in non-human primate animal model showed no pericapsular fibrotic capsule in 6 out of 7 animals and 90.0% islet cell viability was retained after 4-months of implant. Additionally, when glucose stimulated insulin secretion of the encapsulated islets was measured, these islets secreted significantly higher levels of insulin as compared to non-modified alginate microspheres [401].
More recently, zwitterionic polymers bearing CB, SB, and phosphorylcholine have shown ultra-low-fouling properties due to their high resistance to nonspecific protein adsorption and cell attachment. SB-conjugated alginate microcapsules were implanted in the intraperitoneal space of C57BL/6J mice, intraperitoneal space of dogs, and omental pouch of pigs for up to 6 months. SB-alginate microcapsules in mice showed significantly less cellular overgrowth than unmodified alginate microspheres. Similar results were also seen in large animals, including dogs and pigs, indicating the effectiveness of this strategy in various species. Moreover, after encapsulating rat islets, SB-coated microcapsules also showed significantly better long-term glycemic control for up to 200 days in streptozotocin (STZ)-induced diabetic mice [402]. Although zwitterionic hydrogels have great anti-fouling properties, these hydrogels lack mechanical properties. Therefore, triazole moieties have been integrated into a hydrogel monomer to create a more mechanically robust hydrogel that has greater compressive strain and tensile strain. Despite the addition of triazole, the biocompatibility of the hydrogel was unaffected. In fact, encapsulation of islets in triazole-zwitterionic alginate hydrogels showed correction of glycemic levels in diabetic mice and subcutaneous implantation of the hydrogels also showed significantly lower foreign body response as compared to control [403].
Instead of surface conjugation, co-encapsulation of anti-fibrotic drugs is also a possible strategy for mitigating fibrosis. Co-encapsulated GW2580, a colony stimulating factor 1 receptor (CSIF1R) inhibitor, with β cells in diabetic mice showed lower fibrosis in microcapsules containing the drug (Figure 6). GW2580 targets CSIF1R, which is has been shown to play a key role in targeting monocyte/macrophage phenotype polarization and mediating the FBR against implants. Moreover, subcutaneous and intraperitoneal transplantation of rat islets in 0.5 mm alginate microcapsules with and without drug in STZ-induced diabetic C57BL/6 mice showed promising results. After 72 days in the subcutaneous space, control capsules had a collagen-encapsulated sack around the graft while the drug loaded capsules were fibrosis free. Moreover, there was also a difference between encapsulating amorphous and crystalline GW2580-loaded capsules. The study showed that, after ~1.3 years, crystalline drug capsules had higher islet viability, as indicated by ~30-fold higher Pdx1 expression, and lower myofibroblast and fibrosis response, as indicated by 74% lower α-smooth muscle actin expression [390]. Additionally, incorporation of CXCL12, an immunomodulatory cytokine, in sodium alginate microcapsules containing stem cell derived β cells prevented pericapsular fibrotic response, leading to long-term (>150 day) glycemic correction in mice. The presence of CXCL12 also enhanced the glucose-stimulated insulin secretion of the stem cell derived β cells, which helped the treated mice correct hyperglycemia faster than the control group [107, 387]. Co-delivery of other drugs, such as dexamethasone and curcumin, can effectively minimize fibrotic cellular overgrowth as these drugs inhibit activities of inflammatory proteases and reactive oxygen species. In fact, when curcumin was co-encapsulated with rat islets in alginate microcapsules, there was reduced fibrosis around the implant and promoted greater glycemic control in the diabetic mice [102, 275, 396].
Figure 6.
Long-term delivery of drugs in compact crystals can help modulate fibrosis caused by implantation of alginate microcapsules (a) FACs analysis shows that several drugs are able to modulate the effects of alginate microcapsules on recruiting macrophages and neutrophils (b) H&E and Masson Trichrome staining shows that, after 3 months, several crystalline drugs are able to reduce fibrosis typically induced by alginate spheres in the subcutaneous space. (c) In particular, co-encapsulation of crystalline GW250 with alginate microspheres showed little to no fibrotic overgrowth even after 6 months of implantation in the omentum or subcutaneous space. (d) Moreover, transplantation of islets co-encapsulated with crystalline GW250 in diabetic mice are able to maintain euglycemia for a prolonged period as compared to no drug containing microcapsules and amorphous-loaded microcapsules. (e) Photos also showed high collagenous deposition around control capsules with no drug as compared to capsules with crystalline drug after 72 days of transplant in the subcutaneous space [390].
Lastly, co-encapsulation of different cell types with islets can help mitigate fibrotic overgrowth induced by alginate microcapsules as well. Co-encapsulation and co-transplantation of mesenchymal stem cells (MSCs) with islets resulted in significantly lower perivascular fibrotic overgrowth along with improved graft survival and functionality. Additionally, MSCs pre-stimulated with IFN-γ and TNF-α secreted higher quantity of immunomodulatory cytokines and showed slightly lower fibrosis around the graft. Data also suggested that the observed decrease in fibrosis could be attributed to the upregulation of IL-10 and G-CSF, which directly inhibit TNF-α [405]. Sertoli cells are another set of companion cells that have shown higher engraftment and function of islets when co-encapsulated in alginate microcapsules as these cells inhibit T-cell and B-cell proliferation while increasing local IL-12 production. Co-microencapsulation of these Sertoli cells with islets increased local immunosuppressive factors and showed higher islet β cell mitotic function that produced significantly higher insulin release upon glucose stimulation. However, the effect of these cells in directly preventing fibrosis has not been studied yet [246,406].
7.2.2. Other Microcapsules
Microcapsules fabricated from other natural and synthetic polymers have also shown promise in preventing fibrosis of grafts fabricated for T1D. For example, islets encapsulated in 5% agarose hydrogels were able to maintain euglycemia in diabetic mice for more than 100 days. Moreover, histological analysis showed that implantation of these hydrogels for 100, 150, 200, 300, and 400 days induced little to no immune response [137, 399-401]. Collagen microcapsules have also shown minimal fibrosis, however, their high degradation and low mechanical stability limit their application for long-term treatment of T1D [410]. Additionally, bio-composite silica ceramic microcapsules did not induce fibrosis and the encapsulated islets demonstrated high insulin secretion after one month of implantation in the subcutaneous space of diabetic mice [411]. Hyaluronic acid hydrogel have also been used to encapsulate islets, and after 80 days in vivo, not only were the mice non-diabetic due to the functioning encapsulated islets, histological analysis showed little to no cellular overgrowth around the implant [412]. Lastly, although various polymers can also be combined to modulate the immune response, there is a narrow window in chemistry and capsule processing that may limit successful use of multi-polymer microcapsules. In fact, a study showed that subtle changes in the concentration of any of the components in a multi-component polymer capsule fabricated from the combination of sodium alginate, cellulose sulfate, poly (methylene-co-guanidine) hydrochloride, calcium chloride, and sodium chloride can lead to severe biocompatibility issues [311,413].
7.2.3. PEGylation
Polyethylene glycol (PEG) is used for a variety of drug delivery and nanotechnology applications due to its high biocompatibility. Coating the surface of proteins or drugs with PEG molecules, or “PEGylation” is a widely used technique that allows particles to evade the immune system and prolong their circulation time. Addition of the PEG brush layer creates an impermeable layer on the surface of the attached polymer, causing steric hindrance that prevents protein adsorption along with the subsequent immune cell response. This protein resistance depends on PEG brush layer density, length, and conformation [414-416]. Recently, there have been many advances in using this technique for nanoencapsulation of cells, particularly islets.
Successful covalent attachment of PEG to primary rat islets has been demonstrated by several groups, demonstrating that single polymer grafting approach can be used with islets to successful modulate local transplant environment without adversely affecting cell survival or function [82,417]. PEGylated islets have also showed a 90% decrease in antibody binding, making them antigenically silent. Moreover, these islets decreased lymphocyte proliferation when cultured in the presence of lymphocytes in vitro. Thin conformal coating of islets with PEG-alginate using microfluidics is also another PEGylation that can be used to minimize graft size and volume, while preventing fibrosis [82,231,418,419]. However, in most cases, results showed that PEGylation only delays the rejection of allogeneic and xenogeneic grafts in rodent models, indicating that further enhancement of this therapy is necessary to completely prevent the post-transplant immune reaction [420]. A study showed that transplantation of PEGylated islets led to 60% normoglycemia in diabetic mice for more than 100 days, while local delivery of anti-LFA-1 antibody alone resulted in 50% euglycemia in diabetic mice. The combination of these two strategies, in which PEGylated islets were transplanted along with anti-LFA-1 antibody, however, showed synergistic effects, with 78% of the grafts exhibiting euglycemia at 100 days [415]. Co-transplantation of PEGylated islets with combination therapy of cyclosporine A (CsA) and anti-CD4 monoclonal antibody (OX-38), also again showed synergistic effects after 30 days in vivo [406, 413]. In fact, the combinatorial approach of sub-therapeutic dosage of CsA and PEGylated islets provided a semi-permanent effective therapy for at least 1 year. The non-fasting blood glucose levels of mice treated with the combinatorial approach showed no significant change at day 100, day 200, and 1 year. Although the insulin production levels decreased after 1-year, further refinement of this therapy can offer great promise in long-term protection of islets [414]. Moreover, a recently published study included rapamycin monotherapy alongside allotransplantation of PEGylated islets in non-human primate model. Even at the lowest islet dosage (4160 IEQ/kg), animals with PEGylated islets had significantly higher glycemic function, higher fasting C-peptide levels, and required no exogenous insulin supply as compared to the untreated controls [416].
A potential method to enhance islet protection through PEGylation is by increasing PEGylation layers. Increasing the amount of PEG conjugated to islet surfaces through multiple PEGylation or by amplifying PEGylation using poly-l-lysine, poly(allylamine), or poly(ethyleneimne) can completely shield islets. Although increased PEGylation with these molecules caused greater islet cell toxicity, the overall cell viability and function were unaffected. In fact, 100 days after allotransplant, 3 out of the 7 mice showed survival of triple PEGylated islets in diabetic mice. The 4 transplants that were rejected still showed immune cell protection at day 20, while the control, naked islet grafts, did not even survive 1 week [422]. Another method to enhance PEGylation involves attaching nanoparticles to PEGylated islets. A group showed that long-term (>100 days) euglycemia in 30% of PEGylated grafts, 43% of PEGylated grafts with empty nanoparticles, and 57% of PEGylated grafts with leukemia inhibitory factor (a factor that promotes adaptive immune tolerance and regulated pancreatic β cell mass). The addition of nanoparticles on the PEGylated islets expand the potential of this therapy as they allow for local, sustained delivery of immunomodulatory drugs [82, 415].
7.3. Macroencapsulation
Macroencapsulation is another promising strategy for islet replacement as it allows encapsulation of large number of islets in a retrievable and replenishable device. These larger, micron-scaled, semi-permeable devices may allow greater selectivity through manipulation of membrane properties. However, due to larger device size, macroencapsulation devices tend to be limited by oxygen and nutrient diffusion that leads a greater loss in cell mass. Currently, there is not a universally accepted macroencaspulation device design, size, or material that has shown to provide maximum cell viability and function while preventing immune cell infiltration. Therefore, many research groups have developed their own encapsulation devices, with carefully chosen materials, pore sizes, and surface coatings to increase the device’s function as a cell encapsulation technology for T1D. However, regardless of these differences, an issue that still remains chemical and mechanical properties of the device can induce a foreign body response post-implant, which eventually leads to implant failure [75,424,425].
The need to modulate fibrosis for macroencapsulation devices was demonstrated through the outcomes of the commercially available TheraCyte device produced by Baxter Healthcare. The device was a planar pouch fabricated from PTFE, designed to promote vasculature around the device while still isolating the encapsulated cells. This was done through the double membranes, in which the outer membrane was 15 μm thick with 5 μm pore size that allowed for angiogenesis and the inner membrane was 30 μm thick with 0.4 μm pore size that allowed for immune protection. After 6 months of implant in rats, severe fibrosis had developed inside the device although no inflammatory cells were observed in close proximity to the outer membranes of the device. However, due to the likelihood of fibrosis, graft survival time post-transplant is shortened and limits the use of this device for clinical applications. This result of fibrosis is attributed to the material itself (PTFE), which has been associated with inducing inflammatory reactions that activate FBR and fibrosis [424,426].
To avoid the results seen from the TheraCyte device, inert synthetic polymers such as polycaprolactone (PCL) can be used to fabricate islet macroencapsulation devices. In fact, it has been shown that porous, thin-film PCL device can support the survival and function of stem-cell derived insulin producing cells for 6 months in vivo. One of the key factors that contributes to the success of these devices is the material itself. PCL is a nontoxic polymer that has been used in numerous FDA-approved medical devices. Compared to other polyesters, it has a lower ratio of esters to carbon, allowing it to have longer degradation times. Moreover, when the ester bonds are degraded through hydrolytic cleavage, the resulting byproduct is caproic acid, which is well tolerated by the body. Histological results show that when implanted in the subcutaneous space for four months, porous PCL shows no deposition of fibrotic tissue along the graft. This contrasts greatly from polypropylene films, which showed collagen deposition around the graft and greater immune cell infiltration. Immunostaining after one month of implant also showed that, overall, porous PCL had increased blood vessel formation and reduced fibrosis and macrophage recruitment when the device was transplanted near the liver and the subcutaneous space [427-430].
A recently published study also reported a new retrievable and scalable cell encapsulation device that can be used for islet transplantation. The body of the device was made of PDMS, and the internal structure, fabricated using photolithography, contained chambers designed specifically for holding encapsulated cells along with an injection port for loading cells. Porous PCTE membrane was chemically bonded to the PDMS chips, allowing for controlled release of small molecules while protecting encapsulated cells from immune attack. To address the issue of biocompatibility, zwitterionic polymers were coated onto the surface of the PCTE membranes through surface-initiated atom transfer radical polymerization, allowing growth of dense brush layers on the surface of the membrane. Their studies found that membranes coated with small molecule tetrahydropyran phenyl triazole (THPT) showed 70% reduction in collagen buildup after 4 weeks of implantation. Gene expression analysis showed that THPT coating suppressed expression of CD146, a marker for neovascularization, as well as TNFα and IL-1β, which are inflammatory cytokines. Overall, there was significantly lower cellular overgrowth and total DNA content around the device as compared to non-coated membranes. Moreover, rat islets in alginate solution were loaded in the uncoated and THPT-coated devices, and results showed that, upon implantation in diabetic mice, the encapsulated device was able to restore euglycemia in mice for over 75 days. Although the devices failed during the late phase of the study, this failure in devices was not due to fibrosis as minimal fibrotic deposition was observed around the device. Additionally, human embryonic kidney cells were also encapsulated in these THPT-coated devices, and even after 130 days, the coated devices provided protection against fibrosis [431].
Instead of using a sturdy, polymeric encapsulation device, efforts have also been focused on increasing the mechanical properties of hydrogels so that they can serve as retrievable encapsulation devices. A group has developed a technique to fabricate nanofiber-enabled encapsulation devices (NEEDs) by impregnating Nylon 6 tubular or planar electrospun nanofiber membranes with hydrogel precursor solution using capillary action. After the hydrogel had completely infiltrated the electrospun nanofiber membranes, the hydrogel precursor solution was crosslinked, resulting in hydrogels of various sizes, porosities, and greater mechanical properties. Not only were these devices able to encapsulate various cell types, they also retained the biocompatible properties of the hydrogels. Moreover, primary islets suspended in Matrigel were encapsulated into NEEDs and transplanted into the peritoneal cavity of diabetic mice. After 8 weeks, the mice showed corrected blood glucose levels with minimal fibrosis around the graft. This strategy allows for the use of versatile polymers such as polycaprolactone (PCL) and polyacrylonitrile (PAN) that can be electrospun into various shapes and sizes as well as various hydrogels such as PEG and collagen that can be impregnated with cells to provide greater survival [432].
Some groups have also shown that natural polymers can be used to enhance islet transplantation as well as mitigate fibrosis in macroencapsulation devices. Engagement with ECM components such as collagen is critical for islets as it has been shown that in vitro culture of islets with collagen promotes islet differentiation, survival [422, 423], and reorganization of pancreatic endocrine cell monolayers into islet-like organoids [419, 424,425]. Using this knowledge, a group explored the use of type I oligomeric collagen scaffolds for encapsulating islets. This group used an oligomer, instead of monomeric collagen, because oligomers are able to retain their natural intermolecular crosslinks that result in interconnected, fibrillar scaffolds of high stiffness and longer degradation times. When islets were encapsulated in these type I oligomeric collagen devices/scaffolds and implanted in the subcutaneous space for 14 days, results showed high biocompatibility with no inflammatory reaction around the implant along with preservation of islet morphology. These qualities of the scaffolds heavily contributed in the ability of the encapsulated islets to correct and maintain low blood glucose levels of diabetic mice for up to 90 days in vivo [437]. Another similar approach of promoting formation of extracellular matrix by developing a fibroblast populated type-I collagen matrix scaffold has been successful as well. This approach showed a significant increase in insulin secretion and also reduced the critical islet mass required to reverse diabetes from 200 islets to 100 islets per recipient. Moreover, due to the fibroblasts embedded in the scaffold, higher production of growth factors and increased cell proliferation was observed. This increase in cell proliferation, however, did not lead to fibroblast over-growth, demonstrating the fabrication of a device that enhances engraftment with controlled fibrotic response [430].
Additionally, the combined use of natural and synthetic materials has also been explored. A group has fabricated alginate hydrogel capsule that is surrounded and bound to a nanoporous, wettable, Ca2+ releasing nylon polymer thread, resulting in in situ crosslinking of the alginate hydrogel (Figure 7). This thread-reinforced alginate fiber for islet encapsulation (TRAFFIC) device provides facile mass transfer while still providing the mechanical stability needed for easy implantation and retrieval. The therapeutic potential of this device for treating T1D was also demonstrated when rat islets were encapsulated and implanted into diabetic C57BL/6 mice and human islets were encapsulated and implanted into SCID-Beige mice. Both the models demonstrated diabetes correction for several months, and additionally, the scaling up of the device in dogs was also proven. Additionally, changing the thickness of the alginate devices so that they have a diameter of ~1.3 mm lowered cellular overgrowth than thinner devices with diameter of 500 μm after 7-month of implantation in the intraperitoneal space. Even implantation in dogs showed no fibrosis or histological evidence of inflammation in the tissue, further confirming the biocompatibility of the device [393].
Figure 7.
Calcium releasing twist-folded nylon sutures which allow in situ crosslinking method of alginate hydrogel have great potential as islet macroencapsulation devices. (a) This thread-reinforced alginate fiber for islet encapsulation (TRAFFIC) device offers a new technology that combines polymeric and hydrogel approach to encapsulate cells. (b) Moreover, the easily modifiable devices showed little to no cellular overgrowth and correction of diabetes in rats after 7-month of implantation in the intraperitoneal cavity. When tested in dogs, not only did the scientists show that this device is easily scalable but histological evidence also showed high biocompatibility, demonstrating the translatability of this device.
Lastly, a new technology that incorporates microencapsulation of islets in a three-dimensional structure to promote a microenvironment that is conducive to survival of islets also shows great potential in providing long term treatment for T1D. Islets in PEG hydrogel were casted into a PDMS mold, and after 12 days of implantation in the epididymal fat, encapsulated islets were able to promote euglycemia. Moreover, after 8 weeks of implantation, histological staining showed a thin layer of connective tissue around the hydrogel, demonstrating that these devices were integrated in the body and did not result in a foreign body response [438].
7.4. Vascular Perfusion Devices
The challenges of inadequate supply of nutrients, hypoxia, and central necrosis in extravascular devices (micro and macro encapsulation) impelled the development of vascular perfusion devices (such as intravascular diffusion chamber and intravascular ultrafiltration chamber) [215, 388]. Intravascular bioartificial pancreas device (iBAP) is anastomosed to blood vessels, allowing blood perfusion through the device [439]. Unlike extravascular devices, iBAPs do not rely on passive diffusion and instead rely on connective mass transfer of glucose and insulin across the immune isolation barrier membrane. This intravascular approach, however, interposes normal blood flow and results in acute implant rejection due to immediate blood mediated inflammation reaction (IBMIR) [82, 215, 229, 388]. Consequently, activation of the coagulation cascade and complement system leads to thrombosis, which reduces the membrane permeability and eventually leading to islet necrosis [82, 102, 127, 215, 229, 429]. Additionally, this approach requires more intensive and invasive surgical procedures that result in significant vessel injuries and tissue trauma, which further exacerbate the immune response to iBAPs.
For vascular perfusion devices, traditionally, a two-prong approach is used to manage immune response to both IBMIR and FBR [103,440]. The two strategies are to, first, select an immune evasive, inert, synthetic, non-degradable biomaterial for fabricating, both, the immune isolation membrane and shell of the iBAP and, second, to immobilize active immune modulators, such as small molecules and other biologics. With these devices, there is a greater risk of coagulation and hemorrhage complications, and this limits the choice of biomaterials used to fabricate the immune isolation membrane of iBAPs. An example of iBAP material that has achieved partial feat is a silicon nanopore membrane [84,86]. This membrane emerged as a preferred choice due to its established bioinert and biocompatible nature. Another example of such a material is alumina, however, complete understanding of its in vivo biocompatibility is still unknown [215, 431-433]. Although, titania nanopores membranes for intravascular device can also be used as titania has been already approved for a few other implants, but more studies need to be conducted on the use of titania nanopores in terms of its immune-isolation properties, pore characteristics, and compatibility with implanted islets [135, 142, 163, 431-433]. Negative photoresist SU-8 2025 and 2075 [255,441,443,444], along with polycarbonate (PC), ePTFE, Dacron, PU, and nylon, have also been evaluated [440,441,443,445,446], but all these materials have sown minimal success as fluid exchange through the nanopore membranes eventually slowed, leading to thrombosis, clotting, and fibrosis.
Due to the limited choices of biomaterials for vascular perfusion devices, more efforts have been placed in developing approaches to modulate the immune system therapeutically. Typically, before encapsulation, islets can be cultured in the presence of small molecules, such as L-arginine [327,447,448] , cyclosporine A [82,246], enalapril[449,450], nicotinamide[451-453], vitamin B derivative, that will suppress the initial inflammatory reaction caused by islet associated tissue factors and other chemotactic factors, such as MCP-1, MIF, IL-8. Monoclonal antibodies, siRNA, and other active site inhibitors are also used to attenuate IBMIR [454].
Inhibition of the coagulation system can also increase long term efficacy of iBAPs. Anticoagulants and antiapoptotic molecules, such as activated protein C (APC) [455-457], thrombin inhibitors (such as melagatran, N-acetylcysteine) [458-461], and platelet inhibitors (anti-GP IIb/IIIa) [462,463], significantly reduce pro-inflammatory cytokines and inhibit IBMIR. Thrombomodulin and human recombinant antithrombin (ATryn) [464-467] can limit thrombosis and reduce deposition of fibrin, infiltration of PMN leukocytes, expression of pro-inflammatory cytokines, and thrombin-antithrombin (TAT) complex [82]. Withaferin-A, an anti-NFκB molecule, can also suppress the inflammatory response to implanted islets and IBMIR [468-472].
Additionally, antibodies from islets and the coagulation process also trigger the complement system, which initiates recruitment of immune cells. Complement inhibitor (compstatin) and anaphylatoxin inhibitory peptide (C5aIP) can effectively manage complement system-induced inflammatory response [458, 463, 464]. Low molecular weight dextran sulfate (LMW-DS) can also successfully inhibit the coagulation and complement cascade. CD39 and soluble form of CR1 can serve as attractive targets to ameliorate the IBMIR and FBR [93, 465, 466].
Also, surface coating is one of the most widely used immune engaging strategies in the context of vascular perfusion devices in which the immune isolation membrane or encapsulated islets are coated with an immune-passivation material. For example, silicon nanopore membranes have been modified with PEG to minimize protein fouling, which prevents IBMIR and FBR [84, 215, 428, 467]. Also, coating poly(sulfobetaine methacrylate) (pSBMA), a zwitterionic polymer, on silane treated silicon-nanopore immune isolation membrane has also demonstrated effective suppression of the immune reaction. Other zwitterionic polymers, such as poly(2-methacryloyloxyethyl phosphorylcholine) (pMPC) and poly oligo(ethylene glycol) methyl ether methacrylate (pOMEGA), can also be coated on silicon nanopore membranes to form brush-like structures change surface hydrophilicity such that steric repulsion prevents protein and cell adhesion, leading to attenuation of FBR [170 ,252, 350, 351, 435].
Lastly, cell surface modification or islet-endothelial cells (EC) co-graft have also been effective in modulating the immune response. The amine group on the surface of islets cans be used to covalently attach an ultrathin heparin-PEG film, which can successfully suppress IBMIR and modulate immune cell response [286, 431, 435]. IBMIR and FBR can also be downregulated by reducing complement and coagulation activation with a coating of biomolecules, such as thrombomodulin, urokinase, APC, and sCR1 [215, 455]. These biomolecules can be linked onto the islet surface using maleimide/thiol bonding or DNA hybridization [82,110]. ECs or colony-forming ECs can also be co-transplanted with islets as ECs are neutral to blood exposure, lower the C3a level and TAT complex formation, preventing IBMIR and FBR [110, 397, 468-470].
7.5. Ancillary strategies
In addition to the work predominantly focused on mitigating the FBR accompanying implantable CGMs, intravascular and extravascular islets encapsulation devices, many independent efforts are made in the field. These efforts to tackle fibrosis and related impediments though in different domains, can be relevantly extended to broaden the available strategies for the implants for managing diabetes mellitus.
We have discussed crucial role of biomechanics in FBR and fibrosis, and several biomechanical design aspects for the implant to resist the FBR [194, 201, 314]. Recently published work on an actuatable soft reservoir for modulating host FBR uses a milli-scale dynamic soft reservoir (DSR) to mechanically oscillate and, subsequently, modulate the implant-tissue interface’s biomechanics for downregulating pro-inflammatory response (Figure 8a). The induced oscillatory motion at the biotic-abiotic interface perturbs fluid flow along with cellular activity in the peri-implant tissue, promoting an anti-inflammatory response and reducing the fibrotic capsule while preserving its coherency and collagen maturity. This is a highly versatile and tunable DSR platform that can be integrated with implantable devices to manage diabetes mellitus, specifically for continuous glucose sensors and cell encapsulation devices, for which rapid diffusion of glucose, insulin, oxygen, and other small molecules are required to improve medical outcomes [481].
Figure 8.
Relevant ancillary approaches for mitigating foreign body response and fibrosis which can be extended to the implants for the diabetes. (a) An implantable and actuatable soft reservoir device modulates host foreign body response and describe milli-scale DSRs that successfully use a mechanoceutical approach to actively modulate the biomechanics of the biotic-abiotic interface by perturbing strain and fluid flow [462]. (b) A wireless resonant magnetoelastic actuators made from metal alloy foils generate a fluid flow on the surfaces of implantable Ahmed glaucoma drainage devices to limit cellular adhesion to the surface of the implant to prevent encapsulation and failure [463]. (c) A micro-engineered non-resorbable nano-patterned biosynthesized cellulose (BC) membrane as conformal wrapping around cardiac implantable electronic device (CIED) creating BC interface between the implant and the surrounding tissue in the surgical pocket which significantly reduce the formation of fibrotic tissue [464]. (d) The crystallized drug with compact lattice structure for the long-term controlled release of drug for local anti-inflammatory effect for the suppression of foreign body reaction [371].
On a similar principle, biomechanoceutical approach using magnetoelastic resonator can also modulate FBR at the biotic-abiotic interface (Figure 8b). This exciting work describes a passive, wireless, resonant magnetoelastic actuator to manipulate the fluid flow on the surface of implantable Ahmed glaucoma drainage devices. The magnetic field, generated by external coils, remotely excites the actuator to resonate, generating perturbations that limit FBR and significantly augment glaucoma drainage devices’ efficacy for lowering intraocular pressure. The ability of the magnetoelastic actuators can be appropriated for fibrosis mitigation in the implants for diabetes treatment [482].
Likewise, the mechanical mismatch, in terms of topography, surface geometry, surface chemistry, between the implant and peri-implant tissue is a driver of FBR. Matching the implant stiffnesses with host tissue and providing surface properties that resist non-specific protein and cell binding significantly reduce pro-inflammatory response. A study exploited these key drivers and incorporated them while designing the sheath for the cardiac implantable electronic device (CIED) (Figure 8c). Scientists micro-engineered a non-resorbable, biosynthesized cellulose (BC) membrane that can be conformally wrapped around CIED implants. The mechanical properties of BC membrane, along with the rendered surface topography and spatial arrangement, synergistically minimized the adhesion and differentiation of the cellular mediators responsible for FBR. This BC conformal sheathing can be effectively borrowed and employed for CGMs, intravascular and extravascular cell encapsulation strategies to extenuate FBR [167, 471, 473].
Additionally, in terms of local delivery of FBR mitigating molecules, the effect of an active modulation of immune response using small and large biomolecules ware off with the depletion of the drug and/or changes in release rate. An exciting study addressed this issue by fabricating crystallized concentrated drug depots of the various anti-inflammatory drugs within implants’ (such as micro, macro extravascular cell-encapsulation devices, and implantable CGMs) material (Figure 8d). These concentrated drug depots offer long-term, sustained release of the active immune modulators, which extends the overall lifespan (more than 12 months) of the implant and functional performance by reducing the fibrosis [390].
8. Concluding Remarks
All current and future treatments for diabetes mellitus require the use of implants or cellular grafts that can detect blood glucose levels and secrete insulin for correction of the detected blood glucose levels. Technologies like CGMs (percutaneous or subcutaneous electrochemical sensors), artificial pancreas, intravascular, extravascular immuno-isolation, and cell encapsulation not only improves the outcome, but also have the potential to be a cure for DM. The long-term function and efficacy of these grafts depends on the activation and regulation of the immune cascade, which consists of two phases: inflammatory phase and reparative phase. In the inflammatory phase, cellular debris is cleared through the secretion of proteolytic enzymes, degradation enzymes, inflammatory cytokines, etc. Once the debris has been cleared, the reparative phase dominates, in which cytokines and chemokines that promote angiogenesis and integration of the implant are secreted. After cells, such as fibroblasts, have completed the tissue repair process through deposition of collagen, the implant has successfully integrated within the body. However, if there are inflammatory cues present that prevent the resolution of the immune cascade, foreign body giant cells form, leading to excessive collagen deposition (fibrosis) and device failure. Therefore, it is essential to develop biomaterials that won’t trigger a strong, inflammatory immune response that leads to fibrotic overgrowth and rejection of implant.
The body’s immune response to an implanted biomaterial can be regulated by protein adsorption, biomechanics of the implant material and tissue, and cellular activation. Protein adsorption on the implant surface occurs almost instantaneously and plays a key role in the immune cell – implant interaction. Biomechanics of the biomaterial as well as the tissue also affects the immune response as mechanical loading and stress at implant site can directly promote secretion of soluble factors by immune cells. Both protein adsorption and biomechanics affect the activation of the immune cascade, in which various cell types are involved. Secretion of soluble factors or expression of surface markers can activate multiple cell types from, both, the innate and the adaptive immune system.
Knowing the factors that play a key role in the activation and progression of foreign body response, biomaterials can be modified such that the foreign body response can be minimized. Immune evasive strategies involve the use of natural biomaterials or relatively inert synthetic materials. Immune engaging strategies, on the other hand, involve changing material surface properties by changing surface chemistry, biofunctionalizing the surface, applying surface coating, adding surface patterning or topography, and changing the materials’ mechanical properties. All these strategies have been applied to implants, particularly those used for diabetes. Many groups have demonstrated the effectiveness of these strategies in mitigating fibrosis. However, due to the intricate nature of the immune response, no strategy has had complete long-term success in vivo.
A potential way to further increase efficacy of implant materials in mitigating fibrosis could be through combinations of the various stand-alone solutions that have been used in the past. By combining different strategies, maybe a greater synergistic effect will be induced. Moreover, the mechanisms by which the immune cells interact with biomaterials have not been completely elucidated. Key details of which factors are contributing to this immune cell activation are crucial for successful design of an anti-fouling implant. More insights on macrophage polarization would also be beneficial for designing biomaterials that promote the reparative M2 macrophage phenotype and subsequent integration of implant.
Increasing knowledge and insight on how the biological events can be modulated by changing chemical and physical properties of implant material, along with recent advances, will introduce highly biocompatible implants that have greater longevity in vivo. The future of the field of diabetes relies on this advancement as more sophisticated implant designs will allow implantable technology for diabetes to reach its full potential.
Figure 4.
Several strategies can be used to mitigate the FBR and resulting fibrotic overgrowth. The three major categories that are affected by changes in material properties are protein adsorption, cell and tissue biomechanics, and cellular interaction. Changes in any of these three categories can induce a favorable immune response towards implants and increase their longevity as well as function in vivo.
Acknowledgments
B.N.K and G.S.C contributed equally to this work, and T.A.D. provided direction and edited the manuscript. Thank you to Daniel A. Bernards for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jiménez PG, Martín-Carmona J, Hernández EL, Diabetes mellitus, Med. (2020). 10.1016/j.med.2020.09.010. [DOI] [Google Scholar]
- [2].Atkinson MA, Eisenbarth GS, Michels AW, Type 1 diabetes, Lancet. (2014). 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark A, Type 1 diabetes mellitus, Nat. Rev. Dis. Prim (2017). 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- [4].Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C, On type 1 diabetes mellitus pathogenesis, Endocr. Connect (2018). 10.1530/EC-17-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Samson SL, Garber AJ, Type 2 diabetes, in: Encycl. Endocr. Dis, 2018. 10.1016/B978-0-12-801238-3.95795-7. [DOI] [Google Scholar]
- [6].DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R, Type 2 diabetes mellitus, Nat. Rev. Dis. Prim (2015). 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- [7].Chatterjee S, Khunti K, Davies MJ, Type 2 diabetes, Lancet. (2017). 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- [8].Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin JR, Aguilar RB, The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell–Centric Classification Schema, Diabetes Care. 39 (2016) 179–186. 10.2337/dc15-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Classification and diagnosis of diabetes, Diabetes Care. (2017). 10.2337/dc17-S005. [DOI] [Google Scholar]
- [10].Usmani-Brown S, Perdigoto AL, Lavoie N, Clark P, Korah M, Rui J, Betancur G, Herold KC, β cell responses to inflammation, Mol. Metab (2019). 10.1016/j.molmet.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wilcox NS, Rui J, Hebrok M, Herold KC, Life and death of β cells in Type 1 diabetes: A comprehensive review, J. Autoimmun (2016). 10.1016/j.jaut.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burrack AL, Martinov T, Fife BT, T cell-mediated beta cell destruction: Autoimmunity and alloimmunity in the context of type 1 diabetes, Front. Endocrinol. (Lausanne). (2017). 10.3389/fendo.2017.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oram RA, Sims EK, Evans-Molina C, Beta cells in type 1 diabetes: mass and function; sleeping or dead?, Diabetologia. (2019). 10.1007/s00125-019-4822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stabler CL, Li Y, Stewart JM, Keselowsky BG, Engineering immunomodulatory biomaterials for type 1 diabetes, Nat. Rev. Mater (2019). 10.1038/s41578-019-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fernández-Real JM, Pickup JC, Innate immunity, insulin resistance and type 2 diabetes, Diabetologia. (2012). 10.1007/s00125-011-2387-y. [DOI] [PubMed] [Google Scholar]
- [16].Pickup JC, Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabletes, Diabetes Care. (2004). 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- [17].Velloso LA, Eizirik DL, Cnop M, Type 2 diabetes mellitus - An autoimmune disease?, Nat. Rev. Endocrinol (2013). 10.1038/nrendo.2013.131. [DOI] [PubMed] [Google Scholar]
- [18].Odegaard JI, Chawla A, Connecting type 1 and type 2 diabetes through innate immunity, Cold Spring Harb. Perspect. Med (2012). 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D, The role of inflammation in diabetes: Current concepts and future perspectives, Eur. Cardiol. Rev (2019). 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piñero-Piloña A, Raskin P, Idiopathic Type 1 diabetes, J. Diabetes Complications (2001). 10.1016/S1056-8727(01)00172-6. [DOI] [PubMed] [Google Scholar]
- [21].N.D.S. Report, National Diabetes Statistics Report, 2020, Natl. Diabetes Stat. Rep (2020). [Google Scholar]
- [22].WHO, Non-Communicable Diseases Fact Sheet, Public Heal. An Action Guid. to Improv. Heal (2018). [Google Scholar]
- [23].WHO, Global action plan for the prevention and control of noncommunicable diseases 2013-2020., World Heal. organ. (2013). https://doi.org/978 92 4 1506236. [Google Scholar]
- [24].WHO, Diabetes Programme, World Heal. organ. (2018). [Google Scholar]
- [25].Internation Diabetes Federation, IDF Diabetes Atlas Ninth, 2019. [Google Scholar]
- [26].Nathan DM, Diabetes: Advances in diagnosis and treatment, JAMA - J. Am. Med. Assoc (2015). 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- [27].Bansal N, Prediabetes diagnosis and treatment: A review, World J. Diabetes (2015). 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].American Diabetes Association, Diagnosing Diabetes and Learning About Prediabetes, Www.Diabetes.Org/Diabetes-Basics/Diagnosis. (2015).
- [29].Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020, Diabetes Care. (2020). 10.2337/dc20-S002. [DOI] [Google Scholar]
- [30].Frid AH, Kreugel G, Grassi G, Halimi S, Hicks D, Hirsch LJ, Smith MJ, Wellhoener R, Bode BW, Hirsch IB, Kalra S, Ji L, Strauss KW, New Insulin Delivery Recommendations, Mayo Clin. Proc 91 (2016) 1231–1255. 10.1016/j.mayocp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- [31].Malik FS, Taplin CE, Insulin therapy in children and adolescents with type 1 diabetes, Pediatr. Drugs (2014). 10.1007/s40272-014-0064-6. [DOI] [PubMed] [Google Scholar]
- [32].Farrar D, Tuffnell DJ, West J, West HM, Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes, Cochrane Database Syst. Rev (2016). 10.1002/14651858.CD005542.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silver B, Ramaiya K, Andrew SB, Fredrick O, Bajaj S, Kalra S, Charlotte BM, Claudine K, Makhoba A, EADSG Guidelines: Insulin Therapy in Diabetes, Diabetes Ther. (2018). 10.1007/s13300-018-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA, Insulin for the treatment of women with gestational diabetes, Cochrane Database Syst. Rev (2017). 10.1002/14651858.CD012037.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pharmacologic approaches to glycemic treatment, Diabetes Care. (2017). 10.2337/dc17-S011. [DOI] [PubMed] [Google Scholar]
- [36].Boughton CK, Hovorka R, The artificial pancreas, Curr. Opin. organ Transplant (2020). 10.1097/MOT.0000000000000786. [DOI] [PubMed] [Google Scholar]
- [37].Boughton CK, Hovorka R, Advances in artificial pancreas systems, Sci. Transl. Med (2019). 10.1126/scitranslmed.aaw4949. [DOI] [PubMed] [Google Scholar]
- [38].Brooker G, The artificial pancreas, in: Handb. Biomechatronics, 2018. 10.1016/B978-0-12-812539-7.00015-5. [DOI] [Google Scholar]
- [39].Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A, Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-Analysis, BMJ. (2018). 10.1136/bmj.k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Blauw H, van Bon AC, Koops R, DeVries JH, Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose co006Etrol at home, Diabetes, Obes. Metab (2016). 10.1111/dom.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thabit H, Hovorka R, Coming of age: the artificial pancreas for type 1 diabetes, Diabetologia. (2016). 10.1007/s00125-016-4022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R, Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: An open-label randomised controlled crossover trial, Lancet Diabetes Endocrinol. (2015). 10.1016/S2213-8587(14)70226-8. [DOI] [PubMed] [Google Scholar]
- [43].Peyser T, Dassau E, Breton M, Skyler JS, The artificial pancreas: Current status and future prospects in the management of diabetes, Ann. N. Y. Acad. Sci (2014). 10.1111/nyas.12431. [DOI] [PubMed] [Google Scholar]
- [44].Hovorka R, Closed-loop insulin delivery: From bench to clinical practice, Nat. Rev. Endocrinol (2011). 10.1038/nrendo.2011.32. [DOI] [PubMed] [Google Scholar]
- [45].Cobelli C, Renard E, Kovatchev B, Artificial pancreas: Past, present, future, Diabetes. (2011). 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, Boettcher C, Kapellen T, Rosenbauer J, Holl RW, Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes, JAMA - J. Am. Med. Assoc (2017). 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bruen D, Delaney C, Florea L, Diamond D, Glucose sensing for diabetes monitoring: Recent developments, Sensors (Switzerland). (2017). 10.3390/s17081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Klonoff DC, Ahn D, Drincic A, Continuous glucose monitoring: A review of the technology and clinical use, Diabetes Res. Clin. Pract (2017). 10.1016/j.diabres.2017.08.005. [DOI] [PubMed] [Google Scholar]
- [49].Rodbard D, Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities, Diabetes Technol. Ther (2016). 10.1089/dia.2015.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, Zethelius B, Avdic T, Landin-Olsson M, Jendle J, Gudbjörnsdóttir S, Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18 168 people with type 1 diabetes: Observational study, BMJ. (2015). 10.1136/bmj.h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nimri R, Nir J, Phillip M, Insulin Pump Therapy, Am. J. Ther (2020). 10.1097/MJT.0000000000001097. [DOI] [PubMed] [Google Scholar]
- [52].Van Enter BJ, Von Hauff E, Challenges and perspectives in continuous glucose monitoring, Chem. Commun (2018). 10.1039/c8cc01678j. [DOI] [PubMed] [Google Scholar]
- [53].Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE), BMJ. (2017) j1285. 10.1136/bmj.j1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Brennan DC, Kopetskie HA, Sayre PH, Alejandro R, Cagliero E, Shapiro AMJ, Goldstein JS, DesMarais MR, Booher S, Bianchine PJ, Long-Term Follow-Up of the Edmonton Protocol of Islet Transplantation in the United States, Am. J. Transplant 16 (2016) 509–517. 10.1111/ajt.13458. [DOI] [PubMed] [Google Scholar]
- [55].Tekin Z, Garfinkel MR, Chon WJ, Schenck L, Golab K, Savari O, Thistlethwaite JR, Philipson LH, Majewski C, Pannain S, Ramachandran S, Rezania K, Hariprasad SM, Millis JM, Witkowski P, Outcomes of Pancreatic Islet Allotransplantation Using the Edmonton Protocol at the University of Chicago, Transplant. Direct (2016). 10.1097/txd.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, Fuller C, Chiou AJ, Barker CF, Luning Prak ET, Naji A, Improvement in β-Cell secretory capacity after human islet transplantation according to the CIT07 protocol, Diabetes. (2013). 10.2337/db12-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Langer RM, Islet Transplantation: Lessons Learned Since the Edmonton Breakthrough, Transplant. Proc (2010). 10.1016/j.transproceed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- [58].Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems J-A, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JRT, International Trial of the Edmonton Protocol for Islet Transplantation, N. Engl. J. Med (2006). 10.1056/nejmoa061267. [DOI] [PubMed] [Google Scholar]
- [59].Ryan EA, Lakey JRT, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AMJ, Clinical outcomes and insulin secretion after islet transplantation with the edmonton protocol, Diabetes. (2001). 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- [60].Odorico J, Markmann J, Melton D, Greenstein J, Hwa A, Nostro C, Rezania A, Oberholzer J, Pipeleers D, Yang L, Cowan C, Huangfu D, Egli D, Ben-David U, Vallier L, Grey ST, Tang Q, Roep B, Ricordi C, Naji A, Orlando G, Anderson DG, Poznansky M, Ludwig B, Tomei A, Greiner DL, Graham M, Carpenter M, Migliaccio G, D’Amour K, Hering B, Piemonti L, Berney T, Rickels M, Kay T, Adams A, Report of the Key Opinion Leaders Meeting on Stem Cell-derived Beta Cells, Transplantation. (2018). 10.1097/TP.0000000000002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dimitrioglou N, Kanelli M, Papageorgiou E, Karatzas T, Hatziavramidis D, Paving the way for successful islet encapsulation, Drug Discov. Today (2019). 10.1016/j.drudis.2019.01.020. [DOI] [PubMed] [Google Scholar]
- [62].Anazawa T, Okajima H, Masui T, Uemoto S, Current state and future evolution of pancreatic islet transplantation, Ann. Gastroenterol. Surg (2019). 10.1002/ags3.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matsumoto S, Shimoda M, Current situation of clinical islet transplantation from allogeneic toward xenogeneic, J. Diabetes 12 (2020) 733–741. 10.1111/1753-0407.13041. [DOI] [PubMed] [Google Scholar]
- [64].Takaki T, Shimoda M, Pancreatic islet transplantation: toward definitive treatment for diabetes mellitus, Glob. Heal. Med 2 (2020) 200–211. 10.35772/ghm.2020.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rickels MR, Paul Robertson R, Pancreatic islet transplantation in humans: Recent progress and future directions, Endocr. Rev (2019). 10.1210/er.2018-00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Graham ML, Schuurman HJ, Pancreatic islet xenotransplantation, Drug Discov. Today Dis. Model (2017). 10.1016/j.ddmod.2017.11.004. [DOI] [Google Scholar]
- [67].Reichart B, Niemann H, Chavakis T, Denner J, Jaeckel E, Ludwig B, Marckmann G, Schnieke A, Schwinzer R, Seissler J, Tönjes RR, Klymiuk N, Wolf E, Bornstein SR, Xenotransplantation of porcine islet cells as a potential option for the treatment of type 1 diabetes in the future, Horm. Metab. Res (2015). 10.1055/s-0034-1395518. [DOI] [PubMed] [Google Scholar]
- [68].Sarny KP, Martin BM, Turgeon NA, Kirk AD, Islet cell xenotransplantation: A serious look toward the clinic, Xenotransplantation. (2014). 10.1111/xen.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Meier RPH, Seebach JD, Morel P, Mahou R, Borot S, Giovannoni L, Parnaud G, Montanari E, Bosco D, Wandrey C, Berney T, Bühler LH, Muller YD, Survival of free and encapsulated human and rat islet xenografts transplanted into the mouse bone marrow, PLoS One. (2014). 10.1371/journal.pone.0091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R, Pig-to-nonhuman primates pancreatic islet xenotransplantation: An overview, Curr. Diab. Rep (2011). 10.1007/s11892-011-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TMS, De Vos P, Hortelano G, Hunkeler D, Lacík I, Shapiro AMJ, Pedraz JL, Cell encapsulation: Promise and progress, Nat. Med (2003). 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- [72].Ernst AU, Wang LH, Ma M, encapsulation Islet, J. Mater. Chem. B (2018). 10.1039/c8tb02020e. [DOI] [PubMed] [Google Scholar]
- [73].Sneddon JB, Tang Q, Stock P, Bluestone JA, Roy S, Desai T, Hebrok M, Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges, Cell Stem Cell. (2018). 10.1016/j.stem.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Korsgren O, Islet encapsulation: Physiological possibilities and limitations, Diabetes. (2017). 10.2337/db17-0065. [DOI] [PubMed] [Google Scholar]
- [75].Desai T, Shea LD, Advances in islet encapsulation technologies, Nat. Rev. Drug Discov (2017). 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Steele JAM, Hallé JP, Poncelet D, Neufeld RJ, Therapeutic cell encapsulation techniques and applications in diabetes, Adv. Drug Deliv. Rev (2014). 10.1016/j.addr.2013.09.015. [DOI] [PubMed] [Google Scholar]
- [77].Kang AR, Park JS, Ju J, Jeong GS, Lee SH, Cell encapsulation via microtechnologies, Biomaterials. (2014). 10.1016/j.biomaterials.2013.12.073. [DOI] [PubMed] [Google Scholar]
- [78].Weir GC, Islet encapsulation: Advances and obstacles, Diabetologia. (2013). 10.1007/s00125-013-2921-1. [DOI] [PubMed] [Google Scholar]
- [79].Farina M, Alexander JF, Thekkedath U, Ferrari M, Grattoni A, Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond, Adv. Drug Deliv. Rev 139 (2019) 92–115. 10.1016/j.addr.2018.04.018. [DOI] [PubMed] [Google Scholar]
- [80].Ernst AU, Wang LH, Ma M, Islet encapsulation J Mater. Chem. B 6 (2018) 6705–6722. 10.1039/c8tb02020e. [DOI] [PubMed] [Google Scholar]
- [81].Hwang PTJ, Shah DK, Garcia JA, Bae CY, Lim D-J, Huiszoon RC, Alexander GC, Jun H-W, Progress and challenges of the bioartificial pancreas, Nano Converg. 3 (2016) 1–11. 10.1186/s40580-016-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution, Adv. Drug Deliv. Rev 67–68 (2014) 35–73. 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- [83].Krishnan R, Alexander M, Robles L, Foster CE, Lakey JRT, Islet and stem cell encapsulation for clinical transplantation, Rev. Diabet. Stud 11 (2014) 84–101. 10.1900/RDS.2014.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Song S, Yeung R, Park J, Posselt AM, Desai TA, Tang Q, Roy S, Glucose-Stimulated Insulin Response of Silicon Nanopore-Immunoprotected Islets under Convective Transport, ACS Biomater. Sci. Eng (2017). 10.1021/acsbiomaterials.6b00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S, An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport, Lab Chip. (2017). 10.1039/c71c00096k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S, Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport, Sci. Rep (2016). 10.1038/srep23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhou Q, Melton DA, Pancreas regeneration, Nature. (2018). 10.1038/s41586-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gamble A, Pepper AR, Bruni A, Shapiro AMJ, The journey of islet cell transplantation and future development, Islets. (2018). 10.1080/19382014.2018.1428511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kieffer TJ, Woltjen K, Osafune K, Yabe D, Inagaki N, Beta-cell replacement strategies for diabetes, J. Diabetes Investig (2018). 10.1111/jdi.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ, Ao Z, Warnock GL, Kieffer TJ, Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice, Diabetes. (2012). 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Farney AC, Sutherland DER, Opara EC, Evolution of Islet Transplantation for the Last 30 Years, Pancreas. (2016). 10.1097/MPA.0000000000000391. [DOI] [PubMed] [Google Scholar]
- [92].Colton CK, Challenges in the Development of Immunoisolation Devices, in: Princ. Tissue Eng. Fourth Ed, 2013. 10.1016/B978-0-12-398358-9.00028-8. [DOI] [Google Scholar]
- [93].Song S, Roy S, Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices, Biotechnol. Bioeng (2016). 10.1002/bit.25895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Y, Vaddiraju S, Gu B, Papadimitrakopoulos F, Burgess DJ, Foreign Body Reaction to Implantable Biosensors, J. Diabetes Sci. Technol (2015). 10.1177/1932296815601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rigla M, Pons B, Rebasa P, Luna A, Pozo FJ, Caixàs A, Villaplana M, Subías D, Bella MR, Combalia N, Human Subcutaneous Tissue Response to Glucose Sensors: Macrophages Accumulation Impact on Sensor Accuracy, Diabetes Technol. Ther (2018). 10.1089/dia.2017.0321. [DOI] [PubMed] [Google Scholar]
- [96].Keselowsky BG, Bridges AW, Burns KL, Tate CC, Babensee JE, LaPlaca MC, García AJ, Role of plasma fibronectin in the foreign body response to biomaterials, Biomaterials. (2007). 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kastellorizios M, Tipnis N, Burgess DJ, Foreign body reaction to subcutaneous implants, Adv. Exp. Med. Biol (2015). 10.1007/978-3-319-18603-0_6. [DOI] [PubMed] [Google Scholar]
- [98].Major MR, Wong VW, Nelson ER, Longaker MT, Gurtner GC, The Foreign Body Response: At the Interface of Surgery and Bioengineering, Plast. Reconstr. Surg (2015). 10.1097/PRS.0000000000001193. [DOI] [PubMed] [Google Scholar]
- [99].Wang Y, Vaddiraju S, Gu B, Papadimitrakopoulos F, Burgess DJ, Foreign body reaction to implantable biosensors: Effects of tissue trauma and implant size, J. Diabetes Sci. Technol (2015). 10.1177/1932296815601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].De Groot M, Schuurs TA, Van Schilfgaarde R, Causes of limited survival of microencapsulated pancreatic islet grafts, J. Surg. Res (2004). 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- [101].Gifford R, Continuous glucose monitoring: 40 years, what we’ve learned and what’s next, ChemPhysChem. (2013). 10.1002/cphc.201300172. [DOI] [PubMed] [Google Scholar]
- [102].Jones K, Fibrotic Response to Biomaterials and all Associated Sequence of Fibrosis, in: Host Response to Biomater. Impact Host Response Biomater. Sel, 2015. 10.1016/B978-0-12-800196-7.00009-8. [DOI] [Google Scholar]
- [103].Corradetti B, Impact T, The Immune Response to Implanted Materials and Devices, 2017. 10.1007/978-3-319-45433-7. [DOI] [Google Scholar]
- [104].Ratner BD, The Biocompatibility of Implant Materials, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00003-7. [DOI] [Google Scholar]
- [105].Klopfleisch R, Macrophage reaction against biomaterials in the mouse model – Phenotypes, functions and markers, Acta Biomater. (2016). 10.1016/j.actbio.2016.07.003. [DOI] [PubMed] [Google Scholar]
- [106].Chung L, Maestas DR, Housseau F, Elisseeff JH, Key players in the immune response to biomaterial scaffolds for regenerative medicine, Adv. Drug Deliv. Rev 114 (2017) 184–192. 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- [107].Veiseh O, Vegas AJ, Domesticating the foreign body response: Recent advances and applications, Adv. Drug Deliv. Rev 144 (2019). 10.1016/j.addr.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang Y, Vaddiraju S, Gu B, Papadimitrakopoulos F, Burgess DJ, Foreign body reaction to implantable biosensors: Effects of tissue trauma and implant size, J. Diabetes Sci. Technol 9 (2015) 966–977. 10.1177/1932296815601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kyriakides TR, Molecular Events at Tissue-Biomaterial Interface, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00005-0. [DOI] [Google Scholar]
- [110].Chen W, Yung BC, Qian Z, Chen X, Improving long-term subcutaneous drug delivery by regulating material-bioenvironment interaction, Adv. Drug Deliv. Rev (2018). 10.1016/j.addr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Desai TA, Tang Q, Islet encapsulation therapy — racing towards the finish line?, Nat. Rev. Endocrinol 14 (2018) 630–632. 10.1038/s41574-018-0100-7. [DOI] [PubMed] [Google Scholar]
- [112].Desai T, Shea LD, Advances in islet encapsulation technologies, Nat. Rev. Drug Discov 16 (2017) 338–350. 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Dolgin E, Encapsulating the problem, Nature. 540 (2016) S60–62. [DOI] [PubMed] [Google Scholar]
- [114].Gray M, Meehan J, Ward C, Langdon SP, Kunkler IH, Murray A, Argyle D, Implantable biosensors and their contribution to the future of precision medicine, Vet. J (2018). 10.1016/j.tvjl.2018.07.011. [DOI] [PubMed] [Google Scholar]
- [115].Mason McClatchey P, McClain ES, Williams IM, Malabanan CM, James FD, Lord PC, Gregory JM, Cliffel DE, Wasserman DH, Fibrotic encapsulation is the dominant source of continuous glucose monitor delays, Diabetes. (2019). 10.2337/db19-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wood A, O’Neal D, Furler J, Ekinci EI, Continuous glucose monitoring: a review of the evidence, opportunities for future use and ongoing challenges, Intern. Med. J (2018). 10.1111/imj.13770. [DOI] [PubMed] [Google Scholar]
- [117].Li J, Liang JY, Laken SJ, Langer R, Traverso G, Clinical Opportunities for Continuous Biosensing and Closed-Loop Therapies, Trends Chem. (2020). 10.1016/j.trechm.2020.02.009. [DOI] [Google Scholar]
- [118].Teymourian H, Barfidokht A, Wang J, Electrochemical glucose sensors in diabetes management: an updated review (2010–2020), Chem. Soc. Rev (2020). 10.1039/d0cs00304b. [DOI] [PubMed] [Google Scholar]
- [119].Stout PJ, Racchini JR, Hilgers ME, Noujaim SE, Continuous glucose monitoring: key challenges to replacing episodic SMBG, Diabetes Res. Clin. Pract (2006). 10.1016/S0168-8227(06)70007-9. [DOI] [Google Scholar]
- [120].Scarritt ME, Londono R, Badylak SF, Host response to implanted materials and devices: An overview, in: Immune Response to Implant. Mater. Devices Impact Immune Syst. Success an Implant, Springer International Publishing, 2016: pp. 1–14. 10.1007/978-3-319-45433-7_1. [DOI] [Google Scholar]
- [121].Corradetti B, The immune response to implanted materials and devices: The impact of the immune system on the success of an implant, Springer International Publishing, 2016. 10.1007/978-3-319-45433-7. [DOI] [Google Scholar]
- [122].Williams DF, On the mechanisms of biocompatibility, Biomaterials. 29 (2008) 2941–2953. 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- [123].Anderson JM, Jiang S, Implications of the acute and chronic inflammatory response and the foreign body reaction to the immune response of implanted biomaterials, in: Immune Response to Implant. Mater. Devices Impact Immune Syst. Success an Implant, Springer International Publishing, 2016: pp. 15–36. 10.1007/978-3-319-45433-7_2. [DOI] [Google Scholar]
- [124].Santambrogio L, Biomaterials in Regenerative Medicine and the Immune System, Springer International Publishing, 2015. 10.1007/978-3-319-18045-8. [DOI] [Google Scholar]
- [125].Yu T, Tutwiler VJ, Spiller K, The role of macrophages in the foreign body response to implanted biomaterials, in: Biomater. Regen. Med. Immune Syst, Springer International Publishing, 2015: pp. 17–34. 10.1007/978-3-319-18045-8_2. [DOI] [Google Scholar]
- [126].McKiel LA, Woodhouse KA, Fitzpatrick LE, The role of Toll-like receptor signaling in the macrophage response to implanted materials, MRS Commun. 10 (2020) 55–68. 10.1557/mrc.2019.154. [DOI] [Google Scholar]
- [127].Franz S, Rammelt S, Scharnweber D, Simon JC, Immune responses to implants – A review of the implications for the design of immunomodulatory biomaterials, Biomaterials. (2011). 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- [128].Christo SN, Diener KR, Bachhuka A, Vasilev K, Hayball JD, Innate Immunity and Biomaterials at the Nexus: Friends or Foes, Biomed Res. Int 2015 (2015) 1–23. 10.1155/2015/342304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Davenport Huyer L, Pascual-Gil S, Wang Y, Mandla S, Yee B, Radisic M, Advanced Strategies for Modulation of the Material–Macrophage Interface, Adv. Funct. Mater (2020). 10.1002/adfm.201909331. [DOI] [Google Scholar]
- [130].Anderson J, Cramer S, Perspectives on the Inflammatory, Healing, and Foreign Body Responses to Biomaterials and Medical Devices, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00002-5. [DOI] [Google Scholar]
- [131].Chandorkar Y, Ravikumar K, Basu B, The Foreign Body Response Demystified, ACS Biomater. Sci. Eng 5 (2019) 19–44. 10.1021/acsbiomaterials.8b00252. [DOI] [PubMed] [Google Scholar]
- [132].Geerlings SE, Hoepelman AIM, Immune dysfunction in patients with diabetes mellitus (DM), FEMS Immunol. Med. Microbiol (1999). 10.1016/S0928-8244(99)00142-X. [DOI] [PubMed] [Google Scholar]
- [133].Shoelson SE, Lee J, Goldfine AB, Inflammation and insulin resistance, J. Clin. Invest (2006). 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Lackey DE, Olefsky JM, Regulation of metabolism by the innate immune system, Nat. Rev. Endocrinol (2016). 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- [135].Espinoza-Jiménez A, Peón AN, Terrazas LI, Alternatively activated macrophages in types 1 and 2 diabetes, Mediators Inflamm. (2012). 10.1155/2012/815953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kopan C, Tucker T, Alexander M, Mohammadi MR, Pone E, Lakey JRT, Approaches in immunotherapy, regenerative medicine, and bioengineering for type 1 diabetes, Front. Immunol 9 (2018). 10.3389/fimmu.2018.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].De Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, Spagnuolo MI, Colamatteo A, Fusco C, Micillo T, Bruzzaniti S, Ceriello A, Puca AA, Matarese G, Type 2 diabetes: How much of an autoimmune disease?, Front. Endocrinol. (Lausanne) (2019). 10.3389/fendo.2019.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R, State of the union between metabolism and the immune system in type 2 diabetes, Genes Immun. (2011). 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Donath MY, Shoelson SE, Type 2 diabetes as an inflammatory disease, Nat. Rev. Immunol (2011). 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- [140].Wolf G, Aumann N, Michalska M, Bast A, Sonnemann J, Beck JF, Lendeckel U, Newsholme P, Walther R, Peroxiredoxin III protects pancreatic β cells from apoptosis, J. Endocrinol (2010). 10.1677/JOE-09-0455. [DOI] [PubMed] [Google Scholar]
- [141].Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K, The Effects of Type 2 Diabetes Mellitus on organ Metabolism and the Immune System, Front. Immunol (2020). 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zaccardi F, Webb DR, Yates T, Davies MJ, Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective, Postgrad. Med. J (2016). 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- [143].Bosi E, Braghi S, Maffi P, Scirpoli M, Bertuzzi F, Pozza G, Secchi A, Bonifacio E, Autoantibody Response to Islet Transplantation in Type 1 Diabetes, Diabetes. 50 (2001) 2464–2471. 10.2337/diabetes.50.11.2464. [DOI] [PubMed] [Google Scholar]
- [144].Zorec B, Jelenc J, Miklavčič D, Pavšelj N, Ultrasound and electric pulses for transdermal drug delivery enhancement: Ex vivo assessment of methods with in vivo oriented experimental protocols, Int. J. Pharm 490 (2015) 65–73. 10.1016/j.ijpharm.2015.05.035. [DOI] [PubMed] [Google Scholar]
- [145].Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E, Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells, J. Clin. Invest 118 (2008) 1806–1814. 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pugliese A, Reijonen HK, Nepom J, Burke GW, Recurrence of autoimmunity in pancreas transplant patients: research update, Diabetes Manag. 1 (2011) 229–238. 10.2217/dmt.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A, Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?), Nat. Rev. Endocrinol (2020). 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D, The role of inflammation in diabetes: Current concepts and future perspectives, Eur. Cardiol. Rev . 14 (2019) 50–59. 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, Venketaraman V, Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis, J. Clin. Med (2019). 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kumar Nathella P, Babu S, Influence of diabetes mellitus on immunity to human tuberculosis, Immunology. (2017). 10.1111/imm.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li C, Guo C, Fitzpatrick V, Ibrahim A, Zwierstra MJ, Hanna P, Lechtig A, Nazarian A, Lin SJ, Kaplan DL, Design of biodegradable, implantable devices towards clinical translation, Nat. Rev. Mater (2020). 10.1038/s41578-019-0150-z. [DOI] [Google Scholar]
- [152].Kyriakides TR, Molecular Events at Tissue-Biomaterial Interface, in: Host Response to Biomater. Impact Host Response Biomater. Sel, 2015. 10.1016/B978-0-12-800196-7.00005-0. [DOI] [Google Scholar]
- [153].Stieglitz T, Schuettler M, Material-tissue interfaces in implantable systems, in: Implant. Sens. Syst. Med. Appl, 2013. 10.1533/9780857096289.1.39. [DOI] [Google Scholar]
- [154].Horbett TA, Adsorbed Proteins on Biomaterials, in: Biomater. Sci. An Introd. to Mater. Third Ed., 2013. 10.1016/B978-0-08-087780-8.00036-X. [DOI] [Google Scholar]
- [155].Vogler EA, Protein adsorption in three dimensions, Biomaterials. (2012). 10.1016/j.biomaterials.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, Lendlein A, Ballauff M, Haag R, Protein interactions with polymer coatings and biomaterials, Angew. Chemie - Int. Ed (2014). 10.1002/anie.201400546. [DOI] [PubMed] [Google Scholar]
- [157].Camilloni C, Bonetti D, Morrone A, Giri R, Dobson CM, Brunori M, Gianni S, Vendruscolo M, Towards a structural biology of the hydrophobic effect in protein folding, Sci. Rep (2016). 10.1038/srep28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Dyson HJ, Wright PE, Scheraga HA, The role of hydrophobic interactions in initiation and propagation of protein folding, Proc. Natl. Acad. Sci. U. S. A (2006). 10.1073/pnas.0605504103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Schmidt DR, Waldeck H, Kao WJ, Protein Adsorption to Biomaterials, in: Biol. Interact. Mater. Surfaces, Springer CIS, 2009: pp. 1–18. 10.1007/978-0-387-98161-1_1. [DOI] [Google Scholar]
- [160].Dahal YR, Olvera de la Cruz M, Controlling protein adsorption modes electrostatically, Soft Matter. (2020). 10.1039/d0sm00632g. [DOI] [PubMed] [Google Scholar]
- [161].Hühn D, Kantner K, Geidel C, Brandholt S, De Cock I, Soenen SJH, Riveragil P, Montenegro JM, Braeckmans K, Müllen K, Nienhaus GU, Klapper M, Parak WJ, Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge, ACS Nano. (2013). 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- [162].Zhou HX, Pang X, Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation, Chem. Rev (2018). 10.1021/acs.chemrev.7b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Guseman AJ, Speer SL, Perez Goncalves GM, Pielak GJ, Surface Charge Modulates Protein-Protein Interactions in Physiologically Relevant Environments, Biochemistry. (2018). 10.1021/acs.biochem.8b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Jokinen V, Kankuri E, Hoshian S, Franssila S, Ras RHA, Superhydrophobic Blood-Repellent Surfaces, Adv. Mater 30 (2018). 10.1002/adma.201705104. [DOI] [PubMed] [Google Scholar]
- [165].Anderson H, Llopis-Hernandez V, Sweeten P, Donnelly H, Gurden R, Orapiriyakul W, Salmeron-Sanchez M, Dalby MJ, Tsimbouri MP, 4.11 Nanoscale Surface Cues and Cell Behavior, Elsevier, 2017. 10.1016/B978-0-12-803581-8.10226-7. [DOI] [Google Scholar]
- [166].McNamara LE, McMurray RJ, Dalby MJ, Tsimbouri PM, Surfaces and Cell Behavior, Elsevier, 2011. 10.1016/B978-0-08-055294-1.00010-6. [DOI] [Google Scholar]
- [167].Di Cio S, Gautrot JE, Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype, Acta Biomater. 30 (2016) 26–48. 10.1016/j.actbio.2015.11.027. [DOI] [PubMed] [Google Scholar]
- [168].Robotti F, Bottan S, Fraschetti F, Mallone A, Pellegrini G, Lindenblatt N, Starck C, Falk V, Poulikakos D, Ferrari A, A micron-scale surface topography design reducing cell adhesion to implanted materials, Sci. Rep 8 (2018) 10887. 10.1038/s41598-018-29167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ross AM, Jiang Z, Bastmeyer M, Lahann J, Physical aspects of cell culture substrates: Topography, roughness, and elasticity, Small. 8 (2012) 336–355. 10.1002/smll.201100934. [DOI] [PubMed] [Google Scholar]
- [170].Ruprecht V, Monzo P, Ravasio A, Yue Z, Makhija E, Strale PO, Gauthier N, Shivashankar GV, Studer V, Albiges-Rizo C, Viasnoff V, How cells respond to environmental cues – insights from bio-functionalized substrates, J. Cell Sci 130 (2016) 51–61. 10.1242/jcs.196162. [DOI] [PubMed] [Google Scholar]
- [171].Damodaran VB, Murthy SN, Bio-inspired strategies for designing antifouling biomaterials, Biomater. Res (2016). 10.1186/s40824-016-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Li J, Liang JY, Laken SJ, Langer R, Traverso G, Clinical Opportunities for Continuous Biosensing and Closed-Loop Therapies, Trends Chem. 2 (2020) 319–340. 10.1016/j.trechm.2020.02.009. [DOI] [Google Scholar]
- [173].Le LV, Mkrtschjan MA, Russell B, Desai TA, Hang on tight: reprogramming the cell with microstructural cues, Biomed. Microdevices 21 (2019) 1–17. 10.1007/s10544-019-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Smith Q, Gerecht S, Stem Cell Fate Is a Touchy Subject, Cell Stem Cell. 19 (2016) 289–290. 10.1016/j.stem.2016.08.015. [DOI] [PubMed] [Google Scholar]
- [175].Mahon OR, Browe DC, Gonzalez-Fernandez T, Pitacco P, Whelan IT, Von Euw S, Hobbs C, Nicolosi V, Cunningham KT, Mills KHG, Kelly DJ, Dunne A, Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner, Biomaterials. (2020). 10.1016/j.biomaterials.2020.119833. [DOI] [PubMed] [Google Scholar]
- [176].Karazisis D, Petronis S, Agheli H, Emanuelsson L, Norlindh B, Johansson A, Rasmusson L, Thomsen P, Omar O, The influence of controlled surface nanotopography on the early biological events of osseointegration, Acta Biomater. 53 (2017) 559–571. 10.1016/j.actbio.2017.02.026. [DOI] [PubMed] [Google Scholar]
- [177].Abagnale G, Sechi A, Steger M, Zhou Q, Kuo CC, Aydin G, Schalla C, Müller-Newen G, Zenke M, Costa IG, van Rijn P, Gillner A, Wagner W, Surface Topography Guides Morphology and Spatial Patterning of Induced Pluripotent Stem Cell Colonies, Stem Cell Reports. 9 (2017) 654–666. 10.1016/j.stemcr.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ermis M, Antmen E, Hasirci V, Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective, Bioact. Mater 3 (2018) 355–369. 10.1016/j.bioactmat.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Galdiero MR, Mantovani A, Macrophage Plasticity and Polarization: Relevance to Biomaterials, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00006-2. [DOI] [Google Scholar]
- [180].Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M, Macrophages, foreign body giant cells and their response to implantable biomaterials, Materials (Basel). 8 (2015) 5671–5701. 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lee JK, Choi IS, Oh TI, Lee E, Cell-Surface Engineering for Advanced Cell Therapy, Chem. - AEur. J (2018). 10.1002/chem.201801710. [DOI] [PubMed] [Google Scholar]
- [182].Londono R, Badylak SF, Factors Which Affect the Host Response to Biomaterials, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00001-3. [DOI] [Google Scholar]
- [183].Anderson HJ, Sahoo JK, Ulijn RV, Dalby MJ, Mesenchymal Stem Cell Fate: Applying Biomaterials for Control of Stem Cell Behavior, Front. Bioeng. Biotechnol 4 (2016) 38. 10.3389/fbioe.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Burugapalli K, Wijesuriya S, Wang N, Song W, Biomimetic electrospun coatings increase the in vivo sensitivity of implantable glucose biosensors, J. Biomed. Mater. Res. - Part A 106 (2018) 1072–1081. 10.1002/jbm.a.36308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Rigla M, Pons B, Rebasa P, Luna A, Pozo FJ, Caixàs A, Villaplana M, Subías D, Bella MR, Combalia N, Human Subcutaneous Tissue Response to Glucose Sensors: Macrophages Accumulation Impact on Sensor Accuracy, Diabetes Technol. Ther 20 (2018) 296–302. 10.1089/dia.2017.0321. [DOI] [PubMed] [Google Scholar]
- [186].Carnicer-Lombarte A, Barone DG, Dimov IB, Hamilton RS, Prater M, Zhao X, Rutz AL, Malliaras GG, Lacour SP, Bryant CE, Fawcett JW, Franze K, Mechanical matching of implant to host minimises foreign body reaction, Bioarxiv. (2019) 1–41. [Google Scholar]
- [187].Prasadh S, Ratheesh V, Wong R, Impact of biomaterial mechanics on cellular and molecular responses, in: Handb. Biomater. Biocompat, Elsevier, 2020: pp. 85–109. 10.1016/b978-0-08-102967-1.00006-2. [DOI] [Google Scholar]
- [188].Lenzini S, Devine D, Shin JW, Leveraging Biomaterial Mechanics to Improve Pluripotent Stem Cell Applications for Tissue Engineering, Front. Bioeng. Biotechnol 7 (2019) 260. 10.3389/fbioe.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Gilbert PM, Weaver VM, Cellular adaptation to biomechanical stress across length scales in tissue homeostasis and disease, Semin. Cell Dev. Biol (2017). 10.1016/j.semcdb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A, Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response, Trends Biotechnol. (2016). 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- [191].Wang L, Wang C, Wu S, Fan Y, Li X, Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges, Biomater. Sci 8 (2020) 2714–2733. 10.1039/d0bm00269k. [DOI] [PubMed] [Google Scholar]
- [192].Carver W, Goldsmith EC, Regulation of tissue fibrosis by the biomechanical environment, Biomed Res. Int (2013). 10.1155/2013/101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bergeron JJM, Di Guglielmo GM, Dahan S, Dominguez M, Posner BI, Spatial and Temporal Regulation of Receptor Tyrosine Kinase Activation and Intracellular Signal Transduction, Annu. Rev. Biochem 85 (2016) 573–597. 10.1146/annurev-biochem-060815-014659. [DOI] [PubMed] [Google Scholar]
- [194].Dharmarajan A, Floren M, Cox L, Ding Y, Johnson R, Tan W, Mechanochemical Effects on Extracellular Signal-Regulated Kinase Dynamics in Stem Cell Differentiation, Tissue Eng. - Part A 24 (2018) 1179–1189. 10.1089/ten.tea.2017.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Carver W, Esch AM, Fowlkes V, Goldsmith EC, The biomechanical environment and impact on tissue fibrosis, in: Immune Response to Implant. Mater. Devices Impact Immune Syst. Success an Implant, Springer International Publishing, 2016: pp. 169–188. 10.1007/978-3-319-45433-7_9. [DOI] [Google Scholar]
- [196].Farge E, Mechanotransduction in Development, 2011. 10.1016/B978-0-12-385065-2.00008-6. [DOI] [PubMed] [Google Scholar]
- [197].Chen W, Kim DH, Lim CT, Special Issue: Biomaterials for Cell Mechanobiology, ACS Biomater. Sci. Eng 5 (2019) 3685–3687. 10.1021/acsbiomaterials.9b01123. [DOI] [PubMed] [Google Scholar]
- [198].Zadpoor AA, Biomaterials and tissue biomechanics: A match made in heaven?, Materials (Basel). 10 (2017). 10.3390/ma10050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Burdick JA, García AJ, Special Issue: Biomaterials in Mechanobiology, Adv. Healthc. Mater 9 (2020) 2000412. 10.1002/adhm.202000412. [DOI] [PubMed] [Google Scholar]
- [200].Waldeck HM, Guerra AD, Kao WJ, Extracellular Matrix: Inspired Biomaterials, Compr. Biomater. II (2017) 132–146. 10.1016/B978-0-12-803581-8.10147-X. [DOI] [Google Scholar]
- [201].Jansen KA, Donato DM, Balcioglu HE, Schmidt T, Danen EHJ, Koenderink GH, A guide to mechanobiology: Where biology and physics meet, Biochim. Biophys. Acta - Mol. Cell Res 1853 (2015) 3043–3052. 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- [202].Janson IA, Putnam AJ, Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms, J. Biomed. Mater. Res. - Part A 103 (2015) 1246–1258. 10.1002/jbm.a.35254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Paul CD, Hruska A, Staunton JR, Burr HA, Jiang N, Tanner K, Kim J, Decoupling cellular response to topography and stiffness in three dimensions, Food, Pharm. Bioeng. Div 2018 - Core Program. Area 2018 AIChE Annu. Meet. 2 (2018) 617–618. [Google Scholar]
- [204].Jansen KA, Atherton P, Ballestrem C, Mechanotransduction at the cell-matrix interface, Semin. Cell Dev. Biol 71 (2017) 75–83. 10.1016/j.semcdb.2017.07.027. [DOI] [PubMed] [Google Scholar]
- [205].Helton KL, Ratner BD, Wisniewski NA, Biomechanics of the sensor-tissue interface - Effects of motion, pressure, and design on sensor performance and the foreign body response - Part I: Theoretical framework, in: J. Diabetes Sci. Technol, 2011. 10.1177/193229681100500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ekdahl KN, Lambris JD, Elwing H, Ricklin D, Nilsson PH, Teramura Y, Nicholls IA, Nilsson B, Innate immunity activation on biomaterial surfaces: A mechanistic model and coping strategies, Adv. Drug Deliv. Rev 63 (2011) 1042–1050. 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Brennan TV, Lunsford KE, Kuo PC, Innate pathways of immune activation in transplantation., J. Transplant 2010 (2010). 10.1155/2010/826240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Christo SN, Diener KR, Bachhuka A, Vasilev K, Hayball JD, Innate Immunity and Biomaterials at the Nexus: Friends or Foes, Biomed Res. Int (2015). 10.1155/2015/342304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Andorko JI, Jewell CM, Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine, Bioeng. Transl. Med (2017). 10.1002/btm2.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Dellacherie MO, Seo BR, Mooney DJ, Macroscale biomaterials strategies for local immunomodulation, Nat. Rev. Mater 4 (2019) 379–397. 10.1038/s41578-019-0106-3. [DOI] [Google Scholar]
- [211].Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Semin. Immunol 20 (2008) 86–100. 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M, Localized immunosuppressive environment in the foreign body response to implanted biomaterials, Am. J. Pathol 175 (2009) 161–170. 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kelly SH, Shores LS, Votaw NL, Collier JH, Biomaterial strategies for generating therapeutic immune responses, Adv. Drug Deliv. Rev (2017). 10.1016/j.addr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Swartzlander MD, Blakney AK, Amer LD, Hankenson KD, Kyriakides TR, Bryant SJ, Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels, Biomaterials. (2015). 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Gattazzo F, Urciuolo A, Bonaldo P, Extracellular matrix: A dynamic microenvironment for stem cell niche, Biochim. Biophys. Acta - Gen. Subj 1840 (2014) 2506–2519. 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Galvan FR, Barranco V, Galvan JC, Batlle S, Sebastian FeliuFajardo, García, Biomaterials for Tissue Engineering Applications in Diabetes Mellitus, Intech. i (2016) 13. [Google Scholar]
- [217].Walters NJ, Gentleman E, Evolving insights in cell-matrix interactions: Elucidating how non-soluble properties of the extracellular niche direct stem cell fate, Acta Biomater. 11 (2015) 3–16. 10.1016/j.actbio.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Mariani E, Lisignoli G, Borzì RM, Pulsatelli L, Biomaterials: Foreign bodies or tuners for the immune response?, Int. J. Mol. Sci (2019). 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lewis JS, Roy K, Keselowsky BG, Materials that harness and modulate the immune system, MRS Bull. (2014). 10.1557/mrs.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].The Immune Response to Implanted Materials and Devices, 2017. 10.1007/978-3-319-45433-7. [DOI] [Google Scholar]
- [221].Lopresti ST, Brown BN, Host Response to Naturally Derived Biomaterials, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00004-9. [DOI] [Google Scholar]
- [222].Sarkar K, Xue Y, Sant S, Host response to synthetic versus natural biomaterials, in: Immune Response to Implant. Mater. Devices Impact Immune Syst. Success an Implant, 2016. 10.1007/978-3-319-45433-7_5. [DOI] [Google Scholar]
- [223].Slaughter G, Improving the Biocompatibility of Implantable Bioelectronics Devices, Implant. Bioelectron 9783527335 (2014) 265–283. 10.1002/9783527673148.ch13. [DOI] [Google Scholar]
- [224].Gasperini L, Mano JF, Reis RL, Natural polymers for the microencapsulation of cells, J. R. Soc. Interface 11 (2014) 20140817–20140817. 10.1098/rsif.2014.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Marchioli G, Van Gurp L, Van Krieken PP, Stamatialis D, Engelse M, Van Blitterswijk CA, Karperien MBJ, De Koning E, Alblas J, Moroni L, Van Apeldoom AA, Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation, Biofabrication. 7 (2015). 10.1088/1758-5090/7/2/025009. [DOI] [PubMed] [Google Scholar]
- [226].De Vos P, Lazarjani HA, Poncelet D, Faas MM, Polymers in cell encapsulation from an enveloped cell perspective, Adv. Drug Deliv. Rev 67–68 (2014) 15–34. 10.1016/j.addr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- [227].Stabler CL, Li Y, Stewart JM, Keselowsky BG, Engineering immunomodulatory biomaterials for type 1 diabetes, Nat. Rev. Mater 4 (2019) 429–450. 10.1038/s41578-019-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].De Vries R, Stell A, Mohammed S, Hermanns C, Martinez AH, Jetten M, van Apeldoorn A, Bioengineering, biomaterials, and β-cell replacement therapy, in: Transplantation, Bioeng. Regen. Endocr. Pancreas, 2020. 10.1016/b978-0-12-814831-0.00033-6. [DOI] [Google Scholar]
- [229].Adly N, Weidlich S, Seyock S, Brings F, Yakushenko A, Offenhäusser A, Wolfrum B, Printed microelectrode arrays on soft materials: from PDMS to hydrogels, Npj Flex. Electron 2 (2018) 1–9. 10.1038/s41528-018-0027-z. [DOI] [Google Scholar]
- [230].Kumar M, Nandi SK, Kaplan DL, Mandal BB, Localized Immunomodulatory Silk Macrocapsules for Islet-like Spheroid Formation and Sustained Insulin Production, ACS Biomater. Sci. Eng (2017). 10.1021/acsbiomaterials.7b00218. [DOI] [PubMed] [Google Scholar]
- [231].Song S, Roy S, Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: Cells, biomaterials, and devices, Biotechnol. Bioeng 113 (2016) 1381–1402. 10.1002/bit.25895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, Yeh CC, Xing Y, Isa D, Ghani S, Li J, Landry C, Bader AR, Olejnik K, Chen M, Hollister-Lock J, Wang Y, Greiner DL, Weir GC, Strand BL, Rokstad AMA, Lacik I, Langer R, Anderson DG, Oberholzer J, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques, Nat. Biomed. Eng 2 (2018). 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Marchioli G, Di Luca A, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, Van Apeldoorn AA, Moroni L, Hybrid Polycaprolactone/Alginate Scaffolds Functionalized with VEGF to Promote de Novo Vessel Formation for the Transplantation of Islets of Langerhans, Adv. Healthc. Mater 5 (2016) 1606–1616. 10.1002/adhm.201600058. [DOI] [PubMed] [Google Scholar]
- [234].Strand BL, Coron AE, Skjak-Braek G, Current and future perspectives on alginate encapsulated pancreatic islet, Stem Cells Transl. Med 6 (2017) 1053–1058. 10.1002/sctm.16-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Llacua LA, Hoek A, de Haan BJ, de Vos P, Collagen type VI interaction improves human islet survival in immunoisolating microcapsules for treatment of diabetes, Islets. 10 (2018). 10.1080/19382014.2017.1420449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Taraballi F, Corradetti B, Minardi S, Powel S, Cabrera F, Van Eps JL, Weiner BK, Tasciotti E, Biomimetic collagenous scaffold to tune inflammation by targeting macrophages, J. Tissue Eng (2016). 10.1177/2041731415624667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Llacua LA, Faas MM, de Vos P, Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets, Diabetologia. 61 (2018). 10.1007/s00125-017-4524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Llacua LA, de Haan BJ, de Vos P, Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets, J. Tissue Eng. Regen. Med (2018). 10.1002/term.2472. [DOI] [PubMed] [Google Scholar]
- [239].Zakeri Siavashani A, Mohammadi J, Maniura-Weber K, Senturk B, Nourmohammadi J, Sadeghi B, Huber L, Rottmar M, Silk based scaffolds with immunomodulatory capacity: Anti-inflammatory effects of nicotinic acid, Biomater. Sci (2020). 10.1039/c9bm00814d. [DOI] [PubMed] [Google Scholar]
- [240].Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, Marelli B, Mitropoulos AN, Omenetto FG, Kaplan DL, Highly Tunable Elastomeric Silk Biomaterials, Adv. Funct. Mater 24 (2014) 4615–4624. 10.1002/adfim.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Fishman JM, Wiles K, Wood KJ, The Acquired Immune System Response to Biomaterials, Including Both Naturally Occurring and Synthetic Biomaterials, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00008-6. [DOI] [Google Scholar]
- [242].Nyitray CE, Chang R, Faleo G, Lance KD, Bernards DA, Tang Q, Desai TA, Polycaprolactone Thin-Film Micro- and Nanoporous Cell-Encapsulation Devices, ACS Nano. 9 (2015) 5675–5682. 10.1021/acsnano.5b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Rios PD, Skoumal M, Liu J, Youngblood R, Kniazeva E, Garcia AJ, Shea LD, Evaluation of encapsulating and microporous nondegradable hydrogel scaffold designs on islet engraftment in rodent models of diabetes, Biotechnol. Bioeng (2018). 10.1002/bit.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Manzoli V, Villa C, Bayer AL, Morales LC, Molano RD, Torrente Y, Ricordi C, Hubbell JA, Tomei AA, Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol, Am. J. Transplant 18 (2018). 10.1111/ajt.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Bobrowski T, Schuhmann W, Long-term implantable glucose biosensors, Curr. Opin. Electrochem 10(2018) 112–119. 10.1016/j.coelec.2018.05.004. [DOI] [Google Scholar]
- [246].Qi M, Transplantation of Encapsulated Pancreatic Islets as a Treatment for Patients with Type 1 Diabetes Mellitus, Adv. Med 2014 (2014) 1–15. 10.1155/2014/429710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Wang Y, Papadimitrakopoulos F, Burgess DJ, Polymeric “smart” coatings to prevent foreign body response to implantable biosensors, J. Control. Release 169 (2013) 341–347. 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- [248].Kette F, Rojas-Canales D, Drogemuller C, McInnes S, Toby Coates P, Modification of Polyurethane Scaffolds for Localised Immunosuppression of Subcutaneous Islet Transplantation., Transplantation. 102 (2018) S77. 10.1097/01.tp.0000542659.73899.92. [DOI] [Google Scholar]
- [249].Soto RJ, Privett BJ, Schoenfisch MH, In vivo analytical performance of nitric oxide-releasing glucose biosensors, Anal. Chem 86 (2014) 7141–7149. 10.1021/ac5017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Skrzypek K, Groot Nibbelink M, Van Lente J, Buitinga M, Engelse MA, De Koning EJP, Karperien M, Van Apeldoorn A, Stamatialis D, Pancreatic islet macroencapsulation using microwell porous membranes, Sci. Rep 7 (2017) 9186. 10.1038/s41598-017-09647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Song J, Millman JR, Economic 3D-printing approach for transplantation of human stem cell-derived β-like cells, Biofabrication. 9 (2017). 10.1088/1758-5090/9/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, Scaglione F, Sabek OM, Horner P, Thekkedath U, Gaber OA, Grattoni A, 3D Printed Vascularized Device for Subcutaneous Transplantation of Human Islets, Biotechnol. J (2017). 10.1002/biot.201700169. [DOI] [PubMed] [Google Scholar]
- [253].Lecomte A, Descamps E, Bergaud C, A review on mechanical considerations for chronically-implanted neural probes, J. Neural Eng 15 (2018). 10.1088/1741-2552/aa8b4f. [DOI] [PubMed] [Google Scholar]
- [254].Richbourg NR, Peppas NA, Sikavitsas VI, Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications, J. Tissue Eng. Regen. Med (2019) 0–2. 10.1002/term.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Ward WK, A review of the foreign-body response to subcutaneously-implanted devices: The role of Macrophages and cytokines in biofouling and fibrosis, J. Diabetes Sci. Technol 2 (2008) 768–777. 10.1177/193229680800200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Peloso A, Citro A, Zoro T, Cobianchi L, Kahler-Quesada A, Bianchi CM, Andres A, Berishvili E, Piemonti L, Berney T, Toso C, Oldani G, Regenerative medicine and diabetes: Targeting the extracellular matrix beyond the stem cell approach and encapsulation technology, Front. Endocrinol. (Lausanne) 9 (2018) 1–9. 10.3389/fendo.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Badylak SF, Host response to biomaterials: The impact of host response on biomaterial selection, 2015. [Google Scholar]
- [258].Baker DW, Zhou J, Tang L, Methods Used to Evaluate the Host Responses to Medical Implants In Vivo, Elsevier Inc., 2015. 10.1016/B978-0-12-800196-7.00014-1. [DOI] [Google Scholar]
- [259].Hattori K, Sugiura S, Kanamori T, Scaffold fabrication in a perfusion culture microchamber array chip by O2 plasma bonding of poly(dimethylsiloxane) protected by a physical mask, Biomicrofluidics. 5 (2011) 022204. 10.1063/1.3576933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Caldeira J, Sousa A, Sousa DM, Barros D, Extracellular matrix constitution and function for tissue regeneration and repair, Pept. Proteins as Biomater. Tissue Regen. Repair (2018) 29–72. 10.1016/B978-0-08-100803-4.00002-4. [DOI] [Google Scholar]
- [261].Frantz C, Stewart KM, Weaver VM, No Title, 123 (2010). 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Nichols SP, Koh A, Brown NL, Rose MB, Sun B, Slomberg DL, Riccio DA, Klitzman B, Schoenfisch MH, The effect of nitric oxide surface flux on the foreign body response to subcutaneous implants, Biomaterials. 33 (2012) 6305–6312. 10.1016/j.biomaterials.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Laschke MW, Augustin V, Kleer S, Tschernig T, Menger MD, Locally applied macrophage-activating lipopeptide-2 (MALP-2) promotes early vascularization of implanted porous polyethylene (Medpor®), Acta Biomater. 10 (2014) 4661–4669. 10.1016/j.actbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- [264].Minardi S, Corradetti B, Taraballi F, Byun JH, Cabrera F, Liu X, Ferrari M, Weiner BK, Tasciotti E, IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response, Ann. Biomed. Eng (2016). 10.1007/s10439-016-1580-z. [DOI] [PubMed] [Google Scholar]
- [265].Kumar M, Gupta P, Bhattacharjee S, Nandi SK, Mandal BB, Immunomodulatory injectable silk hydrogels maintaining functional islets and promoting anti-inflammatory M2 macrophage polarization, Biomaterials. (2018). 10.1016/j.biomaterials.2018.09.037. [DOI] [PubMed] [Google Scholar]
- [266].Lee H, Song C, Baik S, Kim D, Hyeon T, Kim DH, Device-assisted transdermal drug delivery, Adv. Drug Deliv. Rev 127 (2018) 35–45. 10.1016/j.addr.2017.08.009. [DOI] [PubMed] [Google Scholar]
- [267].Lund T, Mangsbo SM, Scholz H, Gjorstrup P, Ttterman TH, Korsgren O, Foss A, Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro, Exp. Clin. Endocrinol. Diabetes (2010). 10.1055/s-0029-1241825. [DOI] [PubMed] [Google Scholar]
- [268].Vériter S, Gianello P, Igarashi Y, Beaurin G, Ghyselinck A, Aouassar N, Jordan B, Gallez B, Dufrane D, Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates., Cell Transplant. 23 (2014) 1349–64. 10.3727/096368913X663550. [DOI] [PubMed] [Google Scholar]
- [269].Yu Q, Zhang Y, Wang H, Brash J, Chen H, Anti-fouling bioactive surfaces, Acta Biomater. 7 (2011) 1550–1557. 10.1016/j.actbio.2010.12.021. [DOI] [PubMed] [Google Scholar]
- [270].Kasoju N, Pátíková A, Wawrzynska E, Vojtišková A, Sedlačik T, Kumorek M, Pop-Georgievski O, Sticová E, Kříž J, Kubies D, Bioengineering a pre-vascularized pouch for subsequent islet transplantation using VEGF-loaded polylactide capsules, Biomater. Sci 8 (2020). 10.1039/c9bm01280j. [DOI] [PubMed] [Google Scholar]
- [271].Smink AM, Li S, Swart DH, Hertsig DT, de Haan BJ, Kamps JAAM, Schwab L, van Apeldoorn AA, de Koning E, Faas MM, Lakey JRT, de Vos P, Stimulation of vascularization of a subcutaneous scaffold applicable for pancreatic islet-transplantation enhances immediate post-transplant islet graft function but not long-term normoglycemia, J. Biomed. Mater. Res. - Part A 105 (2017). 10.1002/jbm.a.36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Tom B, Foreign body giant cells in the foreign body reaction to implanted biomaterials , a systematic review, (n.d.) 1–24. [Google Scholar]
- [273].Le NN, Rose MB, Levinson H, Klitzman B, Implant healing in experimental animal models of diabetes, J. Diabetes Sci. Technol 5 (2011) 605–618. 10.1177/193229681100500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Atala A, Kasper FK, Mikos AG, Engineering Complex Tissues, 4 (2012) 1–11. [DOI] [PubMed] [Google Scholar]
- [275].Jiang K, Chaimov D, Patel SN, Liang JP, Wiggins SC, Samojlik MM, Rubiano A, Simmons CS, Stabler CL, 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture, Biomaterials. 198 (2019). 10.1016/j.biomaterials.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S, Long-term in vivo glucose monitoring using fluorescent hydrogel fibers, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 13399–13403. 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].McMurray RJ, Wann AKT, Thompson CL, Connelly JT, Knight MM, Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells, Sci. Rep 3 (2013) 25–28. 10.1038/srep03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Orive G, Emerich D, Khademhosseini A, Matsumoto S, Hernández RM, Pedraz JL, Desai T, Calafiore R, de Vos P, Engineering a Clinically Translatable Bioartificial Pancreas to Treat Type I Diabetes, Trends Biotechnol. 36 (2018) 445–456. 10.1016/j.tibtech.2018.01.007. [DOI] [PubMed] [Google Scholar]
- [279].Lotti F, Ranieri F, Vadalà G, Zollo L, Di Pino G, Invasive intraneural interfaces: Foreign body reaction issues, Front. Neurosci 11 (2017) 1–14. 10.3389/fnins.2017.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Zhao X, Irvine SA, Agrawal A, Cao Y, Lim PQ, Tan SY, Venkatraman SS, 3D patterned substrates for bioartificial blood vessels – The effect of hydrogels on aligned cells on a biomaterial surface, Acta Biomater. 26 (2015) 159–168. 10.1016/J.ACTBIO.2015.08.024. [DOI] [PubMed] [Google Scholar]
- [281].Lee Y, Matsushima N, Yada S, Nita S, Kodama T, Amberg G, Shiomi J, Revealing How Topography of Surface Microstructures Alters Capillary Spreading, Sci. Rep 9 (2019) 1–11. 10.1038/s41598-019-44243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Kondyurina I, Kondyurin A, Foreign body reaction ( immune respond ) for artificial implants can be avoided, (n.d.). [DOI] [PMC free article] [PubMed]
- [283].Hanson TL, Diaz-Botia CA, Kharazia V, Maharbiz MM, Sabes PN, The “sewing machine” for minimally invasive neural recording, BioRxiv. (2019) 578542. 10.1101/578542. [DOI] [Google Scholar]
- [284].del Campo A, Arzt E, Fabrication approaches for generating complex micro- and nanopatterns on polymeric surfaces, Chem. Rev 108 (2008) 911–945. 10.1021/cr050018y. [DOI] [PubMed] [Google Scholar]
- [285].Hoffman-Kim D, Mitchel JA, Bellamkonda RV, Topography, Cell Response, and Nerve Regeneration, Annu. Rev. Biomed. Eng 12 (2010) 203–231. 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Yoshinari M, Matsuzaka K, Inoue T, Surface modification by cold-plasma technique for dental implants—Bio-functionalization with binding pharmaceuticals, Jpn. Dent. Sci. Rev 47 (2011) 89–101. 10.1016/J.JDSR.2011.03.001. [DOI] [Google Scholar]
- [287].Wang K, Leong KW, Yang Y, Expanding nanopatterned substrates using stitch technique for nanotopographical modulation of cell behavior, J. Vis. Exp 2016 (2016) 6–11. 10.3791/54840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Qin D, Xia Y, Whitesides GM, Soft lithography for micro- and nanoscale patterning, Nat. Protoc 5 (2010) 491–502. 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- [289].Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT, Surface immobilization of neural adhesion molecule L1 for improving the biocompatibility of chronic neural probes: In vitro characterization, Acta Biomater. (2008). 10.1016/j.actbio.2008.02.028. [DOI] [PubMed] [Google Scholar]
- [290].Weaver JD, Headen DM, Hunckler MD, Coronel MM, Stabler CL, García AJ, Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation, Biomaterials. 172 (2018) 54–65. 10.1016/j.biomaterials.2018.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Editor S, Iby T, Biomaterials as Stem Cell Niche, 2010. 10.1007/9783642138935. [DOI] [Google Scholar]
- [292].Giraldo JA, Molano RD, Rengifo HR, Fotino C, Gattás-Asfura KM, Pileggi A, Stabler CL, The impact of cell surface PEGylation and short-course immunotherapy on islet graft survival in an allogeneic murine model, Acta Biomater. 49 (2017). 10.1016/j.actbio.2016.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Sun C, Miao J, Yan J, Yang K, Mao C, Ju J, Shen J, Applications of antibiofouling PEG-coating in electrochemical biosensors for determination of glucose in whole blood, Electrochim. Acta 89 (2013) 549–554. 10.1016/j.electacta.2012.11.005. [DOI] [Google Scholar]
- [294].Nugent WH, Sheppard FR, Dubick MA, Cestero RF, Darlington DN, Jubin R, Abuchowski A, Song BK, Microvascular and Systemic Impact of Resuscitation with PEGylated Carboxyhemoglobin-Based Oxygen Carrier or Hetastarch in a Rat Model of Transient Hemorrhagic Shock, Shock. (2020). 10.1097/SHK.0000000000001370. [DOI] [PubMed] [Google Scholar]
- [295].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, García AJ, Shirwan H, Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance, Nat. Mater 17 (2018) 732–739. 10.1038/s41563-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Skoumal M, Woodward KB, Zhao H, Wang F, Yolcu ES, Pearson RM, Hughes KR, García AJ, Shea LD, Shirwan H, Localized immune tolerance from FasL-functionalized PLG scaffolds, Biomaterials. 192 (2019) 271–281. 10.1016/j.biomaterials.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Lou S, Zhang X, Zhang J, Deng J, Kong D, Li C, Pancreatic islet surface bioengineering with a heparin-incorporated starPEG nanofilm, Mater. Sci. Eng. C 78 (2017). 10.1016/j.msec.2017.03.295. [DOI] [PubMed] [Google Scholar]
- [298].Wallace A, Albadawi H, Patel N, Khademhosseini A, Zhang YS, Naidu S, Knuttinen G, Oklu R, Anti-fouling strategies for central venous catheters, Cardiovasc. Diagn. Ther 7 (2017) S246–S257. 10.21037/cdt.2017.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Weis GCC, Assmann CE, Cadoná FC, Bonadiman B. da S.R., Alves A. de O., Machado AK, Duarte MMMF, da Cruz IBM, Costabeber IH, Immunomodulatory effect of mancozeb, chlorothalonil, and thiophanate methyl pesticides on macrophage cells, Ecotoxicol. Environ. Saf 182 (2019). 10.1016/j.ecoenv.2019.109420. [DOI] [PubMed] [Google Scholar]
- [300].Xu Y, Takai M, Ishihara K, Protein adsorption and cell adhesion on cationic, neutral, and anionic 2-methacryloyloxyethyl phosphorylcholine copolymer surfaces, Biomaterials. (2009). 10.1016/j.biomaterials.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [301].Goda T, Konno T, Takai M, Moro T, Ishihara K, Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization, Biomaterials. (2006). 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- [302].Ishihara K, Ando B, Takai M, Phosphorylcholine group-immobilized surface prepared on polydimethylsiloxane membrane by in situ reaction for its reduced biofouling, Nanobiotechnology. (2007). 10.1007/s12030-008-9006-0. [DOI] [Google Scholar]
- [303].Komatsu H, Gonzalez N, Salgado M, Cook CA, Li J, Rawson J, Omori K, Tai Y, Kandeel F, Mullen Y, A subcutaneous pancreatic islet transplantation platform using a clinically applicable, biodegradable Vicryl mesh scaffold - an experimental study, Transpl. Int 33 (2020) 806–818. 10.1111/tri.13607. [DOI] [PubMed] [Google Scholar]
- [304].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates, Nat. Mater 14 (2015) 643–651. 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Bhushan B, Nanotribology and Materials Characterization of MEMS/NEMS and BioMEMS/BioNEMS Materials and Devices, in: Springer Handb. Nanotechnol, Springer Berlin Heidelberg, 2007: pp. 1575–1638. 10.1007/978-3-540-29857-1_50. [DOI] [Google Scholar]
- [306].Wang GJ, Chen CL, Hsu SH, Chiang YL, Bio-MEMS fabricated artificial capillaries for tissue engineering, in: Microsyst. Technol, Springer, 2005: pp. 120–127. 10.1007/s00542-005-0017-7. [DOI] [Google Scholar]
- [307].Subramani K, Ahmed W, Fabrication of PEG Hydrogel Micropatterns by Soft-Photolithography and PEG Hydrogel as Guided Bone Regeneration Membrane in Dental Implantology, in: Emerg. Nanotechnologies Dent, Elsevier Inc., 2012: pp. 171–187. 10.1016/B978-1-4557-7862-1.00011-0. [DOI] [Google Scholar]
- [308].Cao Y, Zeng X, Cai Z, Duan J, Laser micro/nano-fabrication techniques and their applications in electronics11, in: Adv. Laser Mater. Process. Technol. Res. Appl, Elsevier Inc., 2010: pp. 629–670. 10.1533/9781845699819.7.629. [DOI] [Google Scholar]
- [309].Caldorera-Moore M, Peppas NA, Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems, Adv. Drug Deliv. Rev 61 (2009) 1391–1401. 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Engstrom DS, Porter B, Pacios M, Bhaskaran H, Additive nanomanufacturing - A review, J. Mater. Res 29 (2014) 1792–1816. 10.1557/jmr.2014.159. [DOI] [Google Scholar]
- [311].Steele JAM, Hallé JP, Poncelet D, Neufeld RJ, Therapeutic cell encapsulation techniques and applications in diabetes, Adv. Drug Deliv. Rev 67–68 (2014). 10.1016/j.addr.2013.09.015. [DOI] [PubMed] [Google Scholar]
- [312].Liu R, Qin Y, Wang H, Zhao Y, Hu Z, Wang S, The in vivo blood compatibility of bio-inspired small diameter vascular graft: Effect of submicron longitudinally aligned topography, BMC Cardiovasc. Disord 13 (2013). 10.1186/1471-2261-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Lee S-J, Nowicki M, Harris B, Zhang LG, Fabrication of a Highly Aligned Neural Scaffold via a Table Top Stereolithography 3D Printing and Electrospinning , Tissue Eng. Part A 23 (2017) 491–502. 10.1089/ten.tea.2016.0353. [DOI] [PubMed] [Google Scholar]
- [314].Semnani D, Naghashzargar E, Hadjianfar M, Manshadi FD, Mohammadi S, Karbasi S, Effaty F, Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering, 10.1080/00914037.2016.1190931. (2016). 10.1080/00914037.2016.1190931. [DOI] [Google Scholar]
- [315].Peres C, Matos AI, Conniot J, Sainz V, Zupančič E, Silva JM, Graça L, Sá Gaspar R, Préat V, Florindo HF, Poly(lactic acid)-based particulate systems are promising tools for immune modulation, Acta Biomater. (2017). 10.1016/j.actbio.2016.11.012. [DOI] [PubMed] [Google Scholar]
- [316].McCrea Z, Arnanthigo Y, Cryan SA, O’Dea S, A Novel Methodology for Bio-electrospraying Mesenchymal Stem Cells that Maintains Differentiation, Immunomodulatory and Pro-reparative Functions, J. Med. Biol. Eng 38 (2018) 497–513. 10.1007/s40846-017-0331-4. [DOI] [Google Scholar]
- [317].Joddar B, Sarang-Sieminski AL, Hogrebe NJ, Tennant CJ, Gooch KJ, 5.4 Biomaterials and the Microvasculature ☆, in: Compr. Biomater. II, Elsevier, 2017: pp. 67–87. 10.1016/B978-0-12-803581-8.09820-9. [DOI] [Google Scholar]
- [318].Hughes RA, Menumerov E, Neretina S, When lithography meets self-Assembly: A review of recent advances in the directed assembly of complex metal nanostructures on planar and textured surfaces, Nanotechnology. 28 (2017) 282002. 10.1088/1361-6528/aa77ce. [DOI] [PubMed] [Google Scholar]
- [319].Kharbikar BN, Kumar HS, Kr S, Srivastava R, Hollow silicon microneedle array based trans-epidermal antiemetic patch for efficient management of chemotherapy induced nausea and vomiting, in: Eggleton BJ, Palomba S (Eds.), Micro+Nano Mater. Devices, Syst, SPIE, 2015: p. 96682W. 10.1117/12.2207407. [DOI] [Google Scholar]
- [320].Jell G, Minelli C, Stevens MM, Biomaterial-related approaches: Surface structuring, in: Fundam. Tissue Eng. Regen. Med, Springer Berlin Heidelberg, 2009: pp. 469–484. 10.1007/978-3-540-77755-7_35. [DOI] [Google Scholar]
- [321].Helton KL, Ratner BD, Wisniewski NA, Biomechanics of the sensor-tissue interface - Effects of motion, pressure, and design on sensor performance and foreign body response - Part II: Examples and application, in: J. Diabetes Sci. Technol, 2011. 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution, Adv. Drug Deliv. Rev 67–68 (2014) 35–73. 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- [323].Toda S, Fattah A, Asawa K, Nakamura N, Ekdahl KN, Nilsson B, Teramura Y, Optimization of islet microencapsulation with thin polymer membranes for long-term stability, Micromachines. 10 (2019). 10.3390/mi10110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ, A review of the biocompatibility of implantable devices: Current challenges to overcome foreign body response, J. Diabetes Sci. Technol 2 (2008) 1003–1015. 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Cobelli C, Renard E, Kovatchev B, Artificial pancreas: Past, present, future, Diabetes. 60 (2011) 2672–2682. 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Rege NK, Phillips NFB, Weiss MA, Development of glucose-responsive “smart” insulin systems, Curr. Opin. Endocrinol. Diabetes Obes (2017). 10.1097/MED.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Pareta R, Mcquilling JP, Farney AC, Opara EC, 4 Bioartificial Pancreas: Evaluation of Crucial Barriers to Clinical Application, n.d. www.intechopen.com (accessed November 10, 2018). [Google Scholar]
- [328].Mason McClatchey P, McClain ES, Williams IM, Malabanan CM, James FD, Lord PC, Gregory JM, Cliffel DE, Wasserman DH, Fibrotic encapsulation is the dominant source of continuous glucose monitor delays, Diabetes. 68 (2019) 1892–1901. 10.2337/db19-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Yuan J, Wang K, Xia X, Highly ordered platinum-nanotubule arrays for amperometric glucose sensing, Adv. Funct. Mater 15 (2005) 803–809. 10.1002/adfm.200400321. [DOI] [Google Scholar]
- [330].Holt-Hindle P, Nigro S, Asmussen M, Chen A, Amperometric glucose sensor based on platinum-iridium nanomaterials, Electrochem. Commun 10 (2008) 1438–1441. 10.1016/j.elecom.2008.07.042. [DOI] [Google Scholar]
- [331].Lucisano JY, Routh TL, Lin JT, Gough DA, Glucose Monitoring in Individuals with Diabetes Using a Long-Term Implanted Sensor/Telemetry System and Model, IEEE Trans. Biomed. Eng 64 (2017) 1982–1993. 10.1109/TBME.2016.2619333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Lee H, Hong YJ, Baik S, Hyeon T, Kim DH, Enzyme-Based Glucose Sensor: From Invasive to Wearable Device, Adv. Healthc. Mater 7 (2018). 10.1002/adhm.201701150. [DOI] [PubMed] [Google Scholar]
- [333].Ahmad R, Tripathy N, Ahn MS, Bhat KS, Mahmoudi T, Wang Y, Yoo JY, Kwon DW, Yang HY, Hahn YB, Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode, Sci. Rep 7 (2017) 1–10. 10.1038/s41598-017-06064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Moser O, Münzker J, Korsatko S, Pachler C, Smolle K, Toller W, Augustin T, Plank J, Pieber TR, Mader JK, Ellmerer M, A prolonged run-in period of standard subcutaneous microdialysis ameliorates quality of interstitial glucose signal in patients after major cardiac surgery, Sci. Rep 8 (2018) 1–8. 10.1038/s41598-018-19768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI, A critical review of Glucose biosensors based on Carbon nanomaterials: Carbon nanotubes and graphene, Sensors (Switzerland). 12 (2012) 5996–6022. 10.3390/s120505996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Chen C, Zhao XL, Li ZH, Zhu ZG, Qian SH, Flewitt AJ, Current and emerging technology for continuous glucose monitoring, Sensors (Switzerland). 17 (2017). 10.3390/s17010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Elsherif M, Hassan MU, Yetisen AK, Butt H, Glucose Sensing with Phenylboronic Acid Functionalized Hydrogel-Based Optical Diffusers, ACS Nano. 12 (2018) 2283–2291. 10.1021/acsnano.7b07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Dong Y, Wang W, Veiseh O, Appel EA, Xue K, Webber MJ, Tang BC, Yang XW, Weir GC, Langer R, Anderson DG, Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid-Glucose Complexation, Langmuir. 32 (2016) 8743–8747. 10.1021/acs.langmuir.5b04755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Kajisa T, Sakata T, Glucose-responsive hydrogel electrode for biocompatible glucose transistor, Sci. Technol. Adv. Mater 18 (2017) 26–33. 10.1080/14686996.2016.1257344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Tanne J, Schäfer D, Khalid W, Parak WJ, Lisdat F, Light-controlled bioelectrochemical sensor based on CdSe/ZnS quantum dots, Anal. Chem 83 (2011) 7778–7785. 10.1021/ac201329u. [DOI] [PubMed] [Google Scholar]
- [341].Li Z, Dong C, Tang L, Zhu X, Chen H, Ren J, Aqueous synthesis of CdTe/CdS/ZnS quantum dots and their optical and chemical properties, Luminescence. 26 (2011) 439–448. 10.1002/bio.1250. [DOI] [PubMed] [Google Scholar]
- [342].Zhang C, Zhang Z, Yang Q, Chen W, Graphene-based Electrochemical Glucose Sensors: Fabrication and Sensing Properties, Electroanalysis. 30 (2018) 2504–2524. 10.1002/elan.201800522. [DOI] [Google Scholar]
- [343].Al-Sagur H, Komathi S, Khan MA, Gurek AG, Hassan A, A novel glucose sensor using lutetium phthalocyanine as redox mediator in reduced graphene oxide conducting polymer multifunctional hydrogel, Biosens. Bioelectron 92 (2017) 638–645. 10.1016/j.bios.2016.10.038. [DOI] [PubMed] [Google Scholar]
- [344].Olejnik A, Siuzdak K, Karczewski J, Grochowska K, A Flexible Nafion Coated Enzyme-free Glucose Sensor Based on Au-dimpled Ti Structures, Electroanalysis. 32 (2020) 323–332. 10.1002/elan.201900455. [DOI] [Google Scholar]
- [345].Chen D, Wang C, Chen W, Chen Y, Zhang JXJ, PVDF-Nafion nanomembranes coated microneedles for in vivo transcutaneous implantable glucose sensing, Biosens. Bioelectron 74 (2015) 1047–1052. 10.1016/j.bios.2015.07.036. [DOI] [PubMed] [Google Scholar]
- [346].Vaidya R, Atanasov P, Wilkins E, Effect of interference on the performance of glucose enzyme electrodes using Nafion® coatings, Med. Eng. Phys 17 (1995) 416–424. 10.1016/1350-4533(94)00006-U [DOI] [PubMed] [Google Scholar]
- [347].Nery EW, Kundys M, Jeleń PS, Jönsson-Niedziólka M, Electrochemical glucose sensing: Is there still room for improvement?, Anal. Chem 88 (2016) 11271–11282. 10.1021/acs.analchem.6b03151. [DOI] [PubMed] [Google Scholar]
- [348].Xie X, Doloff JC, Yesilyurt V, Sadraei A, McGarrigle JJ, Omami M, Veiseh O, Farah S, Isa D, Ghani S, Joshi I, Vegas A, Li J, Wang W, Bader A, Tam HH, Tao J, Chen HJ, Yang B, Williamson KA, Oberholzer J, Langer R, Anderson DG, Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer, Nat. Biomed. Eng 2 (2018) 894–906. 10.1038/s41551-018-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH, Biocompatible materials for continuous glucose monitoring devices, Chem. Rev 113 (2013) 2528–2549. 10.1021/cr300387j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Unruh RM, Roberts JR, Nichols SP, Gamsey S, Wisniewski NA, McShane MJ, Preclinical evaluation of Poly(HEMA-co-acrylamide) hydrogels encapsulating glucose oxidase and palladium benzoporphyrin as fully implantable glucose sensors, J. Diabetes Sci. Technol 9 (2015) 985–992. 10.1177/1932296815590439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Wang C, Yu B, Knudsen B, Harmon J, Moussy F, Moussy Y, Synthesis and performance of novel hydrogels coatings for implantable glucose sensors, Biomacromolecules. 9 (2008) 561–567. 10.1021/bm701102y. [DOI] [PubMed] [Google Scholar]
- [352].Keum DH, Kim SK, Koo J, Lee GH, Jeon C, Mok JW, Mun BH, Lee KJ, Kamrani E, Joo CK, Shin S, Sim JY, Myung D, Yun SH, Bao Z, Hahn SK, Wireless smart contact lens for diabetic diagnosis and therapy, Sci. Adv 6 (2020) eaba3252. 10.1126/sciadv.aba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Mugweru A, Clark BL, Pishko MV, Electrochemical sensor array for glucose monitoring fabricated by rapid immobilization of active glucose oxidase within photochemically polymerized hydrogels, J. Diabetes Sci. Technol 1 (2007) 366–371. 10.1177/193229680700100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Quinn CAP, Connor RE, Heller A, Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors, Biomaterials. 18 (1997) 1665–1670. 10.1016/S0142-9612(97)00125-7. [DOI] [PubMed] [Google Scholar]
- [355].Lin P, Lin CW, Mansour R, Gu F, Improving biocompatibility by surface modification techniques on implantable bioelectronics, Biosens. Bioelectron (2013). 10.1016/j.bios.2013.01.071. [DOI] [PubMed] [Google Scholar]
- [356].Song B, Zhang E, Han X, Zhu H, Shi Y, Cao Z, Engineering and Application Perspectives on Designing an Antimicrobial Surface, ACS Appl. Mater. Interfaces 12 (2020) 21330–21341. 10.1021/acsami.9b19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Wang Z, Li J, Jiang L, Xiao S, Liu Y, Luo J, Zwitterionic Hydrogel Incorporated Graphene Oxide Nanosheets with Improved Strength and Lubricity, Langmuir. 35 (2019) 11452–11462. 10.1021/acs.langmuir.9b01640. [DOI] [PubMed] [Google Scholar]
- [358].Chou YN, Chang Y, Wen TC, Applying thermosettable zwitterionic copolymers as general fouling-resistant and thermal-tolerant biomaterial interfaces, ACS Appl. Mater. Interfaces 7 (2015) 10096–100107. 10.1021/acsami.5b01756. [DOI] [PubMed] [Google Scholar]
- [359].Yang W, Xue H, Carr LR, Wang J, Jiang S, Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media, Biosens. Bioelectron 26 (2011) 2454–2459. 10.1016/j.bios.2010.10.031. [DOI] [PubMed] [Google Scholar]
- [360].Sun M, Qiu H, Su C, Shi X, Wang Z, Ye Y, Zhu Y, Solvent-Free Graft-From Polymerization of Polyvinylpyrrolidone Imparting Ultralow Bacterial Fouling and Improved Biocompatibility, ACS Appl. Bio Mater 2 (2019) 3983–3991. 10.1021/acsabm.9b00529. [DOI] [PubMed] [Google Scholar]
- [361].Koschwanez HE, Yap FY, Klitzman B, Reichert WM, In vitro and in vivo characterization of porous poly-L-lactic acid coatings for subcutaneously implanted glucose sensors, J. Biomed. Mater. Res. - Part A 87 (2008) 792–807. 10.1002/jbm.a.31824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Klueh U, Qiao Y, Czajkowski C, Ludzinska I, Antar O, Kreutzer DL, Basement membrane-based glucose sensor coatings enhance continuous glucose monitoring in vivo, J. Diabetes Sci. Technol 9 (2015) 957–965. 10.1177/1932296815598776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Klueh U, Ludzinska I, Czajkowski C, Qiao Y, Kreutzer DL, Crosslinked basement membrane-based coatings enhance glucose sensor function and continuous glucose monitoring in vivo, J. Biomed. Mater. Res. - Part A 106 (2018) 7–16. 10.1002/jbm.a.36206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Wang N, Burugapalli K, Wijesuriya S, Far MY, Song W, Moussy F, Zheng Y, Ma Y, Wu Z, Li K, Electrospun polyurethane-core and gelatin-shell coaxial fibre coatings for miniature implantable biosensors, Biofabrication. (2014). 10.1088/1758-5082/6/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Wang N, Burugapalli K, Song W, Halls J, Moussy F, Ray A, Zheng Y, Electrospun fibro-porous polyurethane coatings for implantable glucose biosensors, Biomaterials. (2013). 10.1016/j.biomaterials.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Lanzalaco S, Molina BG, Polymers and plastics modified electrodes for biosensors: A review, Molecules. 25 (2020). 10.3390/MOLECULES25102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Bridges AW, García AJ, Anti-inflammatory polymeric coatings for implantable biomaterials and devices, in: J. Diabetes Sci. Technol, SAGE Publications Inc., 2008: pp. 984–994. 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Locke AK, Means AK, Dong P, Nichols TJ, Coté GL, Grunlan MA, A Layer-by-Layer Approach to Retain a Fluorescent Glucose Sensing Assay within the Cavity of a Hydrogel Membrane, ACS Appl. Bio Mater 1 (2018) 1319–1327. 10.1021/acsabm.8b00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Fei R, Means AK, Abraham AA, Locke AK, Coté GL, Grunlan MA, Self-Cleaning, Thermoresponsive P(NIPAAm-co-AMPS) Double Network Membranes for Implanted Glucose Biosensors, Macromol. Mater. Eng 301 (2016) 935–943. 10.1002/mame.201600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Means AK, Dong P, Clubb FJ, Friedemann MC, Colvin LE, Shrode CA, Coté GL, Grunlan MA, A self-cleaning, mechanically robust membrane for minimizing the foreign body reaction: towards extending the lifetime of sub-Q glucose biosensors, J. Mater. Sci. Mater. Med 30 (2019) 79. 10.1007/s10856-019-6282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Xu J, Lee H, Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors, Chemosensors. 8 (2020) 66. 10.3390/chemosensors8030066. [DOI] [Google Scholar]
- [372].Bridges AW, García AJ, Anti-inflammatory polymeric coatings for implantable biomaterials and devices, in: J. Diabetes Sci. Technol, SAGE Publications Inc., 2008: pp. 984–994. 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Vallejo-Heligon SG, Klitzman B, Reichert WM, Characterization of porous, dexamethasone-releasing polyurethane coatings for glucose sensors, Acta Biomater. 10 (2014) 4629–4638. 10.1016/j.actbio.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Klueh U, Kaur M, Montrose DC, Kreutzer DL, Infammation and glucose sensors: Use of dexamethasone to extend glucose sensor function and life span in vivo, J. Diabetes Sci. Technol 1 (2007) 496–504. 10.1177/193229680700100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Welch NG, Winkler DA, Thissen H, Antifibrotic strategies for medical devices, Adv. Drug Deliv. Rev (2020). 10.1016/j.addr.2020.06.008. [DOI] [PubMed] [Google Scholar]
- [376].Avula M, Jones D, Rao AN, McClain D, McGill LD, Grainger DW, Solzbacher F, Local release of masitinib alters in vivo implantable continuous glucose sensor performance, Biosens. Bioelectron 77 (2016) 149–156. 10.1016/j.bios.2015.08.059. [DOI] [PubMed] [Google Scholar]
- [377].Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F, Modulation of the foreign body response to implanted sensor models through device-based delivery of the tyrosine kinase inhibitor, masitinib, Biomaterials. 34 (2013) 9737–9746. 10.1016/j.biomaterials.2013.08.090. [DOI] [PubMed] [Google Scholar]
- [378].Avula M, Jones D, Rao AN, McClain D, McGill LD, Grainger DW, Solzbacher F, Local release of masitinib alters in vivo implantable continuous glucose sensor performance, Biosens. Bioelectron 77 (2016) 149–156. 10.1016/j.bios.2015.08.059. [DOI] [PubMed] [Google Scholar]
- [379].Soto RJ, Privett BJ, Schoenfisch MH, In vivo analytical performance of nitric oxide-releasing glucose biosensors, Anal. Chem 86 (2014) 7141–7149. 10.1021/ac5017425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Cha KH, Meyerhoff ME, Compatibility of Nitric Oxide Release with Implantable Enzymatic Glucose Sensors Based on Osmium (III/II) Mediated Electrochemistry, ACS Sensors. 2 (2017) 1262–1266. 10.1021/acssensors.7b00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Cha KH, Wang X, Meyerhoff ME, Nitric oxide release for improving performance of implantable chemical sensors – A review, Appl. Mater. Today. 9 (2017) 589–597. 10.1016/j.apmt.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Zhang WJ, Laue C, Hyder A, Schrezenmeir J, Purity of alginate affects the viability and fibrotic overgrowth of encapsulated porcine islet xenografts, Transplant. Proc (2001). 10.1016/S0041-1345(01)02419-8. [DOI] [PubMed] [Google Scholar]
- [383].Mallett AG, Korbutt GS, Alginate modification improves long-term survival and function of transplanted encapsulated islets, Tissue Eng. Part A 15 (2009) 1301–1309. 10.1089/ten.tea.2008.0118. [DOI] [PubMed] [Google Scholar]
- [384].Kulseng B, Skjåk-Braek G, Ryan L, Andersson A, King A, Faxvaag A, Espevik T, Transplantation of alginate microcapsules: generation of antibodies against alginates and encapsulated porcine islet-like cell clusters, Transplantation. 67 (1999) 978–984. 10.1097/00007890-199904150-00008. [DOI] [PubMed] [Google Scholar]
- [385].Villa C, Manzoli V, Abreu MM, Verheyen CA, Seskin M, Najjar M, Molano RD, Torrente Y, Ricordi C, Tomei AA, Effects of Composition of Alginate-Polyethylene Glycol Microcapsules and Transplant Site on Encapsulated Islet Graft Outcomes in Mice, Transplantation. 101 (2017) 1025–1035. 10.1097/TP.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].S. S, F. Pj, K. O, H. T, S. Ta, L. Ha, B. J, P. R, W. Mm, Biocompatibility of Alginates for Grafting: Impact of Alginate Molecular Weight, Artif. Cells. Blood Substit. Immobil. Biotechnol 31 (2003). 10.1081/bio-120025409. [DOI] [PubMed] [Google Scholar]
- [387].Lum ZP, Krestow M, Tai IT, Vacek I, Sun AM, Xenografts of rat islets into diabetic mice. An evaluation of new smaller capsules, Transplantation. 53 (1992) 1180–1183. 10.1097/00007890-199206000-00002. [DOI] [PubMed] [Google Scholar]
- [388].Schneider S, Feilen PJ, Slotty V, Kampfner D, Preuss S, Berger S, Beyer J, Pommersheim R, Multilayer capsules: A promising microencapsulation system for transplantation of pancreatic islets, Biomaterials. 22 (2001) 1961–1970. 10.1016/S0142-9612(00)00380-X. [DOI] [PubMed] [Google Scholar]
- [389].Park H-S, Kim J-W, Lee S-H, Yang HK, Ham D-S, Sun C-L, Hong TH, Khang G, Park C-G, Yoon K-H, Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation, J. Tissue Eng. Regen. Med 11 (2017) 1274–1284. 10.1002/term.2029. [DOI] [PubMed] [Google Scholar]
- [390].Farah S, Doloff JC, Müller P, Sadraei A, Han HJ, Olafson K, Vyas K, Tam HH, Hollister-Lock J, Kowalski PS, Griffin M, Meng A, McAvoy M, Graham AC, McGarrigle J, Oberholzer J, Weir GC, Greiner DL, Langer R, Anderson DG, Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations, Nat. Mater 18 (2019). 10.1038/s41563-019-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner EL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat. Biotechnol 34 (2016) 345–352. 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Anselmo AC, Gokarn Y, Mitragotri S, Non-invasive delivery strategies for biologies, Nat. Rev. DrugDiscov 18 (2018) 19–40. 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- [393].An D, Chiu A, Flanders JA, Song W, Shou D, Lu Y-CC, Grunnet LG, Winkel L, Ingvorsen C, Christophersen NS, Fels JJ, Sand FW, Ji Y, Qi L, Pardo Y, Luo D, Silberstein M, Fan J, Ma M, Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes, Proc. Natl. Acad. Sci. U. S. A 115 (2017) E263–E272. 10.1073/pnas.1708806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice, Nat. Med 22 (2016) 306–311. 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Alagpulinsa DA, Cao JJL, Driscoll RK, Sîrbulescu RF, Penson MFE, Sremac M, Engquist N, Brauns TA, Markmann JF, Melton DA, Poznansky MC, Alginate-microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression, Am. J. Transplant 19 (2019) 1930–1940. 10.1111/ajt.15308. [DOI] [PubMed] [Google Scholar]
- [396].Citro A, Moser PT, Dugnani E, Rajab KT, Ren X, Evangelista-Leite D, Charest JM, Peloso A, Podesser BK, Manenti F, Pellegrini S, Piemonti L, Ott HC, Biofabrication of a vascularized islet organ for type 1 diabetes, Biomaterials. 199 (2019) 40–51. 10.1016/j.biomaterials.2019.01.035. [DOI] [PubMed] [Google Scholar]
- [397].Vaithilingam V, Kollarikova G, Qi M, Larsson R, Lacik I, Formo K, Marchese E, Oberholzer J, Guillemin GJ, Tuch BE, Beneficial effects of coating alginate microcapsules with macromolecular heparin conjugates-in vitro and in vivo study, Tissue Eng. Part A 20 (2014) 324–334. 10.1089/ten.TEA.2013.0254. [DOI] [PubMed] [Google Scholar]
- [398].Safley SA, Kenyon NS, Berman DM, Barber GF, Willman M, Duncanson S, Iwakoshi N, Holdcraft R, Gazda L, Thompson P, Badell IR, Sambanis A, Ricordi C, Weber CJ, Microencapsulated adult porcine islets transplanted intraperitoneally in streptozotocin-diabetic non-human primates, Xenotransplantation. 25 (2018). 10.1111/xen.12450. [DOI] [PubMed] [Google Scholar]
- [399].Yang HK, Ham D-S, Park H-S, Rhee M, You YH, Kim MJ, Shin J, Kim O-Y, Khang G, Hong TH, Kim J-W, Lee S-H, Cho J-H, Yoon K-H, Long-term Efficacy and Biocompatibility of Encapsulated Islet Transplantation With Chitosan-Coated Alginate Capsules in Mice and Canine Models of Diabetes, Transplantation. 100 (2016) 334–343. 10.1097/TP.0000000000000927. [DOI] [PubMed] [Google Scholar]
- [400].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat. Biotechnol 34 (2016) 345–352. 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, Yeh CC, Xing Y, Isa D, Ghani S, Li J, Landry C, Bader AR, Olejnik K, Chen M, Hollister-Lock J, Wang Y, Greiner DL, Weir GC, Strand BL, Rokstad AMA, Lacik I, Langer R, Anderson DG, Oberholzer J, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques, Nat. Biomed. Eng 2 (2018) 810–821. 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Liu Q, Chiu A, Wang LH, An D, Zhong M, Smink AM, de Haan BJ, de Vos P, Keane K, Vegge A, Chen EY, Song W, Liu WF, Flanders J, Rescan C, Grunnet LG, Wang X, Ma M, Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation, Nat. Commun 10 (2019) 1–14. 10.1038/s41467-019-13238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Liu Q, Chiu A, Wang L, An D, Li W, Chen EY, Zhang Y, Pardo Y, McDonough SP, Liu L, Liu WF, Chen J, Ma M, Developing mechanically robust, triazole-zwitterionic hydrogels to mitigate foreign body response (FBR) for islet encapsulation, Biomaterials. 230 (2020) 119640. 10.1016/j.biomaterials.2019.119640. [DOI] [PubMed] [Google Scholar]
- [404].Dang TT, Thai AV, Cohen J, Slosberg JE, Siniakowicz K, Doloff JC, Ma M, Hollister-Lock J, Tang KM, Gu Z, Cheng H, Weir GC, Langer R, Anderson DG, Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug, Biomaterials. 34 (2013) 5792–5801. 10.1016/j.biomaterials.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Vaithilingam V, Evans MDM, Lewy DM, Bean PA, Bal S, Tuch BE, Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted, Sci. Rep 7 (2017). 10.1038/s41598-017-10359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Luca G, Calafiore R, Basta G, Ricci M, Calvitti M, Neri L, Nastruzzi C, Becchetti E, Capitani S, Brunetti P, Rossi C, Improved function of rat islets upon co-microencapsulation with Sertoli’s cells in alginate/poly-L-ornithine, AAPS PharmSciTech. 2 (2001) E15. 10.1208/pt020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanaga M, Ko S, Nagao M, Nakajima Y, Indefinite islet protection from autoimmune destruction in nonobese diabetic mice by agarose microencapsulation without immunosuppression, Transplantation. 75 (2003) 619–625. 10.1097/01.TP.0000053749.36365.7E. [DOI] [PubMed] [Google Scholar]
- [408].Kobayashi T, Aomatsu H, Iwata T, Kin H, Kanehiro M, Hisanga S, Ko S, Nagao M, Harb G, Nakajima Y, Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice, Cell Transplant. 15 (2006) 359–365. 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- [409].Bratlie KM, York RL, Invernale MA, Langer RL, Anderson DG, Materials for diabetes therapeutics, Adv. Healthc. Mater (2012). 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Yin C, Chia SM, Quek CH, Yu H, Zhuo RX, Leong KW, Mao HQ, Microcapsules with improved mechanical stability for hepatocyte culture, Biomaterials. 24 (2003) 1771–1780. 10.1016/S0142-9612(02)00580-X. [DOI] [PubMed] [Google Scholar]
- [411].Peterson KP, Peterson CM, Pope EJA, Silica Sol-Gel Encapsulation of Pancreatic Islets, Exp. Biol. Med 218 (1998) 365–369. 10.3181/00379727-218-44305. [DOI] [PubMed] [Google Scholar]
- [412].Harrington S, Williams J, Rawal S, Ramachandran K, Stehno-Bittel L, Hyaluronic Acid/Collagen Hydrogel as an Alternative to Alginate for Long-Term Immunoprotected Islet Transplantation, Tissue Eng. - Part A 23 (2017) 1088–1099. 10.1089/ten.tea.2016.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [413].Wang T, Lacík I, Brissová M, Anilkumar AV, Prokop A, Hunkele D, Green R, Snahrokhi K, Powers AC, An Encapsulation System for the Immunoisolation of Pancreatic Islets, Nat. Biotechnol 15 (1997) 358–362. 10.1038/nbt0497-358. [DOI] [PubMed] [Google Scholar]
- [414].Lee DY, Park SJ, Nam JH, Byun Y, A combination therapy of PEGylation and immunosuppressive agent for successful islet transplantation, J. Control. Release 110 (2006) 290–295. 10.1016/j.jconrel.2005.10.023. [DOI] [PubMed] [Google Scholar]
- [415].Giraldo JA, Molano RD, Rengifo HR, Fotino C, Gattás-Asfura KM, Pileggi A, Stabler CL, The impact of cell surface PEGylation and short-course immunotherapy on islet graft survival in an allogeneic murine model, Acta Biomater. 49 (2017) 272–283. 10.1016/j.actbio.2016.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Stabler CL, Giraldo JA, Berman DM, Gattás-Asfura KM, Willman MA, Rabassa A, Geary J, Diaz W, Kenyon NM, Kenyon NS, Transplantation of PEGylated islets enhances therapeutic efficacy in a diabetic nonhuman primate model, Am. J. Transplant 20 (2020) 689–700. 10.1111/ajt.15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Panza JL, Wagner WR, Rilo HLR, Harsha Rao R, Beckman EJ, Russell AJ, Treatment of rat pancreatic islets with reactive PEG, Biomaterials. 21 (2000) 1155–1164. 10.1016/S0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- [418].Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA, Device design and materials optimization of conformal coating for islets of Langerhans, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 10514–10519. 10.1073/pnas.1402216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [419].Korsgren O, Islet encapsulation: Physiological possibilities and limitations, Diabetes. 66 (2017) 1748–1754. 10.2337/db17-0065. [DOI] [PubMed] [Google Scholar]
- [420].Wee YM, Lim DG, Kim YH, Kim JH, Kim SC, Yu ES, Park MO, Choi MY, Park YH, Jang HJ, Cho EY, Cho MH, Han DJ, Cell surface modification by activated polyethylene glycol prevents allosensitization after islet transplantation, Cell Transplant. 17(2008) 1257–1269. 10.3727/096368908787236657. [DOI] [PubMed] [Google Scholar]
- [421].Jeong JH, Yook S, Hwang JW, Jung MJ, Moon HT, Lee DY, Byun Y, Synergistic effect of surface modification with poly(ethylene glycol) and immunosuppressants on repetitive pancreatic islet transplantation into antecedently sensitized rat, Transplant. Proc 45 (2013) 585–590. 10.1016/j.transproceed.2012.02.028. [DOI] [PubMed] [Google Scholar]
- [422].Dong YL, Sang JP, Lee S, Jong HN, Byun Y, Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication, Tissue Eng. 13 (2007)2133–2141. 10.1089/ten.2006.0009. [DOI] [PubMed] [Google Scholar]
- [423].Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, Adams DB, Gao W, Wang H, Immuno-Isolation of Pancreatic Islet Allografts Using Pegylated Nanotherapy Leads to Long-Term Normoglycemia in Full MHC Mismatch Recipient Mice, PLoS One. 7 (2012) e50265. 10.1371/journal.pone.0050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [424].Vaithilingam V, Bal S, Tuch BE, Encapsulated islet transplantation: Where do we stand?, Rev. Diabet. Stud 14 (2017). 10.1900/RDS.2017.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].King A, Microencapsulation of islets of Langerhans: impact of cellular overgrowth, Ups. J. Med. Sci (2001). 10.3109/2000-1967-140. [DOI] [PubMed] [Google Scholar]
- [426].Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A, The theracyte™ device protects against islet allograft rejection in immunized hosts, Cell Transplant. 22 (2013) 1137–1146. 10.3727/096368912X657486. [DOI] [PubMed] [Google Scholar]
- [427].Chendke GS, Faleo G, Juang C, Parent AV, Bernards DA, Hebrok M, Tang Q, Desai TA, Supporting Survival of Transplanted Stem-Cell-Derived Insulin-Producing Cells in an Encapsulation Device Augmented with Controlled Release of Amino Acids, Adv. Biosyst 3 (2019). 10.1002/adbi.201900086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [428].Chang R, Faleo G, Russ HA, Parent AV, Elledge SK, Bernards DA, Allen JL, Villanueva K, Hebrok M, Tang Q, Desai TA, Nanoporous Immunoprotective Device for Stem-Cell-Derived β-Cell Replacement Therapy, ACS Nano. 11 (2017) 7747–7757. 10.1021/acsnano.7b01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Nyitray CE, Chang R, Faleo G, Lance KD, Bernards DA, Tang Q, Desai TA, Polycaprolactone Thin-Film Micro- and Nanoporous Cell-Encapsulation Devices, ACS Nano. 9 (2015) 5675–5682. 10.1021/acsnano.5b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A, Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal, J. Cell. Physiol 226 (2011) 1813–1819. 10.1002/jcp.22515. [DOI] [PubMed] [Google Scholar]
- [431].Bose S, Volpatti LR, Thiono D, Yesilyurt V, McGladrigan C, Tang Y, Facklam A, Wang A, Jhunjhunwala S, Veiseh O, Hollister-Lock J, Bhattacharya C, Weir GC, Greiner DL, Langer R, Anderson DG, A retrievable implant for the long-term encapsulation and survival of therapeutic xenogeneic cells, Nat. Biomed. Eng 4 (2020) 814–826. 10.1038/s41551-020-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].An D, Ji Y, Chiu A, Lu YC, Song W, Zhai L, Qi L, Luo D, Ma M, Developing robust, hydrogel-based, nanofiber-enabled encapsulation devices (NEEDs) for cell therapies, Biomaterials. 37 (2015) 40–48. 10.1016/j.biomaterials.2014.10.032. [DOI] [PubMed] [Google Scholar]
- [433].Daoud J, Rosenberg L, Tabrizian M, Pancreatic islet culture and preservation strategies: Advances, challenges, and future outlook, Cell Transplant. 19 (2010) 1523–1535. 10.3727/096368910X515872. [DOI] [PubMed] [Google Scholar]
- [434].Saleem S, Li J, Yee SP, Fellows GF, Goodyer CG, Wang R, β1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival, J. Pathol 219 (2009) 182–192. 10.1002/path.2577. [DOI] [PubMed] [Google Scholar]
- [435].Montesano R, Mouron P, Amherdt M, Orci L, Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids, J. Cell Biol 97 (1983) 935–939. 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [436].Weber LM, Hayda KN, Anseth KS, Cell–Matrix Interactions Improve β-Cell Survival and Insulin Secretion in Three-Dimensional Culture, Tissue Eng. Part A 14 (2008) 1959–1968. 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [437].Stephens CH, Orr KS, Acton AJ, Tersey SA, Mirmira RG, Considine RV, Voytik-Harbin SL, In situ type I oligomeric collagen macroencapsulation promotes islet longevity and function in vitro and in vivo, Am. J. Physiol. - Endocrinol. Metab 315 (2018) E650–E661. 10.1152/ajpendo.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [438].Rios PD, Zhang X, Luo X, Shea LD, Mold-casted non-degradable, islet macro-encapsulating hydrogel devices for restoration of normoglycemia in diabetic mice, Biotechnol. Bioeng (2016). 10.1002/bit.26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S, An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport, Lab Chip. 17(2017) 1778–1792. 10.1039/c7lc00096k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Nilsson B, Ekdahl KN, Korsgren O, Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment, Curr. Opin. organ Transplant 16 (2011) 620–626. 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- [441].Schweicher J, Nyitray C, Desai TA, Membranes to achieve immunoprotection of transplanted islets, Front. Biosci. - Landmark 19 (2014) 49–76. 10.2741/4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [442].La Flamme KE, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA, Biocompatibility of nanoporous alumina membranes for immunoisolation, Biomaterials. 28 (2007) 2638–2645. 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [443].Mendelsohn A, Desai T, Inorganic nanoporous membranes for immunoisolated cell-based drug delivery, Adv. Exp. Med. Biol 670 (2010) 104–125. 10.1007/978-1-4419-5786-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [444].Kwon J, Trivedi K, Krishnamurthy NV, Hu W, Lee J-B, Gimi B, SU-8-based immunoisolative microcontainer with nanoslots defined by nanoimprint lithography, J. Vac. Sci. Technol. B Microelectron. Nanom. Struct 27 (2009) 2795. 10.1116/1.3258146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Iqbal Z, Moses W, Kim S, Kim EJ, Fissell WH, Roy S, Sterilization effects on ultrathin film polymer coatings for silicon-based implantable medical devices, J. Biomed. Mater. Res. Part B Appl. Biomater 106 (2018) 2327–2336. 10.1002/jbm.b.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Francolini I, Piozzi A, Antimicrobial Polyurethanes for Intravascular Medical Devices, in: Adv. Polyurethane Biomater, Elsevier Inc., 2016: pp. 349–385. 10.1016/B978-0-08-100614-6.00012-3. [DOI] [Google Scholar]
- [447].Rickels MR, Schutta MH, Markmann JF, Barker CF, Naji A, Teff KL, β-Cell function following human islet transplantation for type 1 diabetes, Diabetes. 54 (2005) 100–106. 10.2337/diabetes.54.1.100. [DOI] [PubMed] [Google Scholar]
- [448].Bogdan C, Röllinghoff M, Diefenbach A, The role of nitric oxide in innate immunity, Immunol. Rev (2000). 10.1034/j.1600-065X.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- [449].Hashimoto H, Olson EN, Bassel-Duby R, Therapeutic approaches for cardiac regeneration and repair, Nat. Rev. Cardiol 15 (2018) 585–600. 10.1038/s41569-018-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [450].Carter CS, Giovaninni S, Seo DO, Dupree J, Morgan D, Chung HY, Lees H, Daniels M, Hubbard GB, Lee S, Ikeno Y, Foster TC, Buford TW, Marzetti E, Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 x Brown Norway rats, Age (Omaha). 33 (2011) 167–183. 10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Johansson U, Ria M, Åvail K, Dekki Shalaly N, Zaitsev SV, Berggren P-O, Hedhammar M, Pancreatic Islet Survival and Engraftment Is Promoted by Culture on Functionalized Spider Silk Matrices, PLoS One. 10 (2015) e0130169. 10.1371/journal.pone.0130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [452].Skrzypek K, Groot Nibbelink M, Van Lente J, Buitinga M, Engelse MA, De Koning EJP, Karperien M, Van Apeldoorn A, Stamatialis D, Pancreatic islet macroencapsulation using microwell porous membranes, Sci. Rep 7 (2017) 1–12. 10.1038/s41598-017-09647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [453].Moberg L, Olsson A, Berne C, Felldin M, Foss A, K??llen R, Salmela K, Tibell A, Tufveson G, Nilsson B, Korsgren O, Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: implications for clinical islet transplantation1, Transplantation. 76 (2003) 1285–1288. 10.1097/01.TP.0000098905.86445.0F. [DOI] [PubMed] [Google Scholar]
- [454].Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O, Inflammatory mediators expressed in human islets of Langerhans: Implications for islet transplantation, Biochem. Biophys. Res. Commun (2003). 10.1016/S0006-291X(03)01392-5. [DOI] [PubMed] [Google Scholar]
- [455].Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernández JA, Griffin JH, Eckhoff DE, Activated protein C preserves functional islet mass after intraportal transplantation: A novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death, Diabetes. 53 (2004) 2804–2814. 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- [456].Elizondo DM, Brandy NZD, da Silva RLL, de Moura TR, Ali J, Yang D, Lipscomb MW, Pancreatic islets seeded in a novel bioscaffold forms an organoid to rescue insulin production and reverse hyperglycemia in models of type 1 diabetes, Sci. Rep 10 (2020) 1–11. 10.1038/s41598-020-60947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [457].Narang AS, Mahato RI, Biological and biomaterial approaches for improved islet transplantation, Pharmacol. Rev 58 (2006) 194–243. 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- [458].Webb MA, Dennison AR, James RF, The potential benefit of non-purified islets preparations for islet transplantation, Biotechnol. Genet. Eng. Rev 28 (2012) 101–114. 10.5661/bger-28-101. [DOI] [PubMed] [Google Scholar]
- [459].Jacobson EF, Tzanakakis ES, Human pluripotent stem cell differentiation to functional pancreatic cells for diabetes therapies: Innovations, challenges and future directions, J. Biol. Eng 11 (2017) 21. 10.1186/s13036-017-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [460].Bogdani M, Suenens K, Bock T, Pipeleers-Marichal M, In’t Veld P, Pipeleers D, Growth and functional maturation of β-cells in implants of endocrine cells purified from prenatal porcine pancreas, Diabetes. 54 (2005) 3387–3394. 10.2337/diabetes.54.12.3387. [DOI] [PubMed] [Google Scholar]
- [461].Beuneu C, Vosters O, Ling Z, Pipeleers D, Pradier O, Goldman M, Verhasselt V, N-Acetylcysteine derivative inhibits procoagulant activity of human islet cells., Diabetologia. 50 (2007) 343–347. 10.1007/s00125-006-0529-4. [DOI] [PubMed] [Google Scholar]
- [462].Khosravi-Maharlooei M, Hajizadeh-Saffar E, Tahamtani Y, Basin M, Montazeri L, Khalooghi K, Ashtiani MK, Farrokhi A, Aghdami N, Hashemi Nejad AS, Larijani MB, De Leu N, Heimberg H, Luo X, Baharvand H, Therapy of endocrine disease: Islet transplantation for type 1 diabetes: So close and yet so far away, Eur. J. Endocrinol 173 (2015) R165–R183. 10.1530/EJE-15-0094. [DOI] [PubMed] [Google Scholar]
- [463].Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A, Bioengineering the endocrine pancreas: Intraomental islet transplantation within a biologic resorbable scaffold, Diabetes. 65 (2016) 1350–1361. 10.2337/db15-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [464].Lee S, Sathialingam M, Alexander M, Lakey J, Physical Protection of Pancreatic Islets for Transplantation, in: Biomater. - Phys. Chem. - New Ed., InTech, 2018. 10.5772/intechopen.71285. [DOI] [Google Scholar]
- [465].Cui W, Angsana J, Wen J, Chaikof EL, Liposomal formulations of thrombomodulin increase engraftment after intraportal islet transplantation, Cell Transplant. 19 (2010) 1359–1367. 10.3727/096368910X513964. [DOI] [PubMed] [Google Scholar]
- [466].Gmyr V, Bonner C, Moerman E, Tournoys A, Delalleau N, Quenon A, Thevenet J, Chetboun M, Kerr-Conte J, Pattou F, Hubert T, Jourdain M, Human recombinant antithrombin (ATryn®) administration improves survival and prevents intravascular coagulation after intraportal islet transplantation in a piglet model, Cell Transplant. 26 (2017) 309–317. 10.3727/096368916X693554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [467].Hu S, de Vos P, Polymeric approaches to reduce tissue responses against devices applied for islet-cell encapsulation, Front. Bioeng. Biotechnol (2019). 10.3389/fbioe.2019.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [468].Quintana J, Stinchcomb A, Kostyo J, Robichaud B, Plunk M, Kane R, Chemical Strategies for Improving Islet Transplant Outcomes, OBM Transplant. 2 (2018) 1–1. 10.21926/obm.transplant.1804036. [DOI] [Google Scholar]
- [469].Phelps EA, Templeman KL, Thulé PM, García AJ, Engineered VEGF-releasing PEG–MAL hydrogel for pancreatic islet vascularization, Drug Deliv. Transl. Res 5 (2015) 125–136. 10.1007/s13346-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [470].Wang D, Ding X, Xue W, Zheng J, Tian X, Li Y, Wang X, Song H, Liu H, Luo X, A new scaffold containing small intestinal submucosa and mesenchymal stem cells improves pancreatic islet function and survival in vitro and in vivo, Int. J. Mol. Med 39 (2017) 167–173. 10.3892/ijmm.2016.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [471].Sorelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, Matsumoto S, Levy MF, Lawrence MC, Naziruddin B, Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation, Diabetologia. 56 (2013) 814–824. 10.1007/s00125-012-2813-9. [DOI] [PubMed] [Google Scholar]
- [472].Barra JM, Tse HM, Redox-dependent inflammation in islet transplantation rejection, Front. Endocrinol. (Lausanne) 9 (2018) 175. 10.3389/fendo.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [473].Kumano K, Vasu S, Shabbir R, Darden C, Lawrence M, Naziruddin B, Characterizing and overcoming innate immunity in beta-cell replacement therapy, J. Immunol. Regen. Med (2020) 100034. 10.1016/j.regen.2020.100034. [DOI] [Google Scholar]
- [474].Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglamia AR, Lupu F, Nilsson B, Risitano AM, Ricklin D, Lambris JD, Compstatin: A C3-targeted complement inhibitor reaching its prime for bedside intervention, Eur. J. Clin. Invest 45 (2015)423–440. 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [475].Johansson H, Goto M, Siegbahn A, Elgue G, Korsgren O, Nilsson B, Low molecular weight dextran sulfate: A strong candidate drug to block IBMIR in clinical islet transplantation, Am. J. Transplant 6 (2006) 305–312. 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- [476].Gamble A, Pepper AR, Bruni A, Shapiro AMJ, The journey of islet cell transplantation and future development, Islets. 10 (2018) 80–94. 10.1080/19382014.2018.1428511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [477].Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, Tang Q, Roy S, Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport, Sci. Rep 6 (2016). 10.1038/srep23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [478].Pathak V, Pathak NM, O’Neill CL, Guduric-Fuchs J, Medina RJ, Therapies for Type 1 Diabetes: Current Scenario and Future Perspectives, Clin. Med. Insights Endocrinol. Diabetes 12 (2019) 117955141984452. 10.1177/1179551419844521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [479].Salg GA, Giese NA, Schenk M, Hüttner FJ, Felix K, Probst P, Diener MK, Hackert T, Kenngott HG, The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells., J. Tissue Eng 10 (2019) 2041731419884708. 10.1177/2041731419884708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [480].Skrzypek K, Nibbelink MG, Karbaat LP, Karperien M, van Apeldoorn A, Stamatialis D, An important step towards a prevascularized islet macroencapsulation device—effect of micropatterned membranes on development of endothelial cell network, J. Mater. Sci. Mater. Med 29 (2018) 91. 10.1007/s10856-018-6102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Dolan EB, Varela CE, Mendez K, Whyte W, Levey RE, Robinson ST, Maye E, O’Dwyer J, Beatty R, Rothman A, Fan Y, Hochstein J, Rothenbucher SE, Wylie R, Starr JR, Monaghan M, Dockery P, Duffy GP, Roche ET, An actuatable soft reservoir modulates host foreign body response, Sci. Robot 4 (2019) eaax7043. 10.1126/scirobotics.aax7043. [DOI] [PubMed] [Google Scholar]
- [482].Pepakayala V, Stein J, Gianchandani Y, Resonant magnetoelastic microstructures for wireless actuation of liquid flow on 3D surfaces and use in glaucoma drainage implants, Microsystems Nanoeng. (2015). 10.1038/micronano.2015.32. [DOI] [Google Scholar]
- [483].Robotti F, Sterner I, Bottan S, Monné Rodríguez JM, Pellegrini G, Schmidt T, Falk V, Poulikakos D, Ferrari A, Starck C, Microengineered biosynthesized cellulose as anti-fibrotic in vivo protection for cardiac implantable electronic devices, Biomaterials. 229 (2020) 119583. 10.1016/j.biomaterials.2019.119583. [DOI] [PubMed] [Google Scholar]