Abstract
Cutaneous T-cell lymphomas constitute a rare category of non-Hodgkin lymphomas, which may involve the heart in the timeline of their natural course as an infrequent picture with a poor prognosis. Syncope, either due to outflow obstruction or conduction block, is also an uncommon presentation of cardiac metastasis. We herein describe a 35-year-old man, who presented with weight loss, dyspnea of 6 months’ duration, an indolent skin ulcer in the left flank, lower limb deep vein thrombosis (DVT), and recurrent syncope. He underwent implantation of a permanent pacemaker due to a complete heart block and received anticoagulants for the DVT. Skin biopsy demonstrated a T-cell lymphoma. The syncopal episodes ceased thereafter. Echocardiography and computed tomography scan revealed cardiac metastasis, which responded to systemic chemotherapy. In the first follow-up visit after 3 months, he was still pacemaker-dependent. However, the DVT was partially resolved, and the symptoms had disappeared.
Key Words: Lymphoma, T-cell, Syncope, Neoplasm metastasis, Atrioventricular block, Consolidation chemotherapy, Echocardiography
Introduction
T-cell lymphomas constitute an uncommon type of hematologic malignancy involving the heart. Cardiac tumors, including those with lymphoblastic origins, usually have an insidious course and nonspecific manifestations before a definite diagnosis. Patients may present with fatigue, chest pains, progressive dyspnea, heart failure, pericardial effusion, and syncope. Cutaneous T-cell lymphomas, including mycosis fungoides and Sezary syndrome, comprise a rare subclass of T-cell lymphomas. Sezary syndrome itself is also extremely rare and is characterized by hematogenous metastasis. Alongside these findings, the cardiac metastasis of cutaneous T-cell lymphomas, especially as an early manifestation, is an additional atypical feature of the disease.1-4
Given this background, we herein present a case of a metastatic cutaneous T-cell lymphoma involving cardiac tissues with an interesting presentation. In this case, a major clue for cardiac metastasis was recurrent syncope, accompanied by a complete atrioventricular (AV) block, which necessitated further evaluations.
Case Report
A 35-year-old man, who was a heavy cigarette smoker and an ex-opium addict, presented with malaise, left lower limb edema, and a low-grade fever to the emergency department of Imam Khomeini Hospital. The patient had a history of beta-thalassemia minor. Additionally, he mentioned a long-standing history of weight loss, night sweating, and loss of appetite of 6 months’ duration, accompanied by the indolent growth of an ill-demarcated cutaneous ulcer in his left flank within the recent 3 months. Furthermore, he cited frequent episodes of syncope commencing 2 weeks prior to admission in both sitting and upright positions lasting for less than 1 minute. All the episodes were resolved spontaneously. Dyspnea on exertion (functional class 2) and moderately reduced exercise tolerance were also prominent during this period. There were no obvious clustered swollen lymph nodes in related lymphatic regions. A skin biopsy was taken.
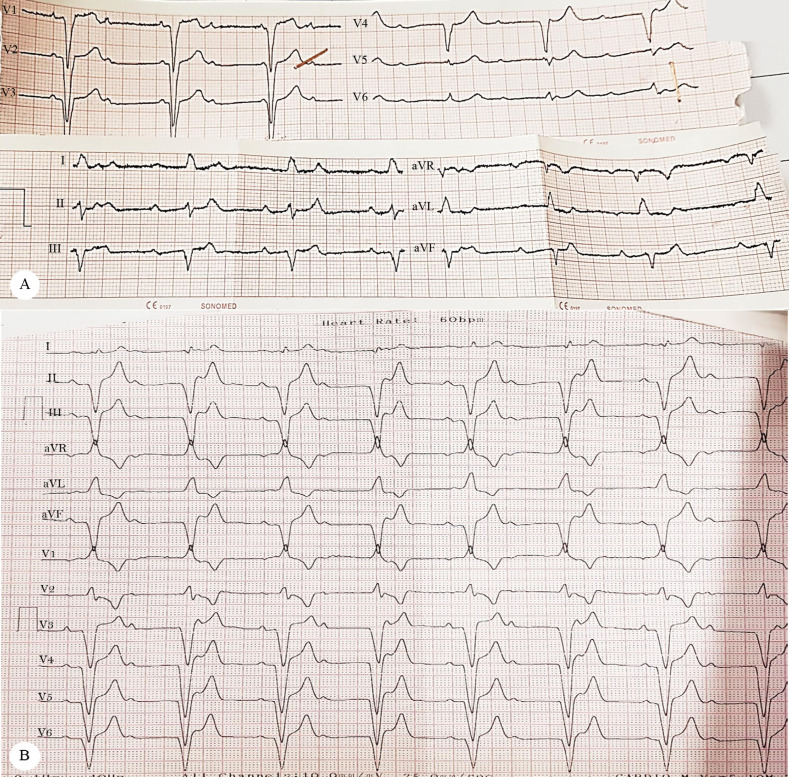
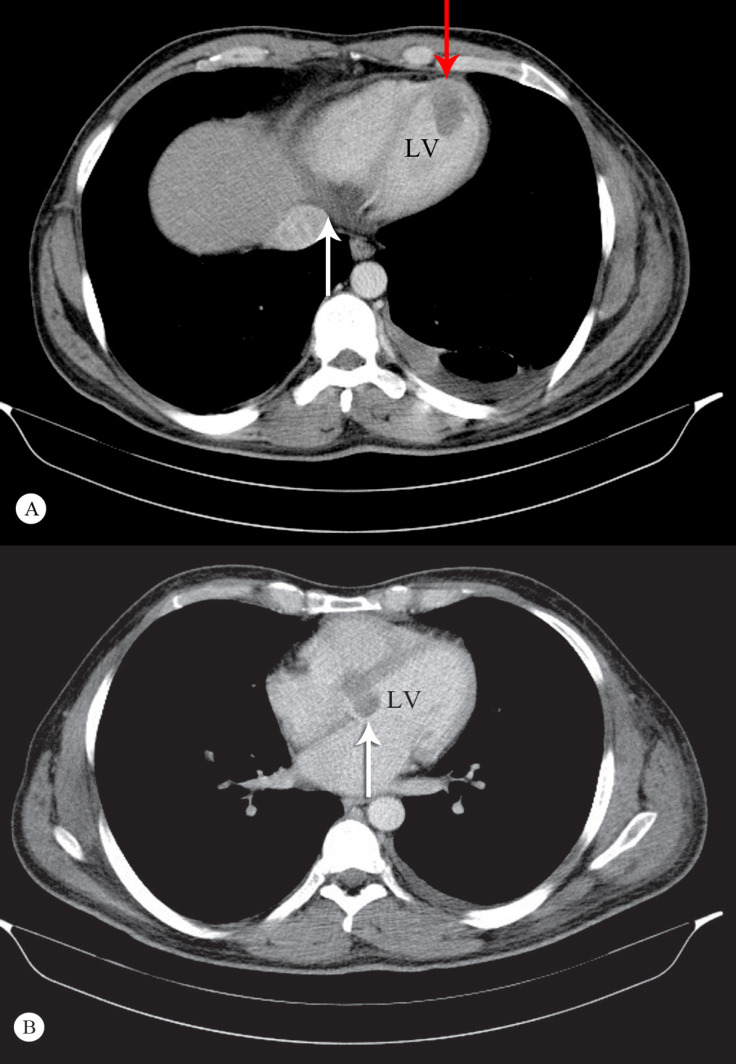
On the patient’s arrival at the emergency department, he had a blood pressure of 110/70 mmHg, a respiratory rate of 26 per minute, a pulse rate of 52 bpm, and a temperature of 39°C. In the emergency department, color Doppler ultrasonography confirmed the presence of acute deep vein thrombosis (DVT) in the left lower limb. Treatment with heparin, followed by warfarin, was planned to achieve appropriate anticoagulation. Chest computed tomography (CT) angiography was also done for further evaluations of pulmonary thromboembolism, which was negative. On the other hand, some filling defects were revealed in the left ventricle (LV) and the left ventricular outflow tract (LVOT) in the subvalvular region, in favor of clot or vegetation. Therefore, echocardiography was a reasonable imaging modality for complementary diagnostic steps. Electrocardiographic strips were obtained; they showed a complete heart block, associated with bradycardia (heart rate = 52 bpm) thereafter. Figure 1A and Figure 1B display the strips recorded in the emergency department and during the index admission. Figure 2 depicts the sectional CT scan showing the cardiac masses.
Figure 1.
Images of the electrocardiographic (ECG) strips obtained in the patient’s first visit (Figure 1A) to the emergency department, as well as the first follow-up visit (Figure 1B). A) ECG tracing, recorded in the patient’s first visit to the emergency department, shows a complete atrioventricular block, accompanied by left axis deviation, left bundle branch block, and bradycardia. B) ECG strip shows the persistence of the advanced atrioventricular block during the analysis of the pacemaker in the follow-up visit (after 3 months).
Figure 2.
A) Cross-sectional image of the metastatic involvement of the heart in computed tomography (CT) scanning. Note the infiltration of the basal posterior (and inferior) walls of the left and right ventricular septa (white arrow), as well as the apical protrusion of the tumor (red arrow). B) Cross-sectional CT scan of the heart and the mediastinum at the level of the atria and the main bronchi. Note that the base of the interventricular septum and the mitral annulus is involved (white arrow).
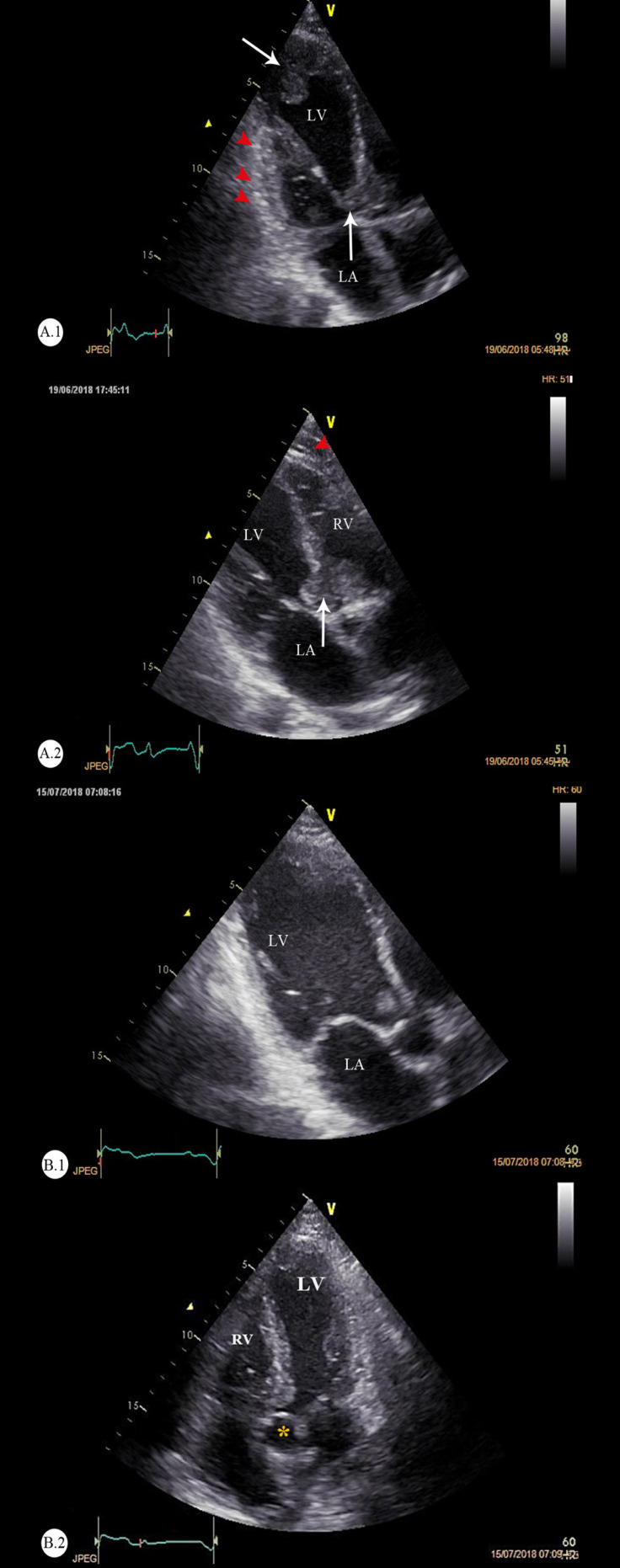
Further evaluations via transthoracic echocardiography demonstrated a large echo-density in the basal portion of the interventricular septum, suggestive of a tumoral mass (2.2 × 2.6 cm). The myocardial tissue appeared tumoral predominantly at the base of the anterior, anteroseptal, and apicolateral walls, as well as the LVOT. Another large (3.6 × 3.5 cm), oval-shaped, and mobile echo-density attached to the LV apex was seen, suggestive of a tumoral mass. Additional findings indicated the acceptable size of both ventricles with mild LV systolic dysfunction. No significant valvular heart disease was noted. The patient had mild pulmonary hypertension (pulmonary artery pressure = 35-40 mmHg). No pericardial effusion was detected. The echocardiographic images of the cardiac tumors are demonstrated in Figure 3, and Figure 4 depicts the cutaneous lesion, as well as the pathologic structure of the tumors.
Figure 3.
Echocardiographic images of the cardiac T-cell lymphoma at baseline (A) and after 3 months (B)
A) Modified apical 3-chamber views: The vertical arrows (white-colored) point to the heavy bulk of the tumor in the basal septum and the LVOT (right side), and the red arrowheads point to the myocardial infiltrations in the posterior and anterolateral walls of the LV. B) The follow-up echocardiography after 3 months. The apical 3-chamber and apical 5-chamber views are illustrated. These figures demonstrate that the cardiac metastases have regressed in response to intensive chemotherapy.
LV, Left ventricle; RV, Right ventricle; LA, Left atrium; LVOT, Left ventricular outflow tract
*Yellow asterisk denotes the aortic root.
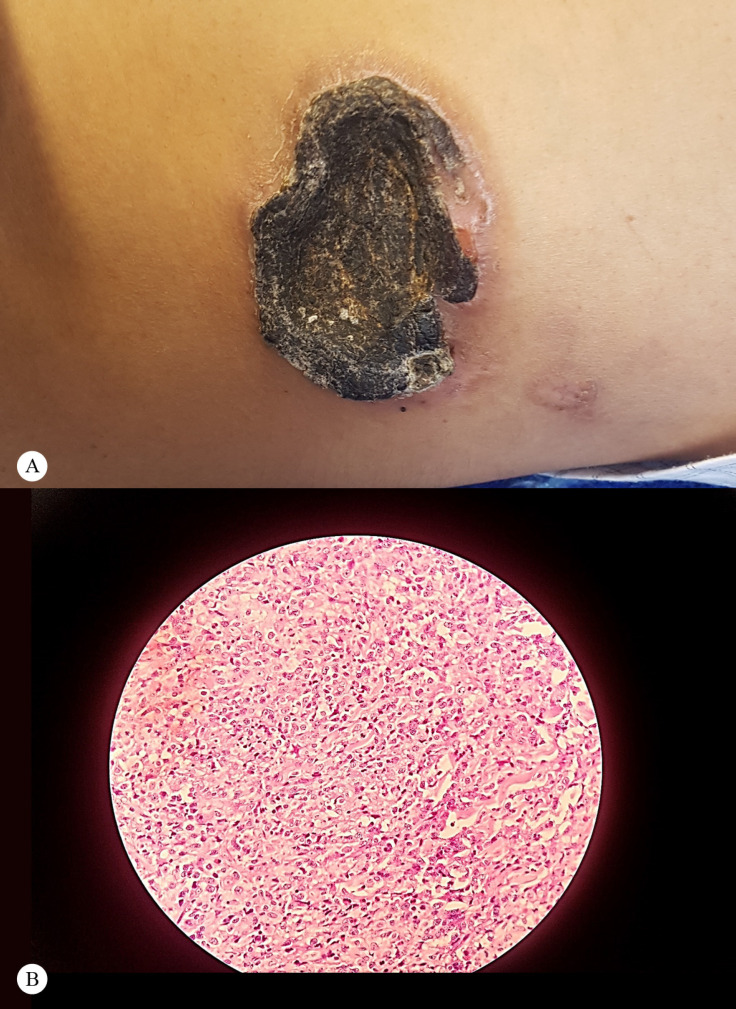
Figure 4.
Gross features and microscopic images of the cutaneous ulcer
A) Large crater-like skin ulcer in the left flank with irregular borders
B) Microscopic image of the biopsy of the cutaneous lesion with 40× magnification
Hematoxylin and eosin (H & E) staining shows small round blue cells with a high nucleus-to-cytoplasm ratio, necrosis, and interstitium, in favor of a lymphoblastic tissue.
The skin biopsy revealed a cutaneous T-cell lymphoma. Immunohistochemical assays determined the subtypes of the malignant T-cells as CD3+, CD4+ (CD: cluster determinant), and Ki67: 70-75%. These markers were compatible with a T-cell origin and a non-Hodgkin lymphoma-derived cell line, respectively. Malignant T-cells were also obtained from peripheral blood samples.
The complete AV block, diagnosed on admission accompanied by an underlying cutaneous T-cell lymphoma associated with cardiac masses, suggested the diagnosis of the metastatic (secondary) involvement of the heart. The patient underwent the standard chemotherapy regimen, which resulted in the partial regression and shrinkage of the cardiac tumors. The chemotherapy protocol was comprised of cyclophosphamide, etoposide, doxorubicin, vincristine, and prednisolone, in conjunction with the administration of granulocyte colony-stimulating factor (G-CSF). The premedication regimen contained ranitidine or a proton pump inhibitor, granisetron as an antiemetic, and fungal/bacterial infection prophylaxis, as required. The chemotherapy regimen caused the regression of the cardiac tumors and the improvement of the cutaneous ulcer. The DVT was resolved, and the patient was encouraged to use anticoagulant treatment. Syncopal episodes did not occur again, but he was still pacemaker-dependent after 3 months. Despite the intensive treatment, however, the complete AV block persisted, warranting the implantation of a permanent pacemaker. A permanent pacemaker was implanted to support effective heart rhythms. Follow-up was done through regular outpatient visits.
Discussion
A review of a 20-year (1972–1991) autopsy series demonstrated that the incidence rate of primary and secondary cardiac tumors was 0.056% and 1.23%, respectively. Although the majority of metastases to the heart originate from the lung, breast carcinomas, soft-tissue sarcomas, renal malignancies, and melanomas, a small proportion of secondary tumors arise from lymphoblastic disorders.1 Cardiac involvement constitutes between 8.7% and 30% of autopsy cases in patients with underlying leukemia or lymphomas. Another literature review showed that the invasion of non-Hodgkin lymphomas to the heart occurs in 18% of patients during a median of 20 months. The anatomic site, tumor size, proliferation rate, extent of invasion, and sensitivity to chemo-radiation affect the clinical picture. Wide varieties of clinical behaviors and heterogeneous manifestations exist that range from being asymptomatic to severely hemodynamically unstable. Cardiac lymphomas may lead to the mechanical or functional obstruction of the outflow tracts, valvular dysfunction, arrhythmias, AV blocks, pericardial effusions, tamponades, the fragmentation and embolization of masses, endocarditis, heart failure, and myocardial infarctions. Nonetheless, a diagnosis of cardiac metastasis is often undermined due to the subclinical progression of lymphomas or indolent courses, in addition to nonspecific manifestations.2
It is exceedingly rare for cutaneous T-cell lymphomas, including mycosis fungoides and Sezary syndrome, to involve the heart as the main extra-cutaneous manifestation.3 A greater proportion of patients with cutaneous T-cell lymphomas have mycosis fungoides (72%) than Sezary syndrome (2.5%). The latter is also called “the leukemic variant”, in which neoplastic CD4+ T lymphocytes tend to have a hematogenous spread, in tandem with infiltrations in the lymphatic tissues and skin.4 Nevertheless, both mycosis fungoides and Sezary syndrome are extremely rare, with an annual incidence rate of 3 to 4 new cases per million.5 The early involvement of the heart in cutaneous T-cell lymphomas denotes a supernumerary, rare finding, which is in contrast to what is observed more frequently in B-cell lymphomas.
The point of interest in our case is the rare association of an indolent cutaneous T-cell lymphoma and cardiac involvement. What is more, the patient’s internal organs, other than the heart, including the liver, spleen, and particularly systemic lymphatics were relatively spared. In addition, syncope was the prominent initial manifestation of cardiovascular involvement, which is an uncommon prodromal feature that is expected to occur late in the course of a cardiac tumor.3
The mechanism of syncope may be either the LVOT obstruction or a transient high-degree AV block. Moreover, the involvement of the pericardium and the appearance of myocardial nodular infiltrates are more frequent than conduction disorders and /or large masses protruding within the chambers.6 We found that one of the ventricular masses originated from the base of the interventricular septum, which is adjacent to the anatomic location of the AV node, supporting the evidence of a direct tumor extension to the conduction pathways. The mass lesions appeared as partially hypoattenuated and heterogeneous structures, while the filling defects were seen in the contrast phase. Myocardial infiltrations were evident at the base of the anterior, anteroseptal, and apicolateral walls, as well as in the LVOT. Multiple infiltrative extensions and wide-based luminal protrusions of the lymphoma were confirmed via CT scanning thereafter. The aforementioned mass-like protrusions exhibited substantial regression in our follow-up echocardiography. This considerable tumor shrinkage occurred after a course of consolidation chemotherapy in a period of 3 to 4 months, while the AV block was still persistent. Several factors may cause AV blocks in patients with primary or secondary cardiac lymphomas; they include direct invasions; infiltrations of the surrounding tissues leading to compression, ischemia, inflammation, or edema; fibrosis; immune responses; and alterations of the electrical characteristics of the bundles. There is not a sufficient body of evidence regarding the implantation of permanent pacemakers or implantable cardioverter-defibrillators in these patients yet.7 The likelihood and timeframe of the recovery of advanced AV blocks following chemotherapy (or treatment with novel agents) also have yet to be fully elucidated.
The induction of hematopoietic aplasia occurs within early post-chemotherapy stages, resulting in susceptibility to infections and bleeding. Weighing the risk of sudden cardiac arrests and arrhythmias against the probable incidence of infections, bleeding, pacing-related cardiomyopathy, myocardial rupture, and tumor embolism remains a challenging subject.8 We opted to implant a permanent pacemaker for our patient; nonetheless, the AV block was irreversible despite the obvious regression of the tumor after systemic treatment. This can be explained by the persistent damage of the AV area or distal structures, including the bundle branches, fascicles, small fibers, and junctions.9 Another challenge in the treatment of our patient was the maintenance of optimal anticoagulation given the underlying malignancy and the history of DVT. In the case of resistance against chemotherapy or recurrence, radiation is an available option with the potential risks of constrictive pericarditis, atherosclerosis, cardiomyopathy, conduction disorders, and diastolic dysfunction.10 In addition to the adverse side effects of mediastinal radiation, particularly fibrosis in the conductive tissues of the heart, other potential hazards also exist. Uncommon toxicity to chemotherapeutic agents such as doxorubicin and arsenic trioxide may also lead to AV blocks.11 Still, chemotherapy remains the most effective treatment strategy in these patients. Notably, the potential risk of anthracycline-mediated cardiac rupture should be taken into consideration in our patient due to considerable tissue involvement. Venous thromboembolism and a chronic nonhealing skin ulcer denote an underlying malignancy, preceding subsequent cardinal presentations such as syncopal episodes. Such findings may reflect an established complete heart block because of tumor invasion to the vicinity of the AV node and conducting bundles, as well as a transient and partial obstruction of the LVOT. Intensive chemotherapy led to the eminent shrinkage of our patient’s malignant tumor bulk; he has, however, remained pace-dependent so far. We propose several interpretations such as permanent damage to the His-Purkinje system, residual microinvasion of the tumor, autoantibodies responsible for blockades, and paradoxical injury due to the chemotherapy regimen.
Conclusion
Cutaneous T-cell lymphomas, as an infrequent subclass of non-Hodgkin lymphomas, may scarcely involve the heart (unlike B-cell and non-cutaneous T-cell lymphomas) through hematogenous or lymphatic metastasis. AV blocks and syncope are uncommon features of the presentation of cardiac lymphomas in the absence of other systemic manifestations. However, physicians should take heed of all signs and symptoms. Transthoracic echocardiography is a valuable imaging modality in that it effectively depicts the characteristics of gross pathologic structures. Supplementary modalities such as transesophageal echocardiography, CT scanning, and magnetic resonance imaging confer a greater resolution and, thus, help determine the morphology, location, and extent of cardiac neoplasms. What remarkably aided us in establishing the diagnosis was contrast enhancement, which illustrated the patient’s vascularity patterns in favor of tumoral infiltrations. Decision-making concerning the implantation of devices such as permanent pacemakers poses a formidable challenge considering the probability of AV block reversals in response to systemic treatment, the limited survival of such patients, and potential complications.
Notes:
This paper should be cited as: Sattarzadeh R, Ghodsi S, Eslami M, Mollazadeh R, Safaei Nodehi R, Hosseini Z. Secondary Cardiac T-Cell Lymphoma Presenting with Syncope and Refractory Complete Atrioventricular Block: A Case Report. J Teh Univ Heart Ctr 2020;15(4):183-188.
References
- 1.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 2.O'Mahony D, Peikarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma. 2008;8:249–252. doi: 10.3816/CLM.2008.n.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servitje O, Limón A, Blanco A, Carmona M, Serrano T, Romagosa V, Gallardo F, García J, Peyrí J. Cardiac involvement and molecular staging in a fatal case of mycosis fungoides. Br J Dermatol. 1999;141:531–535. doi: 10.1046/j.1365-2133.1999.03053.x. [DOI] [PubMed] [Google Scholar]
- 4.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 5.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- 6.Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–1103. doi: 10.1148/radiographics.20.4.g00jl081073. [DOI] [PubMed] [Google Scholar]
- 7.Dellas C, Chapuy B, Schweyer S, Hasenfuß G, Hünlich M. A rare cause of sudden cardiac arrest: primary cardiac lymphoma. Clin Res Cardiol. 2009;98:509–511. doi: 10.1007/s00392-009-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniades L, Eftychiou C, Petrou PM, Bagatzounis A, Minas M. Primary cardiac lymphoma: case report and brief review of the literature. Echocardiography. 2009;26:214–219. doi: 10.1111/j.1540-8175.2008.00757.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho SW, Kang YJ, Kim TH, Cho SK, Hwang MW, Chang W, Rhee KJ, Kim BO, Goh CW, Park KM, Kim JH, Byun YS, Yuh YJ. Primary cardiac lymphoma presenting with atrioventricular block. Korean Circ J. 2010;40:94–98. doi: 10.4070/kcj.2010.40.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucunato M, Cannavà G, Currò A. Complete atrioventricular block can be a long-term complication of radiation therapy. Int J Cardiol. 2015;187:676–677. doi: 10.1016/j.ijcard.2015.03.421. [DOI] [PubMed] [Google Scholar]
- 11.Rudzinski T, Ciesielczyk M, Religa W, Bednarkiewicz Z, Krzeminska-Pakula M. Doxorubicin-induced ventricular arrhythmia treated by implantation of an automatic cardioverter-defibrillator. Europace. 2007;9:278–280. doi: 10.1093/europace/eum033. [DOI] [PubMed] [Google Scholar]