Abstract
Aims:
To examine whether cognitive behavioral therapy for insomnia (CBT-I), delivered by telephone, improves sleep and non-sleep symptoms of Gulf War Illness (GWI).
Main methods:
Eighty-five Gulf War veterans (21 women, mean age: 54 years, range 46–72 years) who met the Kansas GWI case definition, the Centers for Disease Control and Prevention (CDC) case definition for Chronic Multisymptom Illness (CMI), and research diagnostic criteria for insomnia disorder were randomly assigned to CBT-I or monitor-only wait list control. Eight weekly sessions of individual CBT-I were administered via telephone by Ph.D. level psychologists to study participants. Outcome measures included pre-, mid-, and post-treatment assessments of GWI and insomnia symptoms, subjective sleep quality, and continuous sleep monitoring with diary. Outcomes were re-assessed 6-months post-treatment in participants randomized to CBT-I.
Key findings:
Compared to wait list, CBT-I produced significant improvements in overall GWI symptom severity, individual measures of fatigue, cognitive dysfunction, depression and anxiety, insomnia severity, subjective sleep quality, and sleep diary outcome measures. The beneficial effects of CBT-I on overall GWI symptom severity and most individual GWI symptom measures were maintained 6-months after treatment.
Significance:
GWI symptoms have historically been difficult to treat. Because CBT-I, which is associated with low stigma and is increasingly readily available to veterans, improved both sleep and non-sleep symptoms of GWI, these results suggest that a comprehensive approach to the treatment of GWI should include behavioral sleep interventions.
Keywords: Gulf war illness, Chronic multisymptom illness, Insomnia, Cognitive behavioral therapy, Telehealth, Veteran
1. Introduction
Thirty years after the Gulf War (GW), many veterans who served in Operations Desert Shield/Desert Storm continue to suffer from the debilitating symptoms of Gulf War Illness (GWI) [1–3]. A symptomatic disorder, the specific symptoms of GWI varies from veteran to veteran, but the condition typically includes some combination of the following: widespread pain, persistent fatigue, insomnia and other sleep disturbances, memory and concentration problems, chronic headache, gastrointestinal problems, skin abnormalities, and/or mood disturbances [4–7]. Despite nearly three decades of research, there are few evidence-based treatments available for veterans suffering from GWI [8].
Insomnia is a common symptom of GWI [9]. Furthermore, sleep disturbance can influence and/or exacerbate other GWI symptoms of fatigue, pain, impaired mood, cognition, and daily function [10,11]. For this reason, we hypothesized that cognitive behavioral therapy for insomnia (CBT-I), a multi-component behavioral treatment that has emerged as the first-line treatment for chronic insomnia [12], may benefit both sleep and non-sleep symptoms of GWI. CBT-I has never been tested before in veterans with GWI, although there is evidence that CBT-I improves both sleep and non-sleep symptoms in other multi-systemic illnesses [13,14]. For example, fibromyalgia is a multisymptom disorder characterized by widespread pain, cognitive dysfunction, and sleep disturbance (e.g., non-refreshing sleep) [15] that shares overlapping symptoms with GWI. There is suggestive evidence that sleep disorder plays a prominent role in the etiology and symptomatic management of fibromyalgia [16–18], and that CBT-I not only improves sleep disturbance in patients with fibromyalgia [19–21], but also pain intensity, fatigue, anxiety [19], daily function and psychological well-being [22]. Chronic fatigue syndrome (CFS) is another multisymptom disorder that shares many overlapping symptoms with GWI. CFS is characterized by persistent, medically unexplained fatigue, musculoskeletal pain, sleep disturbance, headaches, impaired concentration and short-term memory [23]. Like fibromyalgia, sleep disorder has been suggested to be a maintaining factor in CFS [24]. Furthermore, research suggests that targeting insomnia improves treatment responses in the management of CFS [24]. One study reported that long-term improvements in insomnia severity in patients with CFS were significantly associated with long-term improvements in fatigue [25].
We conducted this proof-of-concept trial to assess the impact of CBT-I on sleep and non-sleep symptoms of GWI in veterans with GWI and comorbid insomnia. CBT-I is a treatment that has been rolled out through the VA system [26], less stigmatizing than other mental health treatments, and acceptable and feasible to veterans [27]. There is evidence and precedence for delivering insomnia interventions by telephone [28,29]. Because veterans with GWI may have physical and functional impairments that can make traveling to and from a therapist’s office difficult [30], we administered CBT-I by telephone to minimize some of the barriers associated with mobility and transportation. The remote delivery of CBT-I also allowed us to include GW veterans with rural residences, a known factor for sleep care disparity in the VA [31].
2. Material and methods
The study was approved by the institutional review boards at the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center and registered with clinicaltrials.gov (NCT02782780). All participants provided informed consent and were paid for participation (up to $355 for completion of all study procedures).
2.1. Participants
Eighty-five eligible GW veterans were randomized to CBT-I or monitor-only wait list (see Fig. 1). All participants were deployed to the Gulf Theater of operations between 1990 and 1991 in accordance with the inclusion/exclusion criteria set forth in the federal definition of GWI as used for the Gulf War Registry [32]. The participants all met the Kansas case definition for GWI [33], the Centers for Disease Control and Prevention (CDC) case definition of Chronic Multisymptom Illness (CMI) [4], and the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) criteria for insomnia disorder [34].
Fig. 1.
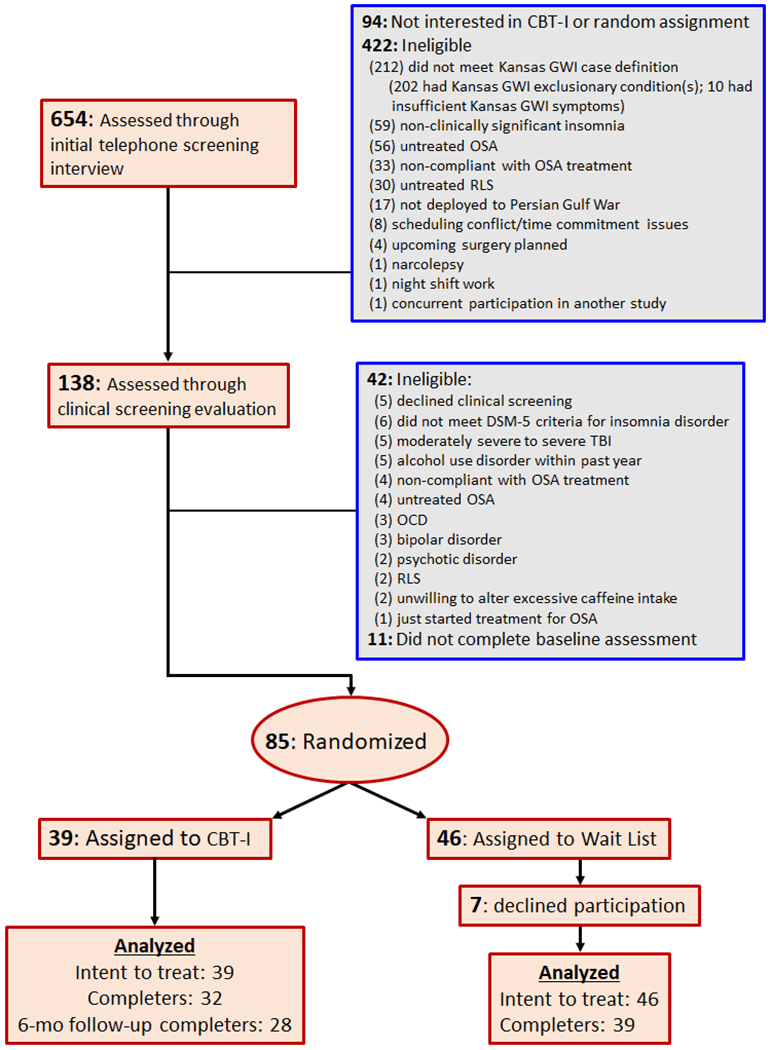
Consort diagram. Flowchart of participant numbers through the trial; Cognitive Behavioral Therapy for Insomnia (CBT-I); Gulf War Illness (GWI); Obstructive Sleep Apnea (OSA); Restless Legs Syndrome (RLS); Diagnostic and Statistical Manual for Mental Disorders (DSM-5); Traumatic Brain Injury (TBI); Obsessive Compulsive Disorder (OCD).
Exclusion criteria included: (1) medical illnesses that may be responsible for the symptoms of GWI, such as autoimmune diseases, congestive heart failure, chronic obstructive pulmonary disease (COPD), anemia, untreated endocrine disorders, liver and/or renal disease; (2) exposure to recent (within the past 3 months) trauma; (3) pregnancy; (4) prominent suicidal or homicidal ideation; (5) history of cognitive or behavioral sleep interventions; (6) concurrent enrollment in another clinical trial; (7) night shift work schedules or extreme morning or evening tendencies; (8) high risk for OSA according to the Snoring, Tiredness during daytime, Observed apnea, and high blood Pressure (STOP) screening questionnaire [35]. Untreated obstructive sleep apnea (OSA) or non-compliance with OSA treatment, defined as less than 4 h of nightly continuous positive airway pressure (CPAP) use [36]. Participants who had been diagnosed OSA but were compliant with CPAP treatment were eligible for inclusion in the study. (9) Untreated or high risk for restless legs syndrome (RLS) according to the restless legs syndrome screening questionnaire (RLSSQ) [37]. (10) Moderately severe to severe traumatic brain injury (TBI), as determined by the Ohio State University Traumatic Brain Injury Identification method (OSU TBI-ID [38]). Veterans currently receiving benzodiazepine or benzodiazepine receptor agonists, anticonvulsants, atypical antipsychotic medication, or non-selective serotonin reuptake inhibitor (SSRI) antidepressant medications such as trazodone were not excluded if they meet the DSM-5 criteria for persistent insomnia disorder and continued with the treatments during the course of the CBT-I protocol. (11) Recent termination of psychotherapy in the last month or plans to start a new psychotherapy during the course of CBT-I. Veterans who have received or were receiving psychotherapy were not excluded if they met the following criteria: For those currently in psychotherapy, they must have been in psychotherapy for at least 6 months, meet symptomatic criteria for study inclusion, and did not discontinue psychotherapy during the course of the CBT-I protocol.
Study participants were recruited from January 2016 to July 2019. Recruitment sources included flyers, online advertising, and direct mailings to GW veterans who participated in past GW-related studies at the San Francisco VA and GW veterans from a list provided by the Department of Defense Manpower Data Reporting Center (https://www.dmdc.osd.mil/dmdcrs/). After giving verbal consent to undergo the initial screening, veterans participated in a brief telephone interview to determine inclusion/exclusion criteria and probable GWI/CMI and insomnia diagnoses. Participants who passed the initial telephone eligibility screening (N = 138) were invited to undergo a second round of clinical screening that consisted of the Structured Clinical Interview for DSM-5 (SCID-5 [34]) to assess DSM-5 diagnostic criteria for mental health disorders, Clinician Administered PTSD Scale (CAPS [39]) to assess PTSD status at baseline, Lifetime Drinking History (LDH [40]) questionnaire to assess the amount, duration, and pattern of lifetime alcohol consumption, the OSU TBI-ID [38] to assess lifetime history of TBI, the STOP screening questionnaire [36] to assess the likelihood of OSA, and the RLSSQ [37] to assess the likelihood of RLS. The clinical interviews were conducted over the telephone by study staff at the San Francisco VA Medical Center who were blind to the treatment condition.
2.2. Procedures
After completing the initial telephone screen, clinical interviews, baseline assessment, and one week of sleep diary, participants were randomized to 8 weeks of CBT-I or 8 weeks of monitor-only wait list control. Randomization was stratified by sex with blind assignment determined by a computer-generated random allocation schedule operated by the study statistician (TJM).
2.3. Study assessment
The Primary Outcomes included measures of insomnia and GWI symptom severity. Insomnia severity was assessed with the Insomnia Severity Index (ISI [41]), a 7-item self-report questionnaire assessing the nature, severity, and impact of insomnia in the last month. Total scores range from 0 to 28, with a higher score indicating greater insomnia severity. It has been suggested that a cutoff score of 10 may be optimal for detecting insomnia cases in community samples [42]. The ISI has excellent internal consistency (Cronbach’s α = 0.74) and has been validated with both sleep diary and polysomnography [41]. Because no single measure of severity addresses all possible presentations of GWI due to its complexity and variability, we adapted the symptom portion of the Kansas Gulf War Military History and Health Questionnaire [43] to assess GWI symptom severity. To assess current GWI symptom severity, participants were asked about the absence, presence, and severity of GWI symptoms over the past 2 weeks instead of over the past 6-months. A GWI Symptom Severity index was derived by summing the answers to 29 questions about fatigue/sleep problems, somatic pain, skin abnormalities, gastrointestinal symptoms, respiratory symptoms, and neurologic/cognitive/mood symptoms. Higher GWI Symptom Severity index scores indicate more symptoms and/or greater symptom severity.
2.4. Secondary outcomes
The following instruments and questionnaires were used to assess individual GWI symptoms: The 9-item Fatigue Severity Scale (FSS [44]) was used to measure fatigue and the consequences of fatigue. FSS scores range from 0 to 7. Higher scores on the FSS indicate greater fatigue and a cutoff score of 4 or more is considered to be indicative of problematic fatigue. The 17-item self-rated Brief Pain Inventory (BPI [45]) was used to assess the severity of pain and its impact on function (i.e., pain interference). BPI pain severity scores range from 0 to 10, with higher scores indicating greater severity. BPI pain interference scores range from 0 to 4, with higher scores indicating greater levels of interference with activity and enjoyment of life. The 38-item self-report Multiple Abilities Self-Report Questionnaire (MASQ [46]) was used to assess cognitive function across 5 domains (i.e., verbal memory, attention, language, visual memory, visuo-perceptual ability). Items on the MASQ reflect the degree to which the respondent has difficulty with cognitive tasks, rated on a Likert scale (0 = Never to 4 = Always). Possible scores range from 0 to 152, with higher scores indicating more cognitive problems. The Hospital Anxiety and Depression Scale (HADS [47]) was used to quantify depression and anxiety. Although initially developed for use in a hospital setting, the HADS has since been validated and widely used with outpatients in different clinical settings [47]. The HADS has a depression and an anxiety subscale. Each subscale has seven items and scoring for the items range from zero to three, with three denoting the highest anxiety or depression level. A subscale score of >8 points out of a possible 21 signifies considerable anxiety or depression symptoms. The Pittsburgh Sleep Quality Index (PSQI [48]) was used to assess overall subjective sleep quality. The global PSQI score can range from 0 to 21, with higher scores indicating worse sleep quality. A global PSQI score of 5 or more indicates poor sleep quality.
To measure subjective reports of sleep duration and sleep continuity, we used the sleep diary employed in the VA CBT-I Training Program as a study outcome measure and for therapists to monitor the participants’ progress. All participants completed a sleep diary twice daily (morning and evening) throughout the study, recording information about their sleep that allowed for the calculation of sleep latency (i.e., how long it takes to fall asleep), wake time after sleep onset (i.e., length of awakening after falling asleep), total sleep time, and sleep efficiency (i.e., percentage of total time actually spent asleep during the night).
2.5. Intervention
Cognitive Behavioral Therapy for Insomnia (CBT-I) was administered via telephone by therapists at the San Francisco VA Medical Center to participants for 8 weekly sessions. CBT-I consists of several sessions of sleep restriction, stimulus control and cognitive restructuring related to sleep concerns. The CBT-I therapists, two post-doctoral clinical psychologists and one licensed clinical psychologist, all had prior training in CBT-I and were supervised by study coauthor Dr. Shira Maguen, a licensed clinical psychologist with over ten years of experience in behavioral sleep medicine treatment and research. The main CBT-I therapist (post-doctoral clinical psychologist, JCK) provided CBT-I to 24 participants. The other study therapists (NC and LDS) each provided CBT-I to four participants.
2.6. Wait list control
The monitor-only wait list control condition consisted of continuous monitoring of sleep with diary and data collection at baseline, weeks 4 and 9. Wait list participants received weekly telephone or email checkins from the study coordinator and were offered CBT-I following their completion of the research protocol.
2.7. Statistical analyses
Linear mixed models with random effects for subjects were used to analyze all available data for the intent-to-treat analysis. Because sex was used as a stratification variable in the randomization procedure, it was not included as a covariate the analyses. Treatment effects were estimated from post-treatment group differences using mixed models adjusting for baseline scores on each outcome. Standardized effect sizes were calculated by dividing the treatment effect on each outcome variable by the pooled standard deviation of the variable at baseline. To compare baseline data to 6-month follow-up data in the CBT-I group, planned contrasts following the mixed models were used. All the outcome variables were normally distributed except for sleep latency and sleep efficiency. The sleep efficiency data was square transformed and the sleep latency was log transformed for analysis. Statistical analyses were carried out using R [49] (version 4.0.1). Mixed models were fitted using the lme4 package [50] (version 1.1-23) and significance tests were based on Kenward-Roger degrees of freedom estimate provided by the pbkrtest package [51] (version 3.1-2).
The primary outcome measures were ISI and overall GWI Symptom Severity Index based on the symptom portion of the Kansas Gulf War Military History and Health Questionnaire [43]. Secondary outcomes included PSQI, sleep diary measures of sleep efficiency, sleep latency, wake after sleep on set, total sleep time, and individual measures of GWI symptoms as assessed by FSS, BPI, MASQ, and HADS. All tests were planned and used two-tailed tests of significance, with p < 0.05 values indicating statistical significance.
3. Results
Sociodemographic and clinical characteristics of the participants are summarized in Table 1. Participants were 25% women, between 46 and 72 years old (mean: 54 ± 6 years), had 15.5 ± 2.8 years of formal education, predominately white, and married. There were no significant demographic differences between the two treatment groups. Approximately 28% of the participants had current PTSD, 18% had current major depressive disorder (MDD), 22% had other psychiatric comorbidities, and 14% of the participants were taking psychotropic medication. There were no significant group differences in mental health diagnoses.
Table 1.
Participant demographic and clinical characteristics by treatment arm
| Wait list (N=46) | CBT-I (N=39) | |
|---|---|---|
| Age, in years, mean (SD) | 54 (6) | 55 (7) |
| Education, in years, mean (SD) | 15.6 (2.4) | 15.5 (3.3) |
| Women, n (%) | 10 (22) | 11 (28) |
| Ethnicity, n (%) | ||
| White | 36 (78) | 25 (64) |
| African American | 8 (17) | 11 (28) |
| Other | 2 (4.3) | 3 (7.7) |
| Marital status, n (%) | ||
| Married | 30 (65) | 29 (74) |
| Divorced/separated | 12 (26) | 7 (18) |
| Single | 3 (6.5) | 3 (7.7) |
| Widowed | 1 (2.2) | 0 (0) |
| Current PTSD, n (%) | 10 (22) | 14 (36) |
| Current MDD, n (%) | 5 (11) | 10 (26) |
| Other psychiatric comorbidity,a n (%) | 8 (17) | 10 (26) |
| Psychotropic medication use, n (%) | 9 (20) | 4 (10) |
panic disorder, agoraphobia, general, or social anxiety disorder
PTSD: posttraumatic stress disorder; MDD: major depressive disorder
3.1. Treatment attrition and adherence
The participants who completed CBT-I attended all eight sessions. Five participants failed to complete CBT-I: three participants dropped out while two were discontinued for protocol violations. Seven wait-list participants dropped out after randomization. There were no significant demographic or clinical differences between study completers and participants who did not complete CBT-I or the wait list condition. Four CBT-I participants were lost to follow-up at the 6-month follow-up. There were no demographic or clinical differences between CBT-I participants who completed the 6-month follow-up and the four who did not.
Digital audio recordings of CBT-I sessions were assessed for treatment fidelity by study coauthor Dr. Maguen. All three CBT-I therapists received high ratings across the constructs assessed: delivery of the individual treatment components, knowledge, attentiveness to the participants, skillfulness, and adherence to the protocol. The mean rating of overall delivery of CBT-I therapy components on a scale of 0–10 was 9.9 (SD = 0.26, range 9–10), indicating excellent delivery of CBT-I.
3.2. Primary outcomes
Fig. 2 shows the change in the primary outcome measures over time. Table 2 summarizes the means, standard deviations, and effect sizes for all the measures of interest. Veterans in the CBT-I group had greater reductions in ISI (effect size: −1.52, p < 0.001) and overall GWI symptom severity (effect size: −0.62, p < 0.01) at post-treatment compared to veterans in the Wait List control group. Planned contrasts showed that the reductions in ISI (p < 0.001) and overall GWI symptom severity (p < 0.001) were maintained in the CBT-I group at the 6-month follow-up. We obtained the same results regardless of whether the GWI Symptom Severity index was derived with or without questions about sleep problems.
Fig. 2.
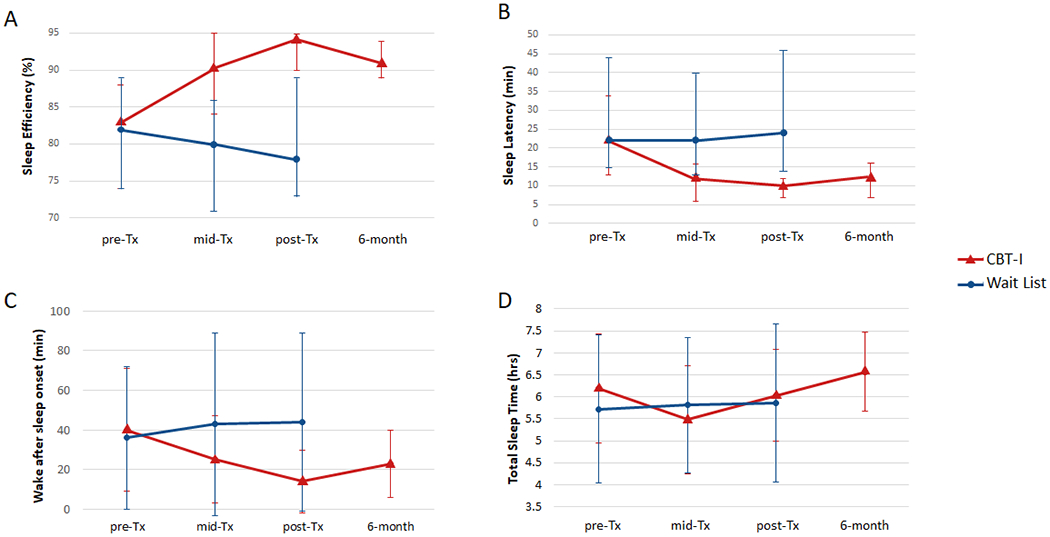
Primary outcome measures in CBT-I (red triangle) and Wait List (blue circle) groups showing means and standard deviations for scores on the Gulf War Illness Severity Index (A) and Insomnia Severity Index (B). Higher scores on both measures indicate more severe symptoms.
Table 2.
Means (SE) and Effect Sizes for CBT-I and Wait List for Intent-To-Treat Analyses
| Baseline | Mid-Tx | Post-Tx | 6-month follow-up | Std. Effect sizea (95% C.I.) | Test of Treatment Effectb | Baseline vs. 6-mo. follow-upc | |
|---|---|---|---|---|---|---|---|
| Primary Outcomes | |||||||
| GWI Symptom Severity Index | |||||||
| CBT-I | 69 (17) | 59 (16) | 54 (16) | 57 (14) | −0.62 (−1.00, −0.27) | t(91.6)=3.21f | t(92.3)=4.10e |
| Wait List | 66 (16) | 63 (16) | 62 (17) | ||||
| Insomnia Severity Index (ISI) | |||||||
| CBT-I | 21.0 (5.0) | 15.0 (5.0) | 11.0 (7.0) | 12.0 (7.0) | −1.52 (−2.00, −1.03) | t(94.7)=6.10e | t(94)=6.67e |
| Wait List | 19.5 (4.7) | 18.3 (4.2) | 18.0 (5.0) | ||||
| Secondary Outcomes | |||||||
| FSS | |||||||
| CBT-I | 5.28 (1.37) | 4.72 (1.57) | 3.87 (1.51) | 4.06 (1.62) | −0.62 (−0.97, −0.26) | t(104)=3.40e | t(91.6)=4.37e |
| Wait List | 4.66 (1.61) | 4.67 (1.47) | 4.47 (1.64) | ||||
| BPI – Pain Severity | |||||||
| CBT-I | 4.88 (2.68) | 4.65 (2.92) | 4.48 (2.39) | 4.50 (2.11) | 0.06 (−0.22, 0.34) | t(110)=−0.41 | t(91.8)=0.94 |
| Wait List | 4.48 (2.52) | 4.08 (2.47) | 4.12 (2.47) | ||||
| BPI – Pain Interference | |||||||
| CBT-I | 5.38 (2.87) | 4.76 (3.13) | 3.92 (2.81) | 4.21 (2.95) | −0.37 (−0.71, −0.02) | t(99.9)=2.09g | t(92.1)=2.56f |
| Wait List | 4.61 (2.79) | 4.40 (2.63) | 4.50 (3.10) | ||||
| MASQ | |||||||
| CBT-I | 60 (20) | 56 (28) | 55 (22) | 58 (20) | −0.48 (−0.86, −0.11) | t(83.7)=2.51g | t(90.9)=0.27 |
| Wait List | 61 (23) | 62 (26) | 68 (28) | ||||
| HADS-Anxiety | |||||||
| CBT-I | 11.3 (3.6) | 9.2 (3.7) | 7.9 (4.2) | 9.2 (4.2) | −0.83 (−1.19, −0.47) | t(105)=4.55d | t(91.3)=3.23f |
| Wait List | 10.3 (4.3) | 10.2 (4.2) | 10.6 (4.7) | ||||
| HADS- Depression | |||||||
| CBT-I | 9.2 (3.7) | 7.7 (4.3) | 5.8 (4.5) | 6.2 (3.7) | −0.67 (−1.05, −0.28) | t(91.6)=3.37f | t(92.1)=3.64e |
| Wait List | 9.0 (4.6) | 8.4 (4.4) | 8.8 (4.4) | ||||
| PSQI | |||||||
| CBT-I | 12.1 (3.5) | 8.0 (4.1) | 6.5 (3.8) | 7.6 (4.1) | −1.34 (−1.82, −0.85) | t(99.2)=5.45d | t(93.4)=6.06e |
| Wait List | 12.1 (3.5) | 11.3 (3.3) | 10.6 (3.0) | ||||
| Sleep Diary Measures | |||||||
| Sleep Efficiency (%)d | |||||||
| CBT-I | 83 (74-88) | 90 (84-95) | 94 (90-95) | 91 (89-94) | 0.90 (0.44, 1.36) | t(76.9)=−4.20e | t(33.1)=6.52e |
| Wait List | 82 (74-89) | 80 (71-86) | 78 (73-89) | ||||
| Sleep Latency (min.)d | |||||||
| CBT-I | 22 (13-34) | 12 (6-16) | 10 (7-12) | 12 (7-16) | −0.48 (−0.75, −0.22) | t(75.1)=3.91e | t(34.5)=4.39e |
| Wait List | 22 (15-44) | 22 (13-40) | 24 (14-46) | ||||
| Wake after sleep onset (min.) | |||||||
| CBT-I | 40 (31) | 25 (22) | 14 (16) | 23 (17) | −0.90 (−1.35, −0.45) | t(75.6)=4.33e | t(34.4)=4.75e |
| Wait List | 36 (36) | 43 (46) | 44 (45) | ||||
| Total Sleep Time (hrs.) | |||||||
| CBT-I | 6.19 (1.24) | 5.48 (1.23) | 6.03 (1.05) | 6.57 (0.90) | 0.18 (−0.26, 0.62) | t(76.2)=−0.11 | t(33.4)=−1.60 |
| Wait List | 5.72 (1.68) | 5.81 (1.54) | 5.86 (1.98) |
Calculated by dividing the treatment effect on the outcome variable at the Post-Tx timepoint by the pooled standard deviation of the variable at baseline.
Contrast between CBT-I and Control at Post-Tx, adjusted for baseline, from mixed model. Degrees of freedom are Kenward-Roger approximations.
Planned contrasts comparing baseline to 6-month follow-up data in the CBT-I group
Median (inter-quartile range) reported because the data was non-normally distributed.
p≤0.001,
p≤0.01,
p<0.05
3.3. Secondary outcomes
Figs. 3 and 4 show the changes in the secondary and sleep diary outcome measures over time. Veterans in the CBT-I group had greater reductions in fatigue (FSS, effect size: −0.62, p < 0.001), pain interference (BPI-pain interference: effect size: −0.37, p < 0.05), cognitive failures (MASQ, effect size: −0.48, p < 0.05), anxiety (HADS-anxiety: effect size: −0.83, p < 0.001), and depression (HADS-depression, effect size: −0.67, p < 0.01), and greater improvements in subjective sleep quality (PSQI, effect size: −1.34, p < 0.001), sleep efficiency (effect size: 0.90, p < 0.001), sleep latency (effect size: −0.48, p < 0.001), and wake after sleep onset (effect size: −0.90, p < 0.001) at post-treatment compared to veterans in the Wait List group. Planned contrasts revealed that the reductions in FSS (p < 0.001), BPI-pain interference (p = 0.01), HADS-anxiety (p = 0.002) and depression (p < 0.001), and the improvements in PSQI (p < 0.001), sleep efficiency (p < 0.001), sleep latency (p < 0.001), and wake after sleep onset (p < 0.001) were maintained in the CBT-I group at the 6-month follow-up.
Fig. 3.
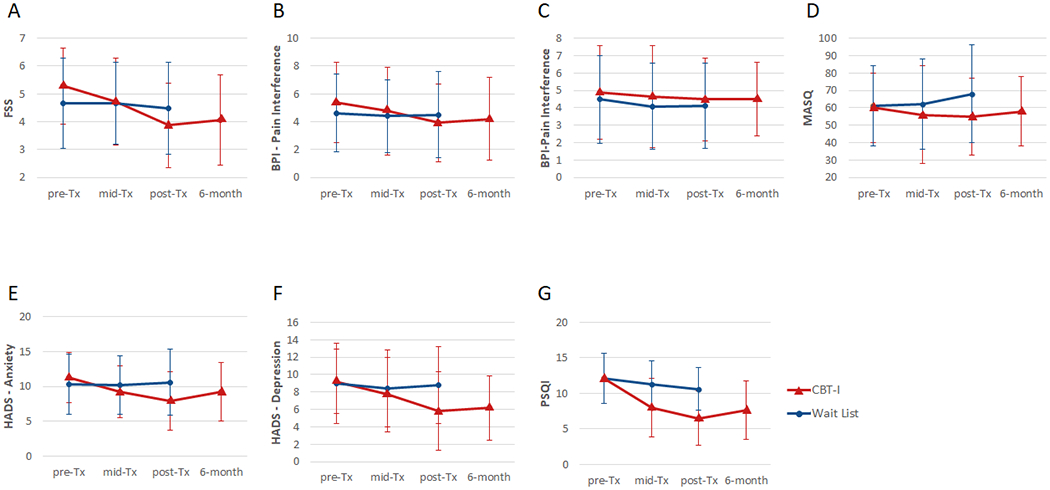
Secondary outcome measures for CBT-I (red triangle) and Wait list (blue circle) groups. The graphs show means and standard deviations intervals for the Fatigue Severity Scale, FSS (A); Brief Pain Inventory, BPI - pain severity index (B); BPI pain interference index (C); Multiple Abilities Self-report Questionnaire, MSAQ (D); Hospital Anxiety and Depression Scale, HADS – anxiety index (E); HADS – depression index (F); Pittsburgh Sleep Quality Index, PSQI (G). Higher scores on all measures indicate more severe or worse symptoms.
Fig. 4.
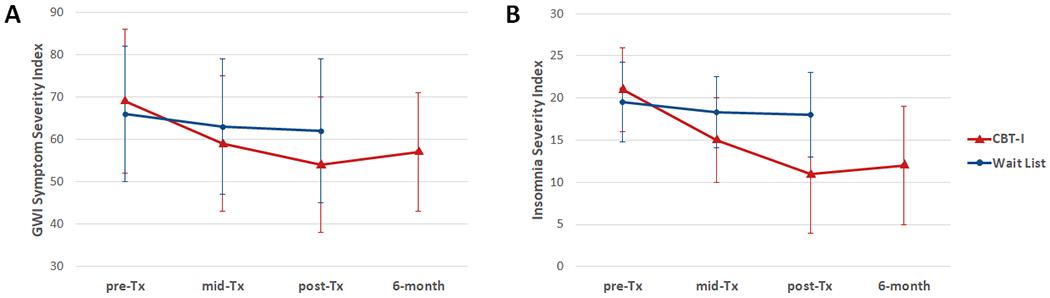
Sleep diary measures for CBT-I (red triangle) and Wait list (blue circle) groups. (A) Sleep Efficiency (SE), higher values are better; (B) Sleep Latency (SL), lower values are better; (C) Wake After Sleep Onset (WASO), lower values are better; (D) Total Sleep Time (TST), higher values are better. SE and SL data were non-normally distributed and transformed for analyses. The graphs show medians and inter-quartile range, converted back to percent and minutes. WASO and TST data were normally distributed; the graphs show means and standard deviations.
4. Discussion
This is the first study to show that CBT-I improves GWI symptom severity as well as independent measures of fatigue, pain interference, cognitive dysfunction, anxiety, and depression compared to a wait list condition. Furthermore, most of these gains were maintained at the six-month follow up. Additionally, but not surprisingly, CBT-I significantly improved insomnia severity, subjective sleep quality, and all sleep diary measures. On average, there was an 11-point improvement in ISI from pre- to post-treatment in the CBT-I group. This is greater than the minimally important difference of a 9-point change ISI for signifying marked improvement in insomnia [42]. These results provide initial support that CBT-I is a viable intervention for GWI comorbid with insomnia.
Although the outcomes of CBT-I have been reported in numerous medical and psychiatric conditions [13,14], CBT-I has never been studied in veterans with GWI. Our finding that CBT-I reduced symptoms of fatigue, anxiety, and depression in veterans with GWI is consistent with other CBT-I studies of patients with persistent insomnia [52], depression [53,54], cancer [55], and posttraumatic stress disorder [56]. Research also suggests that CBT-I may benefit patients with fibromyalgia [19,22] and CFS [57], two medically unexplained syndromes that share many overlapping symptoms with GWI. Specifically, CBT-I has been reported to reduce fibromyalgia symptoms (e.g., fatigue, anxiety, depression, and pain catastrophizing) [19,22] and fatigue symptoms in patients with CFS who were able to adhere with the treatment [57].
Like fibromyalgia and CFS, sleep disturbance is a common symptom of GWI [9] that can influence and/or exacerbate other GWI symptoms [10,11]. This is why we postulated treating insomnia would benefit both sleep and non-sleep GWI symptoms in veterans with GWI and comorbid insomnia. The significance of CBT-I’s effect on reducing GWI symptom severity index did not change when we excluded questions about sleep difficulties in our calculation of the overall GWI symptom severity index. Relatively few studies have investigated treatments for GWI in the 30 years since the Gulf War ended. However, it is noteworthy that two other studies that focused on treating sleep disturbance in GW veterans also reported success in alleviating GWI symptoms. Because there is no consensus diagnosis for GWI, research has relied on a number of different definitions of the symptomatic illness that plagues GW veterans. A pilot study that used the Centers for Disease Control and Prevention (CDC) case definition of Chronic Multisymptom Illness (CMI) [4] reported that three weeks of CPAP treatment improved subjective sleep quality, fatigue, pain, and cognitive symptoms in 18 ill GW veterans who also had comorbid sleep disordered breathing [58]. Another study reported that Mind-Body Bridging (MBB), which trains participants become more aware of dysfunctional mind-body states [59], was more effective for reducing sleep, PTSD, and depression symptoms in ill GW veterans than sleep education [60]. Sleep education is commonly used as an active control condition in studies of CBT-I. Although MBB has some similarities to CBT-based programs, it does not teach participants to identify maladaptive behaviors or thought patterns that perpetuate sleep difficulties or interfere with sleep [59]. Unlike MBB, CBT-I specifically teaches patients to identify and correct maladaptive behaviors and beliefs related to sleep problems and tracks success of behavioral changes with continuous sleep diaries.
In one of the earliest and largest (N = 1092) study of treatments for GWI, the Veterans Affairs (VA) Cooperative Study Program (CSP) #470 investigated the efficacy of a CBT program that focused on teaching behavioral skills to improve physical function and cognitive strategies to overcome barriers to functioning [61]. It is difficult to directly compare results of the current study with CSP #470 because we used different outcome measures. Nevertheless, it is noteworthy that CBT-I produced medium to large effects on overall GWI symptom severity and individual GWI symptoms after 8 weeks. In contrast, the CBT intervention in CSP #470 yielded only modest improvements in cognitive symptoms and mental health functioning after 12 months. When it was paired with aerobic exercise, the CSP #470 CBT intervention moderately improved fatigue, distress, cognitive symptoms, and mental health functioning [61]. Compared to CSP #470, the current study had better follow-up and adherence rates, likely due to the administration of our intervention by telephone. This suggests that we were successful in reducing many of the barriers associated with mobility and transportation by administering CBT-I by telephone. It has previously been suggested that strategies such as telephone sessions may be necessary to reduce attrition and to increase adherence in patients with higher levels of fatigue, pain, depression, anxiety and more severe insomnia [57].
Not only are telephones, particularly mobile phones, a ubiquitous and well-accepted means of communication [62], but mobile devices are increasingly becoming a reliable tool for managing and delivering health care [63]. Research has shown telephone-based interventions to be effective in providing CBT for a variety of mental disorders and symptoms, including insomnia [63–66]. One study that compared face-to-face versus telephone-based CBT for anxiety and depression found both interventions were equally effective. However, the costs of the telephone intervention were approximately a third lower than face-to-face sessions [67]. Although we did not perform health economic analyses in this study, in theory telephone-based therapy may be more cost-effective than in person sessions because of fewer missed visits, which would result in savings for the clinician. Telephone-based therapy also eliminates transportation costs and the need to take time off from work, resulting in savings for the patient. From a practical standpoint, telephone-based therapies may result in lower attrition and afford greater access to therapy to people who have restricted mobility or who live in rural or remote areas. Finally, because telephone-based CBT-I is not limited by geography, it allows for cost-effective upscaling of the intervention to reach more patients.
During the COVID-19 pandemic, the Veterans Health Administration has placed an emphasis on face-to-face care via VA Video Connect (VVC) for replacing in-person visits. While face-to-face interactions via video conferencing may be helpful for building therapeutic alliance and good rapport, we were able to reach a much wider group of GW veterans by telephone (e.g., veterans who live in rural areas with little to no internet access). Furthermore, because VVC requires computer and Wi-Fi access, bug-free software, and tech savviness, we believe there is still an important place for telephone-delivered intervention.
Although the mechanisms that underlie and perpetuate GWI remain unclear, chronic inflammation has been hypothesized to be one cause of GWI-associated symptoms [68]. In a case-control observational study of GW veterans, the blood-based biomarkers that differentiated veterans with GWI from asymptomatic (i.e., GWI-) veterans were all potential indicators of inflammation [69]. In a follow-up study, the same group found that GWI-associated increases in C-reactive protein (CRP) were significantly correlated with increases in plasma Iinterlukin-6 [68,70]. The production of CRP is thought to reflect the activity of proinflammatory cytokines, particularly interleukin-6 [71], a key proinflammatory mediator [72]. Compared to sleep education, CBT-I has been associated with a reduced risk of high CRP levels (>3.0 mg/L) [73]. Insomnia remission has also been associated with lower levels of CRP [73]. Therefore, it is possible that CBT-I may help to alleviate GWI symptoms by decreasing inflammation. Another possible mechanism through which CBT-I relieves GWI symptoms may be the reduction of sleep fragmentation [74–78]. Anecdotally, many veterans with GWI report that they have trouble staying asleep throughout the night. Research has shown that sleep fragmentation can induce hyperalgesia [79], increase fatigue [80–86], elevate energy expenditure and exhaustion [81], and impair cognitive function [82–84]. In this respect, it is noteworthy that the pilot study that reported CPAP treatment improved GWI symptoms in GW veterans with CDC CMI and sleep disordered breathing also found that CPAP treatment reduced sleep fragmentation. Furthermore, changes in GWI symptoms were significantly correlated with changes in sleep stage shifts [58]. There is evidence that CBT-I decreases sleep fragmentation in patients with fibromyalgia [20].
This study had some limitations that should be noted: First, we did not acquire polysomnography data; therefore, future research will be necessary to determine whether improvements in GWI symptoms are directly related to objective decreases in sleep fragmentation. We also did not collect blood-based biomarkers in the current study. Thus, we could not examine CBT-I’s effects on biomarkers of GWI and inflammation. Because this study was designed to be a proof-of-concept trial, we did not employ an active control condition. We also did not adjust for multiple comparisons because we wished to avoid missing potential associations that could lead to additional treatments for GWI [86]. As is common in many clinical studies of insomnia, participants with untreated OSA or non-compliant with OSA treatment were excluded in the current study. However, insomnia and OSA are frequently comorbid [87–89] and OSA is common in veterans with GWI [90]. Moreover, there is suggestive evidence that CBT-I may be effective even in the presence of comorbid OSA [90]. Thus, future research will need to examine whether CBT-I is effective for alleviating GWI symptoms in veterans with untreated comorbid OSA. Although CBT-I was administered via telephone, there is growing evidence that CBT-I can also be successfully delivered through other digital e-health mediums [91–93]. Future studies will be necessary to determine the efficacy CBT-I delivered through these other accessible, affordable mediums for treating GWI with comorbid insomnia.
5. Conclusions
The current proof-of-concept trial provides initial support that CBT-I, a scalable and feasible behavioral intervention for insomnia, has great potential for managing and reducing the severity of the symptoms of Gulf War Illness in veterans with comorbid GWI and insomnia.
Acknowledgments
The authors would like to thank all the GW veterans who participated in this research project.
Funding
This work was supported by the Department of Veterans Affairs grant number 1I21CX001428.
Footnotes
Declaration of competing interest
The authors have no competing interests.
References
- [1].Institute of Medicine, Gulf War and Health: Volume 8 - Health Effects of Serving in the Gulf War, 2010. (Washington, D.C.). [Google Scholar]
- [2].Kang HK, Li B, Mahan CM, Eisen SA, Engel CC, Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years, J Occup Environ Med. 51 (2009) 401–410. [DOI] [PubMed] [Google Scholar]
- [3].Research Advisory Committee on Gulf War Veterans’ Illnesses (RAC-GWVI). Gulf War Illness and the Health of Gulf War Veterans. Washington, D.C., 2008. [Google Scholar]
- [4].Fukuda K, Niesenbaum R, Stewart G, et al. , Chronic multisymptom illness affecting Air Force veterans of the Gulf War, JAMA. 280 (1998) 981–988. [DOI] [PubMed] [Google Scholar]
- [5].Goss Gilroy Inc. Health Study of Canadian Forces Personnel Involved in the 1991 Conflict in the Persian Gulf. Ottawa, Canada: Gulf War Illness Advisory Committee; 1998. [Google Scholar]
- [6].Kang HK, Mahan CM, Lee KY, Magee CA, Murphy FM, Illnesses among United States veterans of the Gulf War: a population-based survey of 30,000 veterans, J Occup Environ Med. 42 (2000) 491–501. [DOI] [PubMed] [Google Scholar]
- [7].Unwin C, Blatchley N, Coker W, et al. , Health of UK servicemen who served in Persian Gulf War, Lancet. 353 (1999) 169–178. [DOI] [PubMed] [Google Scholar]
- [8].Chester JE, Rowneki M, Van Doren W, Helmer DA, Progression of intervention-focused research for Gulf War illness, Mil Med Res. 6 (1) (2019) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Committee on the Development of a Consensus Case Definition for Chronic Multisymptom Illness in 1990–1991 Gulf War Veterans. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. Washington, D.C.: Institute of Medicine, 2014. [PubMed] [Google Scholar]
- [10].Theadom A, Cropley M, Humphrey KL, Exploring the role of sleep and coping in quality of life in fibromyalgia, J Psychosom Res. 62 (2) (2007) 145–151. [DOI] [PubMed] [Google Scholar]
- [11].Fernandez-Mendoza J, Vgontzas AN, Insomnia and its impact on physical and mental health, Curr Psychiatry Rep. 15 (12) (2013) 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buenaver LF, Townsend D, Ong JC, Delivering cognitive behavioral therapy for insomnia in the real world: considerations and controversies, Sleep Med Clin. 14 (2) (2019) 175–181. [DOI] [PubMed] [Google Scholar]
- [13].Smith MT, Huang MI, Manber R, Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders, Clin Psychol Rev. 25 (5) (2005) 559–592. [DOI] [PubMed] [Google Scholar]
- [14].Wu JQ, Appleman ER, Salazar RD, Ong JC, Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis, JAMA Internal Med. 175 (9) (2015) 1461–1472. [DOI] [PubMed] [Google Scholar]
- [15].Wolfe F, Clauw DJ, Fitzcharles MA, et al. , The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity, Arthritis Care Res (Hoboken). 62 (5) (2010) 600–610. [DOI] [PubMed] [Google Scholar]
- [16].Shah MA, Feinberg S, Krishnan E, Sleep-disordered breathing among women with fibromyalgia syndrome, J Clin Rheumatol. 12 (6) (2006) 277–281. [DOI] [PubMed] [Google Scholar]
- [17].Roizenblatt S, Neto NS, Tufik S, Sleep disorders and fibromyalgia, Curr Pain Headache Rep. 15 (5) (2011) 347–357. [DOI] [PubMed] [Google Scholar]
- [18].Meresh ES, Artin H, Joyce C, et al. , Obstructive sleep apnea co-morbidity in patients with fibromyalgia: a single-center retrospective analysis and literature review, Open Access Rheumatol. 11 (2019) 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lami MJ, Martinez MP, Sanchez AI, et al. , Gender differences in patients with fibromyalgia undergoing cognitive-behavioral therapy for insomnia: preliminary data, Pain Pract. 16 (2) (2016) E23–E34. [DOI] [PubMed] [Google Scholar]
- [20].Sanchez AI, Diaz-Piedra C, Miro E, Martinez MP, Galvez R, Buela-Casal G, Effects of cognitive-behavioral therapy for insomnia on polysomnographic parameters in fibromyalgia patients, Int J Clin Health Psychol. 12 (2012) 39–53. [Google Scholar]
- [21].Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR, Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial, Arch Intern Med. 165 (2005) 2527–2535. [DOI] [PubMed] [Google Scholar]
- [22].Martinez MP, Miro E, Sanchez AI, et al. , Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial, J Behav Med. 37 (4) (2014) 683–697. [DOI] [PubMed] [Google Scholar]
- [23].Larun L, Brurberg KG, Odgaard-Jensen J, Price JR, Exercise therapy for chronic fatigue syndrome, Cochrane Database Syst Rev. 2017 (4) (2017), CD003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kallestad H, Jacobsen HB, Landro NI, Borchgrevink PC, Stiles TC, The role of insomnia in the treatment of chronic fatigue, J Psychosom Res. 78 (5) (2015) 427–432. [DOI] [PubMed] [Google Scholar]
- [25].Vethe D, Kallestad H, Jacobsen HB, Landro NI, Borchgrevink PC, Stiles TC. The relationship between improvement in insomnia severity and long-term outcomes in the treatment of chronic fatigue. Front Psychol. 2018;9:1764.:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;April 15. [DOI] [PubMed] [Google Scholar]
- [27].Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R, National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans, Int J Geriatr Psychiatry. 30 (3) (2015) 308–315. [DOI] [PubMed] [Google Scholar]
- [28].Arnedt JT, Cuddihy L, Swanson LM, Pickett S, Aikens J, Chervin RD, Randomized controlled trial of telephone-delivered cognitive behavioral therapy for chronic insomnia, Sleep. 36 (2013) 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McCurry SM, Guthrie KA, Morin CM, et al. , Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial, JAMA Internal Med. 176 (7) (2016) 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pepin R, Segal DL, Coolidge FL, Intrinsic and extrinsic barriers to mental health care among community-dwelling younger and older adults, Aging Ment Health. 13 (5) (2009) 769–777. [DOI] [PubMed] [Google Scholar]
- [31].Sarmiento KF. VA’s TeleSleep Program: Understanding Disparities in Rural Sleep Care. Seminar Presented at the San Francisco VA Health Care System; San Francisco, CA, March 14, 2019. [Google Scholar]
- [32].U.S. Department of Veterans Affairs, Gulf War (including operation Iraqi freedom) registry (GWR) program (formerly Persian Gulf registry program). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5416, 2007.
- [33].Steele L, Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service, Am J Epidemiol. 152 (2000) 992–1002. [DOI] [PubMed] [Google Scholar]
- [34].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders. Vol 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- [35].Chung F, Yegneswaran B, Liao P, et al. , STOP questionnaire: a tool to screen patients for obstructive sleep apnea, Anesthesiology. 108 (2008) 812–821. [DOI] [PubMed] [Google Scholar]
- [36].Weaver TE, Grunstein RR, Adherence to continuous positive airway pressure therapy the challenge to effective treatment, Proc Am Thorac Soc. 5 (2) (2008) 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stiasny-Roister K, Möller JC, Heinzel-Gutenbrunner M, Baum E, Ries V, Oertel WH, Validation of the Restless Legs Syndrome Screening Questionnaire (RLSSQ), Somnologie - Schlafforschung und Schlaftnedizin. 13 (1) (2009) 37–42. [Google Scholar]
- [38].Corrigan JD, Bogner JA, Initial reliability and validity of the OSU TBI Identification Method, J Head Trauma Rehabil. 22 (2007) 318–329. [DOI] [PubMed] [Google Scholar]
- [39].Blake DD, Weathers FW, Nagy LM, et al. , The development of a clinican-administered PTSD scale, J Trauma Stress. 8 (1995) 75–90. [DOI] [PubMed] [Google Scholar]
- [40].Skinner HA, Sheu WJ, Reliability of alcohol use indices: the Lifetime Drinking History and the MAST, Journal of Studies on Alcohol. 43 (1982) 1157–1170. [DOI] [PubMed] [Google Scholar]
- [41].Bastien CH, Vallieres A, Morin CM, Validation of the Insomnia Severity Index as an outcome measure for insomnia research, Sleep Medicine. 2 (2001) 297–307. [DOI] [PubMed] [Google Scholar]
- [42].Morin CM, Belleville G, Belanger L, Ivers H, The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response, Sleep 34 (2011) 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Steele L, Sastre A, Gerkovich MM, Cook MR, Complex factors in the etiology of Gulf War Illness: wartime exposures and risk factors in veteran subgroups, Environ Health Perspect. 120 (2012) 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD, The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus, Arch Neurol. 46 (1989) 1121–1123. [DOI] [PubMed] [Google Scholar]
- [45].Cleeland CS, Ryan KM, Pain assessment: global use of the Brief Pain Inventory, Ann Acad Med Singapore. 23 (1994) 129–138. [PubMed] [Google Scholar]
- [46].Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A, Development and validation of a Multiple Ability Self-Report Questionnaire, J Clin Exp Neuropsychol. 16 (1994) 93–104. [DOI] [PubMed] [Google Scholar]
- [47].Zigmond AS, Snaith RP, The hospital anxiety and depression scale, Acta Psychiatr Scand. 67 (1983) 361–370. [DOI] [PubMed] [Google Scholar]
- [48].Buysse DJ, Renolyds CF 3rd., Monk TH, Berman SR, Kupfer DJ, The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research, Psychiatry Research. 28 (1989) 193–213. [DOI] [PubMed] [Google Scholar]
- [49].R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- [50].Bates D, Maechler M, Bolker B, Walker S, Fitting linear mixed-effects models using lme4, J Stat Softw. 67 (1) (2015) 1–48. [Google Scholar]
- [51].Halekoh U, Hojsgaard S, A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models - the R package pbkrtest, J Stat Softw. 59 (9) (2014) 1–30.26917999 [Google Scholar]
- [52].Morin CM, Beaulieu-Bonneau S, Belanger L, et al. , Cognitive-behavior therapy singly and combined with medication for persistent insomnia: impact on psychological and daytime functioning, Behav Res Ther. 87 (2016) 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Manber R, Edinger JD, Gress JL, et al. , Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia, Sleep. 31 (2008) 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Taylor DJ, Lichstein KL, Weinstock J, Sanford S, Temple JR, A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression, Behav Ther. 38 (1) (2007) 49–57. [DOI] [PubMed] [Google Scholar]
- [55].Fleming L, Randall K, Harvey CJ, Espie CA, Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. 23 (6) (2014) 679–684. [DOI] [PubMed] [Google Scholar]
- [56].Talbot LS, Maguen S, Metzler TJ, et al. , Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial, Sleep. 37 (2014) 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gotts Z, Deary V, Newton JL, Ellis J, Treatment of insomnia reduces fatigue in chronic fatigue syndrome in those able to comply with the intervention, Fatigue: Biomedicine, Health & Behavior. 4 (4) (2016) 208–216. [Google Scholar]
- [58].Amin MM, Gold MS, Broderick JE, Gold AR, The effects of nasal continuous positive airway pressure on the symptoms of Gulf War illness, Sleep Breath. 15 (2011) 579–587. [DOI] [PubMed] [Google Scholar]
- [59].Nakamura Y, D.L. L, Kanarowski E, McCormick T, Sutherland D, Melow-Murchie M. Investigating impacts of incorporating an adjuvant mind-body intervention method into treatment as usual at a community-based substance abuse treatment facility: a pilot randomized controlled study. SAGE Open. 2015;5(1). [Google Scholar]
- [60].Nakamura Y, Lipschitz DL, Donaldson GW, et al. , Investigating clinical benefits of a novel sleep-focused mind-body program on gulf war illness symptoms: a randomized controlled trial, Psychosom Med. 79 (6) (2017) 706–718. [DOI] [PubMed] [Google Scholar]
- [61].Donta ST, Clauw DJ, Engel CC Jr., et al. , Cognitive behavioral therapy and aerobic exercise for Gulf War Veterans’ illnesses: a randomized controlled trial, JAMA. 289 (2003) 1396–1404. [DOI] [PubMed] [Google Scholar]
- [62].Bastawrous A, Hennig B, Livingstone I, mHealth possibilities in a changing world. Distribution of global cell phone subscriptions, Journal of Mobile Technology in Medicine. 2 (1) (2013) 22–25. [Google Scholar]
- [63].Watzke B, Haller E, Steinmann M, et al. , Effectiveness and cost-effectiveness of telephone-based cognitive-behavioural therapy in primary care: study protocol of TIDe – telephone intervention for depression, BMC Psychiatry. 17 (1) (2017) 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bastien CH, Morin CM, Ouellet MC, Blais FC, Bourchard S, Cognitive behavioral therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations, J Consult Clin Psychol. 72 (2004) 653–659. [DOI] [PubMed] [Google Scholar]
- [65].Brenes GA, Miller ME, Williamson JD, McCall WV, Knudson M, Stanley MA, A randomized controlled trial of telephone-delivered cognitive-behavioral therapy for late-life anxiety disorders, Am J Geriatr Psychiatry. 20 (8) (2012) 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brenes GA, Danhauser SC, Lyles MF, Anderson A, Miller ME, Effects of telephone-delivered cognitive-behavioral therapy and nondirective supportive therapy on sleep, health-related quality of life, and disability, Am J Geriatr Psychiatry. 24 (10) (2016) 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hammond GC, Croudace TJ, Radhakrishnan M, et al. , Comparative effectiveness of cognitive therapies delivered face-to-face or over the telephone: an observational study using propensity methods, PLoS One. 7 (9) (2012), e42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Butterick TA, Trembley JH, Hocum Stone LL, Muller CJ, Rudquist RR, Bach RR, Gulf War Illness-associated increases in blood levels of interleukin 6 and C-reactive protein: biomarker evidence of inflammation, BMC Research Notes. 12 (2019) 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR, Blood biomarkers of chronic inflammation in Gulf War illness, PLoS One. 11 (6) (2016), e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Del Giudice M, Gangestad SW, Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters, Brain Behav Immun. 70 (2018) 61–75. [DOI] [PubMed] [Google Scholar]
- [71].Sproston NR, Ashworth JJ, Role of C-reactive protein at sites of inflammation and infection, Front Immunol. 9 (2018) 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Suyasa IK, Kawiyana IKS, Bakta IM, Widiana IGR, Interleukin-6 and ratio of plasma interleukin-6/interleukin-10 as risk factors of symptomatic lumbar osteoarthritis, World J Orthop. 8 (2) (2017) 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Irwin MR, Olmstead R, Carrillo C, et al. , Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial, Sleep. 37 (9) (2014) 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE, Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial, JAMA. 285 (2001) 1856–1864. [DOI] [PubMed] [Google Scholar]
- [75].Espie CA, Inglis SJ, Tessier S, Harvey L, The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice, Behav Brain Res. 39 (2001) 45–60. [DOI] [PubMed] [Google Scholar]
- [76].Morin CM, Colecchi C, Stone J, Sood R, Brink D, Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial, JAMA. 281 (1999) 991–999. [DOI] [PubMed] [Google Scholar]
- [77].Verbeek I, Schreuder K, Dederck G, Evaluation of short-term nonpharmacological treatment of insomnia in a clinical setting, J Psychosom Res. 47 (1999) 369–383. [DOI] [PubMed] [Google Scholar]
- [78].Cervena K, Dauvilliers Y, Espa F, et al. , Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia, J Sleep Res. 13 (2004) 385–393. [DOI] [PubMed] [Google Scholar]
- [79].Iacovides S, George K, Kamerman P, Baker FC, Sleep fragmentation hypersensitizes healthy young women to deep and superficial experimental pain, J Pain. 18 (7) (2017) 844–854. [DOI] [PubMed] [Google Scholar]
- [80].Yue HJ, Bardwell W, Ancoli-Israel S, Loredo JS, Dimsdale JE, Arousal frequency is associated with increased fatigue in obstructive sleep apnea, Sleep Breath. 13 (2009) 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hursel R, Rutters F, Gonnissen HKJ, Martens EAP, Westerterp-Plantenga MS, Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber, Am J Clin Nutr. 94 (3) (2011) 804–808. [DOI] [PubMed] [Google Scholar]
- [82].Martin SE, Brander PE, Deary IJ, Douglas NJ, The effect of clustered versus regular sleep fragmentation on daytime function, J Sleep Res. 8 (4) (1999) 305–311. [DOI] [PubMed] [Google Scholar]
- [83].Blackwell T, Yaffe K, Ancoli-Israel S, et al. , Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures, J Gerontol A Biol Sci Med Sci. 61 (4) (2006) 405–410. [DOI] [PubMed] [Google Scholar]
- [84].Spira AP, Stone KL, Redline S, et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep. 2017;40(8):zsx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bender R, Lange S, Adjusting for multiple testing — when and how? J Clin Epidemiol. 54 (2001) 343–349. [DOI] [PubMed] [Google Scholar]
- [86].Luyster FS, Buysse DJ, Strollo PJ Jr., Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research, J Clin Sleep Med. 6 (2010) 196–204. [PMC free article] [PubMed] [Google Scholar]
- [87].Cho YW, Kim KT, Moon HJ, Korostyshevskly VR, Motamedi GK, Yang KI, Comorbid insomnia with obstructive sleep apnea: clinical characteristics and risk factors, J Clin Sleep Med. 14 (3) (2018) 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sweetman A, Lack L, Bastien C. Co-Morbid Insomnia and Sleep Apnea (COMISA): prevalence, consequences, methodological considerations, and recent randomized controlled trials. Brain Sci. 2019;9(12):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chao LL, Abadjian L, Esparza IL, Reeb R, Insomnia severity, subjective sleep quality, and risk for obstructive sleep apnea in veterans with Gulf War illness, Mil Med. 181 (9) (2016) 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sweetman A, Lack L, Lambert S, Gradisar M, Harris J, Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 39 (2017) 38–46. [DOI] [PubMed] [Google Scholar]
- [91].Espie CA, Emsley R, Kyle SD, et al. , Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial, JAMA Psychiatry. 76 (2018) 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Koffel E, Kuhn E, Petsoulis N, et al. , A randomized controlled pilot study of CBT-I Coach: feasibility, acceptability, and potential impact of a mobile phone application for patients in cognitive behavioral therapy for insomnia, Health Informatics J. 24 (1) (2018) 3–13. [DOI] [PubMed] [Google Scholar]
- [93].Peter L, Reindl R, Zauter S, Hillemacher T, Richter K, Effectiveness of an online CBT-I intervention and a face-to-face treatment for shift work sleep disorder: a comparison of sleep diary data, Int J Environ Res Public Health. 16 (17) (2019) 3081. [DOI] [PMC free article] [PubMed] [Google Scholar]


