Abstract
Background:
Climbing fibers (CFs) innervate Purkinje cells (PCs) with 1:1 relationship to ensure proper cerebellar function. Although CFs abnormally extend into the parallel fiber domain of PC dendrites in essential tremor (ET), the architecture of CFs in relation to PCs has yet to be investigated in detail.
Objective:
The aim of this work was to study the architecture of CFs in relation to PCs in ET.
Methods:
The number of PC somas and PC dendrites that a single CF crossed was quantified in the postmortem cerebellum of 15 ET cases and 15 control cases.
Results:
In ET, CFs crossed a greater number of PC somas and PC dendrites than in control cases, raising the possibility that there is abnormal CF wiring onto the PCs. Interestingly, the increase in CF-PC crossings positively correlated with tremor severity.
Conclusions:
Patients with ET have increased CF crossings on PC dendrites. This abnormal architectural arrangement may contribute to synchronous brain activity and tremor.
Keywords: essential tremor, cerebellum, climbing fiber, Purkinje cell, pathology
Patients with essential tremor (ET) have kinetic tremor, a rhythmic and oscillatory involuntary movement. The physiological underpinning of tremor is thought to be related to synchronized, oscillatory neuronal activity in the cerebellum and the downstream thalamocortical loop.1-3 However, the structural alterations in the ET cerebellum underlying the oscillatory neuronal activity are still not well understood.
One of the brain intrinsic oscillators is inferior olive (IO), which sends climbing fibers (CFs) to innervate Purkinje cells (PCs). CFs form synaptic connections with PCs on the thick, proximal part of PC dendrites, whereas parallel fibers (PFs) form synapses on the thin, spiny PC dendritic branchlets.4,5 We previously used vesicular glutamate transporter type 2 antibody to label CF synapses and found that the ET cerebellum has CF synapses extending into the PF territory,6-8 suggesting a disruption of cerebellar synaptic distribution. The number of CF synapses in the PF territory inversely correlates with tremor severity,6,9 indicating that this pathological feature might only partially contribute to tremor.
Another important feature of CFs and PCs is that there is a 1:1 relationship of innervation (ie, one CF innervates one PC).10 These multiple PC innervations by CFs might contribute to synchronous and oscillatory neuronal activity in the cerebellum.11 Our prior studies of CF synaptic pathology6-9,12 only allowed us to visualize synaptic puncta without tracing the CFs across multiple PCs. To further evaluate the interface between CFs and PCs, we developed a dual immunohistochemical method in thick tissue sections from postmortem human cerebellar cortex to visualize CFs and PCs, and we quantified the extent that a CF crosses multiple PC somas and dendrites. We examined whether these pathological metrics distinguished ET versus control cases and whether these metrics correlated with tremor severity.
Subjects and Methods
Postmortem Human Brains
We obtained ET brains from the Essential Tremor Centralized Brain Repository, a joint effort between investigators from Yale and Columbia Universities.13 The diagnosis of ET was previously described.14 A total tremor score (range, 0–36) was assigned to each case based on videotaped neurological examination, although data were incomplete in one.9 No ET cases had a history of heavy ethanol use as previously defined.15 We excluded ET cases who had undergone deep brain stimulation, which can possibly change CF synaptic pathology.12 Control cases were followed at the Alzheimer’s Disease Research Center at Columbia University. During life, all study subjects signed informed consent approved by their respective University Ethics Boards. We performed a power analysis based on data on CF synaptic pathology.6 Assuming two-sided testing and alpha = 0.05, a sample size of 11 per group would be powered at 90% to detect differences of the magnitude previously described.6 Assuming there would be some brains with inadequate staining, we initially selected the available 20 control cases and frequency-matched these to 20 ET cases based on age. Five ET cases and five control cases had inadequate staining, despite at least three attempts, and these were excluded, yielding a final sample of 15 per group in the initial cohort. In addition, we created a small replicate cohort by staining five ET cases and five control cases, for which three ET cases and three control cases were successfully stained.
Cerebellar Immunohistochemistry
We examined the anterior lobe of the lateral hemisphere of the cerebellum in both ET and control cases, which corresponds to the motor cerebellum, as previously described.6 CFs express cocaine and amphetamine-regulated transcript (CART),16 whereas PCs express calbindin. We performed dual immunohistochemistry of CART and calbindin in 100-μm-thick vibratome sections. Brain sections were incubated with Proteinase K (#3115879001; Millipore Sigma, Burlington, MA) at 37°C, followed by 1% hydrogen peroxide (#H1009; Millipore Sigma, Burlington, MA). Brain sections were subsequently treated with suppression block of 10% normal goat serum and 1% Triton (#005-000-121; Jackson ImmunoResearch, West Grove, PA) at room temperature for 1 hour. Sections were first labeled with mouse anti-calbindin (1:250, #300; Swant, Marly, Switzerland) at 4°C overnight, followed by alkaline phosphatase-conjugated anti-mouse IgG (1:200, #1:111-055-146; Jackson ImmunoResearch, West Grove, PA) for 2 hours, and staining was visualized with ImmPACT™ Vector® Red (#SK-5105; Vector Lab, Burlingame, CA). Sections were then incubated with rabbit anti-CART antibody (1:2500, #H-003-62; Phoenix Pharmaceuticals, Burlingame, CA) at 4°C overnight, followed by biotinylated secondary antibody (1:200, #BA-1000; Vector Lab, Burlingame, CA) at room temperature for 2 hours, and staining was visualized with 3, 3′-diaminobezidine precipitation (#3468; Dako, Carpinteria, CA).
CF–PC Crossing Assessment
We assessed the CF–PC crossings by tracing individual CART-labeled CFs in the molecular layer in three sections for each subject. First, we quantified the number of visualized PC dendrites that a single CF crosses. Second, we measured the number of PC somas that a single CF crosses by tracing to the origin the PC dendrites. Because the longer the CF length, the more likely a CF will cross more PC dendrites or PC somas, we adjusted the outcome measures by dividing the number of PC dendrites or PC somas that a CF crosses by the CF length (number of crossings per millimeter). We randomly selected and quantified five CFs in each case and used the average value. The rater (Y.-C.W.) was blinded to the diagnosis when quantifying the outcome measures.
Statistical Analyses
Data were analyzed in Prism (v8.3.0). Our primary analyses were the number of PC dendrites and the number of PC somas that a CF crossed. We further assessed the correlation between these measures and tremor severity. Kolmogorov–Smirnov test was used to determine the normality of continuous variables. Student independent samples t test and Mann–Whitney U tests were used to compare groups of normally and nonnormally distributed variables, respectively. We used Pearson correlation coefficient and Spearman rank correlation coefficient to study the association between normally distributed variables and nonnormally distributed variables, respectively. We performed linear regression analyses to assess the number of PC dendrites or PC somas that a CF crossed after adjusting for age, sex, postmortem interval (PMI), and PC counts.
Results
ET and control cases were similar in age and sex. ET cases had a longer PMI, lower PC counts, and a higher percentage of CFs extending into the PF territory (Table 1), consistent with previous studies.6-9,17
TABLE 1.
Demographics and pathological features of patients with ET and control cases
| Variables | Controls | ET Cases | P Value |
|---|---|---|---|
| n | 15 | 15 | |
| Age at death (yr) | 83.9 ± 10.0 | 87.5 ± 5.2 | 0.750a |
| Median = 87.0 | Median = 88.0 | ||
| Age of tremor onset (yr) | NA | 38.5 ± 23.9 | |
| Duration of tremor (yr) | NA | 48.7 ± 25.1 | |
| Sex | 0.273b | ||
| Men | 9 (60.0%) | 6 (40.0%) | |
| Women | 6 (40.0%) | 9 (60.0%) | |
| Postmortem intervals (hr) | 14.1 ± 9.1 | 29.9 ± 14.5 | <0.001a |
| Median = 10.96 | Median = 31.4 | ||
| Braak AD stagec | 0.248b | ||
| 0 | 2 (14.3%) | 0 (0.0%) | |
| 1 | 1 (7.1%) | 0 (0.0%) | |
| 2 | 5 (35.7%) | 4 (26.7%) | |
| 3 | 4 (28.6%) | 5 (33.3%) | |
| 4 | 1 (7.1%) | 5 (33.3%) | |
| 5 | 1 (7.1%) | 0 (0.0%) | |
| 6 | 0 (0.0%) | 1 (7.1%) | |
| CERAD scores | 0.736b | ||
| 0 | 7 (46.7%) | 9 (60.0%) | |
| A | 4 (26.7%) | 3 (20.0%) | |
| B | 4 (26.7%) | 2 (13.3%) | |
| C | 0 (0.0%) | 1 (6.7%) | |
| Braak PD stage | 0.0 ± 0.0 | 0.1 ± 0.5d | 1.000e |
| PC counts | 11.9 ± 1.7 | 8.8 ± 1.5 | <0.001e |
| Torpedo counts | 6.9 ± 7.0 | 15.2 ± 12.9 | 0.041e |
| Percentage of CFs extending into PF territory | 17.5 ± 9.0 | 30.0 ± 6.1 | <0.001e |
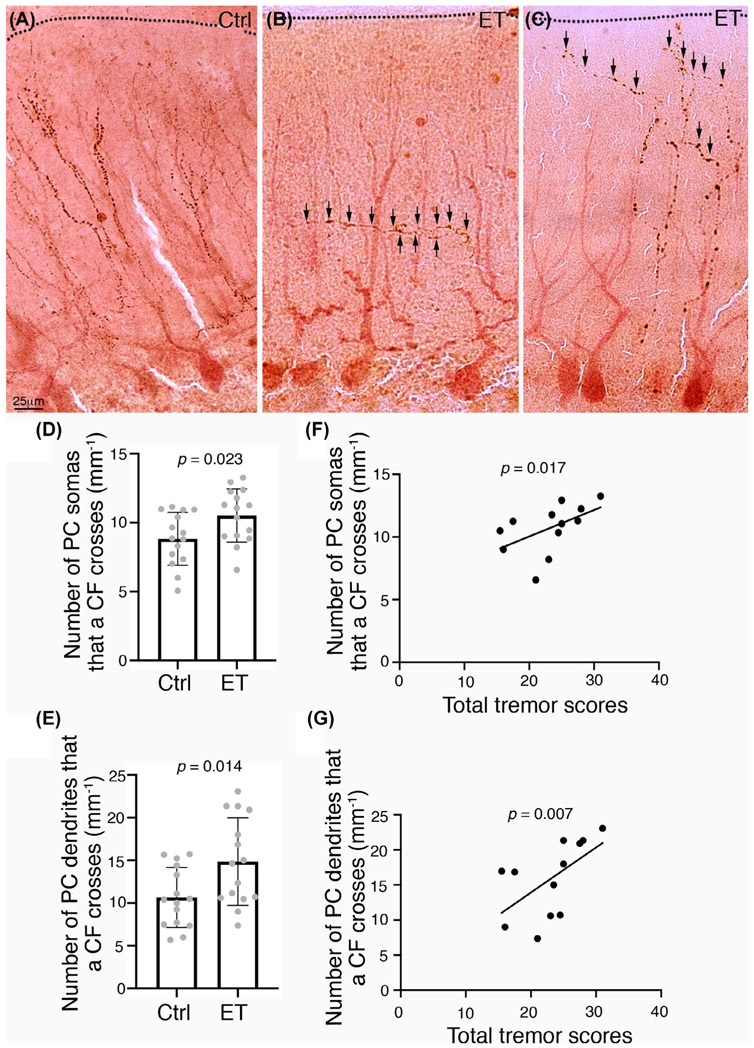
| Number of PC somas that a single CF crosses (mm−1) | 8.8 ± 1.9 | 10.5 ± 1.9 | 0.023e |
| Number of PC dendrites that a single CF crosses (mm−1) | 10.7 ± 3.5 | 14.9 ± 5.1 | 0.014e |
Values represent mean ± standard deviation or number (percentage), and for variables with a nonnormal distribution, the median is reported as well. Postmortem intervals indicate the time from death to the time of brain placed in −80°C. Mean number of PC counts per 100× microscopic field, among 15 sampled fields in a section, and normalized to the PC layer length. The number of PC axonal torpedoes was quantified throughout each cerebellar section and normalized to the PC layer length.
Abbreviations: ET, essential tremor; NA, not applicable; AD, Alzheimer’s disease; CERAD, consortium to establish a registry for Alzheimer’s disease; PD, Parkinson’s disease; PC, Purkinje cell; CF, climbing fiber; PF, parallel fiber.
Independent samples Mann–Whitney U test.
Fisher’s exact test.
One Braak AD stage value in control group is a missing value.
One ET case has incidental Lewy body with Braak PD stage of 2.
Independent samples t test.
In control cases, the number of PC somas or PC dendrites that a CF crossed did not correlate with PMI (P = 0.149 and 0.504, respectively), indicating that PMI is not a significant confounding factor.
In comparison with control cases, a CF in ET cases crossed 19% more PC somas (8.8 ± 1.9 vs 10.5 ± 1.9; P = 0.023) and crossed 39% more PC dendrites (10.7 ± 3.5 vs 14.9 ± 5.1; P = 0.014) (Table 1 and Fig. 1A-E). Even after removing the lowest control value for the number of PC somas that a CF crossed, the ET versus control difference still remained significant (P = 0.044). Because ET cases also have modest PC loss, we further performed linear regression analysis to adjust for PC counts, in addition to age, sex, and PMI. Compared with control cases, ET cases still had a significantly higher number of PC somas or PC dendrites that a CF crossed (P = 0.037 and P = 0.003, respectively).
FIG. 1.
Climbing fibers (CFs) pathology in the essential tremor (ET) cerebellar cortex. (A–C) Representative images of CFs, labeled by cocaine and amphetamine-regulated transcript (CART) (brown), and Purkinje cells (PCs), labeled by calbindin (red), in a control (A) and three ET cases (B, C). Arrows, CART-labeled CF crossings. Dashed lines, the pial surface of the cerebellar cortex. Scale bar: 25 μm. (D, E) ET cases have CFs crossing more PC somas and PC dendrites in comparison with control cases. (F, G) The number of PC somas or the number of PC dendrites crossed by a CF correlates with tremor severity. Error bars represent the standard deviation.
We assessed the association between tremor severity and increased CF-PC crossings in ET; both measures correlated with total tremor score (Pearson r = 0.623, P = 0.017 and r = 0.684, P = 0.007, respectively) (Fig. 1F,G). Because patients with ET may have asymmetric tremor, we additionally assessed correlations between the number of PC somas or PC dendrites that a CF crosses with the ipsilateral tremor scores, and the results remained similar (Pearson r = 0.546, P = 0.044 and r = 0.635, P = 0.015, respectively).
We studied a small replicate cohort of three ET cases and three control cases (age: 87.3 ± 4.5 vs 87.0 ± 10.6 years, respectively). In this cohort, in comparison with control cases, a CF in ET cases crossed 13% more PC somas (11.6 ± 3.6 in ET cases vs 10.3 ± 1.1 in control cases) and crossed 54% more PC dendrites (17.1 ± 9.1 in ET cases vs 11.1 ± 0.9 in control cases). Hence both the direction of the difference (ET cases > controls) and the magnitude of difference (13%/54% in replicate cohort and 19%/39% in initial cohort) remained similar. Statistical testing was not feasible, though, given the small size of the replicate cohort. The degree of differences is similar to the original cohort.
We further assessed the association between CF crossings with other PC pathological features previously identified in ET. The number of PC somas or PC dendrites that a CF crossed in ET cases did not correlate with either PC torpedo counts (Pearson r = −0.395, P = 0.182 and r = −0.359, P = 0.229, respectively) or CF synapses in the PF synaptic territory (Pearson r = 0.155, P = 0.630 and r = 0.146, P = 0.651, respectively).
Discussion
We found that CFs in ET cases extend laterally across more PC somas and PC dendrites than in control cases. Our observation is even more remarkable given that ET cases have lower PC counts17 and less prominent PC dendritic arborization than control cases.14 Interestingly, these pathological features robustly and positively correlated with tremor severity, suggesting a possible structural alteration contributing to tremor.
The pathophysiological implications of our findings are not certain and deserve further study. There are several possibilities. One is that the increased crossings are not accompanied by increased synaptic contacts between CFs and PCs, although this is unlikely due to the close physiological relationship between the two cell types. The second possibility is that this represents a rewiring, with increased synaptic contacts between CFs and PCs. Indeed, increases in crossings are seen in certain pathological states in rodents in which there are multiple PC innervations by a CF.4,11,18
Several additional aspects of a potential rewiring are now discussed. First, this could be a primary event in ET pathophysiology. The IO is the source of CFs and is a robust oscillation generator. Thus, multiple CF–PC innervations could enhance the coupling between IO and PCs to set up oscillatory activity in the cerebellum.11,18 In addition, ET cases also have CFs extending to the distal part of PC dendrites,2,6-9 further increasing the influence of IO on PC physiology. It is possible that the combination of these structural alterations can entrain PC activity into a more synchronous and rhythmic mode, leading to tremor.
Alternatively, the potential rewiring could be a secondary event. Thus, in the setting of PC and/or IO damage, CFs could have compensatory sprouting to innervate multiple PCs.19-21 We have previously demonstrated a loss of CF synaptic density on proximal PC dendrites in ET.6-8 If CF–PC synapses are focally lost due to PC degeneration, adjacent intact CFs develop collateral branches to reinnervate PC dendrites, thereby disturbing the normal 1:1 ratio of CF to PC innveration.19-21 Therefore, it is possible that PC degeneration in ET, which has been observed in several studies,17,22 leads to multiple PC innervations by CFs and thereby potentially alters the cerebellar physiology linked to tremor.
This study had limitations. First, CART staining only partially visualized CFs; therefore, we were not able to trace the entire course of CFs. Nonetheless, we performed blinded analysis to eliminate the assessors’ bias as much as possible. Second, this dual immunohistochemistry method is technically challenging, with ~25% failure rate in sample staining. Nonetheless, the significant benefit of this technique is an enhanced ability to visualize CF trajectories in thick tissue sections. Third, we do not know whether CFs that cross multiple PCs actually form synaptic connections. Triple immunofluorescence of calbindin, vesicular glutamate transporter type 2, and CART might help us to understand this issue; however, this has been proved to be technically difficult and will be an important future direction. Fourth, our study focused only on the cerebellar hemisphere. Future studies should investigate the CF crossing pathology in additional regions of the cerebellum, such as vermis and paravermis, to elucidate any regional differences. Fifth, a CF crossing multiple PC somas or PC dendrites is best visualized in the coronal sections in rodent studies,4,5 whereas sagittal sections, as in our study, are likely to underestimate the amount PC somas or PC dendrites crossed by a CF, leading to a potential bias toward the null hypothesis. Nonetheless, we still detected the case–control differences. Future studies on coronal sections might yield even more robust case–control differences.23 Finally, the sample size of study remains modest; a larger study will be important to explore disease heterogeneity in ET.
In conclusion, we identified a novel CF pathology in the ET cerebellum, with increased CF–PC crossings that raise the possibility of multiple PC innervations by CFs. This represents an additional change in cerebellar neuronal morphology that is likely linked to changes in cerebellar circuitry, contributing to tremor.
Supplementary Material
Acknowledgments:
E.D.L. has received research support from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) #R01 NS094607 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University). P.L.F. has received funding from the National Institutes of Health, NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). M.-K.P. has received funding from the Ministry of Science and Technology in Taiwan [grants MOST 104-2314-B-002-076-MY3 (principal investigator), MOST 107-2321-B-002-020 (principal investigator), MOST 108-2321-B-002-011 (principal investigator), and MOST 108-2321-B-002-059-MY2 (principal investigator)], National Taiwan University Hospital (grants 105-N3227 and 108-039), and Yin-Lin branch of the hospital (grant NTUHYL104.N007). S.-H.K. has received funding from the National Institutes of Health (NINDS #R01 NS104423 (principal investigator), NINDS #R01 NS118179 (principal investigator), NINDS #R03 NS114871 (principal investigator), the Louis V. Gerstner Jr. Scholar Award, National Ataxia Foundation, Parkinson’s Foundation, International Essential Tremor Foundation, and Brain Research Foundation.
Footnotes
Relevant conflicts of interest/Financial disclosures: Nothing to report.
Financial Disclosures of All Authors (for the Preceding 12 Months)
Dr. Kuo has served as a site PI for clinical trials of Biohaven and Sage Therapeutics, and have served as a scientific advisor for unique and Praxis.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord 2009;24:1629–1635. [DOI] [PubMed] [Google Scholar]
- 2.Pan MK, Li YS, Wong SB, et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med 2020;12:eaay1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filip P, Lungu OV, Manto MU, Bareš M. Linking essential tremor to the cerebellum: physiological evidence. Cerebellum 2016;15:774–780. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa R, Miyazaki T, Kano M, et al. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J Neurosci 2002;22:8487–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki T, Yamasaki M, Takeuchi T, Sakimura K, Mishina M, Watanabe M. Ablation of glutamate receptor GluRδ2 in adult Purkinje cells causes multiple innervation of climbing fibers by inducing aberrant invasion to parallel fiber innervation territory. J Neurosci 2010;30:15196–15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137:3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo SH, Lin CY, Wang J, et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 2017;133:121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee D, Gan SR, Faust PL, Louis ED, Kuo SH. Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord 2018;51:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis RJ, Lin CY, Faust PL, Koeppen AH, Kuo SH. Climbing fiber synaptic changes correlate with clinical features in essential tremor. Neurology 2015;84:2284–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kano M, Watanabe T, Uesaka N, Watanabe M. Multiple phases of climbing fiber synapse elimination in the developing cerebellum. Cerebellum 2018;17:722–734. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Katoh A, Ohtsuki G, Mishina M, Hirano T. Oscillating Purkinje neuron activity causing involuntary eye movement in a mutant mouse deficient in the glutamate receptor delta2 subunit. J Neurosci 2004;24:2440–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo SH, Lin CY, Wang J, et al. Deep brain stimulation and climbing fiber synaptic pathology in essential tremor. Ann Neurol 2016;80:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis ED, Lee M, Babij R, Ma K, Cortés E, Vonsattel JP, Faust PL. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014;137:3142–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 2013;136:3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res 2001;25:228–235. [PubMed] [Google Scholar]
- 16.Reeber SL, Sillitoe RV. Patterned expression of a cocaine- and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. J Comp Neurol 2011;519:1781–1796. [DOI] [PubMed] [Google Scholar]
- 17.Choe M, Cortés E, Vonsattel JP, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 2016;31:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good JM, Mahoney M, Miyazaki T, et al. Maturation of cerebellar Purkinje cell population activity during postnatal refinement of climbing fiber network. Cell Rep 2017;21:2066–2073. [DOI] [PubMed] [Google Scholar]
- 19.Rossi F, Wiklund L, van der Want JJ, Strata P. Reinnervation of cerebellar Purkinje cells by climbing fibres surviving a subtotal lesion of the inferior olive in the adult rat. I. Development of new collateral branches and terminal plexuses. Comp Neurol 1991;308:513–535. [DOI] [PubMed] [Google Scholar]
- 20.Strata P, Tempia F, Zagrebelsky M, Rossi F. Reciprocal trophic interactions between climbing fibres and Purkinje cells in the rat cerebellum. Prog Brain Res 1997;114:263–282. [DOI] [PubMed] [Google Scholar]
- 21.Rossi F, van der Want JJ, Wiklund L, Strata P. Reinnervation of cerebellar Purkinje cells by climbing fibres surviving a subtotal lesion of the inferior olive in the adult rat. II. Synaptic organization on reinnervated Purkinje cells. J Comp Neurol 1991;308:536–554. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–3307. [DOI] [PubMed] [Google Scholar]
- 23.Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure and functions. Boca Raton: CRC Press, Inc; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.