Abstract
The COVID-19 pandemic has resulted in the increased use of cryopreserved grafts for allogeneic hematopoietic cell transplantation (HCT). However, information about the effect of cryopreservation on outcomes for patients receiving allogeneic donor grafts is limited.
We evaluated outcomes of HCT recipients who received either fresh or cryopreserved allogeneic bone marrow or peripheral blood stem cell (PBSC) grafts reported to the CIBMTR. A total of 7,397 patients were included in the analysis. Recipients of cryopreserved graft were divided into three cohorts based on graft source: HLA matched related PBSC (n=1,051), matched unrelated PBSC (n=678), and matched related or unrelated bone marrow donors (n=154). These patients were propensity score matched with 5,514 patients who received fresh allografts. The primary endpoint was engraftment.
Multivariate analyses showed no significant increased risk of delayed engraftment, relapse, NRM, or survival with cryopreservation of marrow grafts. In contrast, cryopreservation of related donor PBSC grafts was associated with decreased platelet recovery (HR=0.73, CI=0.68–0.78, p<0.001) and an increased risk of grade II-IV (HR-1.27, CI=1.09–1.48, p=0.002) and grade III-IV (HR=1.48, CI=1.19–1.84, p<0.001) acute GVHD. Cryopreservation of unrelated PBSC grafts was associated with delayed engraftment of neutrophils (HR=0.77, CI=0.71–0.84, p<0.001) and platelets (HR=0.61, CI=0.56–0.66, p<0.001) as well as an increased risk of NRM (HR=1.4, CI=1.18–1.66, p<0.001) and relapse (HR=1.32, CI=1.11–1.58, p=0.002) and decreased PFS (HR=1.36, CI=1.20–1.55, p<0.001) and OS (HR=1.38, CI=1.22–1.58, p<0.001). Reasons for cryopreservation were not routinely collected, however in a subset of unrelated donor HCT the reason was typically a change in patient condition. Products cryopreserved for patient reasons were significantly associated with inferior OS in MVA (HR=0.65, CI=0.44–0.96, P=0.029).
We conclude that cryopreservation is associated with slower engraftment of PBSC grafts which may be associated with inferior transplant outcomes in some patient populations. However the small numbers in the cryopreserved BM cohort and the lack of information on the reason for cryopreservation in all patients suggests that these data should be interpreted with caution, particularly in the context of the risks associated with unexpected loss of a graft during the pandemic. Future analyses addressing outcomes when cryopreservation is universally applied are urgently required.
Keywords: cryopreservation, Peripheral blood stem cell graft, bone marrow graft
INTRODUCTION
Donor grafts in allogeneic hematopoietic cell transplants (HCT) are generally administered fresh.1 Cryopreservation of the donor graft is typically performed only if there are difficulties in coordinating the procurement of the graft or a situation develops in the recipient where the graft cannot be given immediately after collection, often due to unexpected clinical findings. There is an abundance of data on the safety and efficacy of cryopreserved marrow2 and PBSC3 grafts in the autologous setting. The limited data that exist on the effect of cryopreservation of the graft on both engraftment and survival4–9 from allogeneic donors suggest that cryopreservation does not appear to have a significant impact on survival or incidence of graft-vs-host disease (GVHD) regardless of donor source with the exception of a recent publication that showed higher one-year mortality in patients receiving cryopreserved marrow grafts for aplastic anemia10.
The COVID-19 pandemic adversely affected the ability to infuse fresh donor cells, largely due to travel restrictions, both domestically and internationally, but also due to potential delays related to donor availability for a variety of reasons including quarantines and infection of the donor with SARS-CoV-2. To ensure HCT grafts were available at the scheduled time of infusion, the American Society for Transplantation and Cellular Therapy (ASTCT) and National Marrow Donor Program (NMDP) strongly recommended that all unrelated donor (URD) products be cryopreserved (at the transplant center) prior to starting the recipient’s conditioning regimen.11, 12 This became mandatory in the US on March 30, 2020, when the NMDP specified that cryopreservation was required of all URD grafts. Although no such guidelines exist for related donors, many transplant centers started cryopreserving these products, for the same concerns. Historically, some transplant centers are known to routinely cryopreserve related donor products.
With the increased utilization of cryopreserved grafts, the CIBMTR rapidly published two studies addressing the impact of cryopreservation on outcomes. The first analysis in HCT patients receiving post-transplant cyclophosphamide for hematologic malignancies as GVHD prophylaxis, and who mostly received PBSC grafts, found no impact on survival or engraftment,9 but another in patients transplanted for severe aplastic anemia using mostly bone marrow grafts found, as noted above, an adverse impact of cryopreservation on graft failure and survival10
Here we evaluate the impact of cryopreservation on engraftment and other key outcomes, in related and unrelated donor HCT recipients performed for hematologic malignancies using conventional calcineurin inhibitor based GVHD prophylaxis.
METHODS
The CIBMTR® is a research affiliation between the NMDP and the Medical College of Wisconsin (MCW) collecting detailed data on transplant recipients from more than 320 transplantation centers worldwide. Participating centers are requested to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The NMDP, Institutional Review Board (IRB), which is the IRB of record for the CIBMTR’s database protocols, approved this study.
Data Sources
Detailed patient-, disease-, and treatment data were retrieved from the CIBMTR database. Additional data concerning graft origin, transit times and reasons for cryopreservation (in URD) were obtained from the NMDP.
Patients
All patients undergoing an allogeneic HCT from 2013 through 2018 for hematologic malignancies were included in this analysis. Diagnoses was limited to acute leukemias in first or second complete remission (CR1/CR2), chronic leukemias or myelodysplastic syndrome (with <5% blasts at HCT) and lymphomas. Donors included HLA-identical siblings and matched or mismatched URD. Mismatched related donors were excluded. Grafts were either bone marrow or PBSC. Grafts that were T-cell depleted or GCSF stimulated were excluded. Umbilical cord blood grafts, due to universal cryopreservation, and patients who received post-transplant cyclophosphamide (± calcineurin inhibitor and/or mycophenolate mofetil) as GVHD prophylaxis were also excluded from the analysis.
Definitions and Study Endpoints
The primary endpoint was time to engraftment. Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count (ANC) ≥500/μL. Platelet recovery is defined as a platelet count 20,000/μL or higher in the absence of platelet transfusion for 7 consecutive days. Primary graft failure was defined as lack of neutrophil recovery before 28 days. Secondary graft failure was not assessed. All total nucleated cells (TNC) and CD34+ cell content of the graft were calculated at time of graft infusion. Secondary endpoints included acute and chronic GVHD, non-relapse mortality (NRM), progression/relapse and progression-free survival (PFS) and overall survival (OS). NRM was defined as death without evidence of disease relapse/progression; relapse/progression was considered a competing risk. Relapse/progression was defined as morphologic, cytogenetic, or molecular disease recurrence for leukemias and myeloid malignancies, or as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of relapse/progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. For OS, death from any cause was considered an event and surviving patients were censored at last contact. The intensity of allogeneic HCT conditioning regimens was categorized as myeloablative (MAC) or reduced-intensity/non-myeloablative conditioning (RIC/NMA) using consensus criteria.13 Disease risk index (DRI) was assigned as previously reported.14 Acute GVHD15 was graded using standard criteria. For neutrophil and platelet recovery and calculation of incidence of acute GVHD, death without the event was considered a competing risk.
Statistical analysis
Analyses were done separately for three cohorts of BM grafts, PB grafts with matched related donor [MRD], and PB grafts with unrelated donor [URD]. A total of 1,883 patients were identified who met the eligibility criteria described above who received cryopreserved grafts were matched with 5,514 patients who received fresh grafts using a mixed method of direct matching and propensity score matching. The propensity score is the probability of a given patient to receive the cryopreserved graft, based on the observed covariates of the patient and was predicted for each patient using logistic regression accounting for following risk factors: recipient age, race, ethnicity, Karnofsky Performance Score (KPS) (≥90 vs. <90%), HCT-comorbidity index (0 vs. 1–2 vs. ≥3), disease histology (acute myeloid leukemia vs. acute lymphocytic leukemia vs. chronic myeloid leukemia vs. chronic lymphocytic leukemia vs. myelodysplastic syndrome vs. non-Hodgkin lymphoma vs. Hodgkin lymphoma), DRI (low risk vs. intermediate risk vs. high risk), interval from diagnosis to transplant, conditioning intensity (MAC vs. RIC/NMA), use of total body irradiation (TBI), GVHD prophylaxis, donor -recipient cytomegalovirus (CMV) matching, donor-recipient sex matching, year of transplant, and use of in vivo T-cell depletion (anti-thymocyte globulin (ATG) or Campath). Two patients with equal propensity scores meant they had similar probabilities of receiving a cryopreserved graft. The distributions of estimated propensity scores between cryopreserved and fresh grafts were examined. Within each of the three donor type/graft type cohorts, we matched each recipient of a cryopreserved graft with up to 3 controls receiving fresh grafts, using exact matching on DRI and recipient age (within 5 years), and then performing greedy (or nearest neighbor) matching among potential exact matches using the propensity score (restricted within 1 standard deviation (SD))16.
Almost all cases were matched to 3 controls. One-hundred fifteen cases had 2 controls and 7 cases had one control. Fifty-two cases were removed due to missing covariate data (50 cases) or inability to match (2 cases).
Patient-, disease- and transplant-related factors were compared between matched cases and controls using the Chi-square test for categorical and Mann-Whitney test for continuous variables. The Kaplan-Meier estimator was used to evaluate the probability of OS and PFS.17 Cumulative incidence rates were calculated for hematopoietic recovery, GVHD, NRM and relapse, while accounting for competing events.18 The marginal Cox model was applied to evaluate the main treatment effect, while adjusting for the potential correlation within each matched pair. Stepwise variable selection was used to identify additional covariates to adjust for among the same list of variables as used in the propensity score model. The assumption of proportional hazards for the main risk factor (cryopreserved graft vs. fresh graft) for each outcome was tested by adding a time-dependent covariate. Hazard ratios (HR) (95%CI) and p-values were reported for each clinical outcomes of interest comparing the cryopreserved graft treatment group with the fresh graft group. E-values were also presented to assess potential impact of unmeasured confounders on the estimated effect of cryopreservation; these are defined as the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome, conditional on the measured covariates, to fully explain away a specific exposure–outcome association.19. Due to the large number of comparisons required, p-values < 0.01 were considered statistically significant, except for the subgroup analysis of the reason for cryopreservation were p<0.05 (minimal comparisons). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics
A total of 7,397 patients were included in the analysis. Outcomes for patients receiving cryopreserved PBSC grafts were analyzed separately by donor source, HLA-identical sibling (n=1051) or URD (n=678). As analysis of cryopreserved related and unrelated bone marrow grafts did not show a difference between donor sources, the cohorts were combined (n=154) (data not shown). The baseline patient, donor, and transplant related characteristics are shown in Tables 1–3. Overall, the patient and donor characteristics of the cryopreserved and fresh cohorts were similar.
Table 1.
Baseline Characteristics
| BM | Related PBSC | Unrelated PBSC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Fresh | Cryo | P | Fresh | Cryo | P | Fresh | Cryo | P |
| No. of patients | 456 | 154 | 3030 | 1051 | 2028 | 678 | |||
| Median age at transplant (y) | 32.5 (0.6–79.8) | 34.7 (0.4–75.1) | .74 | 56.8 (3.7–76) | 56.9 (7.6–77.7) | .52 | 54.7 (0.9–83.4) | 54.9 (0.9–77.9) | .93 |
| Male Sex (%) | 235 (52) | 80 (52) | .73 | 1767 (58) | 645 (61) | .03 | 1220 (60) | 394 (58) | .21 |
| Race (%) | .29 | .03 | .007 | ||||||
| Caucasian | 374 (82) | 120 (78) | 2440 (81) | 871 (83) | 1795 (89) | 581 (86) | |||
| African-American | 21 (5) | 10 (6) | 161 (5) | 60 (6) | 72 (4) | 32 (5) | |||
| Other | 22 (4) | 10 (7) | 228 (7) | 60 (6) | 70 (3) | 31 (5) | |||
| Missing | 39 (9) | 14 (9) | 201 (7) | 60 (6) | 91 (4) | 34 (5) | |||
| KPS >=90, n(%) | 289 (63) | 102 (66) | .57 | 1688 (56) | 612 (58) | .16 | 1114 (55) | 353 (52) | .24 |
| HCT-CI | .39 | .04 | .97 | ||||||
| 0 | 163 (36) | 54 (35) | 675 (22) | 193 (18) | 384 (19) | 133 (20) | |||
| 1–2 | 139 (30) | 42 (27) | 872 (29) | 305 (29) | 574 (28) | 193 (28) | |||
| 3+ | 154 (34) | 58 (38) | 1483 (49) | 553 (53) | 1070 (53) | 352 (52) | |||
| Primary disease | .95 | .66 | .87 | ||||||
| AML | 201 (44) | 64 (42) | 1230 (41) | 454 (43) | 906 (45) | 291 (43) | |||
| ALL | 115 (25) | 43 (28) | 461 (15) | 151 (14) | 316 (16) | 111 (16) | |||
| CML | 23 (5) | 8 (5) | 82 (3) | 24 (2) | 72 (4) | 24 (4) | |||
| MDS/MPD | 82 (18) | 29 (19) | 855 (28) | 274 (26) | 447 (22) | 160 (24) | |||
| Other acute leukemia | 14 (3) | 5 (3) | 31 (1) | 14 (1) | 32 (2) | 10 (1) | |||
| NHL | 13 (3) | 3 (2) | 304 (10) | 113 (11) | 208 (10) | 69 (10) | |||
| Hodgkin lymphoma | 8 (2) | 2 (1) | 67 (2) | 21 (2) | 47 (2) | 13 (2) | |||
| DRI, n(%) | |||||||||
| Adult | .92 | .74 | .58 | ||||||
| Low | 25 (5) | 8 (5) | 196 (6) | 72 (7) | 163 (8) | 56 (8) | |||
| Intermediate | 149 (33) | 49 (32) | 1734 (57) | 582 (55) | 997 (49) | 335 (49) | |||
| High | 69 (15) | 21 (14) | 762 (25) | 277 (26) | 507 (25) | 172 (25) | |||
| Very high | 13 (3) | 5 (3) | 104 (3) | 36 (3) | 123 (6) | 42 (6) | |||
| Pediatric (adult definition) | .93 | .76 | .08 | ||||||
| Low | 5 (1) | 2 (1) | 1 (0) | 1 (0) | 11 (1) | 2 (0) | |||
| Intermediate | 70 (15) | 24 (16) | 13 (0) | 2 (0) | 23 (1) | 5 (1) | |||
| High | 67 (15) | 25 (16) | 15 (0) | 5 (0) | 12 (1) | 1 (0) | |||
| Very high | 0 | 0 | 1 (0) | 0 | 2 (0) | 1 (0) | |||
HCT-CI indicate Hematopoietic Cell Transplantation Comorbidity index; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; MDS/MPD, myelodysplastic disease/myeloproliferative disease; NHL, non-Hodgkin’s lymphoma
Table 3.
Transplant Characteristics
| BM | Related PBSC | Unrelated PBSC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Fresh | Cryo | P | Fresh | Cryo | P | Fresh | Cryo | P |
| No. of patients | 456 | 154 | 3030 | 1051 | 2028 | 678 | |||
| MAC, n(%) | 390 (86) | 131 (85) | .66 | 1622 (54) | 624 (59) | .004 | 1161 (57) | 378 (56) | .54 |
| TBI, n(%) | 132 (29) | 45 (29) | .89 | 647 (21) | 165 (16) | <.001 | 427 (21) | 168 (25) | .19 |
| GVHD prophylaxis, n(%) | .74 | <.001 | .60 | ||||||
| TAC + MMF ± other(s) | 49 (11) | 17 (11) | 331 (11) | 157 (15) | 323 (16) | 98 (14) | |||
| TAC + MTX ± other(s) | 315 (69) | 108 (70) | 1855 (61) | 632 (60) | 1280 (63) | 456 (67) | |||
| TAC + other(s) | 7 (2) | 2 (1) | 362 (12) | 62 (6) | 139 (7) | 41 (6) | |||
| TAC alone | 8 (2) | 3 (2) | 47 (2) | 32 (3) | 66 (3) | 20 (3) | |||
| CSA + MMF ± other(s) | 13 (3) | 4 (3) | 128 (4) | 26 (2) | 58 (3) | 19 (3) | |||
| CSA + MTX ± other(s) | 63 (14) | 19 (12) | 290 (10) | 137 (13) | 143 (7) | 40 (6) | |||
| CSA + other(s) | 0 | 1 (1) | 5 (0) | 0 | 8 (0) | 1 (0) | |||
| CSA alone | 1 (0) | 0 | 12 (0) | 5 (0) | 323 (16) | 98 (14) | |||
| ATG/alemtuzumab | 190 (42) | 61 (40) | .64 | 245 (8) | 73 (7) | .83 | 875 (43) | 308 (45) | .37 |
| Time from diagnosis to HCT, mo, median (IQR) | 7 (1.4–182.9) | 6.9 (1–111.1) | .25 | 6.3 (0.2–556.3) | 6.8 (0.5–507.5) | .002 | 7.4 (0.7–490.5) | 8.4 (1.7–302.2) | .005 |
| Follow-up, mo, median (IQR) | 35.9 (0.3–76) | 36.3 (0.5–73.6) | 36.1 (0.1–75.5) | 36 (0.4–73.7) | 35.7 (0.1–75.2) | 35 (0–73.4) | |||
| Cell dose, median (IQR) | |||||||||
| Nucleated cell dose, × 108/kg | 3.02 (0.03–19.9) | 2.67 (0.01–11.36) | .007 | – | – | – | – | – | – |
| CD34 cell dose, × 106/kg | 3.3 (0–37547.9) | 3.1 (0–96.3) | .73 | 5.6 (0–57272.7) | 5.3 (0–8863.8) | <.001 | 6.2 (0–262984.7) | 5.9 (0–51.7) | <.001 |
| RBC depletion, n(%) | 152 (33) | 56 (36) | .68 | 36 (1) | 7 (1) | .04 | 39 (2) | 10 (1) | .33 |
| Time to recovery, d, median (IQR) | |||||||||
| Neutrophil | 17.5 (1–77) | 18.5 (1–37) | .02 | 14 (1–113) | 14 (1–107) | <0.001 | 14 (1–89) | 14 (1–96) | <0.001 |
| Platelets | 24 (1–246) | 26 (1–471) | .07 | 16 (0–348 | 17 (1–253) | <0.001 | 17 (1–307) | 19 (1–240) | <0.001 |
MAC indicates myeloablative conditioning; TBI, total body irradiation; TAC, tacrolimus; MMF, mycophenolate mofetil; MTX, methotrexate; CSA cyclosporine; ATG, antithymocyte globulin.
Significant differences in graft and transplant characteristics were seen in the three cohorts. In the BM cohort, cryopreserved BM donor grafts had a lower median total nucleated cell dose (TNC) compared to fresh BM grafts (2.67×108 cells/kg vs. 3.02×108 cells/kg, p=0.007) However, no statistically significant difference in CD34+ cell dose was seen between the two groups (3.1×106 cells/kg vs. 3.3×106 cells/kg, p=0.73).
In the MRD PBSC cohort, cryopreserved graft recipients were more likely to receive MA conditioning (59% vs. 54%, p=0.004) and were less likely to receive TBI (16% vs 21%, p<0.001) as part of their conditioning regimen. Donors of cryopreserved MRD PBSC products were more likely to be Caucasian (75% vs. 71%, p=0.002) then donors of fresh products. There was also a longer interval between diagnosis and transplant with cryopreserved MRD PBSC grafts (6.8 months vs. 6.3 months, p=0.002) and cryopreserved graft recipients received a lower CD34+ cell dose overall (5.3×106 cells/kg vs. 5.6×106 cells/kg, p<0.001).
With the URD PBSC cohort, recipients of cryopreserved grafts were less likely to be Caucasian (86% vs. 89%, p=0.007) and had a longer interval of time between diagnosis and transplant. (8.4 months vs. 7.4 months, p=0.005). The median CD34+ cell dose of cryopreserved grafts was also lower compared to fresh grafts (5.9×106 cells/kg vs. 6.2×106 cells/kg, p<0.001).
Engraftment
The effect of cryopreservation on engraftment varied according to donor source (Table 4). For bone marrow and MRD PBSC grafts, there were no differences in rates of graft failure between cryopreserved and fresh grafts. There was also no significant difference in neutrophil recovery at 28 days in either cohort. However, there was a significantly lower likelihood of platelet recovery at day 100 in the MRD PBSC setting with cryopreservation in univariate analysis (92% vs. 96%, p<0.001; Table 4) that remained significant in multivariate analysis (Table 5).
Table 4.
Univariate Analysis of Major Endpoints in All Cohorts
| Outcome | BM | Related PBSC | Unrelated PBSC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Cryo | Fresh | Cryo | Fresh | Cryo | ||||||||||
| N | % (95% CI) | N | % (95% CI) | P | N | % (95% CI) | N | % (95% CI) | P | N | % (95% CI) | N | % (95% CI) | P | |
| Primary graft failure (day 28) | 456 | 35 (8) | 154 | 14 (9) | .76 | 3030 | 42 (1) | 1051 | 13 (1) | .57 | 2028 | 36 (2) | 678 | 33 (5) | <.001 |
| Neutrophil recovery (day 28) | 456 | 91 (88–94) | 154 | 90 (85–94) | .23 | 3018 | 98 (97–98) | 1048 | 98 (97–99) | .08 | 2024 | 97 (97–98) | 674 | 93 (91–95) | <.001 |
| Platelet recovery (day 100) | 452 | 87 (84–90) | 153 | 84 (78–89) | .05 | 3017 | 96 (95–97) | 1047 | 92 (90–94) | <.001 | 2016 | 94 (93–95) | 671 | 87 (84–90) | <.001 |
| Relapse/PFS | 445 | 149 | .05 | 2919 | 1012 | .09 | 1930 | 645 | <.001 | ||||||
| 6 mo | 74 (70–78) | 68 (61–75) | 71 (69–73) | 70 (67–73) | 72 (70–74) | 61 (57–65) | |||||||||
| 1 yr | 63 (59–68) | 56 (48–64) | 61 (59–62) | 56 (52–59) | 60 (58–62) | 48 (44–52) | |||||||||
| 2 yr | 58 (54–63) | 48 (40–57) | 51 (49–53) | 49 (46–52) | 51 (48–53) | 41 (37–46) | |||||||||
| 3 yr | 56 (51–61) | 44 (36–54) | 48 (46–50) | 45 (42–49) | 46 (43–48) | 38 (34–42) | |||||||||
| Relapse/Progression | 445 | 149 | .17 | 2919 | 1012 | .57 | 1930 | 645 | .09 | ||||||
| 6 mo | 14 (11–17) | 20 (14–27) | 20 (19–22) | 19 (17–22) | 14 (13–16) | 19 (16–22) | |||||||||
| 1 yr | 22 (18–26) | 26 (19–33) | 26 (25–28) | 26 (23–29) | 20 (18–22) | 24 (21–28) | |||||||||
| 2 yr | 25 (21–30) | 31 (24–40) | 31 (29–33) | 30 (27–32) | 25 (23–27) | 28 (24–31) | |||||||||
| 3 yr | 27 (22–31) | 34 (26–43) | 33 (31–35) | 32 (29–35) | 27 (24–29) | 29 (25–32) | |||||||||
| Treatment related mortality | 445 | 149 | 0.30 | 2919 | 1012 | 0.007 | 1930 | 645 | 0.002 | ||||||
| 6 mo | 12 (9–15) | 11 (7–17) | 9 (8–10) | 11 (9–13) | 13 (12–15) | 20 (17–23) | |||||||||
| 1 yr | 15 (12–18) | 18 (12–25) | 13 (12–15) | 19 (16–21) | 20 (18–22) | 27 (24–31) | |||||||||
| 2 yr | 16 (13–20) | 20 (14–27) | 18 (17–20) | 21 (19–24) | 25 (23–27) | 31 (27–35) | |||||||||
| 3 yr | 18 (14–22) | 21 (15–29) | 20 (18–21) | 23 (20–26) | 28 (26–30) | 33 (29–37) | |||||||||
| OS | 456 | 154 | .02 | 3030 | 1051 | .02 | 2028 | 678 | <.001 | ||||||
| 6 mo | 82 (78–85) | 78 (72–85) | 84 (83–86) | 82 (80–85) | 80 (78–82) | 71 (67–74) | |||||||||
| 1 yr | 73 (69–77) | 64 (56–72) | 72 (70–74) | 66 (63–69) | 67 (65–69) | 56 (52–60) | |||||||||
| 2 yr | 64 (59–69) | 55 (46–63) | 60 (58–62) | 56 (53–59) | 57 (55–59) | 46 (42–50) | |||||||||
| 3 yr | 60 (55–65) | 50 (41–59) | 54 (52–57) | 53 (49–56) | 50 (48–53) | 40 (36–45) | |||||||||
| aGVHD at 100 d | 456 | 154 | 3030 | 1051 | 2028 | 678 | |||||||||
| Any grade | 223 (49) | 70 (45) | .04 | 1327 (44) | 507 (48) | .08 | 1162 (57) | 381 (56) | .86 | ||||||
| Grade II-IV | 151 (33) | 47 (31) | .03 | 906 (30) | 369 (35) | .01 | 803 (40) | 265 (39) | .88 | ||||||
| Grade III-IV | 54 (12) | 13 (8) | .04 | 295 (10) | 143 (14) | <.001 | 282 (14) | 102 (15) | .40 | ||||||
Table 5.
Summary of Multivariate Analysis of Study Endpoints in All Cohorts
| Endpoint | BM (Fresh = 456, Cryo = 154) | Related PBSC (Fresh = 3030, Cryo = 1051) | Unrelated PBSC (Fresh = 2028, Cryo = 678) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P | E-value | HR | CI | P | E-value | HR | CI | P | E-value | |
| Neutrophil recovery (day 28)* | 0.87 | 0.73–1.03 | .109 | 1.44 | 1.06 | 0.99–1.14 | .104 | 1.25 | 0.77 | 0.71–0.84 | <.001 | 1.69 |
| Platelet recovery (day 100)† | 0.81 | 0.66–0.98 | .028 | 1.58 | 0.73 | 0.68–0.78 | <.001 | 1.79 | 0.61 | 0.56–0.66 | <.001 | 2.16 |
| Treatment-related mortality‡ | 1.28 | 0.84–1.94 | .25 | 1.66 | 1.22 | 1.04–1.44 | .015 | 1.56 | 1.4 | 1.18–1.66 | <.001 | 1.84 |
| Relapse/Progression§ | 1.28 | 0.90–1.83 | .164 | 1.66 | 0.96 | 0.84–1.10 | .524 | 1.20 | 1.32 | 1.11–1.58 | .002 | 1.72 |
| Relapse/PFSǁ | 1.25 | 0.95–1.65 | .107 | 1.61 | 1.07 | 0.97–1.19 | .168 | 1.27 | 1.36 | 1.20–1.55 | <.001 | 1.78 |
| OSǀ | 1.34 | 1.00–1.80 | .052 | 1.75 | 1.12 | 1.00–1.25 | .045 | 1.38 | 1.38 | 1.22–1.58 | <.001 | 1.81 |
| aGVHD (day 100) | ||||||||||||
| Grade II-IV# | 1.09 | 0.73–1.65 | .668 | 1.32 | 1.27 | 1.09–1.48 | .002 | 1.64 | 0.96 | 0.81–1.15 | .683 | 1.2 |
| Grade III-IV** | 1.44 | 0.80–2.57 | .222 | 1.89 | 1.48 | 1.19–1.84 | <.001 | 1.95 | 1.12 | 0.87–1.44 | .381 | 1.38 |
Additional covariates adjusted for in multivariate analysis for neutrophil engraftment: BM: recipient age, donor age, donor type/matching, and GVHD prophylaxis; related PBSC: ATC/Campath use. conditioning regimen intensity, disease, donor-recipient CMV status, GVHD prophylaxis, KPS, use of TBI.
Additional covariates adjusted for in multivariate analysis for platelet engraftment: BM: donor type/matching, KPS; related PBSC: recipient age, disease, GVHD prophylaxis, HCT-CI, KPS, use of TBI; unrelated PBSC: recipient age, disease, donor-recipient CMV status, DRI, GVHD prophylaxis, HCT-CI, interval from diagnosis to HCT. KPS, year of HCT.
Additional covariates adjusted for in multivariate analysis for treatment-related mortality: BM: recipient age, donor type/matching, ethnicity; related PBSC: recipient age, disease, donor-recipient CMV status, DRI.ethnicity, GVHD prophylaxis, HCT-CI; unrelated PBSC: recipient age, conditioning regimen intensity, donor age, donor type/matching, donor-recipient CMV status, GVHD prophylaxis, HCT-CI, interval from diagnosis to HCT, KPS.
Additional covariates adjusted for in multivariate analysis for relapse/progression: BM: DRI, TBI use; related PBSC: disease, donor-recipient CMV status, DRI, HCT-CI, interval from diagnosis to HCT, year of HCT; unrelated PBSC: disease, ORI.interval from diagnosis o HCT, year of HCT.
Additional covariates adjusted for in multivariate analysis for relapse/PFS: BM: recipient age, DRI, ethnicity; related PBSC: recipient age, disease, DRI, donor-recipient sex matching, ethnicity, HCT-CI, interval from diagnosis to HCT, KPS, year of HCT; unrelated PBSC: recipient age, conditioning regimen intensity, donor age, disease, donor type/matching, donor-recipient CMV status, DRI, GVHD prophylaxis, HCT-CI, interval from diagnosis to HCT, KPS.
Additional covariates adjusted for in multivariate analysis for OS: BM: recipient age, DRI, ethnicity; related PBSC: recipient age, DRI, ethnicity, GVHD prophylaxis. HCT-CI, interval from diagnosis to HCT, KPS; unrelated PBSC: recipient age, conditioning regimen intensity, donor age, donor type/matching, donor-recipient CMV status, DRI, GVHD prophylaxis, HCT-CI, interval from diagnosis to HCT, KPS.
Additional covariates adjusted for in multivariate analysis for aGVHD grade II-IV: BM: donor type/matching, disease. GVHD prophylaxis, ATG/Campath use; related PBSC: race, disease, GVHD prophylaxis, conditioning regimen intensity. ATG/Campath use; unrelated PBSC: disease, conditioning regimen intensity, donor age, ATG/Campath use.
Additional covariates adjusted for in multivariate analysis for aGVHD grade III-IV: BM: donor type/matching; related PBSC: disease, GVHD prophylaxis; unrelated PBSC: sex, conditioning regimen intensity, donor type/matching, donor age, donor-recipient CMV status, ATG/Campath use.
In the URD PBSC cohort, there was an increase in primary graft failure with cryopreservation (5% vs. 2%, p < 0.001) on univariate analysis. There was also a significantly lower likelihood of day 28 neutrophil (93% vs. 97% p<0.001) and day 100 platelet (87% vs. 94% p<0.001) recovery in univariate (Table 4) and multivariate analyses (Table 5).
GVHD and Relapse
There was no difference in the incidence of acute GVHD (aGVHD) of grades II-IV and III-IV at 100 days between fresh and cryopreserved grafts in the BM and URD PBSC cohorts. With matched related donors, cryopreservation was associated with a modest increase in incidence of Grades II-IV (35% vs. 30%, p=0.01) and III-IV (14% vs. 10%, p< 0.001) aGVHD in univariate analyses, and remained significant in multivariate analysis (Table 5). There were no statistically significant differences in relapse between fresh and cryopreserved grafts in any of the cohorts in univariate analyses. However, in multivariate analysis, there was a statistically significant increase in relapse with cryopreserved URD PBSC grafts (HR=1.32, CI=1.11–1.58, p=0.002).
PFS, OS and NRM
Overall, there were no significant differences between cryopreserved and fresh grafts in NRM, OS, or PFS in the bone marrow cohort or in PFS and OS in the MRD PBSC cohort (Figure 1). There was a significant increase in rates of NRM in favor of fresh grafts in the MRD PBSC cohort in univariate analysis; however, these differences were not statistically significant on multivariate analysis.
Figure 1.
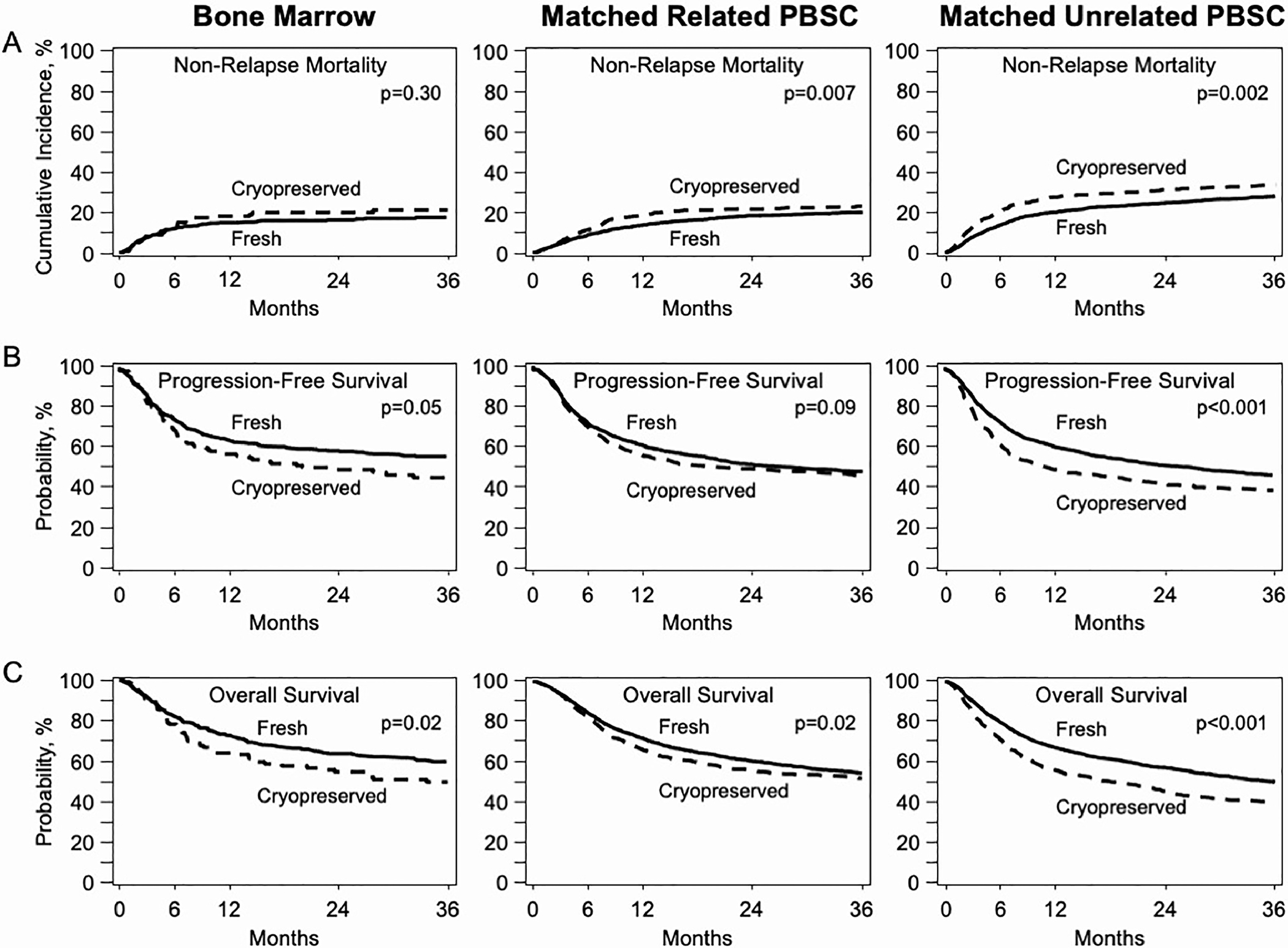
Kaplan-Meier curves in BM, MRD, and URD grafts. (A) Treatment-related mortality. (B) PFS. (C) OS.
In the URD PBSC cohort, however, a significant increase in mortality was associated with cryopreserved compared to fresh grafts (2 year OS: 46% vs. 57%, p< 0.001). The most common cause of death with both cryopreserved and fresh grafts was relapse of the primary disease. Univariate analyses found significant differences in NRM (p=0.002), PFS (p<0.001), and OS (p<0.001) at all timepoints (Table 4). These differences remained significant in multivariate analyses (Table 5).
Because cryopreservation of URD PBSC grafts produced outcomes that were significantly different in comparison to the other groups, we performed an in-depth analysis of this cohort. Information regarding the time between collection of the graft and receipt at the transplant center (transit time) was obtained for 1235 fresh and 398 cryopreserved domestic URD PBSC grafts. Transit time was not available for international URD PBSC donor grafts. Median time of transit was similar between the two cohorts (8.68 hours vs. 8.82 hours respectively) and was not statistically significant. Analysis of survival endpoints between domestic and international URD PBSC grafts revealed no statistically significant differences (data not shown).
A subset analysis of the reason for cryopreserving URD PBSC grafts in 299 donors where this information was available revealed patient condition, including change in disease status, reaction to conditioning regimen and infection, was the most common reason for cryopreservation (56%, Table 6). Multivariable analysis showed significantly inferior OS in patients whose products were cryopreserved due to patient condition compared to other reasons (HR=0.65, CI=0.44–0.96, P=0.029).
Table 6.
Reason for cryopreservation for NMDP products collected between 2016 and 2018
| Reason | Number (%) |
|---|---|
| Total | 299 |
| Clinical Schedule* | 81 (27) |
| Donor availability | 25 (8) |
| PBSC or BM only donor | 27 (9) |
| Patient condition† | 166 (56) |
Clinical schedule: reasons such as insurance delay, dental work, issues related to the treatment schedule (eg, holidays, work disruptions, prep scheduling), or any issue related to patient’s availability on a given date (eg, weddings, funerals, other life events).
Patient condition: infection, adverse reaction to prep, or any change in status (eg, relapse, induction failure, waiting on additional tests, waiting on count recovery).
DISCUSSION
This is the largest study about the effect of cryopreservation on HCT outcomes using allogeneic donor grafts. In bone marrow and related PBSC grafts, the impact of cryopreservation appears to be minimal, with delayed platelet engraftment and an increased risk of grade II-IV and grade III-IV aGVHD at 100 days seen in cryopreserved related PBSC grafts. No statistically significant effect on NRM, relapse, PFS, and OS was observed with either cohort. This is similar to previously published reports.4–7 Early studies comparing matched related donor cryopreserved bone marrow grafts with fresh marrow grafts revealed no differences in engraftment, however, it was unclear whether there was an effect on the incidence of acute GVHD.4,5 With related PBSC grafts, there appears to be no effect of cryopreservation on engraftment from earlier studies, however, the effect of cryopreservation on GVHD was not consistent, with one trial reporting no difference in GVHD6, while another showed a statistically significant increase in acute GVHD in the cryopreserved cohort.7
Information on the effect of cryopreservation in unrelated donor grafts was not readily available from earlier reports as most studies combined unrelated and related donors. One report of 76 cryopreserved PBSC allograft recipients, of whom 19 were from unrelated donors, revealed delayed platelet engraftment and an increased incidence of chronic GVHD.8 There was no effect of cryopreservation observed on relapse and survival. Another report of 274 patients who received cryopreserved allogeneic grafts (mostly PBSC grafts) followed by post-transplant cyclophosphamide prophylaxis found no significant effect of cryopreservation on engraftment or survival.9 In contrast, an analysis of 52 recipients of cryopreserved BM and PBSC allografts for patients with aplastic anemia found inferior 1-year survival in the cryopreserved cohort.10 In our analysis, cryopreservation was associated with a statistically significant negative impact in URD PBSC grafts, resulting in more primary graft failures, slower engraftment of neutrophils and platelets, as well as increased NRM, relapse and decreased PFS and OS.
It is unclear why cryopreservation was associated with inferior outcomes in unrelated in contrast to related PBSC grafts. One possibility is there may be a delay between collection and cryopreservation due to the additional transit time between the donor center and transplant center and delays in cryopreservation might have reduced the product’s hematopoietic potency. At most centers, allogeneic donor grafts are typically delivered unmanipulated to the transplant center, which then cryopreserves the graft. In contrast, with related donors, the donor center and the transplant center are either at the same site or are very close to each other, allowing for cryopreservation within hours of procurement of the graft. In the unrelated donor setting, however, donor grafts can be in transit for a significant amount of time. Additionally, there may be delays in cryopreservation if arrival occurs at night. Our analysis did not show any significant differences in transit time between fresh and cryopreserved unrelated PBSC grafts, however, time between receipt of the graft and cryopreservation was not available. In cord blood grafts, it is known that delays in cryopreservation results in decreased mononuclear cells, particularly in the granulocyte and mature B- and T-cell subsets.20 Significant declines in CFU recovery is also observed post-thaw, which is more pronounced the longer the interval between collection and cryopreservation.21,22 Additionally, there is a negative impact of increased transit time on platelet recovery and mortality with unrelated bone marrow graft recipients.23 In allogeneic PBSC grafts, cryopreservation has been shown to decrease CFU post-thaw,6 however, the effect of the duration of cryopreservation on grafts is unknown. Information concerning allogeneic graft composition in this study was restricted to parameters tested at infusion. Unfortunately, information about graft-composition and graft viability post-thaw was not available for this analysis.
The observation of an increase in relapse and decreased PFS and OS in cryopreserved unrelated PBSC grafts was unexpected. It is likely that patients who require cryopreserved grafts had multiple reasons for delays in transplantation, such as infection or disease relapse. A review from Aziz, et al. found the most common reason for cryopreservation was due to patient related (infection, relapse, deconditioning, etc.) or donor related (availability, workup, etc.) issues.24 Although there is limited data from the NMDP about the reason for cryopreservation, our analysis supports this observation. It is possible delays for patient factors, such as infection, result in recipients becoming more “fragile” compared to their matched controls. If extra courses of chemotherapy were required for relapse or inadequate control of the underlying disease, this may indicate a more biologically aggressive disease compared to their corresponding controls, resulting in the increased relapse and decreased survival in this cohort. Multivariate analysis of the reason for cryopreservation in our analysis did show significantly decreased OS in grafts which were cryopreserved for patient factors supporting the hypothesis that these patients differ in characteristics to those receiving fresh grafts. Cryopreservation may also affect expression of surface molecules in mononuclear cells, in particular CD62L, which is found in CD34+ cells and lymphocytes, and is decreased after cryopreservation.25–27 CD62L also contributes to the activity of regulatory T-cells, which exerts a protective effect against GVHD and may be associated with the graft-vs.-tumor effect and decreased relapse rates.28 Another factor to consider is the process of cryopreservation introduces a cryoprotective agent into the recipient which can result in adverse reactions and can potentially impact survivals.29
There are several strengths as well as significant limitations with this study. This is the largest number of cases yet studied and case matching provides control for a number of potential confounding variables, providing much more precise estimates of outcomes than previously available. It must be acknowledged, however, that despite the large size, there are still a relatively small number of cases in some cohorts, especially the BM cohort, which limits the power of those analyses. Other limitations include lack of information concerning the reasons for cryopreservation and the time between graft procurement and cryopreservation. Thus, the differences seen, despite adjusting for multiple known covariates, may be a surrogate for other factors accounting for inferior outcomes, rather than graft injury from the cryopreservation. Note that many of the E-values are in the plausible range of a potential unmeasured confounder, which could account for the apparent effects of cryopreservation. Additional studies assessing quantitative and qualitative changes in the graft before and after cryopreservation and their impact on clinical outcomes are needed.
In summary, although some differences in outcomes were seen, cryopreservation of allogeneic donor grafts is a suitable option for transplant recipients, especially in cases where there is difficulty with coordinating the administration of donor grafts without modification or if there are recipient factors which preclude the immediate use of the donor graft. That we do not know the reason for cryopreservation (in a population of patients where this is not the norm) is a significant limitation of this study and suggests that the results should be interpreted with caution. Balancing the risk of not having an available graft in a myeloablated patients against the risk of possible effects on clinical outcomes is critical. The use of routine cryopreservation during the COVID-19 pandemic may help in further defining the effects of cryopreservation on allogeneic transplant outcomes, and analyses to study this are urgently needed.
Table 2.
Donor Characteristics
| BM | Related PBSC | Unrelated PBSC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Fresh | Cryo | P | Fresh | Cryo | P | Fresh | Cryo | P |
| No. of patients | 456 | 154 | 3030 | 1051 | 2028 | 678 | |||
| Donor type, n(%) | .59 | .58 | |||||||
| HLA-identical sibling | 68 (15) | 24 (16) | |||||||
| Unrelated, 8/8 | 318 (70) | 106 (69) | 1696 (84) | 566 (83) | |||||
| Unrelated, 7/8 | 70 (15) | 24 (16) | 332 (16) | 112 (17) | |||||
| Donor age, yr, median (IQR) | 29 (9–67) | 28.8 (8.6–72.3) | .94 | 55 (0–78.4) | 54.9 (1.8–79.6) | .12 | 28 (18–64.9) | 28.3 (18.6–60.5) | .19 |
| Donor race, n(%) | <.001 | .002 | <.001 | ||||||
| Caucasian | 279 (61) | 87 (56) | 2157 (71) | 785 (75) | 1137 (56) | 353 (52) | |||
| African-American | 14 (3) | 11 (7) | 142 (5) | 53 (5) | 46 (2) | 22 (3) | |||
| Other | 66 (15) | 37 (25) | 277 (9) | 63 (6) | 368 (18) | 114 (17) | |||
| Missing | 97 (21) | 19 (12) | 454 (15) | 150 (14) | 477 (24) | 189 (28) | |||
| Donor/recipient CMV serostatus, n(%) | .98 | .01 | .04 | ||||||
| +/+ | 173 (38) | 60 (39) | 1348 (44) | 474 (45) | 598 (29) | 219 (32) | |||
| +/− | 61 (13) | 20 (13) | 349 (12) | 105 (10) | 232 (11) | 85 (13) | |||
| −/+ | 144 (32) | 47 (31) | 709 (23) | 291 (28) | 696 (34) | 207 (31) | |||
| −/− | 78 (17) | 27 (18) | 624 (21) | 181 (17) | 502 (25) | 167 (25) | |||
| Donor/recipient sex match, n(%) | .91 | .15 | .43 | ||||||
| Male-Male | 154 (34) | 51 (33) | 956 (32) | 337 (32) | 888 (44) | 300 (44) | |||
| Male-Female | 128 (28) | 41 (27) | 657 (22) | 224 (21) | 541 (27) | 188 (28) | |||
| Female-Male | 81 (18) | 29 (19) | 811 (27) | 308 (29) | 332 (16) | 94 (14) | |||
| Female-Female | 93 (20) | 33 (21) | 606 (20) | 182 (17) | 267 (13) | 96 (14) | |||
| Donor/recipient ABO match, n(%) | .34 | .23 | .26 | ||||||
| Matched | 104 (23) | 28 (18) | 1099 (36) | 356 (34) | 347 (17) | 106 (16) | |||
| Minor mismatch | 24 (5) | 14 (9) | 267 (9) | 71 (7) | 158 (8) | 58 (9) | |||
| Major mismatch | 36 (8) | 12 (8) | 237 (8) | 94 (9) | 125 (6) | 54 (8) | |||
| Bi-directional | 4 (1) | 5 (3) | 57 (2) | 20 (2) | 47 (2) | 20 (3) | |||
| Not available | 287 (63) | 95 (62) | 1369 (45) | 510 (49) | 1349 (67) | 438 (65) | |||
| Missing | 1 (0) | 0 | 1 (0) | 0 | 2 (0) | 2 (0) | |||
CMV indictates cytomegalovirus.
Highlights.
Cryopreservation of Bone Marrow grafts is not associated with stasticially significant delays in engraftment or inferior survivals.
Cryopreservation of related PBSC grafts is associated with delayed platelet engraftment and increased risk of acute Grade II-IV and III-IV GVHD.
Cryopreservation of unrelated PBSC grafts is associated with delayed engraftment, increased NRM and relapse, and decreased survival.
Difference in survival between cryopreserved vs. fresh unrelated donor grafts may be due to difference in recipient factors, highlighting the need for further studies addressing outcomes during the COVID era.
ACKNOWLEDGEMENTS
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, Inc.; Adienne SA; Allovir, Inc.; Amgen, Inc.; Angiocrine Bioscience; Astellas Pharma US; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Incyte Corporation; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Stemcyte; Takeda Pharma; Vor Biopharma; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the “cold shoulder”? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38(6):399–405. [DOI] [PubMed] [Google Scholar]
- 2.Attarian H, Feng Z, Buckner CD, MacLeod B, Rowley SD. Long-term cryopreservation of bone marrow for autologous transplantation. Bone Marrow Transplant. 1996;17(3):425–430. [PubMed] [Google Scholar]
- 3.Akkök ÇA, Liseth K, Melve GK, et al. Is there a scientific basis for a recommended standardization of collection and cryopreservation of peripheral blood stem cell grafts? Cytotherapy. 2011;13(8):1013–1024. [DOI] [PubMed] [Google Scholar]
- 4.Eckardt JR, Roodman GD, Boldt DH, et al. Comparison of engraftment and acute GVHD in patients undergoing cryopreserved or fresh allogeneic BMT. Bone Marrow Transplant. 1993;11(2):125–131. [PubMed] [Google Scholar]
- 5.Stockschläder M, Krüger W, Kroschke G, et al. Use of cryopreserved bone marrow in allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995;15(4):569–572. [PubMed] [Google Scholar]
- 6.Kim DH, Jamal N, Saragosa R, et al. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol. Blood Marrow Transplant 2007;13(10):1233–1243. [DOI] [PubMed] [Google Scholar]
- 7.Parody R, Caballero D, Márquez-Malaver FJ, et al. To freeze or not to freeze peripheral blood stem cells prior to allogeneic transplantation from matched related donors. Eur. J. Haematol 2013;91(5):448–455. [DOI] [PubMed] [Google Scholar]
- 8.Medd P, Nagra S, Hollyman D, Craddock C, Malladi R. Cryopreservation of allogeneic PBSC from related and unrelated donors is associated with delayed platelet engraftment but has no impact on survival. Bone Marrow Transplant. 2013;48(2):243–248. [DOI] [PubMed] [Google Scholar]
- 9.Hamadani M, Zhang M-J, Tang X-Y, et al. Graft Cryopreservation Does Not Impact Overall Survival after Allogeneic Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2020;26(7):1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eapen M, Zhang M-J, Tang X-Y, et al. Hematopoietic Cell Transplantation with Cryopreserved Grafts for Severe Aplastic Anemia. Biol. Blood Marrow Transplant. 2020;26(7):e161–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://www.astct.org/communities/public-home?CommunityKey=d3949d84-3440-45f4-8142-90ea05adb0e5.
- 12. https://network.bethematchclinical.org/news/nmdp/be-the-match-response-to-covid-19/
- 13.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol. Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 16.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. [DOI] [PubMed] [Google Scholar]
- 19.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. Journal of the American Medical Association. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 20.Louis I, Wagner E, Dieng MM, et al. Impact of storage temperature and processing delays on cord blood quality: discrepancy between functional in vitro and in vivo assays. Transfusion. 2012;52(11):2401–2405. [DOI] [PubMed] [Google Scholar]
- 21.Pereira-Cunha FG, Duarte ASS, Reis-Alves SC, et al. Umbilical cord blood CD34(+) stem cells and other mononuclear cell subtypes processed up to 96 h from collection and stored at room temperature maintain a satisfactory functionality for cell therapy. Vox Sang. 2015;108(1):72–81. [DOI] [PubMed] [Google Scholar]
- 22.Schwandt S, Liedtke S, Kogler G. The influence of temperature treatment before cryopreservation on the viability and potency of cryopreserved and thawed CD34+ and CD45+ cord blood cells. Cytotherapy. 2017;19(8):962–977. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus HM, Kan F, Tarima S, Champlin RE, Confer DL, Frey N, Gee AP, Wagner JE, Horowitz MM, Eapen M. Rapid transport and infusion of hematopoietic cells is associated with improved outcome after myeloablative therapy and unrelated donor transplant. Biol Blood Marrow Transplant. 2009;15(5):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz J, Morris G, Rizk M, et al. Cryopreservation of adult unrelated donor products in hematopoietic cell transplantation: the OneMatch experience and systematic review of the literature. Transfusion. 2017;57(11):2782–2789. [DOI] [PubMed] [Google Scholar]
- 25.Costantini A, Mancini S, Giuliodoro S, et al. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods. 2003;278(1–2):145–155. [DOI] [PubMed] [Google Scholar]
- 26.De Boer F, Dräger AM, Van der Wall E, Pinedo HM, Schuurhuis GJ. Changes in L-selectin expression on CD34-positive cells upon cryopreservation of peripheral blood stem cell transplants. Bone Marrow Transplant. 1998;22(11):1103–1110. [DOI] [PubMed] [Google Scholar]
- 27.Florek M, Schneidawind D, Pierini A, et al. Freeze and Thaw of CD4+CD25+Foxp3+ Regulatory T Cells Results in Loss of CD62L Expression and a Reduced Capacity to Protect against Graft-versus-Host Disease. PLoS One. 2015;10(12):e0145763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu G, Yang J-Z, Zhang J-Q, Sun L-X. Regulatory T cells in allogeneic hematopoietic stem cell transplantation: From the lab to the clinic. Cell Immunol. 2019;346:103991. [DOI] [PubMed] [Google Scholar]
- 29.Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. 2014;49(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]


