Abstract
Background:
Breast cancer incidence in women age ≥70 is steadily increasing and many are choosing to undergo post-mastectomy breast reconstruction (PMBR). We aimed to identify factors associated with PMBR, describe reconstruction types, and assess post-operative mortality and re-admission rates in women ≥70.
Methods:
The NCDB was examined between 2004-2015 for women age ≥70 with breast cancer who underwent mastectomy. Statistical analysis was performed by chi-squared tests and multivariate logistic regression to select the best models for predicting PMBR and if patients underwent contralateral prophylactic mastectomy (CPM) with reconstruction.
Results:
73,973 patients met inclusion criteria and 4,552 (6.1%) underwent PMBR, of which 25% had a CPM. 48% had implant reconstruction, 36.2% underwent autologous reconstruction, and 15.1% received combination reconstruction. PMBR was more likely to be performed in patients who were White, lower comorbidities, treated in the Northeast in metropolitan areas, and with lower tumor stage (p<0.001). CPM was more likely to be performed in patients who were White, treated in community hospitals in the South and West in rural areas. (p<0.05). While 30-day readmission rates were higher in PMBR patients (3.5% vs 2.8%, p<0.001), 30 and 90-day mortality rates were lower: 0.03 and 0.2% vs 0.3 and 0.9% (p<0.001).
Conclusion.
While it is understandable that intrinsic tumor characteristics influence the role of PMBR, further research and interventions should be aimed to eliminate the differences that are seen in patient race and geographic location. Readmission and post-op mortality rates are overall low and comparable to that of younger patients.
Article Summary:
This NCDB analysis describes rates of post-mastectomy breast reconstruction (PMBR) in women age ≥70. The importance of this report is that both patient demographic and tumor-related factors are associated with PMBR and most patients undergo unilateral, implant-based reconstruction with overall low post-operative readmission and mortality rates.
Introduction:
Breast cancer is the most commonly diagnosed cancer in older female patients, with one in every three new breast cancer diagnoses being made in women ≥70 years of age. Women in their eighth decade of life have the highest age-specific incidence of breast cancer when compared to all other age groups.1 While patients in this age group are more likely to undergo breast conserving surgery when compared to younger cohorts, an increasing proportion of those undergoing mastectomy are choosing to undergo post-mastectomy breast reconstruction (PMBR).2
In recent years, societal guidelines and randomized-controlled trials have formally defined the ‘older age demographic’ as age ≥70, and therefore, this patient demographic has been used as the cutoff point for de-escalation of treatment in early favorable breast cancers.3-5 Evaluation of satisfaction and quality of life after PMBR in women ≥70 years of age have previously been demonstrated to be quite favorable and even higher scores than younger patients.6 A single institution survey-study reported that in patients ≥70 who underwent PMBR, 88.5% reported they would choose to undergo reconstruction again if they were to face a similar situation in the future.7 While novel techniques have been developed to reconstruct the breast after mastectomy, conventional implant and autologous-based reconstruction remain the most commonly used methods.8
Previous analyses have found several factors that influence whether patients undergo PMBR. These include patient race, age, tumor stage and size, preoperative comorbidities, use of adjuvant radiation therapy, year at diagnosis, geographic location, and hospital facility type.2,9-11 However, despite the unique de-escalation opportunities that exist in women ≥70 with breast cancer, rates of mastectomy and PMBR continue to be elevated in older adults and there is insufficient data on PMBR within this age group.2 By using the American College of Surgeon’s National Cancer Database (NCDB), we aimed to analyze which factors are associated with PMBR in women ≥70, describe the types of reconstruction patients receive, and describe patient post-operative readmission and mortality rates.
Materials and Methods:
The NCDB participant user file (PUF) was queried for all patients age 70 and older with a primary breast malignancy without metastatic disease, including those with ductal carcinoma in situ (DCIS), from the years 2004 to 2015. The NCDB is a national, hospital-based cancer database administered by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The database records approximately 70% of all cancer cases in the United States and provides information on patient demographics, cancer staging, and treatment(s). NCDB data is compliant with the privacy requirements of the Health Insurance Portability and Accountability Act (HIPAA). Geographic treatment location within the NCDB PUF is defined by United States Census divisions. Patients who were male, underwent breast conserving surgery or had missing surgical data, had T4 tumors or Stage 4 disease, or unknown tumor laterality (unknown if tumor(s) were bilateral or unilateral) were excluded given that these variables may influence the decision for PMBR.
Chi-squared tests were used to evaluate univariate analysis and then further tested for significance by multivariate logistic regression in conjunction with Akaike information criterion (AIC) to select the best models for predicting PMBR, unilateral or CPM, and type of reconstruction. Statistical significance was determined at p=0.05 prior to statistical analysis. P-values were adjusted appropriately given the large number of variables on multivariate analysis and Bonferroni correction was used to assure that values remained significant. Prior to data analysis, this study was reviewed by our Institutional Review Board office and determined exempt from formal committee review.
Results:
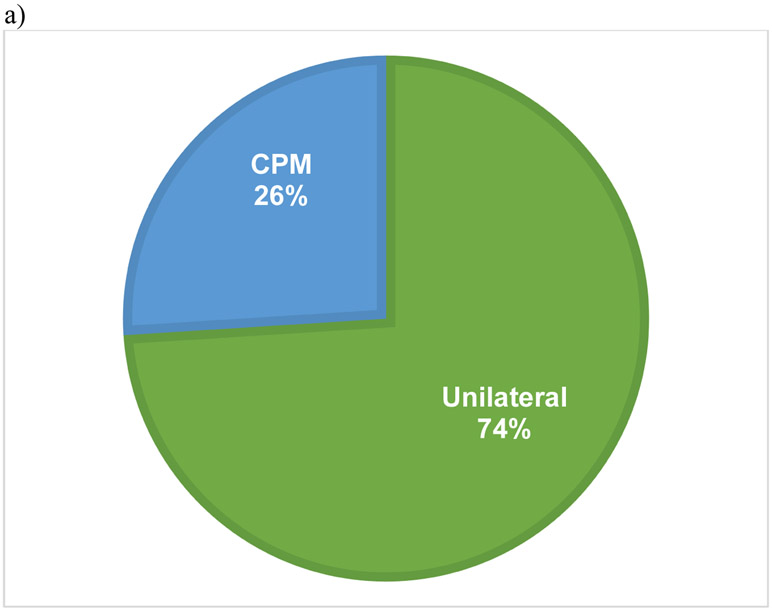
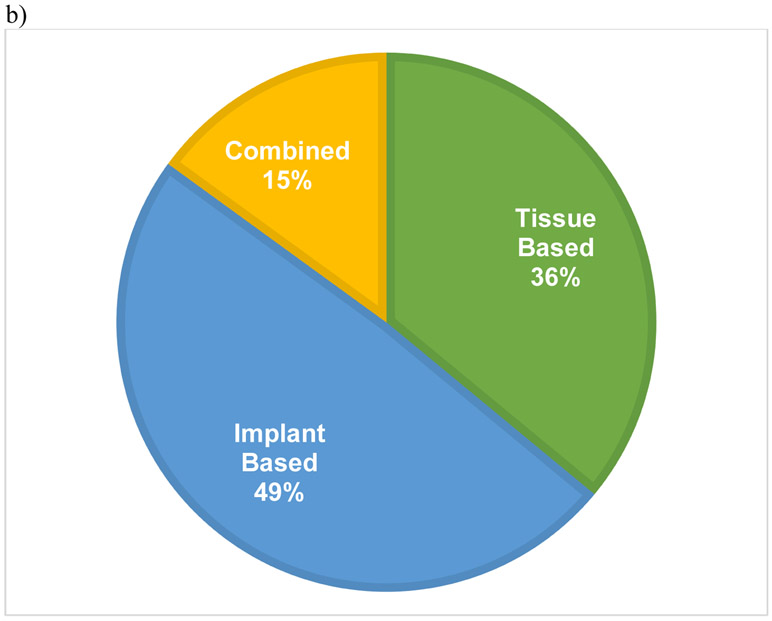
The initial inquiry of the dataset of women ≥70 years of age revealed 274,724 individuals, of which 73,973 met inclusion criteria. The majority (93.8%) of the cohort did not undergo PMBR while 4,552 (6.2%) did undergo PMBR (Figure 1). In those who underwent PMBR, 74% had unilateral surgery and 26% received a CPM (Figure 2a). Details on type of reconstruction were available for 3,732 PMBR patients (82%) which revealed 48.7% (1,817) had implant-based reconstruction, 36.2% (1,350) had tissue-based reconstruction, and 15.1% (565) had combination-based reconstruction (Figure 2b).
Figure 1.
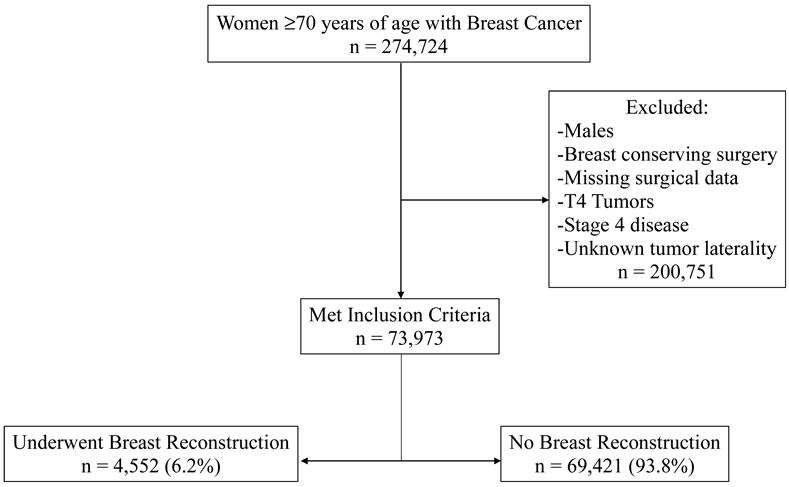
Study inclusion criteria schema.
Figure 2.
Pie charts demonstrating percentage of patients who underwent unilateral versus contralateral prophylactic mastectomy (CPM) with reconstruction (a) along with type of reconstruction (b).
Clinical and demographic data of the cohort are listed in Table 1. PMBR rates were separated before and after the year 2009 given that 2009 served as the midpoint timeframe within this data series and because there was highly publicized media coverage on celebrities undergoing PMBR in the year 2008.2,12 Most patients were treated at a comprehensive community cancer program (CCC) (53.2%), were treated in the Northeast (39.5%), were age 70-74 (36.2%), were White (86.5%), had government-issued insurance (88.2%), were treated in a metropolitan area (82.1%), had a Charlson-Deyo-score of 0 (73.7%), and were treated prior to the year 2009 (51.1%). The majority of tumors were unilateral (99.9%), grade 2 (42.6%), invasive (88.9%), estrogen-receptor (ER) (76.5%) and progesterone receptor (PR) (63.6%) positive, Stage 2 (40.0%), and did not have nodal involvement (60.0%). Most patients did not receive systemic chemotherapy (75.8%) or radiation therapy (83.0%).
Table 1.
Demographic and tumor characteristics of the cohort.
| Variable | Cohort n=73,973 n (%) |
|
|---|---|---|
| Facility Type | ||
| Community | 10,139 (13.7) | |
| CCC | 39,333 (53.2) | |
| Academic | 16,374 (22.1) | |
| INCC | 8,127 (11.0) | |
| Geographic Location | ||
| Northeast | 29,247 (39.5) | |
| Midwest | 20,106 (27.2) | |
| South | 13,285 (18.0) | |
| West | 11,335 (15.3) | |
| Age Group | ||
| 70 - 74 | 26,796 (36.2) | |
| 75 - 79 | 21,482 (29.0) | |
| 80 - 84 | 15,457 (21.0) | |
| 85 - 90 | 10,238 (13.8) | |
| Race | ||
| White | 63,961 (86.5) | |
| Black | 7,031 (9.5) | |
| Other | 2,981 (4.0) | |
| Insurance Status | ||
| Not Insured | 327 (0.4) | |
| Private Insurance | 8,122 (11.0) | |
| Government Provided | 65,265 (88.2) | |
| Unknown | 845 (1.1) | |
| Facility Setting | ||
| Metropolitan | 59,149 (82.1) | |
| Urban | 11,153 (15.5) | |
| Rural | 1,709 (2.4) | |
| CD Score | ||
| 0 | 54,476 (73.7) | |
| 1 | 14,999 (20.3) | |
| 2+ | 4,408 (6.0) | |
| Diagnosis Year | ||
| < 2009 | 37,831 (51.1) | |
| > 2009 | 36,142 (48.9) | |
| Tumor Laterality | ||
| Unilateral | 73,957 (99.9) | |
| Bilateral | 16 (0.01) | |
| DCIS vs IC | ||
| DCIS | 8,267 (11.2) | |
| IC | 65,706 (88.9) | |
| Tumor Grade | ||
| Grade 1 | 13,444 (18.2) | |
| Grade 2 | 31,482 (42.6) | |
| Grade 3 | 22,907 (30.1) | |
| Grade 4 | 521 (0.01) | |
| Unknown | 5,619 (7.6) | |
| Tumor Size | ||
| < 2cm | 34,844 (47.1) | |
| 2 – 5cm | 29,572 (40.0) | |
| > 5cm | 6,591 (9.0) | |
| Unknown | 2,966 (4.0) | |
| Nodal Status | ||
| Negative | 44,385 (60.0) | |
| Positive | 24,617 (33.3) | |
| Unknown | 4,971 (6.7) | |
| Stage | ||
| 0 | 8,423 (11.4) | |
| 1 | 25,328 (34.2) | |
| 2 | 29,646 (40.0) | |
| 3 | 10,576 (14.3) | |
| ER Status | ||
| ER (−) | 13,895 (18.8) | |
| ER (+) | 56,596 (76.5) | |
| Unknown | 3,482 (4.7) | |
| PR Status | ||
| PR (−) | 22,786 (30.8) | |
| PR (+) | 47,056 (63.6) | |
| Unknown | 4,131 (5.6) | |
| Radiation Therapy | ||
| No | 61,410 (83.0) | |
| Yes | 11,552 (15.6 | |
| Unknown | 1,011 (1.4) | |
| Chemotherapy | ||
| No | 56,083 (75.8) | |
| Yes | 15,238 (20.6) | |
| Unknown | 2,652 (3.6) | |
| 30-Day Readmission | ||
| None | 70,300 (95.9) | |
| Readmission | 2,028 (2.7) | |
| Unknown | 1,645 (2.2) | |
| 30-Day Mortality | ||
| Alive | 67,671 (91.5) | |
| Died | 197 (0.2) | |
| Unknown | 6,105 (8.3) | |
| 90-Day Mortality | ||
| Alive | 67,099 (90.7) | |
| Died | 578 (0.8) | |
| Unknown | 6,296 (8.5) | |
Key: CCC=Comprehensive Community Cancer Program, INCC= Integrated Network Cancer Center, DCIS = ductal carcinoma in situ, IC=invasive cancer, ER=estrogen receptor, PR= progesterone receptor, CD = Charlson/Deyo
PMBR:
Univariate analysis revealed that all variables were statistically significant when examining those patients who underwent PMBR compared to those that did not undergo PMBR, with the single exception of tumor laterality (bilateral versus unilateral tumors) (Table 2). Regarding laterality, there was only one patient who had bilateral disease who underwent PMBR. Overall 30-day readmission rate was 2.7% and was higher for those patients that underwent PMBR (3.4%) compared to those that did not (2.7%) (p<0.001). 30 and 90-day morality rates were low at 0.03% and 0.2%, respectively. Those patients who did not undergo PMBR had higher 30-day mortality rate (0.3% vs 0.03%) and a 90-day mortality rate (0.9% vs 0.2%), compared to those who had PMBR (p<0.001).
Table 2.
Demographic and tumor characteristics separated by PMBR, unilateral vs bilateral reconstruction, and type of reconstruction. All p-values are based on chi-squared analysis.
| Variable | No Reconstruction vs Reconstruction | Unilateral vs CPM with Reconstruction | Type of Reconstruction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Reconstruction n (%) |
Reconstruction n (%) |
p value | Unilateral n (%) |
CPM n (%) |
p value | Tissue n (%) |
Implant n (%) |
Combined n (%) |
p value | ||
| 69,421 (93.8) | 4,552 (6.1) | 3,346 (74) | 1,206 (26) | 1,350 (36.2) | 1,817 (48.7) | 565 (15.1) | |||||
| Facility Type | <0.001 | <0.001 | <0.001 | ||||||||
| Community | 9,844 (90.6) | 295 (9.4) | 215 (72.9) | 80 (27.1) | 92 (40.7) | 95 (42.0) | 39 (17.3) | ||||
| CCC | 37,093 (94.3) | 2,240 (5.7) | 1,604 (71.6) | 636 (28.4) | 640 (35.2) | 911 (50.1) | 268 (14.7) | ||||
| Academic | 14,963 (91.4) | 1,411 (8.6) | 1,096 (77.7) | 315 (22.3) | 468 (39.8) | 526 (44.7) | 183 (15.5) | ||||
| INCC | 7,521 (92.5) | 606 (7.5) | 431 (71.1) | 175 (28.9) | 150 (29.4) | 285 (55.9) | 75 (14.7) | ||||
| Geographic Location | <0.001 | <0.001 | <0.001 | ||||||||
| Northeast | 27,052 (92.5) | 2,195 (7.5) | 1,680 (76.5) | 515 (23.5) | 709 (39.3) | 849 (47.1) | 245 (13.6) | ||||
| Midwest | 19,079 (94.9) | 1,027 (5.1) | 775 (75.5) | 252 (24.5) | 264 (30.7) | 458 (53.3) | 137 (15.9) | ||||
| South | 12,635 (95.1) | 650 (4.9) | 432 (66.5) | 218 (33.5) | 213 (40.9) | 219 (42.0) | 89 (17.1) | ||||
| West | 10,655 (94.0) | 680 (6.0) | 459 (67.5) | 221 (32.5) | 164 (29.9) | 291 (53.0) | 94 (17.1) | ||||
| Age Group | <0.001 | <0.001 | 0.004 | ||||||||
| 70 - 74 | 23,703 (88.5) | 3,093 (11.5) | 2,184 (70.6) | 909 (29.4) | 931 (36.3) | 1,240 (48.4) | 393 (15.3) | ||||
| 75 - 79 | 20,376 (94.9) | 1,106 (5.1) | 856 (77.4) | 250 (22.6) | 305 (33.9) | 452 (50.3) | 142 (15.8) | ||||
| 80 - 84 | 15,174 (98.2) | 283 (1.8) | 243 (85.9) | 40 (14.1) | 83 (37.7) | 110 (50.0) | 27 (12.3) | ||||
| 85 - 90 | 10,168 (99.3) | 70 (0.7) | 63 (90) | 7 (10) | 31 (63.3) | 15 (30.6) | 3 (6.1) | ||||
| Race | <0.001 | <0.001 | <0.001 | ||||||||
| White | 59,909 (93.7) | 4,052 (6.3) | 2,925 (72.2) | 1,127 (27.8) | 1,167 (35.0) | 1,660 (49.8) | 505 (15.2) | ||||
| Black | 6,686 (95.1) | 345 (4.9) | 298 (86.4) | 47 (13.6) | 121 (44.5) | 107 (39.3) | 44 (16.2) | ||||
| Other | 2,826 (94.8) | 155 (5.2) | 123 (79.4) | 32 (20.6) | 62 (48.4)) | 50 (39.1) | 16 (12.5) | ||||
| Insurance Status | <0.001 | 0.36 | <0.001* | ||||||||
| Not Insured | 318 (97.2) | 9 (2.8) | 6 (66.7) | 3 (33.3) | 4 (5.5) | 1 (1.4) | 68 (93.2) | ||||
| Private | 6,950 (92.2) | 586 (7.8) | 425 (72.5) | 161 (27.5) | 181 (20.1) | 229 (25.4) | 492 (54.5) | ||||
| Insurance Government | 61,343 (94.0) | 3,922 (6.0) | 2,885 (73.6) | 1,037 (26.4) | 1,154 (42.2) | 1,578 (57.7) | 5 (0.2) | ||||
| Provided Unknown | 810 (95.9) | 35 (4.1) | 30 (85.7) | 5 (14.3) | 0 | 0 | 0 | ||||
| Facility Setting | <0.001 | 0.016 | 0.10 | ||||||||
| Metropolitan | 55,158 (93.3) | 3,991 (6.7) | 2,951 (73.9) | 1,040 (26.1) | 1,204 (36.7) | 1,584 (48.3) | 494 (15.1) | ||||
| Urban | 10,755 (96.4) | 398 (3.6) | 283 (71.1) | 115 (28.9) | 106 (33.0) | 158 (49.2) | 57 (17.8) | ||||
| Rural | 1,660 (97.1) | 49 (2.9 | 28 (57.1) | 21 (42.9) | 12 (27.9) | 28 (65.1) | 3 (7.0) | ||||
| CD Score | <0.001 | 0.71 | 0.094 | ||||||||
| 0 | 50,908 (93.3) | 3,568 (6.7) | 2,695 (73.7) | 963 (26.3) | 1,111 (36.9) | 1,440 (47.9) | 456 (15.2) | ||||
| 1 | 14,266 (95.1) | 733 (4.9) | 537 (73.3) | 196 (26.7) | 201 (33.7) | 312 (52.3) | 83 (13.9) | ||||
| 2+ | 4,247 (96.3) | 161 (3.7) | 114 (70.8) | 47 (29.2) | 38 (29.5) | 65 (50.4) | 26 (20.2) | ||||
| Diagnosis Year | <0.001 | <0.001 | 0.001 | ||||||||
| < 2009 | 36,415 (96.3) | 1,416 (3.7) | 1,153 (81.4) | 263 (18.6) | 436 (39.9) | 520 (17.6) | 136 (12.5) | ||||
| > 2009 | 33,006 (91.3) | 3,136 (8.7) | 2,193 (69.9) | 943 (30.1) | 914 (34.6) | 1,297 (49.1) | 429 (16.2) | ||||
| Tumor Laterality | 1 | 0.59 | N/A | ||||||||
| Unilateral | 69,406 (93.8) | 4,551 (6.2) | 3,346 (73.5) | 1,205 (26.5) | |||||||
| Bilateral | 15 (93.8) | 1 (6.2) | 0 (0.0) | 1 (100) | |||||||
| DCIS vs IC | <0.001 | 1.0 | 0.77 | ||||||||
| DCIS | 7,434 (89.9) | 833 (10.1) | 612 (73.5) | 221 (26.5) | 244 (35.4) | 336 (48.7) | 110 (15.9) | ||||
| Invasive Cancer | 61,987 (94.3) | 3,719 (5.7) | 2,734 (73.5) | 985 (26.5) | 1,106 (36.4) | 1,481 (48.7) | 455 (15.0) | ||||
| Tumor Grade | <0.001* | 0.63† | 0.15† | ||||||||
| Grade 1 | 12,560 (93.4) | 884 (6.6) | 639 (72.3) | 245 (27.7) | 255 (34.6) | 370 (50.2) | 112 (15.2) | ||||
| Grade 2 | 29,484 (93.7) | 1,998 (6.3) | 1,471 (73.6) | 527 (26.4) | 620 (37.9) | 773 (47.2) | 243 (14.9) | ||||
| Grade 3 | 21,664 (94.6) | 1,243 (5.4) | 919 (73.9) | 324 (26.1) | 356 (35.0) | 505 (49.7) | 155 (15.3) | ||||
| Grade 4 | 485 (93.1) | 36 (6.9) | 30 (83.3) | 6 (16.6) | 7 (24.1) | 19 (65.5) | 3 (10.3) | ||||
| Unknown | 5,228 (93.0) | 391 (7.0) | 287 (73.4) | 104 (26.6) | 112 (35.7) | 150 (47.8) | 52 (16.6) | ||||
| Tumor Size | <0.001* | <0.001* | 0.069† | ||||||||
| < 2cm | 32,273 (92.6) | 2,571 (7.4) | 1,828 (71.1) | 743 (28.9) | 739 (35.1) | 1045 (49.6) | 324 (15.4) | ||||
| 2 – 5cm | 28,172 (95.3) | 1,400 (4.7) | 1,059 (75.6) | 341 (24.4) | 423 (36.9) | 550 (48.0) | 173 (15.1) | ||||
| > 5cm | 6,268 (95.1) | 323 (4.9) | 267 (82.7) | 56 (17.3) | 102 (40.0) | 115 (45.1) | 38 (14.9) | ||||
| Unknown | 2,708 (91.3) | 258 (8.7) | 192 (74.4) | 66 (25.6) | 86 (38.6) | 107 (48.0) | 30 (13.5) | ||||
| Nodal Status | <0.001* | 0.002* | 0.046* | ||||||||
| Negative | 41,259 (93) | 3,126 (7) | 2,253 (72.1) | 873 (27.9) | 909 (35.1) | 1,300 (50.2) | 382 (14.7) | ||||
| Positive | 23,426 (95.2) | 1,191 (4.8) | 905 (76.0) | 286 (24.0) | 377 (39.3) | 434 (45.3) | 148 (15.4) | ||||
| Unknown | 4,736 (95.3) | 235 (4.7) | 188 (80.0) | 47 (20.0) | 64 (35.2) | 83 (45.6) | 35 (19.2) | ||||
| Stage | <0.001 | <0.001* | 0.032 | ||||||||
| 0 | 7,568 (89.8) | 855 (10.2) | 625 (73.1) | 230 (26.9) | 245 (34.7) | 349 (49.4) | 113 (16.0) | ||||
| 1 | 23,464 (92.6) | 1,864 (7.4) | 1,317 (70.7) | 547 (29.3) | 539 (35.1) | 762 (49.6) | 236 (15.4) | ||||
| 2 | 28,231 (95.2) | 1,415 (4.8) | 1,081 (76.4) | 334 (23.6) | 419 (36.1) | 574 (49.5) | 167 (14.4) | ||||
| 3 | 10,158 (96) | 418 (4) | 323 (77.3) | 95 (22.7) | 147 (44.8) | 132 (40.2) | 49 (14.9) | ||||
| ER Status | <0.001* | 0.96† | 0.31† | ||||||||
| ER (−) | 13,144 (94.6) | 751 (5.4) | 13,144 (94.6) | 751 (5.4) | 217 (34.6) | 328 (52.3) | 82 (13.1) | ||||
| ER (+) | 52,962 (93.6) | 3,634 (6.4) | 52,962 (93.6) | 3,634 (6.4) | 1,088 (36.5) | 1427 (47.9) | 464 (15.6) | ||||
| Unknown | 3,315 (95.2) | 167 (4.8) | 3,315 (95.2) | 167 (4.8) | 45 (35.7) | 62 (49.2) | 19 (15.1) | ||||
| PR Status | <0.001* | 0.51† | 0.12† | ||||||||
| PR (−) | 21,543 (94.5) | 1,243 (5.5) | 918 (73.9) | 325 (26.1) | 367 (35.8) | 520 (50.7) | 138 (13.5) | ||||
| PR (+) | 43,965 (93.4) | 3,091 (6.6) | 2,261 (73.1) | 830 (26.9) | 924 (36.4) | 1,212 (47.7) | 404 (15.9) | ||||
| Unknown | 3,913 (94.7) | 218 (5.3) | 167 (76.6) | 51 (23.4) | 59 (35.3) | 85 (50.9) | 23 (13.8) | ||||
| 30-Day Readmission | <0.001* | <0.001* | 0.34† | ||||||||
| None | 65,965 (93.8) | 4,335 (6.2) | 3,204 (93.8) | 1,131 (6.2) | 1,293 (36.4) | 1,735 (48.8) | 529 (14.9) | ||||
| Readmission | 1,875 (92.5) | 153 (7.5) | 95 (92.5) | 58 (7.5) | 43 (33.1) | 61 (46.9) | 26 (20.0) | ||||
| Unknown | 1,581 (96.1) | 64 (3.9) | 47 (96.1) | 17 (3.9) | 14 (31.1) | 21 (46.7) | 10 (22.2) | ||||
| Radiation Therapy | 0.05 * | <0.001* | 0.020 | ||||||||
| No | 57,573 (93.8) | 3,837 (6.2) | 2,780 (72.5) | 1,057 (27.5) | 1,127 (35.3) | 1,579 (49.5) | 486 (15.2) | ||||
| Yes | 10,890 (94.3) | 662 (5.7) | 519 (78.4) | 143 (21.6) | 208 (41.5) | 218 (43.5) | 75 (15.0) | ||||
| Unknown | 958 (94.8) | 53 (5.2) | 47 (88.7) | 6 (11.3) | 15 (38.5) | 20 (51.3) | 4 (10.3) | ||||
| Chemotherapy | <0.001* | 0.91 | 0.031* | ||||||||
| No | 52,813 (94.2) | 3,270 (5.8) | 2,398 (73.3) | 872 (26.7) | 946 (35.3) | 1,305 (48.7) | 426 (15.9) | ||||
| Yes | 14,108 (92.6) | 1,130 (7.4) | 835 (73.9) | 295 (26.1) | 368 (39.5) | 440 (47.3) | 123 (13.2) | ||||
| Unknown | 2,500 (94.3) | 152 (5.7) | 113 (74.3) | 39 (25.7) | 36 (29.0) | 72 (58.1) | 16 (12.9) | ||||
| 30-Day Mortality | <0.001* | <0.001‡ | N/A | ||||||||
| Alive | 63,737 (94.2) | 3,934 (5.8) | 2,939 (74.7) | 995 (25.3) | 1,175 (36.4) | 1,559 (48.3) | 494 (15.3) | ||||
| Died | 196 (99.5) | 1 (0.5) | 1 (100) | 0 (0.0) | 0 | 0 | 0 | ||||
| Unknown | 5,488 (89.9) | 617 (10.1) | 406 (65.8) | 211 (34.2) | 175 (34.7) | 258 (51.2) | 71 (14.1) | ||||
| 90-Day Mortality | <0.001* | 0.27† | 0.31† | ||||||||
| Alive | 63,179 (94.2) | 3,920 (5.8) | 2,930 (74.7) | 990 (25.3) | 1,171 (36.4) | 1,554 (48.3) | 493 15.3) | ||||
| Died | 571 (98.8) | 7 (1.2) | 7 (100) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 1 (33.1) | ||||
| Unknown | 5,671 (90.1) | 625 (9.2) | 409 (65.4) | 216 (34.6) | 177 (34.6) | 263 (51.5) | 71 (13.9) | ||||
Key:
p-value remains significant when controlling for unknowns
p-value remains >0.05 when controlling for unknowns
given that only 1 patient died, there is likely no clinical significance
CPM=contralateral prophylactic mastectomy, CCC=Comprehensive Community Cancer Program, INCC= Integrated Network Cancer Center, DCIS = ductal carcinoma in situ, IC=invasive cancer, ER=estrogen receptor, PR= progesterone receptor, CD = Charlson/Deyo
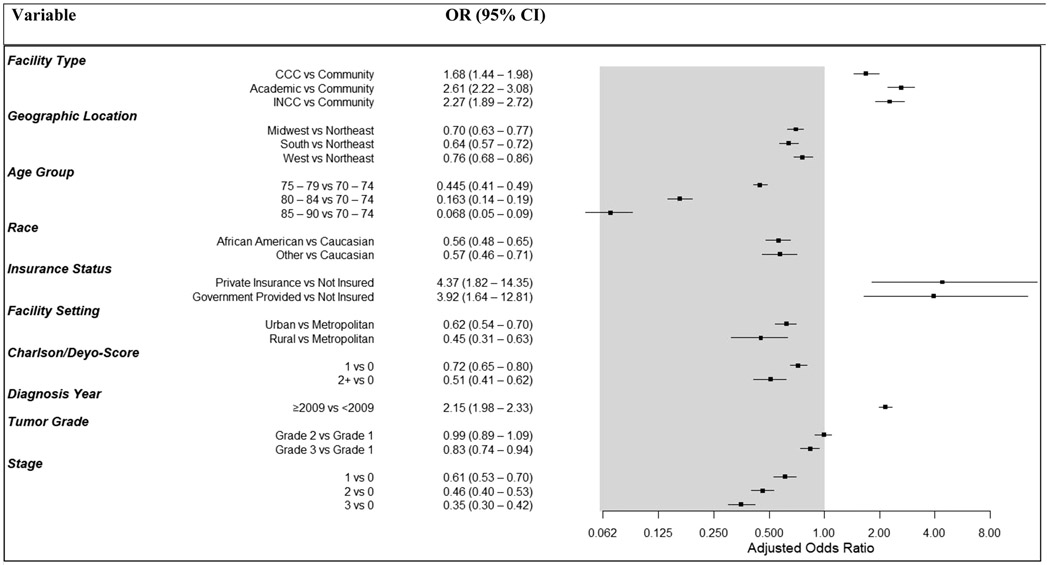
Multivariate logistic regression analysis revealed that patients treated at non-community hospital sites were more likely to undergo PMBR (p<0.001), with patients treated at an academic center most commonly receiving PMBR, with an odds ratio (OR) of 2.61 (95% CI 2.22 – 3.08) (Table 3 and Figure 3). Differences were also seen in facility location, with patients treated in the Northeast more likely to undergo PMBR (p<0.001), as well as those in metropolitan areas (p<0.001). As patient age increased, the likelihood of PMBR simultaneously decreased. Women ≥75 years of age were half as likely to undergo reconstruction compared with those 70-75 (p<0.001). Black patients were half as likely to undergo reconstruction compared to White patients, with an OR of 0.56 (95% CI 0.48 – 0.65; p<0.001). Increasing Charlson-Deyo-Score demonstrated a lower likelihood to receive PMBR (p<0.001), as did those with non-private insurance (p<0.001). Women diagnosed during the year 2009 and after were twice as likely to undergo PMBR, compared to those diagnosed prior to 2009 (OR 2.15, 95% CI 1.98 – 2.33; p<0.001). Tumor characteristics were also influential, with patients with both increasing tumor grade and stage both linked to a decrease in the rates of receiving PMBR (p<0.001).
Table 3.
Multivariate logistic regression analysis of women age ≥70 who underwent mastectomy and odds of undergoing PMBR.
| Variable | Odds Ratio (95% CI) | p-value | |
|---|---|---|---|
| Facility Type | |||
| Community | 1.0 (Reference) | ||
| CCC | 1.68 (1.44 – 1.98) | < 0.001 | |
| Academic | 2.61 (2.22 – 3.08) | < 0.001 | |
| INCC | 2.27 (1.89 – 2.72) | < 0.001 | |
| Geographic Location | |||
| Northeast | 1.0 (Reference) | ||
| Midwest | 0.70 (0.63- 0.77) | < 0.001 | |
| South | 0.64 (0.57 – 0.72) | < 0.001 | |
| West | 0.76 (0.68 – 0.86) | < 0.001 | |
| Age Group | |||
| 70 – 74 | 1.0 (Reference) | ||
| 75 – 79 | 0.445 (0.41 – 0.49) | < 0.001 | |
| 80 – 84 | 0.163 (0.14 – 0.19) | < 0.001 | |
| 85 – 90 | 0.068 (0.05 – 0.09) | < 0.001 | |
| Race | |||
| White | 1.0 (Reference) | ||
| Black | 0.56 (0.48 – 0.65) | < 0.001 | |
| Other | 0.57 (0.46 – 0.71) | < 0.001 | |
| Insurance Status | |||
| Not Insured | 1.0 (Reference) | ||
| Private Insurance | 4.37 (1.82 – 14.35) | 0.004 | |
| Government- Provided | 3.92 (1.64 – 12.81) | 0.008 | |
| Facility Setting | |||
| Metropolitan | 1.0 (Reference) | ||
| Urban | 0.62 (0.54 – 0.70) | < 0.001 | |
| Rural | 0.45 (0.31 – 0.63) | < 0.001 | |
| CD Score | |||
| 0 | 1.0 (Reference) | ||
| 1 | 0.72 (0.65 – 0.80) | < 0.001 | |
| 2+ | 0.51 (0.41 – 0.62) | < 0.001 | |
| Diagnosis Year | |||
| < 2009 | 1.0 (Reference) | ||
| > 2009 | 2.15 (1.98 – 2.33) | < 0.001 | |
| Tumor Grade | |||
| Grade 1 | 1.0 (Reference) | ||
| Grade 2 | 0.99 (0.89 – 1.09) | 0.770 | |
| Grade 3 | 0.83 (0.74 –0.94) | 0.003 | |
| Stage | |||
| 0 | 1.0 (Reference) | ||
| 1 | 0.61 (0.53 – 0.70) | < 0.001 | |
| 2 | 0.46 (0.40 – 0.53) | < 0.001 | |
| 3 | 0.35 (0.30 – 0.42) | < 0.001 | |
Key: PMBR=post-mastectomy breast reconstruction, CCC=Comprehensive Community Cancer Program, INCC= Integrated Network Cancer Center, DCIS = ductal carcinoma in situ, ER=estrogen receptor, PR= progesterone receptor, CD = Charlson/Deyo
Figure 3. Forest plot of odds of women age ≥70 undergoing PMBR based on multivariate analysis.
Key: OR = Odds Ratio, CI= Confidence Interval, CCC =Comprehensive Cancer Center, INCC= Integrated Network Cancer Center
Unilateral vs CPM with reconstruction:
Univariate analysis revealed several significant variables in who received a CPM with reconstruction (Table 2). Furthermore, multivariate analysis demonstrated that patients in the South (OR 1.7, 95% CI 1.3 - 2.1, p <0.001) and Western (OR 1.6, 95% CI 1.2 – 2.0, p<0.001) US were more likely to undergo bilateral reconstruction compared to those in other parts of the country (Table 4). Patients treated in rural areas were more likely to undergo bilateral reconstruction compared to those in metropolitan or urban areas (OR 2.0, 95% CI 1.01 – 3.8, p=0.41). As patient age increased, the odds of undergoing bilateral reconstruction decreased. White patients were more likely to receive bilateral reconstruction compared to Black patients (OR 0.5, 95% CI 0.3-0.7, p<0.001) and other races (OR 0.5, 95% CI 0.3-0.9, p=0.023). Patients diagnosed after 2009 were more likely to receive bilateral reconstruction (OR 1.7, 95% CI 1.4 – 2.0, p<0.001). Patients with tumors larger than 5cm were less likely to undergo bilateral reconstruction compared to those with smaller tumors (OR 0.5, 95% CI 0.3 – 0.8, p=0.002). Neither tumor stage nor receiving local radiation therapy remained significant on multivariate analysis. Thirty-day readmission rates were higher for those patients who underwent a CPM with reconstruction compared to those with unilateral reconstruction (5.1% vs 3.0%, p <0.001). There was no statistical difference in 30 or 90-day mortality between the two groups.
Table 4.
Multivariate logistic regression analysis of women age ≥70 who underwent PMBR and odds of undergoing CPM.
| Variable | Odds Ratio (95% CI) |
p-value | |
|---|---|---|---|
| Facility Type | |||
| Community | 1.0 (Reference) | ||
| CCC | 1.0 (0.7 – 1.4) | 0.90 | |
| Academic | 0.8 (0.5 – 1.1) | 0.13 | |
| INCC | 1.0 (0.7 – 1.5) | 0.81 | |
| Geographic Location | |||
| Northeast | 1.0 (Reference) | ||
| Midwest | 1.1 (0.85 – 1.3) | 0.58 | |
| South | 1.7 (1.3 – 2.1) | < 0.001 | |
| West | 1.6 (1.2 – 2.0) | < 0.001 | |
| Age Group | |||
| 70 – 74 | 1.0 (Reference) | ||
| 75 – 79 | 0.7 (0.6 – 0.8) | < 0.001 | |
| 80 – 84 | 0.3 (0.2 – 0.5) | < 0.001 | |
| 85 – 90 | 0.3 (0.1 – 0.6) | 0.005 | |
| Race | |||
| White | 1.0 (Reference) | ||
| Black | 0.5 (0.3 – 0.7) | < 0.001 | |
| Other | 0.5 (0.3 – 0.9) | 0.023 | |
| Facility Setting | |||
| Metropolitan | 1.0 (Reference) | ||
| Urban | 1.1 (0.9 – 1.4) | 0.419 | |
| Rural | 2.0 (1.01 – 3.8) | 0.041 | |
| Diagnosis Year | |||
| < 2009 | 1.0 (Reference) | ||
| > 2009 | 1.7 (1.4 – 2.0) | < 0.001 | |
| Tumor Size | |||
| < 2cm | 1.0 (Reference) | ||
| 2 – 5cm | 1.0 (0.8 – 1.3) | 0.90 | |
| > 5cm | 0.5 (0.3 – 0.8) | 0.002 | |
| Stage | |||
| 0 | 1.0 (Reference) | ||
| 1 | 0.9 (0.7 – 1.2) | 0.61 | |
| 2 | 0.8 (0.6 – 1.01) | 0.61 | |
| 3 | 1.1 (0.7 – 1.7) | 0.66 | |
| Radiation Therapy | |||
| No RT | 1.0 (Reference) | ||
| Received RT | 0.8 (0.6 – 1.1) | 0.11 | |
Key: CPM=contralateral prophylactic mastectomy, CCC=Comprehensive Community Cancer Program, INCC= Integrated Network Cancer Center, DCIS = ductal carcinoma in situ, ER=estrogen receptor, PR= progesterone receptor
Type of Reconstruction:
Nearly half (48.7%) of patients received implant PMBR which was most commonly performed at CCC and INCCs, while tissue PMBR was more commonly performed in academic centers (p<0.001). Patients treated in the South had the highest rate of tissue PMBR (40.9%, p<0.001) (Table 2). Women under the age of 85 most often received implant PMBR, while those ≥85 most often received tissue PMBR. Patients with private insurance or no insurance had the highest rates of combined (tissue and implant) PMBR while those with government-based insurance more often received implant PMBR (p<0.001). Before the year 2009, only 17.6% of patients had implant PMBR while after 2009, this percentage increased to 49.1% (p=0.001). While univariate analysis revealed several significant associations for type of PMBR patients underwent, multivariate logistic regression modeling did not reveal significant differences with statistical reliability. While 30-day readmission rates were 5.0% for those that had combined reconstruction, 3.5% for those with implant-based reconstruction, and 3.3% for those with tissue-based reconstruction, this was not statistically significant (p=0.34). There were no deaths at 30 days and 90-day mortality revealed only one death in the combination group, 2 deaths in the tissue group, and no deaths in the implant group (p=0.31).
Discussion:
The results of this analysis reveal that in women ≥70 years of age who undergo mastectomy for breast cancer, those patients who are non-White, non-insured, living outside the Northeast, outside of a metropolitan area, with an increasing Charlson-Deyo score, diagnosed before 2009, with grade 3 invasive tumors, are less likely to undergo PMBR. Women who are closer to the age of 70, who are White, with smaller tumors, and who live in the South and West in rural areas are more likely to undergo a CPM with reconstruction. This information demonstrates a clear difference in lower rates of PMBR amongst breast cancer patients age ≥70 with regard to race, geographic location, facility type and setting, year of diagnosis, tumor stage, and patient comorbidities. This is the first study in women age ≥70 that describes geographic differences in PMBR, the types of PMBR these women undergo, report on the perioperative readmission and mortality in this cohort.
Both increasing patient age and Black race have previously been identified through several series to be associated with lower rates of PMBR in both the immediate and delayed settings..2,9,10,13-18 These findings may represent age bias towards older individuals with the assumption that they may not want to pursue immediate reconstruction. A study utilizing the Surveillance, Epidemiology and End Results (SEER) dataset from 2000-2014 found that Black patients were overall less likely to undergo immediate PMBR compared to White patients and that this racial finding persisted when solely examining patients age ≥70.19 This racial disparity remains constant, even in the setting of controlling for insurance type.10 When accounting for other factors that may influence PMBR within this dataset, Black patients were still only half as likely to undergo PMBR compared to White patients. This disparity continues to be consistently seen amongst national datasets and within all age groups.15,20,21. Racial differences that are seen may represent a lack of access to plastic surgeons and/or social determinants of health such as education level, economic situation, social support, and transportation, which may impact the ability to complete the multiple appointments involved in pursuing reconstruction and its post-operative care. Racial differences may also attributable to racial bias towards non-White women when healthcare providers discuss surgical options.
Insurance status has long been associated with PMBR; however, compared to previous studies, a unique aspect of this cohort is that the majority of patients had government-provided insurance (88.2%).10,16,22-24 A recent multi-institutional retrospective analysis found that insurance type was significantly associated with receiving immediate PMBR.10,25 While we found that rates of PMBR were highest in women who had private insurance, this was not statistically significant when compared to those with government-based insurance.
Similar to several previous studies, our data demonstrates that patients in the Northeast continue to have the highest rate of PMBR compared to other geographic locations in the U.S.15,17,19-27 Additionally, this analysis revealed that women in the South and West undergo CPM with reconstruction more frequently when compared to those in the Northeast and Midwest. While tissue-based reconstruction rates are highest in the South, implant-based reconstruction is highest in the Midwest and West. Type of reconstruction may be related to factors such as body-mass index and tobacco use, both of which are not included within the NCDB. Patients treated in non-rural settings had higher rates of PMBR and while some studies suggest this may be an intrinsic institutional issue, other authors suggest this difference may be accounted for by the density of plastic surgeons in these areas or the geographic distance to a plastic surgeon.2,10,18,22,27
In general, PMBR rates decrease as patient age increases, which is consistent with the increase in frailty with age. A systematic review performed in 2016 examined 42 studies to specifically identify rates of PMBR and quality of life in older patients and found that PMBR rates were overall lower in the older population but with similar rates in patient-reported quality of life compared to younger cohorts.11 Although the rates of PMBR are overall lower in women ≥70 of age, a progressively increasing percentage of patients in all age groups have been opting for PMBR in recent years.2 This is most notably seen beginning in 2009 and may coincide with media coverage of celebrities choosing to undergo PMBR.2,12 As survival from breast cancer improves due to more effective therapies, patients are living longer and there is an increasing focus on quality of life, and older patients are more likely considering PMBR as part of their care plan.
Thirty-day readmissions rates, along with 30 and 90-day mortality rates, were overall fairly low for the cohort. Post-operative complication rates after immediate PMBR have previously been found to be similar when comparing younger patients to older patients.28,29 Mortality rates were statistically higher in our cohort for those patients who did not undergo reconstruction, which suggests that the decision to forego PMBR may have been multifactorial and possibly due to careful patient selection by surgeons.28 This finding is further highlighted by lower rates of PMBR in patients with higher Charlson-Deyo scores in this analysis. While patients who had a CPM with reconstruction had slightly higher readmission rates compared to those that did not, this is to be expected given the increased risk with a bilateral operation.
In women age ≥70, those with DCIS received PMBR at higher rates compared to patients with invasive disease. In DCIS, the only adjuvant therapy that is routinely recommended after unilateral mastectomy is endocrine therapy for patients with ER and/or PR positive disease. In contrast, those patients who have invasive disease after mastectomy may be offered radiation and/or systemic chemotherapy depending on final surgical pathology and tumor profile. Patients with higher grade tumors and higher stage disease are more likely to undergo adjuvant therapies; therefore, the correlation with decreasing rates of use of PMBR in those with invasive disease and increasing tumor stage and grade, is likely due to the need for further adjuvant therapies. The possibility of surgical complications causing delay in time to adjuvant treatment(s) may hinder some patients from moving forward with PMBR. Additionally, radiation therapy effects may have adverse cosmetic effects and factor into the pre-operative decision for PMBR.
Several limitations to this study exist. The retrospective nature of the database lends itself to intrinsic human errors on data collection, data entry, and therefore incompleteness. Those entries that are documented as “unknown” or “other” also have the potential to impact the data and its interpretation. Notably, some variables such as body mass index, obesity, and smoking status are factors that are recommended for surgeons to take into consideration for PMBR, but none are available through this dataset. Patient preference cannot be underestimated in the decision-making process for PMBR and may be influenced by personal patient experience, cultural influences, and psychosocial factors; however, this qualitative variable is not captured by the NCDB. Additionally, low sample sizes for several variables, such as readmission, mortality rates, rates of CPM in rural areas, and the types of reconstruction in women ≥85 years of age, calls for caution in the clinical interpretation and significance of the statistical differences that were found. However, strengths of this analysis include the large number of patients in this national database, especially those in this specific age group.
Conclusion:
This is the first study in women age ≥70 utilizing the NCDB that describes geographic differences in PMBR, the types of PMBR these women undergo, and reports on the perioperative readmission and mortality rates. Thirty and ninety-day morality rates are lower for those who undergo PMBR and suggests that surgeons are appropriately selecting patients. However, it is clear that geographic location, facility type and setting, year of diagnosis, tumor stage, and patient comorbidities all significantly impact the decision for PMBR. Healthcare providers must be mindful of implicit biases that they may hold that may hinder their recommendations for PMBR. While it is understandable that intrinsic tumor characteristics influence the role of PMBR, further research and interventions should be aimed to eliminate the differences that are seen in patient race and geographic location. Additionally, both patient preference and social determinants of health likely also have substantial impacts on the decision for PMBR and are opportunities for future investigation. With an increasingly older patient population that is being diagnosed with breast cancer, further data and both patient education and physician education is needed to optimize and standardize pre-operative decision-making algorithms for PMBR, regardless of race and geographic location of the treating facility.
Acknowledgments
Project Funding: The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), Award Number UL1TR001436. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement/Disclosures: The authors do not have any conflicts of interest and do not have any disclosures related to this work.
References
- 1).United States Cancer Statistics. Centers for Disease Control and Prevention. Accessed May 14, 2020. <https://gis.cdc.gov/Cancer/USCS/DataViz.html>
- 2).Gibreel WO, Day CN, Hoskin TL, Boughey JC, Habermann EB, Hieken TJ. Mastectomy and Immediate Breast Reconstruction for Cancer in the Elderly: A National Cancer Data Base Study. J Am Coll Surg. 2017;224(5):895–905. doi: 10.1016/j.jamcollsurg.2016.12.051 [DOI] [PubMed] [Google Scholar]
- 3).Don’t routinely use sentinel node biopsy in clinically node negative women ≥70 years of age with early stage hormone receptor positive, HER2 negative invasive breast cancer. Choosing Wisely® and Society of Surgical Oncology, July 12, 2016. Accessed May 21, 2020. < https://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/> [Google Scholar]
- 4).Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Martelli G, Miceli R, Daidone MG, Vetrella G, Cerrotta AM, Piromalli D, Agresti R. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–133. doi: 10.1245/s10434-010-1217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Girotto JA, Schreiber J, Nahabedian MY. Breast reconstruction in the elderly: preserving excellent quality of life. Ann Plast Surg. 2003;50(6):572–578. doi: 10.1097/01.SAP.0000069064.68579.19 [DOI] [PubMed] [Google Scholar]
- 7).Bowman CC, Lennox PA, Clugston PA, Courtemanche DJ. Breast reconstruction in older women: should age be an exclusion criterion?. Plast Reconstr Surg. 2006;118(1):16–22. doi: 10.1097/01.prs.0000220473.94654.a4 [DOI] [PubMed] [Google Scholar]
- 8).Maruccia M, Di Taranto G, Onesti MG. One-stage muscle-sparing breast reconstruction in elderly patients: A new tool for retaining excellent quality of life. Breast J. 2018;24(2):180–183. doi: 10.1111/tbj.12860 [DOI] [PubMed] [Google Scholar]
- 9).Butler PD, Nelson JA, Fischer JP, Wink JD, Chang B, Fosnot J, et al. Racial and age disparities persist in immediate breast reconstruction: an updated analysis of 48,564 patients from the 2005 to 2011 American College of Surgeons National Surgery Quality Improvement Program data sets. Am J Surg. 2016;212(1):96–101. doi: 10.1016/j.amjsurg.2015.08.025 [DOI] [PubMed] [Google Scholar]
- 10).Butler PD, Familusi O, Serletti JM, Fox JP. Influence of race, insurance status, and geographic access to plastic surgeons on immediate breast reconstruction rates. Am J Surg. 2018;215(6):987–994. doi: 10.1016/j.amjsurg.2017.09.037 [DOI] [PubMed] [Google Scholar]
- 11).Oh DD, Flitcroft K, Brennan ME, Spillane AJ. Patterns and outcomes of breast reconstruction in older women - A systematic review of the literature. Eur J Surg Oncol. 2016;42(5):604–615. doi: 10.1016/j.ejso.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 12).Applegate Underwent Breast Removal to Stop Cancer. ABC News, August 19, 2008. Accessed Jul 1, 2020. < https://abcnews.go.com/GMA/story?id=5606034&page=1> [Google Scholar]
- 13).Tseng JF, Kronowitz SJ, Sun CC, Perry AC, Hunt KK, Babiera GV, Newman LA, et al. The effect of ethnicity on immediate reconstruction rates after mastectomy for breast cancer. Cancer. 2004;101(7):1514–1523. doi: 10.1002/cncr.20529 [DOI] [PubMed] [Google Scholar]
- 14).Morrow M, Mujahid M, Lantz PM, Janz NK, Fagerlin A, Schwartz K, et al. Correlates of breast reconstruction: results from a population-based study. Cancer. 2005;104(11):2340–2346. doi: 10.1002/cncr.21444 [DOI] [PubMed] [Google Scholar]
- 15).Nelson JA, Nelson P, Tchou J, Serletti JM, Wu LC. The ethnic divide in breast reconstruction: a review of the current literature and directions for future research. Cancer Treat Rev. 2012;38(5):362–367. doi: 10.1016/j.ctrv.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 16).Yang RL, Newman AS, Lin IC, Reinke CE, Karakousis GC, Czerniecki BJ,et al. Trends in immediate breast reconstruction across insurance groups after enactment of breast cancer legislation. Cancer. 2013;119(13):2462–2468. doi: 10.1002/cncr.28050 [DOI] [PubMed] [Google Scholar]
- 17).Alderman AK, Wei Y, Birkmeyer JD. Use of breast reconstruction after mastectomy following the Women's Health and Cancer Rights Act. JAMA. 2006;295(4):387–388. doi: 10.1001/jama.295.4.387 [DOI] [PubMed] [Google Scholar]
- 18).In H, Jiang W, Lipsitz SR, Neville BA, Weeks JC, Greenberg CC. Variation in the utilization of reconstruction following mastectomy in elderly women. Ann Surg Oncol. 2013;20(6):1872–1879. doi: 10.1245/s10434-012-2821-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Nayyar A, Strassle PD, Reddy KG, et al. Variations in the utilization of immediate post-mastectomy breast reconstruction. Am J Surg. 2019;218(4):712–715. doi: 10.1016/j.amjsurg.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 20).Nayyar A, Strassle PD, Reddy KG, Jameison DI, Moses CG, Roughton MC, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population-based study. J Clin Oncol. 2009;27(32):5325–5330. doi: 10.1200/JCO.2009.22.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer. 2006;106(4):957–965. doi: 10.1002/cncr.21680 [DOI] [PubMed] [Google Scholar]
- 22).Roughton MC, DiEgidio P, Zhou L, Stitzenberg K, Meyer AM. Distance to a Plastic Surgeon and Type of Insurance Plan Are Independently Predictive of Postmastectomy Breast Reconstruction. Plast Reconstr Surg. 2016;138(2):203e–11e. doi: 10.1097/PRS.0000000000002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Christian CK, Niland J, Edge SB, Ottesen RA, Hughes ME, Theriault R, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Ann Surg. 2006;243(2):241–249. doi: 10.1097/01.sla.0000197738.63512.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Kruper L, Xu X, Henderson K, Bernstein L. Disparities in reconstruction rates after mastectomy for ductal carcinoma in situ (DCIS): patterns of care and factors associated with the use of breast reconstruction for DCIS compared with invasive cancer. Ann Surg Oncol. 2011;18(11):3210–3219. doi: 10.1245/s10434-011-2010-y [DOI] [PubMed] [Google Scholar]
- 25).Restrepo DJ, Boczar D, Huayllani MT, Sisti A, Gabriel E, McLaughlin SA, et al. Influence of Race, Income, Insurance, and Education on the Rate of Breast Reconstruction. Anticancer Res. 2019;39(6):2969–2973. doi: 10.21873/anticanres.13428 [DOI] [PubMed] [Google Scholar]
- 26).Chiu AS, Thomas P, Killelea BK, Horowitz N, Chagpar AB, Lannin DR. Regional variation in breast cancer surgery: Results from the National Cancer Database (NCDB). Am J Surg. 2017;214(5):907–913. doi: 10.1016/j.amjsurg.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 27).Jagsi R, Jiang J, Momoh AO, Alderman A, Giordano SH, Buchholz TA, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. doi: 10.1200/JCO.2013.52.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Mays S, Alabdulkareem H, Christos P, Simmons R, Moo TA. Surgical outcomes in women ≥70 years undergoing mastectomy with and without reconstruction for breast cancer. Am J Surg. 2017;214(5):904–906. doi: 10.1016/j.amjsurg.2017.03.041 [DOI] [PubMed] [Google Scholar]
- 29).Sada A, Day CN, Hoskin TL, Degnim AC, Habermann EB, Hieken TJ. Mastectomy and immediate breast reconstruction in the elderly: Trends and outcomes. Surgery. 2019;166(4):709–714. doi: 10.1016/j.surg.2019.05.055 [DOI] [PubMed] [Google Scholar]