Abstract
Telomeres are protective caps on chromosome ends that shorten with each cell division. Telomere length (TL) predicts the onset of cellular senescence and correlates with longevity and age-related disease risk. Previous research suggests that adults display fixed ranking and tracking of TL by age 20 years, supporting the importance of TL at birth and attrition during childhood. However, longitudinal research examining telomere dynamics during early life is sparse. Here, we used monochrome multiplex quantitative polymerase chain reaction to measure relative TL in leukocytes isolated from cord blood and child blood collected at ages 3, 5, 7, and 9 years among 224 minority children enrolled in a New York City-based birth cohort. We also measured maternal TL at delivery in a subset of 197 participants with a biobanked blood sample. TL decreased most rapidly in the first years of life (birth to 3 years), followed by a period of maintenance into the pre-puberty period. Mothers with longer telomeres gave birth to newborns with longer telomeres that remained longer across childhood, suggesting that the fixed ranking and tracking of TL observed among adults may extend to early childhood or even the prenatal period with a potential transgenerational basis. We did not find significant sex differences in the pattern of child TL change across development. These findings emphasize the need to understand factors and mechanisms that determine TL during early childhood.
Keywords: telomere length, prenatal, childhood, development, epidemiology
1.1. Introduction
Telomeres are repetitive, non-coding T2AG3 sequences and protein structures located at eukaryotic chromosome ends (Moyzis et al., 1988). During cell division, chromosomes erode owing to limitations of DNA replication machinery, thus telomeres serve a self-sacrificing role against damage and degradation of protein coding regions, among other functions (Bonetti et al., 2013). Circulating leukocytes are replenished from hematopoietic stem cells (HSCs) in the bone marrow, which express telomerase, a reverse transcriptase capable of extending T2AG3 sequences (Bonetti et al., 2013; Werner et al., 2015). Over time, telomere erosion exceeds elongation, which is thought to contribute to the onset of cellular senescence. The accumulation of senescent cells limits the potential for growth and repair and drives the process of tissue aging (Collado et al., 2007). Indeed, telomere length (TL) has been linked with several age-related pathologies and is widely considered to serve as an indicator of healthy aging and the propensity for disease development (Fitzpatrick et al., 2007; Grodstein et al., 2008; Valdes et al., 2007).
In order to sustain cellular health across the human lifespan, TL is reset during blastocyst genesis, as indicated by a marked increase in telomerase activity (Turner et al., 2010; Wright et al., 2001), which remains highly expressed throughout the remainder of gestation (Cheng et al., 2013; Ulaner and Giudice, 1997; Wright et al., 1996). Cross-sectional research suggests that leukocyte telomere attrition peaks during the first years of life, as the HSC pool is established, and that erosion continues at a faster rate throughout childhood compared to adult life (Aubert and Lansdorp, 2008; Frenck et al., 1998; Rufer et al., 1999). Recently, a study of adult twin pairs concluded that heritability and the early life environment, beginning in utero, are likely the primary determinants of TL during adulthood (Hjelmborg et al., 2015). Likewise, a study of four adult cohorts (n=1156) found inter-individual ranking and tracking of TL remained fixed over six decades, supporting that the majority of telomere variability originates early in life (Benetos et al., 2013). More recent research has also shown that TL tracking extends back to the second decade of life (Benetos et al., 2019).
Animal models of TL across generations and over the life course further support that TL at birth and rate of attrition during early life better predict lifespan compared to attrition later in life (Asghar et al., 2015; Bateson et al., 2015; Heidinger et al., 2012). Genome-wide association studies (GWAS) on the genetic determinants of TL have also found that variants involved in the maintenance of telomeres differ between adults and children, providing the possibility for different TL regulation by life stage (Stathopoulou et al., 2015; Zeiger et al., 2018). Yet, despite the putative importance of the early environment in determining telomere dynamics across the lifespan, the majority of studies investigating telomeres during childhood have been cross-sectional by design and have thus been limited in their ability to characterize TL change during key phases of growth and development.
In the present study, we characterized telomere dynamics over the early life course using biobanked blood samples collected repeatedly from children between birth and age 9 years and examined associations between maternal and cord blood TL in paired samples collected at delivery. We also considered the impact of several sociodemographic and lifestyle factors on TL.
2.1. Materials and Methods
2.1.1. Study cohort
Primary analyses included 224 of the 722 mother-child pairs enrolled in the Columbia Center for Children’s Environmental Health (CCCEH) Mothers and Newborns birth cohort, which recruited pregnant women from two prenatal clinics in Northern Manhattan and the South Bronx between 1998 and 2006. Women were excluded if they were outside the ages of 18–35, initiated prenatal care after the 20th week of pregnancy, used tobacco products or illicit drugs, or had diabetes, hypertension or HIV. All pregnancies were low-risk and 97% of infants included in the analysis were born at term (≥37 weeks gestation). During pregnancy, trained research workers collected information about sociodemographics and other lifestyle characteristics from the mother through a structured, in-person interview. Maternal pre-pregnancy body mass index (BMI) was calculated from self-reported height and weight. Household material hardship was considered present if the mother reported inadequate access to food, housing, or clothing. Exposure to environmental tobacco smoke was determined based on maternal report of a smoker in the home in combination with serum cotinine levels as previously described (Rauh et al., 2004). After delivery, the child’s sex, birthweight, and gestational age were abstracted from hospital medical records. Mothers and children completed follow-up study visits at the CCCEH laboratory at ages 3, 5, 7, and 9 years and participants were included in the present analysis if they had a biobanked blood sample available 1) at birth (cord blood), 2) at age 3 years, and 3) at age 5 years, 7 years or 9 years. For the majority of children, the mother’s blood at the time of delivery was also biobanked. We found no substantial differences in sociodemographic or lifestyle characteristics considered between children included vs. excluded from the analytic sample at any follow-up period (Appendix Table 1). All study procedures were approved by the Institutional Review Board of Columbia University Medical Center. Before each study visit, mothers were informed about all study rocedures and gave written informed consent for herself and for her child in her preferred language; after age 7 years, children additionally provided informed assent.
2.1.2. Blood collection, processing and storage
Immediately following delivery, blood was drawn from the umbilical cord by a member of the research staff. Maternal blood was collected following delivery by venipuncture and child blood was collected at 3, 5, 7 and 9-year follow-up visits by a pediatric phlebotomist. All samples were collected using green-top tubes containing sodium heparin as an anticoagulant. Buffy coat containing blood leukocytes was separated from whole blood by centrifugation, processed, and stored at −80C in the CCCEH laboratory. At the time of telomere analysis, cord blood samples from the earliest enrolled child had been stored for 18.5 years and age 9 year blood from the most recently enrolled child had been stored for 1.3 years, providing an opportunity to not only investigate changes in telomere length with age, but also to consider the impact of storage duration on TL in biobanked samples. Unfortunately, information on the number of freeze-thaw cycles was not available. Prior research has shown that relative TL measured in blood remains stable through up to seven freeze-thaw cycles (Zanet et al., 2013); however, we acknowledge that differences in the number of freeze-thaw cycles across samples could have introduced non-biological variation into our measures.
2.1.3. Relative telomere length measurement.
For all samples (n=1213 from 230 mother-child pairs), genomic DNA (100–500 ng) was isolated from leukocytes using an identical standard phenol-chloroform protocol. The purity of cord blood DNA only was determined via 260/280 ratios using a Nanodrop 1000 spectrophotometer (ThermoFisher Scientific, Waltham, MA); 4 of the 227 cord blood samples had a 260/280 ratio of 1.7; all other samples had a ratio of 1.8 or greater (range 1.7–1.9). Telomere repeat sequence copy number (T) relative to single gene (S: albumin) copy number was measured using an adaptation of the multiplex monochrome quantitative polymerase chain reaction (MMqPCR) protocol developed by Cawthon et al. 2009 (Cawthon, 2009). This approach provides a measure of relative leukocyte telomere length (rLTL) proportional to the average telomere length across all chromosomes for all cells sampled and has been shown to produce estimates that are highly correlated with terminal restriction fragment (TRF) lengths measured by Southern blot (Cawthon, 2009). MMqPCR was performed on a Bio-Rad CFX96 lightcycler (Bio-Rad Laboratories, Hercules, CA) at the Columbia University Laboratory of Precision Environmental Health. The final reaction mix contained 1x iQ SYBR Green Supermix (BioRad), 600 nM forward telomere primer (Life Technologies), 600 nM reverse telomere primer (Life Technologies), 250 nM forward albumin primer (Life Technologies), 250 nM reverse albumin primer (Life Technologies), and 5 ng DNA in a 20 μL reaction. The telomere primer sequences were: forward primer 5′-TGT TAG GTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA ACA-3′, reverse primer 5′-ACA CTA AGG TTT GGG TTT GGG TTT GGG TTT GGG TTA GTG T-3′. The albumin primer sequences were: forward primer 5′-GCC CGG CCC GCC GCG CCC GTC CCG CCG GAA AAG CAT GGT CGC CTG TT-3′, reverse primer 5′-CGG CGG CGG GCG GCG CGG GCT GGG CGG AAA TGC TGC ACA GAA TCC TTG-3′. All within-family (mother and repeated child) samples were plated together in triplicate, along with a positive control (pooled DNA in triplicate), a negative control (water in triplicate), and a 6-point standard curve derived from serially diluted reference DNA in triplicate (S1: 150 ng/well, S2: 50 ng/well, S3: 16.7 ng/well, S4 5.55 ng/well, S5 1.85 ng/well, S6: 0.62 ng/well). The thermal cycling profile consisted of three stages: Stage 1: 3min at 95°C; Stage 2: 2 cycles of 15s at 94°C, 15s at 49°C; and Stage 3: 32 cycles of 15s at 94°C, 10s at 62°C, 10s at 74°C (with telomere signal acquisition), 10s at 84°C, 10s at 88°C (with albumin signal acquisition). Two standard curves, one for the telomere signal and one for the albumin signal, were generated using CFX Manager software. The average PCR efficiency (E) and R2 for the standard curves were: telomere E= 115.7%, R2 = 0.994 and albumin: E=121.5%, R2 = 0.990. Of the 1213 samples assayed, 9 (0.7%) failed to produce a signal in two of three replicates and were excluded. If the coefficient of variation (CV) across triplicate Cq values exceeded 5% for the telomere or the albumin amplicon then the average of the two closest duplicates was taken; 4 (0.3%) samples had a CV that remained above 5% and were excluded from further analyses. Additionally, we excluded all data from 6 mother-child pairs missing the cord blood or age 3 year sample, leaving a final dataset of 1175 samples from 224 mother-child pairs. After these steps, the average CV of telomere and albumin Cq values were 1.61% and 0.97%, respectively. We calculated T/S ratios, hereafter referred to as rLTL, using the formula:
Where E tel and E alb are the mean reaction efficiencies for the respective amplicon groups across all samples on a given plate. Cq tel calibrator and Cq alb calibrator are the average Cqs of the pooled sample for the respective amplicons, and tel sample and alb sample are the average of the triplicate Cqs for the unknown sample for the respective amplicons. We additionally calculated intra-class correlation coefficients (ICC) for the positive control samples as measures of intra-plate and inter-plate reliability using separate two-way random effects models. Specifically, the intra-plate ICC was 0.897 calculated using absolute agreement and multiple raters per measurement and the inter-plate ICC was 0.918 calculated using consistency and multiple raters per measurement. These values indicate good and excellent reliability, respectively, based on conventional interpretation guidelines (Koo and Li, 2016; Shrout and Fleiss, 1979).
2.1.4. Statistical analysis
We first examined descriptive statistics for rLTL within each follow-up period and visualized the distribution of rLTL at each age using histograms and boxplots. We next examined the bivariate association between storage time and rLTL within each follow-up period using separate linear regression models. We then ran a linear mixed effects model with a cubic B-spline basis for follow-up age (continuous in years) and a random intercept to estimate the non-linear change in rLTL across childhood adjusting for storage duration, which was negatively associated with rLTL. We visualized these dynamics by plotting predicted rLTL from the mixed effects model on both the population level and the individual level with a random effect. When plotting, we fixed storage duration for all samples and all follow-up ages in order to better visualize the true relationship between age and rLTL. We also calculated the percent of children with increasing rLTL, defined as a positive change between sequential episodes of greater than 15%.
Given the unique longitudinal nature of our data, the focus of this work was to investigate telomere dynamics across childhood. However, given a general interest in understanding the dependency of telomere length change on baseline length, in secondary analyses we examined whether we could account for artifact resulting from potential regression to the mean (RTM) using the approach established by Verhulst et al. (Verhulst et al., 2013) and previously applied in models of TL change among adults (Meier et al., 2019). Briefly, this involves adjusting the difference between sequential measurements by subtracting the change that is expected as a result of the regression effect, which is estimated from the mean, standard deviation and correlation of measurements.
An additional goal of this analysis was to examine the relationship between maternal rLTL at delivery and newborn rLTL. We calculated Pearson correlations to compare maternal versus cord blood rLTL among the subsample of 197 mother-child dyads for whom paired samples were available and quantified the proportion of variation in cord blood rLTL attributable to maternal rLTL using bivariate linear regression. Next, we ran our previously described mixed effects model with a cubic spline, and included continuous maternal rLTL, as well as storage time, as covariates. To visualize the effect of maternal rLTL on child rLTL dynamics, we plotted predicted values from a storage time-adjusted mixed effects model with a cubic spline that included the interaction between maternal rLTL (high vs. low based on dichotomization at the distribution median) and child age (continuous in years).
We also examined maternal and cord blood rLTL in relation to several sociodemographic, lifestyle, and obstetric characteristics using multivariable linear regression. The characteristics considered included: maternal age at delivery (continuous in 10 year intervals), race/ethnicity (African American vs. Dominican), education (<high school vs. high school or equivalent), ETS exposure during pregnancy (yes vs. no), maternal pre-pregnancy overweight/obesity (BMI≥25 kg/m2 vs. BMI<25 kg/m2), material hardship (self-reported inadequate access to food, clothing or housing vs. adequate access to all), newborn sex (male vs. female), gestational age (continuous in weeks), birthweight (continuous in kilograms) and season of birth (winter/spring/fall vs. summer). We examined each variable in a separate model and combined into a single, mutually-adjusted model. Lastly, we evaluated the influence of sex on child rLTL dynamics by including sex and the interaction between sex and child age (continuous in years) in our cubic spline mixed effects model also adjusting for storage time.
3.1. Results
3.1.1. Study cohort
The study sample is comprised of 224 low-income, minority (64% Dominican, 36% African American) mother-child pairs recruited from Northern Manhattan and the South Bronx in New York City. At delivery, mothers were on average 25 years old, 35% had less than a high school education, and 41% reported experiencing material hardship. The average gestational length was 39 weeks with 97% of infants born at term (≥ 37 weeks). Before pregnancy, 47% of mothers were overweight or obese, defined as a body mass index of 25 kg/m2 or greater. Table 1 provides additional sociodemographic and lifestyle characteristics of study participants. No characteristics considered substantially varied between the sub-sample of participants with data at each follow-up period (Appendix Table 1).
Table 1.
Characteristics and relative leukocyte telomere length (rLTL) among a sample of mother-child pairs enrolled in the CCCEH Mothers and Newborns birth cohort (n=224).
| Mean±SD or N (%) | |
|---|---|
| African American | 81 (36) |
| Dominican | 143 (64) |
| Maternal age (years) | 25.3±5.2 |
| <High school education or equivalent | 78 (35) |
| Material hardship | 91 (41) |
| Maternal pre-pregnancy over weighta | 99 (47) |
| Prenatal environmental tobacco smoke | 79 (35) |
| Child sex (girl) | 126 (56) |
| Gestational age (weeks) | 39.4±1.3 |
| Birthweight (kg) | 3.4±0.5 |
| Relative LTL | |
| Maternal (n=197, 25.3±5.2 yearsc) | 1.01±1.13b |
| Cord blood (n=224, birthc) | 2.47±1.00b |
| Age 3 (n=224, 2.9±0.4 yearsc) | 2.06±1.02b |
| Age 5 (n=177, 5.0±0.2 yearsc) | 1.99±1.00b |
| Age 7 (n=168, 7.1±0.2 yearsc) | 1.99±1.00b |
| Age 9 (n=185, 9.0±0.2 yearsc) | 1.97±1.00b |
N=220, defined as BMI > 25 kg/m2
Adjusted for storage time from a cubic spline mixed effect model with storage time fixed at the mean for all samples and all follow-up ages.
Mean±standard deviation age at blood draw
3.1.2. Telomere dynamics across childhood
At the time of the MMqPCR assay, biobanked samples had been stored for a mean±standard deviation (SD) of 10.3±3.9years (range: 1.3 – 18.5 years). Storage time was significantly associated with rLTL within follow-up periods (Appendix Table 2). rLTL was approximately normally distributed at each follow-up period (birth, age 3 years, age 5 years, age 7 years, age 9 years), and as expected, was longest in cord blood with a storage time-adjusted mean±SD of 2.47±1.00 (Table 1). In our mixed effects model with a cubic spline examining rLTL dynamics between birth and age 9 years, predicted rLTL on the population level showed a non-linear pattern of change such that the rate of decline was greatest between birth and the first follow-up period at age 3 years, after which it remained approximately stable through age 9 years when the study period ended (Figure 1). The proportion of children with telomere lengthening, defined as an increase greater than 15% between sequential follow-up visits, was as follows: birth to age 3 years: 10.3%, 3 years to 5 years: 20.5%, 5 years to 7 years: 29.8%, 7 years to 9 years: 16.1%.
Figure 1.
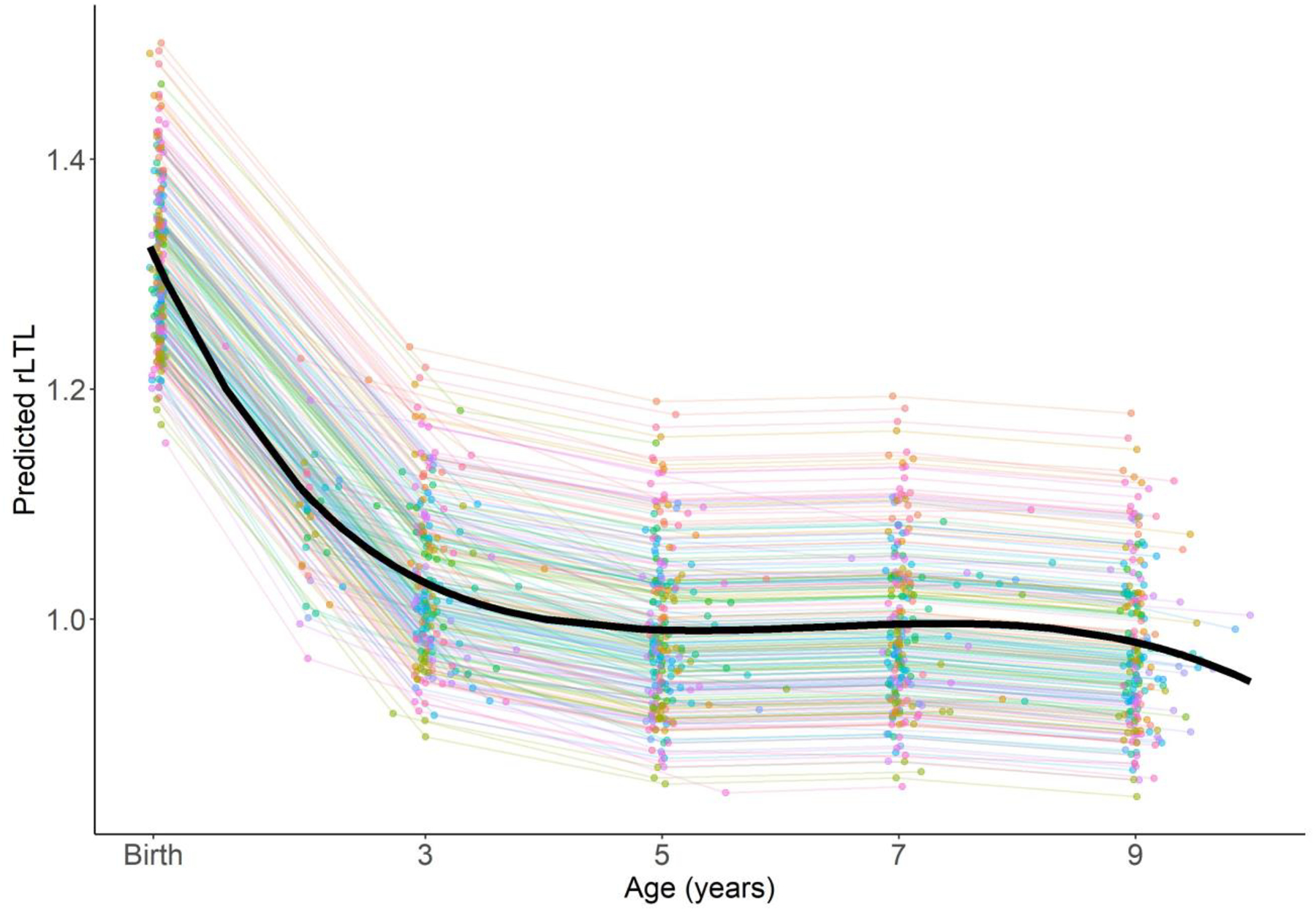
Relative leukocyte telomere length across age (n=224) predicted from a mixed effects model with a cubic spline. Individual lines correspond to children and the black line reflects the population-level mean. Storage duration is fixed at the mean for all samples and all follow-up ages.
In our secondary analysis examining potential RTM, we observed that baseline rLTL influenced the rate of change in the subsequent follow-up period and that this artifact could be accounted for by subtracting the change that is expected as a result of the regression effect from the difference between sequential measurements, as described in the methods (Appendix Figure 1)
3.1.3. Telomere length in paired maternal-newborn samples
Among mothers and children with paired samples (n=197), maternal rLTL collected at the time of her child’s birth (mean±SD: 1.01±1.13) was, on average, shorter than cord blood rLTL (Table 1). Maternal and newborn rLTL were moderately correlated (RPearson= 0.25, p-value = 0.0004) and maternal rLTL explained 6.2% of the variability (R2) in cord blood rLTL (Figure 2). Children born to mothers with shorter rLTL (<50th percentile) at delivery not only had shorter rLTL in cord blood, but also displayed shorter rLTL throughout early life (Figure 3); however, the interaction between dichotomous maternal rLTL and continuous child age was not significant (p-value = 0.53). Continuous maternal rLTL was significant when included in our cubic spline mixed effects model of child rLTL dynamics, with a beta coefficient of 0.35 (95% CI: 0.22, 0.47).
Figure 2.
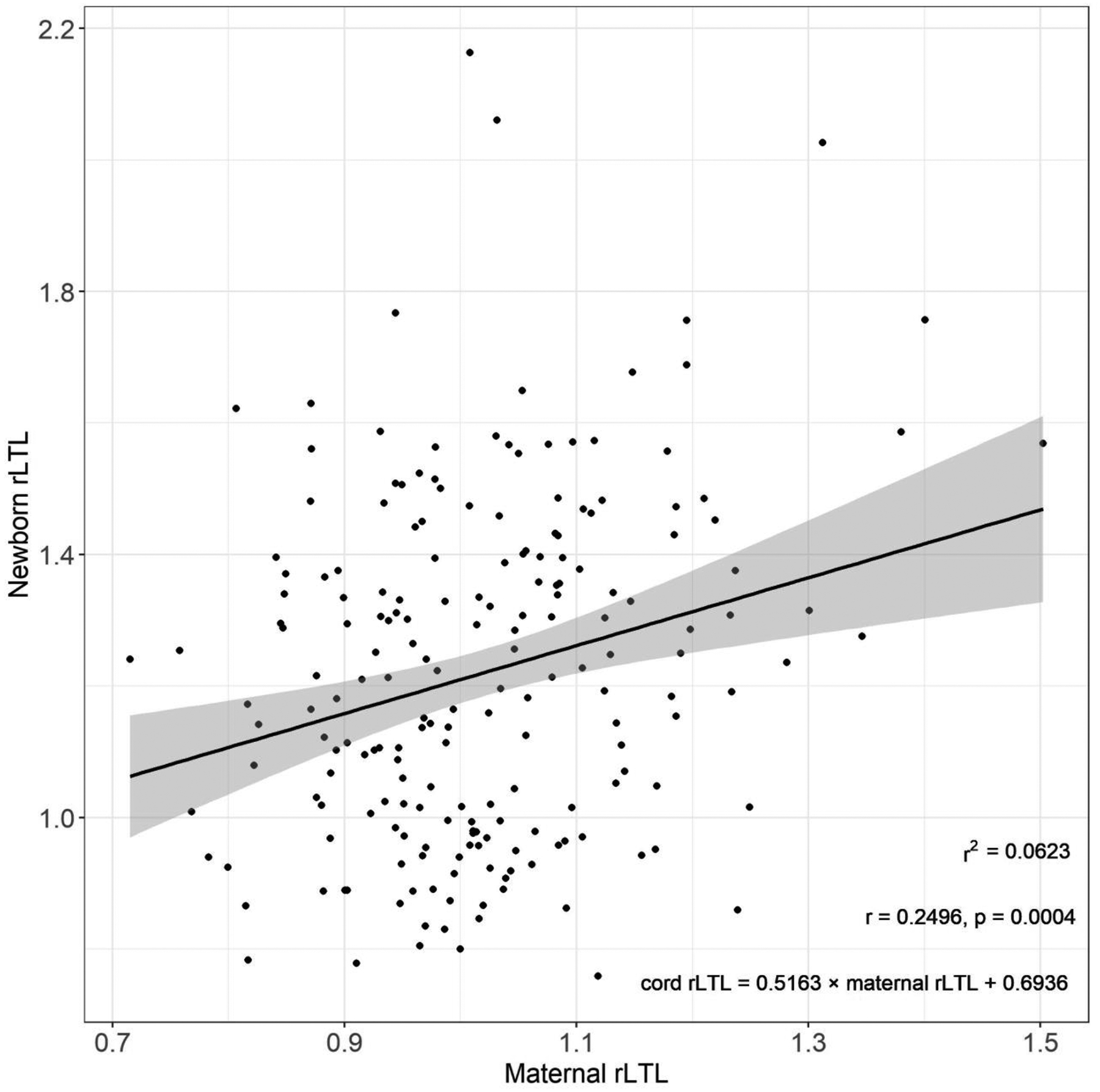
Relationship between maternal and cord blood relative leukocyte telomere length (rLTL) among 197 mother-newborn pairs. Shading reflects 95% confidence interval around the predicted line.
Figure 3.
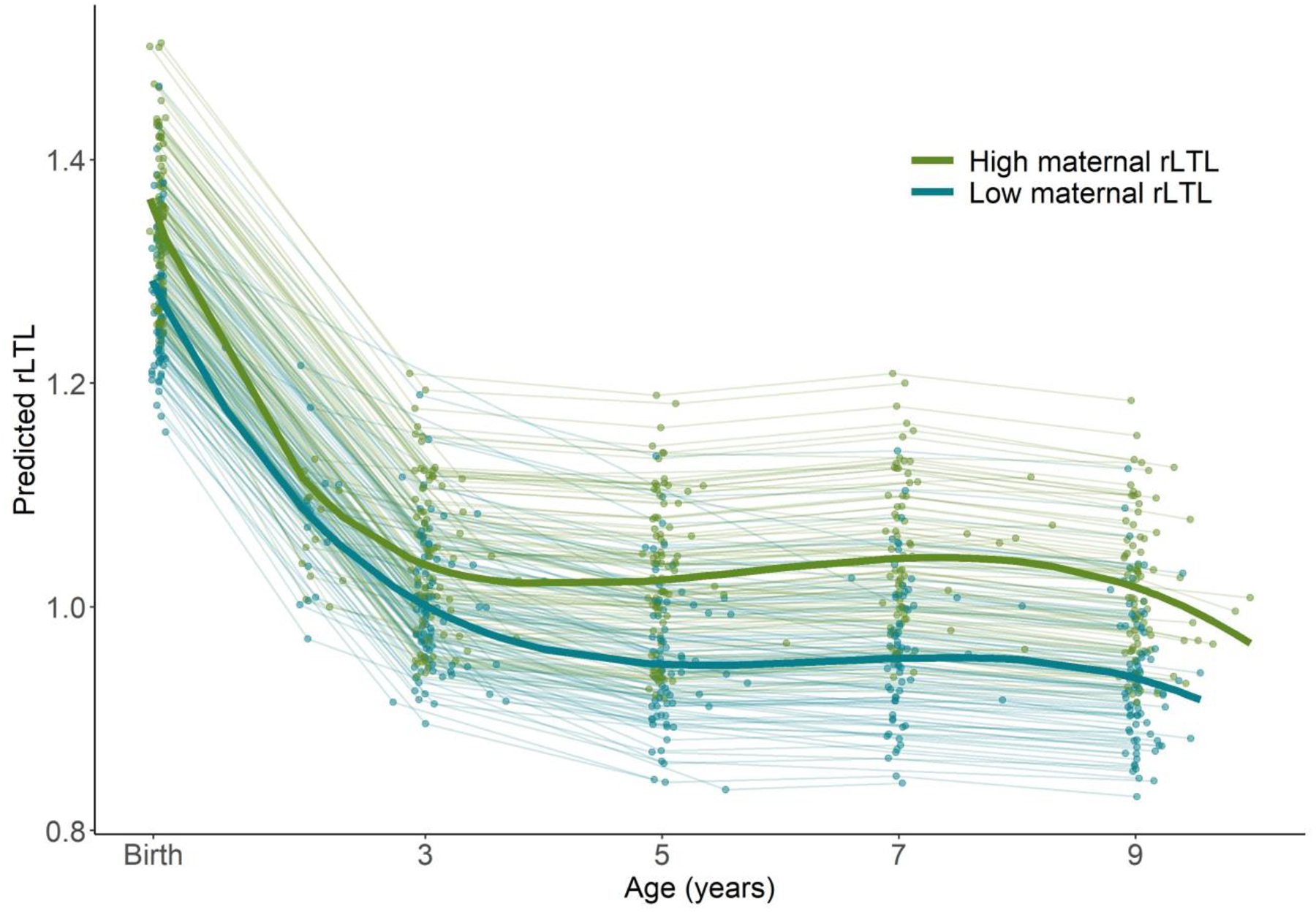
Child relative leukocyte telomere length across age (n=224) predicted from a cubic spline mixed effects model stratified by high vs. low maternal rLTL at delivery based on dichotomization at the maternal rLTL median. Individual lines correspond to children and the bold lines reflects the population-level means for each group. Storage duration is fixed at the mean for all samples and all follow-up ages.
3.4. Influence of sociodemographic and other characteristics on telomere length
Age at delivery was inversely associated with rLTL measured in maternal blood (change per 10 year increase = −0.029, 95% CI: −0.061, 0.004) and cord blood (−0.006, 95% CI: −0.071, 0.059), however, the associations did not reach statistical significance. Similarly, maternal rLTL was not significantly associated with race/ethnicity, education, material hardship, environmental tobacco smoke (ETS) exposure, overweight/obesity, child sex, birthweight, gestational age, or season of delivery (Figure 4). Results were similar in individual and mutually-adjusted models (Figure 4). We observed a slight sex-differential pattern of change in rLTL across childhood, such that telomere length, on average, remained approximately flat between ages 3 and 9 years among boys, but displayed some fluctuation among girls (Figure 5); however, the interaction between sex and child age was not significant (p-value=0.75).
Figure 4.
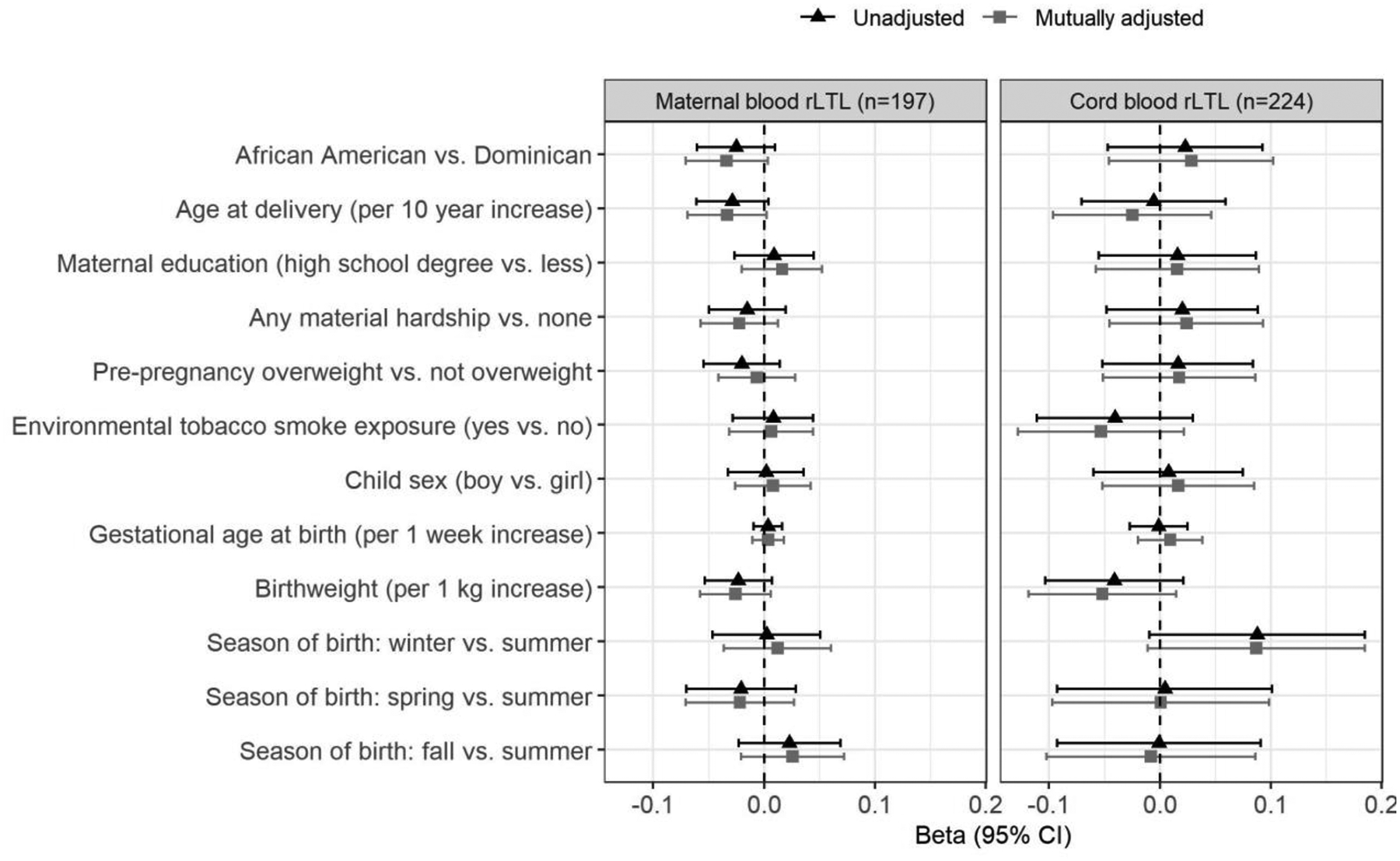
Associations between participant characteristics and relative leukocyte telomere length (rLTL) in maternal and cord blood collected after delivery. All models are adjusted for sample storage duration. Bars around each marker reflect 95% confidence intervals.
Figure 5.
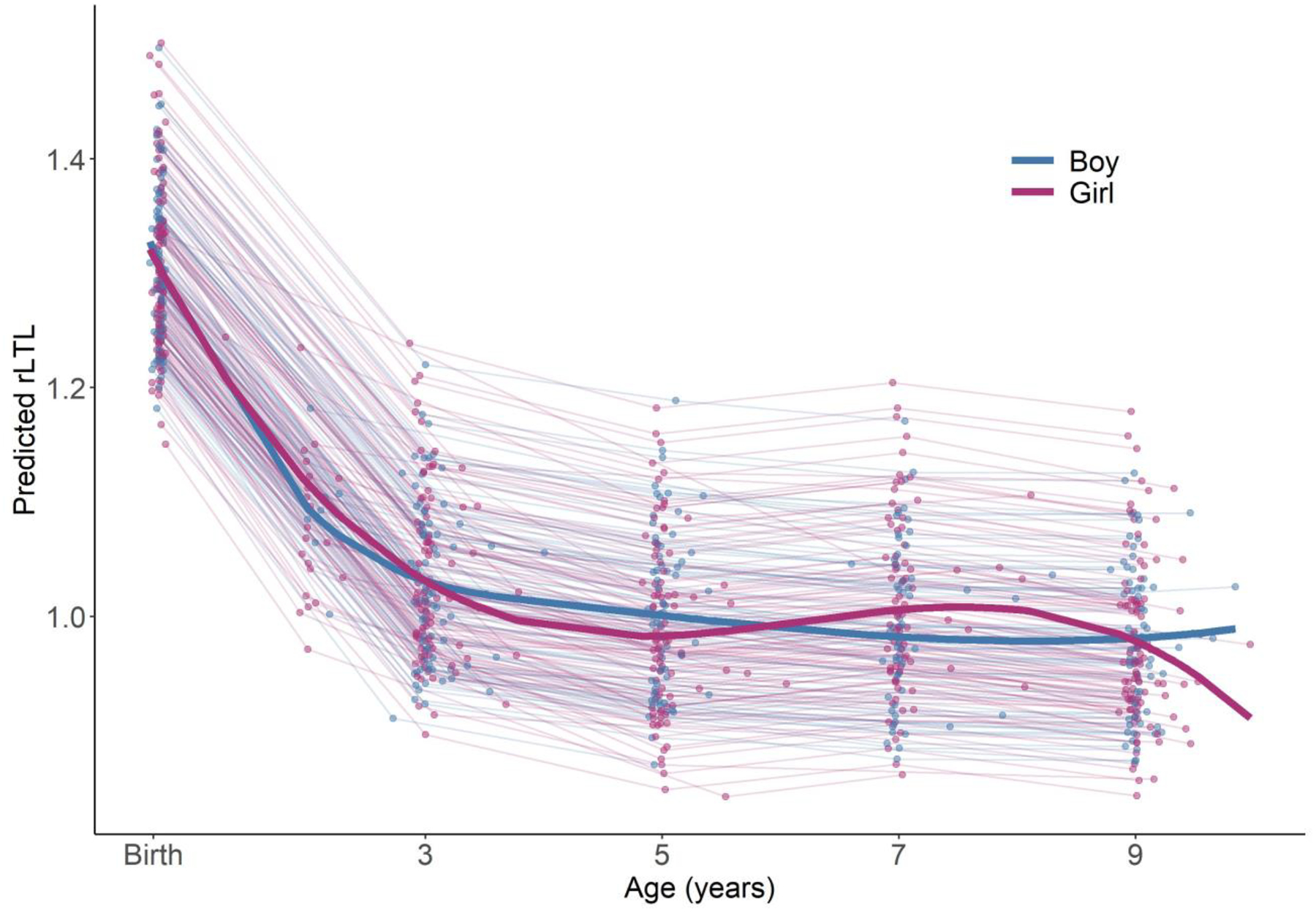
Child relative leukocyte telomere length (rLTL) across age (n=224) predicted from a cubic spline mixed effects model stratified by child sex. Individual lines correspond to children and the bold lines reflects the population-level change for each group. Storage duration is fixed at the mean for all samples and all follow-up ages.
4.1. Discussion
In the present study, we found that rLTL decreased rapidly in the first years of life, followed by a period of maintenance into the pre-puberty period. These findings are consistent with previous cross-sectional research and support the premise of accelerated TL attrition after birth as the HSC pool expands. Notably, the rapid rate of telomere attrition between birth and age 3 years may render telomeres particularly susceptible to environmental influences during this developmental window and given the relative stability of rLTL after toddler years, it is plausible that telomere dynamics during the early postnatal period set the stage for later life telomere length, potentially influencing life-long health and longevity.
We are aware of five studies that have previously investigated longitudinal changes in TL during childhood. These studies are generally limited by small sample sizes and measurement of TL at a few time points. In the earliest study, LTL was measured by Southern blot in samples collected between birth and age 36 months from nine uninfected children born to mothers with HIV. The majority of children showed maintenance of TL until at least 18 months of age, after which TL either remained stable or decreased through age 36 months (Zeichner et al., 1999). A more recent study measured buccal cell TL by MMqPCR at two (n=8), three (n=28), or four (n=14) times points among a sample of 50 children with or without a history of early institutional care between ages 6 and 15 years. The researchers found significant telomere attrition across development, although the change between specific age periods was not reported (Humphreys et al., 2016). In a third study, rTL was measured by qPCR in dried blood spots collected at ages 4 and 5 years among a sample of 77 Latino children. The authors reported that the majority of children displayed telomere lengthening (31%), defined as an increase of 10% of more, or maintenance (66%) over the one year study period (Wojcicki et al., 2016). A fourth study measured TL by qPCR in buccal cells collected from 236 children enrolled in the Environmental-Risk Longitudinal Twin Study at ages 5 and 10 years. On average, rLTL decreased between the two age periods, with 17% of children displaying telomere lengthening, defined as a greater than 15% increase in rLTL between the two follow-up periods (Shalev et al., 2013). In the largest study of 630 healthy children with at least one measure of buccal cell rTL during early life, rTL was shown to decrease between infancy and age 2 years, but remain stable between ages and 2 and 3 years, with a majority of children showing both increases and decreases across the study period (Bosquet Enlow et al., 2020). Cross sectional studies also suggest that the rate of telomere shortening during childhood may vary by developmental period (Frenck et al., 1998).
There are several possible explanations for the lengthening observed among some children in our study and by prior research. With the exception of certain conditions that promote polyclonal expansion, such as infection, circulating leukocytes do not replicate in the periphery, but rather are replenished by HSCs in the bone marrow (Werner et al., 2015). HSCs and their progenitors express telomerase, a reverse transcriptase that buffers telomere erosion by adding nucleotide repeats to chromosome ends (Shore and Bianchi, 2009). It is plausible that telomerase in the bone marrow is upregulated during certain phases of child development, such as pre-puberty or adrenarche, which could translate to longer telomeres in circulating leukocytes. This possibility is supported by evidence demonstrating that HSC migration into different bone marrow niches (e.g., ostoblastic to vascular) can trigger proliferation of previously quiescent HSCs, resulting in the resetting of LTL (Yin and Li, 2006). While the factors promoting HSC migration are not well understood, they could include signals related to growth or maturation. In addition, while it is widely suspected that telomerase activity is lost in somatic cells after embryonic differentiation (Hiyama and Hiyama, 2007), little research has examined telomerase expression or activity during specific phases of accelerated child growth, raising the possibility that changes in TL could reflect telomerase activation in peripheral leukocytes. Alternatively, it is possible that the apparent telomere lengthening that we and others have observed among a subset of individuals reflects changing cell type distributions across development. Leukocytes comprise a heterogenic group of cells that vary in average telomere length (Aubert and Lansdorp, 2008) and cell type distribution, including of leukocytes, is known to shift across age (Kverneland et al., 2016), including during childhood and the pre-pubescent period (Nah et al., 2018). Unfortunately, we were not able to phenotype leukocyte cell distributions due to feasibility constraints. It is also possible that ‘Alternative Lengthening of Telomeres’ (ALT) mechanisms, which encompass a group of non-telomerase dependent telomere elongation pathways, could be at play; however, while it is thought that ALT mechanisms reflect a dysregulated version of typical processes, they have thus far only been observed in disease states (Cesare and Reddel, 2010). Finally, it is possible that changes in TL reflect measurement error attributable to our study design. We plated all within family samples together to reduce intra-family technical variability due to batch, however, this design resulted in samples that were collected and stored for varying periods of time. To the extent possible, we accounted for the influence of storage duration by adjusting for this factor when examining change in rLTL across age.
Interestingly, we found that maternal rLTL not only predicted her newborn’s cord blood rLTL, but also served as a determinant of rLTL across childhood, supporting the high heritability of TL. . In this study, other sociodemographic, lifestyle and obstetric factors considered did not significantly predict maternal or newborn rLTL and rLTL did not significantly vary by sex across the follow-up period. Unfortunately, we did not collect data on several characteristics that have been previously identified as putatively important determinants of TL, such as maternal stress, sleep quality, diet, physical activity or paternal age.
We measured rLTL by MMqPCR, which is advantageous as it can be performed at high throughput and low-cost since half as many reactions are needed, thereby allowing for a relatively large sample size. In addition, given that telomere and single copy gene signals are collected within each reaction, measurement error related to variation in the quantity of pipetted DNA is reduced. However, we note that while qPCR has been shown to correlate highly with Southern blot, it nonetheless remains more prone to measurement error and does not provide an absolute measure of TL (Aviv et al., 2011). Here, we observed high repeatability, with intra-plate and inter-plate ICCs of 9.0 and above. Consistent with previous studies measuring rLTL by qPCR, we found that baseline rLTL influenced the rate of change in the subsequent follow-up period (Aviv et al., 2009; Aviv and Shay, 2018; Ehrlenbach et al., 2009; Nordfjall et al., 2009; Shalev et al., 2013). Simulation studies suggest this pattern is likely in part attributable to regression to the mean (Nettle et al., 2019), which we showed can be addressed by calculating RTM-corrected rLTL change between follow-up periods. The availability of paired mother-newborn samples for the majority of participants was also a strength of our study. Unfortunately, not all children had a biobanked sample at each follow-up period, although at a minimum, all children had a cord blood sample, an age 3 year sample, and an age 5, 7, or 9 year sample, allowing a longitudinal picture to be drawn. Finally, our study sample included low-income, minority women, which we consider a strength as this demographic has largely been understudied in telomere biology research. Previous studies have demonstrated that storage conditions can impact TL (Dagnall et al., 2017; Meier et al., 2019; Reichert et al., 2017; Tolios et al., 2015), however, to the best of our knowledge, no study has investigated the effect of long-term storage (>5 years) on human TL measured by qPCR measurement. Given the longitudinal design of this study, samples were stored for varying lengths of time, which we found was a significant, negative predictor of rLTL. While we adjusted our models for storage duration, residual confounding could have contributed to the apparent lengthening between follow-up periods for some children. Uncertainty about the precise impact of sample storage duration has important implications for future longitudinal research aiming to investigate telomere length in archived samples and a systematic examination is needed to fully understand the influence of storage time on the qPCR TL assay.
5.1. Conclusions
Given the putative importance of TL in cellular health and aging, it is critical to understand the dynamics of telomeres over the early life course. Consistent with cross-sectional research, in this sample we found that rLTL decreased most rapidly in the first years of life, followed by a period of maintenance into early adolescence. Future research is needed to understand the biological mechanisms driving variability in the rate of TL change during the first years of life as well as modifiable environmental factors that contribute to shifts in the rate of attrition.
Supplementary Material
Highlights.
Telomere length (TL) declines most rapidly during early childhood.
Some children display telomere lengthening between sequential follow-up periods.
Maternal TL predicts newborn TL and tracks with child TL through pre-adolescence.
Longitudinal TL measures are sensitive to storage time and regression to the mean.
Funding:
This research was supported by: the Passport, Forsythia, and Fine Foundations and NIH P50ES009600, R03ES026416, and P30ES009089. During preparation of this manuscript, WC was supported by NIH T32 ES023772, NIH T32 ES007322 and EPA FP-91779001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S, 2015. Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM, 2008. Telomeres and aging. Physiol Rev 88, 557–579. [DOI] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, 2009. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol 169, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E, 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res 39, e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Shay JW, 2018. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D, 2015. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proc Biol Sci 282, 20142140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A, 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Verhulst S, Labat C, Lai TP, Girerd N, Toupance S, Zannad F, Rossignol P, Aviv A, 2019. Telomere length tracking in children and their parents: implications for adult onset diseases. FASEB J 33, 14248–14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti D, Martina M, Falcettoni M, Longhese MP, 2013. Telomere-end processing: mechanisms and regulation. Chromosoma. [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, Kane-Grade F, De Vivo I, Petty CR, Nelson CA, 2020. Patterns of change in telomere length over the first three years of life in healthy children. Psychoneuroendocrinology 115, 104602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, 2009. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR, 2010. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11, 319–330. [DOI] [PubMed] [Google Scholar]
- Cheng G, Kong F, Luan Y, Sun C, Wang J, Zhang L, Jiang B, Qi T, Zhao J, Zheng C, Xu D, 2013. Differential shortening rate of telomere length in the development of human fetus. Biochem Biophys Res Commun 442, 112–115. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M, 2007. Cellular senescence in cancer and aging. Cell 130, 223–233. [DOI] [PubMed] [Google Scholar]
- Dagnall CL, Hicks B, Teshome K, Hutchinson AA, Gadalla SM, Khincha PP, Yeager M, Savage SA, 2017. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS One 12, e0184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A, 2009. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol 38, 1725–1734. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A, 2007. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165, 14–21. [DOI] [PubMed] [Google Scholar]
- Frenck RW Jr., Blackburn EH, Shannon KM, 1998. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A 95, 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I, 2008. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PLoS One 3, e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P, 2012. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109, 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K, 2007. Telomere and telomerase in stem cells. Br J Cancer 96, 1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmborg JB, Dalgard C, Moller S, Steenstrup T, Kimura M, Christensen K, Kyvik KO, Aviv A, 2015. The heritability of leucocyte telomere length dynamics. J Med Genet 52, 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Esteves K, Zeanah CH, Fox NA, Nelson CA 3rd, Drury SS, 2016. Accelerated telomere shortening: Tracking the lasting impact of early institutional care at the cellular level. Psychiatry Res 246, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TK, Li MY, 2016. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kverneland AH, Streitz M, Geissler E, Hutchinson J, Vogt K, Boes D, Niemann N, Pedersen AE, Schlickeiser S, Sawitzki B, 2016. Age and gender leucocytes variances and references values generated using the standardized ONE-Study protocol. Cytometry A 89, 543–564. [DOI] [PubMed] [Google Scholar]
- Meier HCS, Hussein M, Needham B, Barber S, Lin J, Seeman T, Diez Roux A, 2019. Cellular response to chronic psychosocial stress: Ten-year longitudinal changes in telomere length in the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 107, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR, 1988. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85, 6622–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah EH, Kim S, Cho S, Cho HI, 2018. Complete Blood Count Reference Intervals and Patterns of Changes Across Pediatric, Adult, and Geriatric Ages in Korea. Ann Lab Med 38, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D, Seeker L, Nussey D, Froy H, Bateson M, 2019. Consequences of measurement error in qPCR telomere data: A simulation study. PLoS One 14, e0216118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G, 2009. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet 5, e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP, 2004. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 26, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert S, Froy H, Boner W, Burg TM, Daunt F, Gillespie R, Griffiths K, Lewis S, Phillips RA, Nussey DH, Monaghan P, 2017. Telomere length measurement by qPCR in birds is affected by storage method of blood samples. Oecologia 184, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM, 1999. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A, 2013. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 18, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D, Bianchi A, 2009. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J 28, 2309–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL, 1979. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86, 420–428. [DOI] [PubMed] [Google Scholar]
- Stathopoulou MG, Petrelis AM, Buxton JL, Froguel P, Blakemore AI, Visvikis-Siest S, 2015. Genetic determinants of leucocyte telomere length in children: a neglected and challenging field. Paediatr Perinat Epidemiol 29, 146–150. [DOI] [PubMed] [Google Scholar]
- Tolios A, Teupser D, Holdt LM, 2015. Preanalytical Conditions and DNA Isolation Methods Affect Telomere Length Quantification in Whole Blood. PLoS One 10, e0143889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Wong HP, Rai J, Hartshorne GM, 2010. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod 16, 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaner GA, Giudice LC, 1997. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod 3, 769–773. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD, 2007. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int 18, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD, 2013. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur J Epidemiol 28, 859–866. [DOI] [PubMed] [Google Scholar]
- Werner B, Beier F, Hummel S, Balabanov S, Lassay L, Orlikowsky T, Dingli D, Brummendorf TH, Traulsen A, 2015. Reconstructing the in vivo dynamics of hematopoietic stem cells from telomere length distributions. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcicki JM, Shiboski S, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E, 2016. Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol Genet Genomics 291, 1379–1389. [DOI] [PubMed] [Google Scholar]
- Wright DL, Jones EL, Mayer JF, Oehninger S, Gibbons WE, Lanzendorf SE, 2001. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod 7, 947–955. [DOI] [PubMed] [Google Scholar]
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW, 1996. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18, 173–179. [DOI] [PubMed] [Google Scholar]
- Yin T, Li L, 2006. The stem cell niches in bone. J Clin Invest 116, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet DL, Saberi S, Oliveira L, Sattha B, Gadawski I, Cote HC, 2013. Blood and dried blood spot telomere length measurement by qPCR: assay considerations. PLoS One 8, e57787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, Goodenow M, Biggar R, Dimitrov D, 1999. Rapid telomere shortening in children. Blood 93, 2824–2830. [PubMed] [Google Scholar]
- Zeiger AM, White MJ, Eng C, Oh SS, Witonsky J, Goddard PC, Contreras MG, Elhawary JR, Hu D, Mak ACY, Lee EY, Keys KL, Samedy LA, Risse-Adams O, Magana J, Huntsman S, Salazar S, Davis A, Meade K, Brigino-Buenaventura E, LeNoir MA, Farber HJ, Bibbins-Domingo K, Borrell LN, Burchard EG, 2018. Genetic Determinants of Telomere Length in African American Youth. Sci Rep 8, 13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


