Abstract
In persistent pulmonary hypertension of the newborn (PPHN) the ratio of pulmonary vascular (PVR) to systemic vascular resistance (SVR) is increased. Extrapulmonary shunts (patent ductus arteriosus and patent foramen value) allow for right-to-left shunting and hypoxemia. Systemic hypotension can occur in newborns with PPHN due to variety of reasons such as enhanced peripheral vasodilation, impaired left ventricular function and decreased preload. Systemic hypotension can lead to end organ injury from poor perfusion and hypoxemia in the newborn with PPHN. Thus, it must be managed swiftly. However, not all newborns with PPHN and systemic hypotension can be managed the same way. Individualized approach based on physiology and echocardiographic findings are necessary to improve perfusion to essential organs. Here we present a review of the physiology and mechanisms of systemic hypotension in PPHN, which can then guide treatment.
Introduction
The fetal circulatory system is characterized by high pulmonary vascular resistance (PVR) and low systemic vascular resistance (SVR). The high PVR is due to relative hypoxemia, lack of alveolar ventilation with fluid-filled lungs and circulating pulmonary vasoconstrictors.1 The low SVR is secondary to low-resistance umbilical and placental circuit. High PVR and low SVR lead to right-to-left shunts at the level of the ductus arteriosus (PDA) and foramen ovale (PFO). At birth, alveolar ventilation and oxygenation reduces PVR with an eight-to-tenfold increase in pulmonary blood flow, and cord clamping increases SVR. The high SVR and low PVR with a systemic blood pressure significantly higher than pulmonary arterial pressure is a life-long feature of postnatal circulation unless altered by disease. Failure to achieve this transition leads to persistence of high PVR in the postnatal period leading to persistent pulmonary hypertension of the newborn (PPHN).2 This review outlines the etiology and management of systemic hypotension in PPHN.
Pathophysiology of PPHN3
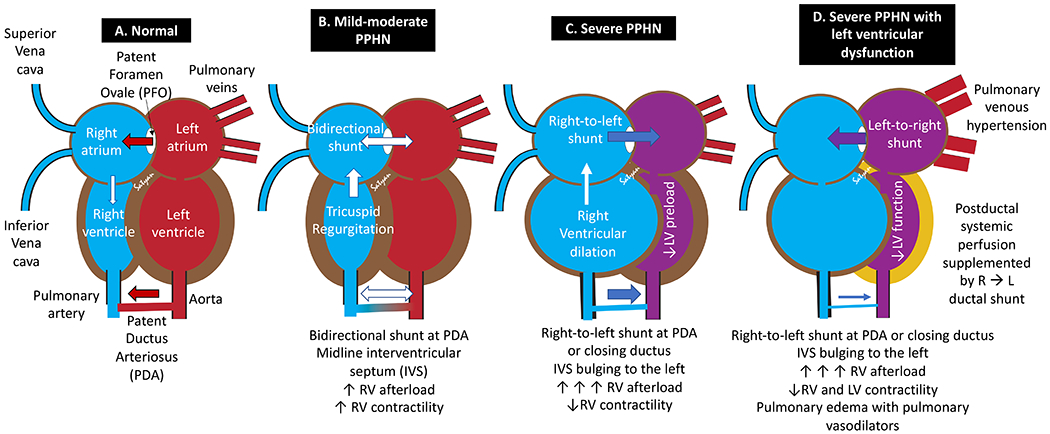
Elevated PVR in PPHN can be idiopathic/primary, secondary to lung disease (either parenchymal, alveolar space disease or pulmonary hypoplasia) or cardiac dysfunction. Normal cardiac physiology verses that of PPHN is shown in figure 1A–1D. Increased PVR leads to bidirectional or right-to-left shunting at the PFO and PDA and increases right ventricular (RV) afterload.4 The right-to-left shunting at the PFO reduces RV preload. The RV afterload can be transiently reduced by a right-to-left PDA shunt if the ductus remains open. Initial increases in afterload cause RV hypertrophy and increases contractility; however, persistent and excessive elevation of PVR is associated with myocardial stretching, ischemia, hypoxemia and acidosis leading to RV dysfunction (table 1).5 Left ventricular (LV) dysfunction can lead to pulmonary venous hypertension and increase pulmonary arterial pressure and reduce systemic output. LV preload decreases secondary to low pulmonary venous return and/or bulging of the interventricular septum to the left. Additionally, hypoxemic respiratory failure can be a primary driver for left ventricular dysfunction. Abnormal interaction between the two ventricles, ischemia, hypoxemia and metabolic acidosis contribute to LV dysfunction.4 Left ventricular dysfunction can also exacerbate and lead to inhaled nitric oxide (iNO)-resistant PPHN by increasing pulmonary venous hypertension.6 Arteriovenous malformations – commonly either intracranial (vein of Galen malformation) or hepatic can present with PPHN and systemic hypotension and should be included in the differential diagnosis.7–9 Rarely, the clinical characteristics (phenotype) of PPHN can be mimicked in situations with normal PVR and reduced SVR due to conditions such as sepsis.10,11
Figure 1. Cardiac pathophysiology in PPHN.
(A) A normal postnatal heart has left-to-right shunting at PFO and PDA with the interventricular septum (IVS) bulging to the right. (B) In mild-to-moderate PPHN, the IVS can be midline with bidirectional shunts at PFO/PDA. Increased right ventricular (RV) afterload is compensated by increased RV contractility. High velocity tricuspid regurgitation (TR) is observed. (C) In severe PPHN, the PFO and PDA shunt right-to-left with IVS bulging to the left decreasing left ventricular (LV) preload. Extremely high RV afterload leads to uncoupling of RV function leading to RV dilation. An open PDA might benefit the RV by providing a pop-off mechanism to reduce RV afterload. (D) When severe PPHN is associated with LV dysfunction, pulmonary venous hypertension and high pressure in the LA leads to left-to-right shunt at PFO but right-to-left shunt at PDA. Inhaled nitric oxide (NO), as well as other therapies that lower pulmonary vascular resistance, can precipitate pulmonary edema in pulmonary venous hypertension if LV failure or other forward flow obstructions are present. In the setting of LV dysfunction, postductal systemic perfusion is supplemented by a transductal right to left shunt. Copyright Satyan Lakshminrusimha.
Table 1.
Cardiac function and shunt changes in persistent pulmonary hypertension of the newborn (see figure 1)
| Parameter | Normal | Mild-to-moderate PPHN | Severe PPHN |
|---|---|---|---|
| RV afterload | Normal | ↑ | ↑↑↑ |
| RV contractility | Normal | ↑↑ with RV hypertrophy | ↓↓ (uncoupling) with RV dilation |
| RV diastolic function | Normal | Normal / ↓ | ↓↓* |
| Septal position | Bulging to the right | Midline | Bulging to the left |
| PFO shunt | Left-to-right | Bidirectional or right-to-left | Right-to-left (or left-to-right with LV dysfunction)** |
| PDA shunt (if open) | Left-to-right | Bidirectional or right-to-left | Right-to-left (or closing ductus) |
| LV preload | Normal | Normal / ↓ | Normal / ↓ |
| Systemic blood pressure | Normal | Normal / ↓ | ↓↓↓↓ |
In the presence of moderate to severe PPHN, RV diastolic function (lusitropy) is significantly reduced. In such states, it is important to avoid chronotropic agents (which will exacerbate diastolic dysfunction) and use agents which specifically target diastolic function (e.g. milrinone).
In some patients with severe PPHN, a bidirectional atrial shunt or a left-to-right shunt may be observed even in the absence of LV dysfunction.12
Normal blood pressure
The optimal systemic blood pressure during management of PPHN is not clear. There are several studies evaluating normal systemic systolic, diastolic and mean pressure during the first week after birth in term infants. Results from one such study are shown in table 2.13 However, instead of exclusively focusing on blood pressure numbers, the clinician should look for signs of hypoperfusion (figure 2).
Table 2.
Normal range of systemic blood pressure in mmHg in term infants (numbers in parentheses are 2 standard deviations below the mean; these can be considered the lower limit of normal during PPHN management).
| Age | Systolic | Mean | Diastolic |
|---|---|---|---|
| 6 to 18 h | 80 ± 13 (54) | 57 ± 12 (33) | 43 ± 10 (23) |
| 18 to 30 h | 83 ± 12 (59) | 60 ± 11(38) | 46 ± 10 (26) |
| 3 d ± 6 h* | 84 ± 14 (56) | 60 ± 12 (36) | 48 ± 13 (22) |
| 7 d ± 1 d | 91 ± 15 (61) | 67 ± 13 (41) | 52 ± 11 (30) |
For practical purposes, systolic pressure of 55, mean of 35 and diastolic of 25 may be considered as lower limits of normal systemic blood pressure for term infants during the first postnatal week.
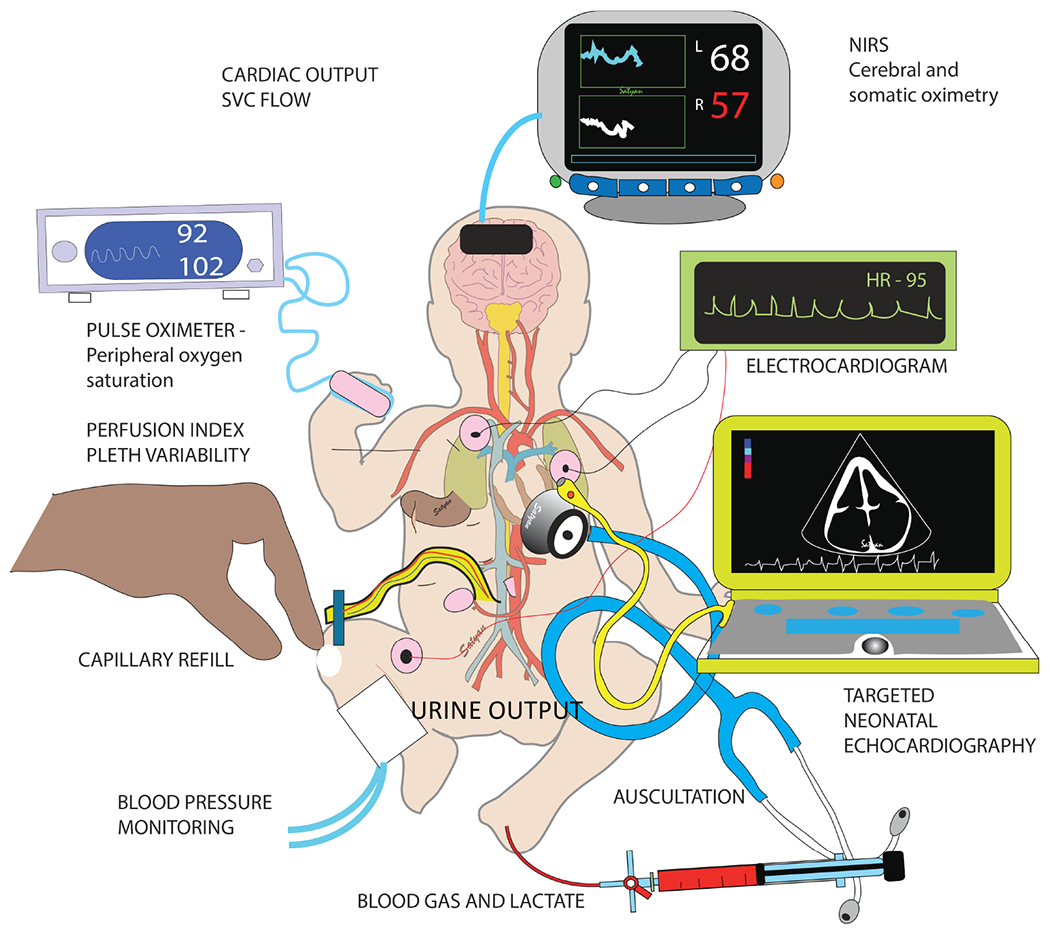
Figure 2. Assessment of perfusion:
clinical signs such as mental status (consciousness), capillary refill, blood pressure and urine output coupled with pulse oximetry, perfusion index, pleth variability index, targeted bedside echocardiography, electrocardiography (EKG), near-infrared spectroscopy (NIRS) and invasive monitoring with blood gas and lactate should be collectively interpreted to assess perfusion. Copyright Satyan Lakshminrusimha.
Hemodynamic assessment – flow, perfusion vs. pressure
Systemic blood pressure and heart rate are the most commonly used parameters for assessment of systemic perfusion. However, blood pressure does not correlate well with perfusion in neonates. Mean and diastolic pressures correlate poorly with LV output.14 Pulse pressure and, to a less extent, systolic blood pressure correlate better with LV output.14 Clinical signs such as mental status, capillary refill and urine output are compromised during late phases of systemic hypoperfusion. Non-invasive monitoring of perfusion index (PI), pleth variability index (PVI), pulse oximetry (SpO2), and near-infrared spectroscopy (NIRS) can detect changes in systemic perfusion.15 Blood gas analysis and serum lactate are invasive mechanisms to assess systemic perfusion. In the last decade, bedside targeted neonatal echocardiography has become a popular mode of hemodynamic assessment.16
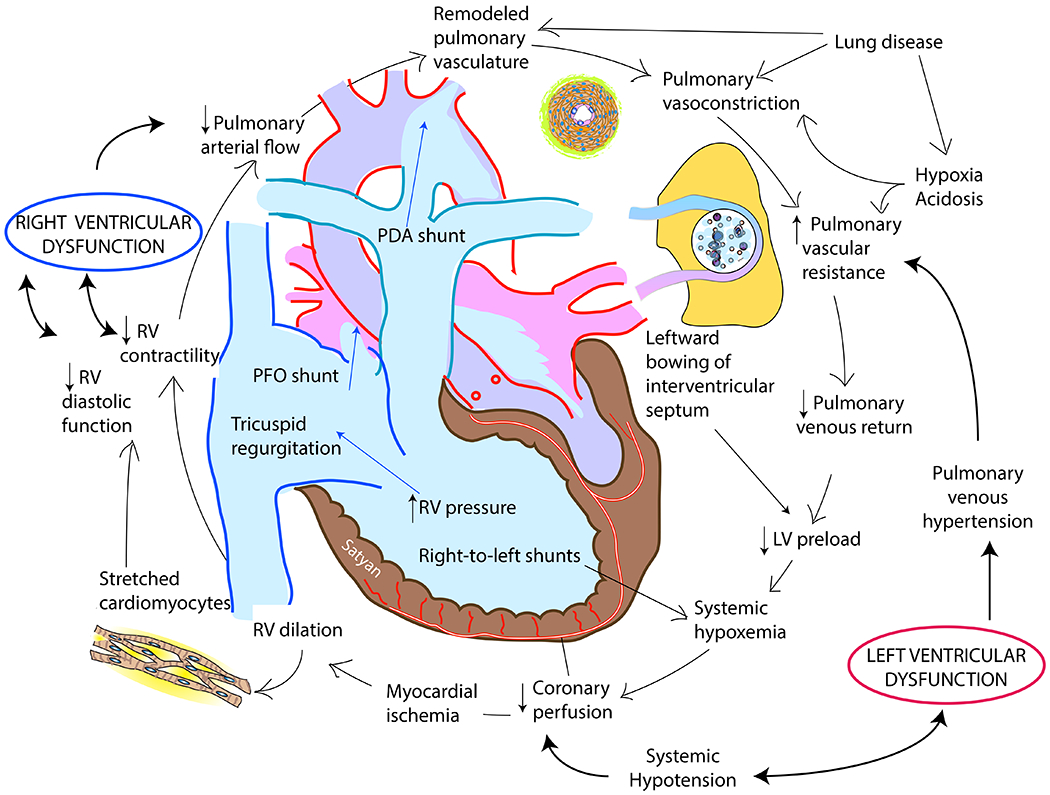
Causes of systemic hypotension in PPHN
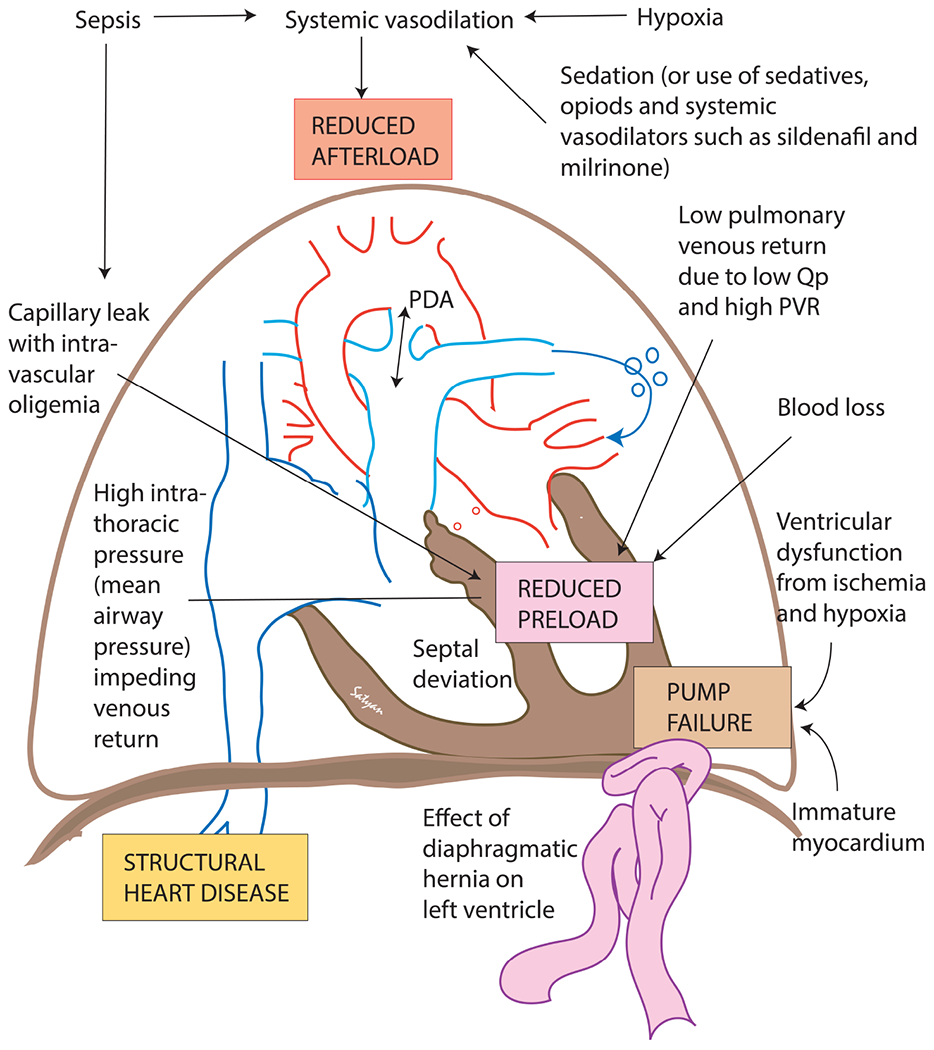
Low SVR and systemic hypotension requiring treatment are common in infants with PPHN. Two-thirds of infants with PPHN requiring ventilation and 87% infants requiring ECMO are on 3 or more inotropes.17 The etiology of hypotension in PPHN is summarized in table 3 and figure 3.
Table 3.
Etiology of systemic hypotension in persistent pulmonary hypertension of the newborn
| Mechanism | Causes |
|---|---|
| Reduced LV afterload | systemic vasodilation from sepsis, hypoxia, sedation or vasodilators such as milrinone, prostacyclin analogs or sildenafil |
| Reduced LV preload | low pulmonary venous return, high intrathoracic pressure impeding venous return, blood loss, dehydration and septal deviation to the left |
| Pump failure | ventricular dysfunction from hypoxemia, acidosis, ischemia or inflammation |
| Structural heart disease | coexisting left ventricular hypoplasia with congenital diaphragmatic hernia (CDH) or severe asymmetric septal hypertrophy due to uncontrolled maternal diabetes |
Figure 3. Etiology of systemic hypotension in PPHN.
Please see table 3 for details. Copyright Satyan Lakshminrusimha.
Fluid management
Many infants with systemic hypotension and PPHN receive fluid boluses prior to initiation of vasoactive agents.17 The relationship between intravascular blood volume and blood pressure is not clear in neonates.18 Additionally, the mechanism of hypotension in PPHN is often not due to hypovolemia. In fact, routine administration of fluid boluses to an infant with PPHN without clinical evidence of hypovolemia, may potentially further exacerbate RV failure. Thus, fluid bolus administration should be targeted and only administered if there are other indications of hypovolemia, such as low central venous pressure (CVP), documented excessive losses or concerns for increased insensible losses.
Vasoactive infusions
If the hypotension or poor perfusion does not appear to be due to hypovolemia, vasoactive infusions are commonly initiated. The goal of vasoactive therapy is to improve oxygen delivery and perfusion (figure 4). The use of vasoactives to increase systemic blood pressure to supraphysiological levels (higher than the means shown in table 2) to minimize right-to-left shunt is not appropriate. Such practice increases RV afterload and hastens RV failure.19 Also, in the setting of LV dysfunction, postductal systemic perfusion is supplemented by a transductal right-to-left shunt. Additionally, if an infant appears to have evidence of adequate oxygen delivery (i.e. normal lactate, normal urine output, no lethargy and without evidence of end-organ injury), despite having hypotension, then tolerating permissive hypotension may be appropriate. It is important to focus on evaluation and correction of hypoperfusion and not manage blood pressure numbers during management of PPHN.
Figure 4. The vicious cycle of pulmonary hypertension, systemic hypotension and cardiac dysfunction in PPHN.
Selective pulmonary vasodilators to treat pulmonary hypertension, selective systemic vasoconstrictors to treat systemic hypotension and/or reducing afterload / optimizing preload are important strategies to interrupt this vicious cycle. Modified from an unpublished e-review by Dany Weisz, Patrick McNamara and Amish Jain. Copyright Satyan Lakshminrusimha.
Several vasoactive medications may be useful to treat hypotension associated with PPHN in neonates. All vary in mechanisms of action (figure 5) and may provide benefit or harm in different physiologic circumstances. For example, vasoactive infusions can have the following effects: inotropy (heart contractility), chronotropy (heart rate), lusitropy (heart relaxation), vasoconstriction (or vasopressors) and/or vasodilation. Some vasoactive medications have more than one effect and the effects can vary depending on dose. Understanding the physiology behind the neonate’s hypotension and the medication’s mechanism of action is necessary to choose the best treatment. A synopsis of each medication is provided in Boxes 1–8 and figure 6.
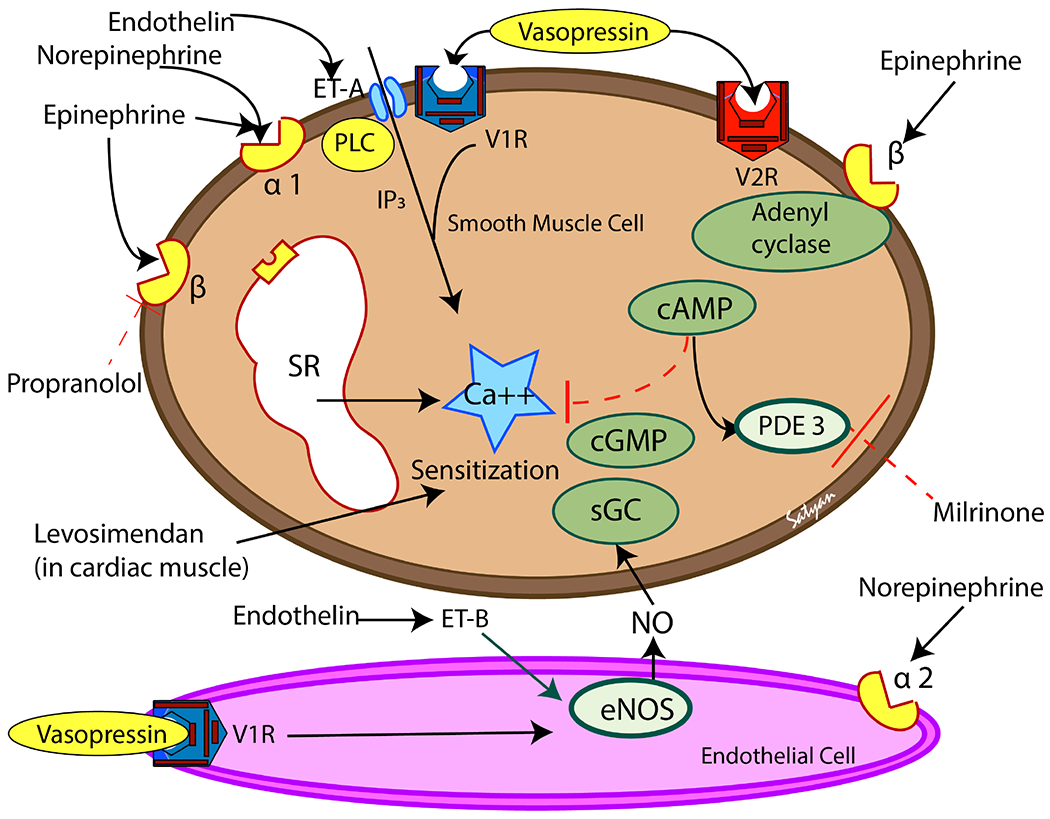
Figure 5. Mechanism of action of vasoactive medications in vascular smooth muscle (brown-top) and endothelial cell (pink-bottom).
V1R and V2R and vasopressin receptors. ET-A and ET-B are endothelin receptors, α1, α2, β1 and β2 are adrenergic receptors. PLC – phospholipase C, IP3 – inositol triphosphate; eNOS – endothelial nitric oxide synthase, NO – nitric oxide, sGC – soluble guanylyl cyclase, PDE3 – phosphodiesterase 3. Most of the vasoconstrictor mediators result in increase in cytosolic ionic calcium concentrations in the smooth muscle. Vasopressin (through V1 receptors), endothelin (through ET-B receptors) and norepinephrine (through α2 receptors) can act on pulmonary endothelium and stimulate NO production leading to pulmonary vasodilation. Copyright Satyan Lakshminrusimha.
Box 1. Dopamine.
| Indication | Decreased cardiac function, hypotension, hypoxemia |
| Dose | 2 to 20 mcg/kg/min (doses > 10-15 mcg/kg/min likely to have pulmonary vasoconstriction) |
| Precautions | May increase pulmonary pressure further adding to RV afterload; Watch for extravasation – (extravasation will need therapy with Phentolamine) |
| Contraindications | Supra-physiologic systemic pressures |
| Class of Recommendation | Class IIb – Usefulness/efficacy is less well established by evidence/opinion. May be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 8. Hydrocortisone.
| Indication | Catecholamine-resistant systemic hypotension Refractory pulmonary hypertension not responsive to inhaled nitric oxide |
| Dose | Acute crisis: 1 mg/kg/dose q 6 h Maintenance: 1 mg/kg/day in divided doses Severe PPHN not due to infection – 4 mg/kg/dose x 1 followed by 1 mg/kg q 6h x 4 doses followed by maintenance |
| Precautions | Occult infection – fungal, viral (herpes simplex, CMV etc.,), bacterial can be exacerbated with steroid therapy |
| Contraindications | Known or suspected infection |
| Class of Recommendation | Class IIb – Usefulness/efficacy is less well established by evidence/opinion. May be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
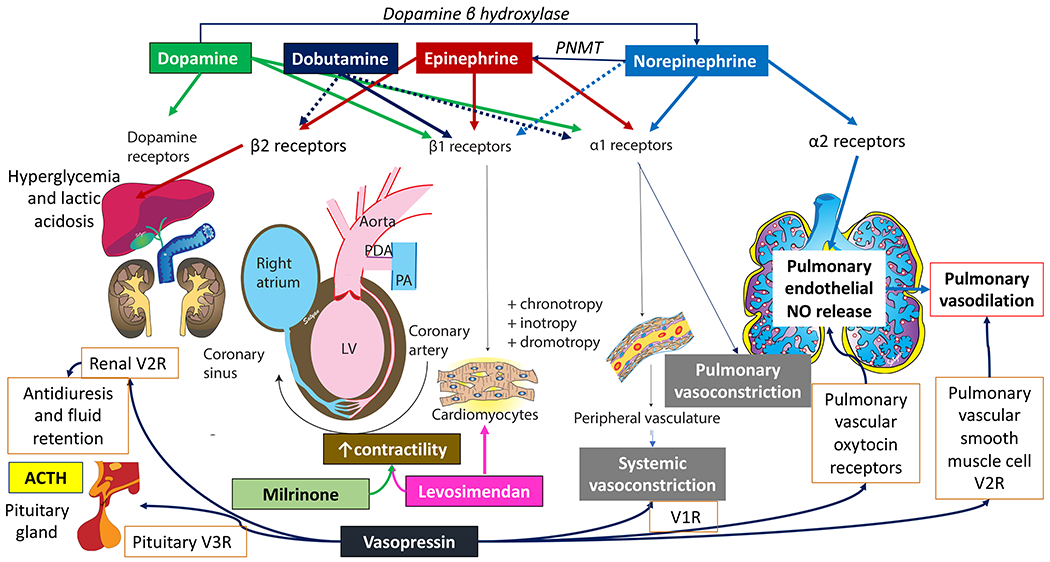
Figure 6. Vasoactive agents and their receptor distribution and action in various systems.
Dopamine is a precursor of norepinephrine. Norepinephrine is converted to epinephrine by Phenylethanolamine methyl transferase (PNMT). Dopamine has a dose-dependent effect on dopamine (D1 and D2) receptors, β1 and α1 receptors. Epinephrine is equally effective on β1 and α1 receptors. Norepinephrine predominantly acts on α1 receptors causing systemic vasoconstriction. It is thought to stimulate α2 receptors in pulmonary vascular endothelium and release NO leading to pulmonary vasodilation. Milrinone and levosimendan increases cardiac contractility. Vasopressin acts on V1 receptors to induce systemic vasoconstriction, V2 causing fluid retention in the kidneys and V3 leading to pituitary stimulation to produce ACTH. Vasopressin also stimulates pulmonary vascular endothelial V1 receptor and pulmonary vascular smooth muscle V2 receptor to induce pulmonary vasodilation. Copyright Satyan Lakshminrusimha.
Dopamine
Dopamine has conventionally been the first line therapy for hypotension in neonates including management of septic shock.20 However, its non-specific mechanism of action and effects on pulmonary versus systemic circulatory system has special implications in PPHN. Dopamine is a central neurotransmitter and is also a precursor to norepinephrine. It directly stimulates D1 and D2 receptors and directly (or through metabolism to norepinephrine) stimulates α1, β1 and β2 receptors. Therefore, dopamine can result in vasoconstriction, vasodilation, inotropy, and/or chronotropy depending on the dose.21 Additionally, the effect dopamine has on systemic versus pulmonary circulation varies with dose.22 For example, in a neonatal lamb model, dopamine selectively increased systemic arterial pressure at lower doses without significantly increasing pulmonary arterial pressure, thus increasing pulmonary blood flow in lambs without PPHN.2 However, in lambs with PPHN, the pulmonary vasculature is remodeled and pulmonary arterial pressure is more sensitive to vasoconstrictor effects of dopamine, and therefore dopamine did not increase pulmonary blood flow.2
In human neonates, the varying effect of dopamine on pulmonary arterial pressure has also been shown.22 In a study of 18 preterm newborns, the ratio of pulmonary/systemic arterial pressure increased (a preferential increase in pulmonary arterial pressure) in half of the newborns.22 This same study demonstrated changes in direction of blood flow across the PDA. After dopamine initiation, 2 of 11 newborns had PDA flow change from initially left-to-right to bidirectional shunting suggesting further elevation of PVR.22. Thus, in a newborn with PPHN requiring dopamine infusion, monitoring of the directionality of PDA flow and pulmonary arterial pressure with serial echocardiograms is necessary.
Norepinephrine
Norepinephrine is more selective than dopamine with regards to receptor stimulation, acting primarily on α1 receptors resulting in vasoconstriction and minimal inotropic effect on β1 receptors. The vasoconstriction mechanism could affect both systemic and pulmonary arterial pressure. Interestingly, fetal lamb models have shown norepinephrine may decrease the basal pulmonary vascular tone through stimulation of α2 receptors and nitric oxide release.23 In newborns with PPHN, norepinephrine has been shown to increase pulmonary arterial pressure; however, unlike dopamine, the ratio of pulmonary/systemic arterial pressure decreased following norepinephrine infusion (0.98 to 0.87, p < 0.001).24 This study also noted decreased oxygen requirement and increased post-ductal oxygen saturation supporting the notion of increased pulmonary blood flow following norepinephrine infusion.24
Epinephrine
Epinephrine is less selective than norepinephrine and its stimulation on α and β receptors vary by dose. At lower doses epinephrine has predominant β effect causing chronotropy and inotropy. Thus, for an infant with depressed myocardial function, epinephrine may be useful. In pediatric trials, epinephrine has been shown to be superior to dopamine with faster resolution of shock and lower mortality.25,26 However, in neonatal trials, epinephrine and dopamine were comparable.27,28 However, epinephrine was associated with more metabolic disturbances such as hyperglycemia and lactic acidosis.27
Dobutamine
Dobutamine is predominantly a β1 agonist resulting in significant inotropic effect. Thus, it may be useful for neonates with decreased cardiac function. However, the potential chronotropic effect must be considered. In a review of dobutamine, neonatal studies noted increased heart rate for all doses evaluated (5, 10, and 20 mcg/kg/min – studies of 2.5 and 7.5 mcg/kg/min did not report on heart rate).29 Studies also noted both increase and decrease in PVR with dobutamine.29 Specifically in neonates, a study among premature infants with depressed myocardial function noted decreased fraction of inspired oxygen (FiO2) following dobutamine initiation, potentially reflecting increased pulmonary blood flow.30 Additionally, studies have shown dobutamine improves and maintains systemic blood pressure better than dopamine and with less left ventricular stress.31,32 While dobutamine is predominately a β1 agonist, it is also a mild β2 and α1 agonist. The β2 effects of dobutamine are thought to reduce SVR, however the reduction in SVR more likely due to sympathetic withdrawal once cardiac function has improved. The overall net effect is expected to be significant inotropy, such that the other effects of dobutamine are likely trivial in comparison. However, it most suitable for patient with LV dysfunction as the primary or major contributing factor leading to poor perfusion.
Milrinone
Milrinone is a phosphodiesterase 3 inhibitor which results in increased levels of cyclic adenosine monophosphate (cAMP). The increase in cAMP results in inotropic effect on myocardium and vasodilation, which may benefit neonates with PPHN and impaired myocardial function. In neonatal lambs, milrinone resulted in relaxation of pulmonary arteries in both controls and lambs with PPHN.33 In a study of 11 newborns with PPHN, milrinone has been shown to improve PaO2 and reduce FiO2, iNO, and mean airway pressure needs – suggesting improved pulmonary blood flow.34 Additionally, while the newborns experienced transient decrease in systemic arterial pressures, overall hemodynamics improved as noted by decreased lactic acid and a trend towards decreased inotropic score.34 However, this study excluded infants with systemic hypotension.34 The systemic vasodilator effects of milrinone need to be considered, particularly in the setting of PPHN. Additionally, studies have generally only evaluated outcomes during the first 72 hours of milrinone initiation. Thus, while it is likely safe for longer administration, the effects beyond this in patients with PPHN are unknown.34,35 There are current two multicenter trials underway evaluating the use of milrinone in CDH36 and PPHN.37
Levosimendan
See Web Appendix for levosimendan details.
Vasopressin
There are three subtypes of vasopressin receptors, V1-3. V1 receptors are located in the vasculature beds and commonly known for their potent vasoconstrictive properties on systemic vasculature with minimal increase/decrease in PVR leading to a decrease in the pulmonary/systemic arterial pressure ratio.39 The mechanism of vasodilation in the pulmonary vasculature is thought to be from stimulation of oxytocin endothelial receptors and subsequent NO pathway activation.40 Siehr et al. evaluated three vasoactive medications (phenylephrine, vasopressin and epinephrine) in 15 pediatric patients with pulmonary hypertension, and vasopressin was the only infusion that consistently decreased the pulmonary/systemic arterial pressure ratio.39
Specifically in newborns, Mohamed et al. reported a case series of newborns with PPHN in which vasopressin was used as a “rescue” therapy for refractory pulmonary hypertension and systemic hypotension despite iNO and vasoactive infusions.41 In this series, vasopressin resulted in improved oxygenation index and reduced iNO.41 Three of the 10 newborns were also receiving milrinone. However, the improvement in oxygenation index persisted when excluding the newborns that received milrinone from the analysis.41 However, it is notable that 4 of the 10 newborns died, and two required extracorporeal life support (ECLS), which may reflect their severity of illness at the time vasopressin was initiated as a rescue therapy in this series.41 Acker et al. reported a case of 13 newborns with CDH that received vasopressin for refractory hypotension. All 13 newborns in this series met ECLS criteria prior to vasopressin initiation. Eleven patients received vasopressin prior to initiation of ECLS (the other two received vasopressin concurrently with ECLS initiation). In 6 of those 11, ECLS was no longer indicated due to overall improved hemodynamics.42 In both the Acker and Mohamed series, vasopressin was used essentially as a rescue therapy after other interventions failed. Thus, earlier initiation of vasopressin in newborns with pulmonary hypertension warrants additional research.
However, it is notable that prior studies did not elaborate on the mechanism of hypotension in the newborns that received vasopressin. Assumingly, when considering its mechanism of action, vasopressin could be beneficial for a patient with low SVR and good LV function and potentially harmful for a newborn with poor LV dysfunction by increasing SVR (or LV afterload). Contrary to this assumption, low dose vasopressin has been beneficial in the post-operative management of newborns following the Norwood procedure or arterial switch operation (less fluid resuscitation needs and lower inotropic scores in the first 24 hours in the vasopressin group).43 The low cardiac output state of these newborns is multifactorial, but likely involves some degree of LV dysfunction. The lack of deleterious effects from vasopressin in these situations may be related to improved coronary perfusion.44 None the less, more information on the pathophysiology of newborns with PPHN treated with vasopressin will be useful in future studies to help better determine situations in which it should be used.
The effect vasopressin has on sodium and water balance has to be considered prior to initiation. Vasopressin can alter sodium and water balance via two mechanisms – V1 receptors result in peripheral vasoconstriction and thus improved renal blood flow, and V2 receptors have antidiuretic effects resulting in reabsorption of free water. This last mechanism has the potential to cause hyponatremia. In the Acker et al. series, they reported hyponatremia complications for the newborns that did not require ECLS, which they explain was due to differences in vasopressin doses used in the two groups. All 6 of the newborns experienced a decrease in serum sodium (average nadir 117.8 mmol/L, range 111-121) and were also treated for hyponatremia.42 Mohamed et al. reported “negligible change in serum sodium” among their newborn series.41 The degree of shock and mechanism of shock likely impacts the effect vasopressin has on sodium balance. For example, a review noted minimal or no cases of hyponatremia among studies of adults and children when vasopressin was used in settings of vasodilatory shock, however hyponatremia was commonly noted when vasopressin was used for indications other than vasodilatory shock.45 None the less, use of vasopressin in neonates requires monitoring of serum sodium and research evaluating the effect on serum sodium at varying doses and varying clinical indications in neonates may be helpful.
Hydrocortisone
Corticotrophin-releasing hormone (CRH), the hypothalamic peptide regulating the hypothalamus-pituitary-adrenal (HPA) axis in response to stress, is expressed in abundance in the human placenta and contributes to fetal cortisol during late gestation.46 This source of CRH is lost following birth. Late preterm and term infants requiring vasopressor support have low cortisol levels but a normal response to exogenous ACTH.47 Randomized trials to evaluate cortisol in term infants are warranted but are difficult to conduct.48,49 The optimal indication and dose of hydrocortisone in neonatal hypotension is not clear.48,49 However, cases demonstrating response to exogenous glucocorticoids in catecholamine-resistance hypotension are reported.50
The mechanisms of cardiovascular effects of hydrocortisone administration are not completely understood, but both genomic and nongenomic steroidal effects seem to play a role. 51 Hydrocortisone administration to preterm and term infants with vasopressor-resistant hypotension is associated with an improvement in BP, stoke volume (and a trend to increase in cardiac output) with a decrease in heart rate and need for vasoactive medications.51 Genomic upregulation of cardiovascular adrenergic and angiotensin receptors and inhibition of inducible nitric oxide synthase and prostaglandins are potential mechanisms. In addition, non-genomic effects such as better capillary integrity, inhibition of catecholamine metabolism and increase in intracellular calcium may be mainly responsible for the rapid onset of the hydrocortisone-induced BP improvement.51
In addition to increasing blood pressure, glucocorticoids might have an effect on the lung in PPHN. Glucocorticoids may have beneficial effects in meconium aspiration syndrome52 but there is a concern of exacerbation of infection and lack of multicenter randomized trials.53 Animal studies have shown that high dose hydrocortisone inhibits phosphodiesterase 5 enzyme (PDE 5) and enhances oxygenation response in PPHN.54,55 A single-center case series using 4 mg/kg loading dose followed by 1 mg/kg/dose q 6h in PPHN resistant to conventional therapy showed significant improvement in oxygenation and blood pressure with a reduction in the need for vasopressors.56
Special considerations
Infants born to mothers with diabetes (IDM) may have hypertrophic cardiomyopathy and impaired cardiac output that must be considered when choosing vasoactive medications. See Web Appendix for more.
Extracorporeal Life Support (ECLS)
Extracorporeal life support (ECLS) is a treatment option for newborns with PPHN, of which more information is in the Web Appendix.
CONCLUSION
PPHN may be characterized by a multitude of physiologic imbalances such an increased PVR to SVR ratio, or due to LV dysfunction or increased pulmonary blood flow that result in increased pulmonary artery pressure. Systemic hypotension is extremely common among infants with PPHN and should be identified early. Systemic hypotension is almost invariably a component of the clinical syndrome of PPHN but may have a variety of mechanisms that require thoughtful and targeted assessment and therapy. Further trials evaluating various vasoactive agents in PPHN are warranted.
Supplementary Material
Box 2. Norepinephrine.
| Indication | Hypotension without decreased cardiac function, hypoxemia |
| Dose | 0.05 to 0.5 mcg/kg/min – occasionally may need up to 1 mcg/kg/min in PPHN with severe hypotension24 |
| Precautions | Increased systemic vasoconstriction may decrease mesenteric perfusion and tolerance/safety of feeding Watch for extravasation – (extravasation will need therapy with Phentolamine) |
| Contraindications | Significant cardiac dysfunction unless using with an inotropic agent as well |
| Class of Recommendation | Class IIa – Weight of evidence/opinion is in favor of usefulness/efficacy. Should be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 3. Epinephrine.
| Indication | Decreased cardiac function, hypotension, hypoxemia |
| Dose | 0.05 to 0.3 mcg/kg/min |
| Precautions | Metabolic disturbances, arrhythmias |
| Contraindications | Potentially prematurity due to propensity to metabolic disturbances |
| Class of Recommendation | Class IIb – Usefulness/efficacy is less well established by evidence/opinion. May be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 4. Dobutamine.
| Indication | Decreased cardiac function, hypotension, hypoxemia |
| Dose | 2 to 25 mcg/kg/min (doses 10 > mcg/kg/min likely to have chronotropic effect) |
| Precautions | Arrythmias, tachycardia |
| Contraindications | Normal cardiac function |
| Class of Recommendation | Class IIa – Weight of evidence/opinion is in favor of usefulness/efficacy. Should be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 5. Milrinone.
| Indication | Hypoxemia, decreased cardiac function, normal blood pressure or hypotension that is either mild or can be treated with another agent |
| Dose | 0.25 to 1.0 mcg/kg/min (a loading dose is optional 25-75 mcg/kg and carries a higher risk of hypotension) |
| Precautions | May cause or worsen systemic hypotension due to vasodilation |
| Contraindications | Significant systemic hypotension or vasodilatory shock Renal dysfunction |
| Class of Recommendation | IIb38 – Usefulness/efficacy is less well established by evidence or opinion |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 6. Levosimendan.
| Indication | Decreased cardiac function with risk of arrhythmia |
| Dose | 6 to 12 mcg/kg loading dose over 30 min followed by 0.1 mcg/kg/min for 6 h and then increase to 0.2 mcg/kg/min if tolerated |
| Precautions | Reduce milrinone dose if used concomitantly |
| Contraindications | Asphyxia due to potential altered affects in the pulmonary vasculature following asphyxia |
| Class of Recommendation | Class IIb – Usefulness/efficacy is less well established by evidence/opinion. May be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Box 7. Vasopressin.
| Indication | Refractory pulmonary hypertension and systemic hypotension, however no literature in earlier use |
| Dose | 0.1 to 2 milliunits (mU)/kg/min |
| Precautions | Hyponatremia, systemic vasoconstriction may affect mesenteric perfusion and tolerance/safety of feeding, and may be deleterious in the setting of LV dysfunction. |
| Contraindications | Hyponatremia |
| Class of Recommendation | Class IIb – Usefulness/efficacy is less well established by evidence/opinion. May be considered |
| Level of Evidence | C – Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
ACKNOWLEGMENTS
FUNDING
Dr. Siefkes’s effort was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) (through grant UL1 TR001860 and linked award KL2 TR001859) and by Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) (1R21 1HD099239-01). Dr. Lakshminrusimha was supported by NICHD, NIH (5R01 HD072929-09). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH. Funded by the National Institutes of Health (NIH).
Footnotes
COMPETING INTERESTS
The authors have no other financial relationships relevant to this article to disclose.
References
- 1.Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26(3):601–619. doi: 10.1016/s0095-5108(18)30039-3 [DOI] [PubMed] [Google Scholar]
- 2.Lakshminrusimha S. The pulmonary circulation in neonatal respiratory failure. Clin Perinatol. 2012;39(3):655–683. doi: 10.1016/j.clp.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair J, Lakshminrusimha S. Update on PPHN: Mechanisms and treatment. Semin Perinatol. 2014;38(2):78–91. doi: 10.1053/j.semperi.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentles TL. The Right Ventricle and Persistent Pulmonary Hypertension of the Newborn Commentary on Patel N et al. Assessment of Right Ventricular Function Using Tissue Doppler Imaging in Infants with Pulmonary Hypertension. Neonatology. 2009;96:200–202. doi: 10.1159/000215586 [DOI] [PubMed] [Google Scholar]
- 5.Arrigo M, Huber LC, Winnik S, et al. Right Ventricular Failure: Pathophysiology, Diagnosis and Treatment. Card Fail Rev. 2019;5(3):140–146. doi: 10.15420/cfr.2019.15.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel N, Lally PA, Kipfmueller F, et al. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2019;200(12):1522–1530. doi: 10.1164/rccm.201904-0731OC [DOI] [PubMed] [Google Scholar]
- 7.Tiwary S, Geethanath RM, Abu-Harb M. Vein of Galen malformation presenting as persistent pulmonary hypertension of newborn (PPHN). BMJ Case Rep. 2013;2013:bcr2013200425. doi: 10.1136/bcr-2013-200425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander CP, Sood BG, Zilberman MV., et al. Congenital hepatic arteriovenous malformation: An unusual cause of neonatal persistent pulmonary hypertension. J Perinatol. 2006;26(5):316–318. doi: 10.1038/sj.jp.7211493 [DOI] [PubMed] [Google Scholar]
- 9.Singh Y, Shore H. Congenital Hepatic Arteriovenous Malformation with Persistent Pulmonary Hypertension: An Unusual Presentation. Infant. 2017;13(6):242–244. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=126328994&%0AIang=fr&site=ehost-live [Google Scholar]
- 10.Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139(1). doi: 10.1542/peds.2016-1165 [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Farooki ZQ, Desai R. β-Hemolytic Streptococcal Infection Appearing as Persistent Fetal Circulation. Am J Dis Child. 1982;136(8):725–727. doi: 10.1001/archpedi.1982.03970440069020 [DOI] [PubMed] [Google Scholar]
- 12.Wehrmann M, Patel S, Haxel C, et al. Implications of Atrial-Level Shunting by Echocardiography in Newborns with Congenital Diaphragmatic Hernia. J Pediatr. 2020;219:43–47. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento MCVA, Xavier CC, Goulart EMA. Arterial blood pressure of term newborns during the first week of life. Brazilian J Med Biol Res. 2002;35(8):905–911. doi: 10.1590/S0100-879X2002000800007 [DOI] [PubMed] [Google Scholar]
- 14.Kharrat A, Rios Dl, Weisz DE, et al. The Relationship between blood pressure parameters and left ventricular output in neonates. J Perinatol. 2019;39(5):619–625. doi: 10.1038/s41372-019-0337-6 [DOI] [PubMed] [Google Scholar]
- 15.Sahni R. Continuous noninvasive monitoring in the neonatal ICU. Curr Opin Pediatr. 2017;29(2): 141–148. doi: 10.1097/MOP.0000000000000459 [DOI] [PubMed] [Google Scholar]
- 16.Giesinger RE, Stanford AH, Rios DR, et al. Targeted neonatal echocardiography in the United States of America: the contemporary perspective and challenges to implementation. Pediatr Res. 2019;85(7):919–921. doi: 10.1038/s41390-019-0338-3 [DOI] [PubMed] [Google Scholar]
- 17.Mydam J, Zidan M, Chouthai NS. A Comprehensive Study of Clinical Biomarkers, Use of Inotropic Medications and Fluid Resuscitation in Newborns with Persistent Pulmonary Hypertension. Pediatr Cardiol. 2015;36(1):233–239. doi: 10.1007/s00246-014-0992-5 [DOI] [PubMed] [Google Scholar]
- 18.Barr PA, Bailey PE, Sumners J, et al. Relation Between Arterial Blood Pressure and Blood Volume and Effect of Infused Albumin in Sick Preterm Infants. Pediatrics. 1977;60(3):282–289. [PubMed] [Google Scholar]
- 19.Lakshminrusimha S, Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews. 2015;16(12):e680–e694. doi: 10.1542/neo.16-12-e680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061–1093. doi: 10.1097/CCM.0000000000002425 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Penny DJ, Kim NS, et al. Mechanisms of blood pressure increase induced by dopamine in hypotensive preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81(2):F99–F104. doi: 10.1136/fn.81.2.F99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liet J-M, Boscher C, Gras-Leguen C, et al. Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J Pediatr. 2002;140(3):373–375. doi: 10.1067/mpd.2002.123100 [DOI] [PubMed] [Google Scholar]
- 23.Jaillard S, Elbaz F, Bresson-Just S, et al. Pulmonary vasodilator effects of norepinephrine during the development of chronic pulmonary hypertension in neonatal lambs. Br J Anaesth. 2004;93(6):818–824. doi: 10.1093/bja/aeh278 [DOI] [PubMed] [Google Scholar]
- 24.Tourneux P, Rakza T, Bouissou A, et al. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr. 2008;153(3):345–349. doi: 10.1016/j.jpeds.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 25.Ventura AMC, Shieh HH, Bousso A, et al. Double-Blind Prospective Randomized Controlled Trial of Dopamine Versus Epinephrine as First-Line Vasoactive Drugs in Pediatric Septic Shock. Crit Care Med. 2015;43(11):2292–2302. doi: 10.1097/CCM.0000000000001260 [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy KN, Singhi S, Jayashree M, et al. Double-Blind Randomized Clinical Trial Comparing Dopamine and Epinephrine in Pediatric Fluid-Refractory Hypotensive Septic Shock*. Pediatr Crit Care Med. 2016;17(11):e502–e512. doi: 10.1097/PCC.0000000000000954 [DOI] [PubMed] [Google Scholar]
- 27.Valverde E, Pellicer A, Madero R, et al. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: Analysis of systemic effects and neonatal clinical outcomes. Pediatrics. 2006;117(6). doi: 10.1542/peds.2005-2108 [DOI] [PubMed] [Google Scholar]
- 28.Baske K, Saini SS, Dutta S, et al. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur J Pediatr. 2018;177(9):1335–1342. doi: 10.1007/s00431-018-3195-x [DOI] [PubMed] [Google Scholar]
- 29.Mahoney L, Shah G, Crook D, et al. A Literature Review of the Pharmacokinetics and Pharmacodynamics of Dobutamine in Neonates. Pediatr Cardiol. 2016;37:14–23. doi: 10.1007/s00246-015-1263-9 [DOI] [PubMed] [Google Scholar]
- 30.Robel-Tillig E, Knüpfer M, Pulzer F, et al. Cardiovascular impact of dobutamine in neonates with myocardial dysfunction. Early Hum Dev. 2007;83(5):307–312. doi: 10.1016/j.earlhumdev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 31.Osborn DA, Evans N, Kluckow M. Left ventricular contractility in extremely premature infants in the first day and response to inotropes. Pediatr Res. 2007;61(3):335–340. doi: 10.1203/pdr.0b013e318030d1e1 [DOI] [PubMed] [Google Scholar]
- 32.Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr. 2002;140(2):183–191. doi: 10.1067/mpd.2002.120834 [DOI] [PubMed] [Google Scholar]
- 33.Lakshminrusimha S, Porta NFM, Farrow KN, et al. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009;10(1):106–112. doi: 10.1097/PCC.0b013e3181936aee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara PJ, Shivananda SP, Sahni M, et al. Pharmacology of Milrinone in Neonates With Persistent Pulmonary Hypertension of the Newborn and Suboptimal Response to Inhaled Nitric Oxide*. Pediatr Crit Care Med. 2013;14(1):74–84. doi: 10.1097/PCC.0b013e31824ea2cd [DOI] [PubMed] [Google Scholar]
- 35.James A, Corcoran J, Mcnamara P, et al. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension Vasopressin as a Rescue Therapy for Refractory Pulmonary Hypertension in Neonates: Case Series View project Article C. Cardiol Young. 2016;26:90–99. doi: 10.1017/S1047951114002698 [DOI] [PubMed] [Google Scholar]
- 36.Lakshminrusimha S, Keszler M, Kirpalani H, et al. Milrinone in congenital diaphragmatic hernia – a randomized pilot trial: study protocol, review of literature and survey of current practices. Matern Heal Neonatol Perinatol. 2017;3(1):1–15. doi: 10.1186/s40748-017-0066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EL-Khuffash A, McNamara PJ, Breatnach C, et al. The use of milrinone in neonates with persistent pulmonary hypertension of the newborn - a randomised controlled trial pilot study (MINT 1): study protocol and review of literature. Matern Heal Neonatol Perinatol. 2018;4(1):1–12. doi: 10.1186/s40748-018-0093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Hear Lung Transplant. 2019;38(9):879–901. doi: 10.1016/j.healun.2019.06.022 [DOI] [PubMed] [Google Scholar]
- 39.Siehr SL, Feinstein JA, Yang W, et al. Hemodynamic effects of phenylephrine, vasopressin, and epinephrine in children with pulmonary hypertension: A pilot study. Pediatr Crit Care Med. 2016;17(5):428–437. doi: 10.1097/PCC.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 40.Thibonnier M, Conarty DM, Preston JA, et al. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140(3):1301–1309. doi: 10.1210/endo.140.3.6546 [DOI] [PubMed] [Google Scholar]
- 41.Mohamed A, Nasef N, Shah V, et al. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr Crit Care Med. 2014;15(2):148–154. doi: 10.1097/PCC.0b013e31829f5fce [DOI] [PubMed] [Google Scholar]
- 42.Acker SN, Kinsella JP, Abman SH, et al. Vasopressin improves hemodynamic status in infants with congenital diaphragmatic hernia. J Pediatr. 2014;165(1):53–58.e1. doi: 10.1016/j.jpeds.2014.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alten JA, Borasino S, Toms R, et al. Early initiation of arginine vasopressin infusion in neonates after complex cardiac surgery. Pediatr Crit Care Med. 2012;13(3):300–304. doi: 10.1097/PCC.0b013e31822f1753 [DOI] [PubMed] [Google Scholar]
- 44.Romanowski BGL, Pharm D. Congenital Heart Defects, Heart Surgeries, Low Cardiac Output Syndrome. Published online 2020. [Google Scholar]
- 45.Salazar M, Hu BB, Vazquez J, et al. Exogenous Vasopressin-Induced Hyponatremia in Patients With Vasodilatory Shock: Two Case Reports and Literature Review. J Intensive Care Med. 2015;30(5):253–258. doi: 10.1177/0885066613507410 [DOI] [PubMed] [Google Scholar]
- 46.McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121(4):493–501. doi: 10.1530/rep.0.1210493 [DOI] [PubMed] [Google Scholar]
- 47.Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol. 2008;28(12):797–802. doi: 10.1038/jp.2008.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez EF, Watterberg KL. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009;29(2):S44–S49. doi: 10.1038/jp.2009.24 [DOI] [PubMed] [Google Scholar]
- 49.Watterberg KL, Fernandez E, Walsh MC, et al. Barriers to enrollment in a randomized controlled trial of hydrocortisone for cardiovascular insufficiency in term and late preterm newborn infants. J Perinatol. 2017;37(11):1220–1223. doi: 10.1038/jp.2017.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tantivit P, Subramanian N, Garg M, et al. Low serum cortisol in term newborns with refractory hypotension. J Perinatol. 1999;19(5):352–357. doi: 10.1038/sj.jp.7200202 [DOI] [PubMed] [Google Scholar]
- 51.Noori S, Friedlich P, Wong P, et al. Hemodynamic changes after low-dosage hydrocortisone administration in vasopressor-treated preterm and term neonates. Pediatrics. 2006;118(4):1456–1466. doi: 10.1542/peds.2006-0661 [DOI] [PubMed] [Google Scholar]
- 52.Tripathi S, Saili A. Effect of Steroids on the Clinical Course and Outcome of Neonates with Meconium Aspiration Syndrome | Journal of Tropical Pediatrics | Oxford Academic. J Trop Pediatr. 2007;53:8–12. Accessed August 4, 2020. https://academic.oup.com/tropej/article/53/1/8/1667616 [DOI] [PubMed] [Google Scholar]
- 53.Ward MC, Sinn JK. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev. 2003;(4). doi: 10.1002/14651858.cd003485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez M, Lakshminrusimha S, Wedgwood S, et al. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Cell Mol Physiol. 2012;302(6):L595–L603. doi: 10.1152/ajplung.00145.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez M, Wedgwood S, Lakshminrusimha S, et al. Hydrocortisone normalizes phosphodiesterase-5 activity in pulmonary artery smooth muscle cells from lambs with persistent pulmonary hypertension of the newborn. Pulm Circ. 2014;4(1):71–81. doi: 10.1086/674903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alsaleem M, Malik A, Lakshminrusimha S, et al. Hydrocortisone Improves Oxygenation Index and Systolic Blood Pressure in Term Infants With Persistent Pulmonary Hypertension. Clin Med Insights Pediatr. 2019;13:1–4. doi: 10.1177/1179556519888918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


