Abstract
Homogeneous earth-abundant metal catalysis based on well-defined molecular complexes has achieved great advance in synthetic methodologies. However, sophisticated ligand, hazardous activator and multistep synthesis starting from base metal salts are generally required for the generation of active molecular catalysts, which may hinder their broad application in large scale organic synthesis. Therefore, the development of metal cluster catalysts formed in situ from simple earth-abundant metal salts is of importance for the practical utilization of base metal resource, yet it is still in its infancy. Herein, a mixture of catalytic amounts of cobalt (II) iodide and potassium tert-butoxide is discovered to be highly active for selective hydroboration of vinylarenes and dihydroboration of nitriles, affording a good yield of diversified hydroboration products that without isolation can readily undergo further one pot transformations. It should be highlighted that the alkoxide-pinacolborane combination acts as an efficient activation strategy to activate cobalt (II) iodide for the generation of metastable heterotopic cobalt catalysts in situ, which is proposed to be catalytically active species.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology
Homogeneous earth-abundant metal catalysis based on well-defined metal complexes is of interest for organic synthesis, but typically employs expensive catalysts, air sensitive or synthetically challenging chemicals. Here, the authors report an efficient and regio-selective catalytic system for hydroboration of vinylarenes and organic nitriles with HBPin, using commercially available CoI2 and KOtBu under ligand-free conditions.
Introduction
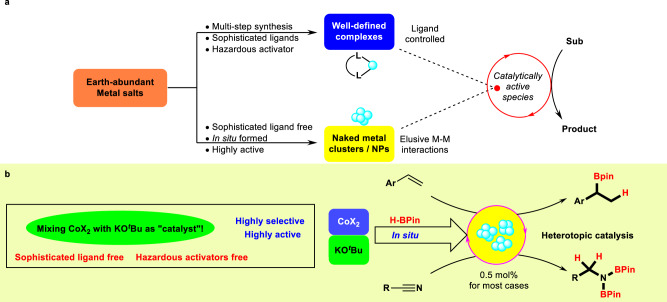
Homogeneous earth-abundant metal catalysis is one of the keys to the sustainable future in organic synthesis benefited from the advantages of cheap, earth-abundant, and less toxic base metals1–5. Their well-defined metal complexes have achieved great advances as homogeneous catalysts in recent years (Fig. 1a). For example, cobalt is abundant, inexpensive and a variety of their salts are commercially available1–5. In the past decade, several well-defined alkene hydroboration catalysts with sophisticated ligands have been developed based on cobalt complexes6–10. Nevertheless, these reactions typically employed catalysts bearing sophisticated ligands, which can be expensive, air sensitive, or difficult to be synthesized. In addition, current methods for the activation of the pre-catalysts to lower oxidation-state catalytic species relied heavily on the use of various hazardous reducing reagents, such as main group organometallics or hydrides11,12. All of these hinder their broad application in large-scale organic synthesis. Complementary to such molecularly defined systems, catalysis by metallic clusters has become a quite recent field of research which attracted the attention from the chemical community13–19. It has appeared that metal clusters which are formed in situ from simple metal salts may fill the gap between the single metal atom with sophisticated ligands and the metal nanoparticles (NPs) (Fig. 1a)20–29. They can also invoke distinct catalytic properties compared to conventional NPs. Taking cobalt again as an example, some ill-defined or nanoparticulate Co catalysts, prepared by in situ reduction of a cobalt salt with a reductant, have been reported to exhibit good hydrogenation activities30–38. Despite this enormous potential in catalysis, the development of metal cluster catalysts based on earth-abundant metals is still in its infancy20–29. From a practical perspective, the development of ligand-free heterotopic cobalt catalysts for synthetically useful alkene hydroboration reaction with HBPin, using readily available cobalt salts would be highly desirable39–46.
Fig. 1. Earth-abundant metal catalysis in organic synthesis.
a Homogenerous earth-abundant metal catalysis in synthetic methodology. b Simple CoX2 activation by KOtBu in hydroboration reactions.
Herein, we disclose a user-friendly catalytic protocol using the mixture of CoI2 and KOtBu for highly active and selective Markovnikov hydroboration of vinylarenes and double hydroboration of nitriles, without using any costly ligand/activator. It should be noted that Markovnikov hydroboration reactions are rarely approached with base-metal catalysts47–58. KOtBu is proposed to act as a masked reducing agent, by reacting with HBPin to form an ate-type complex that can then serve as a reductive pre-catalyst activator11. Preliminary mechanistic studies suggest that the Co(II) salt is most likely to be reduced in situ to some low-valent Co species, which undergo aggregation to form heterotopic Co catalysts responsible for the catalysis (Fig. 1b). Notably, using other strong reductants such as NaBHEt3 or Grignard reagents lead to poor results, demonstrating the formation of the heterotopic species is largely influenced by the reductants and the alkoxide-pinacolborane combination plays a key role in the success of the present catalysis. The as-synthesized hydroboration products can serve as valuable synthons in further synthetic manipulations in a one-pot transformation, demonstrating the practicality and utility of the present methodologies.
Results
Reaction development
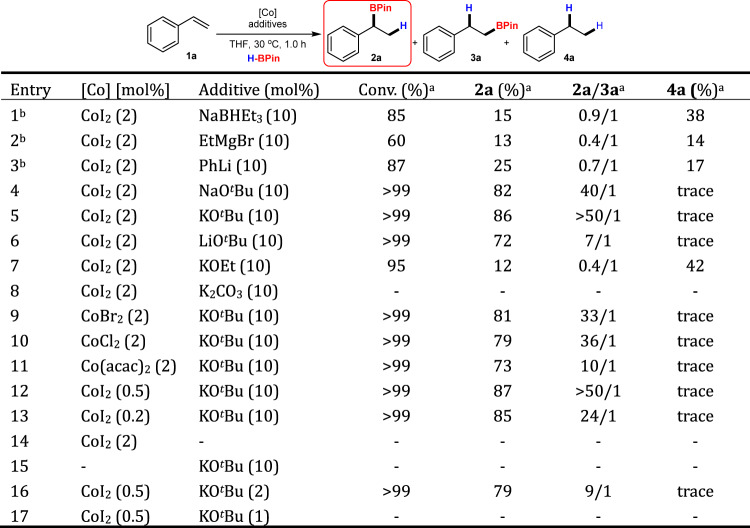
The study was commenced by hydroboration of styrene 1a with HBPin, using commercially available CoI2 as the pre-catalyst in combination with substoichiometric amount of a reductant. The use of NaBHEt3, EtMgBr, or PhLi as the activator in the reaction only gave the desired hydroboration products in low yields with poor regioselectivities (Fig. 2, entries 1–3). Hydrogenated product 4a (ethylbenzene) was also detected in these reaction mixtures, which is consistent with the good hydrogenation activities of the generated nanoparticles30–38. In 2017, Thomas et al. reported that a boron ‘ate’ reductive species can be formed in situ by the reaction between alkoxide and HBPin and activate high-oxidation-state cobalt complexes immediately as hydride donors11,59. Inspired from this pioneering work, a catalytic amount of NaOtBu (10 mol%) was tested in the reaction, leading to a boosting in the yield of hydroboration product 2a to 82% with excellent Markovnikov regioselectivity (40/1), highlighting the importance of the activators in the catalytic activity (Fig. 2, entry 4 vs 1–3). Changing NaOtBu to KOtBu slightly improved the results (Fig. 2, entry 5), whereas use of LiOtBu resulted in a decrease in the regioselectivity (Fig. 2, entry 6). The addition of crown ethers to the reactions turned out to be deleterious for the catalysis (for details, see the Supplementary Information). These results suggested that the cations of the alkoxide salts might be involved in the in situ formed boron ‘ate’ reductive species and active cobalt species, which in turn can affect the catalytic performance. On the other hand, the use of KOEt or K2CO3 gave very poor results (Fig. 2, entries 7 and 8). Other cheap Co(II) salts, such as CoBr2, CoCl2, and Co(acac)2 also worked very well as catalyst precursors, suggesting that this activation mechanism should be less irrelevant to the anions of the cobalt salts (Fig. 2, entries 9–11). To our delight, reducing the loading of CoI2 to 0.5 mol% still afforded complete conversion of 1a in 1.0 h, giving the product 2a in 87% yield with >50/1 Markovnikov regioselectivity (Fig. 2, entry 12). Further diminishing the catalyst loading to 0.2 mol% was also successful, and the product 2a was still obtained in high yield (85%) with a slightly lower regioselectivity (24/1, Fig. 2, entry 13 vs 12). Control experiments showed that no reaction of 1a occurred in the absence of a Co precursor or KOtBu, thus attesting the essential roles of both Co and the base for the catalysis (Fig. 2, entries 14, 15). When only 2 mol% KOtBu was used, the regioselectivity was decreased to 9/1, whereas the reaction did not proceed at all with only 1.0 mol% KOtBu, which was only two times the molar quantity of pre-catalyst (Fig. 2, entries 16, 17). These results seem to be consistent with the significant role of a large excess of reductant formed in situ in the present catalysis.
Fig. 2. Optimization of reaction conditions for Cocatalyzed hydroboration of styrene.
Reaction conditions: HBPin (0.48 mmol) was added in a portion into a mixture of the cobalt salt, the additive and 1a (0.4 mmol) in anhydrous THF (2 mL), and the resulting mixture was stirred under N2 at 30 °C for 1.0 h. aDetermined by GC analysis of the reaction mixture. bThe additive was added in the end.
Substrate scope and synthetic applications
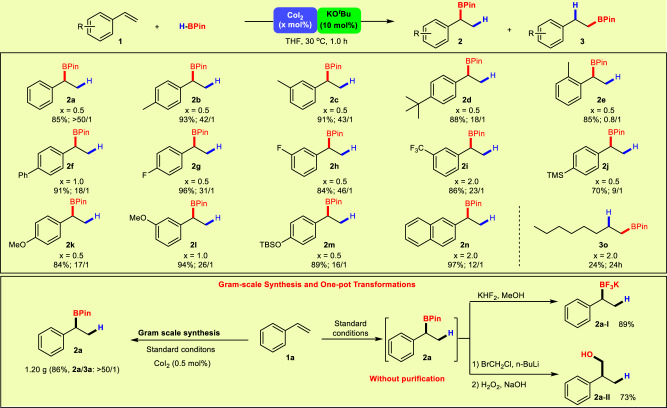
With the optimized reaction conditions in hand, the substrate scope of the Markovnikov hydroboration was investigated (Fig. 3). It is noteworthy that in most cases, the reaction consumed only 0.5 mol% of CoI2 and provided the targeted products in high yields with excellent regioselectivities. Styrene derivatives bearing electron-donating groups such as methyl and tert-butyl groups underwent successful hydroboration in excellent yields with high regioselectivities (2b–2d, 88–93% yields, 18/1–43/1). Styrene with o-methyl group on phenyl ring gave a poor regioselectivity, probably due to the unfavorable steric hinderance (2e). The reaction of 4-phenylstyrene using 1.0 mol% of CoI2 gave the secondary boronic ester 2f in good yield with high regioselectivity (91%, 18/1). In addition, electron-withdrawing substituents including fluoro- and trifluoromethyl- were also well-tolerated, giving the corresponding secondary boronic esters 2g–2i in good to excellent yields and selectivities (84–96%, 23/1–46/1). The reactions also worked efficiently for styrenes bearing trialkylsilyl- or ether substituents, giving good to excellent yields and selectivities of the branched boronic esters 2j–2m (70–94%, 9/1–26/1). The reaction of 2-vinylnaphthalene afforded the branched boronic ester 2n in good yield with high regioselectivity (97%, 12/1). However, the reactions for styrene derivatives bearing a formyl or acetoxy group only resulted in intractable mixtures. Apart from the aromatic alkenes, 1-octene can also be hydroborated using 2.0 mol% CoI2, giving the anti-Markovnikov product 3o in only 24% isolated yield, indicating the relatively poor catalytic performance of the present system towards aliphatic olefins. Gram-scale synthesis using reaction of 1a under 0.5 mol% CoI2 loading also proceeded smoothly, affording the hydroboration product 2a in 86% yield (1.20 g) with excellent Markovnikov regioselectivity (>50/1). Treatment of the in situ generated alkylboronate 2a with aqueous KHF2 afforded the corresponding trifluoroborate 2a-I in 89% yield, which is a valuable synthetic intermediate for Suzuki cross-couplings. In addition, the reaction of the hydroboration mixture with BrCH2Cl and nBuLi, followed by the oxidation, gave the product 2a-II in 73% yield. In these cases, the reactions are performed directly on the hydroboration mixture without isolation and purification of the alkylboronate intermediate, demonstrating the utility of the present methodology.
Fig. 3. CoI2/KOtBu catalyzed Markovnikov hydroboration of vinylarenes.
Reaction conditions: HBPin (1.2 mmol) was added to a mixture of CoI2 (0.5–2.0 mol%), KOtBu (0.1 mmol) and 1 (1.0 mmol) in 5 mL anhydrous THF, and the mixture was stirred at 30 °C for 1.0 h under N2. The yields are for isolated 2 and 3. The ratio of 2/3 was determined by 1H-NMR of the crude product.
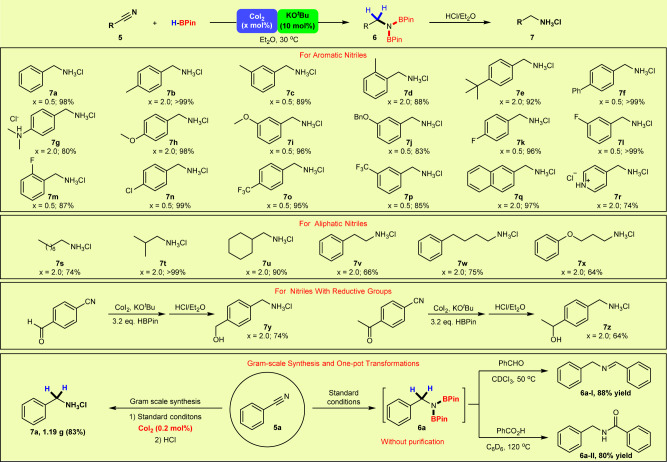
Encouraged by the results obtained in the CoI2/KOtBu mediated hydroboration of styrene derivatives, we extended this ligand-free cobalt catalytic system further into dihydroboration of nitriles60–66. To our delight, using only 0.5 mol% CoI2 and 10 mol% KOtBu, the dihydroboration of benzonitrile 5a worked smoothly in Et2O at 30 °C, giving the corresponding ammonium salt in 98% yield after the treatment with HCl (for details, see the Supplementary Information). In the absence of the cobalt salt, the reaction did not give any of the desired product. It should be noted that Findlater group and von Wangelin group developed double hydroboration of nitriles using NaBHEt3 and lithium bis(trimethylsilyl)amide as catalyst, respectively67,68.
The scope of nitriles for the dihydroboration was next explored and the ammonium salts were generally obtained in good to excellent yields (Fig. 4). The reactions of nitriles with an electron-donating group on the phenyl ring (Me, tBu-, Ph-, Me2N-, MeO-, and BnO-) afforded the corresponding products 7a–7j in 80–>99% yields. Notably, in the case of o-tolunitrile, the methyl group did not adversely affect the catalytic reaction, affording the product 7d in 88% yield. In addition, substrates with electron-withdrawing functional groups on the backbone (F-, Cl-, CF3-) were also well-tolerated, and yields of 85–>99% for 7k–7p were obtained smoothly. The reaction of 2-naphthonitrile delivered the product 7q in 97% yield. For substrate 5r bearing a pyridyl ring, the reaction still furnished an excellent yield of 7r (74%), attesting the notable tolerance of the catalyst to a coordinating group on the substrate. Gratifyingly, the reactions of aliphatic nitriles also proceeded smoothly, leading to the formation of the corresponding products 7s–7x in good to excellent yields (64%–>99%). The hydroboration of nitrile substrates bearing another reducible functional group (formyl or acetyl) was further investigated, and both the cyano and carbonyl groups were reduced under standard conditions, giving a moderate yield of the doubly reduced product 7y (74%) and 7z (64%), respectively. Therefore, aromatic, heterocyclic as well as aliphatic nitriles are well compatible with this ligand-free Co catalyzed dihydroboration, showing the robustness of the protocol. A 10 mmol scale reaction of 5a was successfully carried out in the presence of 0.2 mol% CoI2, and the product 7a was isolated in 83% yield (1.19 g), demonstrating the potential practicability of this method. In addition, several one-pot transformations starting from benzonitrile were further investigated to show the synthetic utility. PhCH2N(BPin)2, produced in situ in the present hydroboration of benzonitrile with HBPin, was subjected to the reaction with benzaldehyde, affording N-benzylidenebenzylamine 6a-I in 88% yield for this one-pot transformation66. Furthermore, a relay process of hydroboration of benzonitrile followed by the reaction with benzoic acid at 120 °C, allowed for the facile synthesis of amide 6a-II in 80% yield without the need for an exogenous coupling reagent65.
Fig. 4. CoI2/KOtBu catalyzed dihydroboration of nitriles.
Reaction conditions: HBPin (2.2 mmol) was added to a mixture of CoI2 (0.5–2.0 mol%), KOtBu (0.1 mmol, 10 mol%) and 5 (1.0 mmol) in anhydrous Et2O (2 mL), and the reaction mixture was stirred under N2 at 30 °C for 4.0–24.0 h. Isolated yield of the corresponding ammonium salt is shown.
Mechanistic studies
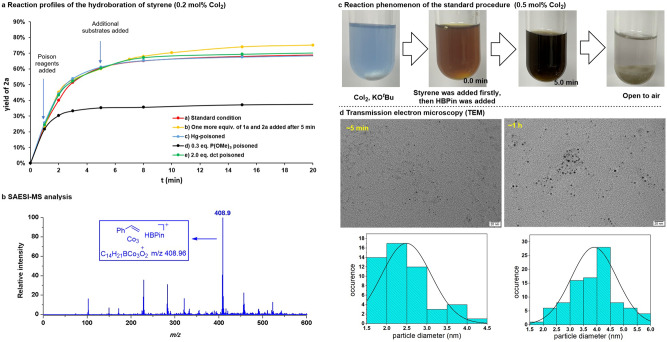
Mechanistic studies were conducted to understand the above hydroboration reactions, specifically on the identification of the catalytically active species. Kinetic profiles for the hydroboration reaction of styrene with 0.2 mol% CoI2 exhibits no obvious induction period, and the yield of 2a reached 61% in 5.0 min, showing a high reactivity during the first 5.0 min (Fig. 5a, curve a). However, the reaction rate was slowed down obviously after that point. Addition of one more equivalent of 1a and HBPin each to the reaction system at 5.0 min resulted in only a slight increase in the yield of the product 2a, suggesting that most of the in situ generated active catalyst deactivated in the first few minutes (Fig. 5a, curve b). Kinetic poisoning studies were further performed to ascertain the topicity of the operating catalyst species69,70. The addition of excess Hg to the reaction system under standard conditions at 1.0 min had almost no effect on the reaction rate (Fig. 5a, curve c). On the other hand, addition of substoichiometric trimethylphosphite [P(OMe)3, 0.3 equiv per Co atom] to the reaction system under standard conditions at 1.0 min led to sharp inhibition of catalytic turnover (Fig. 5a, curve d). The selective homogeneous catalyst poison dibenzo[a,e]cyclo-octatetraene (dct, 2.0 equiv per Co) showed no effect of product formation (Fig. 5a, curve e). Based on the results of these control experiments, we postulate a heterotopic mechanism that involves initial reduction of CoI2 by in situ generated reductant, and rapid aggregation of the resulting low-valent cobalt species to heterotopic Co catalysts as the catalytically active species13–19.
Fig. 5. Data for preliminary mechanistic studies.
a Reaction profiles for hydroboration of styrene with HBPin performed under different conditions. b SAESI-MS spectrum of the sample taken at 0.5 min under the standard reaction conditions. c Visual changes in color and turbidity of the mixture during the reaction course. d TEM images for the samples which were prepared at 5.0 min and 1.0 h.
Solvent-assisted electrospray ionization (SAESI) mass spectrum of the reaction mixture for hydroboration of 1a, collected after 0.5 min on the initiation of the reaction, shows an intense signal at m/z = 408.9 (Fig. 5b, calcd for C14H21BCo3O2+: 408.96), which matches the m/z of [HBpin-Co3-styrene + H]+ (for details, see the Supplementary Information). Although the exact nature of the assembly is not clear at current stage, it is tempting to suggest the peak might be a snapshot of the reacting system, and that the multimetallic species may be the genuine catalyst in the catalytic cycle20–29. However, some efforts to investigate UV/Vis measurements of the formed active species in the reaction mixture failed, which might be due to the high activity, sensitivity, and spontaneous aggregation to larger particles. Co(II)(alkoxide)2 was prepared from the reaction of CoI2 and KOtBu in THF, and the isolated salt did not show good catalytic activity in the hydroboration of styrene, suggesting that bis-tert-butoxide cobalt salt itself is unlikely to be the catalytically active species11. The mix of CoI2 (0.5 mol%) and KOtBu (10.0 mol%) in THF led to a nattier blue suspension (Fig. 5c). When styrene 1a was added to the mixture, the mixture remained unchanged. In contrast, the further addition of HBPin led to a brown solution immediately, indicating that HBPin was involved in the activation of the cobalt salt and some very active cobalt species formed quickly according to the reaction profile11,59. After 5.0 min, the color turned to dark black, suggesting that the cobalt species may aggregate to larger inactive particles. When the reaction system was exposed to air for 30 min, the black color faded and some particles precipitated, supporting that the in situ formed cobalt species in the reaction mixture are air sensitive. Transmission electron microscopy (TEM) showed that the sample which was prepared at 5.0 min after the reaction beginning contained cobalt nanoparticles with an average particle size of 2.5 nm (Fig. 5d). It should be noted that smaller particles are hard to be identified. The average particle size was further increased to 3.9 nm at 1.0 h, supporting that the gradual aggregation of the cobalt species to Co particles with time, which is consistent with the reaction profile and phenomenon. Although the exact underlying reasons are still not clear at the present stage, it is tempting to propose that some metal–metal interactions in the heterotopic Co catalysts might account for the highly catalytic activities20–29.
Discussion
In this work, we develop a highly efficient and regio-selective catalytic system for hydroboration of vinylarenes and organic nitriles with HBPin, using commercially available CoI2 and KOtBu under ligand-free conditions. The alkoxide-pinacolborane combination plays a key role to activate CoI2 to the formation of active heterotopic Co catalysts in the hydroboration of olefins. The practical feature of this ligand-free earth-abundant metal catalysis and mechanistic understanding of this discovery may extend beyond hydroboration itself and provide a useful model for the development of earth-abundant metal catalysis in organic methodologies.
Methods
General procedure for styrene derivatives hydroboration
In a nitrogen-filled glovebox, CoI2 (0.5–2 mol%), KOtBu (11.2 mg, 0.1 mmol, 10 mol%), anhydrous THF (5 mL), and olefin 1 (1.0 mmol) were added to a 10 mL vial equipped with a magnetic stir bar. HBPin (153.6 mg, 174 µL, 1.2 mmol) was then added to the stirring mixture. The reaction mixture was stirred vigorously at 30 °C for 1.0 h and then was quenched by exposing the solution to air. The resulting solution was concentrated in vacuum and the residue was purified by chromatography on silica gel eluting with petroleum ether/Et2O to give the product. The regioselectivity was determined by 1H-NMR of the crude product.
General procedure for nitrile hydroboration
In a nitrogen-filled glovebox, CoI2 (0.5–2 mol%), KOtBu (11.2 mg, 0.1 mmol, 10.0 mol%), anhydrous Et2O (2 mL, 0.5 M), and nitrile 5 (1 mmol) were added to a 10 mL vial equipped with a magnetic stir bar. HBPin (281.6 mg, 319 µL, 2.2 mmol, 2.2 eq.) was then added to the stirring mixture. The reaction mixture was stirred vigorously at 30 °C for 4 h and then was filtered through a pad of celite. The residue was washed with Et2O until no product remained on the celite. HCl (0.6 M in Et2O, 4 mL, 2.4 mmol) was added to the filtrate, affording amines as hydrochloride salts. The resulting suspension was stirred for 1 h and then filtered through a pad of celite. The residue on the celite was washed with MeOH to redissolve the product. After that, volatiles were removed by a rotate evaporator. The product was purified by forming a slurry from the mixture solution of CH2Cl2 and ethyl acetate as a solid.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (21821002) and Shanghai Pujiang Program (19PJ1411500). The authors appreciate the valuable discussion of this work with Prof. Liang Deng, Prof. Zheng Huang at SIOC, and Prof. Zhan Lu at Zhejiang University.
Author contributions
C.L. and X.W. directed the project; C.X., Q.L., and X.W. designed the experiments; C.L. and S.S. performed all the experiments and analyzed all the data. Y.L. and Y.G. performed all the SAESI-MS studies; C.L. and X.W. wrote the manuscript with contributions from all authors.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information file. Any further relevant data are available from the authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Grzegorz Hreczycho, Michael Findlater and the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yinlong Guo, Email: ylguo@sioc.ac.cn.
Xiaoming Wang, Email: xiaoming@sioc.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24117-5.
References
- 1.Bullock RM, et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science. 2020;369:eabc3183. doi: 10.1126/science.abc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holzwarth MS, Plietker B. Biorelevant metals in sustainable metal catalysis—a survey. ChemCatChem. 2013;5:1650–1679. doi: 10.1002/cctc.201200592. [DOI] [Google Scholar]
- 3.Zweig JE, Kim DE, Newhouse TR. Methods utilizing first-row transition metals in natural product total synthesis. Chem. Rev. 2017;117:11680–11752. doi: 10.1021/acs.chemrev.6b00833. [DOI] [PubMed] [Google Scholar]
- 4.Su B, Cao ZC, Shi ZJ. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 2015;48:886–896. doi: 10.1021/ar500345f. [DOI] [PubMed] [Google Scholar]
- 5.Gandeepan P, Cheng CH. Cobalt catalysis involving pi components in organic synthesis. Acc. Chem. Res. 2015;48:1194–1206. doi: 10.1021/ar500463r. [DOI] [PubMed] [Google Scholar]
- 6.Obligacion JV, Chirik PJ. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration: opportunities and assessments. Nat. Rev. Chem. 2018;2:15–34. doi: 10.1038/s41570-018-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Guo J, Lu Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition metal catalysis. Chin. J. Chem. 2018;36:1075–1109. doi: 10.1002/cjoc.201800314. [DOI] [Google Scholar]
- 8.Ai W, Zhong R, Liu X, Liu Q. Hydride transfer reactions catalyzed by cobalt complexes. Chem. Rev. 2019;119:2876–2953. doi: 10.1021/acs.chemrev.8b00404. [DOI] [PubMed] [Google Scholar]
- 9.Wen H, Liu G, Huang Z. Recent advances in tridentate iron and cobalt complexes for alkene and alkyne hydrofunctionalizations. Coord. Chem. Rev. 2019;386:138–153. doi: 10.1016/j.ccr.2019.01.024. [DOI] [Google Scholar]
- 10.Tamang SR, Findlater M. Emergence and applications of base metals (Fe, Co, and Ni) in hydroboration and hydrosilylation. Molecules. 2019;24:3194. doi: 10.3390/molecules24173194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty JH, Peng J, Dominey AP, Thomas SP. Activation and discovery of earth-abundant metal catalysts using sodium tert-butoxide. Nat. Chem. 2017;9:595–600. doi: 10.1038/nchem.2697. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SP, Peng J. Activation strategies for earth-abundant metal catalysis. Synlett. 2020;31:1140–1146. doi: 10.1055/s-0039-1690873. [DOI] [Google Scholar]
- 13.Oliver-Meseguer J, Cabrero-Antonino JR, Domínguez I, Leyva-Pérez A, Corma A. Small gold clusters formed in solution give reaction turnover numbers of 107 at room temperature. Science. 2012;338:1452. doi: 10.1126/science.1227813. [DOI] [PubMed] [Google Scholar]
- 14.Leyva-Perez A, Oliver-Meseguer J, Rubio-Marques P, Corma A. Water-stabilized three- and four-atom palladium clusters as highly active catalytic species in ligand-free C-C cross-coupling reactions. Angew. Chem. Int. Ed. 2013;52:11554–11559. doi: 10.1002/anie.201303188. [DOI] [PubMed] [Google Scholar]
- 15.Rivero-Crespo MA, Leyva-Perez A, Corma A. A ligand-free Pt3 cluster catalyzes the Markovnikov hydrosilylation of alkynes with up to 106 turnover frequencies. Chem. Eur. J. 2017;23:1702–1708. doi: 10.1002/chem.201605520. [DOI] [PubMed] [Google Scholar]
- 16.Buslov I, Song F, Hu X. An easily accessed nickel nanoparticle catalyst for alkene hydrosilylation with tertiary silanes. Angew. Chem. Int. Ed. 2016;55:12295–12299. doi: 10.1002/anie.201606832. [DOI] [PubMed] [Google Scholar]
- 17.Neumeier M, et al. Combined photoredox and iron catalysis for the cyclotrimerization of alkynes. Angew. Chem. Int. Ed. 2020;59:13473–13478. doi: 10.1002/anie.202000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gieshoff TN, Chakraborty U, Villa M, von Wangelin AJ. Alkene hydrogenations by soluble iron nanocluster catalysts. Angew. Chem. Int. Ed. 2017;56:3585–3589. doi: 10.1002/anie.201612548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gieshoff TN, et al. Iron-catalyzed olefin hydrogenation at 1 bar H2 with a FeCl3–LiAlH4 catalyst. Green. Chem. 2015;17:1408–1413. doi: 10.1039/C4GC02368D. [DOI] [Google Scholar]
- 20.Somorjai GA, Contreras AM, Montano M, Rioux RM. Clusters, surfaces, and catalysis. Proc. Natl Acad. Sci. USA. 2006;103:10577–10583. doi: 10.1073/pnas.0507691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018;118:4981–5079. doi: 10.1021/acs.chemrev.7b00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Corma A. Evolution of isolated atoms and clusters in catalysis. Trends Chem. 2020;2:383–400. doi: 10.1016/j.trechm.2020.02.003. [DOI] [Google Scholar]
- 23.Du Y, Sheng H, Astruc D, Zhu M. Atomically precise noble metal nanoclusters as efficient catalysts: a bridge between structure and properties. Chem. Rev. 2020;120:526–622. doi: 10.1021/acs.chemrev.8b00726. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, et al. Determination of the evolution of heterogeneous single metal atoms and nanoclusters under reaction conditions: which are the working catalytic sites? Acs. Catal. 2019;9:10626–10639. doi: 10.1021/acscatal.9b04214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook AW, Hayton TW. Case studies in nanocluster synthesis and characterization: challenges and opportunities. Acc. Chem. Res. 2018;51:2456–2464. doi: 10.1021/acs.accounts.8b00329. [DOI] [PubMed] [Google Scholar]
- 26.Eremin DB, Ananikov VP. Understanding active species in catalytic transformations: from molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017;346:2–19. doi: 10.1016/j.ccr.2016.12.021. [DOI] [Google Scholar]
- 27.Polte J. Fundamental growth principles of colloidal metal nanoparticles—a new perspective. CrystEngComm. 2015;17:6809–6830. doi: 10.1039/C5CE01014D. [DOI] [Google Scholar]
- 28.Buchwalter P, Rosé J, Braunstein P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 2015;115:28–126. doi: 10.1021/cr500208k. [DOI] [PubMed] [Google Scholar]
- 29.Crabtree RH. Resolving heterogeneity problems and impurity artifacts in operationally homogeneous transition metal catalysts. Chem. Rev. 2012;112:1536–1554. doi: 10.1021/cr2002905. [DOI] [PubMed] [Google Scholar]
- 30.Takegami Y, Ueno T, Fujii T. The preparation of heavy metal hydride and its catalytic activity. VII. The hydrogenation of various olefins with a ferric chloride-lithium aluminum hydride or a cobaltous chloride-lithium aluminum hydride catalyst. Bull. Chem. Soc. Jpn. 1965;38:1279–1285. doi: 10.1246/bcsj.38.1279. [DOI] [Google Scholar]
- 31.Schmidt FK, Titova YY, Belykh LB, Umanets VA, Khutsishvili SS. Formation of the cobalt hydrogenation catalysts at the action of lithium aluminum hydride and lithium tri(tert-butoxy)aluminohydride and their properties. Russ. J. Gen. Chem. 2012;82:1334–1341. doi: 10.1134/S1070363212080026. [DOI] [Google Scholar]
- 32.Sandl S, Schwarzhuber F, Pollath S, Zweck J, von Wangelin AJ. Olefin-stabilized cobalt nanoparticles for C=C, C=O, and C=N hydrogenations. Chem. Eur. J. 2018;24:3403–3407. doi: 10.1002/chem.201705366. [DOI] [PubMed] [Google Scholar]
- 33.Dai H, Guan H. Switching the selectivity of cobalt-catalyzed hydrogenation of nitriles. Acs. Catal. 2018;8:9125–9130. doi: 10.1021/acscatal.8b02645. [DOI] [Google Scholar]
- 34.Büschelberger P, et al. Recyclable cobalt(0) nanoparticle catalysts for hydrogenations. Catal. Sci. Technol. 2018;8:2648–2653. doi: 10.1039/C8CY00595H. [DOI] [Google Scholar]
- 35.Timelthaler D, Topf C. Liquid-phase hydrogenation of nitriles to amines facilitated by a Co(II)/Zn(0) pair: a ligand-free catalytic protocol. J. Org. Chem. 2019;84:11604–11611. doi: 10.1021/acs.joc.9b01544. [DOI] [PubMed] [Google Scholar]
- 36.Chen F, et al. Selective catalytic hydrogenation of heteroarenes with N-graphene-modified cobalt nanoparticles (Co3O4-Co/NGr@alpha-Al2O3) J. Am. Chem. Soc. 2015;137:11718–11724. doi: 10.1021/jacs.5b06496. [DOI] [PubMed] [Google Scholar]
- 37.Schwob T, Kempe R. A reusable Co catalyst for the selective hydrogenation of functionalized nitroarenes and the direct synthesis of imines and benzimidazoles from nitroarenes and aldehydes. Angew. Chem. Int. Ed. 2016;55:15175–15179. doi: 10.1002/anie.201608321. [DOI] [PubMed] [Google Scholar]
- 38.Jagadeesh RV, et al. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science. 2017;358:326–322. doi: 10.1126/science.aan6245. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Zhou Y, Wang H, Qu J. FeCl2-catalyzed hydroboration of aryl alkenes with bis(pinacolato)diboron. RSC Adv. 2015;5:73705–73713. doi: 10.1039/C5RA14869C. [DOI] [Google Scholar]
- 40.Wu Y, et al. Catalytic hydroboration of aldehydes, ketones, alkynes and alkenes initiated by NaOH. Green. Chem. 2017;19:4169–4175. doi: 10.1039/C7GC01632H. [DOI] [Google Scholar]
- 41.Bismuto A, Cowley MJ, Thomas SP. Aluminum-catalyzed hydroboration of alkenes. ACS Catal. 2018;8:2001–2005. doi: 10.1021/acscatal.7b04279. [DOI] [Google Scholar]
- 42.Kuciński K, Hreczycho G. Lithium triethylborohydride as catalyst for solvent-free hydroboration of aldehydes and ketones. Green. Chem. 2019;21:1912–1915. doi: 10.1039/C9GC00216B. [DOI] [Google Scholar]
- 43.Ma DH, et al. Catalytic hydroboration of aldehydes, ketones, and alkenes using potassium carbonate: a small key to big transformation. ACS Omega. 2019;4:15893–15903. doi: 10.1021/acsomega.9b01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuciński K, Hreczycho G. Hydrosilylation and hydroboration in a sustainable manner: from Earth-abundant catalysts to catalyst-free solutions. Green. Chem. 2020;22:5210–5224. doi: 10.1039/D0GC01430C. [DOI] [Google Scholar]
- 45.Su W, et al. Ligand-free iron-catalyzed regioselectivity-controlled hydroboration of aliphatic terminal alkenes. ACS Catal. 2020;10:11963–11970. doi: 10.1021/acscatal.0c02731. [DOI] [Google Scholar]
- 46.Wu X, et al. Nickel-catalyzed hydrosilylation of terminal alkenes with primary silanes via electrophilic silicon-hydrogen bond activation. Org. Lett. 2021;23:1434–1439. doi: 10.1021/acs.orglett.1c00111. [DOI] [PubMed] [Google Scholar]
- 47.Obligacion JV, Chirik PJ. Bis(imino)pyridine cobalt-catalyzed alkene isomerization-hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 2013;135:19107–19110. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Zuo Z, Leng X, Huang Z. A cobalt-catalyzed alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 2014;53:2696–2700. doi: 10.1002/anie.201310096. [DOI] [PubMed] [Google Scholar]
- 49.Jones AS, et al. Broad scope hydrofunctionalization of styrene derivatives using iron-catalyzed hydromagnesiation. Org. Lett. 2014;16:5964–5967. doi: 10.1021/ol5029892. [DOI] [PubMed] [Google Scholar]
- 50.Palmer WN, Diao T, Pappas I, Chirik PJ. High-activity cobalt catalysts for alkene hydroboration with electronically responsive terpyridine and α-diimine ligands. ACS Catal. 2015;5:622–626. doi: 10.1021/cs501639r. [DOI] [Google Scholar]
- 51.Reilly SW, Webster CE, Hollis TK, Valle HU. Transmetallation from CCC-NHC pincer Zr complexes in the synthesis of air-stable CCC-NHC pincer Co(III) complexes and initial hydroboration trials. Dalton Trans. 2016;45:2823–2828. doi: 10.1039/C5DT04752H. [DOI] [PubMed] [Google Scholar]
- 52.MacNair AJ, Millet CRP, Nichol GS, Ironmonger A, Thomas SP. Markovnikov-selective, activator-free iron-catalyzed vinylarene hydroboration. ACS Catal. 2016;6:7217–7221. doi: 10.1021/acscatal.6b02281. [DOI] [Google Scholar]
- 53.Espinal-Viguri M, Woof CR, Webster RL. Iron-catalyzed hydroboration: unlocking reactivity through ligand modulation. Chem. Eur. J. 2016;22:11605–11608. doi: 10.1002/chem.201602818. [DOI] [PubMed] [Google Scholar]
- 54.Peng J, Docherty JH, Dominey AP, Thomas SP. Cobalt-catalysed Markovnikov selective hydroboration of vinylarenes. Chem. Commun. 2017;53:4726–4729. doi: 10.1039/C7CC01085K. [DOI] [PubMed] [Google Scholar]
- 55.Zhang G, et al. Cobalt-catalyzed regioselective hydroboration of terminal alkenes. Eur. J. Org. Chem. 2017;2017:5814–5818. doi: 10.1002/ejoc.201701047. [DOI] [Google Scholar]
- 56.Tamang SR, et al. Cobalt-catalyzed hydroboration of alkenes, aldehydes, and ketones. Org. Lett. 2018;20:6695–6700. doi: 10.1021/acs.orglett.8b02775. [DOI] [PubMed] [Google Scholar]
- 57.Teo W, Ge S. Cobalt-catalyzed diborylation of 1,1-disubstituted vinylarenes: a practical access to branched gem-bis(boryl)alkanes. Angew. Chem. Int. Ed. 2018;57:1654–1658. doi: 10.1002/anie.201710389. [DOI] [PubMed] [Google Scholar]
- 58.Fan W, Li L, Zhang G. Branched-selective alkene hydroboration catalyzed by Earth-abundant metals. J. Org. Chem. 2019;84:5987–5996. doi: 10.1021/acs.joc.9b00550. [DOI] [PubMed] [Google Scholar]
- 59.Bage AD, Hunt TA, Thomas SP. Hidden boron catalysis: nucleophile-promoted decomposition of HBpin. Org. Lett. 2020;22:4107–4112. doi: 10.1021/acs.orglett.0c01168. [DOI] [PubMed] [Google Scholar]
- 60.Chong CC, Kinjo R. Catalytic hydroboration of carbonyl derivatives, imines, and carbon dioxide. ACS Catal. 2015;5:3238–3259. doi: 10.1021/acscatal.5b00428. [DOI] [Google Scholar]
- 61.Garduño JA, García JJ. Toward amines, imines, and imidazoles: a viewpoint on the 3d transition-metal-catalyzed homogeneous hydrogenation of nitriles. ACS Catal. 2020;10:8012–8022. doi: 10.1021/acscatal.0c02283. [DOI] [Google Scholar]
- 62.Hayrapetyan D, Khalimon AY. Catalytic nitrile hydroboration: a route to N,N-diborylamines and uses thereof. Chem. Asian J. 2020;15:2575–2587. doi: 10.1002/asia.202000672. [DOI] [PubMed] [Google Scholar]
- 63.Ibrahim AD, Entsminger SW, Fout AR. Insights into a chemoselective cobalt catalyst for the hydroboration of alkenes and nitriles. ACS Catal. 2017;7:3730–3734. doi: 10.1021/acscatal.7b00362. [DOI] [Google Scholar]
- 64.Ben-Daat H, et al. Hydroboration of alkynes and nitriles using an α-diimine cobalt hydride catalyst. Chem. Commun. 2017;53:7333–7336. doi: 10.1039/C7CC02281F. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh C, et al. Efficient cobalt catalyst for ambient-temperature nitrile dihydroboration, the elucidation of a chelate-assisted borylation mechanism, and a new synthetic route to amides. J. Am. Chem. Soc. 2019;141:15327–15337. doi: 10.1021/jacs.9b07529. [DOI] [PubMed] [Google Scholar]
- 66.Gudun KA, Slamova A, Hayrapetyan D, Khalimon AY. Efficient co-catalyzed double hydroboration of nitriles: application to one-pot conversion of nitriles to aldimines. Chem. Eur. J. 2020;26:4963–4968. doi: 10.1002/chem.202000753. [DOI] [PubMed] [Google Scholar]
- 67.Bedi D, Brar A, Findlater M. Transition metal- and solvent-free double hydroboration of nitriles. Green. Chem. 2020;22:1125–1128. doi: 10.1039/C9GC04260A. [DOI] [Google Scholar]
- 68.Ghosh P, von Wangelin AJ. Lithium amide catalyzed hydroboration of nitriles. Org. Chem. Front. 2020;7:960–966. doi: 10.1039/C9QO01507H. [DOI] [Google Scholar]
- 69.Gärtner D, Sandl S, von Wangelin AJ. Homogeneous vs. heterogeneous: mechanistic insights into iron group metal-catalyzed reductions from poisoning experiments. Catal. Sci. Technol. 2020;10:3502–3514. doi: 10.1039/D0CY00644K. [DOI] [Google Scholar]
- 70.Widegren JA, Finke RG. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A: Chem. 2003;198:317–341. doi: 10.1016/S1381-1169(02)00728-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information file. Any further relevant data are available from the authors on request.