Abstract
Background & Aims:
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related death worldwide. Effects of second-line oral antidiabetic medications on incident HCC risk in individuals with type 2 diabetes mellitus remain unclear. This study evaluated associations between sulfonylureas, thiazolidinediones, meglitinides and alpha-glucosidase inhibitors, and incident HCC risk.
Methods:
We systematically reviewed all studies on PubMed, Embase and Web of Science databases. Studies were included if they documented: (1) exposure to oral antidiabetic medication classes; (2) HCC incidence; (3) relative risks/odds ratios (OR) for HCC incidence. Eight eligible observational studies were identified. We performed random-effects meta-analyses to calculate pooled adjusted ORs (aORs) and 95% confidence intervals (CI).
Results:
Thiazolidinedione use (7 studies, 280,567 participants, 19,242 HCC cases) was associated with reduced HCC risk (aOR=0.92, 95% CI=0.86-0.97, I2=43%), including among Asian subjects (aOR=0.90, 95% CI=0.83-0.97), but not Western subjects (aOR=0.95, 95% CI=0.87-1.04). Alpha-glucosidase inhibitor use (3 studies, 56,791 participants, 11,069 HCC cases) was associated with increased HCC incidence (aOR=1.08; 95% CI=1.02-1.14, I2=21%). Sulfonylurea use (8 studies, 281,180 participants, 19,466 HCC cases) was associated with increased HCC risk in studies including patients with established liver disease (aOR=1.06, 95% CI=1.02-1.11, I2=75%). Meglitinide use (4 studies, 58,237 participants, 11,310 HCC cases) was not associated with HCC incidence (aOR=1.19; 95% CI=0.89-1.60, I2=72%).
Conclusions:
Thiazolidinedione use was associated with reduced HCC incidence in Asian individuals with diabetes. Alpha-glucosidase inhibitor or sulfonylurea use was associated with modestly increased HCC risk; future research should determine whether those agents should be avoided in patients with chronic liver disease.
Keywords: Carcinoma, Hepatocellular; Diabetes Mellitus, Type 2; Thiazolidinediones; Alpha-glucosidase inhibitors
1. INTRODUCTION
As the global incidence of hepatocellular carcinoma (HCC) continues to rise, clinical outcomes remain exceptionally poor, due to a combination of inadequate cancer surveillance and a paucity of effective treatment options1. Type 2 diabetes mellitus independently increases the risk of multiple cancers, including HCC2,3,4, and occurs frequently with concurrent liver disease. Informed selection of antidiabetic regimens may enable providers to mitigate risk of HCC development in high-risk patients with diabetes. Given the rapidly increasing prevalence of diabetes, such a strategy could translate to a considerable decline in overall HCC incidence.
Robust evidence from clinical and epidemiological studies indicates that the risk of HCC in diabetic subjects increases with insulin use, and decreases with metformin use5-16. However, the effects of second-line oral antidiabetic agents are less well-characterized, and published data are limited and conflicting. Two prior meta-analyses have reported thiazolidinedione use to be associated with significantly decreased HCC incidence17,18, however, Singh et al. did not identify a significant protective association15, and several recent studies examining thiazolidinediones in relation to HCC incidence were not included in those prior metaanalyses19,20. Similarly, while prior meta-analyses have indicated use of sulfonylureas to be associated with significantly increased risk of incident HCC8,15, they did not include several important, more recent additions to the literature19,21,22. Finally, prior meta-analyses of second-line antidiabetic therapies did not evaluate meglitinides, and while a single meta-analysis reported a trend towards decreased risk of liver cancer in users of alpha-glucosidase inhibitors, compared to non-users23, a subsequent analysis by Bosetti et al.20 found an increased risk of HCC among users of alpha-glucosidase inhibitors. In light of the growing body of primary literature, an updated review of associations between the use of anti-diabetic medications and risk of incident HCC is warranted.
Therefore, we undertook a systematic review of published literature, and performed a series of meta-analyses to characterize the relationships between use of second-line oral antidiabetic medication classes and risk of incident HCC.
2. METHODS
2.1. Search Strategy
A medical librarian (LP) systematically searched PubMed, Embase and Web of Science databases (inception through March 2020), using keywords and controlled vocabulary (Supplementary Table 1). Titles and abstracts of identified studies were screened by two independent reviewers (AA and ZM), and studies that did not investigate the association of interest were excluded. Full texts of the remaining studies were reviewed, and their references examined to identify additional articles of relevance. Any discrepancies were discussed and resolved by consensus (AA and ZM), or with the input of an independent reviewer (TS).
2.2. Selection Criteria
The systematic review and meta-analysis were conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines24. Studies that fulfilled the following criteria were eligible for inclusion: (1) documented exposure to one or more of the following oral antidiabetic medication classes: sulfonylureas, thiazolidinediones, meglitinides, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, sodium glucose co-transporter-2 (SGLT-2) inhibitors, and amylin analogs; (2) reported HCC incidence (outcome) within the patient cohort; (3) reported relative risk (RR)/odds ratio (OR) for HCC incidence, or necessary data for calculation. Concomitant metformin use was adjusted for by individual studies.
Studies were excluded if they met any of the following exclusion criteria: (1) preclinical or nonprimary research, case reports/series; (2) published in abstract form only; (3) evaluated patients who developed HCC prior to treatment with antidiabetic medication; (4) overlapping patient cohorts between studies. If multiple eligible studies utilized overlapping populations, the study with the largest cohort size was included in the analysis and the remaining studies were excluded.
2.3. Assessment of Bias
Study quality was objectively assessed using the modified Newcastle-Ottawa Scale (NOS)25, a well-established tool for assessing the quality of studies included in meta-analyses. Risk of bias was evaluated on the basis of the three predefined components of the NOS: (1) selection of study groups; (2) comparability of the groups; (3) ascertainment of the exposure or outcome of interest (for case-control or cohort studies, respectively). Studies were assigned an overall score ranging from 0-9, with studies scoring ≥7 points deemed to be of high quality.
2.4. Data Abstraction
The following details were collected from each of the included studies, and abstracted onto a pre-specified form: study design, year of publication, geographical region of the study population, inclusion of subjects with known underlying liver disease in the study population, etiology of underlying liver disease, antidiabetic medication exposure, dose and duration of medication exposure (if stated), method of exposure assessment, method of incident HCC ascertainment, latency period between exposure and outcome, study quality, total number of study subjects, number of subjects exposed to each class of medication, number of subjects who developed incident HCC, crude and adjusted RR/OR and 95% confidence intervals (CI), and covariates included in the multivariate analysis.
2.5. Statistical Analysis
We used the DerSimonian and Laird random effects method to estimate the pooled OR and 95% CI26 for the different drug classes included in this study, using adjusted risk estimates from the individual studies, with P<0.05 considered statistically significant. We used Cochran’s Q test and I2 statistic to identify and measure between-study heterogeneity, with I2 statistic >50% and P<0.10 considered statistically significant, as recommended by the Cochrane handbook, given that certain subgroups included a small number of studies27. We conducted a pre-defined set of subgroup analyses, according to geographic region, study design, definition of antidiabetic medication exposure, method of ascertaining HCC outcome, latency period from initiation of antidiabetic therapy to HCC diagnosis, inclusion of patients with liver disease and study quality, with P<0.05 considered statistically significant. Risk of publication bias was quantified using Egger’s test, with P<0.10 considered statistically significant28, and it also was qualitatively assessed by inspection of the funnel plots29. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary NC)
3. RESULTS
3.1. Search Results
Our search strategy yielded 10,105 individual articles, of which 56 were assessed in full for eligibility. Of these, 7 studies were published solely in abstract form, and were excluded. 8 studies utilized the Taiwan National Health Insurance claims database to establish their patient cohort30-37, and 2 studies utilized the South Korean National Health Insurance Service-National Sample Cohort19,38: the 2 studies with the largest respective cohort sizes were included in the analysis, and the rest were excluded. Additional studies were excluded due to incorrect study design (11 articles), intervention (20 articles) or patient population (2 articles). 8 articles were finally included in the study. All included studies were observational in nature. Potential effects of GLP-1 agonists, DPP-4 inhibitors, SGLT-2 inhibitors and amylin analogs were not evaluated using individual meta-analyses, due to insufficient numbers of identified studies. The study selection process is summarized by the MOOSE flow diagram (Supplementary Figure 1). After these exclusions, four separate meta-analyses were conducted to investigate the potential impact of sulfonylureas (8 studies), thiazolidinediones (7 studies), meglitinides (4 studies), and alpha-glucosidase inhibitors (3 studies), respectively, on risk of HCC.
3.2. Characteristics of Included Studies
Baseline characteristics of the 8 included studies are presented in Table 1. The majority of studies accounted for age (5/8)20,22,39,40,41, sex (5/8) 20,22,39,40,41, viral hepatitis (5/8)19,21,30,39,41, cirrhosis (5/8)19,20,30,40,41, and use of other antidiabetic medications (5/8)19,20,21,22,30. A smaller proportion of studies adjusted for statin use (4/8)19,20,21,30, alcohol intake (4/8)22,39,40,41 and smoking (2/8) 22,39. One study accounted for body mass index, fasting plasma glucose and hemoglobin A1c values40. Median NOS was 7 (range 6-9), with 7 studies considered high quality. Complete NOS scoring for included studies is presented in Supplementary Table 2.
Table 1: Baseline Characteristics of Observational Studies Included in Meta-Analysis Variables adjusted for:
1 (viral hepatitis), 2 (cirrhosis), 3 (comorbidities), 4 (household income level), 5 (residential area), 6 (anti-platelets), 7 (statins), 8 (other anti-diabetic medications), 9 (sex), 10 (age), 11 (duration of follow up), 12 (tobacco), 13 (alcohol), 14 (race), 15 (education level), 16 (family history of cancer), 17 (body mass index), 18 (Hemoglobin Alc)
| STUDY (AUTHOR, YEAR) |
REGION | STUDY DESIGN |
MEAN AGE |
HCC CASES* (N) |
SULF GROUP (N) |
TZD GROUP (N) |
MEGLITINIDE GROUP (N) |
ALPHA- GLUCOSIDASE INHIBITOR GROUP (N) |
TOTAL PARTICIPANTS (N) |
MEDICATION ASSESSMENT |
HCC ASSESSMENT |
COVARIATES |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LEE, 2019 | Korea | Case Control | NR | 241 | 1188 | 213 | 124 | NA | 1,446 | Prescription | Billing code | 1, 2, 3, 4, 5, 6, 7, 8 |
| KASMARI, 2017 | US | Retro Cohort | 57.7 | 7,473 | NR† | NR† | NA | NA | 29,583 | Prescription | Billing code | 1, 3, 7, 8 |
| BOSETTI, 2015 | Italy | Case Control | 65.2 | 190 | 2588 | 286 | 330 | 30 | 3,962 | Prescription | Billing code | 2, 3, 6, 7, 8, 9, 10, 11 |
| MIELE, 2015 | Italy | Case Control | NR | 224 | 20 | NA | NA | NA | 613 | Self-report | AFP & Imaging or biopsy | 9, 10, 12, 13 |
| CHANG, 2012 | Taiwan | Case Control | 66 | 10,741 | 181 | 34 | 26 | 32 | 52,588 | Prescription | Billing code | 1, 2, 3, 6, 7, 8 |
| HASSAN, 2010 | US | Case Control | 63 | 420 | 57 | 22 | NA | NA | 1,524 | Self-report | AFP & Imaging or biopsy | 1, 9, 10, 12, 13, 14, 15, 16 |
| KAWAGUCHI, 2010 | Japan | Case Control | 68.8 | 138 | 72 | 5 | 19 | 40 | 241 | NR | AFP & Imaging or biopsy | 2, 8, 9, 10, 13, 17, 18 |
| OLIVERA, 2008 | US | Retro Cohort | 56 | 39 | NR† | NR† | NA | NA | 191,223 | Prescription | AFP & Imaging or biopsy | 1, 2, 9, 10, 13 |
| TOTAL | 19,466 | 4106 | 560 | 499 | 102 | 281,180 |
Abbreviations: Retro=retrospective. NR=not reported. HCC=hepatocellular carcinoma. TZD=thiazolidinediones. AFP=alpha-fetoprotein.
Data on number of HCC cases in each antidiabetic medication user and non-user group was not extracted from included studies
Did not report number of study participants in each exposure group
Effect estimates were directly reported by all studies
Bosetti et al. undertook a nested case-control study on Italian new users versus non-users of sulfonylureas, thiazolidinediones, meglitinides, and alpha-glucosidase inhibitors, who were diagnosed with HCC between 2000 and 2012, and accounted for duration of antidiabetic use20. Chang et al. utilized the Taiwan National Health Insurance claims database to conduct a large nationwide case-control study on users versus non-users of sulfonylureas, thiazolidinediones, meglitinides, and alpha-glucosidase inhibitors with incident HCC, accounting for diabetes duration30. Kawaguchi et al. evaluated associations between use of sulfonylureas, thiazolidinediones, meglitinides, and alpha-glucosidase inhibitors, and the incidence of HCC, in a cohort of Japanese patients with hepatitis C-associated liver disease and type 2 diabetes mellitus40. Lee et al. performed a nationwide, nested case-control study to identify cases of HCC in South Korean sulfonylurea, thiazolidinedione, and meglitinide users and non-users19. Oliveria et al. utilized a large United States (US) population-based database to conduct a retrospective cohort study of subjects on sulfonylurea or thiazolidinedione monotherapy41. Hassan et al. performed a hospital-based case-control study to assess the association between sulfonylurea and thiazolidinedione use and risk of incident HCC39. Kasmari et al. conducted a large retrospective cohort study on users versus non-users of sulfonylureas and thiazolidinediones, who were diagnosed with HCC between 2008 and 2012 using data from the US MarketScan insurance claims database21. Miele et al. evaluated the incidence of HCC among Italian subjects treated with sulfonylurea therapy22.
3.3. Antidiabetic Medication Classes and Risk of HCC
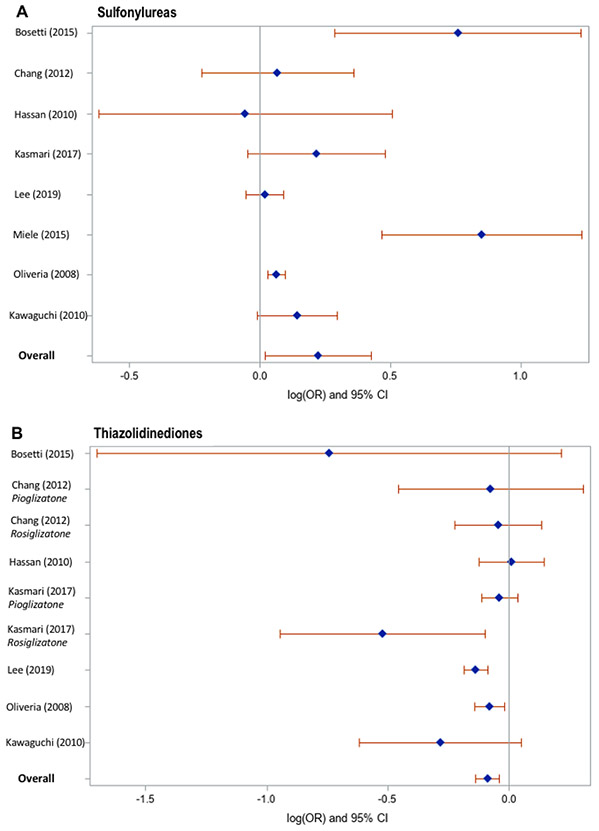
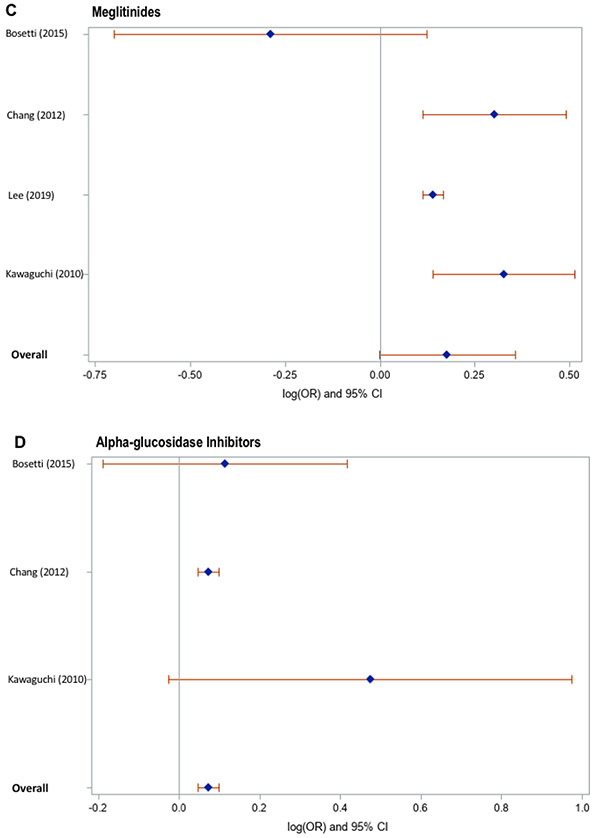
Meta-analysis of the identified studies demonstrated that thiazolidinedione users had an 8% lower risk of incident HCC, compared to non-users (Figure 1), after adjustment for confounding variables (pooled adjusted OR 0.92, 95% CI 0.86-0.97), with low heterogeneity (Cochran’s Q test P = 0.06; I2 = 43%). In contrast, compared to non-users, alpha-glucosidase inhibitor users had an 8% higher risk of incident HCC (pooled adjusted OR 1.08, 95% CI 1.02-1.14), with low heterogeneity (Cochran’s Q test P = 0.28; I2 = 21%). Neither use of sulfonylureas (pooled adjusted OR 1.25, 95% CI 0.98-1.60; Cochran’s Q test P = 0.0002; I2 = 75%) nor meglitinides (pooled adjusted OR 1.19, 95% CI 0.89-1.60; Cochran’s Q test P = 0.01; I2 = 72%) showed a significant overall association with risk of HCC. Likelihood of publication bias was low, based on visual inspection of the funnel plots (Supplementary Figure 2) and quantitative testing, using Egger’s test (thiazolidinediones: p=0.46, sulfonylureas: p=0.56, meglitinides: p=0.44, alpha-glucosidase inhibitors: p=0.32).
Figure 1.
Pooled Odds Ratios for Antidiabetic Medication Use and the Development of Hepatocellular Carcinoma
3.4. Subgroup and Sensitivity Analyses
In order to investigate potential sources of heterogeneity, predefined subgroup analyses were undertaken (Table 2). Thiazolidinedione use was associated with a 10% reduction in HCC risk in Asian subjects (aOR 0.90, 95% CI 0.83-0.97), but this association was not statistically significant in European/US populations (aOR 0.95, 95% CI 0.87-1.04). Their protective association was significant in studies that evaluated individuals with underlying liver disease (aOR 0.92, 95% CI 0.87-0.98), and those that utilized prescription records to ascertain medication exposure (aOR 0.92, 95% CI 0.87-0.98). Furthermore, thiazolidinediones appeared to be chemoprotective when analyses were restricted to studies that ascertained the diagnosis of HCC using biopsy/imaging studies (aOR 0.89, 95% CI 0.82-0.96) as opposed to diagnostic codes (aOR 0.96, 95% CI 0.87-1.06). The association for thiazolidinediones was not significant when analyses were restricted to studies that specified a minimum one year latency period between initiation of therapy and diagnosis of HCC (aOR 0.79, 95% CI 0.44-1.44) versus studies that did not specify a minimum latency period (aOR 0.92, 95% CI 0.86-0.99).
Table 2: Results of Selected Sub-Group Analyses on Sulfonylureas, Thiazolidinediones and Meglitinides.
Abbreviations: OR=odds ratio. CI=confidence interval; HCC=hepatocellular carcinoma
| Subgroup Analysis | No.of Studies |
No.of HCC cases |
Total No.of subjects |
OR (95% CI) | |
|---|---|---|---|---|---|
| SULFONYLUREAS | |||||
| Region | |||||
| Europe / U.S. | 5 | 8346 | 226,905 | 1.21 (0.81, 1.83) | |
| Asia | 3 | 11,120 | 54,275 | 1.33 (0.60, 2.95) | |
| HCC ascertainment | |||||
| Billing code | 3 | 7904 | 34,991 | 2.39 (0.85, 1.41) | |
| Imaging or biopsy | 5 | 11,562 | 246,189 | 1.38 (0.82, 2.32) | |
| Minimum latency | |||||
| 1 year | 4 | 1075 | 7545 | 1.34 (0.76, 2.38) | |
| Not specified | 4 | 18,391 | 273,635 | 1.06 (1.01, 1.12) | |
| Newcastle Ottawa Scale | |||||
| Score ≥7 | 7 | 19,242 | 280,567 | 1.28 (0.97, 1.69) | |
| Study Design | |||||
| Case Control | 6 | 11,954 | 60,374 | 1.36 (0.95, 1.92) | |
| Definition of antidiabetic medication exposure | |||||
| Prescription | 5 | 18,684 | 278,802 | 1.06 (1.02, 1.11) | |
| Evaluated subjects with liver disease | |||||
| Yes | 6 | 18,856 | 275,694 | 1.06 (1.02, 1.11) | |
| THIAZOLIDINEDIONES | |||||
| Region | |||||
| Europe / U.S. | 4 | 8122 | 226,292 | 0.95 (0.87, 1.04) | |
| Asia | 3 | 11,120 | 54,275 | 0.90 (0.83, 0.97) | |
| HCC ascertainment | |||||
| Billing code | 3 | 7904 | 34,991 | 0.96 (0.87, 1.06) | |
| Imaging or biopsy | 4 | 11,338 | 245,576 | 0.89 (0.82, 0.96) | |
| Minimum latency | |||||
| 1 year | 3 | 851 | 6932 | 0.79 (0.44, 1.44) | |
| Not specified | 4 | 18,391 | 273,635 | 0.92 (0.86, 0.99) | |
| Study Design | |||||
| Case control | 5 | 11,730 | 59,761 | 0.89 (0.83, 0.95) | |
| Definition of antidiabetic medication exposure | |||||
| Prescription | 5 | 18,684 | 278,802 | 0.92 (0.87, 0.98) | |
| Evaluated subjects with liver disease | |||||
| Yes | 5 | 18,632 | 275,081 | 0.92 (0.87, 0.98) | |
| MEGLITINIDES | |||||
| Region | |||||
| Asia | 3 | 11,120 | 54,275 | 1.11 (0.61, 2.01) | |
| Definition of antidiabetic medication exposure | |||||
| Prescription | 3 | 11,172 | 57,996 | 1.26 (0.94, 1.68) | |
| Evaluated subjects with liver disease | |||||
| Yes | 3 | 11,120 | 54,275 | 1.11 (0.61, 2.01) |
Although sulfonylurea use did not show a significant overall association with HCC incidence, a marginal increase in HCC risk was identified in studies with patient cohorts that included individuals with underlying liver disease (aOR 1.06, 95% CI 1.02-1.11), and studies that used prescription records (as opposed to self-report) to ascertain history of sulfonylurea exposure (aOR 1.06, 95% CI 1.02-1.11).
Exclusion of each study in turn did not significantly affect the summary estimate. We were unable to conduct subgroup analyses among studies evaluating alpha-glucosidase inhibitors or meglitinides, due to the small number of included studies in each category. Exclusion of studies that reported RR (i.e. Oliveria et al.) did not significantly affect overall summary estimates (sulfonylureas: aOR=1.29, 95% CI=0.96-1.73; thiazolidinediones: aOR=0.91, 95% CI=0.86-0.97) or subgroup summary estimates, with one exception: in the subset of studies that did not specify a minimum latency period from initiation of antidiabetic therapy to HCC diagnosis, the association between sulfonylurea exposure and increased risk of HCC was no longer significant (aOR=1.24, 95% CI=0.52-2.97).
4. DISCUSSION
Type 2 diabetes is projected to continue increasing in prevalence during the coming decade42 and is frequently comorbid with liver disease (in particular non-alcoholic fatty liver disease). Therefore, informed and judicious selection of antidiabetic therapy in patients with concurrent diabetes and liver disease could translate to a meaningful decline in overall rates of incident HCC. We conducted a comprehensive systematic review and a series of meta-analyses, involving more than 19,466 incident cases of HCC in 256,953 participants from both Asian and Western countries. We identified a significant reduction in risk of HCC incidence among users of thiazolidinediones, compared to non-users. This protective effect was significant only among Asian users, who had a 10% lower risk of incident HCC compared to Asian non-users, and only in studies that utilized biopsy or imaging modalities to define HCC. In contrast, sulfonylurea users had a higher risk of developing incident HCC, compared to non-users, particularly in studies evaluating patients with underlying liver disease, or which confirmed HCC diagnoses using imaging or pathologic criteria. We also found that alpha-glucosidase inhibitor use was associated with an increased risk of HCC incidence, compared to non-users, while no significant association was found for meglitinide use. Collectively, these findings support a potential role for thiazolidinedione use in the prevention of incident HCC, while use of sulfonylureas or alpha-glucosidase inhibitors warrant further investigation for potential adverse effects.
Results from preclinical studies have demonstrated the chemoprotective effects of TZDs. TZD treatment inhibited cellular growth and differentiation of various types of cancer cells, either cultured in vitro or implanted in nude mice43. Furthermore, chronic TZD administration in HBV-transgenic mice inhibits hepatocyte proliferation and reduces hepatic tumor incidence, which was associated with induction of tumor suppressor proteins p53 and p2144. Additionally, thiazolidinediones have insulin-sensitizing properties and reduce circulating insulin levels; this may also reduce risk of HCC development, since insulin resistance and hyperinsulinemia have been shown to exert pro-tumorigenic effects4. The protective association observed with thiazolidinedione use reached statistical significance in Asian but not in Western subjects. This could be for several reasons. On the one hand, the efficacy of thiazolidinediones in improving insulin sensitivity has been shown to vary by genetic polymorphism45, while the prevalence of genetic polymorphisms associated with insulin resistance is known to vary by ethnicity46, which may partly account for our findings. Similarly, the prevalence of genetic polymorphisms associated with the pharmacodynamics and metabolism of thiazolidinediones varies by ethnicity47,48, which may partly account for our findings. Notably, a prior study found the incidence of adverse medication effects, including heart failure and edema, to be lower in Asian thiazolidinedione users compared to Caucasian users49. Furthermore, among adults with impaired glucose tolerance, rosiglitazone use was associated with reduced progression to diabetes: this preventative effect was smallest in South Asian subjects50. Hence, it is possible that genetic variations associated with ethnicity may also modulate chemoprotective effects of thiazolidinediones. However, on the other hand, the confidence intervals in these subgroups were overlapping, thus these results should be interpreted cautiously. Current guidelines recommend thiazolidinedione use in selected patients with NAFLD and diabetes51. Accordingly, thiazolidinediones continue to be widely prescribed despite the introduction of newer antidiabetic agents. In several countries, their use has been reported to increase or remain stable in recent years52-54, and they are frequently favored due to their low cost. Further studies will be needed to identify the specific patient populations most likely to benefit from preventative treatment with thiazolidinediones.
Subgroup analyses also revealed a modestly increased risk of incident HCC among sulfonylurea users compared to nonusers, in studies evaluating subjects with established liver disease. These results are supported by findings from preclinical studies, which suggest that sulfonylureas promote secretion of endogenous insulin, with an associated increase in insulinlike growth factor-1 activity, which promotes neoplasia55-57. Interestingly, Kawaguchi et al. identified a significant association between the use of second-generation sulfonylureas and risk of incident HCC, in non-cirrhotic subjects40. In contrast, neither sulfonylureas nor any of the other evaluated anti-diabetic medications (i.e. thiazolidinediones, meglitinides or alpha-glucosidase inhibitors) were significantly associated with HCC incidence in subjects with cirrhosis. Together, these findings suggest that sulfonylureas exert a pro-tumorigenic effect, and potentially should be avoided in patients at increased risk of HCC due to underlying liver disease, particularly those without cirrhosis.
Our study findings differ from those of prior meta-analyses8,15 in several ways. First, we did not observe a significant overall association between sulfonylurea use and risk of incident HCC, although, we identified a modestly increased risk among studies evaluating subjects with liver disease. We accounted for 3 recent studies that were not included in prior meta-analyses19,21,22, which may account for these discrepant findings. Second, previous meta-analyses have yielded conflicting findings regarding the effect of thiazolidinedione use on risk of incident HCC: some have reported reduced HCC incidence17,18, while others have shown no significant association15. We identified a protective association, which was significant among Asian subjects but not Western subjects. Our study included 2 recent studies that were not part of prior metaanalyses19,20. Third, in contrast to our findings, a previous meta-analysis identified a nonsignificant trend towards lower risk of liver cancer with alpha-glucosidase inhibitor use23. Notably, this meta-analysis did not account for findings by Bosetti et al.20, which indicate an increased risk of HCC in users of alpha-glucosidase inhibitors, compared to non-users, and also included 4 studies that derived patient data from the Taiwan National Health Insurance claims database during a similar time period, which may have biased the overall effect estimate30,33,36,37. All 4 of these studies were identified during our literature search, and we included only the study with the largest cohort size to prevent inclusions of overlapping subjects30. Further research will be required to elucidate the potential role of alpha-glucosidase inhibitors in the pathogenesis of HCC.
4.2. Strengths and Limitations
To our knowledge, this study represents the largest and most comprehensive review to examine second line oral antidiabetic agents in relation to risk of HCC development. It includes 256,953 participants from both Asian and Western countries, which enhances the generalizability of our findings. We also conducted numerous sensitivity and subgroup analyses to ascertain risk estimates according to key determinants of HCC risk, including geographic region and presence of chronic liver disease.
We acknowledge several limitations. All included studies were observational in nature, and therefore inherently susceptible to confounding and bias. Variables included in the multivariate analysis were not consistent across studies. Specifically, all except one of the studies40 lacked data on patients’ glycemic control and none of the studies provided data regarding serial HbA1c trends in relation to HCC risk: inadequately controlled blood glucose is a risk factor for HCC development58, and may have confounded the results of these studies, especially since choice of medication regimen often reflects disease severity. However, studies generally matched cases and controls using follow-up duration as a proxy for diabetes severity. In addition, subjects in “non-user” comparator groups were frequently prescribed other antidiabetic medications that could have modified their risk of carcinogenesis, but did not consistently account for use of those other antidiabetic agents. Similarly, several studies failed to adjust for statin use, which independently predicts HCC incidence59. We also cannot exclude the possibility of confounding by indication. However, Chang et al.30 found chronic liver disease to be significantly more prevalent among thiazolidinedione users compared to non-users, indicating that thiazolidinediones were not preferentially prescribed to patients with normal liver function, thus falsely appearing protective. Furthermore, we were unable to address the relationship between antidiabetic medication use and HCC risk according to specific etiologies of chronic liver disease, since an insufficient number of published studies have evaluated these associations in cohorts of patients with NAFLD, alcoholic liver disease, or viral hepatitis B or C infections. In addition, HCC often remains clinically silent until the later stages of disease, and it is unclear whether study subjects were undergoing active surveillance for HCC. A single identified study (Oliveria et al.) reported RR instead of OR, hence we interpreted OR and RR interchangeably for the meta-analyses characterizing associations between the use of thiazolidinediones or sulfonylureas and risk of HCC. We recognize that this entails a theoretical risk of overstating effect size. However, we believe that appreciable divergence between OR and RR would be extremely unlikely in the case of Oliveria et al., since the incidence of HCC in their patient cohort was low (39 HCC cases out of 191,223 study participants). Furthermore, exclusion of Oliveria et al. from the meta-analyses did not significantly affect overall summary estimates.
We lacked sufficient data to analyze potential effects of other anti-diabetic medications, including GLP-1 agonists and DPP-4 inhibitors, and to conduct subgroup analyses on meglitinides and alpha-glucosidase inhibitors. Additionally, dose- and duration-response relationships could not be evaluated by meta-analysis, due to insufficient study numbers. However, Chang et al.30 reported the chemoprotective effect of thiazolidinediones to be stronger for higher cumulative dosage ≥120 daily defined dose (aOR 0.64; 95% CI: 0.56-0.72 for rosiglitazone and aOR 0.80; 95% CI: 0.67-0.95 for pioglitazone), and for cumulative duration of use ≥3 years (aOR 0.64; 95% CI: 0.49-0.85 for rosiglitazone and aOR 0.44; 95% CI: 0.23-0.86 for pioglitazone). In that analysis, dose and duration effects were especially pronounced among patients with prevalent chronic liver disease. In contrast, Bosetti et al. reported no apparent duration effect associated with use of sulfonylureas20.
5. CONCLUSIONS AND FUTURE DIRECTIONS
We conducted a comprehensive systematic review and meta-analysis of studies reporting the risk of incident HCC among users and nonusers of second-line, oral antidiabetic medications. Our findings endorse a protective effect of thiazolidinediones in reducing the risk of HCC development, particularly among Asian subjects. In contrast, use of alpha-glucosidase inhibitors and sulfonylureas were associated with modest but significantly increased risk of developing incident HCC. Collectively, our findings support the need for future well-designed prospective clinical studies focused on thiazolidinedione use across various etiologies of chronic liver disease and in different ethnicities. Prospective studies are also needed to determine whether alpha-glucosidase inhibitors and sulfonylureas should be discouraged in patients at high-risk of developing HCC, such as those with advanced liver disease.
Supplementary Material
Highlights.
Thiazolidinedione use appeared to reduce hepatocellular carcinoma risk in Asians
Alpha-glucosidase inhibitor use appeared to increase hepatocellular carcinoma risk
Sulfonylurea use was associated with increased hepatocellular carcinoma risk
Meglitinide use was not associated with hepatocellular carcinoma incidence
Funding sources/Acknowledgements:
This work was supported by the AASLD Clinical and Translational Research Fellowship in Liver Disease (AA), NIH K23 DK122104 (TGS), Harvard University Center for AIDS Research Career Development Award (TGS), Dana Farber / Harvard Cancer Center GI SPORE Career Enhancement Award (TGS), R01 DK114144 (KEC). Funding sources had no involvement in study design, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the article for publication.
Abbreviations:
- HCC
hepatocellular carcinoma
- OR
odds ratio
- aOR
adjusted odds ratio
- DPP-4
dipeptidyl peptidase-4
- GLP-1
glucagon-like peptide-1
- SGLT-2
sodium glucose co-transporter-2
- RR
relative risk
- NOS
Newcastle-Ottawa Scale
- CI
confidence intervals
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
TGS has served as a consultant to Aetion for work unrelated to this project. KEC serves on the scientific advisory board for Novo Nordisk and BMS and has received grant funding from Boehringer-Ingelheim, BMS and Novartis for work unrelated to this project.
REFERENCES
- 1.Balogh J, Victor D, Asham EH et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon TG, King LY, Chong DQ et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology. 2018;67(5): 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4(3):369–80. [DOI] [PubMed] [Google Scholar]
- 4.Donadon V, Balbi M, Ghersetti M et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. 2009;15(20):2506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat A, Sebastiani G, and Bhat M. Systematic review: Preventive and therapeutic applications of metformin in liver disease. World J Hepatol. 2015;7(12): 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decensi A, Puntoni M, Goodwin P et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3(11):1451–61. [DOI] [PubMed] [Google Scholar]
- 7.Noto H, Goto A, Tsujimoto T et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7(3):e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Kang D, Cao W et al. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–22. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Li H, Tan X et al. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–18. [DOI] [PubMed] [Google Scholar]
- 10.Colmers IN, Bowker SL, Tjosvold LA. Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2012;38(6):485–506. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Wang C, Zheng Y et al. Cancer incidence and mortality in patients with type 2 diabetes treated with human insulin: a cohort study in Shanghai. PLoS One. 2013;8(1):e53411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiang JC, Gane EJ, Bai WW et al. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol. 2015;30(3):591–9. [DOI] [PubMed] [Google Scholar]
- 13.Karlstad O, Starup-Linde J, Vestergaard P et al. Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf. 2013;8(5):333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Wu H, Zhao L et al. Association between insulin therapy and risk of liver cancer among diabetics: a meta-analysis of epidemiological studies. Eur J Gastroenterol Hepatol. 2018;30(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Singh PP, Singh AG et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(6):881–91. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Zhu G, Liu T et al. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci Rep. 2016. September 19;6:33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosetti C, Rosato V, Buniato D et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18(2):148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Zhao S, Zhang M et al. Decreased risk of liver cancer with thiazolidinediones therapy in patients with type 2 diabetes: Results from a meta-analysis. Hepatology. 2013;58(2):835–6. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Jang S, Nam CM et al. Incident Hepatocellular Carcinoma Risk in Patients Treated with a Sulfonylurea: A Nationwide, Nested, Case-Control Study. Sci Rep. 2019;9(1):8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosetti C, Franchi M, Nicotra F et al. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: a nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol Drug Saf. 2015;24(7):771–8. [DOI] [PubMed] [Google Scholar]
- 21.Kasmari AJ, Welch A, Liu G et al. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med. 2017; 130(6):746.e1–746.e7. [DOI] [PubMed] [Google Scholar]
- 22.Miele L, Bosetti C, Turati F et al. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterol Res Pract. 2015;2015:570356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Wang Y, Lou H et al. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Oncotarget. 2017;8(46):81027–81039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O'Connell D et al. (2019). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [online]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 23rd September 2019].
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Thomas J, eds.Cochrane Handbook for Systemic Reviews of interventions https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm. Accessed September 15, 2020. [Google Scholar]
- 28.Lin L, Chu H, Murad MH et al. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J Gen Intern Med. 2018;33(8):1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Easterbrook PJ, Berlin JA, Gopalan R et al. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. [DOI] [PubMed] [Google Scholar]
- 30.Chang C, Lin J, Wu L et al. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55(5):1462–72. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Lin J, Wu L et al. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(7):E1170–5. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Shieh J, Chang C et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62(4):606–15. [DOI] [PubMed] [Google Scholar]
- 33.Chiu C, Huang C, Chen Y et al. Increased risk of gastrointestinal malignancy in patients with diabetes mellitus and correlations with anti-diabetes drugs: a nationwide population-based study in Taiwan. Intern Med. 2013;52(9):939–46. [DOI] [PubMed] [Google Scholar]
- 34.Huang M, Chung C, Chang W et al. The role of thiazolidinediones in hepatocellular carcinoma risk reduction: a population-based cohort study in Taiwan. Am J Cancer Res. 2017;7(7):1606–1616. [PMC free article] [PubMed] [Google Scholar]
- 35.Kao C, Sun L, Chen P et al. A population-based cohort study in Taiwan--use of insulin sensitizers can decrease cancer risk in diabetic patients? Ann Oncol. 2013;24(2):523–530. [DOI] [PubMed] [Google Scholar]
- 36.Lai S, Chen P, Liao K et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107(1):46–52. [DOI] [PubMed] [Google Scholar]
- 37.Lin C, Huang H, Chu F et al. Association between Gastroenterological Malignancy and Diabetes Mellitus and Anti-Diabetic Therapy: A Nationwide, Population-Based Cohort Study. PLoS One. 2015;10(5):e0125421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim G, Jang S, Han E et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int J Cancer. 2017;140(4):798–806. [DOI] [PubMed] [Google Scholar]
- 39.Hassan MM, Curley SA, Li D et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116(8):1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi T, Taniguchi E, Morita Y et al. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30(3):479–86. [DOI] [PubMed] [Google Scholar]
- 41.Oliveria SA, Koro CE, Yood MU et al. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2008;2(1):47–57. [Google Scholar]
- 42.Rowley WR, Bezold C, Arikan Y et al. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017;20(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galli A, Mello T, Ceni E et al. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin Investig Drugs. 2006;15(9):1039–49. [DOI] [PubMed] [Google Scholar]
- 44.Galli A, Ceni E, Mello T et al. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor gamma-independent regulation of nucleophosmin. Hepatology. 2010;52(2):493–505. [DOI] [PubMed] [Google Scholar]
- 45.Della-Morte D, Palmirotta R, Rehni AK et al. Pharmacogenomics and pharmacogenetics of thiazolidinediones: role in diabetes and cardiovascular risk factors. Pharmacogenomics. 2014;15(16):2063–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carulli L, Rondinella S, Lombardini S et al. Review article: diabetes, genetics and ethnicity. Aliment Pharmacol Ther. 2005;22(S2):16–19. [DOI] [PubMed] [Google Scholar]
- 47.Martis S, Peter I, Hulot J et al. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J. 2013;13(4):369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Martin E, Martinez C, Ladero JM et al. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther. 2006;10(1):29–40. [DOI] [PubMed] [Google Scholar]
- 49.Roughead EE , Chan EW, Choi N et al. Variation in Association Between Thiazolidinediones and Heart Failure Across Ethnic Groups: Retrospective analysis of Large Healthcare Claims Databases in Six Countries. 2015;38(9):823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyko EJ, Gerstein HC, Mohan V et al. Effects of ethnicity on diabetes incidence and prevention: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabet Med. 2010;27(11):1226–32. [DOI] [PubMed] [Google Scholar]
- 51.Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 52.Leal I, Romio SA, Schuemie M et al. Prescribing pattern of glucose lowering drugs in the United Kingdom in the last decade: a focus on the effects of safety warnings about rosiglitazone. Br J Clin Pharmacol. 2013;75(3):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold SV, Inzucchi SE, Echouffo-Tcheugui JB et al. Understanding Contemporary Use of Thiazolidinediones. Circ Heart Fail. 2019;12(6):e005855. [DOI] [PubMed] [Google Scholar]
- 54.Masoudi FA, Wang Y, Inzucchi SE et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290(1):81–5. [DOI] [PubMed] [Google Scholar]
- 55.La Vecchia C Diabetes mellitus, medications for type 2 diabetes mellitus, and cancer risk. Metabolism. 2011;60(10):1357–8. [DOI] [PubMed] [Google Scholar]
- 56.Pasello G, Urso L, Conte P et al. Effects of sulfonylureas on tumor growth: a review of the literature. Oncologist. 2013; 18(10): 1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore MA, Park CB, Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. Eur J Cancer Prev. 1998;7(2):89–107. [PubMed] [Google Scholar]
- 58.Han H, Zhang T, Jin Z et al. Blood glucose concentration and risk of liver cancer: systematic review and meta-analysis of prospective studies. Oncotarget. 2017; 8(30): 50164–50173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh S, Singh PP, Singh AG et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.