Abstract
Background:
Given the lack of consensus in the surgical treatment of anal adenocarcinoma, practice-patterns demonstrate utilization of organ-preserving techniques. The adequacy of local excision compared to abdominoperineal resection (APR) as a surgical approach for stage II disease is unknown. Our study examines the utilization of local excision in the treatment of stage II anal adenocarcinoma, rates of R0 resection, and differences in overall survival compared to APR.
Materials and Methods:
Using the National Cancer Database (2004–2016), we retrospectively analyzed patients diagnosed with clinical stage II anal adenocarcinoma who received chemoradiation and surgery. Patient cohorts were assigned based on the surgical procedure they received. Propensity score matching was used to offset selection bias and confounding factors. Treatment approach, pathologic margin status, and overall survival were assessed.
Results:
Overall, 359 patients underwent resection of clinical stage II anal adenocarcinoma and received chemoradiation therapy. Of these patients, 87 (24%) underwent local excision, whereas 272 (76%) received an abdominoperineal resection. In a propensity score-matched cohort, patients who underwent local excision were less likely to achieve an R0 resection (40% vs 90%), and more likely to receive adjuvant instead of neoadjuvant chemoradiation. Overall survival was not significantly different between the propensity-matched groups. Surgical approach and pathologic margin status were not independently associated with overall survival.
Conclusions:
Among patients with clinical stage II anal adenocarcinoma who received chemotherapy and radiation, complete resection was significantly less likely with local excision compared to abdominoperineal resection, however, overall survival was not affected. Prospective studies of neoadjuvant chemoradiation followed by local excision are warranted.
Keywords: Colorectal Cancer, Anal Cancer, Trans-anal Excision, Abdominoperineal Resection, Local control
Micro-abstract
There is considerable practice-pattern heterogeneity in the resection of anal adenocarcinoma. Using the National Cancer Database, we analyzed the outcomes of local excision versus abdominoperineal resection for the treatment of clinical stage II anal adenocarcinoma. In propensity score-matched patients who received chemoradiation, complete resection was less likely with local excision compared to abdominoperineal resection, however, overall survival was not affected.
Introduction
Adenocarcinoma of the anus is a rare but aggressive malignancy.1 The National Comprehensive Cancer Network (NCCN) guidelines recommend treatment of anal adenocarcinoma according to the paradigm established for rectal adenocarcinoma, despite substantial differences in their staging paradigms. In anal adenocarcinoma, T-stage is determined by tumor diameter (T1: 2cm, T2: 2–5cm, T3: >5cm, T4: invasion of adjacent organs), whereas rectal adenocarcinoma is staged by depth of invasion.2 The anatomic proximity of rectal and anal malignancies should not disguise the fact that they may differ significantly in cells of origin (i.e. rectal mucosa versus anal glands) and thereby their tumor biology. Despite such considerations, given the rarity of anal adenocarcinoma, separate treatment guidelines have not been developed. The NCCN panel encourages patient participation in clinical trials, underscoring the lack of data to strongly support any particular treatment strategy.3 As a result, there is considerable practice pattern heterogeneity surrounding anal adenocarcinoma management, particularly in the setting of resectable (stage I-III) disease.1, 3–5 Although some experts advocate for definitive surgical treatment in the form of an abdominoperineal resection (APR), others suggest that chemoradiation therapy alone is sufficient, with APR reserved as a salvage measure.6 The majority of studies, however, show that neoadjuvant chemoradiation followed by APR offers superior overall survival.7–11
Encouraging outcomes with organ preservation for early-stage disease have led to increasing use of local excision (LE) in the treatment of rectal adenocarcinoma.12, 13 This approach may offer treatment-naive patients with T1 tumors equivalent long-term and oncologic outcomes without the morbidity and potentially decreased quality of life associated with low anterior resection (LAR) or APR.14 Additionally, clinical trials suggest potential benefits of LE for highly-selected patients with T2 rectal adenocarcinoma following neoadjuvant chemoradiation.12, 13 For peri-anal squamous cell carcinoma, anal melanoma, and other less common histologic subtypes of cancer, LE has long been considered first-line surgical therapy, exhibiting equivalent overall survival compared to APR.15–17 Furthermore, LE has demonstrated equivalent cause-specific survival compared to definitive chemoradiation, which is standard of care in small, well-differentiated anal squamous cell carcinomas.18 Extrapolating from these findings in other anal cancers, LE may be a reasonable treatment approach for localized anal adenocarcinoma, provided that it is able to achieve an acceptable oncologic outcome.
We first sought to characterize patients who underwent chemoradiation and either LE or APR for stage II anal adenocarcinoma. Next, we aimed to examine the adequacy of LE as a surgical approach, as assessed by rate of R0 resection, when compared to APR. Finally, we sought to compare the overall survival of patients who received LE to those who underwent APR.
Materials & Methods
Data Source
The National Cancer Database participant user files (NCDB PUFs) were the source of all data in our study. The NCDB is a nationwide repository of de-identified patient data related to cancer care metrics and outcomes in the United States derived from the submissions of over 1,500 Commission on Cancer (CoC)-accredited programs.19 The NCDB captures over 70% of new cancer diagnoses in the United States per year. The CoC is a multidisciplinary association maintained by the American College of Surgeons and the American Cancer Society that accredits US hospitals based on various aspects of cancer care. Due to our study’s inclusion of only de-identified data, it was exempt from institutional review board review.
Patient Selection
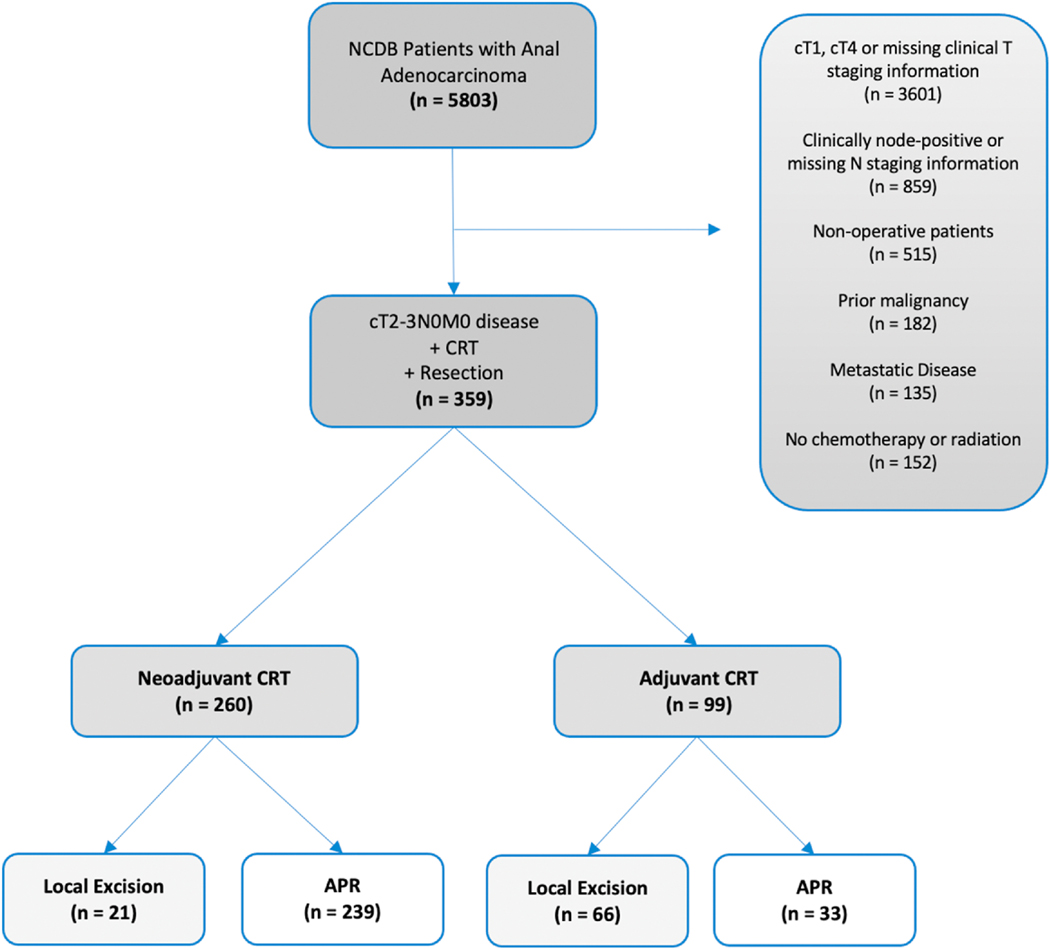
The NCDB was queried for patients from 2004 to 2016 with adenocarcinoma of the anus using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography codes (C210, C211, C212, C218) and morphology codes (8140, 8210, 8215, 8255, 8260, 8261, 8263, 8480, 8481, 8490, 8560). Patient cohort selection is outlined in Figure 1. We isolated those with clinical stage II (cT2N0M0 and cT3N0M0) disease per the American Joint Committee on Cancer (AJCC), 7th Edition clinical staging system who received neoadjuvant or adjuvant chemoradiotherapy and a surgical procedure. LE was defined as local tumor excision with or without local tumor destruction, denoted by Facility Oncology Registry Data Standards site-specific procedure codes 20–27. Patients who received an APR were captured using site-specific procedure codes 60–63. We excluded patients with a prior history of malignancy or insufficient staging information. We collected information on clinicopathologic and treatment characteristics. Patients were divided into cohorts based on receipt of LE or APR.
Figure 1.
Exclusion criteria and case selection for the patient cohort. Abbreviations: NCDB, National Cancer Database; CRT, Chemoradiation Therapy; APR, Abdominoperineal Resection.
Statistical Analysis
Descriptive statistics were calculated for clinicopathologic and treatment variables and analyzed by chi-square, Fisher’s exact, or Mann-Whitney U test, as appropriate, to determine associations with surgical procedure. We performed greedy nearest-neighbor matching with a caliper width of 0.05 times the standard deviation of the logit of the propensity score using patient age, Charlson-Deyo score, and clinical T stage as covariates. Cox proportional hazards regression was performed using the matched cohort to evaluate the independent association of clinicopathologic variables with overall survival. Additionally, we estimated overall survival using the Kaplan-Meier method and compared groups using the log-rank test. Statistical analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 25.0 (IBM Corp., Armonk, N.Y., USA).
Results
Of the 5,803 patients with a diagnosis of anal adenocarcinoma, 359 received an operation and chemoradiation for clinical stage II disease (Figure 1). Most of the unmatched cohort (n = 359) underwent an APR (n = 272, 76%), while the remainder underwent LE (n = 87, 24%) (Table 1). Groups were well balanced with respect to patient sex, age, race, and Charlson-Deyo co-morbidity index. Patients who received LE were more likely to have cT2 disease and receive adjuvant chemoradiation (p < 0.05), whereas those who underwent an APR were more likely to have cT3 disease and receive neoadjuvant chemoradiation (p < 0.05). Median pathologic tumor size was correspondingly larger in the APR cohort, whereas other clinicopathologic factors, namely lymphovascular invasion and tumor grade, were similar between patient groups. Patients who underwent local excision were more likely to have positive resection margins than those who underwent APR (p < 0.05).
Table 1.
Summary of unmatched patients and propensity score-matched patients from the NCDB with stage II anal adenocarcinoma.
| UNMATCHED | MATCHED | |||||
|---|---|---|---|---|---|---|
| Local Excision [n = 87] | APR [n = 272] | p-value [ASD] | Local Excision [n = 78] | APR [n = 78] | p-value [ASD] | |
| Sex | 0.978 | 0.623 | ||||
| Female | 51 (59%) | 159 (59%) | 49 (63%) | 46 (59%) | ||
| Male | 36 (41%) | 113(41%) | 29 (37%) | 32 (41%) | ||
| Age (years)* | 0.012 | 0.685 | ||||
| Median (interquartile range) | 65 (20) | 62 (16) | [0.32] | 64 (18) | 63 (19) | [0.09] |
| Race | 0.843 | 0.757 | ||||
| Caucasian | 72 (83%) | 219 (81%) | 64 (82%) | 63 (81%) | ||
| African American | 12 (14%) | 40 (15%) | 11 (14%) | 10 (13%) | ||
| Other | 3(3%) | 13 (4%) | 3(4%) | 5 (6%) | ||
| CDCC* | 0.045 | 0.335 | ||||
| 0 | 64 (72%) | 221 (81%) | [0.09] | 60 (77%) | 67 (86%) | [0.09] |
| 1 | 14 (16%) | 39 (14%) | [0.02] | 11 (14%) | 6 (8%) | [0.06] |
| ≥2 | 10 (12%) | 12 (5%) | [0.07] | 7(9%) | 5 (6%) | [0.03] |
| Tumor Location | <0.001 | 0.019 | ||||
| Anus | 31 (36%) | 47 (17%) | 29 (37%) | 18 (23%) | ||
| Anal Canal | 29 (33%) | 78 (29%) | 26 (33%) | 20 (26%) | ||
| Overlapping | 27 (31%) | 147 (54%) | 23 (30%) | 40 (51%) | ||
| Clinical T Stage* | <0.001 | 0.572 | ||||
| 2 | 67 (77%) | 123 (45%) | [0.32] | 58 (74%) | 61 (78%) | [0.04] |
| 3 | 20 (23%) | 149 (55%) | 20 (26%) | 17 (22%) | ||
| Tumor Size (mm) | 0.020 | 0.029 | ||||
| Median (interquartile range) | 30 (25) | 62 (16) | 30 (26) | 37.5 (25) | ||
| Grade | 0.211 | 0.484 | ||||
| Well Differentiated | 14 (16%) | 25 (9%) | 11 (14%) | 6 (8%) | ||
| Moderately Differentiated | 39 (45%) | 150 (55%) | 36 (46%) | 44 (56%) | ||
| Poorly Differentiated | 16 (19%) | 43 (16%) | 14 (8%) | 12 (15%) | ||
| Unknown | 18 (20%) | 54 (20%) | 17 (22%) | 16 (21%) | ||
| Lymphovascular Invasion | 0.061 | 0.195 | ||||
| Absent | 25 (29%) | 113(42%) | 23 (30%) | 32 (41%) | ||
| Present | 4 (5%) | 17 (6%) | 3(4%) | 5 (6%) | ||
| Unknown | 22 (66%) | 142 (52%) | 52 (66%) | 41 (53%) | ||
| Margin Status | <0.001 | <0.001 | ||||
| R0 | 35 (40%) | 242 (89%) | 31 (40%) | 70 (90%) | ||
| R1/R2 | 37 (43%) | 23 (9%) | 33 (42%) | 7 (9%) | ||
| Unknown | 15 (17%) | 7 (2%) | 14 (18%) | 1 (1%) | ||
| Chemoradiation | <0.001 | <0.001 | ||||
| Neoadjuvant | 21 (24%) | 239 (88%) | 20 (26%) | 74 (95%) | ||
| Adjuvant | 66 (76%) | 33 (12%) | 58 (74%) | 4 (5%) | ||
Variable used for propensity score-matching.
ASD = Absolute Standardized Difference.
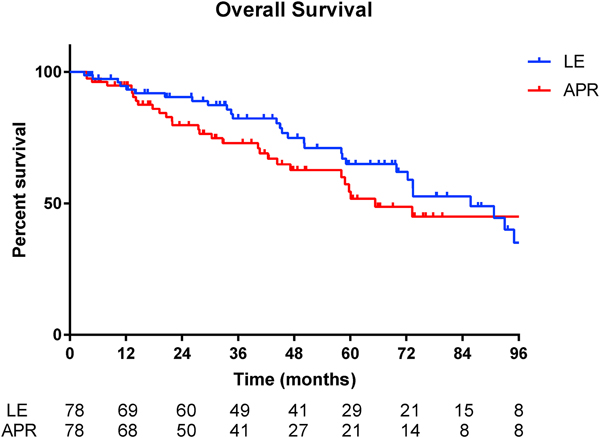
Propensity matching accounted for pre-matching differences in clinical T stage and pathologic tumor size and maintained pre-matching similarities in other patient and clinicopathologic factors (Table 1). In the propensity score-matched cohort, median overall survival was 85.8 months in the LE group versus 65.3 months in the APR group (Figure 2, p = 0.36).
Figure 2.
Kaplan-Meier survival curve for propensity score-matched patients with Stage II anal adenocarcinoma (p=0.36)
Univariable and multivariable Cox regression analyses are shown in Table 2. Factors independently associated with decreased overall survival included more advanced T stage (cT3) and poorly differentiated or undifferentiated tumors. Surgical approach, sequence of treatments, and margin status were not associated with overall survival on either univariable or multivariable analysis of the matched cohort. In a sub-group analysis, there was no association between sequence of treatment and margin status in patients who underwent LE (p = 0.096). Of those who received a margin-positive LE, 81.8% received adjuvant chemoradiation and exhibited improved median overall survival (93 months vs. 46.6 months, log rank p = 0.036) compared to patients receiving neoadjuvant chemoradiation.
Table 2.
Cox regression survival analysis of propensity-matched patients.
| Univariable | Multivariable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | Lower | Upper | Sig | HR | Lower | Upper | Sig | ||
| Age | 156 | 1.03 | 1.01 | 1.05 | 0.017 | 1.02 | 1.00 | 1.04 | 0.055 | |
| Sex | Male | 95 | ref | - | - | - | ||||
| Female | 61 | 0.95 | 0.56 | 1.62 | 0.846 | |||||
| Race | White | 127 | ref | - | - | - | ||||
| African American | 21 | 1.37 | 0.69 | 2.72 | 0.367 | |||||
| Other/Unknown | 8 | 0.64 | 0.16 | 2.64 | 0.536 | |||||
| Surgical Procedure | Local Excision | 78 | ref | - | - | - | ref | - | - | - |
| APR | 78 | 1.27 | 0.76 | 2.13 | 0.360 | 1.11 | 0.54 | 2.27 | 0.782 | |
| Charlson-Deyo Score | 0 | 127 | ref | - | - | - | ref | - | - | - |
| 1 | 17 | 1.76 | 0.88 | 3.52 | 0.110 | 1.81 | 0.82 | 3.99 | 0.139 | |
| ≥2 | 12 | 1.79 | 0.80 | 4.00 | 0.157 | 1.69 | 0.74 | 3.91 | 0.216 | |
| Tumor Location | Anus | 47 | ref | - | - | - | ref | - | - | - |
| Anal Canal | 46 | 1.63 | 0.79 | 3.38 | 0.187 | 1.61 | 0.75 | 3.46 | 0.225 | |
| Overlapping sites of Anus/Anal Canal/Rectum | 63 | 1.83 | 0.95 | 3.54 | 0.072 | 1.83 | 0.90 | 3.71 | 0.096 | |
| Tumor Size | 130 | 1.00 | 1.00 | 1.00 | 0.938 | |||||
| Clinical T Stage | cT2 | 58 | ref | - | - | - | ref | - | - | - |
| cT3 | 98 | 1.93 | 1.08 | 3.46 | 0.027 | 2.44 | 1.26 | 4.75 | 0.009 | |
| Grade | Well Differentiated | 17 | ref | - | - | - | ref | - | - | - |
| Moderately Differentiated | 80 | 3.04 | 0.93 | 9.99 | 0.067 | 2.08 | 0.61 | 7.07 | 0.241 | |
| Poorly/Undifferentiated | 26 | 3.70 | 1.01 | 13.52 | 0.048 | 3.73 | 1.01 | 13.81 | 0.049 | |
| Unknown | 33 | 3.38 | 0.98 | 11.70 | 0.054 | 2.54 | 0.72 | 8.99 | 0.150 | |
| Lymphovascular Invasion | Absent | 55 | ref | - | - | - | ||||
| Present | 8 | 1.31 | 0.38 | 4.56 | 0.673 | |||||
| Unknown | 93 | 0.55 | 0.65 | 2.25 | 0.546 | |||||
| Margin Status | R0 | 101 | ref | - | - | - | ||||
| R1/R2 | 40 | 1.34 | 0.76 | 2.35 | 0.309 | |||||
| Unknown | 15 | 0.69 | 0.21 | 2.23 | 0.533 | |||||
| CRT Sequence | Adjuvant CRT | 88 | ref | - | - | - | ref | - | - | - |
| Neoadjuvant CRT | 62 | 1.92 | 1.09 | 3.39 | 0.024 | 1.63 | 0.76 | 3.51 | 0.210 | |
Discussion
We evaluated the outcomes of LE or APR in addition to chemoradiation in patients with clinical stage II anal adenocarcinoma. Patients who underwent LE were more likely to have cT2 disease, yet they had a lower rate of margin-negative resection. Furthermore, patients who underwent LE were more likely to receive adjuvant CRT. This suggests that efforts were made to spare patients with small (<5cm) anal adenocarcinomas from an APR, with perhaps post hoc decision-making regarding CRT. In contrast, patients who underwent APR had more advanced disease, for which they underwent neoadjuvant CRT followed by resection. Despite these treatment differences, we did not observe survival differences associated with either surgical approach or margin status among a propensity score-matched patient cohort. While only 40% of LE patients were able to undergo R0 resection, those who were unable to achieve complete resection still demonstrated similar overall survival with the receipt of adjuvant chemoradiation. Given the lack of association between sequence of treatment and complete resection with LE, these data argue for extending recent clinical trials of neoadjuvant CRT followed by LE for rectal adenocarcinoma to include patients with anal canal adenocarcinoma and allow for adjuvant CRT.12, 20, 21 LE may provide adequate surgical treatment for localized low rectal cancer, sparing patients from APR and its attendant morbidity and quality-of-life impact, namely genitourinary dysfunction and permanent ostomy.22 Similarly, our results suggest that LE may be a reasonable surgical option for patients with appropriately sized, clinically node-negative anal adenocarcinoma.
While current recommendations endorse APR as the surgical procedure of choice for stage II-III anal adenocarcinoma, the data supporting this approach specifically for stage II disease are limited. Prior studies suggest that LE confers unacceptable oncologic outcomes, but these conclusions were drawn from limited, single-institution series that included patients with more advanced locoregional disease.8, 9 Perhaps most importantly, since their survival is limited primarily by distant recurrence and progression of metastatic disease, patients in these studies were treated prior to the advent of contemporary chemotherapy regimens. When compared to prior anal adenocarcinoma series, our findings suggest that, in the setting of modern systemic therapy, LE and CRT could spare patients the considerable morbidity of a more radical operation while conferring similar long-term survival, despite a low rate of complete resection.8, 9 This is important since OS is dictated by systemic relapse, not local recurrence.8 Additionally, APR remains available as a salvage therapy to patients who recur locally following LE for stage II disease. Furthermore, for patients who experience diminished quality of life due to the sequelae of anal chemoradiation therapy such as fecal incontinence, diverting ostomy or APR would be therapeutic options.23 The decreased morbidity of LE, along with the lack of survival benefits from more aggressive surgical treatment, argues against APR for early stage anal cancer.
Despite our use of a large national database, our study is limited by its small sample size, and thus, may be underpowered to detect an overall survival difference. The retrospective nature of our analysis can be particularly problematic due to missing data, selection bias, and non-standardized follow-up. The rarity of anal adenocarcinoma may preclude the possibility of a randomized controlled trial, but prospective data collection would avoid much of the aforementioned bias. Notably, although it did not appear to impact overall survival, the disparity in R0 resections between groups may be clinically impactful on a larger scale. The NCDB does not track recurrence data or salvage therapy, which would have added value in assessing the adequacy of local control in these patients.
Based on our findings, we suggest that LE can be considered as a surgical option for highly-selected patients with stage II anal adenocarcinoma, particularly those with cT2 tumors, when coupled with chemoradiation. Although APR offers a higher chance of local control, patients often suffer life-limiting distant disease, which should prompt thoughtful patient selection when considering its morbidity. Finally, prospective evaluation of neoadjuvant chemoradiation and LE for localized cT2 anal adenocarcinoma may increase rates of local control using LE to be comparable to those of APR. Quality of life assessments would be important to optimally assess outcomes of this or another approach. In the absence of such data, however, current practice will rely on expert consensus opinion and data from large cohort studies such as ours.
Clinical Practice Points
Current clinical practice guidelines recommend treatment of anal adenocarcinoma according to the paradigm established for rectal adenocarcinoma, despite substantial differences in their staging paradigms. This is largely due to the rarity of anal adenocarcinoma, and thus, the absence of randomized, controlled trials assessing its management. The rising popularity of organ-preservation treatment strategies has led to the increasing use of local excision in the treatment of early-stage rectal, and correspondingly, anal adenocarcinoma. In the absence of clinical trial data comparing the two, current practice will rely on expert consensus opinion and data from large cohort studies. Our retrospective, propensity score-matched analysis of patients with clinical stage II anal adenocarcinoma did not detect an overall survival difference between those who underwent local excision versus abdominoperineal resection, in addition to chemoradiation. These data suggest that local excision can be considered as a surgical option for highly-selected patients, particularly those who are unable or decline to undergo an abdominoperineal resection. Although the standard of care abdominoperineal resection offers a higher chance of local control, it carries a higher morbidity and patients may suffer life-limiting distant disease. Prospective evaluation of local excision will permit concurrent assessment of abdominoperineal resection as a salvage therapy. However, at this time, ours remains the largest analysis of local excision in anal adenocarcinoma to date.
Highlights.
There is practice-pattern heterogeneity in the resection of anal adenocarcinoma.
Organ-preserving treatment strategies are becoming more widely adopted.
Retrospective analysis of patients with stage II anal adenocarcinoma was performed.
Survival following local excision was comparable to abdominoperineal resection.
Complete resection was less likely following local excision.
Acknowledgments
Funding:
This research was supported by the Intramural Research Program of the National Institutes of Health’s National Cancer Institute.
Footnotes
Each of the above co-authors played an essential role in conception and design of the study, analysis and interpretation of relevant data, contribution of intellectual content, and final approval of the manuscript for publication.
Disclosures: the authors of this publications have no financial conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anwar S, Welbourn H, Hill J, Sebag-Montefiore D. Adenocarcinoma of the anal canal - a systematic review. Colorectal Dis 2013;15:1481–8. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB, American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging manual. Eight edition / editor-in-chief, Amin Mahul B. ; editors, Edge Stephen B. and 16 others ; Gress Donna M.- Technical editor ; Meyer Laura R. - Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer, 2017. [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen Y- J, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. 2018;16:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malakhov N, Kavi AM, Lee A, Adedoyin P, Sheth N, Lederman AJ, Schreiber D. Patterns of Care and Comparison of Outcomes Between Primary Anal Squamous Cell Carcinoma and Anal Adenocarcinoma. Dis Colon Rectum 2019;62:1448–57. [DOI] [PubMed] [Google Scholar]
- 5.Bertelson N, Blumetti J, Cintron J, Harrison J, Chaudhry V, Abcarian H. Anal Adenocarcinoma: Outcomes in an Uncommon Malignancy. Am Surg 2015;81:1114–7. [PubMed] [Google Scholar]
- 6.Kounalakis N, Artinyan A, Smith D, Mojica-Manoso P, Paz B, Lai LL. Abdominal perineal resection improves survival for nonmetastatic adenocarcinoma of the anal canal. Ann Surg Oncol 2009;16:1310–5. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Shinde A, Fakih M, Sentovich S, Melstrom K, Nelson R, Glaser S, Chen YJ, Goodman K, Amini A. Impact of Surgical Resection on Survival Outcomes After Chemoradiotherapy in Anal Adenocarcinoma. J Natl Compr Canc Netw 2019;17:1203–10. [DOI] [PubMed] [Google Scholar]
- 8.Chang GJ, Gonzalez RJ, Skibber JM, Eng C, Das P, Rodriguez-Bigas MA. A twenty-year experience with adenocarcinoma of the anal canal. Dis Colon Rectum 2009;52:1375–80. [DOI] [PubMed] [Google Scholar]
- 9.Beal KP, Wong D, Guillem JG, Paty PB, Saltz LL, Wagman R, Minsky BD. Primary adenocarcinoma of the anus treated with combined modality therapy. Dis Colon Rectum 2003;46:1320–4. [DOI] [PubMed] [Google Scholar]
- 10.Joon DL, Chao MW, Ngan SY, Joon ML, Guiney MJ. Primary adenocarcinoma of the anus: a retrospective analysis. Int J Radiat Oncol Biol Phys 1999;45:1199–205. [DOI] [PubMed] [Google Scholar]
- 11.Belkacemi Y, Berger C, Poortmans P, Piel G, Zouhair A, Meric JB, Nguyen TD, Krengli M, Behrensmeier F, Allal A, De Looze D, Bernier J, Scandolaro L, Mirimanoff RO, Rare Cancer N. Management of primary anal canal adenocarcinoma: a large retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys 2003;56:1274–83. [DOI] [PubMed] [Google Scholar]
- 12.Jawitz OK, Adam MA, Turner MC, Gilmore BF, Migaly J. Neoadjuvant chemoradiation followed by transanal local excision for T2 rectal cancer confers equivalent survival benefit as traditional transabdominal resection. Surgery 2019;165:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr., Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atallah C, Taylor JP, Lo BD, Stem M, Brocke T, Efron JE, Safar B. Local excision for T1 rectal tumours: are we getting better? Colorectal Dis 2020; 22:2038–2048. [DOI] [PubMed] [Google Scholar]
- 15.Perez DR, Trakarnsanga A, Shia J, Nash GM, Temple LK, Paty PB, Guillem JG, Garcia-Aguilar J, Bello D, Ariyan C, Carvajal RD, Weiser MR. Locoregional lymphadenectomy in the surgical management of anorectal melanoma. Ann Surg Oncol 2013;20:2339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullard KM, Tuttle TM, Rothenberger DA, Madoff RD, Baxter NN, Finne CO, Spencer MP. Surgical therapy for anorectal melanoma. J Am Coll Surg 2003;196:206–11. [DOI] [PubMed] [Google Scholar]
- 17.Kiran RP, Rottoli M, Pokala N, Fazio VW. Long-term outcomes after local excision and radical surgery for anal melanoma: data from a population database. Dis Colon Rectum 2010;53:402–8. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Goffredo P, Kahl AR, Charlton ME, Weigel RJ, Hassan I. Chemoradiation versus local excision in treatment of stage I anal squamous cell carcinoma: A population-based analysis. Eur J Surg Oncol 2020. September;46(9):1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki T, Ito Y, Ohue M, Kanemitsu Y, Kobatake T, Ito M, Moriya Y, Saito N. Postoperative Chemoradiotherapy After Local Resection for High-Risk T1 to T2 Low Rectal Cancer: Results of a Single-Arm, Multi-Institutional, Phase II Clinical Trial. Dis Colon Rectum 2017;60:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra-Aracil X, Pericay C, Golda T, Mora L, Targarona E, Delgado S, Reina A, Vallribera F, Enriquez-Navascues JM, Serra-Pla S, Garcia-Pacheco JC, group T-Ts. Non-inferiority multicenter prospective randomized controlled study of rectal cancer T2-T3s (superficial) N0, M0 undergoing neoadjuvant treatment and local excision (TEM) vs total mesorectal excision (TME). Int J Colorectal Dis 2018;33:241–9. [DOI] [PubMed] [Google Scholar]
- 22.Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients with Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann Surg Oncol 2019;26:2497–506. [DOI] [PubMed] [Google Scholar]
- 23.Sterner A, Derwinger K, Staff C, Nilsson H, Angenete E. Quality of life in patients treated for anal carcinoma-a systematic literature review. Int J Colorectal Dis 2019;34:1517–28. [DOI] [PubMed] [Google Scholar]