Abstract
Objective:
The COVID-19 pandemic has strained many healthcare systems. In response, U.S. hospitals altered their care delivery systems, but there are few data regarding specific structural changes. Understanding these changes is important to guide interpretation of outcomes and inform pandemic preparedness. We sought to characterize emergency responses across hospitals in the U.S. over time and in the context of local case rates early in the COVID-19 pandemic.
Design:
We surveyed hospitals from a national acute care trials group regarding operational and structural changes made in response to the COVID-19 pandemic from January–August 2020. We collected pre-pandemic characteristics and changes to hospital system, space, staffing, and equipment during the pandemic. We compared the timing of these changes to county-level COVID-19 case rates.
Setting and participants:
U.S. hospitals participating in the PETAL Network COVID-19 Observational (CORAL) Study. Site investigators at each hospital collected local data.
Interventions:
None
Measurements and Main Results:
Forty-five sites participated (94% response rate). System-level changes (incident command activation and elective procedure cancellation) occurred at nearly all sites, preceding rises in local case rates. The peak inpatient census during the pandemic was greater than the prior hospital bed capacity in 57% of sites with notable regional variation. Nearly half (49%) expanded ward capacity and 63% expanded ICU capacity, with nearly all bed expansion achieved through re-purposing of clinical spaces. Two-thirds of sites adapted staffing to care for patients with COVID-19, with 48% implementing tiered staffing models, 49% adding temporary physicians, nurses, or respiratory therapists, and 30% changing the ratios of physicians or nurses to patients.
Conclusions:
The COVID-19 pandemic prompted widespread system-level changes, but front-line clinical care varied widely according to specific hospital needs and infrastructure. Linking operational changes to care delivery processes are a necessary step to understand the impact of the COVID-19 pandemic on patient outcomes.
Keywords: Intensive Care Units, Health Workforce, Capacity Building, COVID-19, Pandemics
INTRODUCTION
Coronavirus disease 2019 (COVID-19) strained healthcare systems, demanding substantial operational innovations for the provision of inpatient care. Initial reports describing emergency responses implemented in reaction to COVID-19 were limited to cities or regions that experienced surges of critically ill patients early in the course of the pandemic, including Wuhan, China, the Lombardy region of Italy, and New York City (1–4). These reports provided limited snapshots of possible responses to inform care for subsequent surge events—whether from recrudescent COVID-19 in the absence of universal vaccination, or from other emerging infectious or non-infectious threats.
While federal agencies and professional organizations issued recommendations for surge capacity planning and resource allocation that were informed by past outbreaks and trauma/mass casualty events (5–8), these provided inadequate guidance for multiple waves of a continuing pandemic. Managers, planners, and regulators faced a profound challenge— absent widespread empirical evaluations of the safety and efficacy of alternative responses, hospital leadership may reasonably ask: What did my colleagues do? Such “wisdom of the crowd” may usefully supplement expert opinion, and identify options and barriers not obvious in tabletop or academic planning exercises (9).
We sought to characterize surge responses to the COVID-19 pandemic across a large network of U.S. academic and community hospitals, in relation to pre-existing organizational structures and the dynamic local burden of disease. To inform current and future surge responses, we asked about mechanisms used to free-up or prioritize existing capacity, changes in the availability of the space, staff, and equipment needed to care for COVID-19 patients with respiratory failure, and the timing of these changes during the first wave of the pandemic.
MATERIALS AND METHODS
We surveyed hospitals participating in the National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network to obtain data regarding the different emergency activation plans and structural changes made in response to the COVID-19 pandemic. This survey was a part of the Network’s multicenter, multimethod, multiphase cohort study called the COVID-19 Observational (CORAL) Study.
Study Design & Measurement
We iteratively developed survey items informed by literature review and consensus among PETAL investigators. We created a survey with the following domains: a) hospital and ICU characteristics before and during the pandemic; b) ICU staffing models before and during the pandemic, including the use of tiered staffing models (a structure in which a critical care physician oversees care provided by non-ICU providers (10)); c) emergency responses planned and implemented in response to the pandemic for both ICU and ward patients (7, 9, 11, 12). We asked for maximum patient census from March through August 2020 for both ICUs and wards, as well as specific initiation dates for each hospital operational change. To maximize accuracy and completeness of the survey, data elements were limited to those that would be readily available from hospital leadership, bed management, and Incident Command Center administration.
The final survey version included 41 questions. We piloted the survey among four PETAL intensivists and two non-PETAL ICU directors to improve clinical sensibility. We also solicited feedback regarding clarity and face validity from the CORAL Steering Committee (33 PETAL investigators). (See Supplemental Digital Content Table S1 for the final instrument.) The survey was sent out via email in July 2020 by the PETAL Network Clinical Coordinating Center to all the PETAL Site Principal Investigators (n=48) for hospitals participating in the CORAL study, with completion occurring over a two–month period. Site Principal Investigators were encouraged to solicit input from team members, leadership, administrators, as needed to complete the survey, with one complete document to be submitted per site. Study data were collected and managed using REDCap at Vanderbilt University (13, 14).
The Vanderbilt University Medical Center Institutional Review Board (IRB), which acts as the Central IRB for the PETAL Network, determined this work qualified as public health surveillance as defined in 45 CFR 46.102(l)(2), and therefore this study was exempted from full review and did not require approval.
Analysis
We reported all categorical variables as absolute counts and percentages, while continuous variables were reported as means and standard deviation if normally distributed or median (interquartile range, IQR) if skewed.
We grouped data for individual sites by region, with linkage to hospital-matched county-level data obtained from an ongoing publicly accessible repository of data on COVID-19 cases and deaths developed by The New York Times (15). Case definitions and methodologies of the Centers of Disease Control and Prevention (CDC) defined features for reporting the aggregated cumulative counts of COVID-19 cases at the county-level over time since January 1, 2020 (16, 17). The locations of reported cases indicated where the patients received diagnosis or treatment. We matched each hospital to its respective county and compared to publicly available time series data to generate a choropleth map and heat map for visual inspection of county-level COVID-19 case rates (defined as the absolute number as a percentage of county population). Survey-reported hospital operational changes as part of the COVID-19 emergency response were matched to the county case rates from January to August 2020.
RESULTS
A total of 45 sites completed the survey (94% response rate), with representation from all census regions of the country. Contributors to survey data included site physicians (n=45), nurses (n=13), respiratory therapists (n=6), research staff/data analysts (n=12), and/or administrative staff/leadership (n=6).
Regional COVID-19 infection rates varied during study period, with county-level case rates ranging up to five new weekly reported cases per 1000 residents depending on location (Figure 1). In the Northeast and Midwest regions, infection rates peaked during the first half of the study period while in other regions infection rates peaked at a later point. The majority of hospitals (64%) are located in counties that experienced declines in new case rates by August 24–30, 2020.
Figure 1: Choropleth map of surveyed hospital locations and county-level case rates of COVID-19 from March through August 2020.
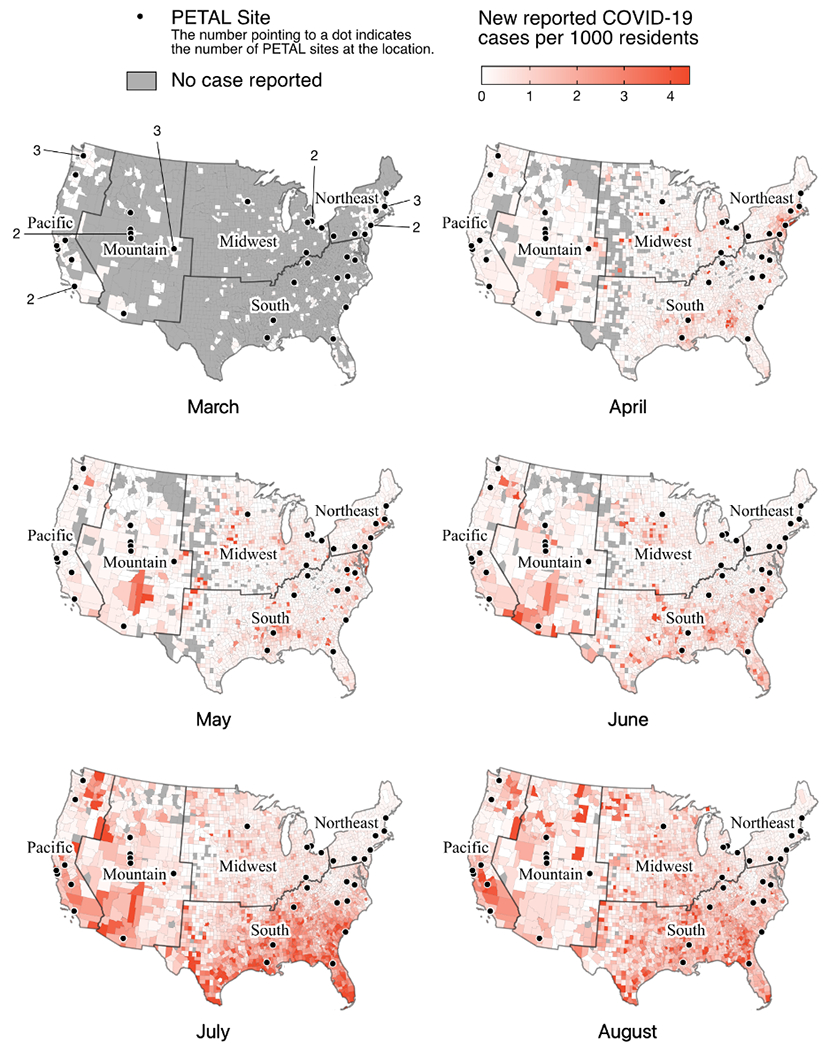
This choropleth map illustrates spatial and temporal variation of weekly county-level COVID-19 cases rates (defined as the absolute number as a percentage of county population). Case rates from the third week of each month are shown in each panel.
Organizational Responses to the COVID-19 Pandemic
Starting in January through the first wave of the pandemic, hospital changes in response to the COVID-19 pandemic included incident command center activation (97.8%), elective procedure cancellation (95.6%), and hospital-wide triage systems (active 84.4%; being developed, 8.9%) (Figure 2). County case rates varied regionally, with operational changes occurring both pre- and during rising infection rates (Supplemental Table S2). By April 1, 2020, nearly all participating sites had cancelled elective surgeries irrespective of their local case rates (Table 1). Triage systems were already in place for the majority of sites and were utilized for managing ICU admission decisions (75.6%), equipment utilization or extracorporeal membrane oxygenation therapy (77.8%), outside and within system hospital transfers (84.4%), and other processes (e.g., staffing, medication distribution, elective procedures) (8.9%). The number and timing of emergency response varied considerably across sites and regions (Table 1, Figure 3).
Figure 2: Hospital operational changes from January to August 2020, matched with county-level COVID-19 case rates.
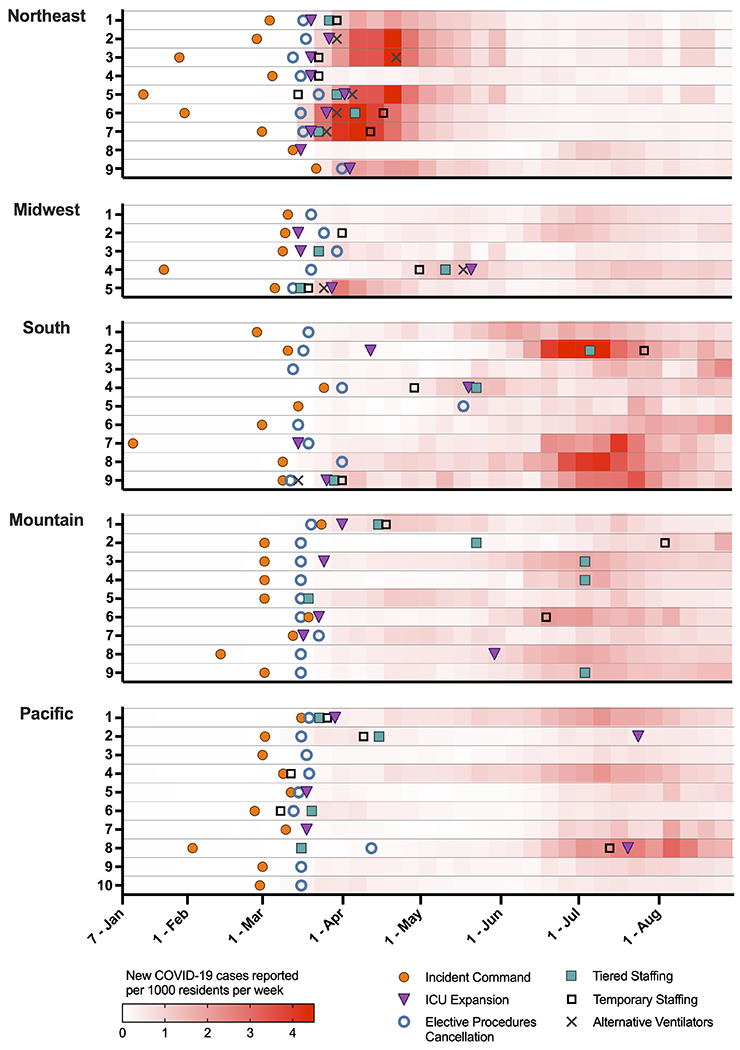
Timing of hospital operational changes varied over the course of the first eight months of the pandemic, with regional variability. The red shading represents the rate of county-level COVID-19 cases per 1000 residents per week, matched to the county of each hospital. Overlying this heat map are six operational changes which show significant similarities across sites (e.g., elective procedure cancellation, [blue open circle]) and heterogeneity both pre-surge (e.g., incident command system activation, [orange solid circle]) and during the rise in cases (e.g., ICU expansion, [purple triangle]; tiered staffing models, [green solid square]; temporary staffing, [black open square]; alternative ventilator usage, [gray cross]). (Three hospitals had incomplete data for these changes).
Table 1:
Median and interquartile ranges for county case rates of COVID-19 infection (number of new weekly cases per 1000 county residents) at the time of implementation of hospital operational changes, stratified by region
| Region |
Incident Command System N=44 |
Elective Procedure Cancellation N=43 |
ICU Expansion N=26 |
Tiered Staffing Model N=20 |
Temporary Staffing Hires N=21 |
Alternative Ventilator Strategies N=9 |
|---|---|---|---|---|---|---|
| Northeast | 0.00 (0.00, 0.00) |
0.16 (0.03, 1.03) |
1.03 (0.12, 1.44) |
0.28 (0.13, 2.98) |
1.60 (0.88, 2.73) |
2.95 (2.17, 4.11) |
| Midwest | 0.00 (0.00, 0.00) |
0.08 (0.04, 0.09) |
0.67 (0.06, 1.30) |
0.29 (0.28, 0.48) |
0.54 (0.40, 0.92) |
1.28 (1.27, 1.29) |
| South | 0.00 (0.00, 0.02) |
0.05 (0.01, 0.27) |
0.23 (0.12, 0.53) |
0.46 (0.39, 1.46) |
1.14 (0.73, 2.98) |
0.00 (0.00, 0.00) |
| West – Mountain | 0.00 (0.00, 0.00) |
0.06 (0.01, 0.14) |
0.21 (0.13, 0.60) |
0.81 (0.79, 1.12) |
0.98 (0.30, 1.25) |
NA |
| West – Pacific | 0.01 (0.00, 0.03) |
0.09 (0.03, 0.15) |
0.04 (0.03, 0.60) |
0.15 (0.03, 0.58) |
0.16 (0.03, 0.30) |
NA |
Abbreviations: ICU – Intensive Care Unit; N = number; NA = not applicable
Figure 3: Frequency of combinations of specific hospital operational changes across the PETAL Network between January-August 2020.
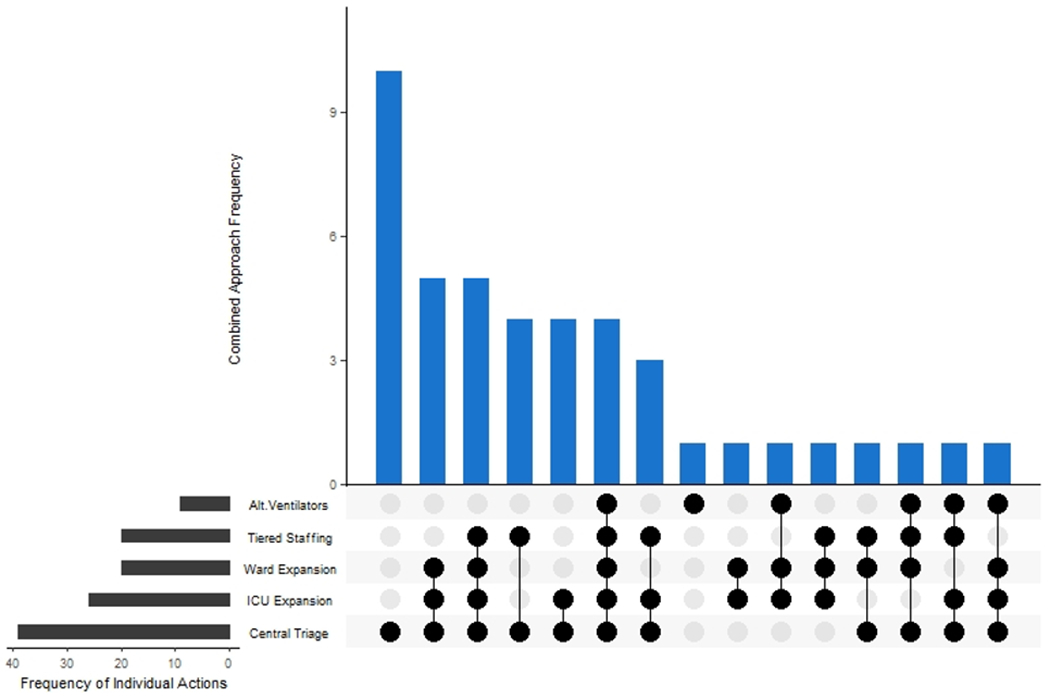
Hospitals across the PETAL Network implemented various combinations of operational changes. Steps included in this figure are limited to those implemented, not just planned at individual hospitals, including centralized triage systems, ICU and ward bed expansion, tiered staffing models, and alternative ventilator usage. The highest frequency of sites implemented only centralized triage processes. These changes are in addition to activation of an incident command center and elective procedure cancellation, which were in place at the majority of sites.
Modifications in ICU and Inpatient Bed Capacity and Resources
Prior to the COVID-19 pandemic, hospitals had a median 561 (IQR 451–875) operational adult inpatient beds, with over half of sites reporting greater than 90% average occupancy on a typical day. Hospitals reported 94 (IQR 70–119) ICU beds, which comprised a median 15.4% (IQR 12.6–20.0%) of the total inpatient beds. Similarly, half of the sites reported greater than 90% average ward occupancy. Respondents estimated a median of 35 (IQR 21–50) patients on invasive mechanical ventilation on a given day, excluding those undergoing mechanical ventilation for procedures. (See Supplemental Table S3.)
Thirty-five hospitals reported peak census data for the study period (n=10 missing). ICU capacity was exceeded in 20 hospitals (57.1% of those who provided data), with a median increase of 25.8% (IQR 9.9–88.8%) over pre-pandemic bed numbers, though regional variability was evident (Supplemental Figure S1a). The South was the only region during the study period where peak ward census exceeded operational inpatient bed availability in all contributing hospitals (Supplemental Figure S1b). Planning for ward bed expansion into additional clinical areas (e.g., perioperative areas, procedure suites, outpatient clinics) began in all hospitals reporting data (n=41), with only half (n=20) implementing this change. Similarly, 97.6% of hospitals planned for extending critical care delivery into clinical spaces not typically utilized for ICU care (e.g., stepdown units, wards, perioperative areas), with 63.4% implementing this expansion. Expansion into non-clinical areas (e.g., hallways, lobbies, off-site facilities) for ICU and ward patients occurred in only two hospitals, though planning for this contingency took place in an additional 16 and 7 hospitals, respectively (Supplemental Figure S2).
Plans for use of alternative ventilator devices and strategies were common (n=38, 84.4%), but implemented in only nine hospitals. The implemented devices included transport ventilators (n=7), anesthesia/operating room machines (n=6), and non-invasive ventilator machines modified for use via artificial airways (n=6). Though plans for multiple patients on single ventilators were developed, this strategy was not deployed at any of the participating sites.
ICU Staffing Model Adaptation
Before the pandemic, surveyed hospitals reported that their Medical ICUs were high intensity units chiefly closed units, (93.3% closed and involving an intensivist as the primary attending; 4.3% requiring an ICU consult) (Table 2). Sites employed medical ICU attending staff with diverse training backgrounds, including Pulmonary–Critical Care Medicine (PCCM) (95.7% of sites), Internal Medicine (IM)–Critical Care Medicine (CCM) (58.7%), Emergency Medicine–Critical Care (28.2%), Critical Care Anesthesiology (19.6%), Surgical Critical Care (10.9), Neurosurgical Critical Care (4.3%), Nephrology Critical Care (4.4%), and Cardiology/Thoracic Surgery (2.2%). The majority of teams were structured with attending physicians, fellows, and other trainees. Additionally, 54.3% of Medical ICUs also utilized advanced practice practitioners (APPs, nurse practitioners and physician assistants).
Table 2:
Medical Intensive Care Unit staffing pre- and during COVID-19 pandemic, for 45 sites participating in survey
| Pre-pandemic (n=45) |
During pandemic (n=43)* |
|
|---|---|---|
| Changes made to staffing model for ICU patients with COVID-19 | -- | 30 (69.8) |
| Intensivist role (n, %) | ||
| Primary attending | 42 (93.3) | 39 (90.7) |
| Consultant | 2 (4.4) | 4 (9.3) |
| Intensivist training (n, %) | ||
| PCCM | 45 (100) | 43 (100) |
| IM–CCM | 26 (57.8) | 29 (67.4) |
| Non–IM CCM | 17 (37.8) | 22 (51.2) |
| No formal CCM training | 1 (2.2) | 2 (4.7) |
| Other | 1 (2.2) | 2 (4.7) |
| Team members (n, %) | ||
| Fellows | 40 (88.9) | 38 (88.4) |
| Residents | 43 (95.6) | 40 (93.0) |
| APPs | 26 (57.8) | 29 (67.4) |
| Other | 5 (11.1) | 3 (7.0) |
| Temporary Workforce (n, %) | ||
| Physicians | -- | 9 (20.9) |
| Former/retired physicians | -- | 2 (4.7) |
| Outpatient physicians | -- | 5 (11.6) |
| Research, admin, non-clinical physicians | -- | 2 (4.7) |
| Physicians from outside system | -- | 4 (9.3) |
| Registered nurses | -- | 19 (44.2) |
| Respiratory therapists | -- | 7 (16.3) |
| No temporary staff | -- | 22 (51.2) |
Two sites did not report details on staffing during the pandemic. All percentages in this column are out of the 43 sites that completed these data.
Abbreviations: APP, Advanced Practice Practitioner; CCM, Critical Care Medicine; IM, Internal Medicine; PCCM, Pulmonary and Critical Care Medicine
After March 2020, almost two-thirds of hospitals adapted their ICU staffing models. Tiered staffing models were implemented at 26 sites, with an additional 16 hospitals planning for this potential need. Fourteen hospitals (38.9%) developed tiered staffing models for physicians, 17 (47.2%) for nurses, and 6 (16.7%) for respiratory therapists, occurring primarily in the Northeast. Half (48.8%) added temporary staff of physicians, nurses, or respiratory therapists. Four hospitals (9.7%) deployed physicians from outside the health system as temporary staff. While an intensivist remained the primary attending in most sites, team structure changed to include more APPs and temporary staff (Table 2). ICU patient-to-physician and patient-to-nurse ratios increased for 14 sites (30.4%).
DISCUSSION
In this first national assessment of hospital COVID-19 response, we found substantial regional variability across the U.S. in both the type and timing of specific operational changes. The majority of hospitals implemented system-level steps, including activating an incident command center to coordinate care, cancelling elective surgeries early on in the pandemic, and activating a centralized triage system to aid in the allocation of resources (9, 18). Even in areas with low case rates, hospitals proactively planned for clinical space expansion, staffing, and equipment in the event of surge, in line with society consensus recommendations for surge care (7, 11), though implementation varied widely across the country. This heterogeneity across sites in the timing of these front-line changes, seemingly unrelated to local COVID-19 case rates, supports the need to examine the safety and efficacy of hospital responses to inform preparedness for future emergencies.
The wide range of responses identified in this study underscores the call to investigate, establish, and adopt process metrics to better evaluate the effect of hospital responses on outcomes, as previously described (19–22). While this study is insufficiently structured to effectively understanding the causal relationships between operational changes and outcomes, it provides a key first step in that direction, by outlining the various hospital-level responses undertaken as a result of the pandemic. Other studies have examined mortality as it relates to case volume and ICU bed number (23, 24). However, our survey results demonstrate that hospitals pragmatically responded as needed, and critical care services may have been available even if the patient was not admitted to an “official” ICU.
Most surveyed hospitals cancelled elective procedures during March 2020, regardless of their local case rates. These decisions, made at a time when infectivity and transmission rate were poorly understood, were likely informed by recommendations from the American College of Surgeons (25), in an effort to ensure the availability of beds for patients with COVID-19 and conserve personal protective equipment supply. However, the decision to cancel elective surgical cases is a complicated one with many potential downstream effects, including patient harm due to delayed care and lost revenue for hospitals (26). Most hospitals across the U.S. resumed elective procedures after the first wave in 2020, and are now again facing the burden of deciding how to proceed with surgical procedures in the midst of a surge in COVID-19 cases nationwide (27). More detailed research of operational efforts across hospital systems is needed to identify the threshold at which the benefits of cancelling elective surgeries outweighs the risks, with the goal of providing high quality care to all patients, irrespective of their COVID-19 status.
As hospitals plan for the growing staffing shortage associated with subsequent waves of the pandemic, the space and staffing expansion changes reported by our sites may help inform which changes to prioritize. We found that most sites were able to plan for and provide critical care services in non-ICU settings. Fortunately, the burden of the pandemic’s first wave only required ICU expansion into clinical service areas at surveyed hospitals, but the feasibility of mobilizing their staff and resources to operationalize wards, step-down units, and other locations to provide ICU-level care is notable (10). Although our study is limited in its capture of the details of the scope and structure of this non-ICU critical care delivery, this component of hospital emergency activation warrants further exploration to garner actionable targets for improvement of care for decompensating patients when ICUs are at capacity (28).
Recognizing variation in the structure of ICU staffing across the U.S. (29, 30), several approaches to augmenting the critical care pool may exist. To address increased physician need, many study hospitals utilized both IM-CCM and non-IM-CCM physicians in the care of patients with COVID-19. Though others have recommended training non-intensivists to augment medical ICU care (31, 32), when it was available, surveyed hospitals chose to employ the larger pool of CCM-trained physicians rather than extending ICU care responsibilities to non-intensivists (33–35). In hospitals with open ICUs or where full-time intensivists are few in number, reliance on tiered staffing models using hospitalists and other specialties, as well as the expanded use of advanced practice practitioners, may help offset staffing shortages. Our study demonstrates that one approach to staffing cannot meet the needs of every institution (30).
Additionally, many hospitals reported increasing patient-to-staff ratios during the pandemic and common use of tiered staffing models, reinforcing the risk for excessive workload and contributing to the rising rates of clinical burnout in critical care (36). Though we do not have details on the degree to which staffing ratios changed (the number of patients per clinician or nurse), the optimal staffing ratios in times of surge warrants further research. While many hospitals reported utilizing temporary staffing from less-burdened areas to augment care, temporary staffing may become more scarce as case numbers increase across the U.S. as seen in subsequent waves of the pandemic and with the threat of coronavirus variant strains. As demand for critical care services has been high, there is a need to think strategically about how to distribute workload in alternative staffing models, perhaps by increasing use of ICU telemedicine or through regionalizing care (37).
Though we were unable to quantify daily healthcare capacity strain or causal effect of operational changes on patient outcomes, our study highlights variability in both proactive and responsive actions to the COVID-19 pandemic in the context of local case volume. A number of these responses are based on regional and local factors, and our findings support cautious interpretation of clinical studies lacking adjustment for hospital-level context. Such temporal heterogeneity in peak case rates likely explains the variability in decisions to implement tiered staffing models and mobilize temporary staff. It also suggests that some hospitals had substantially more time to mobilize resources and adapt to the operational demands of the pandemic. These operational processes affect access to resources or therapies and patient outcomes (24, 38, 39). With adjustment for COVID-19 hospital context, additional investigation is needed to identify specific hospital-level responses that improved patient outcomes (10, 40).
Several limitations to this study exist. Though this study provides a broad snapshot of academic medical centers and community hospitals in both urban and suburban regions across the U.S., we administered the survey only to PETAL Network hospitals with a sizable number of existing ICU beds, almost all of which benefited from staffing with fellow and resident physicians, thereby limiting generalizability to smaller and/or non-teaching hospitals. Additionally, while the geographic representation of the PETAL sites aligns with areas with high county case rates during the study period, we are limited in our observations of the Midwest and Southern regions, which have been affected by subsequent pandemic waves. There were also missing data amongst some of the key variables on census and COVID-19 volume. This limited the interpretation of the true burden of the pandemic experienced by hospitals, though we partially accounted for this limitation by using county-level case rates from public datasets. Additionally, reporting responsibility may have varied among states, leading to county case rates inadequately representing true hospital surge. As the survey asked for retrospective information, there is the risk of recall bias, albeit limited, since we structured our survey to capture data logged by hospital administrators and incident command centers. We also did not capture the frequency of triage system usage or allocation of scarce resources, nor did we garner data on the source of the temporary staffing (e.g., locums tenens), limiting our ability to identify those hospitals in crisis modes.
We must evaluate variation in hospital responses in the context of the pandemic. For subsequent waves of the pandemic, health care systems must learn to adapt to surges without compromising care for their patients, both with and without COVID-19 (24). A standardized approach across all U.S. regions may not be appropriate, especially as the structure, staffing, and resources differ between hospitals. Our study demonstrates that planning for and making operational changes can occur in a rapid fashion, but work remains to understand the effects of these responses and to establish a long-term flexible response system that identifies the most suitable plan for each hospital and its patients.
CONCLUSIONS
Serving as a lens into the operations of COVID-19 care for the critically ill, this study demonstrates that hospitals enacted similar system-level changes, but they varied in timing and in the front-line operations of team structure, bed expansion, and equipment availability. Our findings support the importance of considering the type and heterogeneity of hospital structure, context, and operational processes when evaluating the safety and efficacy of care provided during the COVID-19 pandemic and beyond.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Mr. Xiaoqi Bao and Ms. Alison Pollock for assistance in data visualization.
Conflicts of Interest and Sources of Funding:
Dr. Mathews reports grants from National Institutes of Health (NIH) / National Heart Lung and Blood Institute (NHBLI) (K23HL130648) and serves on a steering committee of the BREATHE trial, funded by Roivant/Kinevant Sciences, outside of the submitted work. Dr. Vranas reports grants from the Department of Veterans Affairs during the conduct of the study. Dr. Valley reports grants from the NHLBI (K23HL140165) and the Agency for Healthcare Research and Quality (R01HS028038). Dr. Harhay reports editorial positions at the American Thoracic Society and receiving grants from the National Institutes of Health, outside the submitted work. Dr. Chang reported receiving personal fees from PureTech and LaJolla Pharmaceuticals, outside the submitted work. Dr. Hough reports grants from the NIH during the conduct of the study. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This study was supported by grants from the NHLBI (3U01HL123009-06S1, U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020). The REDCap data tools used for this study were supported by the NIH/NCATS UL1TR000445.
The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
Copyright Form Disclosure:
Drs. Matthews, Valley, Zhao, and Hough’s institutions received funding from the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institution (NHLBI) 3U01HL123009-06S1, U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020; the REDCap data tools used for this study were supported by the NIH/NCATS UL1TR000445. Drs. Matthews, Valley, Zhao, Gundel, and Hough received support for article research from the NIH. Dr. Harhay received funding from the NIH; he disclosed having editorial positions at the American Thoracic Society. Dr. Chang received funding from La Jolla Pharmaceuticals and PureTech. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Xie J, Tong Z, Guan X, et al. : Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med 2020; 46: 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carenzo L, Costantini E, Greco M, et al. : Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia 2020; 75: 928–934. [DOI] [PubMed] [Google Scholar]
- 3.Spina S, Marrazzo F, Migliari M, et al. : The response of Milan’s emergency medical system to the COVID-19 outbreak in Italy. Lancet 2020; 395: e49–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin KM, Karas MG, Ivascu NS, et al. : Hospital preparedness for COVID-19: A practical guide from a critical care perspective. Am J Respir Crit Care Med 2020; 201: 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Office of the Assistant Secretary of Preparedness and Response Hospital Preparedness Program. Healthcare Preparedness Capabilities: National Guidelines for Healthcare System Preparedness. Washington, D.C.: U.S. Department of Health and Human Services; 2012. Available from: https://www.phe.gov/preparedness/planning/hpp/reports/documents/capabilities.pdf. Accessed: 2020 Dec 17. [Google Scholar]
- 6.U.S. Department of Homeland Security. Pandemic influenza preparedness, response, and recovery guide for critical infrastructure and key resources. Washington, D.C.; 2006. Available from: https://www.dhs.gov/sites/default/files/publications/cikrpandemicinfluenzaguide.pdf. Accessed: 2020 Dec 17. [Google Scholar]
- 7.Hick JL, Einav S, Hanfling D, et al. : Surge capacity principles: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146: e1S–e16S. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations, Institute of Medicine. Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. Washington, D.C.: National Academies Press (US); 2012. [PubMed] [Google Scholar]
- 9.Anesi GL, Lynch Y, Evans L. A conceptual and adaptable approach to hospital preparedness for acute surge events due to emerging infectious diseases. Crit Care Explor 2020; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpern NA, Kaplan LJ, Rausen M, et al. : Configuring ICUs in the COVID-19 era. Society of Critical Care Medicine; Updated 2020. June 15. Available from: https://www.sccm.org/Disaster/COVID19. Accessed: 2020 Dec 17.
- 11.Einav S, Hick JL, Hanfling D, et al. : Surge capacity logistics: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014; 146: e17S–43S. [DOI] [PubMed] [Google Scholar]
- 12.Rewa OG, Stelfox HT, Ingolfsson A, et al. : Indicators of intensive care unit capacity strain: a systematic review. Crit Care 2018; 22: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Minor BL, et al. : The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith M, Yourish K, Almuktar S, et al. : COVID in the U.S.: Latest map and case count. The New York Times. New York, NY; 2020. [Google Scholar]
- 16.Turner K, Davidson SL, Collins J, et al. : Interim-20-ID-01: Standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Updated 2020. April 5. Available from: https://www.cste.org/. Accessed: 2020 Nov 15
- 17.National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases. Cases, data & surveillance: About CDC COVID-19 data. Updated 2020. November 25. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/about-us-cases-deaths.html. Accessed: 2020 Dec 17.
- 18.Barbisch DF, Koenig KL. Understanding surge capacity: Essential elements. Acad Emerg Med 2006; 13: 1098–1102. [DOI] [PubMed] [Google Scholar]
- 19.Sprung CL, Cohen R, Adini B, et al. : Chapter 1. Introduction. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive Care Med 2010; 36 Suppl 1: S4–10. [DOI] [PubMed] [Google Scholar]
- 20.Barnato AE, Kahn JM, Rubenfeld GD, et al. : Prioritizing the organization and management of intensive care services in the United States: The PrOMIS Conference. Crit Care Med 2007; 35: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 21.Leung S, Gregg SR, Coopersmith CM, et al. : Critical care organizations: Business of critical care and value/performance building. Crit Care Med 2018; 46: 1–11. [DOI] [PubMed] [Google Scholar]
- 22.Wiler JL, Welch S, Pines J, et al. : Emergency department performance measures updates: Proceedings of the 2014 emergency department benchmarking alliance consensus summit. Acad Emerg Med 2015; 22: 542–553. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz LI, Jones SA, Cerfolio RJ, et al. : Trends in COVID-19 risk-adjusted mortality Rates. J Hosp Med 2021; 16: 90–92. [DOI] [PubMed] [Google Scholar]
- 24.Janke AT, Mei H, Rothenberg C, et al. : Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med 2021. [Published ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Surgeons. COVID-19: Recommendations for management of elective surgical procedures. Online 2020. March 13. Available from: https://www.facs.org/covid-19/clinical-guidance/elective-surgery. Accessed: 2020 Nov 12.
- 26.Bose SK, Dasani S, Roberts SE, et al. : The cost of quarantine: Projecting the financial impact of canceled elective surgery on the nation’s hospitals. Ann Surg 2021; [Published Ahead of Print]. [DOI] [PubMed] [Google Scholar]
- 27.Wu K, Smith CR, Lembcke BT, et al. : Elective surgery during the COVID-19 pandemic. N Engl J Med 2020; 383: 1787–1790. [DOI] [PubMed] [Google Scholar]
- 28.Mazurik L, Javidan AP, Higginson I, et al. : Early lessons from COVID-19 that may reduce future emergency department crowding. Emerg Med Australas 2020; 32: 1077–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpern NA, Tan KS, DeWitt M, et al. : Intensivists in U.S. acute care hospitals. Crit Care Med 2019; 47: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angus DC, Shorr AF, White A, et al. : Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med 2006; 34: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 31.Steinbach TC, Albert TJ, Carmona HD, et al. : Just-in-time tools for training non-critical care providers: Troubleshooting problems in the ventilated patient. ATS Scholar 2020; 1: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Society of Critical Care Medicine. Critical care for the non-ICU clinician. 15 December 2020. Available from: https://covid19.sccm.org/nonicu/. Accessed: 2020 Nov 12.
- 33.Chiu WC, Marcolini EG, Simmons DE, et al. : Training dedicated emergency physicians in surgical critical care: Knowledge acquisition and workforce collaboration for the care of critically ill trauma/surgical patients. J Trauma 2011; 71: 43–48. [DOI] [PubMed] [Google Scholar]
- 34.Mayglothling JA, Gunnerson KJ, Huang DT. Current practice, demographics, and trends of critical care trained emergency physicians in the United States. Acad Emerg Med 2010; 17: 325–329. [DOI] [PubMed] [Google Scholar]
- 35.Napolitano LM, Rajajee V, Gunnerson KJ, et al. : Physician training in critical care in the United States: Update 2018. J Trauma Acute Care Surg 2018; 84: 963–971. [DOI] [PubMed] [Google Scholar]
- 36.Wahlster S, Sharma M, Lewis AK, et al. : The Coronavirus-19 pandemic’s impact on critical care resources and providers: A global survey. Chest 2020. September 11; S0012-3692(20)34438-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn JM, Rubenfeld GD. The myth of the workforce crisis. Why the United States does not need more intensivist physicians. Am J Respir Crit Care Med 2015; 191: 128–134. [DOI] [PubMed] [Google Scholar]
- 38.Flaatten H, Van Heerden V, Jung C, et al. : The good, the bad and the ugly: Pandemic priority decisions and triage. J Med Ethics 2020. June 10;medethics-2020-106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell R, Banks C. Emergency departments and the COVID-19 pandemic: making the most of limited resources. Emerg Med J 2020; 37: 258–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zebrowski AM, Branas C, Sims S, et al. : Right-sizing response: The optimization of critical care resources during COVID-19. [abstract.] American College of Emergency Physicians 2020 Research Forum. Virtual Conference; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


