Abstract
Due to the ongoing opioid epidemic, innovative scientific perspectives and approaches are urgently needed to reduce the unprecedented personal and societal burdens of nonmedical and recreational opioid use. One promising opportunity is to focus on the relationship between sleep deficiency and opioid use. In this review, we examine empirical evidence: (1) at the interface of sleep deficiency and opioid use, including hypothesized bidirectional associations between sleep efficiency and opioid abstinence; (2) as to whether normalization of sleep deficiency might directly or indirectly improve opioid abstinence (and vice versa); and (3) regarding mechanisms that could link improvements in sleep to opioid abstinence. Based on available data, we identify candidate sleep-restorative therapeutic approaches that should be examined in rigorous clinical trials.
Keywords: Sleep deficiency, Opioid use, Abstinence, Stress, Relapse, Treatment
1. Responding to the Opioid Epidemic
Opioid overdoses, emergency department visits, and deaths related to opioid use have risen precipitously over the past two decades. These and other adverse consequences of opioid use (e.g. unemployment, social and legal problems) are a major ongoing burden for individuals, families and society,1,2 leading to aggressive federal, state and local policies and funding to combat this crisis including the NIH Helping to End Addiction Long-term (HEAL) Initiative (https://heal.nih.gov). It has been estimated that opioid misuse cost the US more than $2.5 trillion from 2015 through 2018.3
Substantial scientific evidence supports the effectiveness of medications for treating opioid use disorder (MOUD): methadone or buprenorphine are gold-standard agonist options for maintenance treatment and naltrexone is an antagonist option for relapse prevention. Despite the availability of these MOUD alternatives, the rate of treatment engagement for OUD in the US remains unacceptably low: only about 20% of patients with OUD are receiving any form of treatment,4–6 and few in the US receive MOUD.7–9 It is also well established that longer retention in OUD treatment is associated with improved outcomes including higher rates of opioid abstinence, less mortality risk, and lower healthcare utilization/costs.10–13 Nonetheless, an unacceptably high proportion of patients with OUD in treatment drop out; this can be driven by several factors including patient’s non-preference for existing treatments, side effects, or lack of efficacy.14 Patients who receive prompt follow-up care after opioid detoxification are at lower risk of subsequent mortality.15 Long-acting naltrexone is effective for preventing relapse after detoxification, but is presently used less often than opioid agonist therapies.16
The US Food and Drug Administration (FDA) recently emphasized the importance of expanding OUD treatment alternatives, especially non-opioid options that may have less misuse potential.17 Furthermore, the FDA has advocated for a holistic scientific approach to OUD, encouraging researchers to study and validate alternative, clinically meaningful endpoints besides drug abstinence. Among these phenotypes, remediation of sleep deficiency has tremendous potential to improve overall behavioral health and could partly mediate the successful outcome of drug abstinence.
2. Sleep Deficiency and Opioid Use
2a. Nomenclature
Throughout this review, we will use the broad term “sleep deficiency” to encompass non-restorative patterns of sleep including delayed sleep-onset, sleep-maintenance problems (e.g. difficulty returning to sleep after awakening and/or early awakenings), disruption of regular cycling between non-rapid eye movement (NREM) and rapid eye movement (REM) phases, and diagnoses of insomnia and other sleep disorders (e.g. apnea, periodic leg movements). Adopting this broader terminology can help to account for large differences in methodology across studies. However, when describing individual studies, we will refer to the specific characteristics of the sleep deficiency. Also, consistent with the literature and clinical practice, we define the metric “sleep efficiency” as (total sleep time ÷ time in bed); we will refer to improvements in sleep efficiency using this metric, and normalization as reaching 85% or higher during an 8-hr sleep opportunity.18,19
Likewise, we will broadly refer to “opioid use” to encompass the non-medical and recreational use of opioids (i.e. this review will generally not refer to appropriate FDA-indicated use of prescription opioid analgesics, because such behavior would not meet diagnostic criteria for OUD19). Again, when describing individual studies, we will use study-specific language.
2b. Sleep Deficiency and Substance Use Disorders
Across studies of individuals with varying substance use disorders (SUDs), it has been reported or experimentally demonstrated that sleep deficiency is related to increased pain sensitivity,20–24 emotion dysregulation,25–27 and impaired frontal-executive control and decision making.28–33 Furthermore, sleep deficiency is associated with increased propensity for lapse/relapse to substances34 including alcohol,35–38 tobacco/nicotine,26,39–41 cannabis,42–44 and methamphetamine,45 and has been hypothesized to impair extinction of drug-environment conditioning.46 However (as will be discussed in section 3), few clinical studies have explicitly addressed the role of sleep deficiency in opioid craving and relapse risk.
Figure 1 presents an overarching conceptual framework, which is intentionally broader in scope than this focused review, to situate our discussion in a more comprehensive context. There are hypothesized bidirectional relationships between sleep deficiency and opioid craving, opioid use, and severity of OUD,47–51 and between sleep deficiency and nociceptive behaviors52–56; although there are obvious relationships between pain and opioid use, this is not our emphasis here. These clinical problem areas – sleep deficiency, pain, and opioid use – are closely linked via activation of stress-regulatory systems: sympathetic nervous system (SNS), hypothalamic-pituitary-adrenal (HPA) axis, and inflammatory processes.57 We also represent negative mood states in this model, which are associated with these problem areas, but we touch only briefly on these issues.
Figure 1.
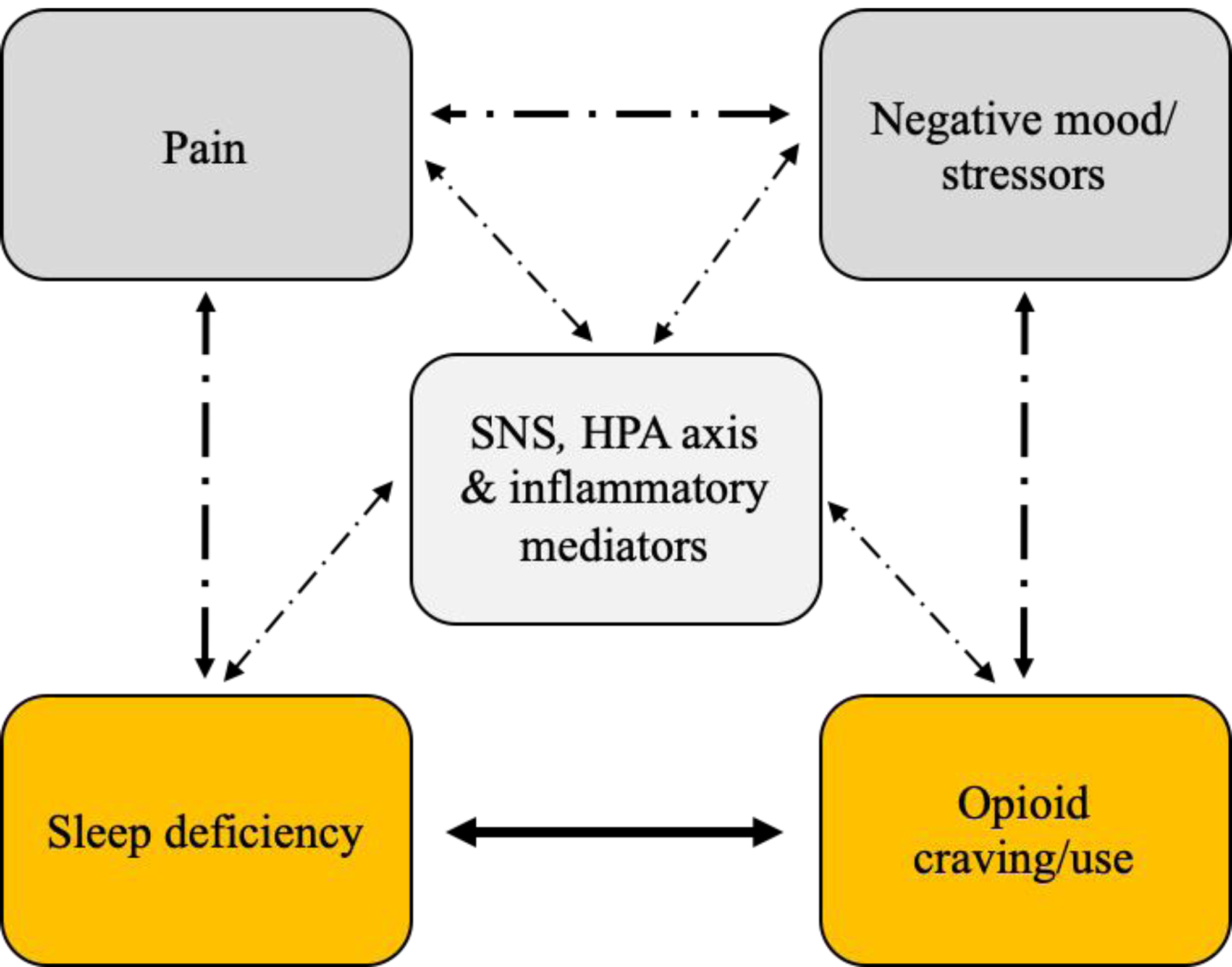
Conceptual model, illustrating hypothesized bidirectional relationships of sleep deficiency (focus of this review), and other salient factors (pain states, negative mood/stressors, physiological mediators), with opioid craving and/or use.
2c. Sleep-Related Effects of Opioid Use and Discontinuation
Starting about 50 years ago, rodent studies of opioid physical dependence and discontinuation were conducted, in which morphine, methadone, or l-alpha-acetyl-methadol were self-administered intravenously while EEG sleep and wake periods were continuously recorded across the 24-hr day; all three opioids disrupted the timing and normal staging of sleep.58 Specifically, sleep and wake episodes were systematically distributed within each inter-injection interval (≈3-hr with morphine and 8 injections per day) irrespective of the 12/12 light-dark cycle. Within each interval, the initial wake period was followed by solely NREM sleep, and then REM sleep intermixed with NREM and wake, and the next injection followed after a brief awakening.
Early clinical studies produced results that paralleled the animal studies above. In three separate double-blind placebo-controlled studies, morphine,59 heroin,60 or methadone61 were administered acutely to recently-abstinent opioid-dependent research participants, who would have otherwise entered a period of opioid withdrawal. These opioid agonists dose-dependently decreased total sleep time, stage 3–4 sleep time, and REM sleep time. All three opioids also increased brief arousals and frequency of sleep-stage changes (i.e. sleep fragmentation).
It is important to recognize that several factors may affect the generalizability of these conclusions. First, morphine, heroin and methadone are full mu-opioid agonists, but they differ in their duration of action and tendencies to produce receptor internalization; it is unknown whether pharmacokinetics or receptor signaling properties of different opioids may influence the degree of tolerance to opioid-related sleep outcomes. Second, we lack systematic data that would enable us to conclude whether misuse of other common opioids (e.g. oxycodone, hydrocodone, tramadol, fentanyl) produce the same effects on objectively-measured sleep efficiency or subjective sleep quality. Third, population differences (e.g. OUD, low back pain, cancer) complicate the interpretation of opioid agonist effects on sleep.
Opioid agonist-related sleep disruptions may also occur by disturbing respiratory mechanisms. Opioids cause hypoventilation, even controlling for cigarette use that is highly prevalent among those with OUD.62 It should also be recognized that other drugs used via smoking/inhalation routes (e.g. marijuana, cocaine) and through delivery vehicles (e.g. vaping devices) that contain respiratory-tract irritants could cause pulmonary inflammation (with possible airway stenosis or coughing) leading to sleep deficiency. Sleep itself normally reduces respiratory drive and, combined with opioids, the risk of sleep-disordered breathing (i.e. central sleep apnea) and the consequent disruption and fragmentation of sleep is heightened.63 In contrast to obstructive sleep apnea, which predominates in obese men and is related to excessive daytime sleepiness, central apnea as a general rule (independent of opioid use) is most typically is associated with insomnia.64 Central apnea is characterized by cessation of both respiratory effort and airflow and is related to awakening and not the brief EEG arousal of obstructed breathing.
Discontinuation of chronic opioid use is associated with protracted rebound of REM sleep, lasting 12 days in rodents65 and with sleep deficiency and disturbed sleep staging that can last many weeks in humans.66–68 Specifically, early-stage and protracted opioid withdrawal are associated with increased sleep latency, decreased total sleep time, decreased slow-wave sleep, and early-phase opioid withdrawal is associated with decreased REM sleep and increased REM latency.69 Notably, these outcomes are qualitatively similar to those produced by acutely administering mu-opioid agonists to recently-abstinent, opioid-dependent individuals (see above). In other words, objective sleep outcomes in states of opioid withdrawal and acute opioid administration (to physically-dependent individuals) are comparable and one pharmacological state is not inherently more deleterious than the other. As discussed in section 3b, evidence is mixed as to whether longer-term opioid agonist maintenance is associated with the development of tolerance to these objective measures of opioid-related sleep deficiency.
When people who were heroin-dependent and maintained on buprenorphine in an outpatient program were discontinued from their treatment both sleep latency and latency to REM sleep were prolonged and percent stage 3–4 sleep was reduced, compared to healthy controls.70 In that study, after a week of buprenorphine 4 mg/day, sleep patterns normalized. The improvement in sleep may be dose-related as 4 mg improved sleep, but 8 mg/day did not.71 In another study, methadone or buprenorphine dose-tapering led to changes in actigraphic recordings (a validated method of monitoring sleep and wake) indicative of disrupted sleep and circadian rhythm.72 Finally, two case reports of patients with chronic pain using opioids and with sleep-related disordered breathing suggest that discontinuation of chronic opioid use may resolve the breathing disturbance.73,74
Notably, the opioid antagonist naltrexone is not expected to resolve opioid detoxification-associated sleep disturbance. Naltrexone treatment has been associated with sleep deficiency (all based on self-reported symptoms) in newly-abstinent patients with opioid dependence.75–78 Therefore, use of naltrexone following opioid detoxification may not be addressing one hypothesized cause of relapse (i.e. continued sleep deficiency). However, some comparative data suggest that individuals maintained on naltrexone may experience less insomnia than individuals maintained on methadone79 or buprenorphine/naloxone.80 The latter two studies used different sleep metrics, i.e. polysomnogram79 vs. symptom reporting,80 which highlights the importance of using common objective sleep metrics in comparing OUD treatment effects on sleep.
2d. Sleep Deficiency and Stress
Insomnia disorder, and probably also comorbid insomnia that occurs in SUDs, is hypothesized to reflect a 24-hr state of hyperarousal.81 Evidence suggests that this physiologic hyperarousal is associated with activation of the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis. People with insomnia show elevated levels of circulating catecholamines,82 increased metabolic rates,83 increased body temperature,84 enhanced cardiovascular risk,85 and smaller resting pupil diameters.86 HPA axis activation in insomnia is indicated by elevated levels of night-time urinary free cortisol proportional to amount of wakefulness during the night.82 An activated SNS and HPA axis suggests a central mechanism, possibly involving corticotropin releasing factor neurons.87,88 Thus, when conducting studies that investigate links between sleep and OUD, it is important to measure biomarkers of HPA axis and SNS function including cortisol and catecholamines. Among patients with chronic insomnia, Vgontzas et al (1998) found that cortisol and catecholamine metabolites positively correlated with total wake time and stage 1 sleep.82
Chronic sleep deficiency may modulate stress-reactivity and its influence on craving and opioid use. In a recent experimental study of persons with OUD, average self-reported nightly sleep duration moderated the relationship between an acute laboratory stressor and opioid cue-induced craving response.89 Likewise, in a prospective observational study with daily measurements, lower-than-usual self-reported sleep quality was associated with greater opioid craving, a relationship that was mediated by decreased positive affect.50
3. Alleviating Sleep Deficiency as a Pathway to Improving Opioid Abstinence
Research findings reviewed above positively connect sleep deficiency with opioid use. This evidence motivates the strategy of using novel sleep-restoration strategies to facilitate opioid abstinence (e.g. in persons receiving opioid agonist treatment) and/or prevent relapse (e.g. following detoxification or treatment with naltrexone). Although the approach of treating sleep deficiency to promote abstinence from substance use has been explored in persons with other SUDs including alcohol,90–92 cocaine,93–97 cannabis,98,99 and tobacco,100 this has not been systematically and thoroughly investigated in persons with OUD. The overall model shown in Figure 1 and the specific sleep–opioid research literature makes a compelling case for exploring, in patients with OUD, non-opioid treatments that may reverse opioid medication-induced sleep deficiency and thereby help patients maintain opioid abstinence.
3a. Does Treatment that Normalizes Sleep Improve Opioid Use Outcomes?
Behavioral approaches.
Although there is substantive evidence that behavioral treatments can reduce sleep deficiency, data are lacking as to whether improved sleep mediates or moderates substance use.101 For instance, cognitive-behavioral treatment for insomnia (CBTi) has been shown to improve sleep in persons without SUDs102,103 but, to our knowledge, only one CBTi interventional study has been conducted in people with OUD. In a small-scale randomized-group study of methadone-maintained patients (11 per group), the authors104 found that, over the 8-week trial, CBTi significantly reduced sleep disturbance (measured with the Pittsburgh Sleep Quality Index [PSQI]105 compared to a behavioral placebo group (which focused on reducing conditioned arousal/frustration associated with not initiating or maintaining sleep). Similarly, mindfulness-based therapy for insomnia (MBTi) has been shown to be effective for improving sleep106 but there are no such studies with OUD. In a small randomized trial, yoga added to buprenorphine/naloxone treatment did not significantly improve sleep over buprenorphine/naloxone alone, and drug use outcomes were not reported.107 To our knowledge, there are no published studies of other behavioral treatments for sleep (e.g. stimulus control, paradoxical intention) that have been used in persons with OUD. In short, we presently lack data from non-pharmacological approaches to test the sleep-efficiency → opioid abstinence hypothesis.
Neuromodulation.
Previous studies have demonstrated that patients with insomnia exhibit heightened cortical excitability and hyperarousal, relative to healthy controls.108–111 Thus, neuromodulation techniques that inhibit cortical excitability/arousal could be useful in treating sleep deficiencies. Guided by this hypothesis, Babiloni et al (2020) systematically reviewed studies that used repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), or transcranial alternating current stimulation (tACS) in sleep-deficient populations.112 These authors concluded that multiple (10–14) sessions of inhibitory (1 Hz, 80–100% of resting motor threshold) rTMS over the dorsolateral prefrontal cortex or right posterior parietal cortex seem to improve subjective sleep quality and objective biomarkers (normalization of sleep efficiency and NREM/REM cycling) in patients with primary insomnia; however, studies reviewed typically had a high risk of bias (e.g. no sham control), tDCS and tACS research is less technically advanced than rTMS, and more rigorous clinical research is needed. Similar conclusions were reached in two other recent reviews of studies of rTMS for treating primary insomnia and other sleep disorders.113,114
Medication approaches.
Sleep deprivation has wide-ranging effects on the function of neurotransmitters and their receptors,115,116 implying that neuropsychopharmacological interventions that reverse these changes could be therapeutic. In the general population, current prescribed medications for treating insomnia include benzodiazepines, ‘z’-drugs (e.g. eszopiclone, zaleplon, zolpidem), gabapentin, antidepressants, melatonin receptor agonists, and atypical antipsychotics.117–123 Yet, these hypnotic agents have limited efficacy, can produce side effects (e.g. cognitive impairment, dizziness), and some display misuse potential.
In persons with OUD, physicians have increasingly eschewed prescribing benzodiazepines to treat sleep deficiency due to the significant added risk of respiratory depression, although some persons with OUD report using benzodiazepines without a prescription for sleep problems.124 Notably, the FDA (2016) issued a boxed warning related to combining opioids and benzodiazepines.125 These concerns of increased risk of respiratory depression or misuse when prescribing medications for insomnia to people with OUD lead to fewer insomnia treatment options available to this population. Misuse of gabapentin, a non-benzodiazepine with sedating and analgesic properties, appears to be greater in combination with opioids than gabapentin alone.126
Findings are mixed concerning the efficacy of medications other than benzodiazepines for improving sleep outcomes during opioid discontinuation. The α2-adrenergic agonist lofexidine has demonstrated some positive effects; however, these findings are not consistent. For patients who had begun opioid detoxification, lofexidine was found to reduce self-reported sleep problems to a greater extent than methadone dose tapering, measured over the course of 23–27 days of inpatient opioid detoxification66; however, that study allowed patients to choose which medication they would receive and assessments were conducted at different times across groups (i.e. two confounds), and other studies have not found this benefit (see review, Gish et al 2010).127 Quetiapine (an atypical antipsychotic with actions at dopamine, serotonin, histamine, muscarinic, and adrenergic receptors) has also been examined for this purpose. In a retrospective analysis of an observational study, 20% of outpatients undergoing opioid discontinuation reported that quetiapine helped to reduce their insomnia.128 Notably, in the same study, patients also reported that quetiapine reduced their opioid craving (74%), anxiety symptoms (48%), and somatic pain (22%), so it possible that quetiapine played an indirect role in reducing insomnia by attenuating these other symptoms.
Slightly more research has examined the efficacy of non-benzodiazepine medications for improving sleep outcomes during opioid agonist treatment. Melatonin may be effective in certain subsets of patients with OUD. Patients maintained on methadone seeking to stop their benzodiazepine misuse underwent 6 weeks of gradual clonazepam dose-tapering alongside either nightly melatonin treatment or placebo. The melatonin group demonstrated significantly improved subjective sleep quality but only among patients who could not discontinue their benzodiazepine use. For patients who discontinued benzodiazepines (i.e. only remained on methadone), the melatonin had no effect on sleep quality (measured with the PSQI); however, this group had a general improvement in sleep quality compared to those who did not discontinue benzodiazepine use.129 Certain medications, included long-term benzodiazepine use, have been associated with decreased levels of endogenous melatonin.130 These findings suggest that melatonin (which drives circadian rhythm) may improve sleep quality in patients receiving opioid agonist treatments, but only when they have concurrent dysfunction in the circadian system. Support for this hypothesis comes from a recent study, in which patients with OUD in methadone treatment received either 12 weeks of melatonin or placebo. Patients receiving melatonin had significant self-reported sleep improvements compared to placebo. Also, those receiving melatonin had reductions in depression and anxiety scores whereas the placebo group saw no change.131 Notably, all participants entering the study scored in the moderate-to-severe range on the Beck Depression Inventory. Depression can impair sleep outcomes and is associated with lower levels of melatonin and dysfunction of the melatonin system, further supporting a role for melatonin within a subset of patients with OUD.132
The atypical antipsychotic quetiapine has also been found to decrease sleep problems among patients with OUD maintained on methadone who also used methamphetamine; however, the study also demonstrated that patients in the quetiapine group (vs. placebo) showed improvements in depression and some cognitive functions. These findings, once again, suggest a role for quetiapine in improving sleep outcomes in patients with OUD, although these sleep-related effects may be indirectly related to improvements in other measures.133 Trazodone (a triazolopyridine derivative with activity at serotonergic, histaminic, and adrenergic receptors), the second most commonly-prescribed medication for treating insomnia in the United States, has shown improvements in sleep latency, sleep efficiency, and sleep duration in patients with insomnia. Although commonly prescribed for insomnia in patients with OUD, a double-blind, placebo-controlled randomized trial with methadone-maintained patients found that one month of trazodone treatment failed to produce subjective or objective sleep-restorative effects when compared to placebo.134 At present, there are no studies that demonstrate trazadone’s efficacy in this population. Zolpidem is the most commonly prescribed sleep aid in the United States; however, it may also be ineffective in this population. A small pilot study examining the efficacy of zolpidem and mirtazapine on sleep outcomes in methadone-maintained patients found that zolpidem had the poorest sleep outcomes even when compared to placebo. This same study showed that mirtazapine (30 mg IR) improved total sleep time, onset latency and sleep efficiency (measured with actigraphy) relative to zolpidem (12.5 mg SR), mirtazapine + zolpidem, and placebo.135 Notably, this study was insufficiently powered to fully compare between these groups, so the results should be considered preliminary. Nonetheless, the findings of this study and previous studies indicate that people with OUD should be considered a unique group when evaluating appropriate medications to improve sleep-related outcomes, and that the findings of studies in clinical groups without OUD may not translate to this population.
3b. Does Treatment of Opioid Use Disorder Improve Sleep Outcomes?
Literature is mixed as to whether sleep problems resolve with extended opioid agonist treatment and, if so, through what mechanisms. Some early studies found tolerance can develop to opioid agonist-induced sleep disruption61,136: in those studies, sleep fragmentation and REM sleep suppressive effects diminished within weeks. However, more recent studies in methadone- or buprenorphine-treated patients suggest that REM sleep remains suppressed70,137 and there can be excessive daytime sleepiness.138,139 Importantly, ongoing sleep disturbance during OUD treatment is often related to comorbid conditions,140,141 which are common in this population. For instance, one uncontrolled study found that self-reported sleep problems improved over 90 days of buprenorphine treatment; however, this could have been secondary to observed reduction in depression symptoms.142 A recent experimental study found that manipulating an exogenous factor, opioid agonist treatment clinic attendance schedule (earlier vs. later daytime hours), can modulate sleep deficiency (measured with electronic diary and actigraphy), with an earlier daily clinic attendance requirement leading to greater sleep disruption.143 Importantly, that study also found ongoing illicit opioid use was related to greater sleep disruption, suggesting that opioid maintenance dosing requirements and ‘on-top’ illicit opioid exposures may independently determine persistence of sleep deficiency (see section 2c). Taken together, findings point to the clinical importance of monitoring sleep problems using multiple methods during opioid agonist treatment,144 while accounting for substance use and co-occurring psychiatric (e.g. depression) and medical (e.g. respiratory) conditions. Given the current state of research evidence, it is not possible to make clear recommendations regarding the timing and/or efficacy of using medications for the treatment of OUD to resolve nonmedical or recreational opioid use-related sleep disturbances.
4. Mechanism-driven approaches for future investigation
Table 1 summarizes promising therapeutic candidates for future rigorous testing at the intersection of sleep deficiency and OUD. When experimental data are available, we highlight examples below of sleep-restorative interventions (cognitive-behavioral, neuromodulation, medication) in people with OUD who are receiving standard medication treatments (buprenorphine, methadone or naltrexone). In essence, this table identifies potential treatment-combination studies that could be designed for efficacy evaluations.
Table 1.
Treatment-combination matrix: testing candidate treatments for sleep deficiency in persons with opioid use disorder (OUD). Examples in the table refer to the few relevant published or ongoing clinical studies (see text for discussion).
| Primary medications for treating OUD | |||
|---|---|---|---|
| Sleep intervention | Buprenorphine | Methadone | Naltrexone |
| Cognitive-Behavioral | |||
| CBTi | [104] | ||
| MBTi | |||
| Yoga | [107] | ||
| Neuromodulation | |||
| rTMS | |||
| tDCS or tACS | |||
| Medications | |||
| 5-HT2,3 receptor antagonists | [133, 135] | ||
| Melatonin1,2 receptor agonists | [129, 131] | ||
| Endocannabinoid modulators | |||
| Orexin1,2 receptor antagonists | NCT04287062 | NCT04287062, NCT04262193 | |
Abbreviations: CBTi, cognitive behavioral treatment for insomnia; MBTi, mindfulness-based therapy for insomnia; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; tACS, transcranial alternating current stimulation.
Evidence reviewed above (section 3a) suggests that medications with primary GABAergic agonist actions are unlikely to be promising (i.e. not first-tier) candidates for managing sleep deficiency in opioid agonist-maintained patients with OUD, due to added risks of respiratory depression and misuse potential, as well as cognitive impairment, accidents, tolerance and rebound insomnia upon discontinuation.145 However, results from one small-scale study suggest a possible benefit of the benzodiazepine prazepam in opioid-detoxified, naltrexone-maintained patients.77 It is noteworthy that persons with OUD who use sedatives only as prescribed appear to experience fewer opioid-related consequences,146 so there is a subset of individuals with OUD for whom carefully-monitored, short-term hypnotic use could be acceptable. Clinical use of sleep-promoting gabapentinoids, which act through voltage-gated calcium channels and NMDA receptors (unlike benzodiazepines), requires careful assessment and risk stratification in the context of chronic opioid use but is not universally contraindicated.147
4a. Monoaminergic drugs
Medications with mixed monoaminergic effects are extensively used to treat neuropsychiatric conditions with affective features, and may alter sleep deficiency (which is correlated with those emotional disturbances). Extant data suggest it may be worthwhile to conduct studies to further investigate preliminary efficacy of the mixed-action D2/5-HT2A antagonist, 5HT1A partial agonist, and alpha1/histamine1 receptor antagonist quetiapine128 and the mixed-action, 5HT2/3 antagonist and alpha2 antagonist, mirtazapine135 in patients with uncomplicated OUD. Quetiapine may also be effective in reducing sleep problems among OUD patients with co-occurring psychostimulant use.133 Common receptor mechanisms linking quetiapine and mirtazapine are the 5HT2 antagonist and 5HT1A agonist actions; the 5HT2/3 receptor antagonism of mirtazapine may restore 5HT1A function that becomes desensitized during chronic sleep deprivation.148
4b. Melatonin receptor agonists
Melatonin is a hormone synthesized by the pineal gland, and functions in the suprachiasmatic nucleus to synchronize circadian cycling of central and peripheral biological functions including sleep/wake rhythm.149 Melatonin has promising effects on treating sleep deficiency and pain54,150,151 but, to our knowledge, only two clinical studies of melatonin have been conducted in relationship to sleep and opioid use. In one double-blind placebo-controlled, within-subject crossover trial, melatonin (5 mg/day prior to bedtime) improved self-reported sleep quality in methadone-maintained outpatients with co-occurring benzodiazepine use; however, melatonin had equivocal effects on benzodiazepine discontinuation.129 A double-blind, placebo-controlled, parallel-group study found melatonin (10mg/day prior to bedtime) supplementation during methadone maintenance improved subjective sleep, as well as depression and anxiety scores.131 The melatonin1/2 agonist ramelteon would also be a reasonable candidate for evaluation in this drug class, although there are no studies to date.
4c. Endocannabinoid modulators
Therapeutically-motivated use of cannabinoid (CB) drugs has been increasing in the general population, often in the absence of clear scientific evidence. It is therefore timely to consider whether drugs that modulate endocannabinoid (eCB) function could be useful in treating sleep deficiency. Δ9-tetrahydrocannabinol (THC), a CB1 receptor-preferring agonist, when administered acutely can decrease sleep onset latency, decrease waking after sleep onset, increase slow-wave sleep and decrease REM sleep time; however, repeated THC exposure can lead to tolerance to these effects, and THC discontinuation can produce rebound insomnia (review by Kesner & Lovinger 2020).152 Thus, orthosteric CB1 receptor-preferring agonists seem unlikely to be promising medications for treating sleep deficiency.
Cannabidiol (CBD) acts via multiple eCB mechanisms including CB1 negative allosteric modulation,153,154 and inhibition of anandamide reuptake,155 thereby increasing eCB signaling. CBD also has non-eCB pharmacological actions including inhibition of adenosine reuptake156 and 5HT1A partial agonism,157 each of which can promote sleep. At higher doses, CBD is sedating.158,159 Thus, although CBD has a complex pharmacology, its synergistic actions (reviews by Campos et al 2012; Izzo et al 2009; Mechoulam et al 2007) are likely to exert multiple sleep-promoting actions.160–162
Literature reviews related to cannabinoid impact on sleep efficiency as a primary outcome identified some initial supportive (but often mixed) evidence; authors have concluded that available studies were not of high quality, but that data offer an adequate ‘signal’ for designing well-controlled studies.163–165 There are virtually no published clinical trials testing cannabinoid impact on sleep efficiency in people with OUD. One interview study found that patients with concurrent DSM-IV cannabis and opioid dependence reported more sleep disturbance than patients with only cannabis dependence166; thus, consistent with evidence noted above regarding chronic THC exposure, excessive use of cannabis may worsen sleep deficiency. A randomized, placebo-controlled trial found – in post hoc analysis – that, among opioid-detoxification patients who received oral THC (dronabinol) in addition to extended-release naltrexone, individuals who smoked marijuana regularly (outpatient) reported less insomnia and anxiety, and were more likely to complete the trial, than those who did not smoke marijuana; dronabinol itself did not improve sleep over placebo.167
4d. Orexin receptor antagonists
Two research groups simultaneously discovered a cluster of 50,000–80,000 neurons located in the lateral hypothalamus that project widely throughout the brain to regulate sleep-wake, feeding, stress-reactivity, and drug-motivated behavior.168–170 These cells express two neuropeptides, named hypocretin by the sleep research group and orexin (OX) by the feeding research group. Lateral hypothalamic OX neurons project to cholinergic and monoaminergic systems, stimulating arousal and motivation. Orexin comes in two isoforms, orexin-A and orexin-B, with ≈50% sequence homology, derived from the same precursor protein, prepro-orexin; these isoforms bind to two subtypes of G protein-coupled receptors, orexin-1 (OX1) and orexin-2 (OX2). Orexin A has slightly higher affinity for OX1 than OX2 receptors, whereas orexin B has much higher affinity for OX2 than OX1 receptors.
The functional/behavioral significance and specificity of OX receptors is not clearly defined.170 Pre-clinical models suggest that OX2 receptors are primarily involved in maintaining wakefulness and arousal.171–175 Clinically, OX receptor antagonists are being developed; the dual OX1/2 antagonist, suvorexant, is the first-in-class FDA-approved hypnotic.176–178 Experiments have not directly parsed whether suvorexant’s OX2 or OX1 antagonism mediates its hypnotic effects; however, the OX2 selective antagonist seltorexant (an investigational drug) improves sleep in people with insomnia, which suggests OX2 antagonism is necessary for suvorexant’s hypnotic effects, as does pre-clinical literature.170,179–181
Suvorexant is effective for treating primary sleep-onset and/or sleep-maintenance insomnia in healthy persons.182,183 Notably, suvorexant improves sleep maintenance via sustained reduction of wake across the 8-hr sleep period, measured electrophysiologically as sleep efficiency (sleep time ÷ time-in-bed) or waking after sleep onset. That effect, in turn, likely will stabilize the ultradian rhythm of NREM-REM sleep, which is disrupted in SUDs (i.e. stage 3–4 and REM disturbance). Efficacy of suvorexant for promoting drug abstinence or preventing drug relapse directly, or indirectly by improving sleep efficiency, has not yet been evaluated among individuals with SUDs. As noted above, evidence indicates OX2 (more so than OX1) receptor antagonism mediates improvements in sleep maintenance. In contrast, OX1 (more so than OX2) antagonism attenuates stress-, cue-, and priming-induced reinstatement of drug seeking.184,185 Few animal studies have tested whether OX2 antagonists block drug reinstatement/ seeking, but some studies demonstrate a positive effect.186–188 Thus, it is possible that both OX1 and OX2 receptors modulate drug relapse-like behaviors, independent of sleep-wake function.
Accordingly, dual OX antagonism could be a unique strategy to target both insomnia and drug relapse. To date, clinical studies on the efficacy of suvorexant have focused solely on treating insomnia disorder; none have addressed the utility of suvorexant to improve sleep as an indirect means of reducing the risk of substance relapse, although this approach has recently been suggested for alcohol use disorder,189 comorbid cocaine and alcohol use disorders,190 and OUD.191,192 Our research team is presently conducting a randomized, placebo-controlled, mechanistic clinical trial to assess whether suvorexant (10 mg and 20 mg at bedtime) dose-dependently modulates sleep efficiency (following opioid detoxification, allowing for extended-release naltrexone maintenance) and subsequent opioid abstinence (across 3 months of weekly outpatient follow-up visits) in people with OUD (NCT04262193). We hypothesize that treatment aimed at improving sleep efficiency (i.e. reducing disruptive effects of wake intrusions on normal sleep staging) is a key mechanism for reducing the risk of drug relapse. Two other ongoing placebo-controlled randomized trials are examining whether suvorexant can increase total sleep time among patients with OUD undergoing supervised withdrawal (NCT03789214), and prevent relapse in recently-abstinent patients who are maintained on extended-release naltrexone or methadone (NCT04287062).
In the alcohol use disorder literature, two predictors of relapse are deficient NREM sleep (i.e. reduced age-corrected NREM stage 3–4 sleep) and shortened REM onset69,193; again, this has not studied with opioids. Both these effects directly relate to sleep maintenance and the normal NREM-REM ultradian rhythm. Normalization of ultradian rhythm by suvorexant (relative to placebo) should be reflected in REM latency of 80–120 min and REM percent of 18–24%.18 Notably, chronic opioid use and discontinuation both disturb sleep and its rhythms in two ways: (1) disruption of circadian timing of sleep such that sleep is tied to the user’s opioid intake irrespective of light-dark cycle; and (2) disruption of ultradian rhythm within sleep, the normal NREM-REM cycling. Thus, in our ongoing project (NCT04262193), we are anchoring sleep to the dark cycle with suvorexant and a fixed light-dark cycle with no daytime sleep, to increase likelihood of normal ultradian rhythmicity by suppressing wake intrusion throughout the 8-hr sleep period. We predict one or both suvorexant doses will produce more rapid resolution of this ultradian disturbance than placebo, i.e. sleep will normalize across assessment nights on the inpatient unit. We will test whether normalization of sleep efficiency predicts weekly opioid abstinence over a 3-month outpatient period.
5. Conclusions
Consistent with our conceptual model (Figure 1), the evidence reviewed here indicates that sleep deficiency and opioid use are likely bidirectionally linked, with additional bidirectional contributions (outside the scope of this review) of pain and affective distress. Although research findings suggest that sustained opioid abstinence can ultimately improve sleep efficiency, opioid agonist or naltrexone treatment for OUD may itself prevent full resolution of sleep deficiency. Thus, it is essential to develop and test non-opioid (including behavioral and neuromodulation) approaches in this clinical population. Presently, there are a few promising medication options including suvorexant, mirtazapine and quetiapine, and other possible targets (e.g. melatonin receptor agonists, eCB modulators) although all require rigorous testing. Furthermore, systematic investigations are needed to establish whether normalization of sleep efficiency is necessary for, or simply contributes to, opioid abstinence and recovery of global function. Such studies will need to account for the influence of demographic factors (e.g. sex, race, age), psychiatric conditions (e.g. depression, anxiety) and medical conditions (particularly, but not limited to, pulmonary disorders). In this way, we will be able to translate findings from mechanism to clinical application.
Acknowledgements
NIH HEAL Initiative grant U01 HL150551 from the National Heart, Lung and Blood Institute (MKG, TAR), the Gertrude Levin Endowed Chair in Addiction and Pain Biology (MKG), Helene Lycaki/Joe Young, Sr. Funds (Michigan Department of Health and Human Services), and the Detroit Wayne Integrated Health Network, supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflict of Interest
All authors have read and accept the journal’s authorship agreement and policy on conflicts of interest. Dr. Greenwald has received compensation as a consultant and speaker for Indivior Inc. and as consultant for Nirsum Labs and Supernus Pharmaceuticals, unrelated to this review. The authors report no conflicts of interest related to the content of this work.
References
- 1.American Society of Addiction Medicine. Opioid Addiction 2016 Facts & Figures.; 2016. http://www.drugabuse.gov/drugs-abuse/opioids. Accessed July 2, 2017.
- 2.Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD; 2016. [Google Scholar]
- 3.Council of Economic Advisers. The Full Cost of the Opioid Crisis: $2.5 Trillion Over Four Years.; 2019. https://www.whitehouse.gov/articles/full-cost-opioid-crisis-2-5-trillion-fouryears/. Accessed December 12, 2020.
- 4.Saloner B, Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013. JAMA. 2015;314(14):1515. doi: 10.1001/jama.2015.10345 [DOI] [PubMed] [Google Scholar]
- 5.Center for Behavioral Health Statistics. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD; 2018. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.pdf. Accessed January 4, 2019. [Google Scholar]
- 6.Wu L-T, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman KA, Ponce Terashima J, McCarty D. Opioid use disorder and treatment: Challenges and opportunities. BMC Health Serv Res. 2019;19(1):884. doi: 10.1186/s12913-019-4751-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011;5(1):21–27. doi: 10.1097/ADM.0b013e3181d41ddb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi: 10.1007/s11920-017-0783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hser Y-I, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23(2):76–89. doi: 10.1097/HRP.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 11.Ronquest N, Willson T, Montejano L, Nadipelli V, Wollschlaeger B. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;Volume 9:59–78. doi: 10.2147/SAR.S150253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiest K, Shaya G, Somaini L, Greenwald M. RBP-6000: A rationally designed prolonged-release buprenorphine formulation. Heroin Addict Relat Clin Probl. 2020;22. [Google Scholar]
- 14.O’Connor AM, Cousins G, Durand L, Barry J, Boland F. Retention of patients in opioid substitution treatment: A systematic review. Latkin CAed. PLoS One. 2020;15(5):e0232086. doi: 10.1371/journal.pone.0232086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt EM, Gupta S, Bowe T, et al. Predictive validity of a quality measure for intensive substance use disorder treatment. Subst Abus. 2017;38(3):317–323. doi: 10.1080/08897077.2016.1212779 [DOI] [PubMed] [Google Scholar]
- 16.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. FDA Takes New Steps to Encourage the Development of Novel Medicines for the Treatment of Opioid Use Disorder | FDA.; 2018. https://www.fda.gov/news-events/press-announcements/fda-takes-new-steps-encourage-development-novel-medicines-treatment-opioid-use-disorder. Accessed December 12, 2020.
- 18.Carskadon M, Dement W. Normal human sleep: an overview. In: M K TR, WC D, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:15–24. [Google Scholar]
- 19.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwell EE, Pfeifer JG, McCauley JL, Moran-Santa Maria M, Back SE. Sleep disturbances and pain among individuals with prescription opioid dependence. Addict Behav. 2014;39(10):1537–1542. doi: 10.1016/j.addbeh.2014.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson RA, Carter JR. Total sleep deprivation and pain perception during cold noxious stimuli in humans. Scand J Pain. 2016;13(1):12–16. doi: 10.1016/j.sjpain.2016.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. doi: 10.1093/sleep/29.2.145 [DOI] [PubMed] [Google Scholar]
- 23.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35(12):1667–1672. doi: 10.5665/sleep.2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardi NF, Lazzarim MK, Guilhen VA, et al. Chronic sleep restriction increases pain sensitivity over time in a periaqueductal gray and nucleus accumbens dependent manner. Neuropharmacology. 2018;139:52–60. doi: 10.1016/j.neuropharm.2018.06.022 [DOI] [PubMed] [Google Scholar]
- 25.Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Med Rev. 2015;24:83–100. doi: 10.1016/j.smrv.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 26.Fillo J, Alfano CA, Paulus DJ, et al. Emotion dysregulation explains relations between sleep disturbance and smoking quit-related cognition and behavior. Addict Behav. 2016;57:6–12. doi: 10.1016/j.addbeh.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klumpp H, Hosseini B, Phan KL. Self-reported sleep quality modulates amygdala resting-state functional connectivity in anxiety and depression. Front Psychiatry. 2018;9. doi: 10.3389/fpsyt.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Liang J, Lin X, et al. Sleep deprivation promotes habitual control over goal-directed control: Behavioral and neuroimaging evidence. J Neurosci. 2017;37(49):11979–11992. doi: 10.1523/JNEUROSCI.1612-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honn KA, Hinson JM, Whitney P, Van Dongen HPA. Cognitive flexibility: A distinct element of performance impairment due to sleep deprivation. Accid Anal Prev. 2019;126:191–197. doi: 10.1016/j.aap.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 30.Massar SAA, Lim J, Sasmita K, Chee MWL. Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia. 2019;123:169–177. doi: 10.1016/j.neuropsychologia.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 31.Mullette-Gillman OA, Kurnianingsih YA, Liu JCJ. Sleep deprivation alters choice strategy without altering uncertainty or loss aversion preferences. Front Neurosci. 2015;9. doi: 10.3389/fnins.2015.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stojanoski B, Benoit A, Van Den Berg N, et al. Sustained vigilance is negatively affected by mild and acute sleep loss reflected by reduced capacity for decision making, motor preparation, and execution. Sleep. 2019;42(1). doi: 10.1093/sleep/zsy200 [DOI] [PubMed] [Google Scholar]
- 33.Trksak GH, Bracken BK, Jensen JE, et al. Effects of sleep deprivation on brain bioenergetics, sleep, and cognitive performance in cocaine-dependent individuals. Sci World J. 2013;2013:1–10. doi: 10.1155/2013/947879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentino RJ, Volkow ND. Drugs, sleep, and the addicted brain. Neuropsychopharmacology. 2020;45(1):3–5. doi: 10.1038/s41386-019-0465-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49(4):417–427. doi: 10.1016/j.alcohol.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24(9):1376–1384. http://www.ncbi.nlm.nih.gov/pubmed/11003203. [PubMed] [Google Scholar]
- 37.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence. JAMA Intern Med. 2014;174(1):70. doi: 10.1001/jamainternmed.2013.11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller MB, Donahue ML, Carey KB, Scott-Sheldon LAJ. Insomnia treatment in the context of alcohol use disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2017;181:200–207. doi: 10.1016/j.drugalcdep.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair US, Haynes P, Collins BN. Baseline sleep quality is a significant predictor of quit-day smoking self-efficacy among low-income treatment-seeking smokers. J Health Psychol. 2019;24(11):1484–1493. doi: 10.1177/1359105317740619 [DOI] [PubMed] [Google Scholar]
- 40.Patterson F, Grandner MA, Malone SK, Rizzo A, Davey A, Edwards DG. Sleep as a target for optimized response to smoking cessation treatment. Nicotine Tob Res. 2019;21(2):139–148. doi: 10.1093/ntr/ntx236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short NA, Mathes BM, Gibby B, Oglesby ME, Zvolensky MJ, Schmidt NB. Insomnia symptoms as a risk factor for cessation failure following smoking cessation treatment. Addict Res Theory. 2017;25(1):17–23. doi: 10.1080/16066359.2016.1190342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babson KA, Boden MT, Harris AH, Stickle TR, Bonn-Miller MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. J Subst Abuse Treat. 2013;44(4):438–443. doi: 10.1016/j.jsat.2012.08.224 [DOI] [PubMed] [Google Scholar]
- 43.Conroy DA, Arnedt JT. Sleep and substance use disorders: An update. Curr Psychiatry Rep. 2014;16(10):487. doi: 10.1007/s11920-014-0487-3 [DOI] [PubMed] [Google Scholar]
- 44.Haney M, Bedi G, Cooper ZD, et al. Predictors of marijuana relapse in the human laboratory: Robust impact of tobacco cigarette smoking status. Biol Psychiatry. 2013;73(3):242–248. doi: 10.1016/j.biopsych.2012.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi-Haghighi S, Haghparast A. Cannabidiol inhibits priming-induced reinstatement of methamphetamine in REM sleep deprived rats. Prog Neuro-Psychopharmacology Biol Psychiatry. 2018;82:307–313. doi: 10.1016/j.pnpbp.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 46.Berro LF, Frussa-Filho R, Tufik S, Andersen ML. Relationships between sleep and addiction: The role of drug-environment conditioning. Med Hypotheses. 2014;82(3):374–376. doi: 10.1016/j.mehy.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 47.Boscarino J, Hoffman S, Han J. Opioid-use disorder among patients on long-term opioid therapy: Impact of final DSM-5 diagnostic criteria on prevalence and correlates. Subst Abuse Rehabil. August 2015:83. doi: 10.2147/SAR.S85667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eacret D, Veasey SC, Blendy JA. Bidirectional relationship between opioids and disrupted sleep: Putative mechanisms. Mol Pharmacol. 2020;98(4):445–453. doi: 10.1124/mol.119.119107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groenewald CB, Law EF, Rabbitts JA, Palermo TM. Associations between adolescent sleep deficiency and prescription opioid misuse in adulthood. Sleep. September 2020. doi: 10.1093/sleep/zsaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lydon-Staley DM, Cleveland HH, Huhn AS, et al. Daily sleep quality affects drug craving, partially through indirect associations with positive affect, in patients in treatment for nonmedical use of prescription drugs. Addict Behav. 2017;65:275–282. doi: 10.1016/j.addbeh.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fathi HR, Yoonessi A, Khatibi A, Rezaeitalab F, Rezaei-Ardani A. Crosstalk between sleep disturbance and opioid use disorder: A narrative review. Addict Heal. 2020;12(2):140–158. doi: 10.22122/ahj.v12i2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain: A tale of two common problems. Chest. 2018;154(5):1249–1259. doi: 10.1016/j.chest.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 53.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–216. doi: 10.1038/s41386-019-0439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lautenbacher S, Kundermann B, Krieg J. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi: 10.1016/j.smrv.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 56.Stroemel-Scheder C, Kundermann B, Lautenbacher S. The effects of recovery sleep on pain perception: A systematic review. Neurosci Biobehav Rev. 2020;113:408–425. doi: 10.1016/j.neubiorev.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 57.Irwin MR, Opp MR. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42(1):129–155. doi: 10.1038/npp.2016.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreton JE, Roehrs T, Khazan N. Drug self-administration and sleep-awake activity in rats dependent on morphine, methadone, or l-alpha-acetylmethadol. Psychopharmacology (Berl). 1976;47:237–241. [DOI] [PubMed] [Google Scholar]
- 59.Kay DC, Eisenstein RB, Jasinski DR. Morphine effects on human REM state, waking state and NREM sleep. Psychopharmacologia. 1969;14(5):404–416. doi: 10.1007/BF00403581 [DOI] [PubMed] [Google Scholar]
- 60.Kay D, Pickworth W, Neider G. Morphine-like insomnia from heroin in nondependent human addicts. Br J Clin Pharmacol. 1981;11(2):159–169. doi: 10.1111/j.1365-2125.1981.tb01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickworth WB, Neidert GL, Kay DC. Morphine like arousal by methadone during sleep. Clin Pharmacol Ther. 1981;30(6):796–804. doi: 10.1038/clpt.1981.240 [DOI] [PubMed] [Google Scholar]
- 62.Greenwald MK. Effects of opioid dependence and tobacco use on ventilatory response to progressive hypercapnia. Pharmacol Biochem Behav. 2004;77(1):39–47. doi: 10.1016/j.pbb.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11(1):35–46. doi: 10.1016/j.smrv.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 64.Zinchuk AV, Thomas RJ. Central sleep apnea: diagnosis and management. In: MH K TR, eds. Principles and Practice of Sleep Medicine. Philadelphia, PA; 2017:1059–1075. [Google Scholar]
- 65.Khazan N, Colasanti B. Protracted rebound in rapid movement sleep time and electroencephalogram voltage output in morphine-dependent rats upon withdrawal. J Pharmacol Exp Ther. 1972;183(1):23–30. http://www.ncbi.nlm.nih.gov/pubmed/4342927. [PubMed] [Google Scholar]
- 66.Beswick T, Best D, Rees S, Bearn J, Gossop M, Stang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: Differences between methadone and lofexidine detoxification treatments. Addict Biol. 2003;8(1):49–57. doi: 10.1080/1355621031000069882 [DOI] [PubMed] [Google Scholar]
- 67.Gossop M, Bradley B. Insomnia among addicts during supervised withdrawal from opiates: A comparison of oral methadone and electrostimulation. Drug Alcohol Depend. 1984;13(2):191–198. doi: 10.1016/0376-8716(84)90058-9 [DOI] [PubMed] [Google Scholar]
- 68.Dijkstra BAG, De Jong CAJ, Krabbe PFM, van der Staak CPF. Prediction of abstinence in opioid-dependent patients. J Addict Med. 2008;2(4):194–201. doi: 10.1097/ADM.0b013e31818a6596 [DOI] [PubMed] [Google Scholar]
- 69.Angarita GA, Emadi N, Hodges S, Morgan PT. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 2016;11(1):9. doi: 10.1186/s13722-016-0056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farney RJ, McDonald AM, Boyle KM, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J. 2013;42(2):394–403. doi: 10.1183/09031936.00120012 [DOI] [PubMed] [Google Scholar]
- 71.Lukas SE, Dorsey CM, Mello NK, et al. Reversal of sleep disturbances in cocaine- and heroin-dependent men during chronic buprenorphine treatment. Exp Clin Psychopharmacol. 1996;4(4):413–420. doi: 10.1037/1064-1297.4.4.413 [DOI] [Google Scholar]
- 72.Pjrek E, Frey R, Naderi-Heiden A, et al. Actigraphic measurements in opioid detoxification with methadone or buprenorphine. J Clin Psychopharmacol. 2012;32(1):75–82. doi: 10.1097/JCP.0b013e31823f91d1 [DOI] [PubMed] [Google Scholar]
- 73.Davis MJ, Livingston M, Scharf SM. Reversal of central sleep apnea following discontinuation of opioids. J Clin Sleep Med. 2012;08(05):579–580. doi: 10.5664/jcsm.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Javaheri S, Patel S. Opioids cause central and complex sleep apnea in humans and reversal with discontinuation: A plea for detoxification. J Clin Sleep Med. 2017;13(06):829–833. doi: 10.5664/jcsm.6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll KM, Nich C, Frankforter TL, et al. Accounting for the uncounted: Physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug Alcohol Depend. 2018;192:264–270. doi: 10.1016/j.drugalcdep.2018.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ndegwa S, Pant S, Pohar S, Mierzwinski-Urban M. Injectable Extended-Release Naltrexone to Treat Opioid Use Disorder. Canadian Agency for Drugs and Technologies in Health; 2016. http://www.ncbi.nlm.nih.gov/pubmed/29400929. Accessed December 12, 2020. [PubMed] [Google Scholar]
- 77.Stella L, D’Ambra C, Mazzeo F, et al. Naltrexone plus benzodiazepine aids abstinence in opioid-dependent patients. Life Sci. 2005;77(21):2717–2722. doi: 10.1016/j.lfs.2005.05.036 [DOI] [PubMed] [Google Scholar]
- 78.Syed YY, Keating GM. Extended-release intramuscular naltrexone (VIVITROL®): A review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs. 2013;27(10):851–861. doi: 10.1007/s40263-013-0110-x [DOI] [PubMed] [Google Scholar]
- 79.Staedt J, Wassmuth F, Stoppe G, et al. Effects of chronic treatment with methadone and naltrexone on sleep in addicts. Eur Arch Psychiatry Clin Neurosci. 1996;246(6):305–309. doi: 10.1007/BF02189023 [DOI] [PubMed] [Google Scholar]
- 80.Latif Z-H, Šaltyte Benth J, Solli KK, et al. Anxiety, depression, and insomnia among adults With opioid dependence treated with extended-release naltrexone vs buprenorphine-naloxone. JAMA Psychiatry. 2019;76(2):127. doi: 10.1001/jamapsychiatry.2018.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roehrs T, Gumenyuk V, Drake C, Roth T. Physiological correlates of insomnia. Curr Top Behav Neurosci. 2014;21:277–290. doi: 10.1007/7854_2014_324 [DOI] [PubMed] [Google Scholar]
- 82.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system. J Psychosom Res. 1998;45(1):21–31. doi: 10.1016/S0022-3999(97)00302-4 [DOI] [PubMed] [Google Scholar]
- 83.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–588. doi: 10.1093/sleep/18.7.581 [DOI] [PubMed] [Google Scholar]
- 84.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23(4):504–510. [PubMed] [Google Scholar]
- 85.Laharnar N, Grote L, Zou D, et al. Overnight pulse wave analysis to assess autonomic changes during sleep in insomnia patients and healthy sleepers. Romigi A, ed. PLoS One. 2020;15(5):e0232589. doi: 10.1371/journal.pone.0232589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichstein KL, Johnson RS. Pupillometric discrimination of insomniacs. Behav Res Ther. 1994;32(1):123–129. doi: 10.1016/0005-7967(94)90093-0 [DOI] [PubMed] [Google Scholar]
- 87.Roth T, Roehrs T, Pies R. Insomnia: Pathophysiology and implications for treatment. Sleep Med Rev. 2007;11(1):71–79. doi: 10.1016/j.smrv.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 88.Sanford LD, Suchecki D, Meerlo P. Stress, arousal, and sleep. Curr Top Behav Neurosci. 2015;25:379–410. doi: 10.1007/7854_2014_314 [DOI] [PubMed] [Google Scholar]
- 89.Teeters JB, Jones JL, Jarnecke AM, Back SE. Sleep moderates the relationship between stress and craving in individuals with opioid use disorder. Exp Clin Psychopharmacol. April 2020. doi: 10.1037/pha0000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: A randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227–233. doi: 10.1016/j.brat.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolla BP, Schneekloth TD, Biernacka JM, et al. Trazodone and alcohol relapse: A retrospective study following residential treatment. Am J Addict. 2011;20(6):525–529. doi: 10.1111/j.1521-0391.2011.00172.x [DOI] [PubMed] [Google Scholar]
- 93.Afshar M, Knapp CM, Sarid-Segal O, et al. The efficacy of mirtazapine in the treatment of cocaine dependence with comorbid depression. Am J Drug Alcohol Abuse. 2012;38(2):181–186. doi: 10.3109/00952990.2011.644002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gómez Pérez LJ, Cardullo S, Cellini N, et al. Sleep quality improves during treatment with repetitive transcranial magnetic stimulation (rTMS) in patients with cocaine use disorder: a retrospective observational study. BMC Psychiatry. 2020;20(1):153. doi: 10.1186/s12888-020-02568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan PT, Angarita GA, Canavan S, et al. Modafinil and sleep architecture in an inpatient–outpatient treatment study of cocaine dependence. Drug Alcohol Depend. 2016;160:49–56. doi: 10.1016/j.drugalcdep.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suchting R, Yoon JH, Miguel GGS, et al. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Res. 2020;1731:146359. doi: 10.1016/j.brainres.2019.146359 [DOI] [PubMed] [Google Scholar]
- 97.Winhusen TM, Theobald J, Lewis DF. Substance use outcomes in cocaine-dependent tobacco smokers: A mediation analysis exploring the role of sleep disturbance, craving, anxiety, and depression. J Subst Abuse Treat. 2019;96:53–57. doi: 10.1016/j.jsat.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haney M, Hart CL, Vosburg SK, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl). 2010;211(2):233–244. doi: 10.1007/s00213-010-1888-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38(8):1557–1565. doi: 10.1038/npp.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soreca I, Conklin CA, Vella EJ, et al. Can exercise alleviate sleep disturbances during acute nicotine withdrawal in cigarette smokers? Exp Clin Psychopharmacol. October 2020. doi: 10.1037/pha0000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chakravorty S, Vandrey RG, He S, Stein MD. Sleep management among patients with substance use disorders. Med Clin North Am. 2018;102(4):733–743. doi: 10.1016/j.mcna.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia. Ann Intern Med. 2015;163(3):191. doi: 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- 103.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Med Rev. 2018;38:3–16. doi: 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 104.Robabeh S, Jafar MM, Sharareh H, Maryam HR, Masoumeh E. The Effect of Cognitive Behavior Therapy in Insomnia due to Methadone Maintenance Therapy: A Randomized Clinical Trial. Iran J Med Sci. 2015;40(5):396–403. http://www.ncbi.nlm.nih.gov/pubmed/26379345. [PMC free article] [PubMed] [Google Scholar]
- 105.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 106.Martires J, Zeidler M. The value of mindfulness meditation in the treatment of insomnia. Curr Opin Pulm Med. 2015;21(6):547–552. doi: 10.1097/MCP.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 107.Lander L, Downs KC, Andrew M, Rader G, Dohar S, Waibogha K. Yoga as an adjunctive intervention to medication-assisted treatment with buprenorphine+naloxone. J Addict Res Ther. 2017;08(06). doi: 10.4172/2155-6105.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 109.Huang Z, Zhan S, Li N, Ding Y, Wang Y. Abnormal recovery function of somatosensory evoked potentials in patients with primary insomnia. Psychiatry Res. 2012;198(3):463–467. doi: 10.1016/j.psychres.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 110.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 111.van der Werf YD, Altena E, van Dijk KD, et al. Is disturbed intracortical excitability a stable trait of chronic insomnia? A study using transcranial magnetic stimulation before and after multimodal sleep therapy. Biol Psychiatry. 2010;68(10):950–955. doi: 10.1016/j.biopsych.2010.06.028 [DOI] [PubMed] [Google Scholar]
- 112.Babiloni AH, Bellemare A, Beetz G, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: A systematic review. Sleep Med Rev. 2021;55:101381. doi: 10.1016/j.smrv.2020.101381 [DOI] [PubMed] [Google Scholar]
- 113.Nardone R, Sebastianelli L, Versace V, et al. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 2020;71:113–121. doi: 10.1016/j.sleep.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 114.Sun N, He Y, Wang Z, Zou W, Liu X. The effect of repetitive transcranial magnetic stimulation for insomnia: A systematic review and meta-analysis. Sleep Med. May 2020. doi: 10.1016/j.sleep.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 115.Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64(7–8):934–946. doi: 10.1007/s00018-007-6457-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Longordo F, Kopp C, Lüthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29(9):1810–1819. doi: 10.1111/j.1460-9568.2009.06719.x [DOI] [PubMed] [Google Scholar]
- 117.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37(2):343–349. doi: 10.5665/sleep.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Everitt H, Baldwin DS, Stuart B, et al. Antidepressants for insomnia in adults. Cochrane Database Syst Rev. 2018;5:CD010753. doi: 10.1002/14651858.CD010753.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaffer KY, Chang T, Vanle B, et al. Trazodone for insomnia: A systematic review. Innov Clin Neurosci. 2017;14(7–8):24–34. doi:29552421 [PMC free article] [PubMed] [Google Scholar]
- 120.Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 2014;15(9):15924–15950. doi: 10.3390/ijms150915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: A meta-analysis of polysomnographic randomized controlled trials. Sleep Med. 2017;34:126–133. doi: 10.1016/j.sleep.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 122.McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep. 2012;14(5):494–502. doi: 10.1007/s11920-012-0302-y [DOI] [PubMed] [Google Scholar]
- 123.Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9(4):728–738. doi: 10.1007/s13311-012-0148-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stein MD, Kanabar M, Anderson BJ, Lembke A, Bailey GL. Reasons for benzodiazepine use among persons seeking opioid detoxification. J Subst Abuse Treat. 2016;68:57–61. doi: 10.1016/j.jsat.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Food and Drug Administration. FDA Drug Safety Communication: FDA Warns about Serious Risks and Death When Combining Opioid Pain or Cough Medicines with Benzodiazepines; Requires Its Strongest Warning | FDA.; 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-serious-risks-and-death-when-combining-opioid-pain-or. Accessed December 12, 2020.
- 126.Peckham AM, Evoy KE, Covvey JR, Ochs L, Fairman KA, Sclar DA. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacother J Hum Pharmacol Drug Ther. 2018;38(4):436–443. doi: 10.1002/phar.2096 [DOI] [PubMed] [Google Scholar]
- 127.Gish EC, Miller JL, Honey BL, Johnson PN. Lofexidine, an α2-Receptor agonist for opioid detoxification. Ann Pharmacother. 2010;44(2):343–351. doi: 10.1345/aph.1M347 [DOI] [PubMed] [Google Scholar]
- 128.Pinkofsky HB, Hahn AM, Campbell FA, Rueda J, Daley DC, Douaihy AB. Reduction of opioid-withdrawal symptoms with quetiapine. J Clin Psychiatry. 2005;66(10):1285–1288. doi: 10.4088/JCP.v66n1011 [DOI] [PubMed] [Google Scholar]
- 129.Peles E, Hetzroni T, Bar-Hamburger R, Adelson M, Schreiber S. Melatonin for perceived sleep disturbances associated with benzodiazepine withdrawal among patients in methadone maintenance treatment: a double-blind randomized clinical trial. Addiction. 2007;102(12):1947–1953. doi: 10.1111/j.1360-0443.2007.02007.x [DOI] [PubMed] [Google Scholar]
- 130.Kabuto M, Namura I, Saitoh Y. Nocturnal enhancement of plasma melatonin could be suppressed by benzodiazepines in humans. Endocrinol Jpn. 1986;33(3):405–414. doi: 10.1507/endocrj1954.33.405 [DOI] [PubMed] [Google Scholar]
- 131.Ghaderi A, Banafshe HR, Mirhosseini N, et al. The effects of melatonin supplementation on mental health, metabolic and genetic profiles in patients under methadone maintenance treatment. Addict Biol. 2019;24(4):754–764. doi: 10.1111/adb.12650 [DOI] [PubMed] [Google Scholar]
- 132.Valdés-Tovar M, Estrada-Reyes R, Solís-Chagoyán H, et al. Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. Br J Pharmacol. 2018;175(16):3200–3208. doi: 10.1111/bph.14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Javdan NS, Ghoreishi FS, Sehat M, Ghaderi A, Banafshe HR. Mental health and cognitive function responses to quetiapine in patients with methamphetamine abuse under methadone maintenance treatment. J Affect Disord. 2019;251:235–241. doi: 10.1016/j.jad.2019.03.078 [DOI] [PubMed] [Google Scholar]
- 134.Stein MD, Kurth ME, Sharkey KM, Anderson BJ, Corso RP, Millman RP. Trazodone for sleep disturbance during methadone maintenance: A double-blind, placebo-controlled trial. Drug Alcohol Depend. 2012;120(1–3):65–73. doi: 10.1016/j.drugalcdep.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stein MD, Kurth ME, Anderson BJ, Blevins CE. A pilot crossover trial of sleep medications for sleep-disturbed methadone maintenance patients. J Addict Med. 2020;14(2):126–131. doi: 10.1097/ADM.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kay DC. Human sleep during chronic morphine intoxication. Psychopharmacologia. 1975;44(2):117–124. doi: 10.1007/BF00420997 [DOI] [PubMed] [Google Scholar]
- 137.Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348–1356. doi: 10.1378/chest.128.3.1348 [DOI] [PubMed] [Google Scholar]
- 138.Hallinan R, Elsayed M, Espinoza D, et al. Insomnia and excessive daytime sleepiness in women and men receiving methadone and buprenorphine maintenance treatment. Subst Use Misuse. 2019;54(10):1589–1598. doi: 10.1080/10826084.2018.1552298 [DOI] [PubMed] [Google Scholar]
- 139.Tripathi R, Dhawan A, Rao R, Mishra AK, Jain R, Sinha S. Assessment of subjective sleep problems in men with opioid dependence maintained on buprenorphine. J Addict Med. 2020;14(2):132–138. doi: 10.1097/ADM.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 140.Dunn KE, Finan PH, Andrew Tompkins D, Strain EC. Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addict Behav. 2018;76:8–14. doi: 10.1016/j.addbeh.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nordmann S, Lions C, Vilotitch A, et al. A prospective, longitudinal study of sleep disturbance and comorbidity in opiate dependence (the ANRS Methaville study). Psychopharmacology (Berl). 2016;233(7):1203–1213. doi: 10.1007/s00213-016-4202-4 [DOI] [PubMed] [Google Scholar]
- 142.Zheng W, Wakim R, Geary R, et al. Self-reported sleep Improvement in buprenorphine MAT (Medication Assisted Treatment) population. Austin J Drug Abus Addict. 2016;3(1):1009. [PMC free article] [PubMed] [Google Scholar]
- 143.Bertz JW, Epstein DH, Reamer D, et al. Sleep reductions associated with illicit opioid use and clinic-hour changes during opioid agonist treatment for opioid dependence: Measurement by electronic diary and actigraphy. J Subst Abuse Treat. 2019;106:43–57. doi: 10.1016/j.jsat.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Assessing sleep in opioid dependence: A comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend. 2011;113(2–3):245–248. doi: 10.1016/j.drugalcdep.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Barker EL, ed. Pharmacol Rev. 2018;70(2):197–245. doi: 10.1124/pr.117.014381 [DOI] [PubMed] [Google Scholar]
- 146.Moses TEH, Lundahl LH, Greenwald MK. Factors associated with sedative use and misuse among heroin users. Drug Alcohol Depend. 2018;185:10–16. doi: 10.1016/j.drugalcdep.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McAnally H, Bonnet U, Kaye AD. Gabapentinoid benefit and risk stratification: Mechanisms over myth. Pain Ther. 2020;9(2):441–452. doi: 10.1007/s40122-020-00189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 149.Srinivasan V, Pandi-Perumal SR, Trahkt I, et al. Melatonin and melatonergic drugs on sleep: Possible mechanisms of action. Int J Neurosci. 2009;119(6):821–846. doi: 10.1080/00207450802328607 [DOI] [PubMed] [Google Scholar]
- 150.Babiloni AH, De Koninck BP, Beetz G, De Beaumont L, Martel MO, Lavigne GJ. Sleep and pain: recent insights, mechanisms, and future directions in the investigation of this relationship. J Neural Transm. 2020;127(4):647–660. doi: 10.1007/s00702-019-02067-z [DOI] [PubMed] [Google Scholar]
- 151.Kaur T, Shyu B-C. Melatonin: A new-generation therapy for reducing chronic pain and improving sleep disorder-related pain. Adv Exp Med Biol. 2018;1099:229–251. doi: 10.1007/978-981-13-1756-9_19 [DOI] [PubMed] [Google Scholar]
- 152.Kesner AJ, Lovinger DM. Cannabinoids, endocannabinoids and sleep. Front Mol Neurosci. 2020;13. doi: 10.3389/fnmol.2020.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br J Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Straiker A, Dvorakova M, Zimmowitch A, Mackie K. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol. 2018;94(1):743–748. doi: 10.1124/mol.118.111864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bisogno T, Hanuš L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mijangos-Moreno S, Poot-Aké A, Arankowsky-Sandoval G, Murillo-Rodríguez E. Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci Res. 2014;84:60–63. doi: 10.1016/j.neures.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 157.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- 158.Brown J, Winterstein A. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) Use. J Clin Med. 2019;8(7):989. doi: 10.3390/jcm8070989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhornitsky S, Potvin S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals. 2012;5(5):529–552. doi: 10.3390/ph5050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc B Biol Sci. 2012;367(1607):3364–3378. doi: 10.1098/rstb.2011.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515–527. doi: 10.1016/j.tips.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 162.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanuš LO. Cannabidiol – Recent advances. Chem Biodivers. 2007;4(8):1678–1692. doi: 10.1002/cbdv.200790147 [DOI] [PubMed] [Google Scholar]
- 163.Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: A review of the literature. Curr Psychiatry Rep. 2017;19(4):23. doi: 10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- 164.Kuhathasan N, Dufort A, MacKillop J, Gottschalk R, Minuzzi L, Frey BN. The use of cannabinoids for sleep: A critical review on clinical trials. Exp Clin Psychopharmacol. 2019;27(4):383–401. doi: 10.1037/pha0000285 [DOI] [PubMed] [Google Scholar]
- 165.Suraev AS, Marshall NS, Vandrey R, et al. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med Rev. 2020;53:101339. doi: 10.1016/j.smrv.2020.101339 [DOI] [PubMed] [Google Scholar]
- 166.Vorspan F, Guillem E, Bloch V, et al. Cannabis withdrawal syndrome in patients with cannabis dependence only, and in patients with cannabis and opioid dependence [Article in French]. Encephale. 2011;37(4):266–272. doi: 10.1016/j.encep.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 167.Bisaga A, Sullivan MA, Glass A, et al. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015;154:38–45. doi: 10.1016/j.drugalcdep.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 170.Tyree SM, Borniger JC, de Lecea L. Hypocretin as a hub for arousal and motivation. Front Neurol. 2018;9. doi: 10.3389/fneur.2018.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. doi: 10.1124/jpet.109.152009 [DOI] [PubMed] [Google Scholar]
- 172.Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8. doi: 10.3389/fnins.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mang GM, Dürst T, Bürki H, et al. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35(12):1625–1635. doi: 10.5665/sleep.2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morairty SR, Revel FG, Malherbe P, et al. Dual hypocretin receptor antagonism Is more effective for sleep promotion than antagonism of either receptor alone. Seifert R, ed. PLoS One. 2012;7(7):e39131. doi: 10.1371/journal.pone.0039131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cao M, Chow M. The hypocretin/orexin system in sleep disorders: preclinical insights and clinical progress. Nat Sci Sleep. March 2016:81. doi: 10.2147/NSS.S76711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: A randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. doi: 10.1212/WNL.0b013e31827688ee [DOI] [PubMed] [Google Scholar]
- 177.Citrome L Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(12):1429–1441. doi: 10.1111/ijcp.12568 [DOI] [PubMed] [Google Scholar]
- 178.Herring WJ, Roth T, Krystal AD, Michelson D. Orexin receptor antagonists for the treatment of insomnia and potential treatment of other neuropsychiatric indications. J Sleep Res. 2019;28(2). doi: 10.1111/jsr.12782 [DOI] [PubMed] [Google Scholar]
- 179.De Boer P, Drevets WC, Rofael H, et al. A randomized phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32(6):668–677. doi: 10.1177/0269881118773745 [DOI] [PubMed] [Google Scholar]
- 180.Hoyer D, Jacobson LH. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand? Neuropeptides. 2013;47(6):477–488. doi: 10.1016/j.npep.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 181.Khoo SY-S, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 2014;28(8):713–730. doi: 10.1007/s40263-014-0179-x [DOI] [PubMed] [Google Scholar]
- 182.Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: A systematic review and meta-analysis. Sleep Med Rev. 2017;35:1–7. doi: 10.1016/j.smrv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 183.Yang LPH. Suvorexant: First global approval. Drugs. 2014;74(15):1817–1822. doi: 10.1007/s40265-014-0294-5 [DOI] [PubMed] [Google Scholar]
- 184.Greenwald MK. Anti-stress neuropharmacological mechanisms and targets for addiction treatment: A translational framework. Neurobiol Stress. 2018;9:84–104. doi: 10.1016/j.ynstr.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Baimel C, Bartlett SE, Chiou L-C, et al. Orexin/hypocretin role in reward: Implications for opioid and other addictions. Br J Pharmacol. 2015;172(2):334–348. doi: 10.1111/bph.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brown RM, Khoo SY-S, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16(9):2067–2079. doi: 10.1017/S1461145713000333 [DOI] [PubMed] [Google Scholar]
- 187.Shoblock JR, Welty N, Aluisio L, et al. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl). 2011;215(1):191–203. doi: 10.1007/s00213-010-2127-x [DOI] [PubMed] [Google Scholar]
- 188.Uslaner JM, Winrow CJ, Gotter AL, et al. Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res. 2014;269:61–65. doi: 10.1016/j.bbr.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 189.Campbell EJ, Marchant NJ, Lawrence AJ. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res. 2020;1731:145902. doi: 10.1016/j.brainres.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 190.James MH, Fragale JE, O’Connor SL, Zimmer BA, Aston-Jones G. The orexin (hypocretin) neuropeptide system is a target for novel therapeutics to treat cocaine use disorder with alcohol coabuse. Neuropharmacology. 2021;183:108359. doi: 10.1016/j.neuropharm.2020.108359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G. Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: Why sleep on this any longer? Neuropsychopharmacology. 2020;45(5):717–719. doi: 10.1038/s41386-020-0619-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Matzeu A, Martin-Fardon R. Targeting the orexin system for prescription opioid use disorder. Brain Sci. 2020;10(4):226. doi: 10.3390/brainsci10040226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roehrs TA, Auciello J, Tseng J, Whiteside G. Current and potential pharmacological treatment options for insomnia in patients with alcohol use disorder in recovery. Neuropsychopharmacol Reports. 2020;40(3):211–223. doi: 10.1002/npr2.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]


