Abstract
Preventing chronic graft versus host disease (GVHD) remains challenging because the unique cellular and molecular pathways that incite chronic GVHD are poorly understood. One major point of intervention for potential prevention of chronic GVHD occurs at the time of transplantation when acute donor anti-recipient immune responses first set the events in motion that result in chronic GVHD. After transplantation, additional insults causing tissue injury can incite aberrant immune responses and loss of tolerance further contributing to chronic GVHD. Points of intervention are actively being identified so that chronic GVHD initiation pathways can be targeted without affecting immune function. The major objective in the field is to continue basic studies and to translate what is learned about etiopathology to develop targeted prevention strategies that decrease the risk of morbid chronic GVHD without increasing the risks of cancer relapse or infection. Development of strategies to predict risk of developing debilitating or deadly chronic GVHD is a high research priority. This Working Group recommends further interrogation into mechanisms underpinning chronic GVHD development, and we highlight considerations for future trial design in prevention trials.
Introduction
An ideal method to prevent chronic graft versus host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT) would be to remove the donor cells that contribute to the risk of chronic GVHD from the graft or allow them to attain immune tolerance of recipient alloantigens that cause chronic GVHD, such that systemic immunosuppression is no longer necessary.1 Recent studies have demonstrated substantial progress in preventing chronic GVHD using strategies that target immune cells with disease-inciting capacity, but prevention has not been fully realized because the underlying mechanisms that initiate chronic GVHD are only partially known. Identification of these mechanisms in human chronic GVHD in the early peri-transplant period remains challenging in part because a minimum of one year follow-up after HCT is needed to assess the chronic GVHD endpoint.
The advent of clinically relevant murine models and innovative measures of immune responses in mice and humans has helped identify the mechanisms that incite chronic GVHD.2, 3 Mechanistic information from murine models, clinical trials and patient samples strongly suggests that pivotal chronic GVHD-inciting events involve certain donor T, B, monocyte, machrophage, innate populations such as ILCs and NK cells, and plasmacytoid dendritic cell subsets, donor and recipient antigen-presenting cells and recipient fibroblastic reticular cells. Chronic GVHD may be prevented by removing certain activated donor lymphoid populations, albeit with immune consequences. The extent of overlap between the antigens that trigger chronic GVHD, graft-versus-tumor (GVT) activity and pathogen defenses is not known. Further, while pathogens are typically cleared through activation of naïve cells that mature to effectors, the recipient alloantigens that trigger chronic GVHD and GVT activity generally persist. The challenge facing the field is to find ways to prevent chronic GVHD without increasing the risks of graft rejection, infection, and recurrent malignancy.
Purpose of this document:
This report reviews our current understanding of the etiologic factors that incite chronic GVHD, identifies knowledge gaps, and suggests cellular targets and mechanisms relevant to the benefits and risks in the design of clinical trials to prevent chronic GVHD. The problem of late acute GVHD is beyond the scope of this report. We distinguish between prevention, which is defined as interventions aimed at initiating or inciting events that occur after HCT but before any clinical or laboratory evidence of active chronic GVHD, and preemption, which is the topic of a future report.
Summary of recommendations:
Regarding the etiology and prevention of chronic GVHD, we reached consensus on the following points:
Moderate to severe chronic GVHD leads to excess long-term morbidity and mortality and should be prevented.
Chronic GVHD is initiated early after HCT and can also be incited late after HCT by additional insults.
Positive predictive values (PPV) for additional insults or secondary inciting events that occur after HCT are unknown, and observational studies should be performed to assess risk.
For patients with hematological malignancies, a risk assessment model should weigh both the risks of cancer relapse and graft rejection against the risk of severe chronic GVHD.
Studies of how damaged primary and secondary lymphoid organs contribute to altered T and B cell recovery and maturation in chronic GVHD genesis are needed.
Additional studies addressing manipulation of the donor graft and host tissue integrity and milieu will be informative.
We should study pediatric hosts and young donor stem cell products, including cord HCT, to define mechanisms of chronic GVHD etiology.
Targeting cells that we agree are not critical in GVT reactions, like certain B cell or T cell subsets or host stromal cell represent prime avenues for further clinical studies.
As the primary endpoint in prevention trials of chronic GVHD, especially in trials intended for regulatory review, we recommend survival without moderate to severe NIH-defined chronic GVHD (chronic GVHD-free survival).
Methods
Each working group was organized to encourage global engagement in the topic (see introduction to the series in this issue of TCT). Four groups worked individually beginning in February 2020 to review the relevant literature and prepare the initial draft of the manuscript. The Steering Committee reviewed and discussed the initial draft and offered recommendations for revisions. Two iterative rounds of comments and revisions were collected before the November 18–20, 2020 Consensus Conference. The manuscript was further revised for submission after additional suggestions from external reviewers, conference participants, and a 30-day public comment period.
I. Primary Insults – Immune cell-driven etiology of chronic GVHD and potential points of intervention
Current Knowledge:
Donor, recipient, and exogenous factors contribute to chronic GVHD genesis, and dynamic interactions between these factors and secondary insults lead to immune dysregulation that manifests subsequently as chronic GVHD.1 Studies in murine models4, 5 and humans6 indicate that events leading to chronic GVHD begin with tissue injury caused by the pretransplant conditioning regimen, which amplifies responses to alloantigens that trigger acute GVHD. Strategies that effectively reduce the risk of acute GVHD, however, have not necessarily reduced the risk of chronic GVHD and vice versa, highlighting the need for further elucidation of the mechanisms that determine these outcomes. Approximately 30% of patients develop chronic GVHD with no prior overt acute GVHD, either through a subacute graft-versus-host reaction or undefined independent mechanisms.7 Considering that GVHD does not occur after autologous HCT, injury due to conditioning regimen alone is not sufficient for ongoing reactivity against recipient tissues. With current methods such as flow cytometry, it is difficult to distinguish the cells that cause chronic GVHD from those that prevent graft rejection, mediate GVT activity, and control infections. Better understanding of functional correlates and molecular drivers of immune cell subsets that mediate chronic GVHD is needed.
Gaps in knowledge and unmet needs; highest priorities:
Figure 1 shows a working model of chronic GVHD inciting factors along with several potential points of clinical intervention that require further study. Clinical results suggest that the risk of chronic GVHD may be decreased by using aspirated marrow cells instead of growth factor-mobilized blood cells, using related donors, using younger donors, avoiding female donors for male patients, and using cord blood donors (Table 2).8–12 Studies to determine whether the incidence of NIH chronic GVHD in adults is lower after CBT compared to PBSCT have yielded inconsistent results,13, 14 but the severity of the disease among those who develop chronic GVHD is lower in cord blood recipients than in those who received mobilized blood cell grafts from HLA-mismatched unrelated donors.15 Thus, donor selection serves as one point of intervention, which may become increasingly relevant as outcomes improve with alternative donor sources. Graft engineering before HCT and in vivo T cell depletion with anti-thymocyte globulin (ATG) or post-transplant cyclophosphamide (PTCy) remain areas of active investigation. Additional targets include B cells, monocytes, and macrhophages.
Figure 1. The etiology of chronic GVHD and the potential points of clinical intervention.
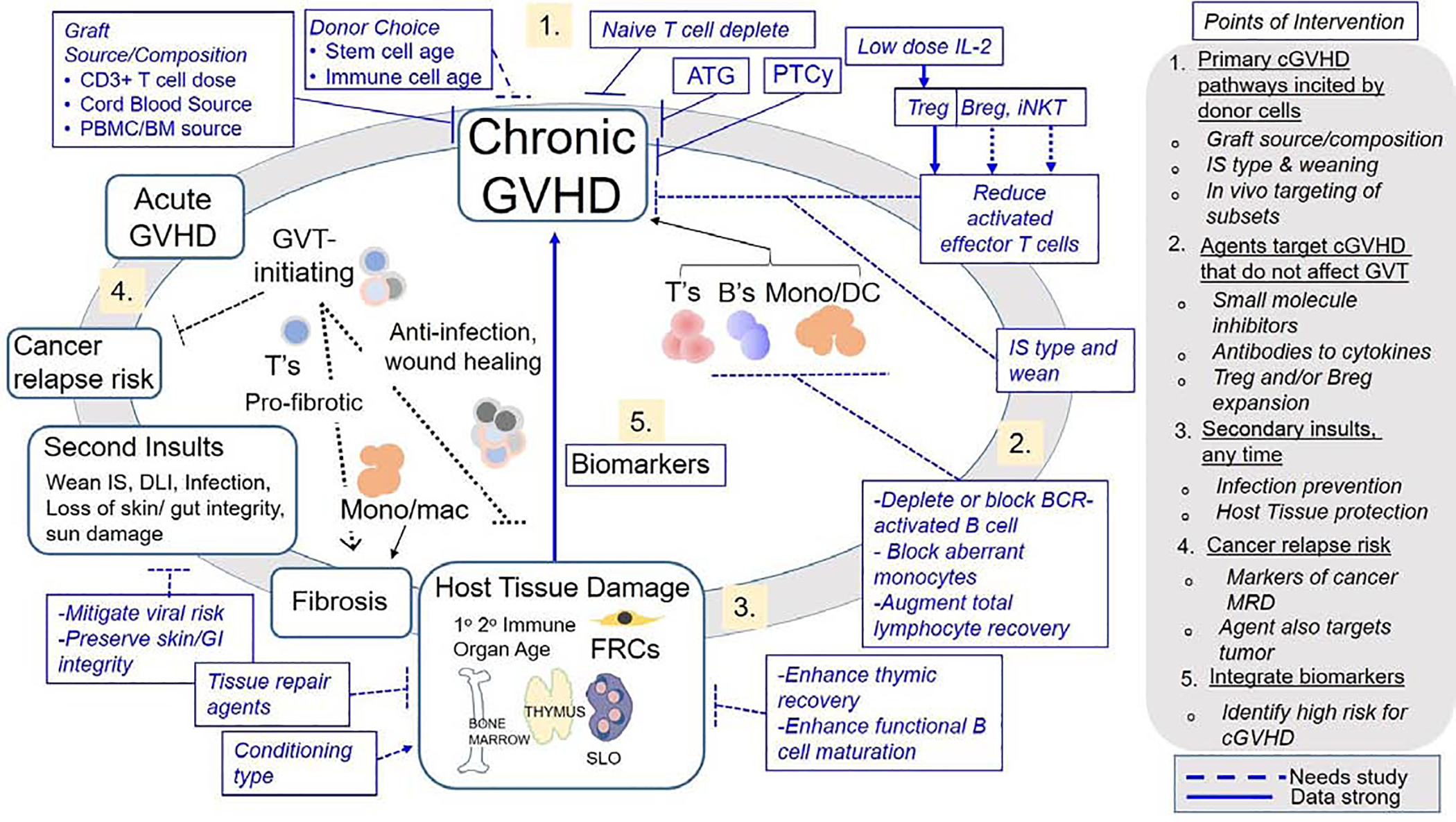
Recipient factors include age, damage to bone marrow stroma, thymus, secondary lymphoid organs (SLO) (i.e., spleen, lymph nodes and other lymphoid tissues), and fibroblastic reticular cells (FRCs). The choice of agents in conditioning regimens and the overall intensity of conditioning regimens influences the extent of damage to these organs. Less robust evidence suggests a role for recovery of these organs in prevention of chronic GVHD, including thymus recovery, functional B cell maturation, and tissue repair. Donor graft factors include donor age, CD3+ T cell dose, and graft source. Donor cell products contain heterogenous cell populations that contribute to acute GVHD, chronic GVHD, graft-versus-tumor activity, pathogen defense and tissue repair. Donor graft points of intervention include non-selective T cell depletion, selective depletion of naïve T cells and other graft engineering. Post-transplant cyclophosphamide (PTCy) may silence alloantigen-activated T cells or induce alloreactive T-cell functional impairment while sparing Treg cells, while low-dose IL-2 could expand Treg. Other graft engineering approaches could target induction of Breg and iNKT cells. Secondary insults occur after infusion of HCT and include withdrawal of immunosuppression, donor lymphocyte infusion, infections, loss of gastrointestinal integrity, and ultraviolet damage to the skin. Potential points of clinical intervention are shown in blue font inside blue boxes. Arrows and block symbols depicted with solid lines indicate strong evidence, while dashed arrows and block symbols represent less robust evidence.
Table 2.
Factors associated with an increased risk of chronic GVHD
Donor T cells subsets
Pre-transplant graft engineering remains an area of active investigation in chronic GVHD prevention since conventional/effector alloreactive T cells (Tcon/Teff) are critical for initiating and mediating chronic GVHD. Evidence suggests that subclinical pathogenic processes begin long before the distinct clinical manifestations of chronic GVHD become apparent. Our understanding of human chronic GVHD etiology is largely based on data derived from in vivo and ex vivo graft manipulation trials to prevent acute GVHD.
Clinical studies demonstrate that higher donor T cell dose is a major risk factor for the development of chronic GVHD. The use of marrow as the stem cell source has an overall chronic GVHD incidence of approximately 30% while mobilized peripheral blood cell products are associated with chronic GVHD in 50% of patients.16 T cell depletion approaches have led to lower chronic GVHD rates of 10–40% for overall chronic GVHD and 10–20% for moderate and severe chronic GVHD.
In Table 1, we provide existing published data using ex vivo and in vivo approaches that have been linked to lower incidence and severity of chronic GVHD.17–43 Approaches that reduce or deplete disease-initiating T cell populations may reduce chronic GVHD. Studies with CD34 selection, combined TCR alpha-beta+ T cell and CD19+ B cell depletion or naïve T cell depletion from the graft have been highly informative, although graft failure tended to be increased with some of these approaches. Many in vivo T-cell depletion studies have shown that the incidence and severity of chronic GVHD can be decreased by rabbit ATG, alemtuzumab or PTCy.31–41 Potential risks associated with these interventions include graft failure, infections and relapse. The risks differ from one method to the next, requiring further evaluation.
Table 1.
Clinical studies that have informed us about potential early points of intervention and the etiology of chronic GVHD
| Approach | In vivo/ex vivo | Study design | Cells targeted | Backbone GVHD ppx | Cohort | %MRD positive | Donor | Cell source (n) | %Moderate to severe* chronic GVHD | %Any chronic GVHD | %Relapse | %Graft failure | %OS | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CD19 Alpha/β | Ex vivo | Ph1/2 | Alpha/β T, B | ATG pre + rituximab | Ped/HM/TBI | N/A | Haplo | PB (81) | 0 | 0 | 24 | 2 | 71 | 17 |
| Anti-CD19 Alpha/β | Ex vivo | Pilot | Alpha/β T, B | ATG pre + CNI ± MTX | Ped/HM/TBI | 18 | Haplo/MUD | PB (33) | 12 | 30 | 30 | 0 | 67 | 18 |
| Anti-CD19 Alpha/β | Ex vivo | Retro | Alpha/β T, B | ATG pre | Ped/NM | N/A | Haplo | PB (14) | 21 | N/A | N/A | 14 | 84 | 19 |
| Anti-CD19 Alpha/β | Ex vivo | Ph1/2 | Alpha/β T, B | ATG pre + rituximab | Ped/NM | N/A | Haplo | PB (23) | 0 | 0 | N/A | 17 | 91 | 20 |
| ATG vs. None | In vivo | Ph3 | ATG rabbit | CNI+MTX | Adult/HM | N/A | MUD/ MSD | PB (155) | 6 vs. 33 | 27 vs. 64 | 32 vs. 26 | 0 vs. 1 | 74 vs. 78 | 21 |
| ATG vs. None | In vivo | Ph3 | ATG rabbit | CNI+MTX | Adult/HM | N/A | MUD | PB (164)/ BM (37) | 12 vs. 45 | 30 vs. 60 | 33 vs. 28 | N/A. | 55 vs. 43 | 22, 23 |
| ATG vs. None | In vivo | Ph3 | ATG rabbit | CNI+MTX | Adult/HM | N/A | MUD | PB (196)/ BM (49) | 12 vs. 33 | 16 vs.38 | 32 vs. 21 | 21 vs. 6 | 59 vs. 74 | 24 |
| ATG vs. None | In vivo | Ph3 | ATG rabbit | CNI+MTX or MMF | Adult/HM | N/A | MUD/MMUD | PB (173)/ BM (23) | 13 vs. 29 | 22 vs. 33 | 11 vs. 16 | 3 vs. 2 | 74 vs. 79 @6 mo. | 25, 26 |
| ATG vs. None | In vivo | Ph3 | ATG rabbit | CSP+MTX+MMF | Adult/HM | N/A | MSD | BM+PB (101)/ PB (153)/ BM (9) | 8.5 vs. 23 | 28 vs. 53 | 21 vs. 15 | 0 vs. 0 | 69 vs. 70 | 27 |
| AntiCD45RA+ CD34 selec. | Ex vivo | Ph2 | Naive T/ CD34− | TAC | Adult/HM/TBI | 37 | MSD | PB (35) | 3 | 9 | 21 | 0 | 78 | 28 |
| CD34 selec. | Ex vivo | Ph2 | CD34− | None | Adult/HM/TBI | N/A | MSD | PB (44) | 7 | 18 | 24 | 0 | 60 | 29 |
| CD34 selec. | Ex vivo | Retro | CD34− | ATG | Adult/HM/TBI | N/A | MSD≥7/8 | PB (241) | 1 | 5 | 22 | <1 | 57 | 30 |
| PTCy | In vivo | Ph2 | Activated T | CSP | Adult/HM | 49 | MSD/MUD | PB (45) | 30 | N/A | 17 | 2 | 70 | 31 |
| PTCy | In vivo | Retro | Activated T | CNI+MMF | Adult/HM | 58 | Haplo | BM (104) | N/A | 30 | 44 | 10 | 45 | 32 |
| PTCy | In vivo | Retro | Activated T | None | Adult/HM | 58 | MSD/MUD | BM (297) | N/A | 12 | 37 | 5 | 72 | 33 |
| PTCy | In vivo | Ph2 | Activated T | None | Adult/HM | 47 | MSD/MUD | BM (92) | 14 | 14 | 22 | 5 | 67 | 34 |
| PTCy | In vivo | Retro | Activated T | CNI+MMF | Adult/HM | 34 | MUD | BM (150) | 15 | 45 | 24 | 8 | 57 | 35 |
| PTCy | In vivo | Retro | Activated T | None vs. CNI+MTX | Ped/HM/TBI | 45 vs. 22 | MSD | BM (29) | 0 vs. 0 | 0 vs. 6 | 45 vs. 44 | 0 vs. 0 | 54 vs. 58 | 36 |
| PTCy | In vivo | Retro | Activated T | None | Ped/NM | N/A | Haplo | BM (27) | 0 | 24 | N/A | 22 | 78 | 37 |
| PTCy | In vivo | Ph2 | Activated T | CNI+MMF | Both/HM | N/A. | Haplo | BM (68) | 13 | N/A | 51 | 13 | 26 | 38 |
| PTCy | In vivo | Ph1/2 | Activated T | None | Adult/HM | N/A. | MSD/MUD | BM (117) | 3 | 10 | 44 | 3 | 55 | 39 |
| PTCy+MMF vs. BOR+MTX vs. MVC+MTX | In vivo | Ph2 | Activated T | TAC | Adult/HM | N/A. | MSD/MUD≥7/8 | PB (273) | 22 vs. 29 vs. 33 | 28 vs. 39 vs. 43 | 28 vs. 24 vs. 31 | 4 vs. 6 vs. 4 | 71 vs. 68 vs. 66 | 40 |
| PTCy vs. TAC+MTX vs. CD34 selec. +ATG | In vivo | Ph3 | Activated T | None | Adult/HM | N/A. | MSD/MUD | BM (346) | 27 vs. 34 vs. 9 | 42 vs. 44 vs. 23 | 14 vs. 26 vs. 21 | 0 vs. 1 vs. 3 | 76 vs. 76 vs. 60 | 41 |
| Anti-CD20 | In vivo | Ph2 | B cells | N/A | Adult/HM/RIC60% | 46 vs. 41 | MSD MUD | PB (65) | 31 vs. 49 | 48 vs. 60 | 34 vs. 28 | N/A | 71 vs. 56 | 42 |
| CB Treg vs. CB control | Ph1 | Activated T | Siro+MMF | Adult/HM | N/A | MMUD Cord >3/6 | CB (11) | N/A. | 0 vs 14 | 33 vs. 40 | 9 vs. 14 | 81 vs. 61 @ 1 yr | 43 | |
| CB vs. MUD | Retro | CNI+MTX or MMF | Both/HM | 31–39 | Cord >3/6 MUD | CB (140)/ PB (237)/ BM (107) | N/A but no. diff. | N/A but no. diff. | 15 vs. 24 | N/A | 71 vs. 63 | 14 | ||
| CB vs. MUD | Retro | CNI+MTX or MMF | Adult/HM/TBI | N/A | Cord >3/6 MUD | CB (116)/ PB (361)/ BM (185) | 23 vs. 34 | 39 vs. 42 | 22 vs. 25 | 8 vs. 3 | 44 vs. 43 | 46 | ||
| CB vs. MUD/MSD | Retro | CNI+MTX or MMF | Both/HM/TBI | Equiv. | Cord>3/6 MUD/MSD | CB (128)/ PF (275)/ BM (81) | N/A | 26 vs. 43–47 | 15 vs. 37–43 | 10 vs 0 | N/A, LFS: 51 vs.33–48 | 45 | ||
| CB | Ph2 | CNI+MMF | Adult/NM | N/A | Cord >3/6 | CB (26) | 12 | 36 | N/A | 12 | 85 | 47 | ||
| PB vs. BM | Ph3 | CNI+MTX | Adult/HM | MUD/MMUD | PB (273)/ BM (278) | 48 vs. 32 | 53 vs. 41 | 30 vs. 30 | 2 vs. 6 | 46 vs. 51 | 16 | |||
| PB/BM/CB | Retro CIBMTR | All | Pediatric/NM | N/A | All (20% MSD) | All (1696) | N/A | 25 | N/A | <1 | 75 | 141 | ||
| PB/BM/CB | Retro CIBMTR | All | Adult/pediatric Sickle cell | N/A | MMUD/MUD/ Cord >3/6 | All (352) | N/A | 29–32 | N/A | 17–44 | 81–87 | 143 | ||
| PB/BM | Retro CIBMTR | All | Adult/pediatric SAA | N/A | MUD/MMUD | BM (409) | N/A | 28–40 | N/A | 8–15 | 71–80 | 139 | ||
Some studies report extensive chronic GVHD.
MRD, measurable residual disease; PTCy, post-transplant cyclophosphamide; BOR, bortezomib; MVC, maraviroc; TAC, tacrolimus; CB, cord blood; MUD, HLA-matched unrelated donor; MSD, HLA-matched sibling donor; PB, peripheral blood stem cell; BM, bone marrow; ATG, anti-thymocyte globulin; CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; CSP, cyclosporine; Siro, sirolimus; HM, hematological malignancy; NM, non-malignancy; N/A, not available; MMUD, HLA-mismatched unrelated donor.
Data in chronic GVHD and in immune-mediated diseases have identified functionally distinct subsets of proinflammatory Teff/Tcon cells that have pathogenic effects and anti-inflammatory regulatory T cells (Treg) that attenuate disease. Inducing apoptosis of rapidly dividing T cells early after HCT with methotrexate44 attenuates the severity of acute GVHD but does not ameliorate chronic GVHD. Data also reveal that cord blood recipients have decreased incidence and severity of chronic GVHD also with relative increases in graft loss. Recipients of umbilical cord blood transplants may have a lower incidence or severity of chronic GVHD despite major HLA mismatching,43, 45–47 although further studies are needed to evaluate the GVT activity of cord blood cells.14, 45 Early phase clinical trials showed a low incidence of chronic GVHD after depletion of CD45RA-positive naïve T cells and B cells from mobilized blood cell grafts.28 These data are consistent with results showing that both allogeneic donor T cells and recipient alloantigens are necessary to develop chronic GVHD in murine models.48
Emerging data suggest that PTCy may prevent GVHD not by deleting alloantigen-activated T cell subsets but by impairing their functions and possibly by increasing the numbers of Tregs.49–51 As we further refine our understanding of the key T-cell subsets involved in chronic GVHD development and their phenotypic profiles, more selective approaches may be possible, for example, by targeting the pathogenic Th17 cells that have been linked to chronic GVHD.52 Further research is needed to explore the full immunological impact of these acute GVHD prophylaxis methods to determine whether they have selective effects on specific cell populations that cause chronic GVHD.
The balance of Treg vs Teff/Tcon cells has a major role in the development of chronic GVHD. Treg/Teff ratios are low at the onset of chronic GVHD, and in patients without chronic GVHD, low Treg/Teff ratios may predict a high risk for chronic GVHD development.53, 54 Adoptive transfer of ex vivo-expanded Treg or in vivo expansion mediated by low-dose IL-2 could decrease the risk of chronic GVHD through restoration of T cell immunoregulatory homeostasis.55–58 Similarly, PTCy spares Treg.50 Adoptive transfer or in vivo expansion of invariant natural killer T cells (NKT) may offer benefit, since the low numbers of these cells have been associated with low numbers of Tregs, aberrant immune recovery and an increased risk of chronic GVHD.59–61 Taken together, clinical evidence has led to an improved understanding of chronic GVHD. We have learned that targeting T, and possibly B cells, at the time of transplantation prevents chronic GVHD development and severity.
Donor B cell subsets
The presence of allo- and autoantibodies in patients with chronic GVHD suggests a pathogenic role for B cells, and results from murine models have shown that antibody-producing B cells contribute to the disease62, 63 and are necessary in some models.63–66 How antibodies mediate chronic GVHD is an area of active investigation. Proposed mechanisms include damage caused by antibody binding to thymic epithelial cell antigens65 or antibodies to tissues.63 B cells also act as antigen presenting cells after HCT.67 The efficacy of B cell-depleting agents such as rituximab for treatment of chronic GVHD in some patients further suggests that pathogenic B cells contribute to clinical chronic GVHD.68–70
Two prophylaxis studies have suggested that in vivo depletion of CD20+ B cells at 2–3 months after HCT may decrease the risk of chronic GVHD.42, 71 Global depletion of CD20+ B cells can induce prolonged B lymphopenia in mice and in patients. In some patients, eliminating CD20+ B cells paradoxically has resulted in progression of chronic GVHD associated with delayed B cell reconstitution and high concentrations of the survival factor, B cell Activating Factor of the TNF family (BAFF), that support rare aberrantly activated B cells.42, 60, 72–76 B cells that incite chronic GVHD are activated and primed for survival in vivo, and BAFF and alloantigen promote survival and B cell receptor (BCR)-activation of B cells in mice that develop chronic GVHD.77 Potentially pathologic B cells have a lowered BCR signaling threshold that enables hyper-reactivity.78, 79 These results have refined our understanding of these potentially pathogenic B cells and suggest that CD20 may not be the optimal target.75 Selective depletion of constitutively activated, alloreactive B cells could be a more effective strategy to reduce the incidence and severity of chronic GVHD. This approach could attenuate chronic GVHD while allowing immune recovery of a comprehensive, diverse, peripheral B-cell compartment under physiologic homeostatic control.79–81 Alternatively, low numbers of B regulatory cells (Breg) have been associated with aberrant immune recovery and chronic GVHD.60, 61 These data suggest that identifying and implementing strategies to expand these cells in vivo could decrease the risk of chronic GVHD if unique cell surface markers can be identified that distinguish Bregs from plasmablasts.
Donor monocytes/macrophages/dendritic cells
The diverse mechanisms that contribute to T cell dysregulation in chronic GVHD include macrophages and dendritic cells, although their role in humans has been difficult to investigate. Studies of pulmonary chronic GVHD in murine models have suggested a significant role for donor-derived, alternatively activated macrophages (M2) that drive Th2- and Th17-cell activation82 or via TGF-β secretion. Experimental models indicate that defective MHC class II antigen presentation during acute GVHD causes failure of Treg homeostasis in the periphery, thereby leading to chronic GVHD mediated by autoreactive T cells that escape negative selection in the thymus.83 Murine data suggest that plasmacytoid dendritic cells are protective for chronic GVHD, and there is some clinical data that may support this hypothesis as well.84–87 Further research is needed to determine whether agents that selectively expand plasmacytoid dendritic cells in vivo could be used to prevent chronic GVHD in humans.88
Recipient fibroblastic reticular cells
Certain recipient stromal cells with immune functions in secondary lymphoid organs (SLO) have a role in the etiology of chronic GVHD. In mice, lymph node damage impairs T and B cell interactions through loss of fibroblastic reticular cells (FRCs) that are necessary to induce tolerance.5, 89 FRCs, and potentially other recipient stromal cells, can also incite chronic GVHD via Notch ligand interactions that lead to aberrant activation of lymphocytes.62, 90, 91 The high numbers of activated circulating follicular T and B cells in patients with chronic GVHD and the presence of circulating post-germinal center plasmablast-like cells that typically reside in SLO suggest SLO damage.92 These CD4 T follicular helper (Tfh) cells interact with B cells, leading to increased plasma cell-like activation in active clinical chronic GVHD.93 Whether the risk of chronic GVHD could be averted or diminished by optimizing the function of primary and secondary lymphoid organs is unknown, although the co-occurrence of immune recovery with restoration of primary and secondary lymphoid organ function and development of immune tolerance supports this possibility.60, 61, 94
Studies in murine models have shown that interactions between FRCs and B cells in recipient lymphoid organs may cause chronic GVHD.62 FRCs are increased in number early after transplant and they have increased BAFF transcription.77 FRCs may promote chronic GVHD because they are defective in their capacity to present recipient-tissue antigens and unable to mediate deletional tolerance.5 Other myofibroblasts are pathologically activated in chronic GVHD95 and may incite pathways leading to chronic GVHD. The strong association of chronic GVHD with poor recovery of TdT-positive and PAX5-positive cells in the marrow at day 30 after HCT72 supports a need to move beyond cell surface phenotyping and enumeration of blood cells toward in-depth studies of interactions in recipient primary and secondary lymphoid organs and other tissues in animal models.
Roadmap to progress
Despite the many developments in the understanding of immune mediators of chronic GVHD, distinct mechanisms that incite disease remain elusive. Additional bench research is critical for the development of agents that will prevent disease. Understanding the etiopathology of chronic GVHD will also enable future risk stratification strategies.
II. Secondary Insults in chronic GVHD – Damage and dysfunction of recipient immune tissues and organs in chronic GVHD development and potential points of intervention
Current Knowledge:
Loss of immune tolerance in patients with de novo autoimmune diseases is affected by age and infections. In HCT recipients, tissue damage from the conditioning likely induces chronic GVHD through antigen exposure and presentation, with damage propagated by infection, microbiome disruption, and loss of oral, pulmonary, and gastrointestinal mucosal integrity. The ability of target tissue to tolerate these types of injury likely varies between individuals, but further research to delineate mechanisms is needed.96 Tissue signals drive recipient alloantigen presentation in murine models, inciting the aberrant activation cascade that leads to chronic GVHD.4 Approaches to minimize tissue injury in the preparative regimen with agents such as hematopoietic stem progenitor cell depleting anti-c-kit antibody could mitigate this contribution to chronic GVHD.97
Gaps in knowledge and unmet needs; highest priorities
Recipient thymic epithelial cell dysfunction
Impaired thymopoiesis is associated with chronic GVHD.98 The thymus incurs damage from the preparative regimen, immunosuppressive medications and donor T cells.65 In murine models, thymic damage impairs negative selection by medullary epithelial cells, permitting autoreactive donor-derived recent thymic emigrant T cells to target recipient tissues and mediate chronic GVHD.48 Autoimmune regulator (AIRE) gene dysfunction and loss of intrathymic group 3 innate lymphoid cells caused by acute GVHD contribute to failure of negative selection in the thymus.99, 100 The extent to which this mechanism applies in human HCT is not known. In both mice and humans, initial T cell reconstitution after HCT is derived primarily from expansion of mature T cells in the graft that have a restricted TCR repertoire.53, 101–105 These results suggest that lack of thymic recovery and failure to generate a naïve T cell repertoire could contribute to chronic GVHD-associated immune dysfunction, although this causal relationship in humans remains to be proven. The decreased risks of chronic GVHD in children compared to adults raise the question of whether the lower rates of thymic involution positions faster thymic recovery that could have a protective effect by maintaining effective negative selection and robust production of Treg cells, achieving immune tolerance through effective de novo T cell production and immunoregulatory homeostasis.13, 106–108 Additionally, lower risks of chronic GVHD may relate to preferential use of marrow and cord blood products for grafting in children.
Experiments in murine models have supported a role for androgen withdrawal, IGF-1 and keratinocyte growth factor (KGF) in thymic recovery.109–112 These results have motivated trials to test whether androgen suppression, IGF-1 supplementation and KGF can decrease the risk of chronic GVHD. Results with KGF have not been encouraging,113 and analyses of results with other agents are underway.
Non-immune organ tissue damage and chronic GVHD development
Certain exogenous events can be considered second insults that incite chronic GVHD by damaging recipient tissues other than primary or secondary lymphoid organs. In damaged tissues, the release of damage-associated molecular patterns (DAMPs) can trigger a proinflammatory microenvironment that leads to presentation of alloantigens or neo-autoantigens and intracellular antigens that are normally sequestered from the immune system.114 Tissue damage, related either to viral and other infections or to the anti-viral immune responses, may incite chronic GVHD. Decreased amounts of surfactant in the lung before HCT, likely due to injured epithelia, confers an increased risk of lung GVHD.115, 116 Elevated collagen type V, a marker of alveolar epithelial injury, has also been linked to pulmonary chronic GVHD.117–119 Collectively, these examples support studies of agents that promote tissue repair and decrease the impact of viral infections early after HCT.
Data regarding the association of dental hygiene and oral health with the risk of oral chronic GVHD are limited. Periodontal disease leads to both gingival inflammation and breakdown. In one study, oral microbiome changes were associated with oral chronic GVHD.120–122 Likewise, metabolic changes associated with the loss of gastrointestinal microbial diversity may be linked to GVHD.123, 124 These data support studies that address the interaction between specific tissues and the local microbiome on chronic GVHD development.124
Factors that incite sclerotic skin and connective tissue manifestations of chronic GVHD remain less well known. The association of sun exposure and local mechanical stress with focal cutaneous manifestations of chronic GVHD suggest that recipient tissue responses may contribute to development of the disease. Further understanding of mechanisms leading to these outcomes will potentially lead to improved understanding of inciting events in chronic GVHD.
Roadmap to progress
While the contribution of “additional insults” has been suggested in chronic GVHD, research is critical to understand both the biology underpinning of these events in preclinical models and in humans and to elucidate ways to prevent morbid forms such as bronchiolitis obliterans and sclerotic cutaneous chronic GVHD.
III. Based on what we know about chronic GVHD etiology, how might we assess risk of chronic GVHD development?
Balancing the risks of moderate to severe chronic GVHD versus the risks of graft rejection, delayed immune reconstitution, and recurrent or progressive malignancy poses a key issue in designing trials to prevent chronic GVHD. As described in Figure 2, important considerations balance the benefit and risks of interventions at each time point after HCT. While lower intensity of the conditioning regimen may decrease the magnitude of tissue damage that might trigger chronic GVHD, it could also increase the risk of relapse or graft rejection. Interventions that decrease the numbers, activation, or survival of donor T and other functional immune cells could decrease the risk of chronic GVHD but could also decrease their ability to prevent graft rejection and recurrent or progressive malignancy (Table 1). Highly intensive immunosuppressive regimens could decrease the risk of chronic GVHD but could also delay immune recovery, increase susceptibility to infections, and possibly increase the risk of recurrent or progressive malignancy. Although interventions to prevent chronic GVHD could increase the risks of graft rejection, delayed immune reconstitution and opportunistic infections, effects on the risk of recurrent or progressive malignancy or the loss of therapeutic response in non-malignant diseases pose the most significant consideration in trial design.
Figure 2. Factors that influence the emergence of chronic GVHD.
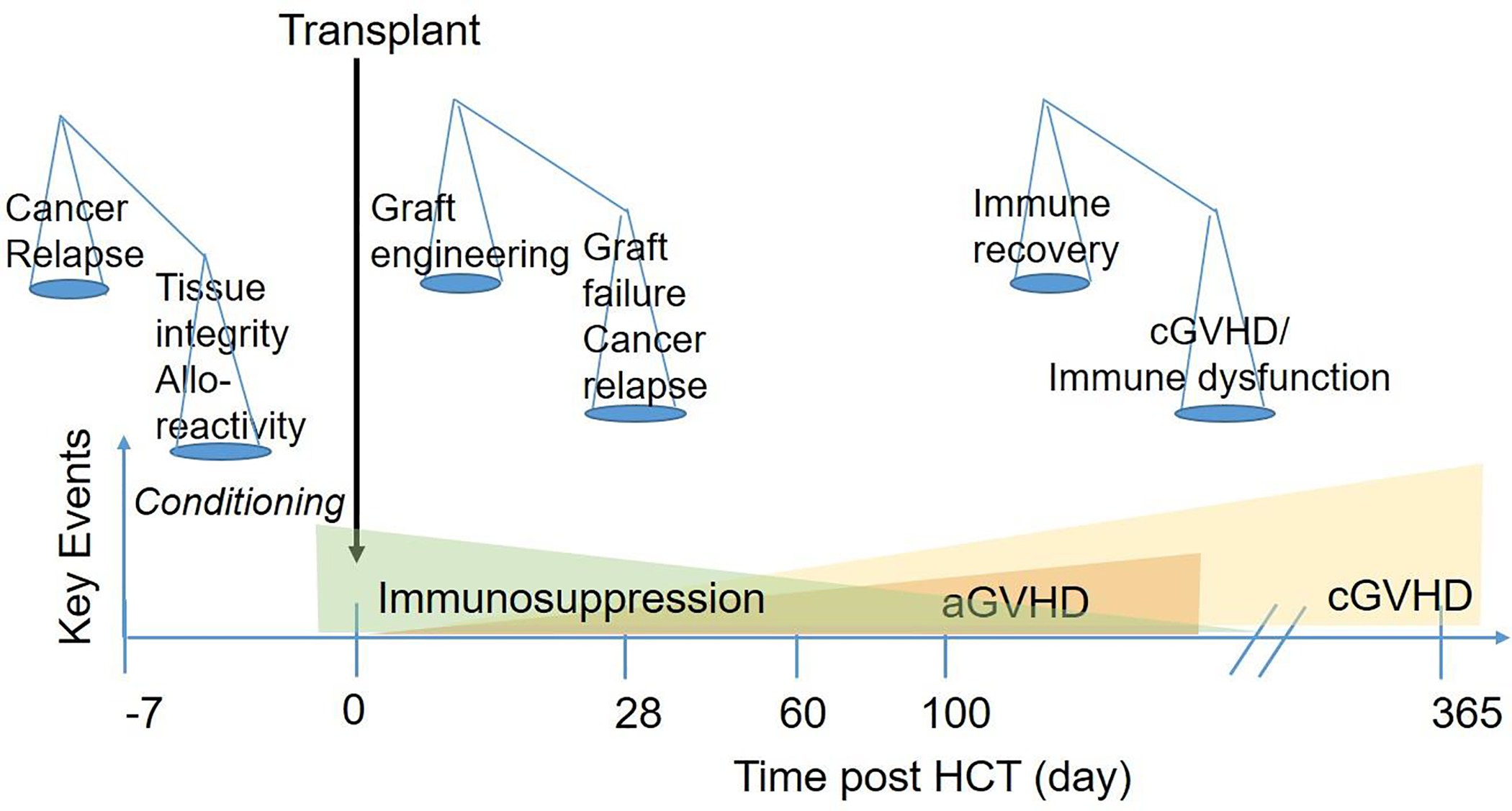
The x-axis shows time after HCT, with key events denoted in shapes. The green triangle indicates gradual tapering of immunosuppressive treatment after HCT. The orange triangle denotes the onset of acute GVHD, and the yellow triangle denotes the onset of chronic GVHD, both of which can be masked by immunosuppressive treatment. High-intensity pre-transplant conditioning regimens can decrease the risk of relapse but increase the risk of chronic GVHD. Depletion of donor T cells can decrease the risk of chronic GVHD but increase the risks of graft rejection, infections due to delayed immune reconstitution and relapse due to loss of GVT activity. Withdrawal of immunosuppression permits immune recovery and protection against infections but can increase the risk of chronic GVHD.
IV. How do we best consider risk of recurrent or progressive malignancy as we consider prevention of chronic GVHD?
Several large studies have shown potent GVT effects associated with the presence of chronic GVHD by NIH criteria and an increased risk of recurrent or progressive malignancy in patients who did not develop chronic GVHD.125–127 Mild chronic GVHD has been associated with improved overall survival for patients with malignant diseases, while moderate to severe chronic GVHD has been associated with an increased risk of non-relapse mortality.128, 129 During the first 18 months after HCT, patients without chronic GVHD who continued immunosuppressive medications had the highest risk of relapse.125 Beyond 18 months after HCT, patients who did not experience chronic GVHD showed the highest risk of relapse even though they had discontinued all immunosuppressive medications, but treatment with immunosuppressive medications had no effect on the risk of relapse in patients who experienced acute or chronic GVHD in this study.125
Some of the therapeutic interventions with the greatest effect in preventing chronic GVHD have been associated with higher relapse rates (Table 1). Limitations of these studies include a lack of data addressing the risk for relapse as indicated by the presence or absence of measurable residual disease (MRD) before transplantation, the intensity of the preparative regimen, and the intrinsic susceptibility of the malignant cells to immunologic control. GVT effects may differ according to disease type and disease status.126, 130 When evaluating the potential success of an intervention to prevent chronic GVHD, it is imperative to consider risk stratification for recurrent or progressive malignancy in the eligibility criteria. As discussed below, the presence of MRD at the time of HCT is associated with an increased risk of relapse after HCT. One approach of great interest would be to test agents that could simultaneously target malignant cells and chronic GVHD.131 Emerging data suggest that adoptive transfer of certain NK subsets132 or invariant NKT133 or gamma delta T cells134 could minimize the risk of chronic GVHD while preserving GVT benefits. Future studies should focus on testing whether adoptive transfer of anti-tumor lymphocytes could mediate critical GVT activity when depletion of donor lymphoid cells is used to prevent chronic GVHD.
V. Critical questions and answers about chronic GVHD prevention trials
Enrollment in prevention trials is based on risk factors known before HCT, regardless of when the intervention is given, while enrollment in preemption trials is based on post-transplant events, signs, symptoms or biomarkers indicating that the risk of chronic GVHD is higher than was previously appreciated. The sections below discuss considerations for prevention trials. Considerations for preemption trials are discussed in a separate report by Working Group 2.
The selection of interventions and approaches to test for prevention of chronic GVHD should be based on an understanding of the underlying mechanisms that initiate the processes leading to development of chronic GVHD, along with consideration of possible off-target effects and the impact on immune reconstitution after HCT. The following sections address three critical questions that should be considered in the design of trials testing new approaches to prevent chronic GVHD.
1). What preventive agents and approaches are most promising?
The key considerations include the strength of the efficacy data for the chronic GVHD intervention approach versus its influence on other HCT outcomes, including prevention of graft rejection, infections and recurrent or progressive malignancy.
Approaches that prevent chronic GVHD without impairing immune function would be ideal. Alternatively, selecting agents that could simultaneously target tumors and dysregulated alloreactivity might mitigate any effect on the risk of relapse or graft rejection. To understand the influence of an investigational product or approach on relapse, it will be crucial to document the underlying pretransplant risk of relapse as accurately as possible. In patients with acute leukemia, the presence of MRD at the time of HCT reflects not only the measurable residual disease burden but also sensitivity of the disease to prior therapies, thereby representing the single strongest predictor of relapse after HCT.135–137 Most reports of chronic GVHD prevention trials have not included information about the presence or absence of MRD at the time of HCT (Table 1). Including this and other information such as the disease risk index (DRI) will be important to understand the impact of specific approaches on the risk of relapse.130 It may be possible to refine graft engineering approaches as we begin to understand the mechanisms leading to chronic GVHD. Alternatively, modification of current graft engineering approaches might improve the risk/benefit ratio, for example, by targeting the dose of ATG to the absolute lymphocyte count138 by adjusting the numbers of T cells in the graft. Additionally, the timing of in vivo cell depletion or expansion strategies and adoptive transfer strategies, or application of ways to improve outcomes with cord blood grafts may improve the risk/benefit ratio.
2). Who should be enrolled in chronic GVHD prevention trials?
Ideally, trial inclusion and exclusion criteria should enrich for patients at high risk of moderate to severe chronic GVHD and exclude those at high risk of graft failure, infections, and relapse. Prevention trials can provide benefit only for the unknown subset of patients who would otherwise develop moderate or severe chronic GVHD. This potential benefit should be carefully weighed against the likelihood that the study intervention could increase the risks of graft rejection, viral reactivation, delayed immune recovery and recurrent or progressive malignancy that apply to all participants (Figure 3). Selecting patients with non-malignant diseases would obviate the risk of relapse, but most patients have malignant diseases. As shown in Table 1, in the absence of measures to prevent chronic GVHD, 25–40% of patients with non-malignant diseases develop this complication, which has no associated GVT benefit in these patients.139–143 Future eligibility criteria could include biomarkers that have a high positive predictive value for development of chronic GVHD. If the risk of a study intervention is low, enrollment of patients with a low risk of developing chronic GVHD may be justified but could require a larger sample size. If the risk of the study intervention is high, however, enrollment of patients with a low risk of developing chronic GVHD may not be justified. Developing prognostic models that quantitate these risk/benefit ratios based on existing data is a high research priority (Table 2).
Figure 3. Critical considerations of risk and benefit in the design of chronic GVHD prevention trials.
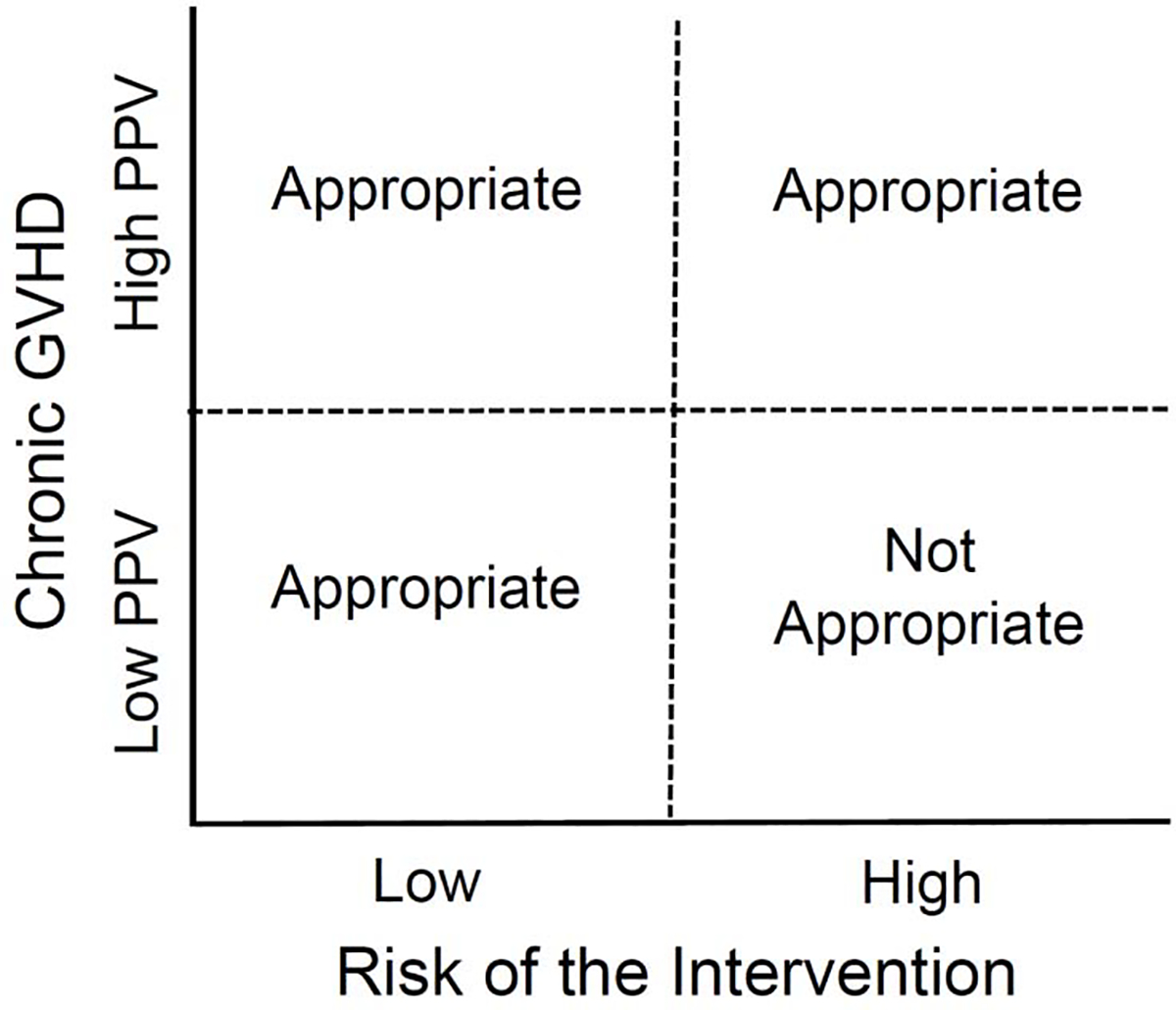
The positive-predictive value (PPV) of a prognostic measure or algorithm before HCT estimates the probability of developing chronic GVHD in patients who have a positive test result. The dashed lines represent hypothetical boundaries between the low and high-risk interventions (X-axis) and between low and high PPV (Y-axis). Patients not destined to develop chronic GVHD cannot benefit in prevention trials. Therefore, clinical trials should be designed to ensure that the harm of the intervention in these patients does exceed the benefit in patients destined to develop chronic GVHD.
3). What are the most appropriate endpoints in chronic GVHD prevention trials?
3.1). Primary efficacy endpoint
Supplementary Table 1 summarizes currently active interventional phase 2 or 3 trials with prevention of chronic GVHD as the primary endpoint from ClinicalTrials.gov. The primary endpoints in these trials vary in terminology, (e.g., “moderate/severe,” “extensive,” “requiring steroid treatment”), incorporation of events other than chronic GVHD (i.e., acute GVHD, recurrent malignancy, and death), specification of time points for assessment, and statistical methods for analysis (e.g., log-rank test, proportional hazards, Gray test).
To move the field toward pivotal chronic GVHD prevention trials intended for regulatory review, we recommend survival without moderate to severe NIH-defined chronic GVHD (chronic GVHD-free survival) as primary endpoint. In two studies that have reported this endpoint, the probabilities of moderate to severe chronic GVHD-free survival were 44% and 47% at 2 years in patients who received standard post-transplant immunosuppression with tacrolimus and methotrexate.24, 41 Cumulative incidence estimates of moderate to severe chronic GVHD are not appropriate as the primary endpoint, because they can be decreased by a high incidence of death or relapse as competing risks. Chronic GVHD requiring systemic treatment could serve as a functional endpoint definition instead of moderate to severe chronic GVHD, although this endpoint depends on providers’ medical judgment regarding the need for systemic treatment, which could be biased. We recommend assessing the primary endpoint at one year after HCT in prevention trials because most chronic GVHD develops within one year, but longer follow-up beyond the primary endpoint at one year would be highly desirable.
In earlier phase studies, an endpoint that captures an effect linked to chronic GVHD prevention would be appropriate as a primary endpoint. For example, if an intervention is intended to increase the number of Tregs or to improve their function as a way to prevent chronic GVHD, an early phase trial could be designed around these immunological endpoints, provided that the established linkage to prevention of chronic GVHD is strong.
3.2). Secondary endpoints
Composite endpoints such as chronic GVHD-free, relapse-free survival (CRFS) or GVHD-free, relapse-free survival (GRFS) have gained popularity as a way of assessing the overall success of HCT. Composite endpoints are most appropriate as secondary endpoints because the onset of failure events other than chronic GVHD (i.e., relapse, death, and severe acute GVHD) can confound the interpretation of the most relevant failure event (i.e., chronic GVHD). GRFS is appropriate if it is anticipated that the study intervention is likely to decrease the incidence of both acute and chronic GVHD. For chronic GVHD prevention studies, CRFS is preferred secondary endpoint over GRFS. In all studies, separate reporting of each component in composite endpoints and tabulation of the causes of death should be included as secondary endpoints to understand the benefits and risks of the study intervention and to understand how these events influence the interpretation of the primary endpoint. We encourage investigators to develop and validate patient-reported measures that could be used for periodic screening to detect the onset of chronic GVHD. Prevention trials also offer an opportunity to collect biospecimens that could be used in exploratory studies to identify biomarker changes that reliably predict the future development of chronic GVHD.2
3.3). Interpretation of results
Pivotal studies intended for regulatory review must be adequate and well controlled, generally by comparing results in the investigational arm versus randomized placebo concurrent controls, no treatment concurrent controls, active treatment concurrent controls or pre-specified historical controls (21CFR§314.126).
To gain efficiency, trials with more than 2 arms can be designed to compare multiple investigational arms against a single control arm. The major disadvantage of this approach is that large numbers of patients are needed to compare the investigational arms against each other, making it difficult to complete them in a timely manner. Another disadvantage is that enrolled patients must be able to receive any of the study interventions, potentially excluding some patients who have contraindications to only one of the study arms. Single arm designs may be appropriate for phase 2 studies, but the interpretation of results is currently limited by the lack of validated risk stratification criteria and benchmarks for the probability of survival without moderate to severe chronic GVHD. Therefore, randomization may be preferred for phase 2 studies, if feasible. Participants in trials to prevent chronic GVHD should generally not co-enroll in trials to prevent other major complications such as relapse, unless stratification is used to balance the study arms in a randomized trial.
The most appropriate control arm in chronic GVHD prevention trials is the standard of care when the trial is designed. Retrospective and prospective studies are needed to develop pre-transplant risk stratification for the incidence of moderate to severe chronic GVHD with standard of care treatment regimens, thereby informing the eligibility criteria for future trials. Studies are also needed to provide benchmarks for the probability of survival without moderate to severe chronic GVHD, thereby informing the design and interpretation of results in future trials.
Roadmap to progress
Clinical trials designed to prevent moderate to severe chronic GVHD should include a risk assessment model for relapse in patients with underlying malignancy with careful consideration of the eligibility criteria for these patients. Investigators should also consider the competing risks of graft failure and infections in those with nonmalignant indications. We recommend survival without moderate to severe NIH-defined chronic GVHD (chronic GVHD-free survival) as the primary endpoint in prevention trials of chronic GVHD.
Below we summarize recommendations for studies over the next 3 years
-
Elucidate the distinct cellular and molecular pathways that induce and maintain immune tolerance, thereby allowing withdrawal of immunosuppressive medications without risk of clinically evident recurrent or progressive chronic GVHD.
-
1.a.
Use primary patient samples and murine models to determine how recipient and donor characteristics incite or attenuate the development of chronic GVHD.
-
1.b.
Define roles for recipient tissues and organs in the initiation of chronic GVHD.
-
1.c.
Determine how additional insults after HCT incite moderate to severe chronic GVHD.
-
1.a.
Integrate studies of patient samples to include potential use of high throughput “multi-omic” approaches and systems immunology approaches to identify predictive risk assessment markers.
Work toward defining a risk stratification strategy that predicts risks of relapse, graft failure, and viral infections, and balances these against the risk of developing morbid forms of chronic GVHD to identify patients who would most benefit from chronic GVHD prevention trials.
Conduct well-designed chronic GVHD prevention trials based on what we know about the balance of benefits and risks to optimize immune reconstitution without impairing GVT activity.
We conclude that well-designed trials to prevent chronic GVHD would best be informed by basic and translational discoveries that have originated from clinical observations. A collaborative bedside-to-bench-to-bedside approach with careful considerations of the risk-benefit ratio regarding diminish morbidity without increasing mortality is needed to prevent chronic GVHD.
Supplementary Material
Consensus Key Point:
Moderate to severe chronic GVHD leads to excess morbidity and mortality and should be prevented.
Despite the advent of effective chronic GVHD prevention strategies, further scientific and clinical research is needed.
T-cell depletion strategies decrease the risk of chronic GVHD but can also impair immune reconstitution and anti-tumor effects after HCT.
Consensus Key Point:
Primary inciting cellular and molecular pathways leading to chronic GVHD arise from donor and host factors and are triggered early after HCT.
Points of Intervention – Mitigation of Risk Factors for chronic GVHD Development
Graft engineering strategies
Modified schedules for weaning immunosuppression (IS) after HCT
Protection of primary and secondary lymphoid organs (SLO)
Maintaining balance between immune effector cells and immune regulatory cells
Consensus Key Point:
Agents that target cells that might not be critical in GVT reactions, such as certain T cell subsets, distinct B cell subsets, monocytes, macrophages, or host lymphoid organ stromal cells represent prime candidates for further clinical studies.
Points of intervention to mitigate chronic GVHD development
Small molecule inhibitors of aberrant B and T cells
Antibodies to cytokines
Key Consensus Point:
Secondary insults may occur at any time after HCT.
Develop Risk Mitigation Strategies based on second insults leading to chronic GVHD
HLA matching
Infection prevention
Tissue damage prevention
Consensus Key Point:
For patients with hematological malignancies, a risk assessment model for moderate to severe chronic GVHD must consider possible effects of the study intervention on the risk of recurrent or progressive malignancy when designing clinical trials.
Points of intervention to mitigate chronic GVHD development
Develop risk stratification tools to guide clinical trial design
Consider interventions that serve dual purposes by targeting both chronic GVHD and malignant cells in the recipient
Consensus Key Point:
Integrate studies of patient samples, to include potential use of high throughput “multi-omic” approaches in a systems immunology approach to aid in chronic GVHD risk stratification
Points of intervention to mitigate chronic GVHD development
Use translational data to identify populations at high risk of chronic GVHD
Use translational data to identify novel targets
Consensus Key Point:
Despite the advent of effective chronic GVHD prevention strategies, further clinical trials are needed.
We recommend moderate to severe chronic GVHD-free survival as the primary endpoint in trials to prevent chronic GVHD.
ACKNOWLEDGMENTS:
Funding and implementation of this Consensus Project is made possible through the support by the Intramural Program of the National Cancer Institute – Center for Cancer Research and the National Institute of Dental and Craniofacial Research, the NIH Intramural and Extramural Research Programs Institutes and Centers. Special acknowledgement goes to the Meredith Cowden GVHD foundation, France Lymphome Espoir, NBMTLink; Anthony Nolan, National Marrow Donor Program, BMT InfoNet and other patient advocacy groups for partnering and collaboration. Thanks go to all working groups and consensus conference participants, professional societies, US government agencies and stakeholders in the field of hematopoietic stem cell transplantation for the generous donation of their work, time, talents and expertise. Particular acknowledgment to the ASTCT and EBMT for their roles in the dissemination, education and implementation of the concepts and best practices evolving from this project.
Special thanks to the independent external peer reviewers who provided they comments and critiques to the 2020 NIH Chronic GVHD Consensus Project: Nicolaus Kroeger, M.D. Professor & Clinical Director of the Department of Stem Cell Transplantation, University of Hamburg, Hamburg, Germany, President EBMT; Ryotaro Nakamura, M.D. Hematologist – Oncologist, Professor & Director of the Center for Stem Cell Transplantation City of Hope Cancer Center, Duarte, California; John DiPersio, M.D., Ph.D. Chief, Division of Oncology; Director, Center for Gene and Cellular Immunotherapy; Deputy Director, Siteman Cancer Center, Washington University School of Medicine, St. Louis, Missouri; Mark Juckett, M.D., Professor & Director of the Blood and Marrow Transplant Program, University of Wisconsin, Madison, Wisconsin; George Chen, M.D., Associate Professor of Medicine, University at Buffalo, Buffalo, New York; Rafael Duarte, M.D., Ph.D., FRCP, Head of Department of Hematology and Director of the Hematopoietic Transplant Program, Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain; Franco Locatelli, M.D. Professor of Pediatrics Università Sapienza, Roma, Head of the Department of Pediatric Hematology and Cell and Gene Therapy, IRCCS Ospedale Pediatrico Bambino Gesù, Roma, Italy; Areej El-Jawahri, M.D., Assistant Professor, Director of the Bone Marrow Transplant Survivorship Program, and Associate Director of the Cancer Outcomes Research and Education Program at Massachusetts General Hospital, Boston, Massachusetts; Robert Soiffer, M.D. Professor & Co-Chief of Stem Cell Transplantation and Cellular Therapies, Chair of the Executive Committee for Clinical Programs, Vice Chair for the Department of Medical Oncology, Chief of the Division of Hematologic Malignancies, Dana Farber Cancer Institute, Boston, Massachusetts; Daniel Weisdorf, M.D., Professor of Medicine & Deputy Director of Clinical Science and Translational Science Institute; Director, Clinical and Translational Research Services, University of Minnesota, Minneapolis, Minnesota; Keith Sullivan. M.D., Professor of Medicine, Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, North Carolina; Catherine Lee, M.D., Assistant Professor, Hematology and Medical Oncology, Huntsman Cancer Institute - University of Utah, Salt Lake City, Utah; Jose Antonio Perez-Simon, M.D., Professor of Hematology, University of Seville, Head of Department of Hematology, University Hospital Virgen del Rocio and Vice director of the Biomedical Research Institute of Seville (IBIS), Seville, Spain; Doris Ponce, M.D., Associate Professor of Medicine, Hematologic Oncologist, Memorial Sloan-Kettering Cancer Center, New York City, New York; Andrew Harris, M.D., Pediatric Hematologist-Oncologist & Assistant Professor of Pediatrics, Pediatric BMT and Cellular Therapy Program, University of Utah/Primary Children’s Hospital, Salt Lake City, Utah.
APPENDIX: 2020 NATIONAL INSTITUTES OF HEALTH CONSENSUS-DEVELOPMENT PROJECT ON CRITERIA FOR CLINICAL TRIALS IN CHRONIC GVHD STEERING COMMITTEE
Chairs: Steven Pavletic, M.D., M.S., National Cancer Institute; Stephanie Lee, M.D., M.P.H., Fred Hutchinson Cancer Research Center; Kirk Schultz, M.D., University of British Columbia; Daniel Wolff, M.D., University of Regensburg
Members: Hildegard Greinix, M.D., University of Graz; Sophie Paczesny, M.D., University of South Carolina; Bruce Blazar, M.D., University of Minnesota; Stefanie Sarantopoulos, M.D., Ph.D., Duke University; Joseph Pidala, M.D., Ph.D., Moffitt Cancer Center; Corey Cutler, M.D., M.P.H., FRCPC, Dana Farber Cancer Institute; Gerard Socie, M,D., Ph.D., St-Louis Hospital, Paris; Paul Martin, M.D., Fred Hutchinson Cancer Research Center; Meredith Cowden, M.A., LPCC-S, Cowden Foundation.
ETIOLOGY AND PREVENTION WORKING GROUP 1
Co-Chairs: Stefanie Sarantopoulos, M.D., Ph.D., Duke University and Gerard Socie, M.D., Ph.D., St-Louis Hospital.
Members: Mukta Arora, M.D., M.S., University of Minnesota; Iskra Pusic, M.D., Washington University School of Medicine; Yoshi (Yoshihiro) Inamoto, M.D., National Cancer Institute-Japan; Leo (Leonido) Luznik, M.D., Johns Hopkins University; Pavan Reddy, M.D., University of Michigan; Jerome Ritz, M.D., Dana-Farber Cancer Institute; Betty Hamilton, M.D., Cleveland Clinic; Kirsten Williams, M.D., Emory School of Medicine; Annie Im, M.D., University of Pittsburgh Medical College; John Koreth, M.D., Ph.D., Dana-Farber Cancer Institute; Paul Carpenter, BSc., MBBS., Fred Hutchinson Cancer Research Center; Jacki (Jacqueline)Mays, DDS., MHSc., Ph.D., NIH National Institute of Dental and Craniofacial Research.
Editor: Paul J. Martin, Fred Hutchinson Cancer Center, The University of Washington
Footnotes
Financial disclosure:
Conflict of interest statement: K.M.W.: none. Y.I.: advisory boards for Novartis, Janssen and Meiji Seika Pharma. A.I.: none. B.H.: advisory board for Syndax. J.K.: research support from Amgen, BMS, Clinigen, Equillium, Miltenyi Biotec, and Regeneron Pharmaceuticals; advisory boards for Cugene and Therakos; consulting income from Biolojic Design, EMD Serono, Equillium, Gentibio, Moderna and Nekonal Oncology. M.A.: consultant for Fate Therapeutics; research funding from Pharmacyclics, Kadmon and Syndax. I.P.: advisory boards for Incyte, Kadmon and Syndax. J.W.M.: none. P.A.C.: consultant for Fate Therapeutics. L.L.: Receives research support from Genentech and Merck, serves on the advisory board: AbbVie, Talaris Therapeutics, PrecisionBiosciences and is a patent holder for WindMIL Therapeutics. P.R.: none. J.R.: research funding from Amgen, Equillium and Kite Pharma; Data Monitoring Committee for Avrobio; consulting income from BMS/Celgene, Infinity Pharmaceuticals, LifeVault Bio, Rheos Medicines, Talaris Therapeutics and TScan Therapeutics. H.G.: none. S.P.: Patent applications (US 20130115232A1 and WO2013066369A3) on “Methods of detection of graft versus host disease” licensed to ViaCore-IBT laboratories. B.R.B.: advisory boards for Magenta Therapeutics and BlueRock Therapeutics. Research funding from BlueRock Therapeutics. Steering committee: Kadmon Corporation. Co-founder Tmunity Therapeutics. J.P.: Consulting and advisory board membership – Syndax, CTI Biopharma, Amgen, Regeneron; Clinical trial support - Novartis, Amgen, Takeda, Janssen, Johnson and Johnson, Pharmacyclics, Abbvie, CTI Biopharma, BMS. C.C.: Consulting/Honoraria from: Incyte, Jazz, CareDx, Mesoblast, Syndax, Omeros, Pfizer. DW: advisory board Novartis and Incyte, DSMB: Novartis and Behring, honoraria from Takeda, Gilead, Pfizer and Neovii. K.R.S: DSMB member BMS/Juno, Advisory board Jazz, Novartis, Janssen. S.Z.P: none. S.J.L.: Research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, Takeda. Steering committee: Incyte. P.J.M.: advisory boards for Mesoblast and Rigel Pharmaceuticals Inc.; honoraria from Janssen. G.S.: advisory boards for Novartis, Incyte, Pharmacyclics, Amgen, and Xenikos. S.S.: advisory board for Rigel Pharmaceuticals Inc.
Disclaimer: The opinions expressed are those of the authors and do not represent the position of the National Cancer Institute, the National Institutes of Health, or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The Biology of Chrnic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23:211–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Davis MM. The science and medicine of human immunology. Science. 2020;369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dertschnig S, Evans P, Santos ESP, Manzo T, Ferrer IR, Stauss HJ, et al. Graft-versus-host disease reduces lymph node display of tissue-restricted self-antigens and promotes autoimmunity. J Clin Invest. 2020;130:1896–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389–96. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, risk factors, and outcomes of sclerosis in patients with chronic graft-versus-host disease. Blood. 2013;121:5098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016;375:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatobene G, Storer BE, Salit RB, Lee SJ, Martin PJ, Cheng GS, et al. Disability related to chronic graft -versus-host disease after alternative donor hematopoietic cell transplantation. Haematologica. 2019;104:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood. 2017;130:677–85. [DOI] [PubMed] [Google Scholar]
- 18.Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant. 2016;51:668–74. [DOI] [PubMed] [Google Scholar]
- 19.Gaziev J, Isgro A, Sodani P, Paciaroni K, De Angelis G, Marziali M, et al. Haploidentical HSCT for hemoglobinopathies: improved outcomes with TCRalphabeta(+)/CD19(+)-depleted grafts. Blood Adv. 2018;2:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–6. [DOI] [PubMed] [Google Scholar]
- 21.Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med. 2016;374:43–53. [DOI] [PubMed] [Google Scholar]
- 22.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. [DOI] [PubMed] [Google Scholar]
- 23.Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–82. [DOI] [PubMed] [Google Scholar]
- 24.Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35:4003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73. [DOI] [PubMed] [Google Scholar]
- 26.Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2020;7:e100–e11. [DOI] [PubMed] [Google Scholar]
- 27.Chang YJ, Wu DP, Lai YR, Liu QF, Sun YQ, Hu J, et al. Antithymocyte Globulin for Matched Sibling Donor Transplantation in Patients With Hematologic Malignancies: A Multicenter, Open-Label, Randomized Controlled Study. J Clin Oncol. 2020;38:3367–76. [DOI] [PubMed] [Google Scholar]
- 28.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125:2677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barba P, Hilden P, Devlin SM, Maloy M, Dierov D, Nieves J, et al. Ex Vivo CD34(+)-Selected T Cell-Depleted Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome Is Associated with Low Incidence of Acute and Chronic Graft-versus-Host Disease and High Treatment Response. Biol Blood Marrow Transplant. 2017;23:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCurdy SR, Kanakry CG, Tsai HL, Gojo I, Smith BD, Gladstone DE, et al. Development of Grade II Acute Graft-versus-Host Disease Is Associated with Improved Survival after Myeloablative HLA-Matched Bone Marrow Transplantation using Single-Agent Post-Transplant Cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiusolo P, Bug G, Olivieri A, Brune M, Mordini N, Alessandrino PE, et al. A Modified Post-Transplant Cyclophosphamide Regimen, for Unmanipulated Haploidentical Marrow Transplantation, in Acute Myeloid Leukemia: A Multicenter Study. Biol Blood Marrow Transplant. 2018;24:1243–9. [DOI] [PubMed] [Google Scholar]
- 36.Jacoby E, Chen A, Loeb DM, Gamper CJ, Zambidis E, Llosa NJ, et al. Single-Agent Post-Transplantation Cyclophosphamide as Graft-versus-Host Disease Prophylaxis after Human Leukocyte Antigen-Matched Related Bone Marrow Transplantation for Pediatric and Young Adult Patients with Hematologic Malignancies. Biol Blood Marrow Transplant. 2016;22:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neven B, Diana JS, Castelle M, Magnani A, Rosain J, Touzot F, et al. Haploidentical Hematopoietic Stem Cell Transplantation with Post-Transplant Cyclophosphamide for Primary Immunodeficiencies and Inherited Disorders in Children. Biol Blood Marrow Transplant. 2019;25:1363–73. [DOI] [PubMed] [Google Scholar]
- 38.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasquini MC, Luznik L, Logan BR, Soiffer R, Wu J, Devine S, et al. Calcineurin Inhibitor-Free Graft-Versus-Host Disease (GVHD) Prophylaxis in Hematopoietic Cell Transplantation (HCT) with Myeloablative Conditioning Regimens (MAC) and HLA-Matched Donors: Results of the BMT CTN 1301 Progress II Trial. The 2021 TCT meetings Abstract LBA1. 2021. [Google Scholar]
- 42.Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen YB, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss G, Osen W, Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. 2002;128:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peffault de Latour R, Chevret S, Jubert C, Sirvent A, Galambrun C, Ruggeri A, et al. Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood. 2018;132:750–4. [DOI] [PubMed] [Google Scholar]
- 48.Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191:488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129:2357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124:2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDonald KP, Blazar BR, Hill GR. Cytokine mediators of chronic graft-versus-host disease. J Clin Invest. 2017;127:2452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120:1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20:2215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–6. [DOI] [PubMed] [Google Scholar]
- 59.Du J, Paz K, Thangavelu G, Schneidawind D, Baker J, Flynn R, et al. Invariant natural killer T cells ameliorate murine chronic GVHD by expanding donor regulatory T cells. Blood. 2017;129:3121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolupaev OV, Dant TA, Bommiasamy H, Bruce DW, Fowler KA, Tilley SL, et al. Impaired bone marrow B-cell development in mice with a bronchiolitis obliterans model of cGVHD. Blood Adv. 2018;2:2307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pierini A, Nishikii H, Baker J, Kimura T, Kwon HS, Pan Y, et al. Foxp3(+) regulatory T cells maintain the bone marrow microenvironment for B cell lymphopoiesis. Nat Commun. 2017;8:15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radojcic V, Paz K, Chung J, Du J, Perkey ET, Flynn R, et al. Notch signaling mediated by Delta-like ligands 1 and 4 controls the pathogenesis of chronic GVHD in mice. Blood. 2018;132:2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng R, Hurtz C, Song Q, Yue C, Xiao G, Yu H, et al. Extrafollicular CD4(+) T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease. Nat Commun. 2017;8:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin H, Ni X, Deng R, Song Q, Young J, Cassady K, et al. Antibodies from donor B cells perpetuate cutaneous chronic graft-versus-host disease in mice. Blood. 2016;127:2249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. [DOI] [PubMed] [Google Scholar]
- 67.Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD. The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia. Science. 1990;249:921–3. [DOI] [PubMed] [Google Scholar]
- 68.Arai S, Pidala J, Pusic I, Chai X, Jaglowski S, Khera N, et al. A Randomized Phase II Crossover Study of Imatinib or Rituximab for Cutaneous Sclerosis after Hematopoietic Cell Transplantation. Clin Cancer Res. 2016;22:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40:273–7. [DOI] [PubMed] [Google Scholar]
- 71.Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fedoriw Y, Samulski TD, Deal AM, Dunphy CH, Sharf A, Shea TC, et al. Bone marrow B cell precursor number after allogeneic stem cell transplantation and GVHD development. Biol Blood Marrow Transplant. 2012;18:968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobson CA, Sun L, Kim HT, McDonough SM, Reynolds CG, Schowalter M, et al. Post-transplantation B cell activating factor and B cell recovery before onset of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mensen A, Johrens K, Anagnostopoulos I, Demski S, Oey M, Stroux A, et al. Bone marrow T-cell infiltration during acute GVHD is associated with delayed B-cell recovery and function after HSCT. Blood. 2014;124:963–72. [DOI] [PubMed] [Google Scholar]
- 75.Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Storek J, Wells D, Dawson MA, Storer B, Maloney DG. Factors influencing B lymphopoiesis after allogeneic hematopoietic cell transplantation. Blood. 2001;98:489–91. [DOI] [PubMed] [Google Scholar]
- 77.Jia W, Poe JC, Su H, Anand S, Matsushima GK, Rathmell JC, et al. BAFF Promotes Heightened BCR Responsiveness and Manifestations of Chronic GVHD after Allogeneic Stem Cell Transplantation. Blood in press. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allen JL, Fore MS, Wooten J, Roehrs PA, Bhuiya NS, Hoffert T, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poe JC, Jia W, Di Paolo JA, Reyes NJ, Kim JY, Su H, et al. SYK inhibitor entospletinib prevents ocular and skin GVHD in mice. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flynn R, Allen JL, Luznik L, MacDonald KP, Paz K, Alexander KA, et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125:4085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leonhardt F, Zirlik K, Buchner M, Prinz G, Hechinger AK, Gerlach UV, et al. Spleen tyrosine kinase (Syk) is a potent target for GvHD prevention at different cellular levels. Leukemia. 2012;26:1617–29. [DOI] [PubMed] [Google Scholar]
- 82.Alexander KA, Flynn R, Lineburg KE, Kuns RD, Teal BE, Olver SD, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leveque-El Mouttie L, Koyama M, Le Texier L, Markey KA, Cheong M, Kuns RD, et al. Corruption of dendritic cell antigen presentation during acute GVHD leads to regulatory T-cell failure and chronic GVHD. Blood. 2016;128:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hassan M, Ulezko Antonova A, Li JM, Hosoba S, Rupji M, Kowalski J, et al. Flt3L Treatment of Bone Marrow Donors Increases Graft Plasmacytoid Dendritic Cell Content and Improves Allogeneic Transplantation Outcomes. Biol Blood Marrow Transplant. 2019;25:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Y, Giver CR, Sharma A, Li JM, Darlak KA, Owens LM, et al. IFN-γ and indoleamine 2,3-dioxygenase signaling between donor dendritic cells and T cells regulates graft versus host and graft versus leukemia activity. Blood. 2012;119:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waller EK, Logan BR, Fei M, Lee SJ, Confer D, Howard A, et al. Kinetics of immune cell reconstitution predict survival in allogeneic bone marrow and G-CSF-mobilized stem cell transplantation. Blood Adv. 2019;3:2250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waller EK, Logan BR, Harris WA, Devine SM, Porter DL, Mineishi S, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32:2365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian Y, Meng L, Wang Y, Li B, Yu H, Zhou Y, et al. Graft-versus-host disease depletes plasmacytoid dendritic cell progenitors to impair tolerance induction. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suenaga F, Ueha S, Abe J, Kosugi-Kanaya M, Wang Y, Yokoyama A, et al. Loss of lymph node fibroblastic reticular cells and high endothelial cells is associated with humoral immunodeficiency in mouse graft-versus-host disease. J Immunol. 2015;194:398–406. [DOI] [PubMed] [Google Scholar]
- 90.Chung J, Ebens CL, Perkey E, Radojcic V, Koch U, Scarpellino L, et al. Fibroblastic niches prime T cell alloimmunity through Delta-like Notch ligands. J Clin Invest. 2017;127:1574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Forcade E, Kim HT, Cutler C, Wang K, Alho AC, Nikiforow S, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127:2489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelly M, Bharadwaj AS, Tacke F, Chao H. Regulatory T cells and immune tolerance to coagulation factor IX in the context of intramuscular AAV1 gene transfer. Mol Ther. 2010;18:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamakawa T, Ohigashi H, Hashimoto D, Hayase E, Takahashi S, Miyazaki M, et al. Vitamin A-coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–85. [DOI] [PubMed] [Google Scholar]
- 96.Wu SR, Reddy P. Tissue tolerance: a distinct concept to control acute GVHD severity. Blood. 2017;129:1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chhabra A, Ring AM, Weiskopf K, Schnorr PJ, Gordon S, Le AC, et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci Transl Med. 2016;8:351ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–66. [DOI] [PubMed] [Google Scholar]
- 99.Dertschnig S, Hauri-Hohl MM, Vollmer M, Hollander GA, Krenger W. Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood. 2015;125:2720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dudakov JA, Mertelsmann AM, O’Connor MH, Jenq RR, Velardi E, Young LF, et al. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease. Blood. 2017;130:933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fukunaga A, Ishikawa T, Kishihata M, Shindo T, Hori T, Uchiyama T. Altered homeostasis of CD4(+) memory T cells in allogeneic hematopoietic stem cell transplant recipients: chronic graft-versus-host disease enhances T cell differentiation and exhausts central memory T cell pool. Biol Blood Marrow Transplant. 2007;13:1176–84. [DOI] [PubMed] [Google Scholar]
- 102.Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182:5846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jenq RR, King CG, Volk C, Suh D, Smith OM, Rao UK, et al. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009;113:1574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jimenez M, Martinez C, Ercilla G, Carreras E, Urbano-Ispizua A, Aymerich M, et al. Clinical factors influencing T-cell receptor excision circle (TRECs) counts following allogeneic stem cell transplantation in adults. Transpl Immunol. 2006;16:52–9. [DOI] [PubMed] [Google Scholar]
- 105.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. 2010;57:297–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuvelier GDE, Nemecek ER, Wahlstrom JT, Kitko CL, Lewis VA, Schechter T, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood. 2019;134:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100:1192–200. [DOI] [PubMed] [Google Scholar]
- 109.Chu YW, Schmitz S, Choudhury B, Telford W, Kapoor V, Garfield S, et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008;112:2836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. [DOI] [PubMed] [Google Scholar]
- 112.Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, et al. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112:3255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nasilowska-Adamska B, Szydlo R, Rzepecki P, Czyz A, Tomaszewska A, Markiewicz M, et al. Palifermin does not influence the incidence and severity of GvHD nor long-term survival of patients with hematological diseases undergoing HSCT. Ann Transplant. 2011;16:47–54. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakane T, Nakamae H, Kamoi H, Koh H, Takeoka Y, Sakamoto E, et al. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;42:43–9. [DOI] [PubMed] [Google Scholar]
- 116.Schott C, Weigt SS, Turturice BA, Metwally A, Belperio J, Finn PW, et al. Bronchiolitis obliterans syndrome susceptibility and the pulmonary microbiome. J Heart Lung Transplant. 2018;37:1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gassas A, Schechter T, Krueger J, Craig-Barnes H, Sung L, Ali M, et al. Serum Krebs Von Den Lungen-6 as a Biomarker for Early Detection of Bronchiolitis Obliterans Syndrome in Children Undergoing Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1524–8. [DOI] [PubMed] [Google Scholar]
- 119.Vanaudenaerde BM, Wuyts WA, Geudens N, Dupont LJ, Schoofs K, Smeets S, et al. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transplant. 2007;7:76–82. [DOI] [PubMed] [Google Scholar]
- 120.Furquim CP, Soares GM, Ribeiro LL, Azcarate-Peril MA, Butz N, Roach J, et al. The Salivary Microbiome and Oral Cancer Risk: a Pilot Study in Fanconi Anemia. J Dent Res. 2017;96:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gurgan CA, Ozcan M, Karakus O, Zincircioglu G, Arat M, Soydan E, et al. Periodontal status and post-transplantation complications following intensive periodontal treatment in patients underwent allogenic hematopoietic stem cell transplantation conditioned with myeloablative regimen. Int J Dent Hyg. 2013;11:84–90. [DOI] [PubMed] [Google Scholar]
- 122.Mester A, Irimie AI, Tanase A, Tranca S, Campian RS, Tomuleasa C, et al. Periodontal disease might be a risk factor for graft versus host disease. A systematic review. Crit Rev Oncol Hematol. 2020;147:102878. [DOI] [PubMed] [Google Scholar]
- 123.Markey KA, Schluter J, Gomes ALC, Littmann ER, Pickard AJ, Taylor BP, et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood. 2020;136:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med. 2020;382:822–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Inamoto Y, Flowers ME, Lee SJ, Carpenter PA, Warren EH, Deeg HJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thepot S, Zhou J, Perrot A, Robin M, Xhaard A, de Latour RP, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia. 2010;24:1852–8. [DOI] [PubMed] [Google Scholar]
- 128.Inamoto Y, Martin PJ, Storer BE, Palmer J, Weisdorf DJ, Pidala J, et al. Association of severity of organ involvement with mortality and recurrent malignancy in patients with chronic graft-versus-host disease. Haematologica. 2014;99:1618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–14. [DOI] [PubMed] [Google Scholar]
- 130.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fowler DH, Pavletic SZ. Syk and tired of current chronic GVHD therapies. Blood. 2015;125:3974–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Killig M, Friedrichs B, Meisig J, Gentilini C, Blüthgen N, Loddenkemper C, et al. Tracking in vivo dynamics of NK cells transferred in patients undergoing stem cell transplantation. Eur J Immunol. 2014;44:2822–34. [DOI] [PubMed] [Google Scholar]
- 133.Mavers M, Maas-Bauer K, Negrin RS. Invariant Natural Killer T Cells As Suppressors of Graft-versus-Host Disease in Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2017;8:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Handgretinger R, Schilbach K. The potential role of γδ T cells after allogeneic HCT for leukemia. Blood. 2018;131:1063–72. [DOI] [PubMed] [Google Scholar]
- 135.Elorza I, Palacio C, Dapena JL, Gallur L, Sanchez de Toledo J, Diaz de Heredia C. Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica. 2010;95:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3:3393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4:e183–e91. [DOI] [PubMed] [Google Scholar]
- 139.Bejanyan N, Kim S, Hebert KM, Kekre N, Abdel-Azim H, Ahmed I, et al. Choice of conditioning regimens for bone marrow transplantation in severe aplastic anemia. Blood Adv. 2019;3:3123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bolaños-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6:e183–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bona K, Brazauskas R, He N, Lehmann L, Abdel-Azim H, Ahmed IA, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137:556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Adv. 2020;4:1770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Eapen M, Brazauskas R, Walters MC, Bernaudin F, Bo-Subait K, Fitzhugh CD, et al. Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 2019;6:e585–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


