Abstract
Background:
Prior studies have measured accelerated aging in people with HIV (PWH) using a DNA methylation (DNAm)-based biomarker of aging, “epigenetic age”, but data are limited in African American (AA) young adults with perinatally-acquired HIV infection (PHIV).
Methods:
We performed a cross-sectional study of AA young adults ages 20–35 years with PHIV (N=31) and seronegative controls (N=30) using DNAm measured in whole blood and cognitive function measured by the NIH Toolbox. Illumina EPIC Array was utilized to measure DNAm age, and accelerated aging markers including epigenetic age acceleration (EAA), as well as extrinsic (EEAA) and intrinsic (IEAA) epigenetic age acceleration.
Results:
PHIV and controls did not differ by sex (45 vs. 43% male), chronological age (26.2 vs. 28.0 years), or ethnicity. Chronological age and DNAm age were correlated (r=0.56, p<0.01). PHIV had a higher mean EAA (2.86±6.5 vs. −2.96±3.9, p<0.01) and EEAA (4.57±13.0 vs. −4.72±6.0, p<0.01) than controls; however, IEAA was not different between groups. Among PHIV, EAA and EEAA were higher in those with HIV viral load ≥50 copies/ml than <50 copies/ml (EEA: 8.1±5.2 vs. 0.11±5.5, p=0<0.01; EEAA: 16.1±10.6 vs. −1.83±9.7, p<0.01). We observed negative correlations (r=−0.36 to −0.31) between EEAA and executive function, attention, and language scores.
Conclusion:
In conclusion, epigenetic age acceleration in blood was observed in AA young adults with PHIV on ART using two measures, including EEAA which up-weights the contribution of immunosenescent cell types. However, there was no evidence of age acceleration with a measure independent of cell type composition.
Keywords: epigenetics, epigenetic aging, biological aging, perinatal HIV, HIV exposure
Introduction
With potent combination antiretroviral therapy (ART) and extended increased life expectancy, aging-related complications have emerged as important clinical, public health, and research concerns for people with HIV (PWH).1 Studies suggest that HIV, directly or indirectly, accelerates aging processes with putative pathways involving chronic immune activation, inflammation, and reactive oxidative stress, leading to cellular senescence and increased vulnerability for comorbidities.2
A number of recent studies have used an algorithm to estimate epigenetic age using patterns of DNA methylation (DNAm)3 in order to determine if epigenetic age acceleration i.e. a greater difference in the gap between epigenetic age and chronological age, is observed in PWH when compared to people without HIV. This question has been evaluated in cross-sectional studies of older men with HIV on ART (mean age 45–51)4–6. Epigenetic age may also be associated with age-related complications, such as declines in cognitive performance. Accelerated epigenetic aging can be observed in brain tissue samples of adults diagnosed pre-mortem with cognitive impairment,7 and a study in children with perinatally-acquired HIV (PHIV) found a negative correlation between epigenetic age acceleration by DNAm and measures of cognitive functioning (executive functioning, working memory, processing speed).8
Despite support from clinical, epidemiologic, and emerging molecular studies for the scientific plausibility of the hypothesis that HIV infection advances or accelerates the aging process, directly or indirectly through immunosenescence pathways, a number of unanswered questions remain. Young adults with PHIV have the greatest potential to experience the cumulative negative effects of HIV infection and chronic inflammation. It is not certain whether increases in epigenetic age among adult PWH have a similar magnitude and consequence in young adults with PHIV. Prior work has found that epigenetic age in children with PHIV under 12 years is accelerated, and associated with cognitive impairments.8,9 However, it is not known whether relationships between epigenetic age acceleration and cognitive functioning observed in children with PHIV under 12 years will persist into young adulthood (20–35 years of age) despite ART.
The goal of this study is to assess if PHIV is associated with accelerated epigenetic age in African American young adults, whether immunologic and virologic status are associated with accelerated epigenetic age in young adults with PHIV on ART, and whether accelerated epigenetic age is associated with neurocognitive impairment among young adults with HIV.
Methods
Study Participants
Participants were recruited for this study from June 2018 through April 2019 at Columbia University Irving Medical Center (CUIMC) in New York, NY. Recruitment was accomplished through two primary avenues: recruitment flyers and introduction of the research study by clinical providers. To be eligible for the study, participants had to identify as African American (AA) and be 20–35 years of age. Participants with PHIV had to have a documented HIV test prior to 5 years of age and be on ART for ≥5 years. Participants without HIV had to have a negative HIV-1 antibody test at enrollment. Participants with hepatitis B or hepatitis C infection, active sexually transmitted infection (STI) or current pre-exposure prophylaxis (PrEP) were excluded from the study. The study was approved by the Institutional Review Board at Columbia University (New York, NY) and written informed consent was obtained from all participants.
Measurements
Demographics and Medical History:
A questionnaire was administered to collect demographic information (sex, age, education, race/ethnicity, neighborhood of residence, sexual orientation). Medications, including multivitamin intake, as well as history of a number of behavioral and social factors, such as drug, alcohol, and tobacco use patterns, were also collected. For PHIV participants, we obtained information on ART treatment history, ART regimen history, ART treatment history, and laboratory measurements (most recent absolute CD4 count as measured by flow cytometry and HIV RNA viral load).
Physical Examination:
Using a standard protocol, trained research staff obtained blood pressure using a digital sphygmomanometer while each participant was seated, taking three measurements which were averaged. Body weight was measured to the nearest 0.1 kg with a balance scale; height without shoes to the nearest 0.5 cm; and waist and iliac circumference with a non-stretchable spring-loaded anthropometric tape measure to the nearest 1 mm. Body mass index (weight/height) and waist to hip ratio were calculated from these measurements.
Cognitive Function Assessment:
The National Institutes of Health (NIH) Toolbox Cognition Battery was used to assess cognitive function across five domains: executive function, attention, working memory, processing speed, and language.10 The battery was self-administered and completed on an iPad. Tests included the Dimensional Change Card Sort Test (executive function), the Flanker Inhibitory Control and Attention Test (attention), the List Sorting Working Memory Test (working memory), the Pattern Comparison Processing Speed Test (processing speed), and the Oral Reading Recognition Test (language). Normative scores included the Age-Corrected Standard Score, for which the normative mean is 100 and the standard deviation (SD) is 15 and the Fully Corrected T-Score, which compares the score of the test-taker to those in the NIH toolbox nationally representative normative sample, while adjusting for key demographic variables including age, gender, race/ethnicity, and educational attainment. The Fully Corrected T-Score has a normative mean of 50 and a SD of 10.
DNA Methylation (DNAm) and DNAm Age:
Blood samples were collected from participants by venipuncture and DNA was extracted using the Qiagen Qiamp DNA Blood Midi Kit (Qiagen, Germantown, MD) and quantified using the Qubit dsDNA BR Assay Kit and Qubit Fluorometer (Thermo Fisher Scientific, Waltham, MA) at CUIMC. DNAm levels were measured using the Infinium MethylationEPIC BeadChip (Illumina, San Diego, CA) at the Genomics Shared Resource at Roswell Park Cancer Institute (Buffalo, NY). Estimated DNAm age (years) was obtained from the DNAm Age Calculator (https://dnamage.genetics.ucla.edu/) developed by Horvath.3 The estimated proportions of six different cell types (B-cells, CD4 T-cells, CD8 T-cells, natural killer cells, granulocytes, monocytes) were estimated using the Houseman method.11
Three measures of epigenetic age acceleration were calculated: (1) epigenetic age acceleration (EAA), (2) extrinsic epigenetic age acceleration (EEAA), and (3) intrinsic epigenetic age acceleration (IEAA) (Table 1). For all measures, positive values indicate that the participant’s biological age is older than expected based on chronological age and negative values indicate that the participant’s biological age is younger than expected based on chronological age. EAA is the (raw) residual resulting from regressing the Horvath-estimated DNAm age estimate on chronological age. EEAA is the residual resulting from regressing the Hannum-estimated DNAm age up-weighted for the contributions of age-related blood cell counts on chronological age.12,13 Since it is dependent on age-related changes in blood cell composition, it strongly reflects aging of the immune system. Finally, IEAA is the residual resulting from regressing the Horvath-estimated DNAm age on chronological age + CD8.naive + CD8pCD28nCD45RAn + PlasmaBlast + CD4T + NK + Mono + Gran. As IEAA is independent of blood cell composition, we included it to better understand potential associations not confounded by differences in circulating blood cell type composition.
Table 1:
Three measures of epigenetic age acceleration
| Measure of epigenetic age acceleration | Residual resulting from regressing: | Description |
|---|---|---|
| Epigenetic age acceleration (EAA) | Horvath DNAm age on chronological age |
|
| Extrinsic epigenetic age acceleration (EEAA) | Hannum DNAmage weighted for contributions of age-related blood cell counts on chronological age |
|
| Intrinsic epigenetic age acceleration (IEAA) | Horvath DNAm age on chronological age and measures of blood cell counts including CD8.naive, CD8pCD28nCD45Ran, PlasmaBlast, CD4T, NK, Mono, Gran |
|
Statistical Analysis
First, demographic characteristics, clinical characteristics, and cognitive function test scores for young adults with PHIV and controls were summarized and compared using Chi-square or Fisher’s exact tests for categorical variables, and t-tests for continuous measures. Next, we compared each of the epigenetic age acceleration measures (EAA, EEAA, and IEAA) between PHIV and controls using t-tests. Then, using linear regression models, we evaluated whether HIV status and other characteristics were associated with each of the epigenetic age acceleration measures. Among young adults with PHIV, we determined if viral load, absolute CD4 count, and DNAm-estimated CD4 and CD8 proportions were associated with each of the epigenetic age acceleration measures. Pearson correlation tests were used for correlating each of the epigenetic age acceleration measures with cognitive function test scores for the entire population and stratified by HIV status. No corrections were made for multiple comparisons. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
A total of 61 African American young adults were enrolled into the study, including 31 with PHIV and 30 confirmed to be HIV-seronegative (controls). Characteristics of the participants are summarized in Table 2. Young adults with PHIV and controls did not differ by sex (45 vs. 43% male), chronological age (26.2 vs. 28.0 years), or ethnicity (90% not Hispanic or Latino in both groups). More controls had a Bachelor’s degree than PHIV (50% vs. 6.5%, p<0.01). Among the young adults with PHIV, 62.1% had a HIV RNA viral load <50 copies/mL. Recent mean CD4 T-cell count was 572 ± 351 cells/mm3. Most (78%) of the young adults with PHIV were on an integrase strand transfer inhibitor (INSTI)-based regimen, including 6/16 who were also taking a protease inhibitor (PI). Twenty percent of the young adults with PHIV were only on a PI-based regimen and 1 individual was on a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen.
Table 2:
Characteristics of 31 young adults with perinatally-acquired HIV (PHIV) and 30 young adults confirmed to be HIV-seronegative (Controls)
| Characteristics | Controls (N=30) | PHIV (N=31) | P |
|---|---|---|---|
| Sex, N (%) | 0.89 | ||
| Male | 13 (43.3) | 14 (45.2) | |
| Female | 17 (56.7) | 17 (54.8) | |
| Age at enrollment (years), Range | 21 – 35 | 20 – 35 | NA |
| Age at enrollment (years), Mean (SD) | 28.0 (3.6) | 26.2 (4.0) | 0.08 |
| Ethnicity, N (%) | 1.00 F | ||
| Hispanic or Latino | 3 (10.0) | 3 (9.7) | |
| Not Hispanic or Latino | 27 (90.0) | 28 (90.3) | |
| Education, N (%) | <0.001 | ||
| Less than high school | 1 (3.3) | 2 (6.5) | |
| High school completion | 3 (10.0) | 18 (58.1) | |
| Some college/Associate’s | 11 (36.7) | 9 (29.0) | |
| Bachelor’s degree or Advanced degree | 15 (50.0) | 2 (6.5) | |
| Tobacco use, N (%) | 0.79 F | ||
| Never | 20 (66.7) | 22 (71.0) | |
| Past | 7 (23.3) | 5 (16.1) | |
| Current | 3 (10.0) | 4 (12.9) | |
| Alcohol use, N (%) | 1.00 F | ||
| Never | 4 (13.3) | 5 (16.1) | |
| Past | 4 (13.3) | 4 (12.9) | |
| Current | 22 (73.3) | 22 (71.0) | |
| Illicit drug use, N (%) | 0.43 | ||
| Never | 16 (53.3) | 13 (41.9) | |
| Past | 7 (23.3) | 6 (19.4) | |
| Current | 7 (23.3) | 12 (38.7) | |
| Sexually active, N (%) | 27 (93.1) | 23 (76.7) | 0.15 F |
| Missing | 1 | 1 | |
| BMI (kg/m2), Range | 18.3 – 52.2 | 11.4 – 38.2 | |
| BMI (kg/m2), Mean (SD) | 28.9 (7.2) | 27.1 (6.5) | 0.32 |
| BP Systolic, Mean (SD) | 119.5 (12.0) | 121.8 (14.5) | 0.52 |
| Missing | 3 | 2 | |
| BP Diastolic, Mean (SD) | 76.1 (9.2) | 75.3 (10.3) | 0.75 |
| Missing | 3 | 2 | |
| Taking multivitamin, N (%) | 9 (30.0) | 7 (22.6) | 0.51 |
| HIV information | |||
| ART class, N (%) | |||
| PI | 6 (19.4) | ||
| INSTI | 18 (58.1) | ||
| INSTI + PI | 6 (19.4) | ||
| NNRTI | 1 (3.2) | ||
| Recent HIV RNA viral load (copies/mL), N (%) | |||
| <50 | - | 18 (62.1) | |
| 50–1000 | 7 (24.1) | ||
| ≥1000 | 4 (13.8) | ||
| Missing | 2 | ||
| Recent CD4 count (cells/mm3), Range | - | 37 – 1295 | |
| Recent CD4 count (cells/mm3), Mean (SD) | 572 (351) | ||
| Recent CD4 count (cells/mm3), N (%) | |||
| <200 | 5 (17.3) | ||
| ≥200 | 24 (82.8) | ||
| Missing | 2 | ||
| Nadir CD4 count, Range | - | 1 – 940 | |
| Nadir CD4 count, Mean (SD) | 268 (222) | ||
| Missing | 7 | ||
| AIDS, N (%) | 13 (41.9) | ||
| DNA Methylation-Estimated Cell Type Proportions | |||
| Proportion CD4 T-cells, Mean (SD) | 0.355 (0.06) | 0.183 (0.13) | <0.001 |
| Proportion CD8 T-cells, Mean (SD) | 0.246 (0.07) | 0.361 (0.12) | <0.001 |
| Proportion Natural Killer cells, Mean (SD) | 0.08 (0.07) | 0.11 (0.10) | 0.25 |
| Proportion B-cells, Mean (SD) | 0.137 (0.04) | 0.168 (0.06) | 0.015 |
| Proportion Monocytes, Mean (SD) | 0.144 (0.06) | 0.143 (0.08) | 0.95 |
| Proportion Granulocytes, Mean (SD) | 0.009 (0.02) | 0.014 (0.06) | 0.62 |
| NIH Toolbox Cognition Battery Scores | |||
| Executive function domain, Mean (SD) | |||
| Age Corrected Standard Score | 89.5 (16.7) | 79.9 (15.5) | 0.023 |
| Fully Corrected T-score | 44.2 (12.3) | 39.8 (10.1) | 0.13 |
| Attention domain, Mean (SD) | |||
| Age Corrected Standard Score | 81.3 (13.0) | 74.4 (10.3) | 0.024 |
| Fully Corrected T-score | 42.8 (8.9) | 38.0 (8.0) | 0.031 |
| Working memory domain, Mean (SD) | |||
| Age Corrected Standard Score | 90.8 (15.5) | 80.3 (11.5) | 0.004 |
| Fully Corrected T-score | 45.2 (11.5) | 38.5 (8.7) | 0.012 |
| Processing speed domain, Mean (SD) | |||
| Age Corrected Standard Score | 100.5 (26.1) | 84.4 (20.1) | 0.010 |
| Fully Corrected T-score | 52.8 (15.8) | 42.7 (13.0) | 0.009 |
| Language domain, Mean (SD) | |||
| Age Corrected Standard Score | 106.1 (19.4) | 81.2 (14.1) | <0.001 |
| Fully Corrected T-score | 57.4 (11.1) | 43.5 (9.3) | <0.001 |
DNAm-estimated blood cell composition differed between PHIV and Controls, largely driven by differential proportions of CD8 (0.36 vs. 0.25, p<0.01) and CD4 T cells (0.18 vs. 0.36, p<0.01). There were significant differences in cognitive ability between the two groups in all measured domains: executive functioning, attention, working memory, processing speed, and language. All Age Corrected Standard Scores were significantly lower and all Fully Corrected T-scores were significantly lower except for the executive function domain (39.8 vs. 44.2, p=0.13) for PHIV young adults compared to the controls.
For this sample of young adults ages 20–35, chronological age and DNAm age were positively correlated (r=0.56, p<0.01) (Figure 1). Young adults with PHIV had a higher mean EAA (2.86 ± 6.5 vs. −2.96 ± 3.9, p<0.01) and EEAA (4.57 ± 13.0 vs. −4.7 2± 6.0, p<0.01) compared to controls. However, although IEAA was higher in PHIV than controls, it was not significantly different between groups (0.10 ± 3.91 vs. −0.10 ± 3.48, p=0.84).
Figure 1:
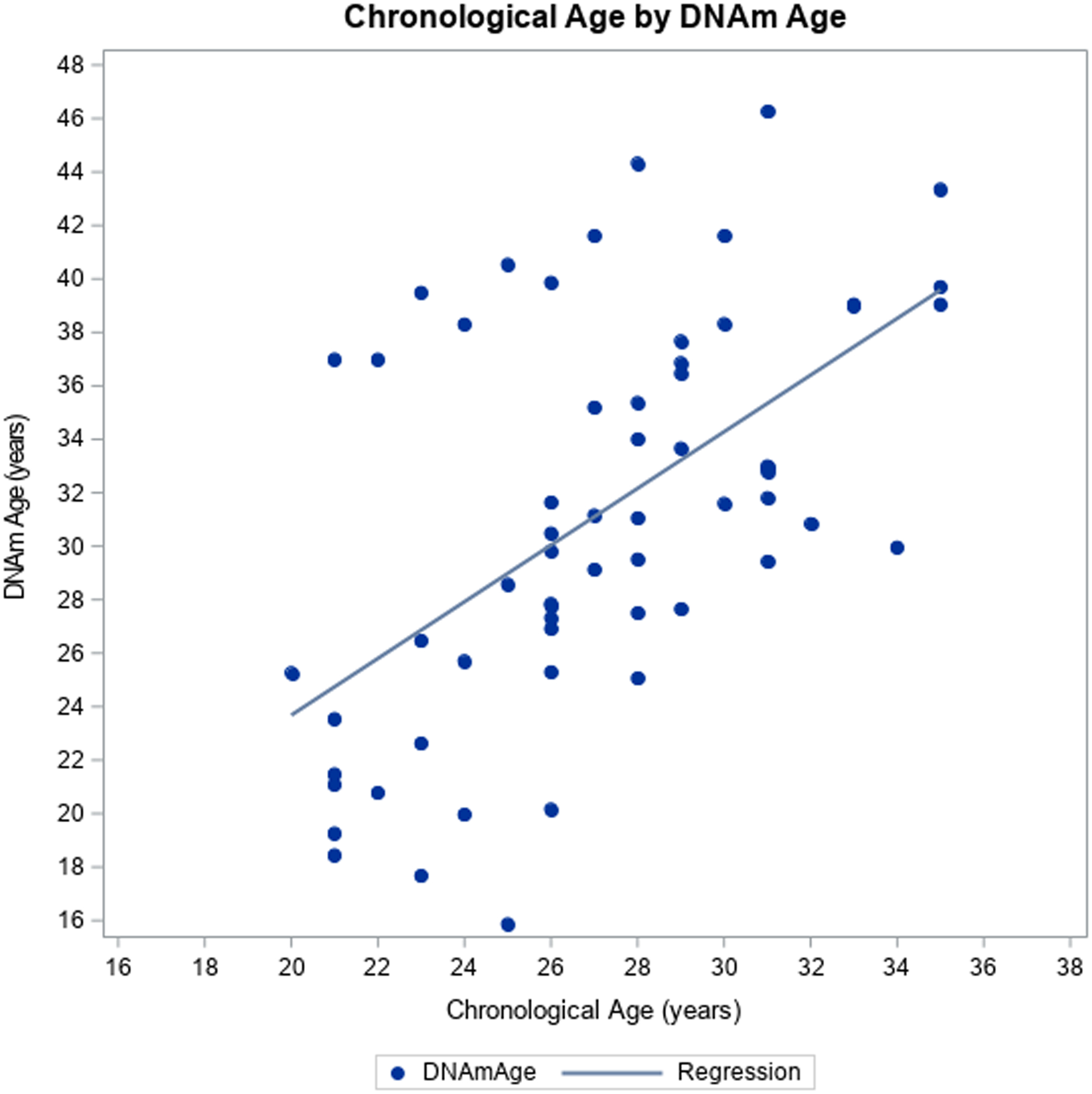
Chronological age vs. DNAm age
In linear regression models, sex, ethnicity, education, tobacco use, alcohol use, illicit drug use, and BMI were not significantly associated with any of the measures of epigenetic age acceleration (Table 3). EAA and EEAA were higher in young adults with PHIV with a detectable HIV RNA viral load ≥50 copies/ml compared to those with a HIV RNA viral load <50 copies/ml (EEA: 8.1 ± 5.2 vs. 0.11 ± 5.5, p=0.0006; EEAA: 16.1 ± 10.6 vs. −1.83 ± 9.7, p<0.0001). EAA and EEEA were also higher in young adults with PHIV with a CD4 count <200 cells/mm3 compared to those with a CD4 count >200 cells/mm3 (EEA: 11.1 ± 1.1 vs. 1.5 ± 6.0, p=0.002; EEAA: 22.3 ± 1.8 vs. 1.4 ± 11.6, p=0.00005). On the continuous scale, a higher CD4 count was associated with a lower EAA (−0.01 cells/mm3; 95% CI: −0.02, −0.009) and EEAA (−0.03 cells/mm3; 95% CI: −0.04, −0.02). In a multivariable model with viral load and CD4, both remained significantly associated with EAA and EEEA. Viral load and CD4 were not associated with IEAA. EAA and EEAA were negatively correlated with DNAm-estimated CD4 cell proportions (r=−0.78, p<0.001; r=−0.91, p<0.0001, respectively) for the young adults with PHIV; IEAA was not.
Table 3:
Factors associated with measures of epigenetic age acceleration in linear regression models
| Epigenetic age acceleration (EAA) | Extrinsic epigenetic age acceleration (EEAA) | Intrinsic epigenetic age acceleration (IEAA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable (All) | Est | 95% CI | P | Est | 95% CI | P | Est | 95% CI | P | |
| HIV status | PHIV | 5.84 | 3.07, 8.58 | <0.0001 | 9.28 | 4.08, 14.49 | 0.0007 | 0.20 | −1.70, 2.10 | 0.84 |
| Control | Ref. | |||||||||
| Sex | Female | −0.29 | −3.46, 2.87 | 0.85 | −0.71 | −6.48, 5.07 | 0.81 | 0.22 | −1.69, 2.13 | 0.82 |
| Male | Ref. | |||||||||
| Ethnicity | Hispanic or Latino | 1.81 | −3.45, 7.06 | 0.49 | 6.00 | −3.50, 15.5 | 0.21 | −1.71 | −4.86, 1.45 | 0.28 |
| Not Hispanic or Latino | Ref. | |||||||||
| Education | High school or less | 0.41 | −2.81, 3.62 | 0.80 | 2.48 | −3.36, 8.31 | 0.40 | −1.44 | −3.35, 0.47 | 0.14 |
| Some college or more | Ref. | |||||||||
| Tobacco use | Past/Current | 1.75 | −1.62, 5.11 | 0.30 | 5.99 | −0.003, 12.0 | 0.05 | −0.96 | −3.00, 1.08 | 0.35 |
| Never | Ref. | |||||||||
| Alcohol use | Past/Current | 1.69 | −2.72, 6.10 | 0.45 | −3.56 | −11.6, 4.47 | 0.38 | 2.08 | −0.54, 4.71 | 0.12 |
| Never | Ref. | |||||||||
| Illicit drug use | Past/Current | −0.42 | −3.57, 2.73 | 0.79 | 0.26 | −5.48, 6.01 | 0.93 | −1.47 | −3.33, 0.40 | 0.12 |
| Never | Ref. | |||||||||
| BMI | 0.06 | −0.17, 0.29 | 0.60 | −0.07 | −0.49, 0.35 | 0.72 | 0.09 | −0.05, 0.23 | 0.19 | |
| Variable (HIV Only) | ||||||||||
| HIV RNA viral load (copies/mL) | ≥50 | 7.96 | 3.54, 12.2 | 0.0006 | 18.0 | 10.1, 25.9 | <0.0001 | 0.21 | −2.91, 3.32 | 0.89 |
| <50 | Ref. | |||||||||
| CD4 count (cells/mm3) | <200 | 9.62 | 4.02, 15.2 | 0.002 | 21.0 | 10.1, 31.8 | 0.0005 | 1.18 | −2.80, 5.16 | 0.55 |
| >200 | Ref. | |||||||||
| CD4 count (cells/mm3) | −0.01 | −0.02, −0.009 | <0.0001 | −0.03 | −0.04, −0.02 | <0.0001 | −0.00 | −0.006, 0.003 | 0.54 | |
We correlated all three measures of epigenetic age acceleration with each of the cognitive function test scores (Table 4) for all participants, and stratified by HIV status. For all participants, there was a negative linear relationship between EAA and scores for executive function, attention, working memory, and language; this was significant for language only (r=−0.36, p=0.005). language domain test score. The negative correlations were observed primarily in the PHIV group. For EEAA, there was a significant negative linear relationship between EEAA and executive function (r=−0.31, p=0.016), attention (r=−0.31, p=0.01), and language scores (r=−0.36, p=0.005). When stratified by HIV group, the negative relationship was observed for executive function (r=−0.33, p=0.07) and attention (r=−0.27, p=0.15) for the PHIV group and language for the controls (r=−0.40, p=0.03). In the full group, no significant correlations were observed between IEAA and cognition test scores. However, of interest, there was a negative correlation between IEAA and the working memory domain test score in PHIV (IEAA: r=−0.32, p=0.08).
Table 4:
Pearson correlations between measures of epigenetic age acceleration and cognitive function domains measured by the NIH Toolbox Cognition Battery Scores for the full sample and stratified by HIV status
| Age acceleration variables | Group | Domain | ||||
|---|---|---|---|---|---|---|
| Executive function | Attention | Working memory | Processing speed | Language | ||
| Epigenetic age acceleration (EAA) | All | r = −0.19 p = 0.14 |
r = −0.22 p = 0.10 |
r = −0.21 p = 0.11 |
r = 0.007 p = 0.96 |
r = −0.36 p = 0.005 |
| Controls | r = −0.003 p = 0.99 |
r = 0.09 p = 0.62 |
r = 0.19 p = 0.30 |
r = 0.16 p = 0.39 |
r = −0.07 p = 0.71 |
|
| PHIV | r = −0.20 p = 0.29 |
r = −0.23 p = 0.22 |
r = −0.27 p = 0.15 |
r = 0.24 p = 0.20 |
r = −0.16 p = 0.40 |
|
| Extrinsic epigenetic age acceleration (EEAA) | All | r = −0.31 p = 0.016 |
r = −0.31 p = 0.01 |
r = =−0.16 p = 0.23 |
r = −0.17 p = 0.19 |
r = −0.36 p = 0.005 |
| Controls | r = −0.19 p = 0.32 |
r = −0.19 p = 0.30 |
r = −0.10 p = 0.61 |
r = −0.13 p = 0.48 |
r = −0.40 p = 0.03 |
|
| PHIV | r = −0.33 p = 0.07 |
r = −0.27 p = 0.15 |
r = 0.01 p = 0.94 |
r = 0.007 p = 0.97 |
r = −0.05 p = 0.79 |
|
| Intrinsic epigenetic age acceleration (IEAA) | All | r = 0.05 p = 0.73 |
r = 0.04 p = 0.78 |
r = −0.02 p = 0.90 |
r = 0.23 p =0.08 |
r = −0.03 p = 0.81 |
| Controls | r = 0.03 p = 0.89 |
r = 0.09 p = 0.65 |
r = 0.26 p = 0.16 |
r = 0.19 p = 0.31 |
r = 0.11 p = 0.57 |
|
| PHIV | r = 0.08 p = 0.67 |
r = 0.007 p = 0.97 |
r = −0.32 p = 0.08 |
r = 0.30 p = 0.11 |
r = −0.15 p = 0.42 |
|
Discussion
Epigenetic age acceleration in blood was observed in a cross-sectional study of African American young adults ages 20–35 years with PHIV on ART in comparison to controls without HIV using two measures: EAA, a standard measure of epigenetic age acceleration and EEAA, a measure that directly incorporates age-related changes in blood cell composition.13 However, using a measure of epigenetic age acceleration independent of blood cell composition (IEAA), no evidence of age acceleration associated with HIV was detected.
Although a study of younger children 4–9 years of age with PHIV who were initiated on ART early before 3 years of age did not find differences in EAA between those with and without HIV,9 the EAA and EEAA findings observed in this study of young adults who started ART later in life are consistent with cross-sectional studies of older men with HIV on ART (mean age 45–51)4–6 and in children with PHIV under 12 years in South Africa, which have reported similar cross-sectional findings of epigenetic age acceleration i.e. a greater difference in the gap between epigenetic age and chronological age.8,9 Taken together with recent evidence that epigenetic age acceleration may be most dramatic prior to ART initiation,14 these findings further emphasize the importance of early ART initiation in young adults with PHIV to minimize epigenetic age acceleration, particularly given links between epigenetic age acceleration and cognition outcomes.
A hallmark of HIV infection is the dysregulation of immune cells.15 This is reflected in our DNAm-estimated cell type proportions, with a decreased proportion of CD4 T-cells and increased proportion of CD8 T-cells in those with HIV. HIV-associated inflammation and immunosenescence has been implicated as a cause of the premature onset of other end organ diseases, and is present in young adults with PHIV.16–18 Our finding of an association between HIV and EEAA indicates a direct association between HIV and an epigenetic age marker that reflects immune system aging. This was also observed in a study of younger children with PHIV ages 9–12 years.8 Consistent with this hypothesis, PHIV young adults with either lower absolute CD4 count as measured by flow cytometry or lower DNAm-estimated CD4 proportion had higher epigenetic age acceleration.
HIV can also damage cells through cellular physiological aging pathways or processes that are relatively independent of its effects on immune cells. Our group has investigated the role of inflammation on osteoblast senescence in HIV, as an explanation for the increased rate of osteoporosis and fracture in PWH. Osteoblast dysfunction is evident in bone biopsies of ART-naïve PWH, with decreased rate of bone formation and mineralization compared to age-matched uninfected controls on histomorphometry.19 We also found lower numbers of circulating osteogenic precursors, a subset of peripheral blood mononuclear cells with characteristics of osteoblasts, and shorter telomere lengths in those circulating osteogenic precursors among PWH compared to uninfected controls.20 These studies suggest that chronic inflammation among PWH may result in senescence of cell lines other than immune cells, which may partially explain the higher prevalence of aging-related comorbidities among PWH. For this reason, we chose to report measures of intrinsic epigenetic aging, which may reflect alternative pathways or processes and reflect epigenetic aging in blood not confounded by differences in circulating blood cell type composition.13 In this study of African American young adults, we did not observe differences in IEAA between young adults with PHIV and controls, suggesting the majority of the HIV effect on epigenetic aging may be reflected in immunosenescence as reflected in the extrinsic measure.
A meta-analysis published in 2016 found working memory, processing speed, and executive function to be the cognitive domains with the greatest impairments in PHIV relative to HIV-uninfected and HIV-unexposed controls, and lesser differences in language or attention.21 We found that African American young adults with PHIV had lower test scores on attention, working memory, processing speed, and language compared to controls. Differences in executive function between groups were attenuated after correcting for age, sex, ethnicity, and education. A strength of our study was the use of the NIH Toolbox Cognitive Battery, a self-administered tablet-based assessment with a nationally representative normative sample. However, this tool was used less often in prior studies of epigenetic aging and cognition in HIV, which may partially explain differences, and limit comparisons.
EEAA, a measure of biological aging and immune system aging, has been found to be associated with cognitive measures and faster decline in cognitive performance among adults in the general population.22,23 Horvath et al. found negative correlations between EEAA and several measures of cognitive functioning, including executive functioning, working memory, and processing speed in children with and without HIV ages 9–12 years (−0.14 to 0.20).8 After restricting the analysis to the PHIV group only, Horvath et al. found significant correlations (−0.14 to −0.15) between EEAA and executive functioning, working memory, and processing speed. For the full sample, we observed stronger correlations (−0.31 to −0.36) in the same direction among young adults with and without HIV for the executive function, attention, and language domains. Correlations between EEAA and working memory and processing speed scores, while in the expected directions, were not statistically significant. After stratifying by HIV status, correlations did not remain statistically significant, but there remained a signal for the PHIV group for executive function (r=−0.33) and attention (r=−0.27). This may be due to a smaller sample size in our study (N=61) compared to the study in South Africa (N=248).8 Of note, we observed a signal that IEAA may be correlated with working memory in the PHIV group, pointing towards a potential association between epigenetic aging and cognition that is not mediated by immune system aging.
Our study was limited by our small sample size (approximately 30 in each group) and cross-sectional study design. Additional follow up of a larger cohort is needed to understand how epigenetic age acceleration may impact trajectories of cognitive decline in adults with HIV. In addition, evaluating the effects of duration, intensity and patterns of health behaviors or life events over time on epigenetic age acceleration are also warranted. Future studies should also consider the inclusion of a comparison group of young adults who acquired HIV in later life to better understand how duration of infection may affect epigenetic age acceleration. Unfortunately, we did not screen for other possible causes of cognitive deficits (e.g. learning disability or prior head injury) and did not have a measure of mood (e.g. depression or anxiety), which could affect cognitive functioning and occur more frequently in the PHIV group. We also did not measure other biomarkers of aging such as telomere length or the neutrophil-to-lymphocyte ratio. Finally, our sample only included African American young adults, and findings may not be generalizable to other racial groups, given existing racial differences in both extrinsic and intrinsic measures of epigenetic aging.24,25
In conclusion, epigenetic age acceleration was observed in African American young adults with PHIV on ART using two measures, including EEAA which reflect immunosenescence. However, there was no evidence of age acceleration independent of cell type composition. There was a signal that EEAA was negatively associated with lower scores on executive function for young adults with PHIV. Future studies should measure DNAm and estimate epigenetic age acceleration in specific cell subsets, including CD4 and CD8 T-cells, and evaluate associations with longitudinal changes in cognitive function outcomes.
Acknowledgements:
This publication was supported by the funding from the National Institutes of Health, through Grant Number UL1 TR001873 (National Center for Advancing Translational Sciences) and Grant Number R21 AG056175. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. SS was supported by R25 MH108389.
References
- 1.Shiau S, Bender AA, O’Halloran JA, et al. The Current State of HIV and Aging: Findings Presented at the 10th International Workshop on HIV and Aging. AIDS Res Hum Retroviruses. August 2020. doi: 10.1089/AID.2020.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabuzda D, Jamieson BD, Collman RG, et al. Pathogenesis of Aging and Age-related Comorbidities in People with HIV: Highlights from the HIV ACTION Workshop. Pathog Immun. 2020;5(1):143–174. doi: 10.20411/pai.v5i1.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross AM, Jaeger PA, Kreisberg JF, et al. Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol Cell. 2016;62(2):157–168. doi: 10.1016/j.molcel.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson KN, Hui Q, Rimland D, et al. Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV-positive, treatment-naive U.S. veterans. AIDS. 2017;31(4):571–575. doi: 10.1097/QAD.0000000000001360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015;212(10):1563–1573. doi: 10.1093/infdis/jiv277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine AJ, Quach A, Moore DJ, et al. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22(3):366–375. doi: 10.1007/s13365-015-0406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horvath S, Stein D, Phillips N, et al. Perinatally acquired HIV infection accelerates epigenetic aging in South African adolescents. Aids. 2018;32(11):1465–1474. doi: 10.1097/QAD.0000000000001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiau S, Strehlau R, Shen J, et al. Biomarkers of Aging in HIV-Infected Children on Suppressive Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2018;78(5):549–556. doi: 10.1097/QAI.0000000000001714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–6. doi: 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8(9):1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehl ME, Rickabaugh TM, Shih R, et al. The Effects of Anti-retroviral Therapy on Epigenetic Age Acceleration Observed in HIV-1-infected Adults. Pathog Immun. 2020;5(1):291–311. doi: 10.20411/pai.v5i1.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0 [DOI] [PubMed] [Google Scholar]
- 16.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV as a Cause of Immune Activation and Immunosenescence. Mediators Inflamm. 2017;2017:6825493. doi: 10.1155/2017/6825493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fastenackels S, Sauce D, Vigouroux C, et al. HIV-mediated immune aging in young adults infected perinatally or during childhood. AIDS. 2019;33(11):1705–1710. doi: 10.1097/QAD.0000000000002275 [DOI] [PubMed] [Google Scholar]
- 19.Ramalho J, Martins CSW, Galvão J, et al. Treatment of Human Immunodeficiency Virus Infection With Tenofovir Disoproxil Fumarate-Containing Antiretrovirals Maintains Low Bone Formation Rate, But Increases Osteoid Volume on Bone Histomorphometry. J Bone Miner Res. July 2019. doi: 10.1002/jbmr.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manavalan JS, Arpadi S, Tharmarajah S, et al. Abnormal Bone Acquisition With Early-Life HIV Infection: Role of Immune Activation and Senescent Osteogenic Precursors. J Bone Miner Res. 2016;31(11):1988–1996. doi: 10.1002/jbmr.2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips N, Amos T, Kuo C, et al. HIV-Associated Cognitive Impairment in Perinatally Infected Children: A Meta-analysis. Pediatrics. 2016;138(5). doi: 10.1542/peds.2016-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beydoun MA, Shaked D, Tajuddin SM, Weiss J, Evans MK, Zonderman AB. Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology. 2020;94(6):e613–e625. doi: 10.1212/WNL.0000000000008756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388–1396. doi: 10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tajuddin SM, Hernandez DG, Chen BH, et al. Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites. Clin Epigenetics. 2019;11(1):119. doi: 10.1186/s13148-019-0722-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


