Abstract
Myelodysplastic/myeloproliferative neoplasms (MDS/MPN) show a male predominance and men with MDS/MPN have worse outcomes but it is unknown if the mutational burden differs between genders. We reviewed 167 MDS/MPN patients and demonstrated that men have worse overall survival (HR 2.09, 95% CI 1.16 – 3.75, P=0.013) independent of subtype, R-IPSS score and age at diagnosis. We analyzed the genomic data on a subset of 100 patients. Men had 0.88 more somatic mutations on average (95% CI 0.20–1.56, P=0.011) independent of subtype, sample source and blast percentage. A higher number of somatic mutations was associated with a higher incidence of transformation to AML (SHR 1.30, 95% CI 1.01 – 1.70, P=0.046). Men had 0.70 more mutations in high-risk genes ASXL1, EZH2, RUNX1, SETBP1, NRAS, STAG2 on average (95% CI 0.11–1.29, P=0.021), and 13 times higher odds of harboring EZH2 mutation (95% CI 1.64 – 102.94, P=0.015). The presence of an EZH2 mutation was associated with worse survival among men (HR 2.98, 95% CI 1.1 – 8.0, P=0.031). Our findings suggest that the worse outcomes in men with MDS/MPN are associated with higher number of somatic mutations, especially in high-risk genes. These results warrant validation in larger cohorts and investigation of the underlying mechanisms.
Keywords: MDS/MPN overlap syndromes, gender differences, outcomes, genomic landscape, mutational burden
Introduction
Myelodysplastic/myeloproliferative neoplasms (MDS/MPN) is a heterogeneous group of malignancies with overlapping features of both MDS and MPN and in adults, includes MDS/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), MDS/MPN unclassifiable (MDS/MPN-U), chronic myelomonocytic leukemia (CMML) and atypical chronic myeloid leukemia (aCML)1. With the exception of MDS/MPN-RS-T, these entities are associated with overall poor outcomes and the therapeutic options for these patients are limited. Thus, the improvement of the understanding of the biology of these neoplasms is a necessity. The karyotype is normal in a majority of MDS/MPN patients but next-generation sequencing (NGS) often reveals a high frequency of mutations1–4. Recent data highlight that the genomic landscape and clonal architecture of these neoplasms are associated with specific phenotypes and have significant prognostic implications5,6.
It is known that these diseases show a male predilection and recent data support that men have a worse survival compared to women2,7. The underlying biologic mechanisms implicated in these gender-related differences in outcomes have not been previously delineated. Our group has demonstrated gender-related differences in the genomic landscape of patients with MPN showing that men have higher number of somatic mutations and particularly higher frequency of high-risk mutations, such as mutations in ASXL1 and U2AF18. Similarly, De-Morgan et al recently found that men with acute myeloid leukemia (AML) have higher frequency of pre-leukemic mutations including mutations in RUNX1, ASXL1 and ZRSR29. However, the comparison of the molecular landscape between genders as a possible explanation of the different outcomes has not been done for patients with MDS/MPN.
Thus, in this study we address this gap in knowledge by comparing the clinical outcomes between genders in our MDS/MPN cohort, exploring gender-specific differences in the mutational patterns as well as mutation burden of MDS/MPN patients and estimating their associations with clinical outcomes.
Methods
Patient Selection
We retrospectively investigated all patients with MDS/MPN, diagnosed based on World Health Organization criteria1, seen at Johns Hopkins University between January 2010 through January 2020. Clinical, laboratory and cytogenetic characteristics at diagnosis and treatment details including history of allogeneic blood or marrow transplantation, were recorded for all the patients. Outcomes of interest were overall survival, and AML-free survival. The study was approved by the Johns Hopkins University Institutional Review Board.
Next-generation sequencing
We identified patients who had NGS performed in their peripheral blood or bone marrow during the chronic phase of the disease. The sequencing was performed using an established 63-genes panel at the Johns Hopkins Molecular Pathology laboratory (Supplementary Table 1) or a commercial 44-genes panel (Genoptix) (Supplementary table 2) as previously described10. DNA was extracted from peripheral blood or bone marrow, was captured with Kapa Roche reagents and integrated DNA Technology probes, and sequenced using Illumina paired end technology. High throughput sequencing detects small base changes, insertions, and deletions in exons and splicing junctions. The analysis was done using human reference sequence genome assembly hg19. An in-house variant caller (MDL VC 10.0), as well as a third-party variant caller (Haplotyper Genome Analysis TK-3.3) based on the Bayesian statistical model were used to generate a list of variants. The variants underwent further filtering utilizing in-house algorithms and annotation utilizing the COSMIC database v90, dbSNP v150, and Annovar (10122020) to confirm mutation status. The 63-genes panel of the Johns Hopkins Molecular Pathology laboratory is certified by the Clinical Laboratory Improvement Amendments.
Variants that are identified in the dbSNP dataset as “common” were not reported.
The following variants were considered somatic mutations and were included in the analysis:
Variants reported in the COSMIC database
Variants that may result in a loss-of-function type of mutation
Variants that are reported in both COSMIC and dbSNP databases and have an allele frequency of <40% or >60%
Variants that have not been reported in either the COSMIC or dbSNP databases and have an allele frequency of <40% or >60%
Finally, the analysis included only variants in genes that were present in both panels.
Statistical analysis
We used Fisher’s exact test to compare the baseline characteristics and frequency of treatments between men and women. Comparison of the percentage of blasts, number of platelets, revised international prognostic scoring system (R-IPSS) score, number of somatic mutations, variant allele frequency, and number of high-risk mutations between men and women were done using univariate and multivariate linear regression analysis. Each of these characteristics were used as outcome variables in the analysis and the different disease subtypes were included in the multivariable analysis. The difference of the frequency of an EZH2 mutation was compared with multivariable logistic regression analysis. We then performed univariate and multivariable cox regression analysis to assess the impact of gender, disease sub-type, age and R-IPSS at diagnosis, number of somatic mutations and high-risk mutations on the overall survival. Kaplan-Meier analysis was used to evaluate the effect of gender, presence of ≤ or > 4 mutations (4 being the median number of the somatic mutations in the cohort) and EZH2 mutation on overall survival. Finally, we conducted competing-risks regression model to assess the impact of gender, number of somatic mutations, high-risk mutations and EZH2 mutation on the transformation to AML. All analyses were performed by using STATA version 13.1 software. P<0.05 was considered significant.
Results
Clinical and cytogenetic features of MDS/MPN at diagnosis
The entire cohort included 62 women and 105 men. The baseline clinical and cytogenetic characteristics and the treatments of the cohort are summarized in Table 1. The median age at diagnosis was 67 (range 44–86) years and men were older compared to women at diagnosis (median 67.92 years vs 64.61 years, P=0.013). The median time from diagnosis until evaluation at our institution was 0 (range 0–72) months and the median follow-up was 26 (1–114) months. Men had a higher percentage of blasts at diagnosis (median 2.9% vs 2%, P=0.029) but after controlling for the specific disease sub-type this difference was not significant (Coef 0.008 P=0.081). In the entire cohort, men had a lower number of platelets at diagnosis compared to women (median 179.95 vs 329.08, P=0.002). Upon stratifying per specific disease subtype, this difference remained significant among patients with MDS/MPN-U (P=0.021) and CMML (P=0.007) (Figure 1A). The karyotype at diagnosis was not significantly different between genders. Finally, fewer men had very low R-IPSS score at diagnosis (23.5% vs 44.8%, P=0.021) compared to women and upon controlling for specific disease subtype, men had overall higher R-IPSS scores at diagnosis (Coef 0.614, 95% CI 0.12 – 1.10, P=0.015) (Figure 1B).
Table 1.
Baseline characteristics and treatments of the entire cohort
| Characteristics | Entire cohort | Women | Men | P value |
|---|---|---|---|---|
| N | 167 (100) | 62 (37.1) | 105 (62.9) | |
| Diagnosis | ||||
| MDS/MPN-RS-T | 17 (10.2) | 8 (12.9) | 9 (8.6) | 0.430 |
| MDS/MPN-U | 41 (24.6) | 19 (30.6) | 22 (21) | 0.193 |
| CMML | 97 (58) | 30 (48.4) | 67 (63.8) | 0.054 |
| aCML | 12 (7.2) | 5 (8.1) | 7 (6.6) | 0.762 |
| History of cytotoxic therapy | 12 (7.1) | 4 (6.5) | 8 (7.6) | 1.000 |
| Age at diagnosis | 66.69 +/− 8.35 | 64.61 +/− 8.73 | 67.92 +/− 7.92 | 0.013 |
| BM blasts (%) at diagnosis | 2.6 +/− 2.5 | 2 +/− 2.18 | 2.9 +/− 2.9 | 0.029 |
| Hemoglobin (g/dl) at diagnosis | 10.59 +/− 2.4 | 10.63 +/− 2.1 | 10.56 +/− 2.5 | 0.853 |
| Absolute neutrophils (106/μl) at diagnosis | 15.93 +/− 18.21 | 16.48 +/− 18.21 | 15.61 +/− 18.29 | 0.772 |
| Platelets at diagnosis (103/μl) | 234.94 +/− 294.97 | 329.08 +/− 365.41 | 179.95 +/− 223.19 | 0.002 |
| Karyotype at diagnosis (N=161) | ||||
| Very good | 6 (3.7) | 4 (6.7) | 2 (2) | 0.196 |
| Good | 120 (74.5) | 45 (75) | 75 (74.2) | 1.000 |
| Intermediate | 26 (16.2) | 9 (15) | 17 (16.8) | 0.827 |
| Poor | 7 (4.3) | 2 (3.3) | 5 (5) | 1.000 |
| Very poor | 2 (1.3) | 0 (0) | 2 (2) | 0.529 |
| R-IPSS score at diagnosis (N=156) | ||||
| Very low | 49 (31.4) | 26 (44.8) | 23 (23.5) | 0.021 |
| Low | 69 (44.2) | 23 (39.7) | 46 (46.9) | 0.408 |
| Intermediate | 19 (12.2) | 4 (6.9) | 15 (15.3) | 0.137 |
| High and Very high | 19 (12.2) | 5 (8.6) | 14 (14.3) | 0.450 |
| Treatments | ||||
| Ruxolitinib | 13 (7.8) | 5 (8.3) | 8 (2.2) | 1.000 |
| HMA | 78 (46.7) | 23 (37.1) | 55 (52.4) | 0.077 |
| Chemotherapy | 23 (13.8) | 7 (11.3) | 16 (15.3) | 0.643 |
| AlloBMT | 39 (23.4) | 11 (18.3) | 28 (26.7) | 0.256 |
Abbreviations:MDS/MPN-RS-T, Myelodysplastic/Myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, MDS/MPN-U, Myelodysplastic/Myeloproliferative neoplasm – unclassifiable; CMML, chronic myelomonocytic leukemia; aCML, atypical chronic myeloid leukemia; BM, bone marrow; R-IPSS, revised-international prognostic scoring system; HMA, hypomethylating agent; AlloBMT, allogeneic bone marrow transplantation; NGS, next generation sequencing.
Figure 1.
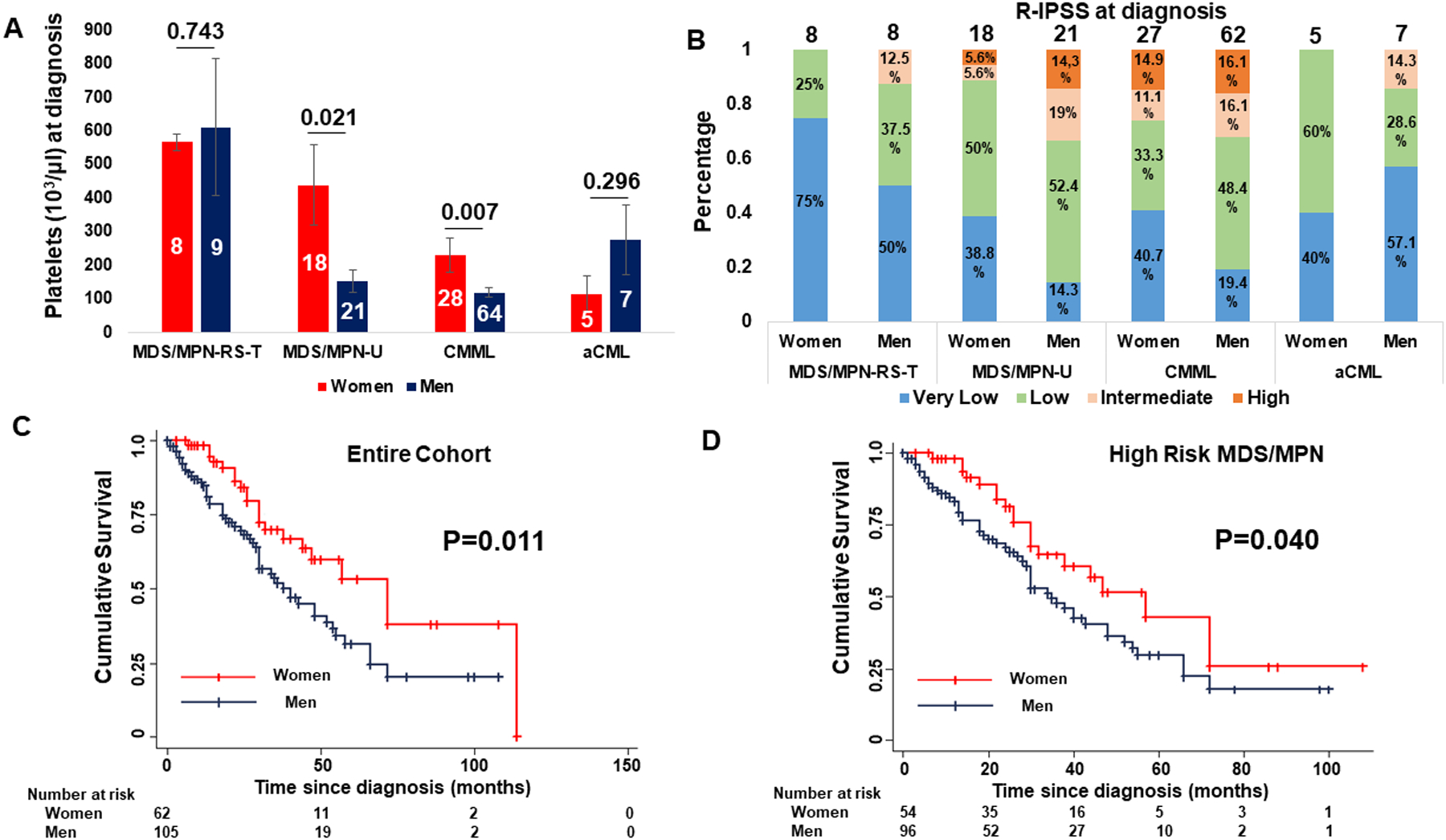
A. Men with MDS/MPN-U (P=0.021) and CMML (P=0.007) have lower platelets compared to women at diagnosis. B. Men have higher R-IPSS at diagnosis (P=0.021) across different MDS/MPN sub-types. C. Men have worse overall survival compared to women (P=0.011) in the entire cohort of patients with MDS/MPN neoplasms. D. Men have worse overall survival compared to women (P=0.040) in the high-risk MDS/MPN cohort including patients with MDS/MPN-U, CMML and aCML.
Male gender is an independent prognostic factor of worse overall survival
Men had a worse mean overall survival compared to women in the entire cohort (48.44 months vs 69.30 months, P=0.011) (Figure 1C) and among the 89 patients with high-risk disease sub-types (MDS/MPN-U, CMML, aCML) (44.02 months vs 58.02 months, P=0.040) (Figure 1D). Multivariable cox-regression analysis showed that male gender is associated with worse overall survival (HR 2.09, 95% CI 1.16 – 3.75, P=0.013) in the entire cohort of 167 patients independent of the specific disease sub-type and R-IPSS and age at diagnosis (Supplementary Table 3). Men were not found to have a higher incidence of transformation to AML based on a competing-risks regression analysis (SHR 1.33, 95% CI 0.66 – 2.67, P=0.431) (Supplementary Figure 1A).
Men have higher number of somatic mutations and increased variant allele frequency
To evaluate for possible gender-related differences in the genomic landscape of patients with MDS/MPN that could be associated with the outcome differences between genders, the frequency of somatic mutations was analyzed in the sub-cohort of 100 patients who had available NGS data. This sub-cohort included 40 women and 60 men and their clinical and cytogenetic characteristics at diagnosis, treatments and sample characteristics are summarized in the Supplementary Table 4. There were no significant differences in the baseline characteristics and treatments between the entire cohort and the NGS sub-cohort (Supplementary Table 5). The variants, which were identified as somatic mutations are shown in the Supplementary Table 6.
Men had more complex genomic landscape in the entire cohort of 100 patients and across different diagnoses (Figure 2, Supplementary Figure 1B). More specifically, men had 0.88 more somatic mutations on average compared to women (95% CI 0.20–1.56, P=0.011), independent of the specific disease sub-type, sample source and blasts percentage (Figure 3A, Supplementary Table 7). Men also had a higher frequency of mutations in genes that are commonly mutated in the accelerated phase of the disease including BCOR, PHF6, ETV6, BRAF, and PTPN1111–14 (Figure 2).
Figure 2.
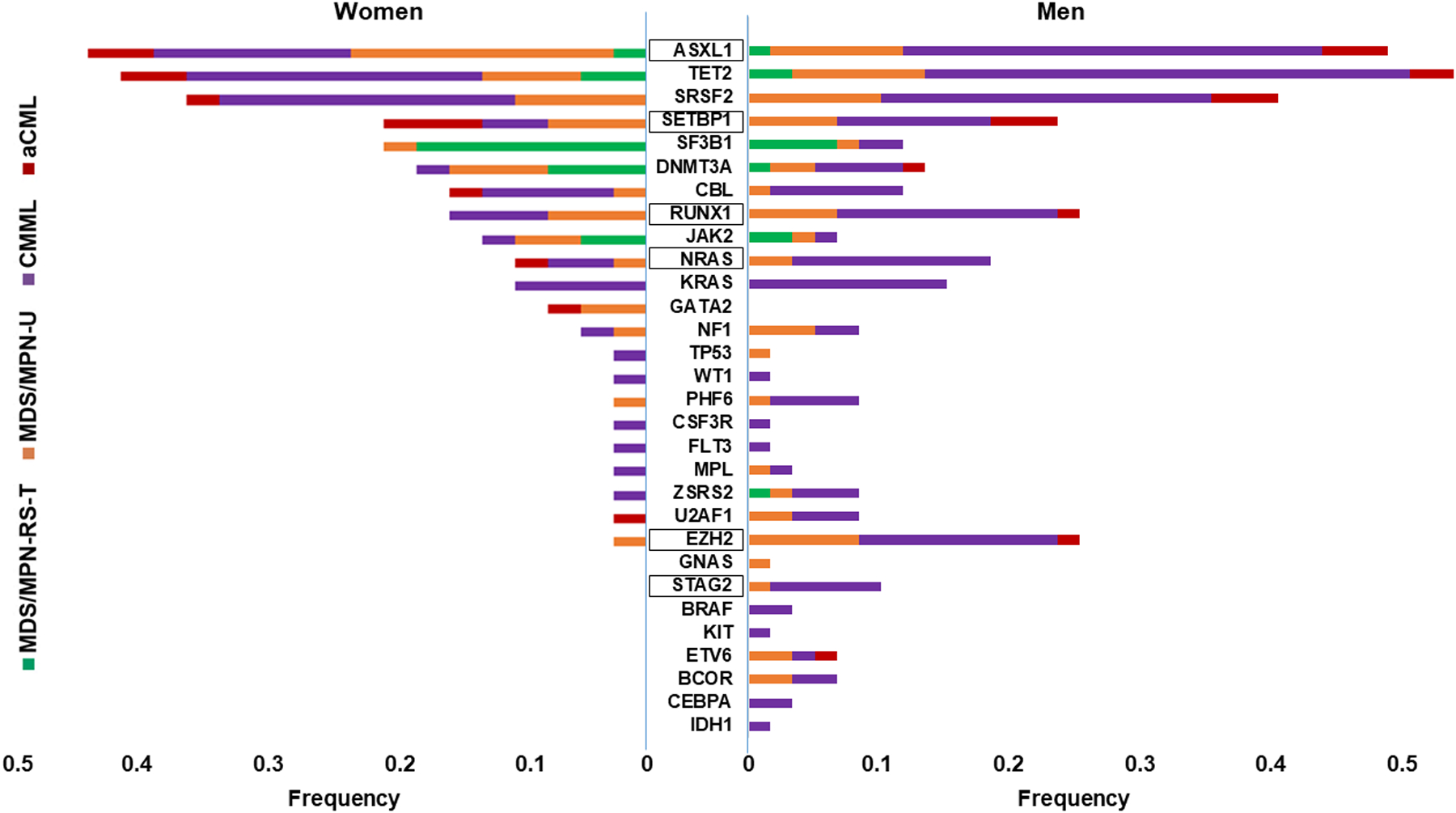
The frequency of mutations in different genes in women and men across the different phenotypes (MDS/MPN-RS-T: MDS/MPN with ring sideroblasts and thrombocytosis, MDS/MPN-U: MDS/MPN-unclassifiable, CMML: chronic mylelomonocytic leukemia, aCML: atypical CML). The high-risk genes are indicated in squares.
Figure 3.
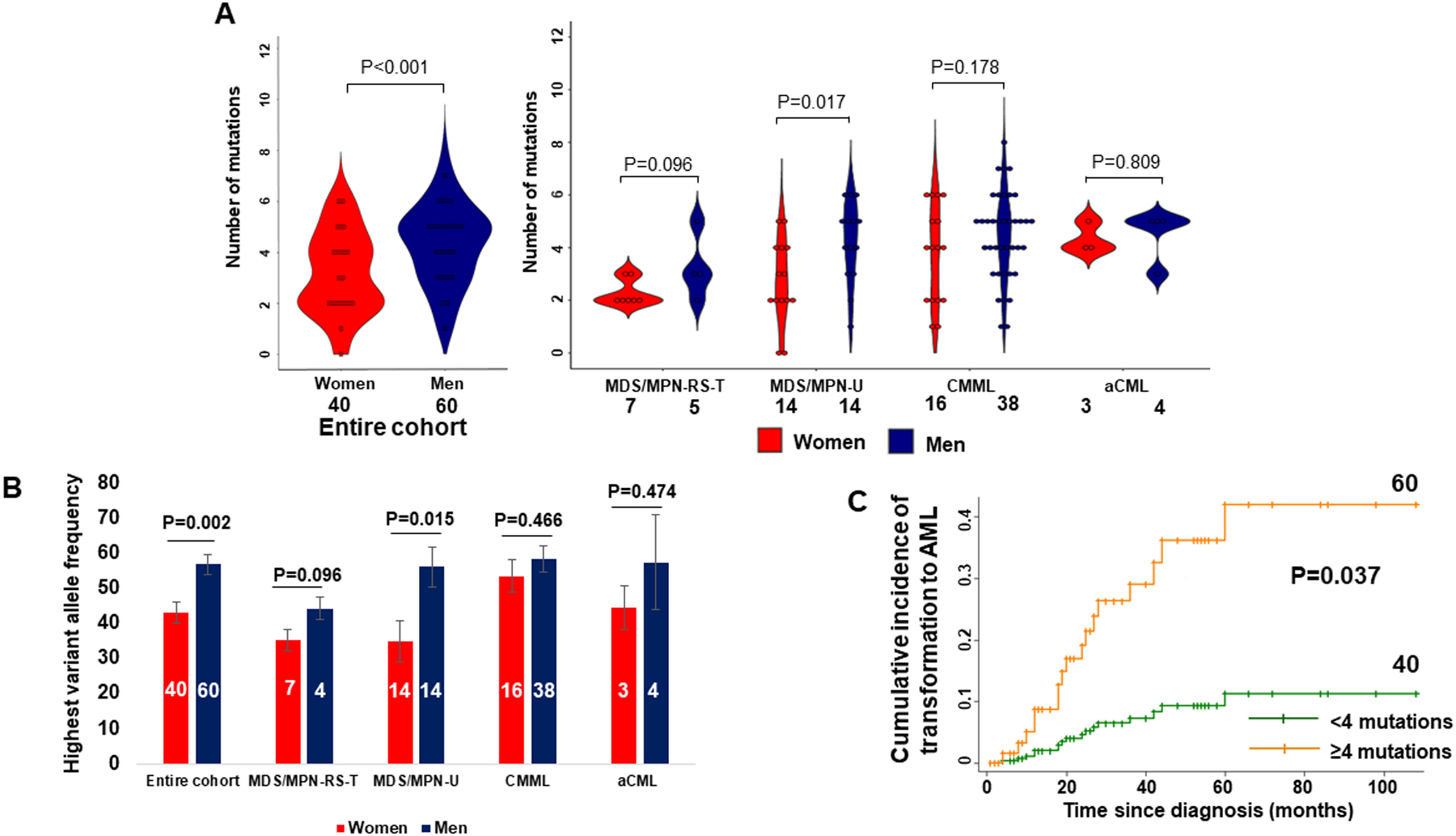
A. Men have significantly higher number of somatic mutations across the entire cohort (P<0.001). The difference was also significant among patients with MDS/MPN-U. B. The VAF is higher among men compared to women across the entire cohort (P=0.002). The difference was also significant among MDS/MPN-U patients. C. Competing-risk regression analysis of MDS/MPN-U, CMML and aCML patients showing the incidence of transformation to AML based on the number of somatic mutations (<4 or ≥4) (4 was the median number of somatic mutations). Patients with 4 or more somatic mutations have higher incidence of transformation to AML (P=0.037).
We further studied whether there was any difference in the mutational burden (variant allele frequency, VAF) by gender. On review of 100 patients with NGS data, VAF was higher in men (56.9% vs 43.1%; Coef 11.07, 95% CI 2.28–19.76 P=0.014) independent of the disease subtype (MDS/MPN-RS-T – ref, MDS/MPN-U – Coef 5.38, 95% CI −9.51 – 20.28, P=0.475, CMML – Coef 14.55, 95% CI 0.43 – 28.68, P=0.044, aCML – Coef 11.01, 95% CI −9.24 – 31.27, P=0.283) (Figure 3B).
Higher number of somatic mutations is associated with higher incidence of transformation to AML
The analysis for impact of the number of mutations on the clinical outcomes did not include patients with MDS/MPN-RS-T as they have significantly better outcomes compared to those of the other three i.e. MDS/MPN-U, CMML, aCML. Thus, henceforth MDS/MPN will denote the 3 overlap neoplasms i.e. MDS/MPN-U, CMML, aCML and the analysis included 89 patients with these neoplasms. A higher number of somatic mutations was associated with a higher incidence of transformation to AML (SHR 1.30, 95% CI 1.01 – 1.70, P=0.046) on a multivariable analysis independent of the specific disease sub-type, and age at diagnosis (Supplementary Table 8). Similarly, competing-risks regression model showed that MDS/MPN patients with at least 4 mutations (median number of somatic mutations) had a significantly higher incidence of transformation to AML (SHR=4.57, 95% CI 1.10–19.08, P=0.037) compared to patients with < 4 somatic mutations (Figure 3C). The association between the number of somatic mutations and overall survival was not statistically significant (HR 1.23, 95% CI 0.98–1.54, P=0.075) on a univariate analysis.
Men have higher number of high-risk somatic mutations, particularly EZH2 mutations, which are associated with shorter overall survival
The number of mutations in high-risk genes, ASXL1, EZH2, RUNX1, SETBP1, NRAS, and STAG2, between genders was compared5,15–18. Men have 0.70 more mutations in these genes on average compared to women (95% CI 0.11–1.29, P=0.021) independent of specific disease sub-type in a multivariable analysis of the 100 patients with NGS data (Figure 4A). In our cohort of 89 patients with MDS/MPN-U, CMML and aCML the number of mutations in these high-risk genes was not statistically significantly associated with higher risk of transformation to AML (SHR 1.34, 95% CI 0.98 – 1.84, P=0.066) or worse overall survival (HR 1.15, 95% CI 0.94 – 1.41, P=0.169).
Figure 4.
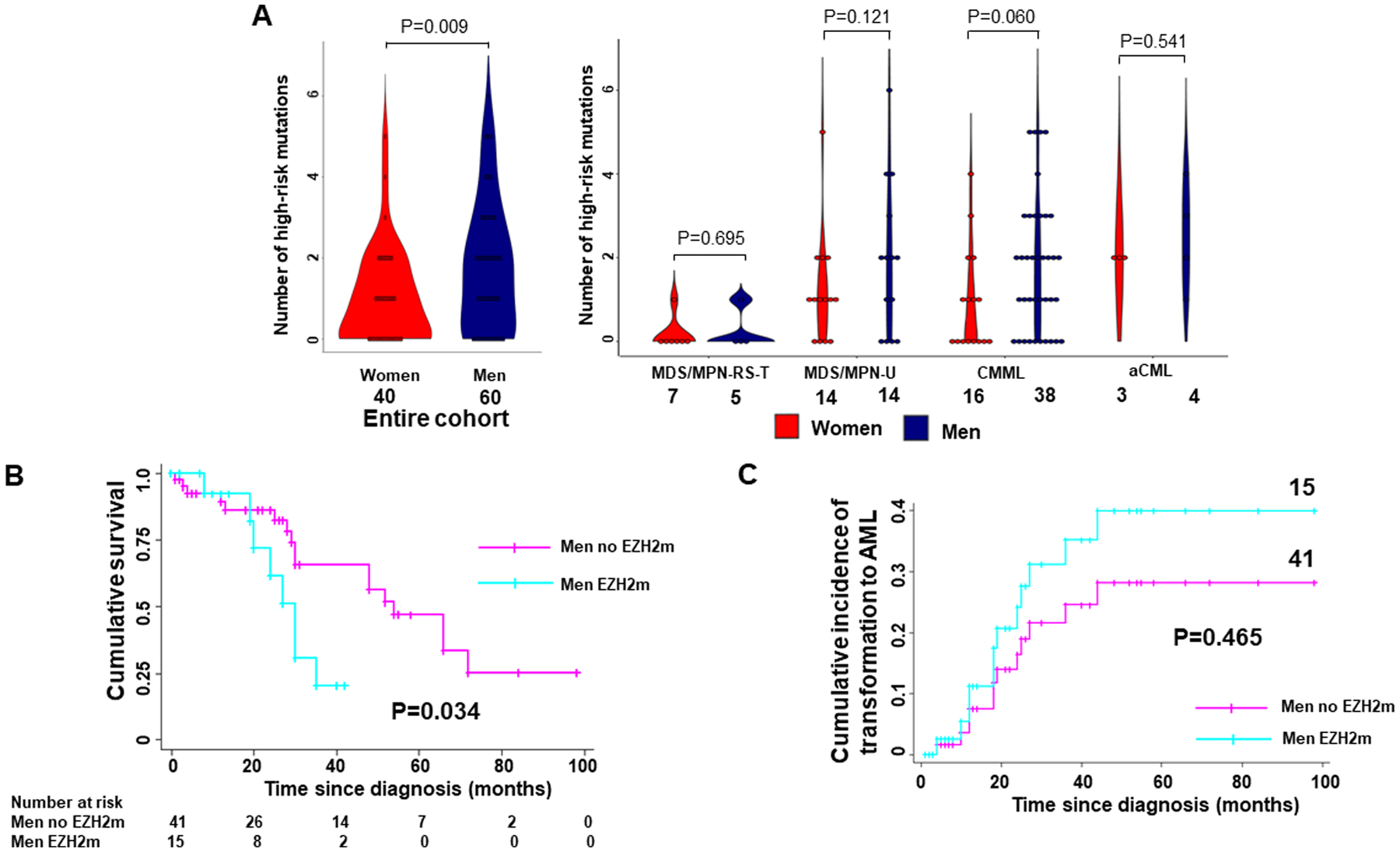
A. Men have significantly higher number of somatic mutations in high-risk genes across the entire cohort (P=0.009) with a more prominent difference among CMML patients. B. Kaplan-Meier analysis showing the cumulative survival of men with and without EZH2 mutation including patients with MDS/MPN-U, CMML and aCML. Men with EZH2 mutation have significantly worse survival (P=0.034) compared to men without EZH2 mutation. C. Competing-risk regression analysis showing the cumulative incidence of transformation to AML among men with and without EZH2 mutation including patients with MDS/MPN-U, CMML and aCML. Men with EZH2 mutation appear to have higher incidence of transformation to AML but difference was not significant (P=0.465).
The most prominent difference was noted in EZH2, with men having 13 times greater odds of an EZH2 mutation compared to women (95% CI 1.64 – 102.94, P=0.015) (Figure 2). Given the significantly higher incidence of EZH2 mutation among men with only one woman carrying an EZH2 mutation we sought to study if EZH2 itself is associated with worse outcomes. Thus, we studied the association of EZH2 mutation with survival among the 56 men with high-risk MDS/MPN. Cox regression analysis showed that men with EZH2 mutation had a worse overall survival compared to men without EZH2 mutation in our cohort (HR 2.98, 95% CI 1.1 – 8.0, P=0.031), independent of the disease subtype and age at diagnosis. This difference was demonstrated by the Kaplan-Meier analysis which showed that men with EZH2 mutation had a significantly worse overall survival (P=0.034) compared to men without EZH2 mutation (Figure 4B). Competing-risks regression showed that men with EZH2 mutation did not have a significantly increased incidence of transformation to AML compared to men without an EZH2 mutation (Figure 4C).
Discussion
Our findings suggest that men with MDS/MPN have overall worse survival independent of the specific sub-types, age and R-IPSS score at diagnosis. The analysis of the molecular landscape in a sub-group of our cohort revealed that men have higher number of somatic mutations and increased disease burden across all MDS/MPN subtypes. Moreover, higher number of somatic mutations was associated with higher incidence of AML transformation. Lastly, men carry high-risk mutations at a higher frequency and have a significantly higher incidence of EZH2 mutation, which was associated with poorer survival among men.
Our study is the first to demonstrate that in MDS/MPN, male gender is associated with worse survival independent of the specific disease sub-type, R-IPSS score and age at diagnosis. The worse clinical outcome in men has been highlighted in other myeloid diseases cohorts in line with our findings in MDS/MPN. In the pre-tyrosine kinase inhibitors era, men with chronic myeloid leukemia were found to have worse overall survival compared to women across different risk groups19. This analysis revealed that men present with bigger spleen, fewer platelets and higher rate of additional cytogenetic abnormalities19. Our group recently reported that men with MPN have worse survival independent of the specific phenotype, age at diagnosis and MPN-specific driver mutation8. Additionally, Barraco et al. showed that men with secondary myelofibrosis have worse survival and lower platelet count at diagnosis20. Finally, Wang et al demonstrated that among patients with various MDS sub-types and MDS/MPN, men have worse overall survival7. However, stratification per different MDS/MPN sub-types and control for R-IPSS score and age at diagnosis was not done in this analysis. The findings of our current study are consistent with these data. We also found that men present with fewer platelets and higher R-IPSS scores compared to women, which are consistent with previously published studies and reflect on a more aggressive biology of MDS/MPN diseases among men19,20. These results highlight the importance of gender-based differences in myeloid diseases suggesting that male gender could be taken into consideration as an independent factor in risk assessment tools and requesting further evaluation of the underlying biologic mechanism.
Our group recently found that men with MPN have higher number of non-driver somatic mutations and particularly high-risk mutations including EZH2 and U2AF1, which are associated with worse outcomes8. These features of male pattern of myeloid disease are also present in our MDS/MPN cohort as we demonstrated that men have more somatic mutations. Higher number of somatic mutations has been correlated with worse response to hypomethylating agents in patients with MDS and MDS/MPN21, further supporting the poor prognosis associated with high number of mutations in MDS/MPN, which appears to be associated with male gender as demonstrated in our study. In our analysis higher number of somatic mutations, which is a feature more prominent among men was associated with higher incidence of transformation to AML. While a small size and limitation of analysis precludes a direct causal association, our results are hypothesis generating that the higher somatic mutational burden in men, is a possible reason for their worse outcomes.
Importantly, mutations that were more frequent among men such as NRAS and STAG2 mutations have been associated with particularly poor outcomes in MDS/MPN15–18. ASXL1 mutation, associated with a poor outcome, was also more common in men with MDS/MPN-U, while DNMT3A and IDH2 mutations were more common among women3. Other mutations that were noted predominantly among men in our study such as BCOR, ETV6, PHF6, and BRAF mutations occur with higher frequency in more advance disease3,11–14,16. The higher frequency of specific pre-leukemic mutations such as mutations in BCOR, RUNX1, U2AF1 and ASXL1 in men with AML was also reported in a recent study by De-Morgan et al9. These findings are consistent with our results in MDS/MPN and support the presence of a distinct molecular profile in men with myeloid diseases potentially mediating their more rapid progression to AML.
EZH2 mutations, which showed the most prominent male predilection, are associated with poor outcomes in myeloid malignancies and they frequently co-exist with mutations in RUNX1 and ASXL1 in MDS/MPN patients5,22,23. The co-existence of EZH2 and ASXL1 mutations in CMML is associated with particularly dismal outcomes17. The analysis of our NGS cohort revealed that the presence of a mutation in EZH2 is associated with worse survival in men independent of the specific sub-type, and age at diagnosis. Men with EZH2 mutation have significantly worse overall survival compared to men without EZH2 mutation. These results suggest that the worse survival of men with MDS/MPN reported by others and confirmed in this study is at least in part attributed to the higher incidence of EZH2 mutations7.
In conclusion, our findings support that men with MDS/MPN diseases have overall worse outcomes compared to the women which are associated with more prominent expansion of higher-risk clones that result in a worse survival. These results warrant further evaluation of possible underlying molecular mechanisms, in a larger cohort. These could provide insight into prognostic and treatment strategies, including early intervention, targeted therapies and timing of stem cell transplantation.
Supplementary Material
Funding:
TK was funded by T32HL007525 NIH/NHLBI.
Competing interests:
ARM has received research funding from Alnylam, and served on advisory boards for PharmEssentia, AED has received research funding from Astex and honoraria from Celgene, and has done consultancy with Celgene, Abbvie and MEI, TJ has done consultancy with Targeted Oncology, advisory board with CareDx and Bristol Myers Squibb. TK, LPG, RJJ have no disclosures or conflicts of interest to report.
References:
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Mughal TI, Cross NC, Padron E, et al. An International MDS/MPN Working Group’s perspective and recommendations on molecular pathogenesis, diagnosis and clinical characterization of myelodysplastic/myeloproliferative neoplasms. Haematologica. 2015;100(9):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose P, Nazha A, Komrokji RS, et al. Mutational landscape of myelodysplastic/myeloproliferative neoplasm-unclassifiable. Blood. 2018;132(19):2100–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patnaik MM, Barraco D, Lasho TL, et al. Targeted next generation sequencing and identification of risk factors in World Health Organization defined atypical chronic myeloid leukemia. American journal of hematology. 2017;92(6):542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomo L, Meggendorfer M, Hutter S, et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elena C, Gallì A, Such E, et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood. 2016;128(10):1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F, Ni J, Wu L, Wang Y, He B, Yu D. Gender disparity in the survival of patients with primary myelodysplastic syndrome. Journal of Cancer. 2019;10(5):1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karantanos T, Chaturvedi S, Braunstein EM, et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood advances. 2020;4(12):2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De-Morgan A, Meggendorfer M, Haferlach C, Shlush L. Male predominance in AML is associated with specific preleukemic mutations. Leukemia. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Gondek LP, Zheng G, Ghiaur G, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damm F, Chesnais V, Nagata Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122(18):3169–3177. [DOI] [PubMed] [Google Scholar]
- 12.Wall M, Rayeroux KC, MacKinnon RN, Zordan A, Campbell LJ. ETV6 deletion is a common additional abnormality in patients with myelodysplastic syndromes or acute myeloid leukemia and monosomy 7. Haematologica. 2012;97(12):1933–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jager RGH, Berg T, Passamonti F, Cazzola M, Rumi E. Deletions of the Transcription Factor Ikaros in Myeloproliferative Neoplasms at Transformation to Acute Myeloid Leukemia. ASH Annual Meeting Abstracts 2009. [DOI] [PubMed] [Google Scholar]
- 14.Klampfl T, Harutyunyan A, Berg T, et al. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood. 2011;118(1):167–176. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SC, Shih LY, Liang ST, et al. Biological Activities of RUNX1 Mutants Predict Secondary Acute Leukemia Transformation from Chronic Myelomonocytic Leukemia and Myelodysplastic Syndromes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(15):3541–3551. [DOI] [PubMed] [Google Scholar]
- 16.Meggendorfer M, Jeromin S, Haferlach C, Kern W, Haferlach T. The mutational landscape of 18 investigated genes clearly separates four subtypes of myelodysplastic/myeloproliferative neoplasms. Haematologica. 2018;103(5):e192–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patnaik MM, Vallapureddy R, Lasho TL, et al. EZH2 mutations in chronic myelomonocytic leukemia cluster with ASXL1 mutations and their co-occurrence is prognostically detrimental. Blood cancer journal. 2018;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thota S, Viny AD, Makishima H, et al. Genetic alterations of the cohesin complex genes in myeloid malignancies. Blood. 2014;124(11):1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger U, Maywald O, Pfirrmann M, et al. Gender aspects in chronic myeloid leukemia: long-term results from randomized studies. Leukemia. 2005;19(6):984–989. [DOI] [PubMed] [Google Scholar]
- 20.Barraco D, Mora B, Guglielmelli P, et al. Gender effect on phenotype and genotype in patients with post-polycythemia vera and post-essential thrombocythemia myelofibrosis: results from the MYSEC project. Blood cancer journal. 2018;8(10):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montalban-Bravo G, Takahashi K, Patel K, et al. Impact of the number of mutations in survival and response outcomes to hypomethylating agents in patients with myelodysplastic syndromes or myelodysplastic/myeloproliferative neoplasms. Oncotarget. 2018;9(11):9714–9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Han Q, Zi J, et al. Mutations in EZH2 are associated with poor prognosis for patients with myeloid neoplasms. Genes & diseases. 2019;6(3):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinke J, Muller JP, Blaess MF, et al. Molecular characterization of EZH2 mutant patients with myelodysplastic/myeloproliferative neoplasms. Leukemia. 2017;31(9):1936–1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


