Abstract
Background & Aims:
Perturbations of intracellular magnesium (Mg2+) homeostasis have implications for cell physiology. The cyclin M family, CNNM, perform key functions in the transport of Mg2+ across cell membranes. Herein, we aimed to elucidate the role of CNNM4 in the development of non-alcoholic steatohepatitis (NASH).
Methods:
Serum Mg2+ levels and hepatic CNNM4 expression were characterised in clinical samples. Primary hepatocytes were cultured under methionine and choline deprivation. A 0.1% methionine and choline-deficient diet, or a choline-deficient high-fat diet were used to induce NASH in our in vivo rodent models. Cnnm4 was silenced using siRNA, in vitro with DharmaFECT and in vivo with Invivofectamine® or conjugated to N-acetylgalactosamine.
Results:
Patients with NASH showed hepatic CNNM4 overexpression and dysregulated Mg2+ levels in the serum. Cnnm4 silencing ameliorated hepatic lipid accumulation, inflammation and fibrosis in the rodent NASH models. Mechanistically, CNNM4 knockdown in hepatocytes induced cellular Mg2+ accumulation, reduced endoplasmic reticulum stress, and increased microsomal triglyceride transfer activity, which promoted hepatic lipid clearance by increasing the secretion of VLDLs.
Conclusions:
CNNM4 is overexpressed in patients with NASH and is responsible for dysregulated Mg2+ transport. Hepatic CNNM4 is a promising therapeutic target for the treatment of NASH.
Lay summary:
Cyclin M4 (CNNM4) is overexpressed in non-alcoholic steatohepatitis (NASH) and promotes the export of magnesium from the liver. The liver-specific silencing of Cnnm4 ameliorates NASH by reducing endoplasmic reticulum stress and promoting the activity of microsomal triglyceride transfer protein.
Keywords: Non-alcoholic steatohepatitis, NASH, Cyclin M4, CNNM4, Magnesium, Therapy, siRNA, Endoplasmic reticulum stress, Microsomal triglyceride transfer protein, MTP
Graphical Abstract
Introduction
Dietary imbalances, such as a low intake of magnesium, are recognised as the root cause of diseases. Although daily magnesium recommended intake is 300–400 mg/day, there is a growing concern about decreased intake in last decade, attributable to changes in dietary habits and food processing.1,2 Its ionic form, Mg2+, is the most abundant divalent cation in the cell and it is required as a cofactor for over 300 enzymatic reactions.3 An intricate network of Mg2+ transporters participate in its uptake and excretion, allowing its flux across cellular membranes, thus impacting Mg2+ homeostasis and distribution.1
Mg2+ supplementation reduces mortality from hepatic complications arising from alcohol intake or steatosis.4 Hypomagnesaemia is present in several comorbidities of non-alcoholic fatty liver disease (NAFLD) such as insulin resistance and type 2 diabetes,5 cardiovascular complications,6 and obesity.7 Moreover, deficiencies in Mg2+ are related to inflammatory responses, mitochondrial dysfunction, and decreased activity of the antioxidant system, all features of liver diseases.8
The term NAFLD encompasses a group of pathologies characterised by chronicity with serial progression from steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis.9 Such progression is mediated by the increased production of reactive oxygen species (ROS), lipotoxicity, mitochondrial dysfunction, and the development of endoplasmic reticulum (ER) stress.10 Remarkably, ER stress has been proposed to be an important mechanism because it disrupts calcium (Ca2+) transport through ATPases11 and leads to protein misfolding.12 It has been widely characterised in both NAFLD and NASH patients, together with impaired VLDL assembly and secretion.13,14
NAFLD is a global health problem with an estimated prevalence of around 25% among the adult population, being expected to increase.9 Changes in lifestyle habits are the most common recommendations for the clinical management of NAFLD, but it is difficult to achieve the long-term compliance of patients, making pharmacological alternatives attractive.
Among the various magnesiotropic proteins, the cyclin M (CNNM) family and their Bateman-module-mediated interactions with phosphatases of regenerating liver (PRLs) are of particular interest.15 PRLs are implicated in tumour progression and metastasis,16,17 including liver cancer, with Mg2+ perturbations reported in such.18 Although the CNNMs emerged as interacting molecular partners of PRLs,17 a new perspective proposes targeting CNNMs to modulate Mg2+ homeostasis more specifically.19 The functions of hepatic CNNMs in this regard are unknown, and their significance as pharmacological targets remains underexplored.
In the present study, we demonstrated the relevance of the differential expression of CNNM4 in NASH development, showing its functional role in transporting hepatic Mg2+. Moreover, we identified CNNM4 as a potential target for NASH treatment.
Patients and methods
Patients
Measurements of serum Mg2+ and hepatic CNNM1-4 mRNA, and immunohistochemical CNNM4 analysis were performed in different cohorts recruited at the Hospital Marqués de Valdecilla, Santander, Spain (Fig. S1A and B).
Animal maintenance and preclinical studies
Mice were maintained with ad libitum access to water and a choline-deficient diet with 0.1% methionine (0.1% MCDD) or a choline-deficient, high-fat diet (CD-HFD). A control group was maintained on a regular diet (SC diet).
Treatment of primary mouse hepatocytes and THLE-2 cells
Upon attachment, isolated mouse primary hepatocytes were transfected by overnight incubation with siRNA using DharmaFECT 1 or a CNNM4 expression plasmid using jetPRIME®. Cells were maintained and incubated for an additional 24 h under different conditions.
Cnnm4 silencing in vivo
Mice fed a 0.1% MCDD for 2 weeks or a CD-HFD for 3 weeks were repeatedly administered an siRNA using Invivofectamine® 3.0 Reagent through tail vein injection. Mice were sacrificed after 4 weeks on 0.1% MCDD and 6 weeks on CD-HFD, respectively. For Cnnm4 GalNAc-siRNA delivery, mice fed for 3 weeks on 0.1% MCDD were treated once, subcutaneously, and sacrificed after a total of 6 weeks on the diet. Samples of liver, white adipose tissue (WAT), and serum were collected.
Statistical analysis
All the experiments were performed at least in triplicate, with n = 3 (in vitro) and n = 4 (in vivo). The data are expressed as mean ± SEM and represent the fold change vs. control mean value when indicated. Statistical significance was determined using Prism 8 (GraphPad Software, San Diego, CA, USA). Groups were compared by 1-way analysis of variance (ANOVA) followed by post hoc Bonferroni tests (for 3 or more groups) or the Student t test (for 2 groups).
Results
CNNM4 is overexpressed in clinical and preclinical NASH
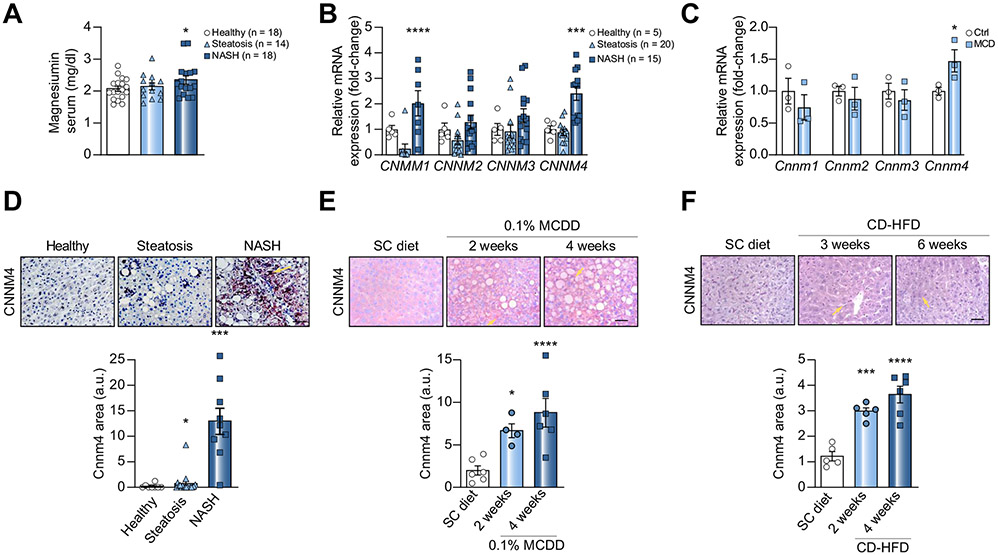
To investigate Mg2+ dysregulation in NASH, we determined Mg2+ levels in sera from a cohort of 50 age- and BMI-matched patients (Fig. S1A), observing increased levels of the cation in NASH patients (Fig. 1A). Mg2+ levels did not correlate with other NASH biomarkers (data not shown).
Fig. 1. CNNM4 is overexpressed in NASH patients and preclinical animal models.
(A) Magnesium determination in serum and (B) hepatic levels of CNNM1-4 mRNA in different cohorts of healthy individuals, patients with steatosis and patients with NASH. (C) Levels of Cnnm1-4 mRNA in primary hepatocytes stimulated with MCD medium. (D) Liver immunohistochemical staining and quantification for CNNM4 in a cohort of healthy individuals, patients with steatosis and patients with NASH, as well as mice fed (E) a 0.1% MCDD or (F) a CD-HFD. Scale bar corresponds to 100 μm. *p <0.05, ***p <0.001, and ****p <0.0001 vs. healthy/ctrl/SC diet. CD-HFD, choline-deficient high-fat diet; MCD, methionine and choline deficient; MCDD, MCD diet; NASH, non-alcoholic steatohepatitis; SC diet, regular diet.
Considering the magnesiotropic role of the CNNM family,19 we sought to assess whether dysregulated CNNM expression could be the cause of the elevated serum Mg2+. The hepatic mRNA levels of each CNNM were assessed in another cohort of 40 patients (Fig. S1B), showing a significant overexpression of CNNM1 and CNNM4 in NASH patients (Fig. 1B). In addition, an increase in Cnnm4 mRNA expression was observed in primary hepatocytes treated with methionine and choline-deficient (MCD) medium (Fig. 1C), an in vitro model that displays features of NASH such as ROS overproduction and lipid accumulation.20,21
Similarly, hepatic CNNM4 overexpression in NASH was confirmed by immunohistochemical protein determination in clinical samples (Fig. 1D) and animal models – mice fed a 0.1% MCDD (Fig. 1E) and CD-HFD (Fig. 1F). These animal models also showed elevated Cnnm4 mRNA, as did primary hepatocytes (Fig. S1C and D). By contrast, Cnnm1 overexpression was observed in neither the in vitro nor in vivo NASH models. To evaluate protein stability, human THLE-2 cells were treated with MLN4924 to inhibit NEDDylation, which prevents proteasome-mediated degradation,22 resulting in decreased CNNM4 expression (Fig. S1E). The expression of other Mg2+ transporters was determined in animal models (Fig. S1F) and clinical samples (Fig. S1G), showing that CNNM4 is unique among Mg2+ transporters in its upregulation in all conditions.
Targeting CNMM4 ameliorates NASH
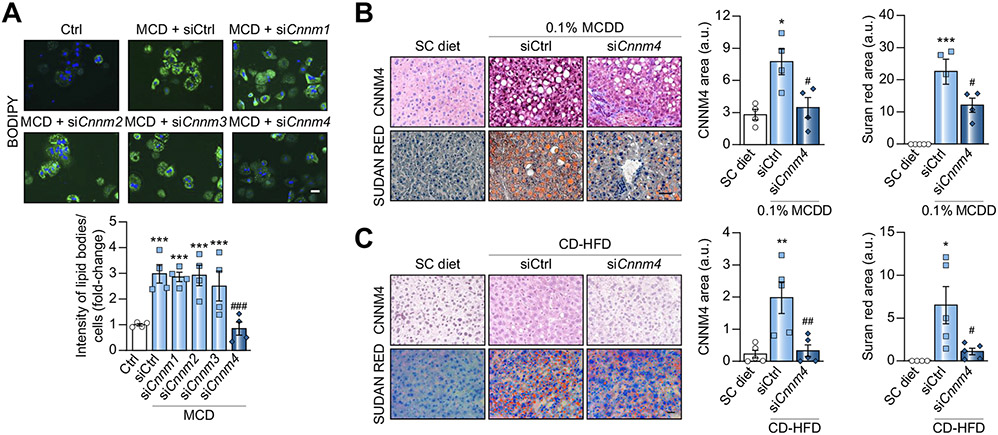
The possible role of CNNM4 in NASH development was examined by in vitro screening by specifically silencing each Cnnm in primary hepatocytes using small interfering RNA (siRNA). Targeting Cnnm4 (siCnnm4) alone effectively reduced MCD-induced lipid accumulation (Fig. 2A). The expression of each Cnnm and Prl mRNA was evaluated, confirming the specific and effective silencing of Cnnm (Fig. S2A) and eliminating the possibility of Prl regulation when targeting Cnnm4 (Fig. S2B). Additionally, CNNM4 silencing in THLE-2 cells also reduced MCD-induced lipid accumulation (Fig. S2C and D).
Fig. 2. Targeting CNNM4 reduces lipid accumulation in in vitro and in vivo NASH models.
(A) BODIPY staining micrographs and quantification in primary hepatocytes transfected with siRNA against Cnnm1-4 (siCnnm1-4) and stimulated with MCD for 24 h. Micrographs of CNNM4 and Sudan Red staining and respective quantification in mice fed (B) a 0.1% MCD diet (0.1% MCDD) or (C) a CD-HFD, and treated with Cnnm4 siRNA (siCnnm4) or an unrelated control (siCtrl). Scale bar corresponds to 50 μm. *p <0.05, **p <0.01, and ***p <0.001 vs. Ctrl/SC diet; #p <0.05, ##p <0.01, and ###p <0.001 vs. MCD + siCtrl/0.1% MCDD + siCtrl/CD-HFD + siCtrl. CD-HFD, choline-deficient high-fat diet; MCD, methionine and choline-deficient; NASH, non-alcoholic steatosis; SC diet, regular diet; siRNA, small interfering RNA.
Taking into consideration the CNNM4 overexpression observed in NASH, and the siCnnm4-derived reduction of lipid accumulation, the therapeutic potential of silencing Cnnm4 in vivo was explored. Mice were fed a 0.1% MCDD to induce steatosis development, increased oxidative stress, inflammation, and fibrosis in a short period of time.23 At 2 weeks, by which steatosis21 and CNNM4 overexpression are observable (Fig. 1E), 0.1% MCDD-fed mice were separated into 2 groups and treated repeatedly with either an siRNA against Cnnm4 (0.1% MCDD + siCnnm4) or an unrelated control (0.1% MCDD + siCtrl), using Invivofectamine 3.0®. The mice were sacrificed at the fourth week. Interestingly, the mice fed the 0.1% MCDD showed hepatic CNNM4 overexpression, whereas Cnnm4 silencing reduced diet-induced steatosis (Fig. 2B and Fig. S2E). Alpha-smooth muscle actin (αSMA) staining revealed that fibrosis, another NASH hallmark, was induced by 0.1% MCDD and attenuated by siCnnm4 (Fig. S2F). Furthermore, the treatment also reduced serum alanine aminotransferase (ALT) levels, a marker of liver damage (Fig. S2G).
In a different NASH murine model, mice were fed a CD-HFD, as this leads to a pattern of pathology more similar to that observed in humans.24 Although studies using this model tend to be performed for longer than 6 weeks,24 our group previously showed that mice developed early NASH phenotypes comprising weight gain, steatosis, and inflammation as early as this point in time.21 After 3 weeks on the diet, at which point CNNM4 was already overexpressed (Fig. 1F), the CD-HFD-fed group was divided and treated with siCnnm4 (CD-HFD + siCnnm4) or siCtrl (CD-HFD + siCtrl). They were sacrificed after 6 weeks and, similarly to in the previous results, the mice fed the CD-HFD showed hepatic CNNM4 overexpression, while the siRNA therapy reduced steatosis (Fig. 2C and Fig. S2H) and fibrosis development (Fig. S2I). Serum ALT levels remained unaltered (Fig. S2J).
CNNM4 acts in the liver as a magnesium exporter
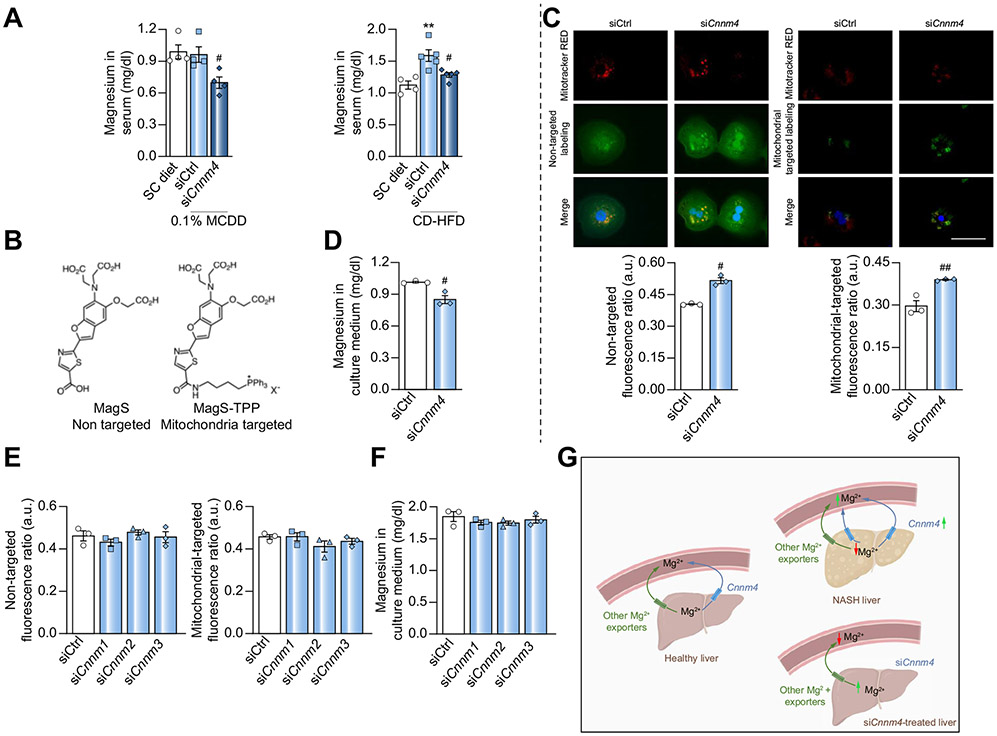
Despite the magnesiotropic role of CNNM4 in kidney epithelia,25 its physiological function in the liver remains largely unknown. The possible relationship between increased serum Mg2+ levels and hepatic CNNM4 overexpression (Fig. 1A and D) led us to anticipate a modulation of the cation in the studied NASH animal models. The measurement of Mg2+ in the serum revealed an increase in CD-HFD-fed mice and a decrease under siCnnm4 treatment in both the CD-HFD and 0.1% MCDD models (Fig. 3A).
Fig. 3. Magnesium distribution after specific silencing of Cnnm4.
(A) Magnesium in serum from mice fed a 0.1% MCDD or CD-HFD with specific Cnnm4 silencing (siCnnm4) compared with non-treated (siCtrl) mice. (B) Biochemical structure of non-targeted MagS and MagS-TPP probes. (C) Micrographs and relative intracellular magnesium levels and (D) extracellular magnesium levels in primary hepatocytes treated with an siRNA against Cnnm4 (siCnnm4). Scale bar corresponds to 100 μm. (E) Intracellular magnesium and (F) extracellular magnesium levels in primary hepatocytes treated with siCnnm1-3. (G) Schematic representation of CNNM4-dependent magnesium fluctuations in liver and in circulation **p <0.01 vs. SC diet; #p <0.05, and ##p <0.01 vs. 0.1% MCDD + siCtrl/CD-HFD + siCtrl/siCtrl. CD-HFD, choline-deficient high-fat diet; CNNM4, cyclin M4; MagS, magnesium-specific; MagS-TPP, mitochondrion-targeted magnesium-specific; MCDD, methionine and choline deficient diet; siRNA, small-interfering RNA.
To confirm the hypothesis of CNNM4 being a hepatic Mg2+ exporter, CNNM4-related Mg2+ flux in hepatocytes was assessed in vitro. Fluorescent staining with the ratiometric probe MagS was used to estimate the relative levels of cytosolic free Mg2+ in live cells treated with siCnnm4. MagS is an analogue of Mag-FURA-2, developed by the Buccela group,26 which displays similar metal recognition but enhanced optical properties. Furthermore, taking into consideration the key role of mitochondria in hepatocyte function,27,28 we sought to explore the mitochondrial Mg2+ levels in the aforementioned siCnnm4 conditions. For this purpose, we developed MagS-TPP, a targeted variant functionalised with a phosphonium group for delivery to the mitochondrial matrix (Fig. 3B). The new dye exhibits a selectivity profile similar to MagS and binds to Mg2+ with an apparent dissociation constant suitable for the detection of typical intracellular concentrations of free Mg2+ (Fig. S3).
Mitochondrion- and non-targeted fluorescent indicators were applied, revealing an increase in both cytosolic and mitochondrial free Mg2+ levels in cells with reduced Cnnm4 expression (Fig 3C), combined with a decrease in the cation in the extracellular medium (Fig 3D). The silencing of other Cnnm family members did not lead to significant alterations in intra- or extracellular Mg2+ (Fig. 3E and F), thus eliminating their possible contribution to Mg2+ homeostasis in the hepatocyte. These results support the notion that the observed decrease in serum Mg2+ of mice treated with siCnnm4 could be a consequence of its accumulation in the liver (Fig. 3G).
CNNM4-mediated magnesium accumulation reduces lipid content
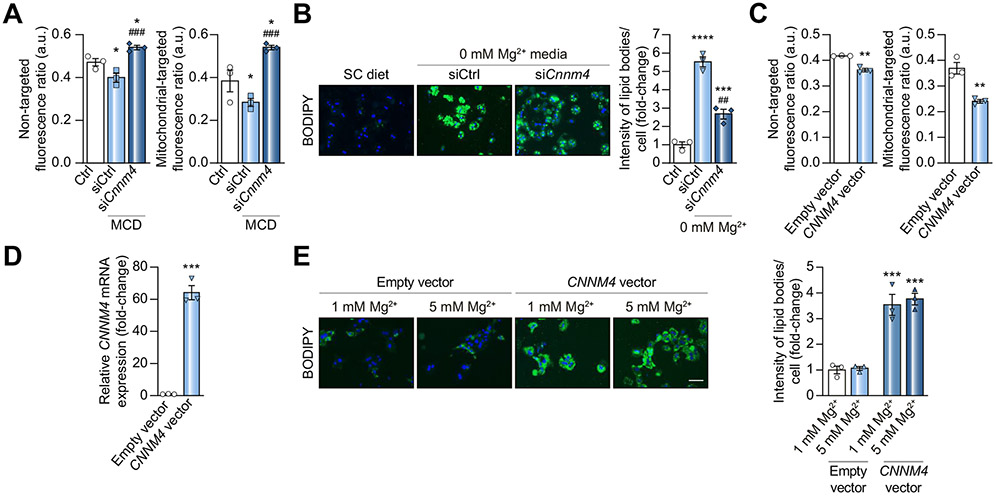
The association between CNNM4 and magnesium efflux prompted us to characterise the contribution of Mg2+ homeostasis to NASH development. The relative content of the cation in primary hepatocytes under MCD conditions was determined, showing an MCD-induced decrease in Mg2+ and an increase upon silencing Cnnm4 (Fig. 4A). Taking the latter into consideration, a possible inverse relationship between hepatic Mg2+ and lipid content was investigated. Magnesium depletion in the cell medium (0 mM Mg2+) resulted in an increased hepatocyte lipid content, while silencing Cnnm4 reduced this effect (Fig. 4B). In line with the results of a clinical trial addressing the beneficial properties of magnesium,4 Mg2+ supplementation (5 mM Mg2+) reverted MCD-induced lipid accumulation in hepatocytes without effects under normal conditions (1 mM Mg2+) (Fig. S4A). Neither Mg2+ depletion nor supplementation affected Cnnm4 expression (data not shown).
Fig. 4. Modulation of intracellular lipid content by CNNM4.
(A) Relative intracellular magnesium determination by MagS and MagS-TPP staining in primary hepatocytes under MCD stimulation and treated with a Cnnm4 siRNA (siCnnm4). (B) BODIPY staining micrographs and quantification in murine hepatocytes maintained for 24 h under magnesium depletion (0 mM Mg2+) and transfected with siCnnm4. (C) Relative intracellular magnesium determination and (D) Cnnm4 mRNA levels in primary hepatocytes transfected for 6 h with an empty or CNNM4 vector. (E) BODIPY staining micrographs and respective quantification in isolated mouse hepatocytes transfected for 6 h with an empty/CNNM4 expression vector and stimulated for 24 h with magnesium (5 mM Mg2+) vs. a control group (1 mM Mg2+). Scale bar corresponds to 100 μm. *p <0.05, **p <0.01, ***p <0.001, and ****p <0.0001 vs. Ctrl/Empty vector; ##p <0.01 and ###p <0.001 vs. MCD + siCtrl/0 mM Mg2+ + siCtrl. CD-HFD, choline-deficient high-fat diet; CNNM4, cyclin M4; MagS, magnesium-specific; MagS-TPP, mitochondrion-targeted magnesium-specific; MCD, methionine and choline deficient; siRNA, small interfering RNA.
To eliminate the possibility of other Mg2+ transporters contributing to siCnnm4-induced lipid reduction, their expression was characterised under various conditions. We observed an increase in the expression of Transient Receptor Protein Melastatin 6 (Trpm6) (Fig. S4B), suggesting that the lipid reduction may also be the result of increased Mg2+ entry. As there is no commercial inhibitor for TRPM6, its isoform 7 was inhibited with 2-aminoethyl diphenyl borinate (2-APB)29 to further investigate the contribution of Mg2+ entry. We observed that the lipid accumulation induced by inhibiting Mg2+ import was normalised by siCnnm4 (Fig. S4C). Finally, the functional relationship between cellular Mg2+ levels and steatosis was investigated by overexpressing CNNM4 via transient transfection, which reduced Mg2+ levels (Fig. 4C and D) and raised lipid contents in hepatocytes (Fig. 4E). Remarkably, Mg2+ supplementation did not attenuate CNNM4-induced lipid accumulation (Fig. 4D and E).
Endoplasmic reticulum and oxidative stress are reduced by silencing cyclin M4
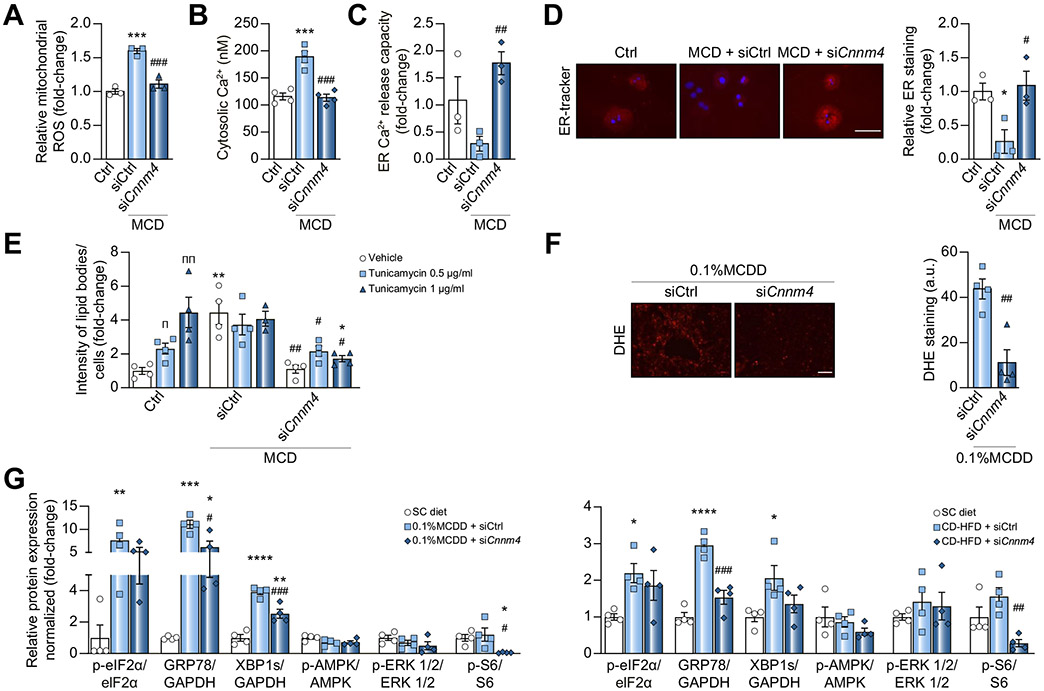
Considering that ROS production and inflammation are major drivers of NASH progression,10,28 mitochondrial ROS were assessed in primary hepatocytes, showing a reduction in MCD-induced ROS production upon silencing Cnnm4 (Fig. 5A). The development of ER stress is also considered a ‘second hit’, linked to oxidative stress30 and with an existing Mg2+ flux between the ER and mitochondria.31 Thus, we hypothesised that ER integrity might be affected by CNNM4-induced Mg2+ fluctuations in NASH. Therefore, cytosolic calcium (Ca2+), an ER-stress indicator, was quantified in primary hepatocytes. The increased [Ca2+]cytosol observed under MCD stimulation and siRNA-derived attenuation (Fig. 5B), together with the partial co-localisation of Mg2+ and ER (Fig. S5A), suggested a protective effect of silencing Cnnm4, not only for mitochondria, but also for the ER. This was further addressed by characterising the release of Ca2+ from the ER by stimulating primary hepatocytes with ATP, reported to promote the P2Y receptor-mediated release of Ca2+ into the cytosol.32 Interestingly, an MCD-induced decreased capacity and then normalisation upon silencing Cnnm4 were observed (Fig. 5C), while ER labelling was decreased under the MCD and recovered by Cnnm4 siRNA (Fig. 5D). The relationship between ER stress and hepatic lipid content was elucidated by treating primary hepatocytes with tunicamycin, an ER-stress inducer.33 A dose-dependent lipid accumulation was observed after 24 h of treatment, while siCnnm4 led to a decrease in lipid content (Fig. 5E and Fig. S5B).
Fig. 5. Specific Cnnm4 silencing reduces oxidative and ER stress in in vitro and in vivo NASH models.
(A) Relative mitochondrial ROS levels, (B) cytosolic Ca2+ levels, (C) Ca2+ release capacity of ER over time and (D) micrographs of ER-tracker red staining and quantification in primary hepatocytes cultured for 24 h in a MCD medium and transfected with a Cnnm4 (siCnnm4) or control (siCtrl) siRNA. Scale bar corresponds to 100 μm. (E) BODIPY determination in primary hepatocytes treated for 24 h with tunicamycin, MCD, or Ctrl medium and treated with siCnnm4 or siCtrl. (F) Representative micrographs (scale bar corresponds to 50 μm) and DHE staining for liver from mice fed a 0.1% MCDD and treated with siCnnm4 or siCtrl. (G) Western blot analysis of binding immunoglobulin protein (BIP/GRP78), x-box binding protein 1 isoform s (XBP1s), phosphoSer51-eukaryotic initiation factor 2α (eIF2α), phosphoThr172-AMP dependent protein kinase (AMPK), phosphoThr202/Thyr204-ERK1/2, and phosphoSer235/236-S6 ribosomal protein (S6). Mouse livers from different groups were compared: healthy (SC diet), 0.1% MCDD, or CD-HFD treated with siCnnm4 or siCtrl. *p <0.05, **p <0.01, ***p <0.001, and ****p <0.0001 vs. Ctrl/Ctrl + vehicle/SC diet; #p <0.05, ##p <0.01, and ###p <0.001 vs. MCD + siCtrl/0.1% MCDD + siCtrl/MCD + vehicle + siCtrl/CDHFD + siCtrl; πp <0.05 and ππp <0.01 for vehicle vs. tunicamycin. CD-HFD, choline-deficient high-fat diet; CNNM4, cyclin M4; DHE, dihydroxyethyl; ER, endoplasmic reticulum; MCD, methionine and choline deficient; MCDD, MCD diet; NASH, non-alcoholic steatohepatitis; ROS, reactive oxygen species; SC, regular diet; siRNA, small interfering RNA.
When characterising ROS production in vivo, siCnnm4-treated mice showed reduced dihydroxiethidium (DHE) staining (Fig. 5F), probably as a result of the oxidative activity measured by various pathways (Fig. S5C–F). Although oxidative stress was not determined in the study with CD-HFD mice, the observed reduction in fatty acid oxidation (Fig. S5G) is consistent with a ROS reduction. The regulation of the oxidative stress response prompted us to evaluate ER stress in the in vivo models of NASH using different markers,34 demonstrating upregulation in both NASH models and reduction under siCnnm4, suggesting that the latter diminished the ER-stress response (Fig. 5G and Fig. S5G). ER-stress associated signalling pathways were also evaluated, showing a decreased activation of S6 ribosomal protein (S6) by phosphorylation.35
CNNM4 inhibition increases MTP activity, promoting VLDL secretion
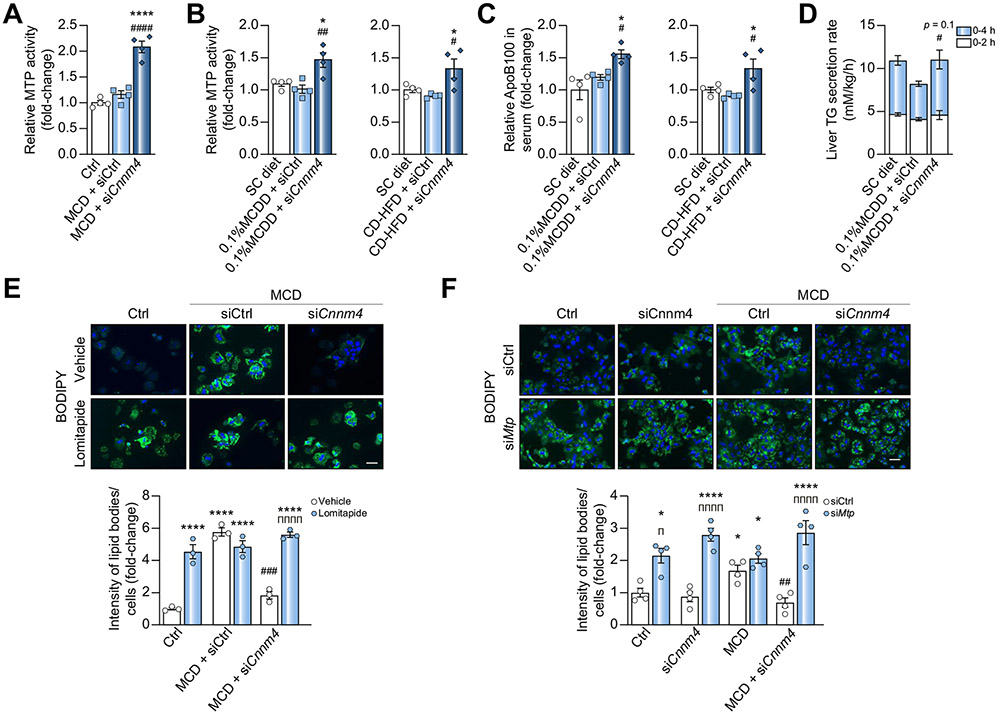
The formation of pre-VLDL, the first step of VLDL assembly, takes place in the ER.36 During this step, a small number of triglycerides (TGs) are associated with an apolipoprotein B (APOB) molecule and embedded in a phospholipid monolayer by the microsomal triglyceride transfer protein (MTP).36 The alterations of ER integrity previously observed under MCD and the reversion of such by siCnnm4 suggested a possible modulation of MTP activity. Remarkably, the protein was increased upon silencing Cnnm4 in both the primary hepatocytes (Fig. 6A) and in vivo rodent NASH models (Fig. 6B).
Fig. 6. Inhibition of CNNM4 expression promotes lipid export by activating MTP activity.
(A) Relative MTP activity in primary hepatocytes cultured for 24 h in a MCD medium and transfected with a Cnnm4 siRNA (siCnnm4) or unrelated control (siCtrl). (B) Relative MTP activity in livers from mice fed a 0.1% MCDD or a CD-HFD and treated with siCnnm4 or siCtrl. (C) Relative apolipoprotein B100 (APOB100) levels in sera from mice fed a 0.1% MCDD or CD-HFD and treated with siCnnm4 or siCtrl. (D) Liver TG secretion rate in mice fed a 0.1% MCDD, treated with siCnnm4 or siCtrl and after poloxamer P407 administration. BODIPY staining micrographs and quantification of primary hepatocytes cultured under MCD and treated with siCtrl or siCnnm4 and (E) a vehicle (DMSO) or 600 nM lomitapide for 24 h or (F) an siRNA against Mtp (siMtp). Scale bar corresponds to 100 μm. *p <0.05 and ****p <0.0001 vs. SC diet/Ctrl + vehicle/Ctrl + siCtrl; #p <0.05, ##p <0.01, ###p <0.001, and ####p <0.0001 vs. 0.1% MCDD + siCtrl/CD-HFD + siCtrl/MCD + vehicle + siCtrl/MCD + siCtrl; πp <0.05 and ππππp <0.0001 for vehicle/siCtrl vs. lomitapide/siMtp. CD-HFD, choline-deficient high-fat diet; MCD, methionine and choline deficient; MCDD, MCD diet; MTP, microsomal triglyceride transfer protein; SC, regular diet; siRNA, small interfering RNA; TG, triacylglyceride.
The serum TGs of mice fed a 0.1% MCDD were found to decrease, owing to intrahepatic lipid accumulation, and were partially restored by Cnnm4 siRNA (Fig. S6A), suggesting an improved VLDL secretion. Therefore, we determined the relative APOB100 concentrations in the serum, an indicator of circulating lipoprotein particles, observing an increase under siCnnm4 conditions (Fig. 6C and Fig. S6B). The contribution of increased lipid secretion mediated by VLDL was further confirmed by measuring APOB100 in serum extracted directly from the cava vein, with VLDLs as the main component, showing an increased APOB100 content (Fig. S6C). The hepatic TG secretion rate, as well as the VLDL content, were determined after administering poloxamer P407 to inhibit lipoprotein lipase.37 Remarkably, both the secretion rate and the VLDL lipid content tended to increase upon silencing Cnnm4 (Fig. 6D and Fig. S6D). MTP was then inhibited in primary hepatocytes by 24-h lomitapide incubation, a selective inhibitor,38 or with a specific siRNA (siMtp). As expected, the lipid reduction observed upon CNNM4 knockdown was absent under lomitapide stimulation (Fig. 6E) or Mtp silencing conditions (Fig. 6F and Fig S6E).
The possible atherogenic secondary effects are a concern when it comes to promoting VLDL secretion. Furthermore, when determining the Mg2+ content in isolated VLDL, the observed restoration in the group treated with the Cnnm4 siRNA (Fig. S7A) suggested a possible impact on the WAT. The measurement of the relative fatty acid oxidation (FAO) capacity of WAT revealed a tendency to increase with the siRNA treatment (Fig. S7B), together with an increased expression of genes involved in lipid oxidation, mitochondrial biogenesis, and thermogenesis (Fig. S7C). This result was corroborated in primary WAT adipocytes, where Mg2+ supplementation increased glycerol production, an indicator of lipolysis without subsequent non-esterified fatty acid (NEFA) production (Fig. S7D). The same result was observed with conditioned medium obtained from primary hepatocytes cultured under MCD and siCnnm4 conditions (Fig. S7E).
The siRNA conjugation with GalNAc offers a potential therapy
Considerable progress has recently been made in the development of oligonucleotide-based therapeutics, whereby the silencing of target genes is being explored in clinical studies.39 To transfer the presented discoveries into a therapeutic approach suitable for clinical development, a newly identified Cnnm4 siRNA candidate was conjugated to an N-acetylgalactosamine (GalNAc) cluster that binds to the asialo-glycoprotein receptors predominantly expressed in hepatocytes. This technology offers a potentially safe, specific, and efficient mode of delivery for targeting therapeutic molecules to hepatocytes.40
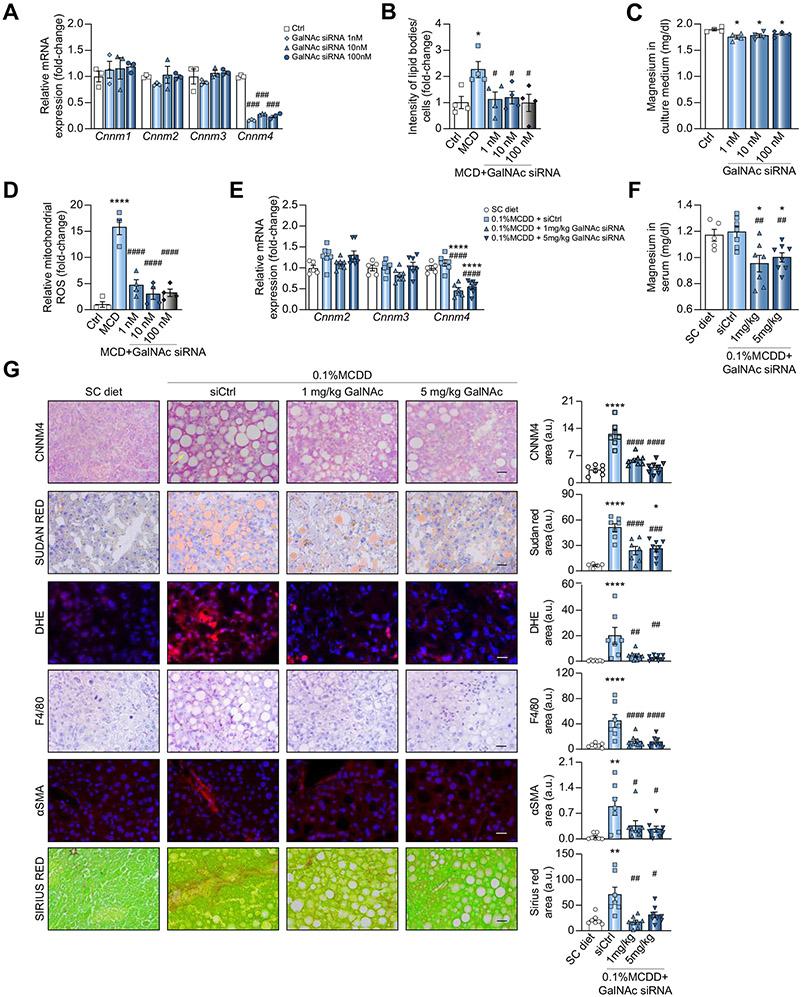
The specific inhibition of Cnnm4 was achieved in primary hepatocytes through receptor-mediated uptake by adding different doses of a GalNAc-conjugated siRNA against Cnnm4 (GalNAc siRNA) directly to the culture medium (Fig. 7A). The lipid accumulation induced by MCD medium was reduced by the GalNAc siRNA treatment (Fig. 7B), and Mg2+ accumulation in hepatocytes was observed indirectly through its decrease in the extracellular medium (Fig. 7C). ROS overproduction upon MCD stimulation was also reduced by treating the hepatocytes with the conjugate (Fig. 7D).
Fig. 7. Inhibition of CNNM4 expression by GalNAc)–siRNA conjugate reduces NASH in preclinical models.
Levels of (A) Cnnm1-3 mRNA and (C) magnesium in extracellular medium from primary hepatocytes treated overnight with a GalNAc-conjugated siRNA against Cnnm4 (GalNAc siRNA) and a MCD medium. Relative determination of (B) lipid accumulation and (D) mitochondrial ROS production in primary hepatocytes treated with an MCD and Ctrl or GalNAc siRNA. Relative levels of (E) Cnnm1-4 mRNA and (F) Mg2+ in serum, and (G) histological characterisation of CNNM4, lipids (Sudan Red), inflammation (F4/80 and DHE) and fibrosis (αSMA and Sirius Red) in livers from mice fed a 0.1% MCDD and treated with siCtrl or GalNAc siRNA. *p <0.05, **p <0.01, and ****p <0.0001 vs. Ctrl/SC diet; #p <0.05, ##p <0.01, ###p <0.001, and ####p <0.01 vs. MCD + siCtrl/0.1% MCDD + siCtrl. CD-HFD, choline-deficient high-fat diet; CNNM4, cyclin M4; DHE, dihydroxyethyl; GalNAc, N-acetylgalactosamine; MCD, methionine and choline deficient; MCDD, MCD diet; NASH, non-alcoholic steatohepatitis; ROS, reactive oxygen species; siRNA, small interfering RNA.
Finally, the effectiveness of Cnnm4 GalNAc siRNA was evaluated in vivo in mice fed a 0.1% MCDD for 6 weeks, leading to a more severe NASH phenotype.21 The treatment was initiated at the 3-week point with a single injection of different doses (1 or 5 mg/kg) of Cnnm4 GalNAc siRNA (0.1% MCDD + GalNAc siRNA) or a control siRNA conjugate (0.1% MCDD + siCtrl). A specific and significant inhibition of Cnnm4 mRNA expression was detected in mice administered either 1 or 5 mg/kg (Fig. 7E), while the Mg2+ levels in serum were reduced in line with a presumed hepatic Mg2+ accumulation (Fig. 7F). Importantly, CNNM4 inhibition by GalNAc siRNA significantly reduced hepatic lipid accumulation and alleviated the inflammatory response and fibrosis development (Fig. 7G).
Discussion
The studies described herein pinpoint CNNM4 as a key regulator of Mg2+ homeostasis in hepatocytes and a potential therapeutic target for NASH. To date, research performed on this cation in liver pathologies has shown a protective effect of Mg2+ supplementation4 and revealed Mg2+ deficiencies in patients with cirrhosis or liver cancer.18 Indeed, hypomagnesaemia is frequently observed in NASH comorbidities.6,7 However, there is still a dearth of knowledge on the implications of Mg2+ perturbations for the development of NASH, and nothing has been reported about magnesiotropic proteins and their modulation in the liver.
Previous studies have mainly focused on PRL, the interacting partner of CNNM, associating its expression with poor prognosis in multiple cancer types including liver,16,17 whereas studies of CNNMs have been limited to their role in transporting Mg2+ across epithelia.25 Herein, we demonstrate that CNNM4 is a contributor to NASH, as it is overexpressed in preclinical models and clinical samples of the pathology. The reduction in CNNM4 expression in THLE-2 cells upon inhibiting NEDDylation suggests the involvement of post-translational mechanisms in the modulation of the stability of CNNM4 during NASH. This is further confirmed by the reduction of MCD-induced lipid accumulation by the specific silencing of Cnnm4, which does not happen upon silencing other Cnnms. Regarding the effect of targeting Cnnm4 on NASH development, preclinical studies in rodent models showed promising effects of an siRNA-based therapy in mice fed either a 0.1% MCDD or CD-HFD. Significantly, both diets increase lipid accumulation and fibrosis development, but the siRNA therapy ameliorates both hallmarks of the disease. The role that CNNM4 may play in other cell populations such as hepatic stellate cells requires more research, as do the possible post-transcriptional and post-translational mechanisms that may contribute to CNNM4 overexpression.
The role of the hepatocyte in NASH and its subsequent progression has been widely characterised.27,28 The silencing of Cnnm4 may reduce oxidative activity, leading to reduced oxidative stress, which could contribute to a reduction in fibrosis development. Although the development of oxidative stress was not characterised in the CD-HFD in vivo study, the observed reduction in FAO and lipid contents prompts to expect a similar reduction. The relevance of the hepatocyte in NASH progression and the implications of modulating CNNM4 were further characterised in mice fed a 0.1% MCDD for 6 weeks. Herein, we also demonstrated that targeting CNNM4 ameliorated NASH, even that with a more severe phenotype.21 To develop a possible therapeutic approach, an siRNA was conjugated with GalNAc allowing stable and liver-specific delivery,40 with results similar to those observed when targeting Cnnm4 with the liposomal siRNA formulation after 4 weeks on the 0.1% MCDD.
Although CNNM4 has been characterised as a Mg2+ transporter in kidney epithelia,41 the CNNM4-mediated flux of the cation in the liver remains unknown. Our research shows CNNM4 to be a Mg2+ exporter in the hepatocyte, as its specific silencing increases intracellular Mg2+. Hepatic siCnnm4-induced Mg2+ accumulation was characterised in all the preclinical studies through the quantification of the cation in serum, currently the only feasible method because of the lack of available tools for directly measuring hepatic Mg2+ in vivo. CNNM4 overexpression in the clinical samples correlated with the increased Mg2+ serum levels observed. The possible contribution of other, non-liver-related factors was eliminated by the BMI and age pairing of the samples. Similarly to clinical NASH, increased serum levels of the cation were observed in mice fed a CD-HFD, whereas in the 0.1% MCDD model, the lower Mg2+ content in the diet may have masked the expected CNNM4-induced increase in serum. The possible involvement of Mg2+ content in NASH-inducing diets might be a topic of interest for future research.
The finding that hepatocyte Mg2+ content is inversely correlated with lipid accumulation also suggests a possible role of the cation in NASH. Decreases in intracellular Mg2+, whether induced by MCD stimulation or transfection with the CNNM4 vector, were accompanied by increased lipid accumulation. Accordingly, Mg2+-depleted culture medium also increased hepatocyte lipid content. Remarkably, the action of CNNM4 in modulating Mg2+ homeostasis prevails over that of other transporters, as the inhibition of Mg2+ import with 2-APB29 leads to an expected lipid accumulation, which is reverted under siCnnm4 treatment. Mg2+ supplementation decreases hepatocyte lipid content under MCD stimulation, whereas under vector-induced CNNM4 overexpression, such supplementation is not sufficient to counteract the increase. These observations suggest the relevance of not only adequate Mg2+ intake, but also the correct balance of the proteins implicated in its homeostasis, such as CNNM4.1,2
Combined with oxidative stress, ER stress has been characterised as an additional driver of NASH progression,10,14 with a demonstrated connection between these 2 ‘hits’.30 In our study, the characterised MCD-induced development of ER stress was reversed by silencing Cnnm4. Despite the current lack of effective methods for determining the Mg2+ content in the ER, the partial co-location observed and the recently characterised Mg2+ flux between the ER and mitochondria31 suggest that the induced Mg2+ accumulation somehow protects the hepatocyte from ER-stress development. Likewise, the development of ER stress by hepatocytes upon tunicamycin stimulation induces lipid accumulation in a dose-dependent manner, and siCnnm4 reduced this effect. In vivo, the ER stress response was shown to increase under NASH development and be attenuated upon silencing Cnnm4, a phenomenon involving the phosphorylation-mediated activation of S6 and independent of other pathways such as AMP-dependent protein kinase or extracellular signal-related protein kinase 1/2.
The reduction of ER stress under Cnnm4 knockdown might be the cause of the increased MTP activity observed. The MTP is located in the ER and catalyses the first step of pre-VLDL formation and transfer to the Golgi for its maturation,36 whereas APOB100 is co-translationally expressed while MTP is functioning.42 Thus, increased MTP activity is accompanied by increased APOB100 in the serum. These findings, together with the restoration of TGs in the serum of treated 0.1% MCDD-fed mice and lipid content in secreted VLDLs, suggest that Cnnm4 knockdown may increase MTP activity in a direct or indirect manner and, consequently, promote VLDL secretion from the liver. This is further supported by the observation that MTP inhibition in hepatocytes blocked the reduction of lipid accumulation by Cnnm4 siRNA treatment. The possible implications of other pathways involved in lipid resolution and the Mg2+-induced changes in metabolic pathways require further investigation.
Interestingly, secondary atherogenic effects from targeting Cnnm4 are not expected as TGs are restored to normal levels rather than above. Moreover, the hepatic Mg2+ accumulation leads to the restoration of the cation in secreted VLDLs, which may promote their catabolism by WAT, as the cation acts as a cofactor in reactions involving ATP.3 Thus, Mg2+ restoration may promote the lipolytic and FAO activities of WAT, as the increase in glycerol production upon stimulating primary adipocytes with Mg2+ or conditioned medium from primary hepatocytes is not accompanied by increased NEFA production. Indeed, the expression in the WAT of several genes involved in lipid catabolism increased in the group of mice treated with the Cnnm4 siRNA. Although the effect of CNNM4 on the oxidative activity of WAT needs to be investigated more deeply, the present study points out a possible anti-atherogenic effect of silencing Cnnm4.
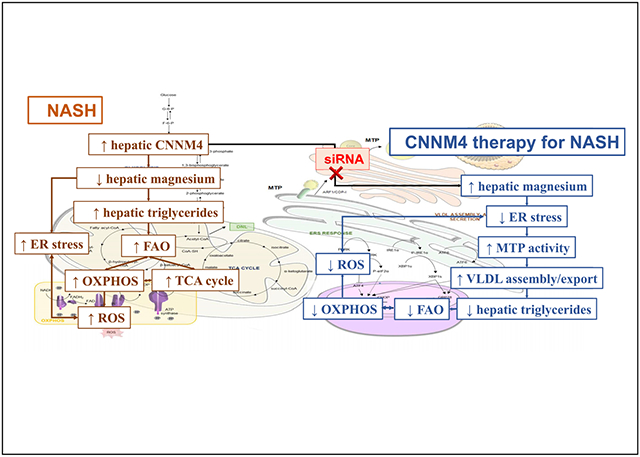
In summary, CNNM4 appears to be a feasible candidate for developing therapies against NASH. CNNM4-based therapy induces hepatic Mg2+ and, consequently, reduces steatosis and other hallmarks of the disease such as oxidative or ER stress, while reducing fibrosis. Additionally, ER recovery might lead to increased MTP activity, promoting VLDL formation, increasing hepatic lipid export, and reducing lipid accumulation (see the Graphical Abstract). Furthermore, the possibility of conjugating the siRNA with a GalNAc molecule and the observed effectiveness in ameliorating NASH, even that of a severe phenotype, points to CNNM4 as an attractive hepatocyte target for treatment.
Supplementary Material
Highlights.
CNNM4 acts as a magnesium exporter in the liver. Its upregulation in NASH leads to elevated magnesium levels in serum.
Liver-specific CNNM4 targeting alleviates steatosis, inflammation, and fibrosis in preclinical NASH models.
siRNA-mediated CNNM4 downregulation promotes hepatic magnesium accumulation and reduces endoplasmic reticulum stress.
Silencing CNNM4 enhances microsomal triglyceride transfer protein activity leading to VLDL assembly and secretion.
Acknowledgments
Financial support
Ministerio de Ciencia e Innovación, Programa Retos-Colaboración RTC2019-007125-1 (for JS and MLM-C); Instituto de Salud Carlos III, Proyectos de Investigación en Salud DTS20/00138 (for JS and MLM-C); Departamento de Industria del Gobierno Vasco (for MLM-C); Ministerio de Ciencia, Innovación y Universidades MICINN: SAF2017-87301-R and RTI2018-096759-A-100 integrado en el Plan Estatal de Investigación Cientifica y Técnica y Innovación, cofinanciado con Fondos FEDER (for MLM-C and TCD, respectively); BIOEF (Basque Foundation for Innovation and Health Research); EITB Maratoia BIO15/CA/014; Asociación Española contra el Cáncer (MLM-C, TCD); Fundación Científica de la Asociación Española Contra el Cancer (AECC Scientific Foundation) Rare Tumor Calls 2017 (for MLM); La Caixa Foundation Program (for MLM); Fundacion BBVA UMBRELLA project (for MLM); BFU2015-70067-REDC, BFU2016-77408-R, and BES-2017-080435 (MINECO / FEDER, UE) and the FIGHT-CNNM2 project from the EJP RD Joint Transnational Call (JTC2019) (Ref. AC19/00073) (for LAM-C); RTI2018-095134-B-100 and Grupos de Investigación del Sistema Universitario Vasco (IT971-16) (for PA); National Institutes of Health under grant CA217817 (for DB); AGL2014-54585-R, AGL-2017-86927-R and EQC2018-004897-P from MINECO; PC0148-2016-0149 and PAI-BIO311 from Junta de Andalucía (for FM). Ciberehd_ISCIII_MINECO is funded by the Instituto de Salud Carlos III. We thank Silence Therapeutics plc. for the financial support provided. We thank MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV-2016-0644).
Abbreviations
- 0.1% MCDD
choline-deficient diet with 0.1% methionine
- 2-APB
2-aminoethyl diphenyl borinate
- αSMA
alpha-smooth muscle actin
- ALT
alanine aminotransferase
- AMPK
AMP-dependent protein kinase
- APOB
apolipoprotein B
- BIP/GRP78
binding immunoglobulin protein
- CD-HFD
choline-deficient, high-fat diet
- CNNM4
cyclin M4
- CNNM
cyclin M family
- DHE
dihydroxiethidium
- eIF2α
(eukaryotic initiation factor 2α)
- ER
endoplasmic reticulum
- ERK 1/2
extracellular signal-related protein kinase 1/2
- FAO
fatty acid oxidation
- MagS
magnesium-specific
- MagS-TPP
mitochondrion-targeted magnesium-specific
- MCD
methionine and choline-deficient
- MTP
microsomal triglyceride transfer protein
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NEFA
non-esterified fatty acid
- GalNAc
N-acetylgalactosamine
- PRLs
phosphatases of regenerating liver
- ROS
reactive oxygen species
- S6
S6 ribosomal protein
- SC diet
regular diet
- siRNA
small interfering RNA
- Trpm6
transient receptor protein melastatin 6
- TGs
triacylglycerides
- WAT
white adipose tissue
- XBP1s
x-box binding protein 1 isoform s
Footnotes
Conflicts of interest
The authors declare no conflicts of interest related to this submitted work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.01.043.
Data availability statement
The data that support the findings of this study are available from the corresponding author, M.L.M.-C. upon reasonable request.
References
- [1].Jahnen-dechent W, Ketteler M. Magnesium basics. Clin Kidney J 2012;5:i3–i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Razzaque MS. Magnesium: are we consuming enough? Nutrients 2018;10:1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].De Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev 2015;1920:1–46. [DOI] [PubMed] [Google Scholar]
- [4].Wu L, Zhu X, Fan L, Kabagambe EK, Song Y, Tao M, et al. Magnesium intake and mortality due to liver diseases: results from the third national health and nutrition examination survey cohort. Sci Rep 2017;7:17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barbagallo M, Di Bella G, Brucato V, D’Angelo D, Damiani P, Monteverde A, et al. Serum ionized magnesium in diabetic older persons. Metabolism 2014;63:502–509. [DOI] [PubMed] [Google Scholar]
- [6].Rosique-Esteban N, Guasch-Ferré M, Hernández-Alonso P, Salas-Salvadó J. Dietary magnesium and cardiovascular disease: a review with emphasis in epidemiological studies. Nutrients 2018;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gonzalez L, Rodrı H, Rodrı M. Hypomagnesemia, insulin resistance, and non-alcoholic steatohepatitis in obese subjects. Arch Med Res 2005;36:362–366. [DOI] [PubMed] [Google Scholar]
- [8].Casas-Grajales S, Muriel P. Antioxidants in liver health. World J Gastro-intest Pharmacol Ther 2015;6:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [10].Sanyal AJ. Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2005;2:46–53. [DOI] [PubMed] [Google Scholar]
- [11].Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci 1998;23:10–14. [DOI] [PubMed] [Google Scholar]
- [12].Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 2005;569:29–63. [DOI] [PubMed] [Google Scholar]
- [13].Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009;58:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 2008;134:568–576. [DOI] [PubMed] [Google Scholar]
- [15].Giménez-Mascarell P, González-Recio I, Fernández-Rodríguez C, Oyenarte I, Müller D, Martínez-Chantar ML, et al. Current structural knowledge on the CNNM family of magnesium transport mediators. Int J Mol Sci 2019;20:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Daouti S, Li W, Qian H, Huang K-S, Holmgren J, Levin W, et al. A selective phosphatase of regenerating liver phosphatase inhibitor suppresses tumor cell anchorage-independent growth by a novel mechanism involving p130Cas cleavage. Cancer Res 2008;68:1162–1169. [DOI] [PubMed] [Google Scholar]
- [17].Gulerez I, Funato Y, Wu H, Yang M, Kozlov G, Miki H, et al. Phosphocysteine in the PRL-CNNM pathway mediates magnesium homeostasis. EMBO Rep 2016;17:1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu M, Yang H, Mao Y. Magnesium and liver disease. Ann Transl Med 2019;7:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen YS, Kozlov G, Fakih R, Funato Y, Miki H, Gehring K. The cyclic nucleotide-binding homology domain of the integral membrane protein CNNM mediates dimerization and is required for Mg(2+) efflux activity. J Biol Chem 2018;293:19998–20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iruarrizaga-lejarreta M, Varela-Rey M, Fernandez-Ramos D, Martinez-Arranz I, Delgado TC, Simon J, et al. Role of aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun 2017;1:911–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Simon J, Nuñez-García M, Fernández-Tussy P, Barbier-Torres L, Fernández-Ramos D, Gómez-Santos B, et al. Targeting hepatic glutaminase 1 ameliorates non-alcoholic steatohepatitis by restoring very-low-density lipoprotein triglyceride assembly. Cell Metab 2020;31:605–622. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zubiete-Franco I, Fernandez-Tussy P, Barbier-Torres L, Simon J, Fernandez-Ramos D, Lopitz-Otsoa F, et al. Deregulated neddylation in liver fibrosis. Hepatology 2017;65:694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, et al. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr 2018;18:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549–564. [DOI] [PubMed] [Google Scholar]
- [25].Yamazaki D, Miyata H, Funato Y, Fujihara Y, Ikawa M, Miki H. The Mg2+ transporter CNNM4 regulates sperm Ca2+ homeostasis and is essential for reproduction. J Cell Sci 2016;129:1940–1949. [DOI] [PubMed] [Google Scholar]
- [26].Afzal MS, Pitteloud J-P, Buccella D. Enhanced ratiometric fluorescent indicators for magnesium based on azoles of the heavier chalcogens. Chem Commun 2014;50:11358–11361. [DOI] [PubMed] [Google Scholar]
- [27].Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 2012;52:59–69. [DOI] [PubMed] [Google Scholar]
- [28].Day CP. From fat to inflammation. Gastroenterology 2006;130:207–210. [DOI] [PubMed] [Google Scholar]
- [29].Chokshi R, Fruasaha P, Kozak JA. 2-Aminoethy diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channel 2012;6:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zeeshan HMA, Lee GH, Kim H-R, Chae H-J. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci 2016;17:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Daw CC, Ramachandran K, Enslow BT, Maity S, Bursic B, Novello MJ, et al. Lactate elicits ER-mitochondrial Mg2+ dynamics to integrate cellular metabolism. Cell 2020;183:474–489. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wan H-X, Hu J-H, Xie R, Yang S-M, Dong H. Important roles of P2Y receptors in the inflammation and cancer of digestive system. Oncotarget 2016;7:28736–28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen J, et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res 2018;37:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011;54:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Park H-W, Park H, Ro S-H, Jang I, Semple IA, Kim DN, et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun 2014;5:4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Olofsson S, Stillemark-billton P, Asp L. Intracellular assembly of VLDL two major steps in separate cell compartments. Trends Cardiovasc Med 2000;10:338–345. [DOI] [PubMed] [Google Scholar]
- [37].Millar JS, Cromley DA, Mccoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res 2005;46:2023–2028. [DOI] [PubMed] [Google Scholar]
- [38].Sirtori CR, Pavanello C, Bertolini S, Sirtori CR, Pavanello C, Bertolini S. Microsomal transfer protein (MTP) inhibition – a novel approach to the treatment of homozygous hypercholesterolemia. Ann Med 2014;46:474. [DOI] [PubMed] [Google Scholar]
- [39].Weng Y, Xiao H, Zhang J, Liang X-J, Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol Adv 2019;37:801–825. [DOI] [PubMed] [Google Scholar]
- [40].Altamura S, Schaeper U, Dames S, Löffler K, Eisermann M, Frauendorf C, et al. SLN124, a GalNAc-siRNA conjugate targeting TMPRSS6, efficiently prevents iron overload in hereditary haemochromatosis type 1. Hemasphere 2019;3:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hirata Y, Funato Y, Takano Y, Miki H. Mg2+-dependent interactions of ATP with the cystathionine-beta-synthase (CBS) domains of a magnesium transporter. J Biol Chem 2014;289:14731–14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gordon DA, Wetterau JR, Gregg RE. Microsomal triglyceride transfer protein: a protein complex required for the assembly of lipoprotein particles. Trends Cell Biol 1995;5:317–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, M.L.M.-C. upon reasonable request.