Abstract
There has been a dramatic increase in illicit fentanyl use in the United States over the last decade. In 2018, more than 31,000 overdose deaths involved fentanyl or fentanyl analogues, highlighting an urgent need to identify effective treatments for fentanyl use disorder. An emerging literature shows that glucagon-like peptide-1 receptor (GLP-1R) agonists attenuate the reinforcing efficacy of drugs of abuse. However, the effects of GLP-1R agonists on fentanyl-mediated behaviors are unknown. The first goal of this study was to determine if the GLP-1R agonist exendin-4 reduced fentanyl self-administration and the reinstatement of fentanyl-seeking behavior, an animal model of relapse, in rats. We found that systemic exendin-4 attenuated fentanyl taking and seeking at doses that also produced malaise-like effects in rats. To overcome these adverse effects and enhance the clinical potential of GLP-1R agonists, we recently developed a novel dual agonist of GLP-1Rs and neuropeptide Y2 receptors (Y2Rs), GEP44, that does not produce nausea-like behavior in drug-naïve rats or emesis in drug-naïve shrews. The second goal of this study was to determine if GEP44 reduced fentanyl self-administration and reinstatement with fewer adverse effects compared to exendin-4 alone. In contrast to exendin-4, GEP44 attenuated opioid taking and seeking at a dose that did not suppress food intake or produce adverse malaise-like effects in fentanyl-experienced rats. Taken together, these findings indicate a novel role for GLP-1Rs and Y2Rs in fentanyl reinforcement and highlight a potential new therapeutic approach to treating opioid use disorders.
Keywords: exendin-4, PYY, opioid, relapse, self-administration, nausea/emesis
1. Introduction
Opioid overdoses, many of which involve illicit fentanyl use, are currently one of the leading causes of death in the United States. In 2018, approximately 47,000 Americans died from opioid overdoses, of which 67% were associated with synthetic opioids including fentanyl and its analogues (Wilson et al., 2020). Despite this overdose epidemic, little is known about how available FDA-approved medications for opioid use disorder (i.e., buprenorphine, methadone, and naltrexone) reduce illicit fentanyl use (Comer and Cahill, 2019). Moreover, there is a paucity of preclinical studies investigating fentanyl-taking and -seeking behaviors and their underlying neurobiological mechanisms (Volkow et al., 2019).
Neuropeptides of the gut-brain axis and their cognate receptors play important roles in drug-mediated behaviors (Hayes and Schmidt, 2016; Hernandez and Schmidt, 2019; Jerlhag, 2018). For example, systemic administration of a GLP-1 receptor (GLP-1R) agonist reduces the rewarding and reinforcing effects of cocaine (Egecioglu et al., 2013b; Graham et al., 2013; Schmidt et al., 2016; Sørensen et al., 2015), nicotine (Egecioglu et al., 2013a; Tuesta et al., 2017), alcohol (Egecioglu et al., 2013c; Shirazi et al., 2013; Sørensen et al., 2016; Thomsen et al., 2017; Vallöf et al., 2020), and amphetamine (Egecioglu et al., 2013b; Sirohi et al., 2016) in rodents. Recently, we expanded this literature and showed that systemic administration of the GLP-1R agonist exendin-4 attenuated voluntary oxycodone taking and seeking in rats (Zhang et al., 2019). While these findings suggest that GLP-1R agonists, which are FDA-approved for treating type II diabetes and obesity, could be re-purposed to treat opioid use disorders, there is some evidence that the efficacy of GLP-1R agonists may differ depending on the opioid self-administered (Bornebusch et al., 2019). Indeed, the pharmacology of fentanyl differs from other μ-opioid receptor agonists and this may impact the efficacy of medications used to treat fentanyl use disorder (Comer and Cahill, 2019; Volpe et al., 2011). Thus, one objective of this study was to determine if exendin-4 attenuates fentanyl taking and seeking in rats.
Type 2 neuropeptide Y receptors (Y2Rs) are expressed throughout the brain (Parker and Herzog, 1999) and are activated by endogenous ligands, including neuropeptide Y (NPY) and peptide YY (PYY) (Brothers and Wahlestedt, 2010; Mittapalli and Roberts, 2014). PYY is a hormone produced by the same L-cells of the distal GI tract that secrete GLP-1 (Batterham and Bloom, 2003). PYY3–36, the most common form of circulating PYY, crosses the blood brain barrier and selectively activates central Y2Rs (Grandt et al., 1994; Murphy and Bloom, 2006; Nonaka et al., 2003). While studies of Y2Rs and behavior have focused mainly on food intake (Batterham and Bloom, 2003; Batterham et al., 2003; Batterham et al., 2002; Batterham et al., 2006; Chandarana and Batterham, 2008), emerging evidence suggests that Y2Rs may also play an important role in mediating behavioral responses to drugs of abuse. For example, repeated infusions of morphine or codeine are associated with decreased levels of NPY in the hypothalamus, striatum, and adrenal glands, suggesting a potential role for Y2Rs in opioid dependence (Pages et al., 1991; Pages et al., 1992). Consistent with these findings, central infusions of PYY attenuated naloxone-precipitated withdrawal from morphine in rats (Woldbye et al., 1998). While these results suggest that Y2Rs may play an important role in opioid-mediated behaviors, the effects of Y2R activation on opioid taking and seeking are unknown.
Recent studies indicate that dual agonists of GLP-1Rs and Y2Rs produce greater behavioral responses and less adverse effects than either monotherapy alone. For example, coadministration of GLP-1 and PYY produced additive effects on food intake in humans (De Silva et al., 2011; Neary et al., 2005; Schmidt et al., 2014), and synergistic effects on blood glucose homeostasis, food intake, and body weight in rodent models (Fenske et al., 2012; Reidelberger et al., 2011; Talsania et al., 2005). In addition, coadministration of exendin-4 and PYY activated neural circuits in a synergistic manner, suggesting that PYY may enhance the effects of GLP-1R agonists (Kjaergaard et al., 2019). Recently, we designed and synthesized a monomeric peptide, GEP44, that binds to and activates both GLP-1Rs and Y2Rs (rat GLP-1R EC50=480 pM; Y2R EC50=10 nM) (Milliken et al., 2021). GEP44 dose-dependently reduced food intake and body weight to a greater extent than exendin-4 alone in drug-naïve rats (Milliken et al., 2021). Importantly, we showed that GEP44 did not produce adverse emetic or malaise-like effects commonly associated with GLP-1R agonists in humans and rodents (Bergenstal et al., 2010; Kanoski et al., 2012; Kendall et al., 2005). Based on these pilot studies, it is provocative to think that combinatorial therapies, like GEP44, that target multiple neuropeptide systems may be more efficacious in reducing substance use disorders than monotherapies targeting single neuropeptide receptors. Thus, a second objective of this study was to screen the efficacy of GEP44 to reduce fentanyl taking and seeking in rats.
Here, we screened the efficacy of novel pharmacotherapies to treat fentanyl use disorder in rodent models of fentanyl taking and seeking and study the neurobiological mechanisms underlying fentanyl reinforcement. The present study had three main goals: 1) to determine whether the FDA-approved GLP-1R agonist exendin-4 attenuates fentanyl self-administration and the reinstatement of fentanyl-seeking behavior in rats; 2) to compare the effects of exendin-4 to our novel GLP-1R/Y2R dual agonist GEP44 in our preclinical models of fentanyl taking and seeking; and 3) to screen potential feeding effects and adverse malaise-like effects of exendin-4 and GEP44 in fentanyl-experienced rats. We hypothesized that both exendin-4 and GEP44 would attenuate fentanyl taking and seeking, but that GEP44 would do so at doses that produced less adverse effects in fentanyl-experienced rats compared to GLP-1R monotherapy.
2. Materials and Methods
2.1. Drugs
Fentanyl citrate was obtained from Covetrus (Dublin, OH) and diluted in bacteriostatic 0.9% saline. Doses of fentanyl used in the self-administration and reinstatement experiments were based on our pilot studies (Figure 1) as well as previously published fentanyl self-administration studies in rats (Ezeomah et al., 2020; Fragale et al., 2019; Townsend et al., 2019; Wade et al., 2015). Buprenorphine hydrochloride (Sigma-Aldrich, MO) was dissolved in bacteriostatic 0.9% saline. Doses and route of administration of buprenorphine were based on previous operant studies in rats (Carrera et al., 1999; Chen et al., 2006; Zhang et al., 2019). Exendin-4 (American Peptide Company, Sunnyvale, CA) was dissolved in bacteriostatic 0.9% saline. GEP44 was synthesized by Genscript (Piscataway, NJ) or in the Doyle lab using a CEM library Blue peptide synthesizer. GEP44 purity and identity were confirmed in-house by reverse-phase HPLC (Zorbax C8; 300 Å column) and electron spray mass spectrometry, respectively (Milliken et al., 2021). Samples were flash-frozen, freeze-dried and shipped as lyophilized powders. GEP44 was dissolved in bacteriostatic 0.9% saline for us in vivo. The doses and time courses of exendin-4 and GEP44 administration were based on our previous experiments in rats (Hernandez et al., 2018; Hernandez et al., 2019; Milliken et al., 2021; Zhang et al., 2019).
Figure 1. Fentanyl self-administration in rats.
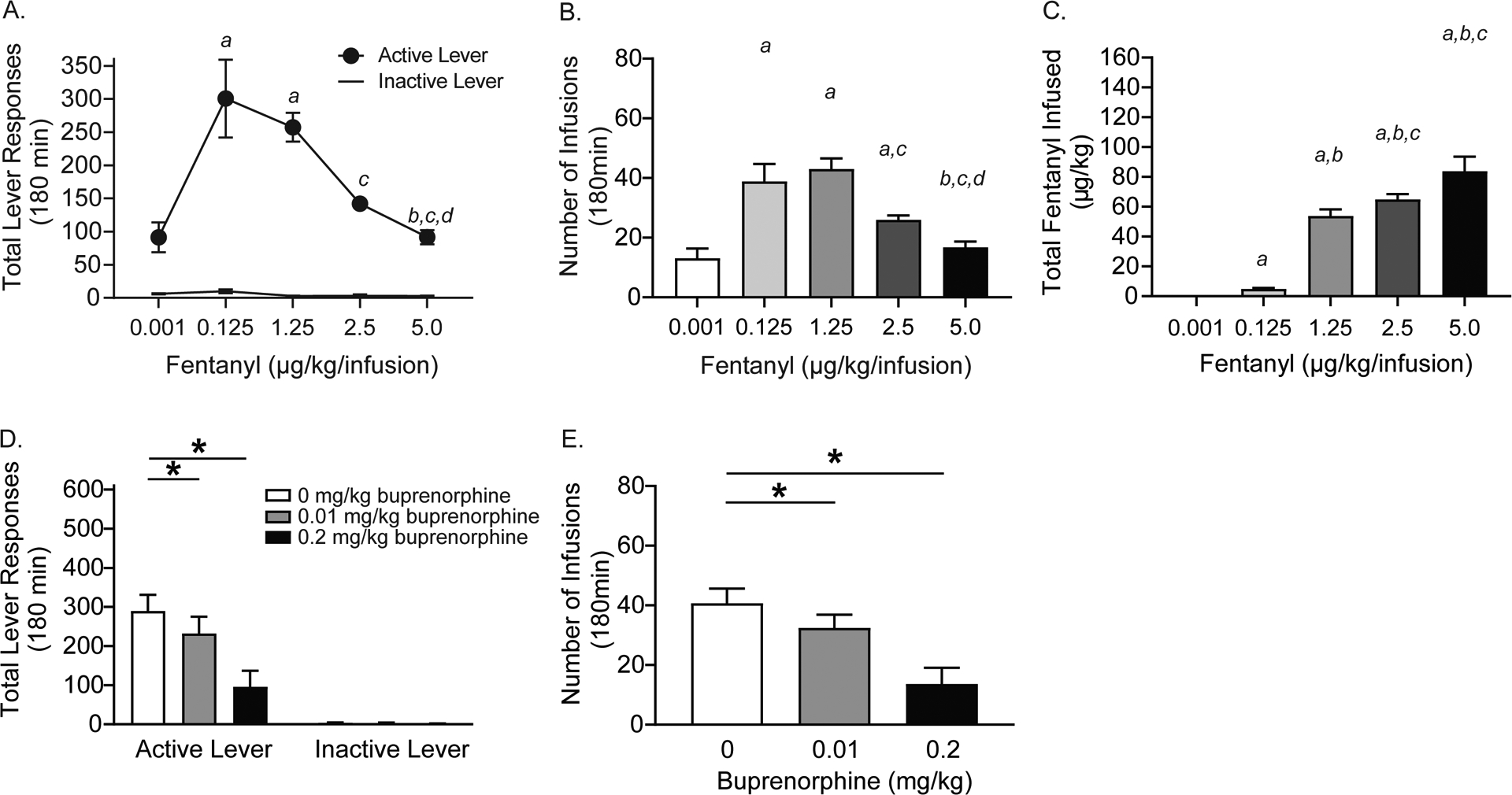
Dose-response curves showing total lever responses (A), total infusions (B), and total fentanyl infused (C) in rats self-administering different unit doses of fentanyl on a FR5 schedule (a=significant increase compared to 0.001 μg/kg/infusion fentanyl; b=significant increase compared to 0.125 μg/kg/infusion fentanyl; c=significant increase compared to 1.25 μg/kg/infusion fentanyl; d=significant increase compared to 2.5 μg/kg/infusion fentanyl: p<0.05, Bonferroni; n=11/treatment). Total lever responses (D) and total fentanyl infusions (E) were significantly decreased in rats (n=11/treatment) pretreated with buprenorphine compared to vehicle-treated controls (*p<0.05, Bonferroni).
2.2. Animals and Housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY, USA). Rats were housed individually with food and water available ad libitum in their home cages. A 12/12 h light/dark cycle was used with the lights on at 1900 h. All behavioral procedures were performed during the dark cycle. The experimental protocols were consistent with the guidelines issued by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.3. Surgery
Rats were handled daily and allowed one week to acclimate to their home cages upon arrival. Rats were then anesthetized using 100 mg/kg ketamine (Midwest Veterinary Supply, Valley Forge, PA) and 10 mg/kg xylazine (Akron Animal Health, Lake Forest, IL). An indwelling catheter (SAI Infusion Technologies, Lake Villa, IL) was inserted into the right jugular vein and sutured in place. The catheter was routed to a mesh backmount platform that was implanted subcutaneously dorsal to the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.2 ml of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA) dissolved in heparinized 0.9% saline. When not in use, catheters were sealed with plastic obturators.
2.4. Fentanyl self-administration
Rats were allowed seven days to recover from surgery before behavioral testing commenced. Initially, rats were placed in operant conditioning chambers and allowed to lever-press for intravenous infusions of fentanyl (2.5 μg/kg/59 μl saline, infused over 5 s) under a fixed-ratio 1 (FR1) schedule of reinforcement. Once a rat achieved >15 infusions of fentanyl in three consecutive self-administration sessions under the FR1 schedule, the subject was switched to a fixed-ratio 3 (FR3) schedule of reinforcement. When a rat achieved >15 infusions of fentanyl in three consecutive sessions under the FR3 schedule, the subject was subsequently switched to and maintained on a fixed-ratio 5 (FR5) schedule of reinforcement. All self-administration sessions were three-hours in duration and were conducted five days per week. Rats continued to respond for fentanyl on a FR5 schedule for ~14 additional days prior to behavioral testing (i.e., a total of 21–28 days of fentanyl self-administration sessions). Each fentanyl infusion was paired with a 20 s contingent light cue illuminated directly above the active lever (i.e., drug-paired lever). For all FR schedules, a 20-s time-out period, during which time active lever responses were tabulated but had no scheduled consequences, followed each fentanyl infusion. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the self-administration sessions.
To comprehensively characterize fentanyl self-administration in rats, a dose-response curve was generated in rats stably self-administering the training dose of fentanyl (2.5 μg/kg/infusion) on a FR5 schedule. Using a between-sessions, within-subjects design rats were allowed to self-administer different unit doses of fentanyl (0, 0.001, 0.125, 1.25, 2.5 and 5.0 μg/kg/infusion) similar to previous opioid self-administration studies (Zhang et al., 2019). Fentanyl doses were counterbalanced, and rats were allowed to self-administer each dose for three consecutive days. After each test dose, rats were returned to the training dose for an additional three days to assess the stability of fentanyl taking at this dose. Total active and inactive lever responses and total infusions were recorded and the three-day mean of each measure for each unit dose of fentanyl was calculated.
To determine the predictive validity of our model, a separate group of rats stably self-administering fentanyl (2.5 μg/kg/infusion) on a FR5 schedule was pretreated with buprenorphine (0, 0.01 and 0. 2 mg/kg, s.c.) 10 minutes prior to self-administration test sessions using a within-subjects, counterbalanced design.
A between-sessions, within-subjects design was used to screen the efficacy of exendin-4 and GEP44 in reducing opioid consumption in separate groups of rats stably self-administering fentanyl on a FR5 schedule. Each test day was separated by 1–2 days of fentanyl self-administration to ensure that drug taking had stabilized between test sessions. Rats were pretreated with vehicle or exendin-4 (0.072 or 0.72 nmol/kg, i.p.) 10 min prior to the beginning of the operant test sessions. Separate rats self-administering fentanyl were pretreated with vehicle or GEP44 (0.3 or 2.4 nmol/kg, s.c.) 30 min prior to the beginning of the operant test sessions.
2.5. Reinstatement of fentanyl-seeking behavior
Following 21–28 days of fentanyl self-administration sessions, drug taking was extinguished by replacing the fentanyl solution with saline and turning off the drug-paired cue light. Daily extinction sessions continued until responding on the active lever was <20% of the total active lever responses completed on the last day of fentanyl self-administration. Typically, it took 6–9 days for rats to meet this criterion. Once fentanyl taking was extinguished, rats entered the reinstatement phase of the experiment. The ability of an acute priming injection of fentanyl (45 μg/kg, i.p.) and re-exposure to the cue light to reinstate drug-seeking behavior was assessed. The priming dose of fentanyl was based on previous reinstatement studies in rats (Wager et al., 2014). During reinstatement test sessions, every 5th lever press resulted in an infusion of saline and illumination of the cue light previously paired with fentanyl infusions during the self-administration phase of the experiment. The effects of vehicle, exendin-4 (0.072 and 0.72 nmol/kg, i.p.) and GEP44 (0.3 and 2.4 nmol/kg, s.c.) on the reinstatement of fentanyl-seeking behavior were investigated using a within-subjects, counterbalanced design. Using a between-sessions reinstatement paradigm, each reinstatement test session was followed by extinction sessions until responding was again <20% of the total active lever responses completed on the last day of fentanyl self-administration. Generally, 1–2 days of extinction were necessary to reach extinction criterion between reinstatement test sessions.
The ability of exendin-4 and GEP44 to reinstate drug-seeking behavior was tested in a separate group of rats. Identical self-administration and extinction procedures were used as described above. Rats were pretreated with vehicle, exendin-4 (0.72 nmol/kg, i.p.) or GEP44 (2.4 nmol/kg) prior to an extinction test session. Light cues previously paired with fentanyl infusions during the self-administration phase were not turned on during the reinstatement test sessions. Thus, the unique ability of exendin-4 and GEP44 to reinstate fentanyl-seeking behavior was assessed in the absence of reinstating stimuli (i.e., conditioned light cues and priming injection of fentanyl).
2.6. Ad libitum food intake
Some of the rats in the aforementioned self-administration and reinstatement experiments were housed in custom-made, hanging wire cages in order to determine if systemic infusions of exendin-4 or GEP44 reduced food intake in fentanyl-experienced rats. Immediately following self-administration and reinstatement tests, rats were returned to the hanging wire cages and given ad libitum access to normal chow (Purina LabDiet 5001, Purina, St. Louis, MO). Food spillage was collected by papers placed beneath the hanging wire cages. Cumulative chow intake was measured as the difference in weight of the food hoppers between two time points minus the weight of crumbs. Feeding measurements were recorded 1, 3, 6, and 21 hours post session (4, 6, 9, and 24 hours post exendin-4 or GEP44 pretreatment). Total body weight and water intake were measured 24 hours post infusion.
2.7. Pica/kaolin intake
Pica, a model of malaise-like behavior (Andrews and Horn, 2006; Kimmey et al., 2014; Mitchell et al., 1976), was also measured in fentanyl-experienced rats housed in hanging wire cages. Pica is the consumption of a non-nutritive substance, such as kaolin clay, in response to an emetic agent. Rats maintained on standard chow were habituated to ad libitum kaolin clay in their hanging wire cages for four days before self-administration and reinstatement test sessions. Kaolin intake was measured before and 24 hours after systemic administration of vehicle, exendin-4 (0.072 and 0.72 nmol/kg, i.p.) or GEP44 (0.3 and 2.4 nmol/kg, s.c.) and subsequent fentanyl self-administration and reinstatement test sessions.
2.8. Statistics
For all fentanyl self-administration and reinstatement experiments, total active and inactive lever responses were analyzed with repeated measures (RM) two-way analyses of variance (ANOVAs). RM one-way ANOVAs were used to analyze total number of infusions and total fentanyl infused. Cumulative chow intake was analyzed with RM two-way ANOVAs. Body weight changes, water intake and kaolin intake were analyzed with separate RM one-way ANOVAs. Pairwise comparisons were made with Bonferroni post hoc tests (p<0.05). All data are presented as mean ± SEM. To ensure robust and reliable results, the behavioral effects of exendin-4 and GEP44 were tested in at least two cohorts of rats per experiment. Experimenters were blinded to treatment assignments during the testing phase of each experiment.
3. Results
3.1. Fentanyl self-administration dose-response curve
Rats (n=11/treatment) acquired and maintained stable fentanyl self-administration on the training dose (2.5 μg/kg/infusion) prior to self-administering different unit doses of fentanyl. Total lever responses are shown in Figure 1A. A RM two-way ANOVA revealed a significant lever × unit dose interaction [F(4,40)=13.02, p<0.01]. Subsequent pairwise analyses revealed that total active lever responses were significantly different between rats self-administering 0.001 versus 0.125 and 1.25 μg/kg/infusion fentanyl, rats self-administering 0.125 versus 5.0 μg/kg/infusion fentanyl, rats self-administering 1.25 versus 2.5 and 5.0 μg/kg/infusion fentanyl, and rats self-administering 2.5 versus 5.0 μg/kg/infusion fentanyl (Bonferroni, p<0.05). No significant differences in inactive lever responding were revealed for any dose. Total number of infusions are shown in Figure 1B and were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(4,40)=21.66, p<0.0001]. Post hoc analyses revealed significantly more infusions earned between rats self-administering 0.001 versus 0.125, 1.25 and 2.5 μg/kg/infusion fentanyl, rats self-administering 0.125 versus 5.0 μg/kg/infusion fentanyl, rats self-administering 1.25 versus 2.5 and 5.0 μg/kg/infusion fentanyl, and rats self-administering 2.5 versus 5.0 μg/kg/infusion fentanyl (Bonferroni, p<0.05). Total fentanyl self-administered is shown in Figure 1C and these data were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(4,40)=78.16, p<0.0001]. Subsequent pairwise analyses revealed that total fentanyl infused was significantly different between rats self-administering 0.001 versus 0.125, 1.25, 2.5 and 5.0 μg/kg/infusion fentanyl, rats self-administering 0.125 versus 1.25, 2.5, and 5.0 μg/kg/infusion fentanyl, and rats self-administering 1.25 versus 2.5 and 5.0 μg/kg/infusion fentanyl (Bonferroni, p<0.05).
To test the predictive validity of our behavioral model, rats (n=11/treatment) were pretreated with buprenorphine, a partial agonist of μ opioid receptors and antagonist of κ opioid receptors. Total lever responses are shown in Figure 1D and were analyzed with a RM two-way ANOVA, which revealed a significant buprenorphine × lever interaction [F(2,20)=48.42, p<0.0001]. Post hoc analyses showed that active lever responses were significantly decreased in rats pretreated with 0.01 and 0.2 mg/kg buprenorphine versus vehicle-treated controls (Bonferroni, p<0.05). Total number of infusions are shown in Figure 1E and were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,40)=49.75, p<0.0001]. Subsequent pairwise analyses showed that total fentanyl infusions were decreased in rats pretreated with 0.01 and 0.2 mg/kg buprenorphine compared to vehicle-treated controls (Bonferroni, p<0.05).
3.2. Systemic administration of exendin-4 attenuates fentanyl self-administration
The efficacy of exendin-4 was tested in rats (n=27/treatment) self-administering fentanyl on a FR5 schedule of reinforcement. Total lever responses are shown in Figure 2A and were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,52)=52.42; p<0.0001]. Total fentanyl infusions are shown in Figure 2B and were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,52)=37.48, p<0.0001]. Separate pairwise analyses indicated that total active lever responses and total fentanyl infusions were significantly decreased in rats pretreated with 0.72 nmol/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p<0.05).
Figure 2. Exendin-4 attenuates fentanyl self-administration in rats.
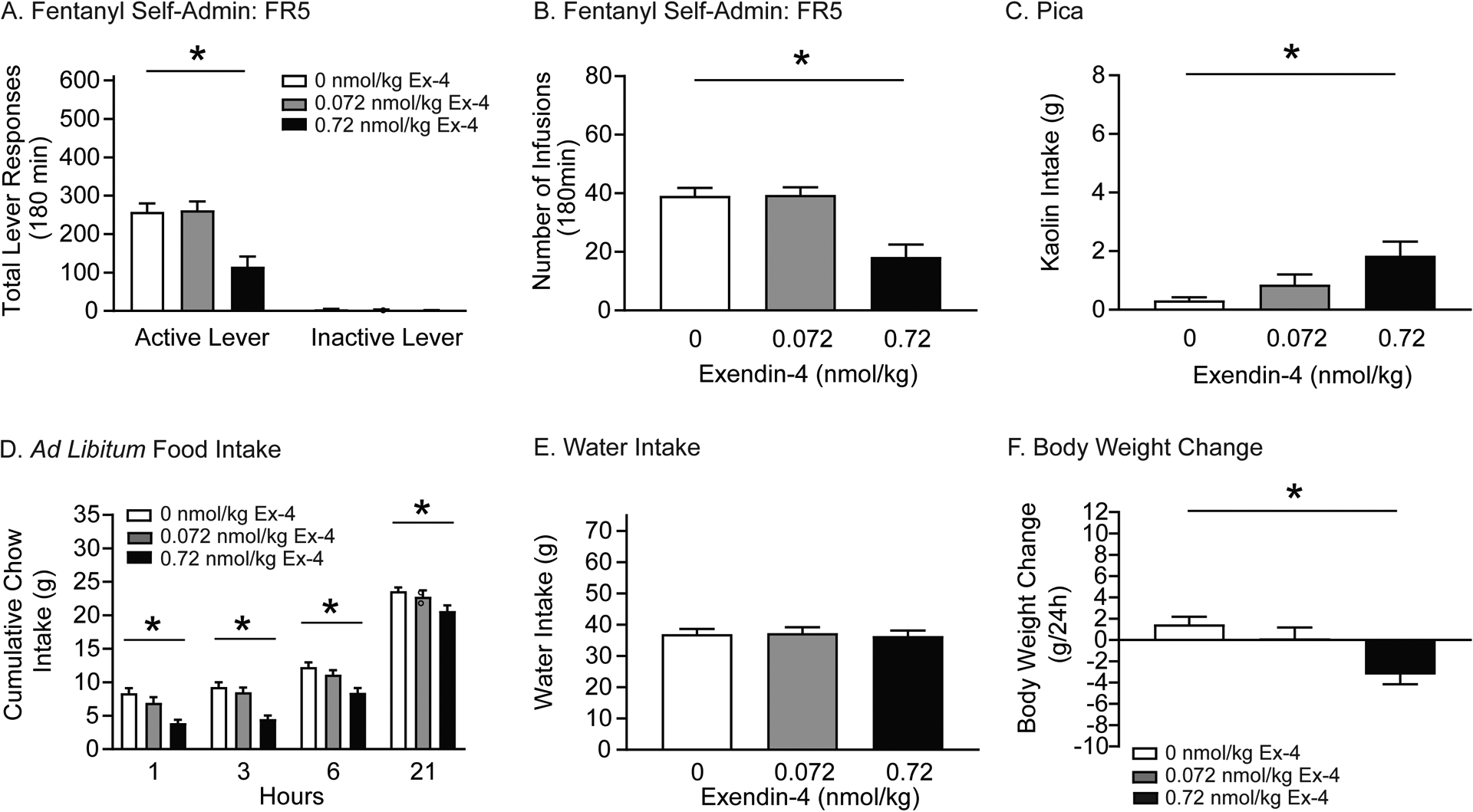
Rats were pretreated with systemic infusions of vehicle or exendin-4 prior to fentanyl self-administration test sessions. Food intake, water consumption, and body weight were measured up to 24 hours post infusion. In self-administration tests, total active lever responses (A) and total fentanyl infusions (B) were significantly decreased in rats pretreated with 0.72 nmol/kg exendin-4 versus vehicle (n=27/treatment; *p<0.05, Bonferroni). (C) 24-hour kaolin intake was significantly increased in rats pretreated with 0.72 nmol/kg exendin-4 compared to vehicle (n=16/treatment; *p<0.05, Bonferroni). (D) Exendin-4 significantly decreased cumulative chow intake in fentanyl-experienced rats compared to controls (n=16/treatment; *p<0.05, Bonferroni). (E) No effects of systemic exendin-4 were found on 24-hour water intake in fentanyl-experienced rats. (F) Exendin-4 significantly decreased body weight in fentanyl-experienced rats compared to controls (n=16/treatment; *p<0.05, Bonferroni).
Systemic administration of exendin-4 has been shown to produce malaise-like effects in drug-naïve rats (Kanoski et al., 2012). Therefore, pica was evaluated by measuring kaolin consumption in a subset of fentanyl-experienced rats (n=16/treatment) used in aforementioned self-administration tests. 24-hour kaolin intake is shown in Figure 2C and was analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,30)=7.492, p=0.0059]. Subsequent pairwise analyses revealed that rats pretreated with 0.72 nmol/kg exendin-4 consumed significantly more kaolin than vehicle-treated controls (Bonferroni, p<0.05).
Exendin-4 decreases food intake and body weight in humans and animal models (Hayes et al., 2010). Next, we examined the effects of systemic exendin-4 on ad libitum chow intake, water intake, and body weight in a subset of rats (n=16/treatment) used in above self-administration tests. Cumulative chow intake is shown in Figure 2D and was analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(2,30)=26.61, p<0.0001] and time [F(3,45)=281.6, p<0.0001]. Subsequent pairwise analyses indicated that food intake was significantly decreased 1, 3, 6, and 21 h post-session in rats pretreated with 0.72 nmol/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p<0.05). No effects of exendin-4 on 24-hour water intake were noted (Figure 2E). 24-hour body weight is shown in Figure 2F. These data were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,30)=8.855, p<0.01]. Subsequent pairwise analyses indicated that 24-hour body weight was significantly decreased in rats pretreated with 0.72 nmol/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p<0.05). Taken together, these results indicate that 0.72 nmol/kg exendin-4 decreased opioid taking, body weight, and ad libitum food intake in fentanyl-experienced rats, effects that were associated with drug-induced malaise.
3.3. Systemic administration of GEP44 attenuates fentanyl self-administration
The efficacy of GEP44 was tested in separate rats (n=24/treatment) stably self-administering fentanyl on a FR5 schedule of reinforcement. Total lever responses are shown in Figure 3A and were analyzed with a RM two-way ANOVA, which revealed a significant dose × lever interaction [F(2,46)=61.85; p<0.0001]. Total fentanyl infusions are shown in Figure 3B and were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,46)=49.83, p<0.0001]. Subsequent pairwise analyses indicated that total active lever responses and total fentanyl infusions were significantly decreased in rats pretreated with 0.3 and 2.4 nmol/kg GEP44 compared to vehicle-treated controls (Bonferroni, p<0.05).
Figure 3. GEP44 attenuates fentanyl self-administration in rats.
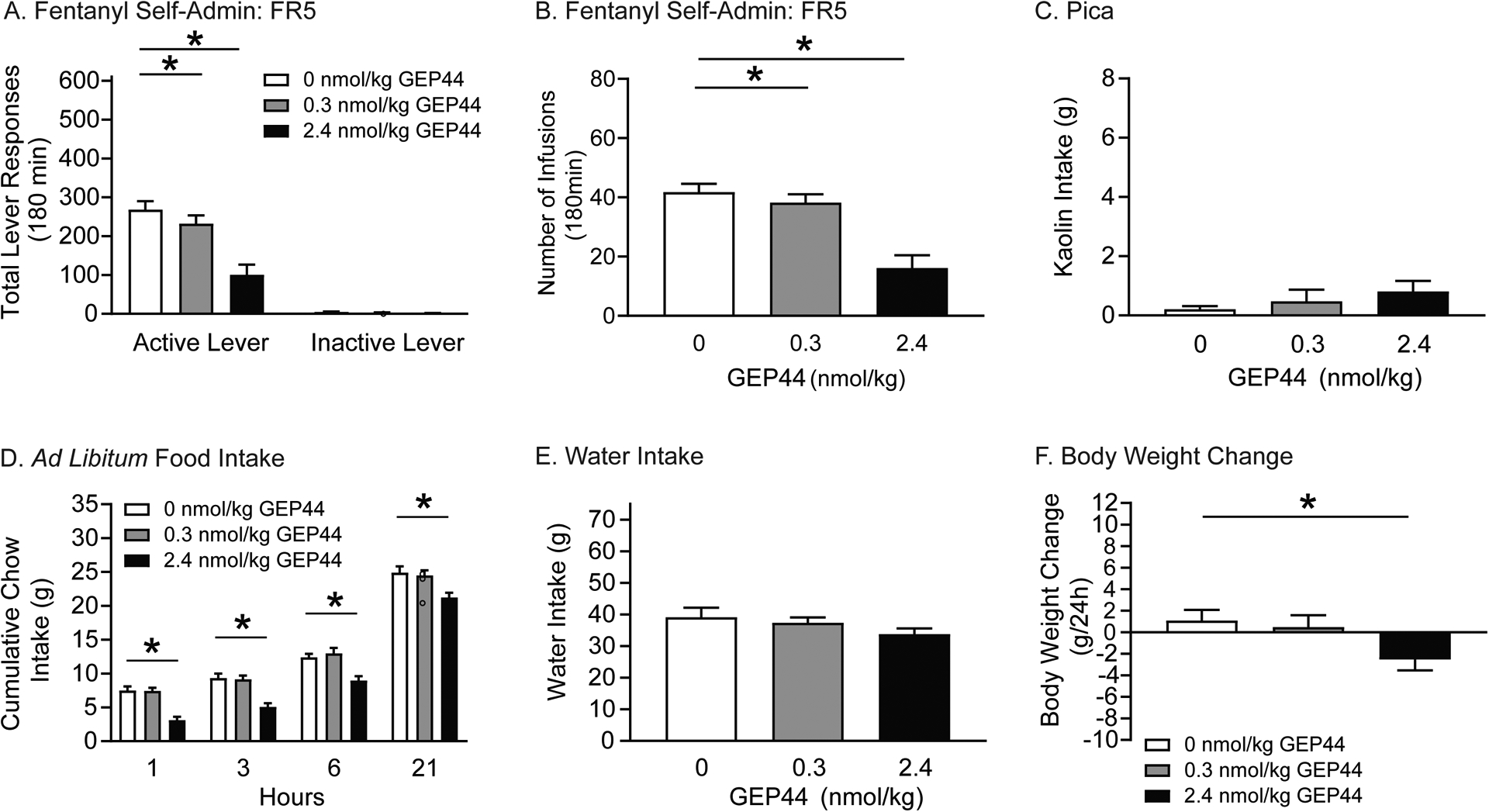
Rats were pretreated with systemic infusions of vehicle or GEP44 prior to fentanyl self-administration test sessions. Food intake, water consumption, and body weight were measured up to 24 hours post infusion. In self-administration tests, total active lever responses (A) and total fentanyl infusions (B) were significantly decreased in rats pretreated with 0.3 and 2.4 nmol/kg GEP44 versus vehicle (n=24/treatment; *p<0.01, Bonferroni). (C) GEP44 did not affect 24-hour kaolin intake in fentanyl-experienced rats (n=10/treatment). (D) GEP44 significantly decreased cumulative chow intake in fentanyl-experienced rats compared to vehicle-treated controls (n=13/treatment; *p<0.05, Bonferroni). (E) No effects of systemic exendin-4 were found on 24-hour water intake in fentanyl-experienced rats. (F) GEP44 significantly decreased body weight in fentanyl-experienced rats compared to vehicle-treated controls (n=13/treatment; *p<0.05, Bonferroni).
Pica was evaluated in a subset of rats (n=10/treatment) used in the aforementioned self-administration tests. There were no differences in 24-hour kaolin intake as shown in Figure 3C. These results indicate that the suppressive effects of GEP44 on operant responding for fentanyl are not due to malaise-like effects in rats.
Effects on food intake were also examined in a subset of rats (n=13/treatment) used in the above self-administration tests. Cumulative chow intake is shown in Figure 3D and was analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(2,24)=39.65, p<0.0001] and time [F(3,36)=444.5, p<0.0001]. Subsequent pairwise analyses indicated that food intake was significantly decreased 1, 3, 6, and 21 h post-session in rats pretreated with 2.4 nmol/kg GEP44 compared to vehicle-treated controls (Bonferroni, p<0.05). No significant effects of GEP44 on 24-hour water intake were noted (Figure 3E). 24-hour body weight is shown in Figure 3F. These data were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,24)=4.325, p<0.05]. Subsequent pairwise analyses indicated that 24-hour body weight was significantly decreased in rats pretreated with 2.4 nmol/kg GEP44 compared to vehicle-treated controls (Bonferroni, p<0.05). Taken together, these results indicate that GEP44 dose-dependently attenuates fentanyl taking and ad libitum food intake in rats but does not induce malaise-like effects.
3.4. Systemic administration of exendin-4 attenuates the reinstatement of fentanyl-seeking behavior
To determine if GLP-1R activation reduces fentanyl-seeking behavior during abstinence, rats (n=13/treatment) were pretreated with exendin-4 prior to reinstatement test sessions. Total lever responses are shown in Figure 4A and were analyzed with a RM two-way ANOVA, which revealed a significant drug × lever interaction [F(2,24)=22.22, p<0.0001]. Total infusions are shown in Figure 4B and were analyzed using a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,24)=30.11, p<0.0001]. Separate pairwise analyses indicated that both total active lever responses and total infusions were significantly different between rats pretreated with 0.072 or 0.72 nmol/kg exendin-4 and vehicle (Bonferroni, p<0.05).
Figure 4. Exendin-4 attenuates fentanyl seeking in rats.
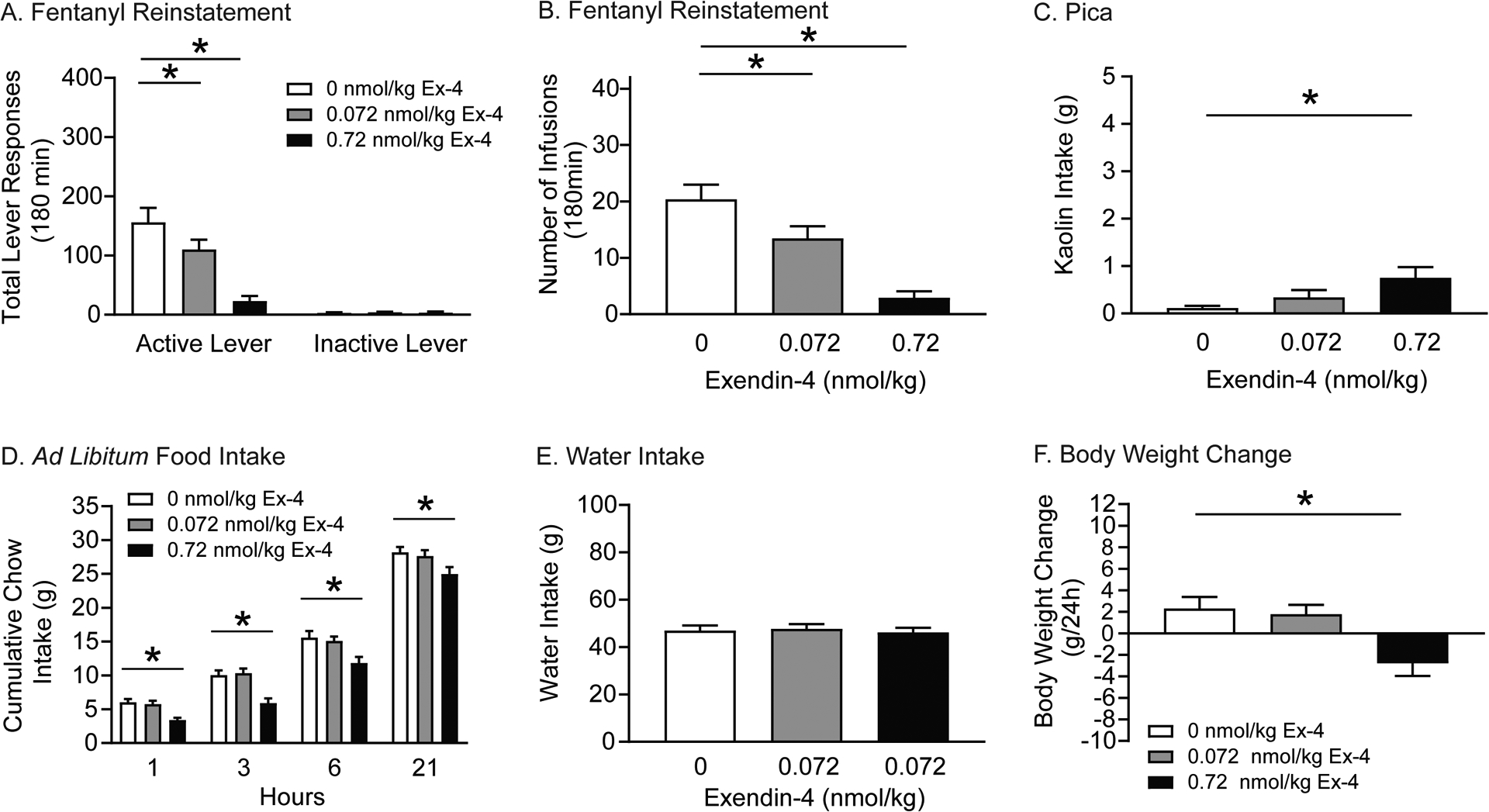
Rats were pretreated with vehicle or exendin-4 prior to fentanyl and cue-primed reinstatement test sessions. Total active lever responses (A) and total number of infusions (B) were attenuated in rats pretreated with 0.072 and 0.72 nmol/kg exendin-4 versus vehicle (n=13/treatment; *p<0.05, Bonferroni). (C) 24-hour kaolin intake was significantly increased in rats pretreated with 0.72 nmol/kg exendin-4 compared with vehicle (n=16/treatment; *p<0.05, Bonferroni). (D) Cumulative chow intake in rats pretreated with 0.72 nmol/kg exendin-4 were significantly decreased compared to vehicle (n=13/treatment; *p<0.05, Bonferroni). (E) 24-hour water intake was not altered in fentanyl-experienced rats (n=13/treatment) pretreated with exendin-4 during abstinence. (F) Body weight in rats pretreated with 0.72 nmol/kg exendin-4 was significantly decreased compared to vehicle (n=13/treatment; *p<0.05, Bonferroni).
The effects of exendin-4 on pica were measured following reinstatement test sessions (n=13/treatment). Total kaolin intake is shown in Figure 4C and was analyzed using a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,24)=4.052, p<0.05]. Post hoc analyses showed that only the high dose of exendin-4 (0.72 nmol/kg) significantly increased 24-hour kaolin intake in fentanyl-experienced rats during abstinence.
We also investigated the effects of exendin-4 on food intake, water intake, and body weight following reinstatement test sessions (n=13/treatment). Cumulative food intake is shown in Figure 4D and was analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(2,24)=26.69, p<0.0001] and time [F(3,36)=605.7, p<0.0001]. Subsequent pairwise analyses indicated that food intake was significantly decreased 1, 3, 6, and 21 hours post session in rats pretreated with 0.72 nmol/kg exendin-4 compared to vehicle-treated controls (Bonferroni, p<0.05). No effects of exendin-4 were found on water intake (Figure 4E). Body weight is shown in Figure 4F and was analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,24)=6.909, p<0.01]. Post hoc analyses revealed a significant decrease in body weight in rats pretreated with 0.72 nmol/kg exendin-4 versus vehicle (Bonferroni, p<0.05). Taken together, these studies highlight a behaviorally selective dose of exendin-4 (0.072 nmol/kg) that attenuated opioid seeking in rats and did not produce adverse malaise-like effects in fentanyl-experienced rats during abstinence.
3.5. Systemic administration of GEP44 attenuates the reinstatement of fentanyl-seeking behavior
The effects of GEP44 on fentanyl seeking were tested in separate rats (n=13/treatment). Total lever responses are shown in Figure 5A and were analyzed with a RM two-way ANOVA, which revealed a significant drug × lever interaction [F(2,24)=20.72, p<0.001]. Subsequent pairwise analyses showed that both 0.3 and 2.4 nmol/kg GEP44 treatments significantly reduced active lever response compared to vehicle-treated controls (Bonferroni, p<0.05). Total number of infusions are shown in Figure 5B and were analyzed with a RM one-way ANOVA, which revealed a significant main effect of treatment [F(2,24)=27.22, p<0.0001]. Post hoc analyses showed that rats pretreated with 0.3 and 2.4 nmol/kg GEP44 self-administered less fentanyl infusions than vehicle-treated controls (Bonferroni, p<0.05).
Figure 5. GEP44 attenuates fentanyl seeking in rats.
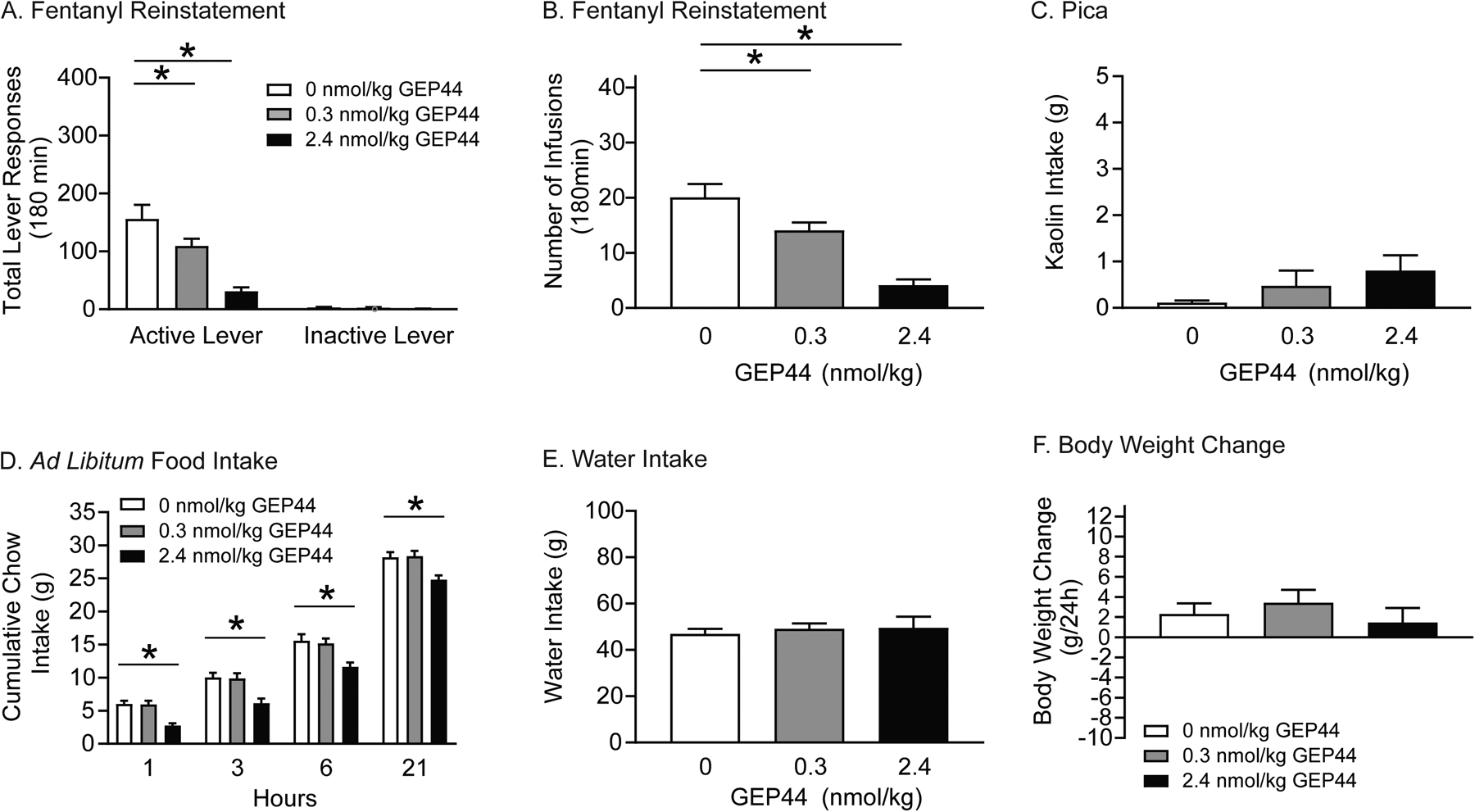
Rats were pretreated with vehicle or GEP44 prior to fentanyl and cue-primed reinstatement test sessions. Total active lever responses (A) and total number of infusions (B) were attenuated in rats pretreated with 0.3 and 2.4 nmol/kg GEP44 versus vehicle (n=13/treatment; *p<0.05, Bonferroni). (C) GEP44 did not affect 24-hour kaolin intake in rats during abstinence (n=13/treatment). (D) Cumulative chow intake was significantly decreased in rats pretreated with 2.4 nmol/kg GEP44 compared to vehicle-treated controls (n=13/treatment, *p<0.05, Bonferroni). Water intake (E) and body weight (F) were not altered in fentanyl-experienced rats (n=13/treatment) pretreated with GEP44 during abstinence.
With regard to pica, no effect of GEP44 was found on 24-hour kaolin intake in fentanyl-experienced rats (n=13/treatment) during abstinence (Figure 5C). Food intake, water consumption, and body weight were also assessed during the reinstatement phase. Cumulative food intake is shown in Figure 5D and was analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(2,24)=21.44, p<0.0001] and time [F(3,36)=698.00, p<0.0001]. Post hoc analyses showed that 2.4 nmol/kg GEP44 significantly reduced food intake 1, 3, 6, and 21 hours post reinstatement test sessions compared to vehicle-treated controls (Bonferroni, p<0.05). No effects of GEP44 were found on water intake (Figure 5E) and body weight (Figure 5F). Collectively, these findings highlight a behaviorally selective dose of GEP44 (0.3 nmol/kg) that reduced fentanyl seeking and did not produce adverse malaise-like effects in rats during abstinence.
3.6. Exendin-4 and GEP44 do not reinstate opioid-seeking behavior during abstinence
Rats (n=11/treatment) were pretreated with vehicle, 0.72 nmol/kg exendin-4 or 2.4 nmol/kg GEP44 prior to extinction test sessions. There were no effects of exendin-4 or GEP44 on total lever responses (Figure 6A) or total number of infusions earned (Figure 6B). These results clearly indicate that exendin-4 and GEP44 do not reinstate drug-seeking behavior in the absence of priming stimuli during abstinence following fentanyl self-administration.
Figure 6. Systemic administration of exendin-4 or GEP44 does not reinstate drug-seeking behavior.
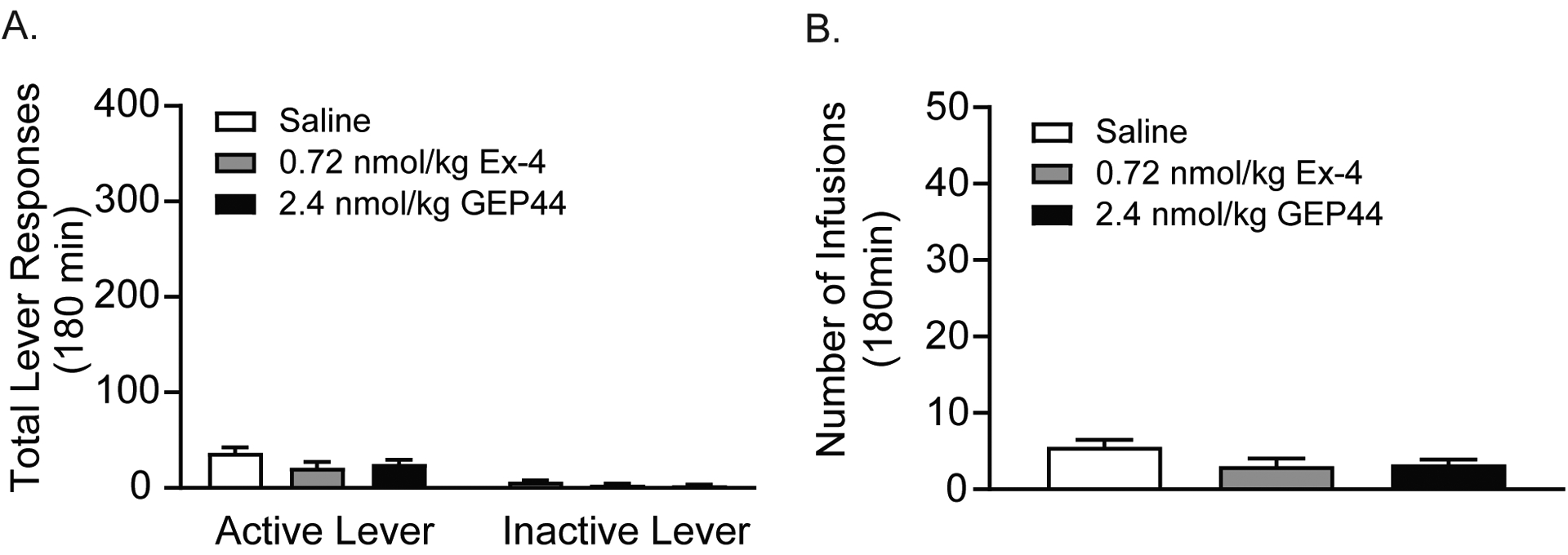
The unique effects of exendin-4 and GEP44 on drug seeking were tested in rats (n=11/treatment) pretreated with vehicle, 0.72 nmol/kg exendin-4, or 2.4 nmol/kg GEP44 prior to extinction test sessions. In the absence of a priming injection of fentanyl and light cues previously paired with fentanyl infusions during the self-administration phase, neither exendin-4 or GEP44 reinstated drug-seeking behavior. There were no effects of either drug on total active lever responses (A) or total number of infusions (B) during reinstatement test sessions.
4. Discussion
To date, preclinical studies of opioid use disorders have focused almost exclusively on morphine and heroin. However, in light of the current fentanyl overdose epidemic, there is an urgent need for studies of fentanyl taking and seeking. In the present study, fentanyl maintained robust self-administration in rats. When given access to different unit doses of fentanyl, rats self-administered intravenous infusions of fentanyl according to an inverted U-shaped dose-response curve, consistent with dose-response studies in humans self-administering opioids (Comer et al., 2008; Haney, 2009; Haney and Spealman, 2008). Peak responding and total infusions were similar to previous fentanyl self-administration studies in rats (Ezeomah et al., 2020; Wade et al., 2015). To further validate our behavioral model, we showed that buprenorphine dose-dependently reduced voluntary fentanyl taking. Buprenorphine is a mixed opioid receptor agonist-antagonist that is FDA-approved for treating opioid use disorders (Strang et al., 2020). Buprenoprhine decreases opioid self-administration in rodents, non-human primates and humans (Chen et al., 2006; Haight et al., 2019; Mello et al., 1983; Mello and Mendelson, 1980; Mello et al., 1982; Mello and Negus, 1998; Zhang et al., 2019). Specifically, buprenorphine shifts the opioid self-administration dose-response curve downward reflecting a decrease in the reinforcing efficacy of opioids (Comer et al., 2001; Mello and Mendelson, 1985). Our buprenorphine results are consistent with these findings and support the predictive validity of our model of fentanyl use disorder (Haney and Spealman, 2008).
Using this preclinical model, we found that both the FDA-approved GLP-1R agonist exendin-4 and our novel dual GLP-1R/Y2R agonist GEP44 attenuated fentanyl self-administration and reinstatement in male rats. However, in contrast to exendin-4, GEP44 did not produce malaise-like effects at doses shown to reduce fentanyl taking and seeking. Given that the training dose of fentanyl is on the descending limb of the self-administration dose-response curve, these results suggest that exendin-4 and GEP44 shift the fentanyl dose-response curve downward. This interpretation is consistent with previous studies showing similar effects of GLP-1R agonists in rodents self-administering licit and illicit drugs (Hernandez and Schmidt, 2019). It should be noted, however, that decreased responding could be indicative of a generalized behavior suppression not picked up in the kaolin test. Collectively, these findings highlight novel roles of GLP-1Rs and Y2Rs in fentanyl-mediated behaviors and suggest that chimeric peptides that simultaneously activate both neuropeptide receptors could serve as potential treatments for opioid use disorders.
4.1. GLP-1 receptors and voluntary opioid taking
GLP-1R agonists attenuate drug-motivated behaviors in a variety of preclinical models of substance use disorders. Systemic infusions of exendin-4 reduce the rewarding and reinforcing effects of alcohol, nicotine, cocaine, and amphetamine in mice and rats (Egecioglu et al., 2013a, b; Egecioglu et al., 2013c; Graham et al., 2013; Hernandez et al., 2018; Hernandez et al., 2019; Schmidt et al., 2016; Sørensen et al., 2015; Thomsen et al., 2017; Tuesta et al., 2017). Consistent with this literature, we recently discovered that systemic exendin-4 attenuates oxycodone self-administration in rats (Zhang et al., 2019). The current study extends our previous findings and reveals that exendin-4 also attenuates fentanyl self-administration in rats. Collectively, these results indicate that activation of GLP-1Rs is sufficient to attenuate the reinforcing efficacy of different classes of abused drugs including opioids.
While our findings clearly show that exendin-4 reduces oxycodone (Zhang et al., 2019) and fentanyl (present study) self-administration in rats, Bornebusch et al. (2019) found no effect of exendin-4 on remifentanil self-administration in mice. These discordant findings could be due to multiple reasons. First, there may be species-specific differences in the efficacy of exendin-4. Central GLP-1R expression is different in mice and rats (Cork et al., 2015a; Graham et al., 2020; Gu et al., 2013; Merchenthaler et al., 1999) and this may affect the ability of GLP-1R agonists to suppress drug-induced dopamine release in the striatum (Fortin and Roitman, 2017; Jensen et al., 2020). In addition, the pharmacokinetic profile of exendin-4 is not as well characterized in mice as in rats (Chen et al., 2013). Thus, there are potential species-specific pharmacokinetic and pharmacodynamic differences that may affect the efficacy of exendin-4 and other GLP-1R agonists in these behavioral models. These opposite findings may also be due to differences in experimental designs. For example, operant responding for remifentanil in Bornebusch et al. (2019) was facilitated by prior food training and varying the dose of remifentanil every 3–4 days during the acquisition phase in mice responding on a FR1 schedule of reinforcement. Moreover, mice did not titrate their responding to maintain remifentanil intake levels when the response requirement was increased and failed to self-administer more remifentanil than saline during subsequent PR tests (Bornebusch et al., 2019). Together with a small sample size (n = 4–7/treatment), these limitations produced “strong variability” in responding that make drawing firm conclusions regarding the efficacy of exendin-4 in reducing opioid-mediated behaviors in mice difficult at best. Future studies investigating the role of GLP-1Rs in opioid-mediated behaviors should carefully consider these factors in their experimental designs.
In our previous oxycodone study, we identified doses of exendin-4 as low as 0.072 nmol/kg that attenuated oxycodone self-administration (Zhang et al., 2019). However, in the present study, 0.072 nmol/kg exendin-4 did not alter fentanyl self-administration. While it is not clear why exactly the efficacy of exendin-4 differs between studies, it is possible and likely that the different pharmacokinetic profiles of opioids may impact therapeutic responses. For example, fentanyl is more lipophilic than oxycodone which enables it to quickly cross over the blood brain barrier (BBB) and produce a rapid onset of action (Comer and Cahill, 2019). In addition, patterns of drug-evoked dopamine release in the nucleus accumbens differ dramatically following intravenous self-administration of different opioids (Vander Weele et al., 2014). The intrinsic efficacies of different opioid agonists also differ resulting in ligand bias at μ opioid receptors (Williams et al., 2013). These findings indicate that opioids exert different effects on the mesocorticolimbic dopamine system that could influence motivated behaviors and treatment responses. Therefore, it is possible that higher doses of exendin-4 (i.e., greater GLP-1R activation) are required to reduced fentanyl versus oxycodone reinforcement in rats. However, as discussed below, higher doses of exendin-4 may produce adverse effects that could limit patient compliance in humans with fentanyl use disorder.
Re-purposing GLP-1R agonists for treating substance use disorders is potentially limited by the ability of these medications to induce nausea/malaise. While not as concerning as the induction of nausea and vomiting, an additional consideration that has been discussed is the fact that acute administration of exendin-4 at doses higher than 0.06 nmol/kg reduces food intake in drug-naïve rats (Hayes et al., 2011a; Kanoski et al., 2012). In the current study, the dose of exendin-4 (0.72 nmol/kg) that reduced fentanyl self-administration also decreased food intake and body weight over 24 h. In contrast, 0.72 nmol/kg exendin-4 transiently suppressed food intake in oxycodone-experienced rats (Zhang et al., 2019). These findings suggest that fentanyl-experienced rats may be more sensitive to the food intake suppressive effects of GLP-1R agonists, which could limit the therapeutic potential of GLP-1R agonists as a monotherapy for treating fentanyl use disorder. Exendin-4 also induces a pica response as measured by increased kaolin intake in rats (Hayes et al., 2011a; Kanoski et al., 2012) and nausea/emesis in humans (Vilsbøll et al., 2012). Our findings indicate that, in addition to reducing fentanyl taking and food intake, 0.72 nmol/kg exendin-4 increased 24-hr kaolin intake in fentanyl-experienced rats. This pica response is a potential confound to interpreting the self-administration data as it suggests that reduced fentanyl consumption may be due to exendin-4-induced malaise-like effects and not direct effects on fentanyl reinforcement.
4.2. GLP-1 receptors and opioid-seeking behavior during abstinence
Consistent with our previous oxycodone studies (Zhang et al., 2019), we found that 0.72 nmol/kg exendin-4 attenuated fentanyl reinstatement. However, this dose of exendin-4 also reduced food intake and produced significant (albeit mild) pica in fentanyl-experienced rats. Interestingly, in contrast to our self-administration studies, we identified a behaviorally selective dose of exendin-4 (0.072 nmol/kg) that reduced fentanyl seeking and did not affect food intake, water consumption or body weight during abstinence. Moreover, 0.072 nmol/kg exendin-4 did not induce pica in fentanyl-experienced rats during abstinence. These findings add to an emerging literature identifying behaviorally selective doses of exendin-4 that reduce drug-seeking behaviors during abstinence/withdrawal (Hernandez et al., 2018; Zhang et al., 2019). It is interesting to note that exendin-4 exerts differential effects on food intake and pica depending on whether a rat is self-administering fentanyl versus after fentanyl taking has been extinguished (i.e., during abstinence).
In our previous cocaine studies, we identified systemic doses of exendin-4 as low as 0.024 nmol/kg that attenuated cocaine seeking in rats (Hernandez et al., 2018). Together with our opioid studies, these results suggest that exendin-4 is more potent in reducing cocaine- versus opioid-seeking behaviors. While we did not test exendin-4 doses lower than 0.072 nmol/kg in our opioid studies, the magnitude of reduced responding in cocaine-experienced rats treated with 0.024 nmol/kg exendin-4 is greater than that seen in opioid-dependent rats treated with 0.072 nmol/kg exendin-4. In addition, the low dose of exendin-4 (0.072 nmol/kg) that attenuated fentanyl seeking did not affect fentanyl self-administration suggesting that higher doses of exendin-4 may be required to suppress compulsive opioid taking versus opioid seeking during abstinence.
4.3. Simultaneous activation of GLP-1Rs and Y2Rs and opioid-mediated behaviors
As discussed above, exendin-4 attenuates fentanyl reinforcement at a dose that elicits nausea-like behavior in rats. This limitation may preclude the development of GLP-1R agonists for treating fentanyl use disorder. One potential alternative strategy is to develop chimeric peptides that simultaneously activate both GLP-1Rs and Y2Rs. Here, we showed that GEP44, a novel dual agonist of GLP-1Rs and Y2Rs, dose-dependently attenuated fentanyl taking and seeking in rats. Importantly, GEP44 was well-tolerated in fentanyl-experienced rats. While the high dose of GEP44 (2.4 nmol/kg) reduced ad libitum food intake in rats during the self-administration phase and during abstinence, the low dose of GEP44 (0.3 nmol/kg) selectively attenuated fentanyl-taking and -seeking behaviors and did not affect food intake, water intake, or body weight in rats. To our knowledge, these are the first studies investigating the role of GLP-1R and Y2R co-activation on opioid reinforcement. Our findings are consistent with recent studies showing that coactivation of GLP-1Rs and Y2Rs produces less adverse effects and greater behavioral responses compared to monotherapy alone (De Silva et al., 2011; Fenske et al., 2012; Milliken et al., 2021; Neary et al., 2005; Reidelberger et al., 2011; Schmidt et al., 2014; Talsania et al., 2005). While the central mechanisms underlying the effects of GEP44 on motivated behaviors including drug taking and seeking are not clear, there is some evidence that suggests that different neural circuits are engaged by simultaneous activation of GLP-1Rs and Y2Rs versus GLP-1R activation alone (Kjaergaard et al., 2019). An interesting future study will be to compare patterns of immediate early gene expression throughout the brains of fentanyl-experienced rats pretreated with GEP44 versus exendin-4 to identify the unique effects of each pharmacotherapy on network-level opioid-induced changes in neuronal activity.
The lack of malaise-like effects in fentanyl-experienced rats treated with GEP44 supports the translational potential of chimeric peptides targeting GLP-1Rs and Y2Rs for treating substance use disorders. The absence of pica in fentanyl-experienced rats treated with GEP44 is likely due to the low potency of GEP44 at GLP-1Rs (Milliken et al., 2021). This hypothesis is supported by studies demonstrating that the adverse behavioral effects of exendin-4 are dose-dependent. For example, exendin-4 administration dose-dependently elicits conditioned taste avoidance and pica and attenuates locomotor activity in drug-naïve rats (Kanoski et al., 2012; Talsania et al., 2005). It is also possible that Y2R agonism reduces GLP-1R-induced pica through interactions between Y2R and GLP-1R signaling pathways. While GLP-1Rs are predominantly coupled to Gs proteins (Hayes et al., 2011b; Holst, 2007), Y2Rs interact with Gi and Gq proteins (Freitag et al., 1995). Depending on the phenotypes of GLP-1R- and Y2R-expressing cells and distributions of these two receptors (Broberger et al., 1997; Cork et al., 2015b; Fetissov et al., 2004; Graham et al., 2020; Gustafson et al., 1997), GLP-1R and Y2R co-activation could enhance or weaken the effects of each monotherapy depending on the neural circuits engaged. It is possible that activation of Y2Rs potentiates the effects of GLP-1R agonists on drug taking and seeking and antagonizes the effects of GLP-1R agonists on nausea-like behavior. Future studies investigating GLP-1R- and Y2R-expressing cell types and intra-cellular signaling pathways will be required to unravel the central mechanisms underlying the effects of GEP44 on motivated behaviors and nausea/emesis.
In addition to regulating food intake (Karra et al., 2009), Y2Rs may also play an important role in drug-mediated behaviors. Our GEP44 results suggest that activation of Y2Rs may reduce the reinforcing efficacy of fentanyl. However, it is not clear whether Y2R activation alone is sufficient to reduce fentanyl taking and seeking or if coactivation of GLP-1Rs is required to produce these behavior responses. To our knowledge, no studies to date have investigated the efficacy of Y2R agonists alone in attenuating opioid-taking and - seeking behaviors. Activation of Y2Rs may regulate drug taking and seeking by altering activity of the mesolimbic dopamine system. Systemic administration of PYY3–36 induces c-fos expression in the nucleus accumbens, dorsal striatum, and central nucleus of the amygdala in mice and rats (Blevins et al., 2008; Stadlbauer et al., 2013; Stadlbauer et al., 2014). Consistent with these preclinical findings, functional magnetic resonance imaging studies in humans demonstrate that Y2R agonism increases neuronal activity in the ventral striatum and orbital frontal cortex (Batterham et al., 2007; Schloegl et al., 2011). Since these brain nuclei are known to regulate the reinforcing effects of drugs of abuse, it is likely that Y2R activation in these circuits affects drug-taking and -seeking behaviors. With regard to opioids, repeated exposure to morphine and codeine is associated with decreased NPY levels in the striatum, hypothalamus, and adrenal glands in rats (Pages et al., 1991; Pages et al., 1992). Moreover, central infusions of NPY and PYY attenuate morphine-related withdrawal phenotypes in rats (Woldbye et al., 1998). Since NPY and PYY are ligands for Y2Rs (Gerald et al., 1995), these results indicate that opioid exposure alters endogenous central Y2R signaling and that activation of Y2Rs may prevent or reduce opioid-withdrawal symptoms. Collectively, these studies suggest that Y2R activation plays an important role in modulating opioid-mediated behaviors, and potentially contributes to the suppressive effects of GEP44 on fentanyl-taking and -seeking behaviors in rats.
5. Conclusion
The present study screened the efficacy of two potentially novel treatments for fentanyl use disorder in rodent models of voluntary fentanyl taking and seeking. We found that exendin-4 reduced fentanyl self-administration and reinstatement but also produced adverse malaise-like effects in opioid-dependent rats. In contrast, GEP44 attenuated fentanyl taking and seeking at a dose that did not elicit nausea-like behavior in rats. While the present study focused on acute administration of exendin-4 and GEP44, future experiments evaluating repeated administration of these drugs are necessary to determine if tolerance develops to both their intake suppressive effects and their adverse effects in fentanyl-dependent rats. Future studies should also examine potential sex differences in therapeutic responses by investigating the efficacy of exendin-4 and GEP44 in female rats. Overall, these findings support further preclinical studies of GLP-1Rs and Y2Rs in opioid-mediated behaviors and highlight the translational potential of dual agonists of GLP-1Rs and Y2Rs to treat opioid use disorders.
Highlights.
Exendin-4 attenuates voluntary fentanyl taking and seeking in rats.
Exendin-4 produces malaise-like effects in fentanyl-experienced rats.
GEP44, an agonist of GLP-1Rs & Y2Rs, reduces fentanyl taking and seeking in rats.
GEP44 does not produce malaise-like effects in fentanyl-experienced rats.
Acknowledgements
Funding:
This work was supported by the following grants from the National Institutes of Health: R01 DA037897 and R21 DA045792 (H.D.S.), and R01 DK021397 (M.R.H.). R.P.D. was supported by a pilot study CUSE award and a Department of Defense grant (6W81XWH201029901). H.D.S. was also supported by the Dr. Dorothy Mereness Endowed Research Fund through a Faculty Grant Award in the School of Nursing. Y.Z. was partially supported by the Jeane B. Kempner postdoctoral fellowship from the University of Texas Medical Branch. A.M. was partially supported by Mary L. and Matthew S. Santirocco Undergraduate Research Grant. S.R. was partially supported by Vagelos Undergraduate Research Grant and the Jumpstart for Juniors Grant. H.D.S. and S.R. were partially supported by a Louis H Castor, M.D., C’48 Undergraduate Research Grant from the Center for Undergraduate Research & Fellowships at the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
R.P.D. receives funding from Balchem Corp. (New Hampton, NY) that was not used to support this study. M.R.H. receives research funding from Eli Lilly & Co. and Boehringer Ingelheim that was not used in support of these studies. All other authors declare no other competing financial interests.
Reference:
- Andrews PL, Horn CC, 2006. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci 125, 100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Bloom SR, 2003. The gut hormone peptide YY regulates appetite. Ann N Y Acad Sci 994, 162–168. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR, 2003. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349, 941–948. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR, 2002. Gut hormone PYY3–36 physiologically inhibits food intake. Nature 418, 650–654. [DOI] [PubMed] [Google Scholar]
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC, 2007. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450, 106–109. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ, 2006. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4, 223–233. [DOI] [PubMed] [Google Scholar]
- Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE, Group D-S, 2010. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376, 431–439. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Chelikani PK, Haver AC, Reidelberger RD, 2008. PYY(3–36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides 29, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornebusch AB, Fink-Jensen A, Wörtwein G, Seeley RJ, Thomsen M, 2019. Glucagon-Like Peptide-1 Receptor Agonist Treatment Does Not Reduce Abuse-Related Effects of Opioid Drugs. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Landry M, Wong H, Walsh JN, Hökfelt T, 1997. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 66, 393–408. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Wahlestedt C, 2010. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO molecular medicine 2, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF, 1999. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology (Berl) 144, 111–120. [DOI] [PubMed] [Google Scholar]
- Chandarana K, Batterham R, 2008. Peptide YY. Curr Opin Endocrinol Diabetes Obes 15, 65–72. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF, 2006. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31, 2692–2707. [DOI] [PubMed] [Google Scholar]
- Chen T, Mager DE, Kagan L, 2013. Interspecies modeling and prediction of human exenatide pharmacokinetics. Pharm Res 30, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Cahill CM, 2019. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev 106, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW, 2001. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology 154, 28–37. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ, 2008. Abuse Liability of Prescription Opioids Compared to Heroin in Morphine-Maintained Heroin Abusers. Neuropsychopharmacology 33, 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, 2015a. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 4, 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S, 2015b. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular Metabolism 4, 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS, 2011. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 14, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013a. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLOS ONE 8, e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013b. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLOS ONE 8, e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E, 2013c. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38, 1259–1270. [DOI] [PubMed] [Google Scholar]
- Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, Paessler S, Cisneros IE, 2020. Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain Behav Immun. [DOI] [PubMed] [Google Scholar]
- Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW, 2012. Exogenous peptide YY3–36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond) 36, 379–384. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Byrne LC, Hassani H, Ernfors P, Hökfelt T, 2004. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. Journal of Comparative Neurology 470, 256–265. [DOI] [PubMed] [Google Scholar]
- Fortin SM, Roitman MF, 2017. Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav 176, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, Aston-Jones G, 2019. The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology 44, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag C, Svendsen AB, Feldthus N, Løssl K, Sheikh SP, 1995. Coupling of the human Y2 receptor for neuropeptide Y and peptide YY to guanine nucleotide inhibitory proteins in permeabilized SMSKAN cells. J Neurochem 64, 643–650. [DOI] [PubMed] [Google Scholar]
- Gerald C, Walker MW, Vaysse PJ, He C, Branchek TA, Weinshank RL, 1995. Expression cloning and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J Biol Chem 270, 26758–26761. [DOI] [PubMed] [Google Scholar]
- Graham DL, Durai HH, Trammell TS, Noble BL, Mortlock DP, Galli A, Stanwood GD, 2020. A novel mouse model of glucagon-like peptide-1 receptor expression: A look at the brain. Journal of Comparative Neurology 528, 2445–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD, 2013. GLP-1 analog attenuates cocaine reward. Mol Psychiatry 18, 961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR, 1994. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept 51, 151–159. [DOI] [PubMed] [Google Scholar]
- Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS, 2013. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol 521, 2235–2261. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Walker MW, Gerald C, Weinshank R, Branchek TA, 1997. Distribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Molecular Brain Research 46, 223–235. [DOI] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, Greenwald MK, Nadipelli VR, Ling W, Heidbreder C, 2019. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 393, 778–790. [DOI] [PubMed] [Google Scholar]
- Haney M, 2009. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R, 2008. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199, 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE, 2010. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ, 2011a. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 19, 1342–1349. [DOI] [PubMed] [Google Scholar]
- Hayes Matthew R., Leichner Theresa M., Zhao S, Lee Grace S., Chowansky A, Zimmer D, De Jonghe Bart C., Kanoski Scott E., Grill Harvey J., Bence Kendra K., 2011b. Intracellular Signals Mediating the Food Intake-Suppressive Effects of Hindbrain Glucagon-like Peptide-1 Receptor Activation. Cell Metab 13, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Schmidt HD, 2016. GLP-1 influences food and drug reward. Curr Opin Behav Sci 9, 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD, 2018. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD, 2019. Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addict Biol 24, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Schmidt HD, 2019. Central GLP-1 receptors: Novel molecular targets for cocaine use disorder. Physiol Behav 206, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ, 2007. The physiology of glucagon-like peptide 1. Physiol Rev 87, 1409–1439. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Galli A, Thomsen M, Jensen KL, Thomsen GK, Klausen MK, Vilsbøll T, Christensen MB, Holst JJ, Owens A, Robertson S, Daws L, Zanella D, Gether U, Knudsen GM, Fink-Jensen A, 2020. Glucagon-like peptide-1 receptor regulation of basal dopamine transporter activity is species-dependent. Neurochem Int 138, 104772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, 2018. Gut-brain axis and addictive disorders: A review with focus on alcohol and drugs of abuse. Pharmacol Ther. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR, 2012. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62, 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra E, Chandarana K, Batterham RL, 2009. The role of peptide YY in appetite regulation and obesity. J Physiol 587, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD, 2005. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28, 1083–1091. [DOI] [PubMed] [Google Scholar]
- Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD, 2014. Donepezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addict Biol 19, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergaard M, Salinas CBG, Rehfeld JF, Secher A, Raun K, Wulff BS, 2019. PYY(3–36) and exendin-4 reduce food intake and activate neuronal circuits in a synergistic manner in mice. Neuropeptides 73, 89–95. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH, 1983. Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J Pharmacol Exp Ther 225, 378–386. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, 1980. Buprenorphine suppresses heroin use by heroin addicts. Science 207, 657–659. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, 1985. Behavioral pharmacology of buprenorphine. Drug and alcohol dependence 14, 283–303. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, 1982. Buprenorphine effects on human heroin self-administration: an operant analysis. Journal of Pharmacology and Experimental Therapeutics 223, 30–39. [PubMed] [Google Scholar]
- Mello NK, Negus SS, 1998. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther 285, 444–456. [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P, 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403, 261–280. [DOI] [PubMed] [Google Scholar]
- Milliken BT, Elfers C, Chepurny OG, Chichura KS, Sweet IR, Borner T, Hayes MR, De Jonghe BC, Holz GG, Roth CL, Doyle RP, 2021. Design and Evaluation of Peptide Dual-Agonists of GLP-1 and NPY2 Receptors for Glucoregulation and Weight Loss with Mitigated Nausea and Emesis. J Med Chem 64, 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK, 1976. Poison induced pica in rats. Physiol Behav 17, 691–697. [DOI] [PubMed] [Google Scholar]
- Mittapalli GK, Roberts E, 2014. Ligands of the neuropeptide Y Y2 receptor. Bioorg Med Chem Lett 24, 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR, 2006. Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859. [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR, 2005. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 146, 5120–5127. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Shioda S, Niehoff ML, Banks WA, 2003. Characterization of blood-brain barrier permeability to PYY3–36 in the mouse. J Pharmacol Exp Ther 306, 948–953. [DOI] [PubMed] [Google Scholar]
- Pages N, Orosco M, Fournier G, Rouch C, Hafi A, Gourch A, Comoy E, Bohuon C, 1991. The effects of chronic administration of morphine on the levels of brain and adrenal catecholamines and neuropeptide Y in rats. Gen Pharmacol 22, 943–947. [DOI] [PubMed] [Google Scholar]
- Pages N, Orosco M, Rouch C, Fournier G, Comoy E, Bohuon C, 1992. Brain and adrenal monoamines and neuropeptide Y in codeine tolerant rats. Gen Pharmacol 23, 159–163. [DOI] [PubMed] [Google Scholar]
- Parker RMC, Herzog H, 1999. Regional distribution of Y-receptor subtype mRNAs in rat brain. European Journal of Neuroscience 11, 1431–1448. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM, 2011. Effects of exendin-4 alone and with peptide YY(3–36) on food intake and body weight in diet-induced obese rats. Obesity (Silver Spring) 19, 121–127. [DOI] [PubMed] [Google Scholar]
- Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M, 2011. Peptide hormones regulating appetite--focus on neuroimaging studies in humans. Diabetes Metab Res Rev 27, 104–112. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, 2016. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology 41, 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JB, Gregersen NT, Pedersen SD, Arentoft JL, Ritz C, Schwartz TW, Holst JJ, Astrup A, Sjödin A, 2014. Effects of PYY3–36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab 306, E1248–1256. [DOI] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP, 2013. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLOS ONE 8, e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, Davis JF, 2016. Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiol Behav 161, 140–144. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Caine SB, Thomsen M, 2016. Effects of the GLP-1 Agonist Exendin-4 on Intravenous Ethanol Self-Administration in Mice. Alcohol Clin Exp Res 40, 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A, 2015. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav 149, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer U, Arnold M, Weber E, Langhans W, 2013. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology 154, 193–204. [DOI] [PubMed] [Google Scholar]
- Stadlbauer U, Weber E, Langhans W, Meyer U, 2014. The Y2 receptor agonist PYY(3–36) increases the behavioural response to novelty and acute dopaminergic drug challenge in mice. Int J Neuropsychopharmacol 17, 407–419. [DOI] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL, 2020. Opioid use disorder. Nat Rev Dis Primers 6, 3. [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL, 2005. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146, 3748–3756. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Dencker D, Wörtwein G, Weikop P, Egecioglu E, Jerlhag E, Fink-Jensen A, Molander A, 2017. The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav 160, 14–20. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML, 2019. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44, 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu XA, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ, 2017. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci 20, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallöf D, Kalafateli AL, Jerlhag E, 2020. Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl Psychiatry 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ, 2014. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. The European journal of neuroscience 40, 3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL, 2012. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344, d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Jones EB, Einstein EB, Wargo EM, 2019. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry 76, 208–216. [DOI] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL, 2011. Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 59, 385–390. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TT, Chandrasekaran RY, Bradley J, Rubitski D, Berke H, Mente S, Butler T, Doran A, Chang C, Fisher K, Knafels J, Liu S, Ohren J, Marconi M, DeMarco G, Sneed B, Walton K, Horton D, Rosado A, Mead A, 2014. Casein kinase 1δ/ε inhibitor PF-5006739 attenuates opioid drug-seeking behavior. ACS Chem Neurosci 5, 1253–1265. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ, 2013. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65, 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H, Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldbye DP, Klemp K, Madsen TM, 1998. Neuropeptide Y attenuates naloxone-precipitated morphine withdrawal via Y5-like receptors. J Pharmacol Exp Ther 284, 633–636. [PubMed] [Google Scholar]
- Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, Schmidt HD, 2019. Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]


