Abstract
Animal and human studies show that cannabis or its derivatives can increase relapse to cocaine seeking following withdrawal. Moreover, cannabis use in humans is associated with impulse control deficits and animal studies implicate endogenous cannabinoids (eCB) in several impulsivity constructs. However, the brain areas where cannabinoids might control impulsivity or cocaine seeking are largely unknown. Here, we assess Lateral Habenula (LHb) involvement on performance in the 5-choice serial reaction time task (5CSRTT) in rats and investigate whether LHb cannabinoid CB1 receptors (CB1R) are involved in these effects. Systemic cocaine increased premature responding, a measure of impulsivity, at a dose (5 mg/kg) that did not alter other measures of task performance. Intra-LHb infusion of the CB1R antagonist AM251 blocked this effect. Systemic injection of the psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC, 1mg/kg), also increased 5CSRTT premature responding at a dose that did not otherwise disrupt task performance. This was blocked by intra-LHb infusion of AM251 in a subgroup of rats showing the largest increases in Δ9-THC-evoked premature responses. Systemic Δ9-THC also prompted impulsive cocaine seeking in a Go/NoGo cocaine self-administration task and this was blocked by intra-LHb AM251. These data show that LHb CB1Rs are involved in deficits in impulse control initiated by cocaine and Δ9-THC, assessed by the 5CSRTT, and play a role in impulsive cocaine seeking during self-administration. This suggests that the LHb eCB system contributes to the control of impulsive behavior, and thus represents a potential target for therapeutic treatment of substance use disorders (SUDs) in humans.
Keywords: Cannabinoids, Habenula, Impulsivity, Cocaine, Addiction
1. Introduction
Impulsive action is associated with the development of an addictive phenotype in humans and in animal models (Anker et al., 2009; Argyriou et al., 2018; Belin et al., 2008; Deroche-Gamonet and Piazza, 2014; Economidou et al., 2009; Jentsch and Pennington, 2014; MacKillop et al., 2016; Wit, 2009). Impulsivity can be observed in animals using behavioral paradigms such as Go/NoGo tasks in which reward availability is signaled and inappropriate responses are not rewarded, and in the 5CSRTT in which executive processes such as attention, reaction time, impulsiveness and motivation can be disambiguated. In general, studies like these agree that impulse control is impaired in humans and animals that seek or have been exposed to drugs that can cause SUDs. Moreover, because impulsivity is both predictive of, and affiliated with SUDs, it is seen as a behavioral trait that is an impediment to drug abstinence (Argyriou et al., 2018; Smith et al., 2014).
Although impulse control has largely been ascribed to inhibition of motor output by frontocortical brain regions, much of this information is also conveyed to downstream targets such as the LHb. Thus, the LHb receives input from the anterior insular, cingulate, prelimbic and infralimbic cortices, and in turn, sends its output to all forebrain projecting midbrain monoaminergic nuclei (Lecourtier and Kelly, 2007; Sutherland, 1982). The LHb thereby constitutes a critical link between brain regions processing executive function and those involved in reward and affect. One example of this is in the inhibition of mid-brain dopamine (DA) neurons by the LHb (Christoph et al., 1986; Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007). This occurs via LHb excitatory efferents that target GABAergic rostromedial tegmental nucleus (RMTg) neurons that, in turn strongly inhibit DA neurons (Hong et al., 2011). As LHb neurons are activated by negative events, such as punishment, omission of expected reward, and by conditioned negative stimuli, this circuit permits interaction between cortical areas involved in prediction and planning, and DA neurons with their influence on reward processing (Matsumoto and Hikosaka, 2009, 2007). In addition to this interaction with reward circuitry, overactivation of the LHb is linked to persistent negative affective states (Proulx et al., 2014), likely via connections with basal forebrain and limbic nuclei, and with behavioral flexibility in learning tasks that require adaptation to changes in external contingencies (Baker et al., 2016; Stopper and Floresco, 2013). With relevance to our current work, there is also evidence that the LHb is involved in aspects of impulse control as it relates to drug seeking behavior (Lecourtier and Kelly, 2005; Zapata et al., 2017). Previously, using a Go/NoGo task in rats trained to use a cue to discriminate cocaine availability, we found that inactivation of the LHb increased NoGo responding for cocaine despite its signaled absence (Zapata et al., 2017). Moreover, this inability to withhold responding with LHb inactivation was observed in rats trained to self-administer cocaine, but not in those trained to self-administer sucrose, suggesting that cocaine experience increased the LHb’s role in this form of impulsivity. Although these experiments implicate the LHb in impulsive action in cocaine seeking, it is not known whether other abused drugs similarly interact with LHb function to modify impulse control, nor what mechanisms are involved in these actions.
The eCB system is present in the LHb where it is involved in regulating neuronal excitability and synaptic plasticity (Park et al., 2017; Valentinova and Mameli, 2016). Moreover, exposure to stressful stimuli recruits the LHb eCB system, leading to altered behavioral strategies (Berger et al., 2018), and eCB control of synaptic plasticity is impaired in an animal model of depression (Park et al., 2017). There is also evidence from recent animal studies to support the idea that the eCB system is involved in impulsive action but not in impulsive choice (Ferland et al., 2018). Thus, systemic CB1R antagonists reduce premature responding in the 5CSRTT, a measure of motor impulsivity, suggesting that eCB tone is responsible for promoting impulsive action (Bruin et al., 2011; Pattij et al., 2007). In humans, the involvement of the eCB system in impulsive action is supported by studies showing that acute Δ9-THC, in both occasional and heavy cannabis users, impairs inhibitory impulse control in stop signal tasks (McDonald et al., 2003; Ramaekers et al., 2008; Theunissen et al., 2012).
To explore a possible relationship between LHb eCB function and drug-associated impulsive action we examine the effects of cocaine and Δ9-THC in the 5CSRTT and Go/NoGo task and ask whether LHb CB1Rs are involved in this impulsive behavior.
2. Methods
2.1. Subjects
Male Long Evans rats (Charles River Laboratories, Wilmington, MA) weighing 300 g at the beginning of experiments were housed 2–3 per cage for at least 1 week before use in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. They were maintained in a temperature- and humidity-controlled environment under a reverse 12 hr light/dark cycle. Experiments were conducted during the dark cycle. At least two days before the beginning of the 5CSRTT experiments, rats were single-housed and food-restricted to 90–95% of their free feeding weight and remained under food restriction for the remainder of the experiments. Rats participating in the Go/NoGo experiments had ad libitum food access throughout the experiments. All procedures were approved by the Institutional Care and Use Committee of the NIDA Intramural Research Program, National Institutes of Health (Baltimore, MD), and conducted in accordance with the Guide for the Care and Use of Laboratory Animals provided by the NIH.
2.2. 5 Choice Serial reaction Time Task
Rats were trained in standard 5CSRTT operant boxes (Lafayette Instruments, Lafayette, Indiana) using established procedures (Bari et al., 2008). Rats were habituated to the chamber and trained to collect food pellets (45 mg, Noyes) from the magazine and the nose poke holes for at least 4 daily 30 min sessions or until they were able to collect all the pellets. Training was then begun on a standard 5CSRTT procedure. Each trial was initiated by illumination of a random nose-poke. A response at the signaled nose poke was recorded as an accurate response and resulted in pellet delivery and illumination of the food magazine. If the animal failed to respond during a specified interval (limited hold) a response omission was recorded. If the animal responded at a nose poke position different than the signaled one, an incorrect response was recorded. Both, omissions and incorrect responses, were penalized by a 5 second time out (signaled by illumination of the house light). Retrieval of the pellet or the end of the time out finalized the trial and started an inter trial interval (ITI). Responses during the ITI were recorded as premature responses and triggered a time out and the end of the trial. During training, stimulus duration and limited hold started at 60 seconds and were progressively decreased to 1 and 5 seconds respectively in successive steps. Rats were required to perform 80% accurate responses with less than 20% omissions in order to progress to the next step. The ITI remained constant at 5 seconds, except on test trials where the effect of a longer ITI (7 seconds) was tested. Each daily session lasted 30 minutes or 100 trials (whichever occurred first). Rats were stabilized for at least 10 sessions at the last training step prior to surgery. After surgery, they were retrained for at least 10 sessions until they showed stable responding, followed by the testing phase. The testing phase used a within subject design in which rats were given a systemic drug or a systemic drug + intra-LHb infusion combination before each test session. Drugs were given in a pseudo-random order, except that vehicle or no drug days were alternated with drug treatment days. If omissions increased above 50%, drug testing stopped until stable performance was again established.
Go/NoGo Cocaine intravenous self-administration (IVSA) Task
Operant training took place as described in detail previously (Zapata et al., 2017). Standard rat operant chambers (Med-Associates; St Albans, VT) were used. Rats were trained to self-administered cocaine (0.75 mg/kg /infusion) for 12 sessions (2 hours or 40 infusions per session) on an FR1 schedule. Following this, they were trained on a Go-NoGo task consisting of 2 hour sessions comprised of 6 × 20 min alternating intervals of cocaine availability (Go) and non–availability (NoGo), signaled by the house light (light on during cocaine availability). During Go intervals, responses were reinforced with cocaine under an FR5 schedule, and during NoGo intervals lever responses did not trigger cocaine infusions. Training progressed until stable discrimination of the Go/NoGo periods were observed (3 consecutive sessions in which NoGo responses were less than 30% of total, 12–14 sessions). After reaching criterion, testing proceeded using a within subject design in which each rat received bilateral infusion of PBS or AM251 (0.7 ug) before intraperitoneal (i.p.) injections of either Δ9-THC or vehicle, in a randomized order, 15 min before each test session.
2.3. Drugs
Δ9-tetrahydrocannabinol (Δ9-THC; in 200 mg/ml EtOH, NIDA drug supply) was dissolved in DMSO/Tween 80/Saline (final vehicle: 20% DMSO, 10% Tween, 70% Saline) and administered 15 min before testing (1 ml/kg, i.p.). Cocaine HCl was dissolved in saline and injected i.p. 5 minutes before testing. For intracranial (i.c.) infusions, AM251 (Tocris) and Δ9-THC were dissolved in DMSO/Tween 80/PBS (5%, 5%, 90% respectively). Rats received bilateral infusions of either Δ9-THC (0.2 or 2 μg) AM251 (0.7 ug) or vehicle into the LHb. A microinjection cannula (C315I, Plastics One, Roanoke VA) which extended 1 mm below the tip of the guide was inserted into the guide cannula and 0.5 μl infused at 1 μl/min. The microinjection cannula was removed 1 min after the infusion to allow for diffusion of the solution into the tissue. The control vehicle solutions contained 0.5% EtOH (i.p. injections) or 0.2% EtOH (i.c. infusions) to match that present in the Δ9-THC solution. All drug doses were chosen based on previous published studies (Ratano et al., 2014; Szkudlarek et al., 2019; Tan et al., 2011; Wiskerke et al., 2011).
2.4. Surgery
Food deprived rats were allowed free feeding for at least 3 days before surgery. Surgical anesthesia was achieved with equithesin (1% pentobarbital, 2% magnesium sulfate, 4% chloral hydrate, 42% propyleneglycol, 11% ethanol, 3 ml/kg, i.p.), diluted to 33% in saline immediately before injection to minimize peritonitis. Anesthetic depth was assessed continuously throughout the procedure. Rats were then stereotaxically implanted with bilateral guide cannulae (C315, Plastics One, Roanoke VA) aimed 1 mm dorsal to the LHb (coordinates: AP: −3.8, L: ±0.6, V: −3.6 mm relative to bregma). Rats in the Go/NoGo IVSA study were also implanted with a polyurethane catheter (Instech, PA) that was inserted 3.5 cm into the right jugular vein. The catheter terminated in a Vascular Access Button™ (Instech, PA) which was subcutaneously mounted dorsally. Rats were monitored daily for signs of adynamic ileus; no such signs were observed in any subjects. All rats resumed normal feeding behavior and demonstrated weight gain during a one-week recovery phase, prior to training in the Go/NoGo task or resuming food deprivation and the 5CSRTT testing phase.
2.5. Histology
At the end of the experiments, the rats were euthanized, and brains removed and frozen on dry ice. Histological verification of the microinjection site was carried out in 50 μm frozen sections. The infusion coordinates were chosen to target the medial aspect of the LHb, and were identical to those used in a previous study in our lab in which the GABA receptor agonists baclofen and muscimol were infused into the LHb (Zapata et al., 2017). In that study we observed that errant cannula placements resulting in infusion of baclofen and muscimol into the 3rd ventricle were rare and associated with marked ataxia, as would be expected with general diffusion of GABA agonists into the cerebrospinal fluid (CSF) via the ventricle. Based upon this experience, the incidence of inaccurate cannula placement and diffusion of solutions into the CSF is rare, and in the present study, data from only two animals had to be discarded due to confirmed inaccurate cannula placements.
2.6. Data Analysis
We analyzed premature responses in the 5CSRTT as a measure of impulsive action, percent response accuracy as a measure of cognitive performance and attention, and response omissions as a measure of behavioral impairment or motivation. For the Go/NoGo experiments we analyzed responses during the Go and NoGo intervals and responses during NoGo intervals as a percentage of total responses. Statistical analysis was conducted using 1- or 2-way repeated measures analysis of variance (RM ANOVA) where appropriate. The main factors were systemic (i.p.) drug injection and intracranial drug infusion. Differences among groups were assessed by Bonferroni’s multiple comparison post-hoc test.
3. Results
3.1. Cocaine effects in the 5CSRTT and involvement of LHb CB1Rs
In our previous study, rats engaged in impulsive cocaine seeking when the LHb was inhibited by infusion of GABA receptor agonists (Zapata et al., 2017). Moreover, this inability to inhibit a response was not observed in rats trained to self-administer sucrose pellets (Zapata et al., 2017). Therefore, we hypothesize that cocaine experience is associated with a lowered threshold for impulsive behavior and that inhibition of LHb activity reveals this increased propensity toward impulsive cocaine seeking in drug-experienced rats. To investigate whether the pro-impulsive effects of cocaine generalize to other measures of impulsive behavior we used the 5CSRTT. Systemic cocaine significantly affected behavior in the 5CSRTT (Fig. 1A). At the highest dose tested (20 mg/kg, i.p.) it increased response omissions (F3,30 = 9.71, p = 0.0001, RM ANOVA) and decreased response accuracy (F3,30 = 6.29, p = 0.002, RM ANOVA), indicating a general disruption of task-relevant behavior. In contrast, a lower dose (5 mg/kg, i.p.) increased only premature responses (F 3,30 = 2.93, p = 0.05, RM ANOVA) with no changes in other task measures, such as omissions or accuracy.
Fig. 1:
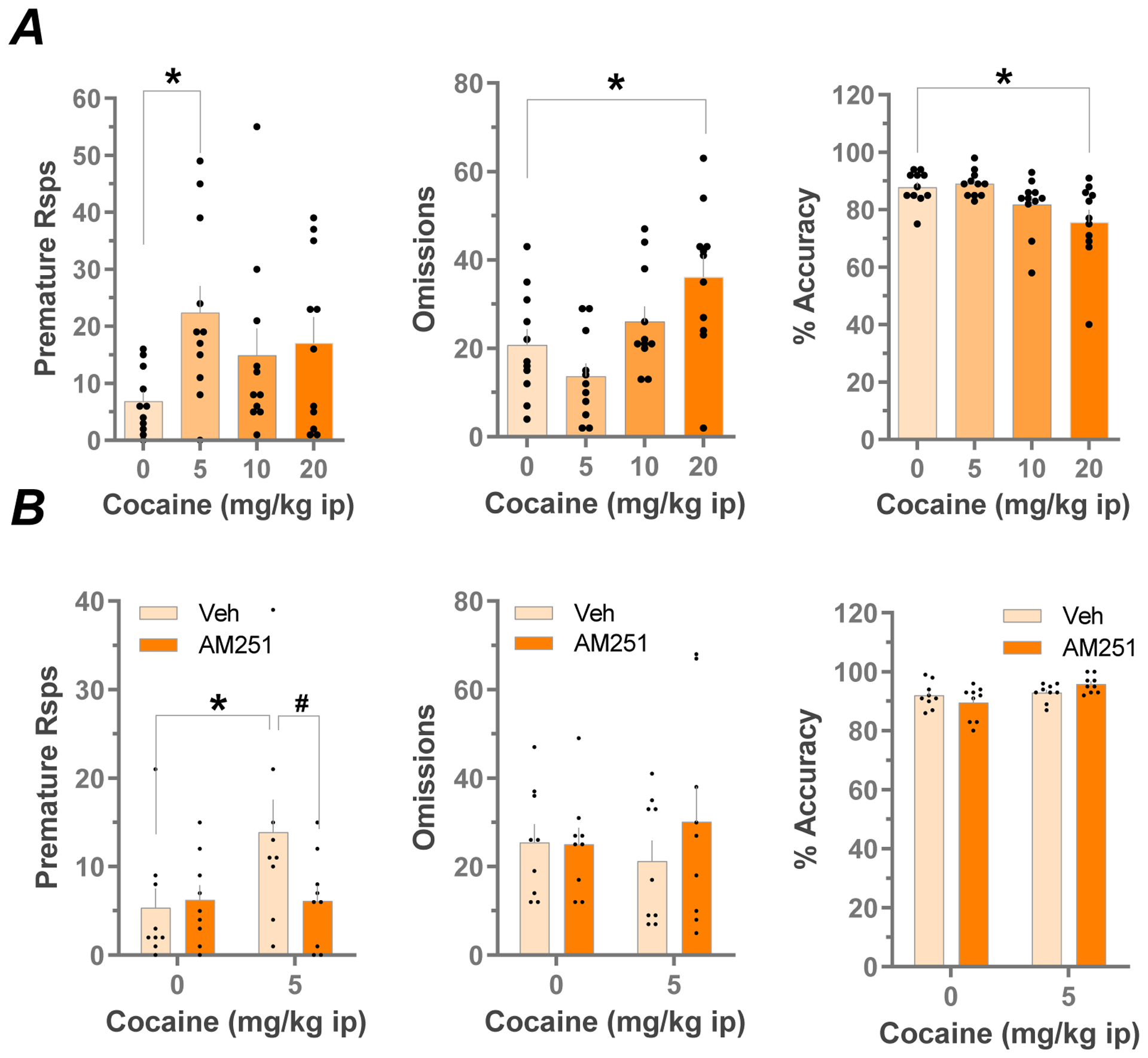
Effects of systemic cocaine on premature responses, omissions and accuracy in the 5CSRTT, and effects of Intra-LHb CB1R antagonism (A) Dose -response relationships for the effects of cocaine on premature responses, response omissions and, % accuracy in the 5CSRTT (n=11, *p<0.05 vs vehicle, Bonferroni’s multiple comparisons test after RM ANOVA). (B) Effects of local LHb infusion of AM251 (0.7 ug) or vehicle on cocaine-altered (5 mg/kg i.p.) behavior in the 5CSRTT (n=9 per group *p<0.05 vs saline, #p<0.05 vs vehicle, Bonferroni’s multiple comparisons test after RM ANOVA). Note that the increase in premature responses caused by 5 mg/kg cocaine was prevented by intra-LHb infusion with AM251.
Recent studies have shown that all components of the eCB system are found in the LHb. Therefore, to determine whether the eCB system is involved in the control of drug-associated impulsive behavior we assessed CB1R involvement in cocaine-induced impulsive responding in the 5CSRTT. Bilateral infusion of the CB1R antagonist/inverse agonist AM251 (0.7 ug) into the LHb did not significantly alter 5CSRTT omissions, accuracy, or premature responses (p>0.05, RM 2-way ANOVA) but significantly prevented cocaine-evoked premature responses (Fig. 1B, AM251 × cocaine interaction, F1,8 = 11.27, p = 0.01, RM 2-way ANOVA).
3.2. Δ9-THC effects in the 5CSRTT and involvement of LHb CB1Rs
As blockade of CB1Rs in the LHb prevented the pro-impulsive effects of cocaine in the 5CSRTT, and prior studies suggested that that the eCB system is involved in impulsive action (Ferland et al., 2018), we next explored the potential involvement of LHb CB1Rs in impulsive responding in the 5CSRTT. Systemic Δ9-THC affected task behavior in a dose-dependent manner. At the 3 mg/kg dose it disrupted task performance as indicated by increased response omissions (Fig. 2A, F3,27 = 5.149, p = 0.006, RM ANOVA), and decreased response accuracy (F3,27 = 3.69, p=0.024, RM ANOVA). However, a moderate dose of Δ9-THC (1 mg/kg) significantly increased impulsive action as measured by premature responses (F3,27 = 3.699, p = 0.0238; RM ANOVA) without disrupting other measures of task performance.
Fig. 2:
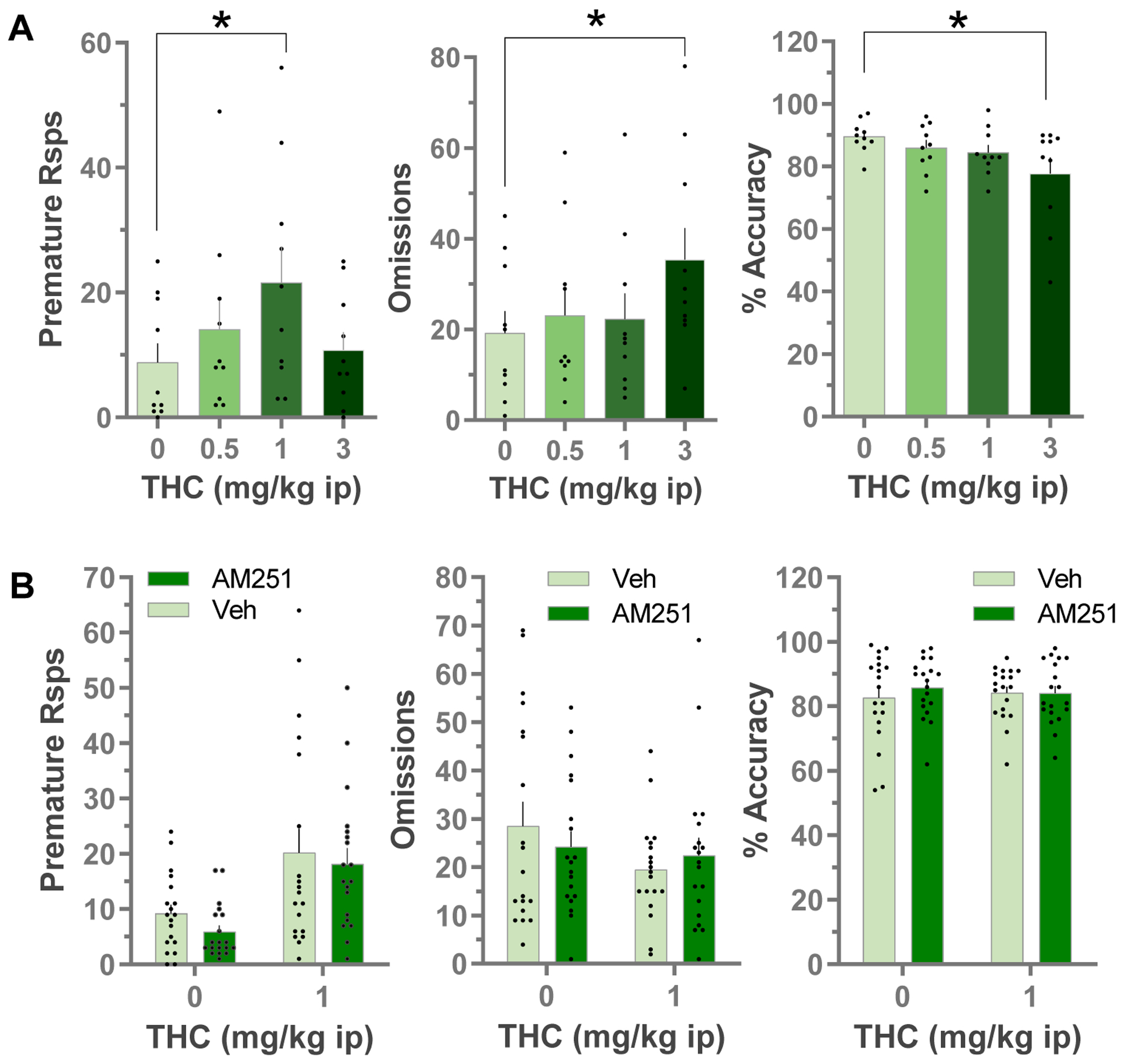
Effects of systemic Δ9-THC on premature responses, omissions and accuracy in the 5CSRTT: dose-response (A). Data are Mean±SEM, individual data points are also shown for premature responses (n=10, *p<0.05 vs vehicle, Bonferroni’s multiple comparisons test after RM ANOVA). B) Effects of local LHb infusions of AM251 (0.7 ug) or vehicle on Δ9-THC (1 mg/kg i.p.) evoked behavior in the 5CSRTT (n=19) Data are Mean ± SEM, individual data points are also shown for premature responses.
We next tested whether LHb CB1Rs are involved in the effects of systemic Δ9-THC on premature responding in the 5CSRTT (Fig. 2B). Systemic Δ9-THC (1 mg/kg) increased premature responding (main effect, F1,18 = 13.64, p = 0.002, RM 2-way ANOVA) without changing response omissions or accuracy. Our initial analysis showed no effects of intra-LHb infusion of AM251 on basal or Δ9-THC-associated premature responding (no significant main effect of AM251 infusion, nor AM251 × Δ9-THC interaction, RM 2-way ANOVA, Fig. 2B, left panel). However, we also noted substantial between subject variability in the data set, where some rats showed a robust increase in premature responding with 1 mg/kg Δ9-THC, and others showed no change or a decrease in this measure (Fig. 2). Pooling the data from all subjects where a heterogeneous response to Δ9-THC exists in the population could mask our ability to detect antagonism by AM251. Therefore, we reanalyzed the data, first by performing a correlational analysis relating the ability of LHb AM251 infusions to inhibit Δ9-THC-evoked premature responses to the magnitude of Δ9-THC-evoked increases in premature responding. We defined the magnitude of the Δ9-THC effect as premature responses emitted following systemic Δ9-THC (1 mg/kg i.p.), minus the premature responses after systemic vehicle injection (THC-evoked: eTHC). We then defined the ability of AM251 to inhibit the Δ9-THC effects as the eTHC after LHb AM251 infusion minus the eTHC after LHb vehicle infusions. With this calculation, negative values indicate that LHb AM251 infusion decreased the systemic Δ9-THC-evoked response, compared to LHb vehicle infusions. This correlational analysis confirmed that AM251 ability to inhibit Δ9-THC effects correlated with the magnitude of Δ9-THC -evoked increases in premature responses (Fig. 3A, Pearson correlation coefficient, r = −0.69, p = 0.001). To further address this, we also performed a median split analysis (Iacobucci et al., 2015) by ranking the 5CSRTT performance of all rats after systemic Δ9-THC (1 mg/kg) or vehicle infusions in the LHb, and then subdividing this group into 2 equal size subgroups based on premature responses either above or below the median (i.e. THC[+] and THC[−] groups, n = 9 rats per group), and discarding the data from the animal at the median (Fig. 3B). We then reanalyzed the data asking whether the effects of LHb AM251 infusion was dependent upon whether the rats showed an initial response to Δ9-THC alone, by including the Δ9-THC response subgroup as a variable in the ANOVA. This analysis, indicated that AM251 infusion into the LHb prevented Δ9-THC-evoked premature responding in the THC[+] subgroup (Δ9-THC response subgroup × AM251 interaction, F1,16 = 9.024, p = 0.0084, RM ANOVA; p < 0.05 vs LHb vehicle infusions, Bonferroni’s post hoc test, Fig. 3C), but had no effect on the subgroup that was unresponsive to Δ9-THC. This analysis also confirmed that individual differences were present in the population with regard to Δ9-THC-induced premature responding in the 5CSRTT (main effect of Δ9-THC response subgroup, F1,16 = 9.245, p = 0.0078, RM ANOVA; *p < 0.05 vs THC[−] subgroup, Bonferroni’s post hoc test, Fig. 3C). However, these subgroups did not differ with regard to the effect of Δ9-THC on response omissions or response accuracy (p>0.05, RM ANOVA, Fig. 3C), nor on any other measures of 5CSRTT performance after systemic vehicle injections (p>0.05, RM ANOVA, Fig. 3D).
Fig. 3:
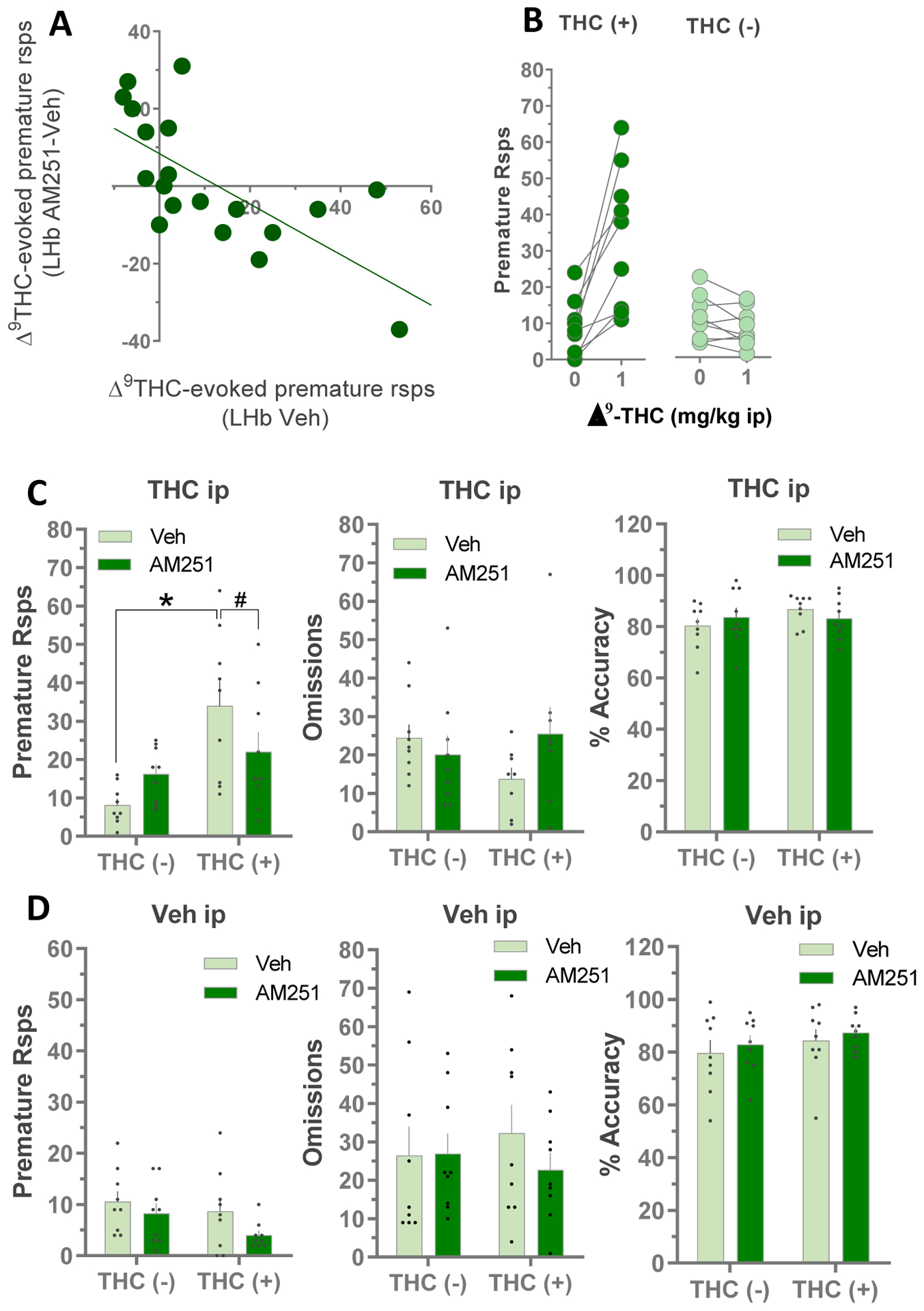
Analysis of Δ9-THC -evoked increase in premature responses and the ability of AM251 LHb infusions to inhibit those effects. A) Correlation between effect of systemic Δ9-THC alone (1 mg/kg i.p.), and systemic Δ9-THC preceded by intra-LHb infusion of AM251 on premature responses in the 5CSRTT. B) Data from Fig. 2 was reanalyzed by dividing the rats (n=19) into 2 equal subgroups (n=9 each) according to their response to Δ9-THC on premature responses (the median animal was discarded). The influence of Δ9-THC response subgroup on the effects of local LHb AM251 infusions on 5CSRTT behavior after systemic Δ9-THC (C) or vehicle (D) administration was assessed (n=9 per group *p<0.05 vs THC(−) group, #p<0.05 vs vehicle, Bonferroni’s multiple comparisons test after RM ANOVA).
To further examine the specificity of the effect of AM251 and LHb CB1Rs in the 5CSRTT, we increased the cognitive demand of the task by increasing the ITI from 4 to 7 seconds, and thus the period of time that each animal was required to withhold a response to receive reward. This manipulation has been shown to selectively increase premature responding without impairing other task variables (Wiskerke et al., 2011). We found that increasing the ITI significantly increased the number of premature responses in the systemic saline control group (27 ± 6 ITI = 7 sec vs 10 ± 2 ITI = 4 sec, control groups in Fig. 4A vs Fig. 2, respectively, t = 2.069, p = 0.005, Student’s t-test). However, in this version of the task, systemic Δ9-THC injections failed to increase premature responding, or affect any other variable measured in the 5CSRTT (p>0.05, RM ANOVA, Fig. 4A). Interestingly, all of the treatments resulting in increased premature responding in our study (cocaine, THC and increased ITI) reached a maximum of 20–30 of these responses. Therefore, this may represent an upper limit to premature responding in this 5CSRTT that cannot be exceeded in the present experimental paradigm. This may explain our observation that Δ9-THC increased premature responding at the 4 sec ITI, but not at the 7 sec ITI. AM251 infusions in the LHb did not alter performance in this task (p>0.05, RM ANOVA, Fig. 4A).
Fig. 4:
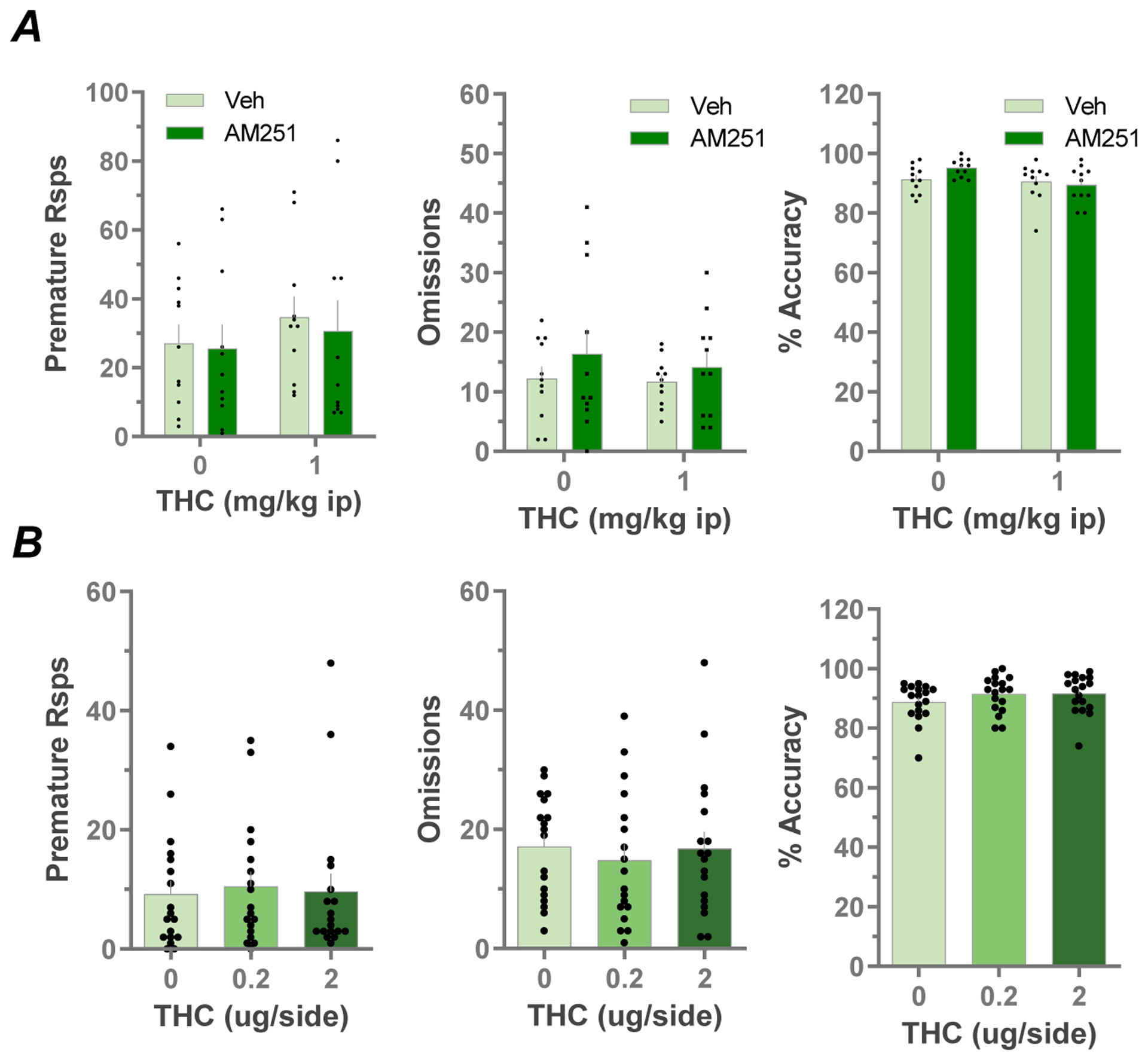
A) Effects of local LHb infusions of AM251 (0.7 ug) or vehicle on Δ9-THC (1 mg/kg i.p.) evoked behavior in a modified version of the 5CSRTT using a longer (7 seconds) ITI (n=11). B) Effects of local LHb Δ9-THC infusions on premature responses, omissions and accuracy in the 5CSRTT: dose-response (n=10).
Finally, we assessed whether local infusions of Δ9-THC (0.2 or 2 μg, 0.5 μl volume, bilateral) into the LHb could alter performance on the 5CSRTT. We found no effects of intra-LHb infusion of Δ9-THC at either concentration on any of the 5CSRTT variables (p>0.05, RM ANOVA, Fig. 4B).
3.3. A role for CB1Rs in the LHb in impulsive responding for cocaine in the Go/NoGo task.
Our above 5CSRTT studies showed that impulsive behavior was increased by systemic cocaine or Δ9-THC, and that LHB CB1Rs are involved in the effects of these drugs. Moreover, our prior study showed that the LHb was also involved in impulsive cocaine seeking in a cocaine self-administration Go/NoGo self-administration task (Zapata et al., 2017). Therefore, we wondered whether systemic Δ9-THC also increased impulsive responding in this task, and whether LHb CB1Rs are involved. We found that systemic Δ9-THC, at a dose that did not disrupt response accuracy or omissions in the 5CSRTT (1 mg/kg, i.p.), did evoke a small but statistically significant decrease in responding during Go intervals (main effect F1,19 = 4.81, p = 0.041, RM 2-way ANOVA, Fig 5). However, this same dose of Δ9-THC dramatically increased responding during the NoGo intervals (Δ9-THC = 44.5 ± 8.6, vehicle = 7.65 ± 1.49 responses). This was apparent in both the number of NoGo responses (main effect of Δ9-THC, F1,19 = 32.0, p < 0.0001, RM 2-way ANOVA), and in NoGo responses as a percentage of total responses (main effect of Δ9-THC, F1,19 = 46.35, p < 0.0001, RM 2-way ANOVA). To determine the potential involvement of LHb CB1Rs in the effects of systemic Δ9-THC we bilaterally infused AM251 into the LHb before systemic Δ9-THC. We found that infusions of AM251 into the LHb prevented the increase in operant responding for cocaine during the NoGo periods caused by systemic Δ9-THC (% NoGo responses, Δ9-THC × AM251 interaction, F1,19 = 6.77, p = 0.018, RM 2-way ANOVA). This trend was also apparent in the analysis of the absolute number of NoGo responses, but this failed to reach significance (Δ9-THC × AM251 interaction, F1,19 = 3.91, p = 0.063, RM 2-way ANOVA). Moreover, we observed that infusion of AM251 into the LHb in the absence of systemic Δ9-THC did not significantly change NoGo responding for cocaine (AM251 main effect, F1,4 = 2.63, p = 0.180, RM 2-way ANOVA). Together, these data suggest that systemic Δ9-THC increases impulsive responding for cocaine, and that LHb CB1Rs are involved in this behavior.
Fig. 5:
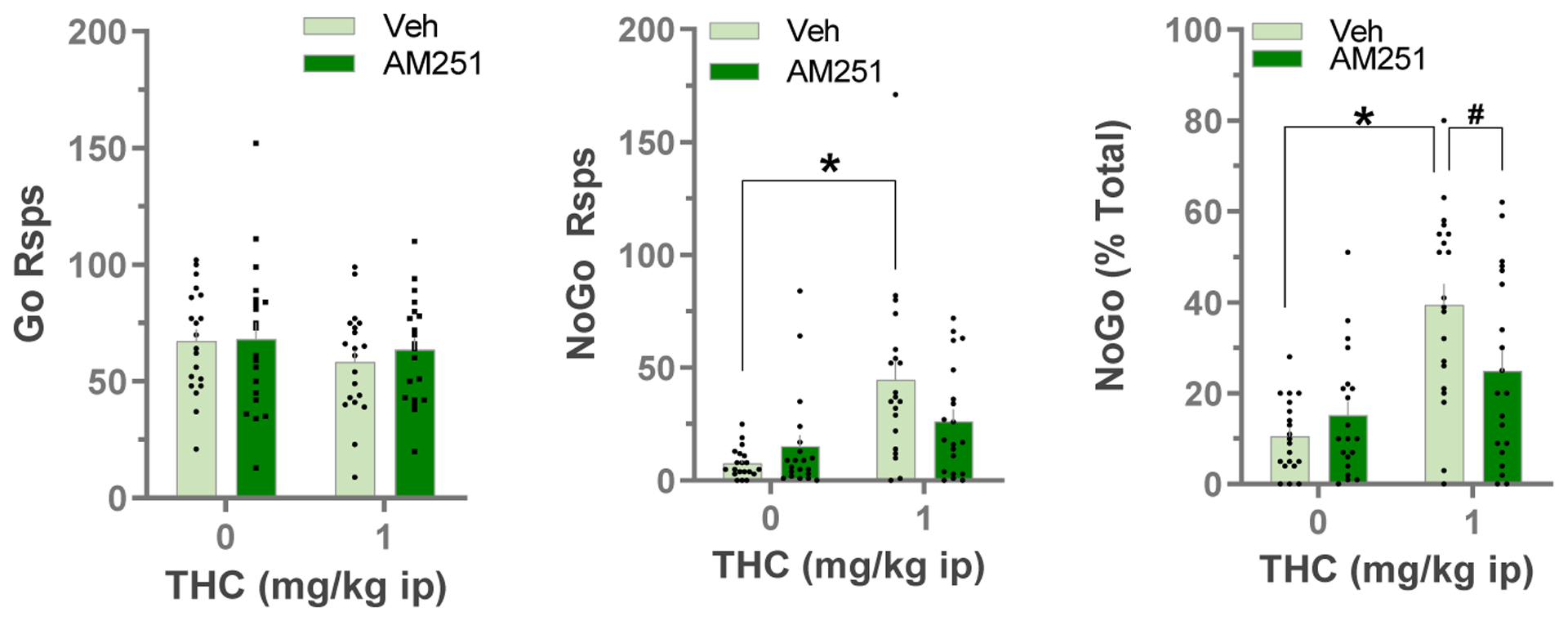
Effects of local LHb infusions of AM251 (0.7 ug) or vehicle on Δ9-THC (1 mg/kg i.p.) evoked behavior in the Go/NoGo Cocaine IVSA task (n=20). Data are Mean ± SEM. *p<0.05 vs vehicle i.p. group, #p<0.05 vs vehicle LHb infusions, Bonferroni’s multiple comparisons test after RM ANOVA.
4. Discussion
This study provides evidence that LHb CB1Rs are involved in cocaine and Δ9-THC-evoked increases in impulsive action, as measured by premature responses in the 5CSRTT and drug seeking during NoGo periods in a Go/NoGo task. Our results generally agree with studies showing that systemic CB1R antagonists can prevent deficits in impulse control caused by amphetamine (Wiskerke et al., 2011), cocaine (Hernandez et al., 2014), or nicotine (Wiskerke et al., 2012), and extend this by implicating the LHb eCB system in these effects. More generally, our study adds to a growing body of evidence implicating the eCB system in impulsive psychostimulant seeking and suggests that the LHb may be involved in this aspect of behavioral control.
Impulsive behavioral traits are of interest because of their high concordance with addiction and relapse risk (Dalley et al., 2011; Stevens et al., 2015; Thomsen et al., 2018; Wit, 2009), and because they are implicated in several psychiatric disorders (Amlung et al., 2019). In rats, high trait impulsivity is associated with increased vulnerability to development of compulsive drug seeking and taking (Belin et al., 2008), and with an increased propensity for relapse after punishment-induced drug abstinence (Economidou et al., 2009). An extensive literature implicates a top-down circuit model in which impulse control involves inhibition of basal ganglia output by prefrontal cortex (Chambers et al., 2009; Dalley et al., 2011; Dalley and Robbins, 2017), and modulation of this behavior by monoaminergic pathways (Pattij and Vanderschuren, 2008). Cocaine is thought to influence this circuitry through its ability to inhibit monoamine transporters (Amara and Sonders, 1998), and long-term use of this drug is linked to deficits in impulse control, as measured by response inhibition in humans (Ersche et al., 2011; Fernández-Serrano et al., 2012; Fillmore and Rush, 2002; Kaufman et al., 2003). In rodents, amphetamine, cocaine, and nicotine impair response inhibition, as measured by increased premature responding in the 5CSRTT, and this is blocked by dopamine D2 receptor antagonists (Gaalen et al., 2006). Moreover, dopaminergic tone in the ventral striatum (nucleus accumbens) is strongly implicated in impulsive action (Pattij and Vanderschuren, 2020).
In humans, there is good agreement that cannabis use is related to deficits in motor impulse control (McDonald et al., 2003; Ramaekers et al., 2008; Theunissen et al., 2012). However, studies in rodents are inconsistent, with some showing decreased motor impulsivity after systemic administration of CB1R antagonists and others showing no effects of Δ9-THC or selective CB1 agonists (Pattij et al., 2007; Wiskerke et al., 2011). In contrast to these studies, and in agreement with evidence in humans, we observed deficits in impulse control in the 5CSRTT after systemic administration of Δ9-THC. The reasons why our results differ from these earlier studies are not clear, although one possibility could be the strain of rats used as experimental subjects. In earlier studies where motor impulsivity was not increased by cannabinoids Wistar rats were used, whereas Long Evans rats were used in our study. Wistar rats are particularly sensitive to the anxiogenic properties of cannabinoids (Arnold et al., 2010; Ferland et al., 2018), which may relate to differences in performance in the 5CSRTT. Moreover, Long Evans and Wistar rats also differ in the ability of amphetamine to elicit impulsive responding in a modified gambling task (Zeeb et al., 2009)(Ferland et al., 2018). Procedural differences may also be involved in the inconsistent effects of cannabinoids on impulsive responding. For example, studies failing to detect CB1R agonist effects on motor impulsivity used short limited-hold periods (2 seconds) in which responses could be rewarded (Pattij et al., 2007; Wiskerke et al., 2011). However, studies showing CB1R agonist-induced increased premature responding used a longer 5 second limited-hold period (present study and Arguello and Jentsch, 2004). Therefore, differences among rat strains or experimental procedures may explain the discrepancies in cannabinoid effects on impulsive behavior.
Our study also found considerable individual variability in impulsive responding caused by systemic Δ9-THC in the 5CSRTT, where approximately half of the individuals in our sample failed to exhibit this behavior. However, when the magnitude of the initial Δ9-THC-induced premature responding in the 5CSRTT was considered in subsequent analyses, we found that intra-LHb injections of AM251 prevented this response. Inter-subject variability in cannabinoid-associated impulsive behavior has been reported previously where systemic injection of the CB1R agonist WIN55,212–2 increased premature responding in a subset of rats, but not in the larger population in a modified Iowa Gambling Task (Ferland et al., 2018). Therefore, individual differences in sensitivity to cannabinoid-induced impulsiveness appear likely to exist within rat populations. In our study, the cannabinoid-sensitive subpopulation showed increased premature responding at a dose of Δ9-THC (1 mg/kg) that did not interfere with other measures of task performance such as response omissions or choice accuracy. However, a modest increase in the dose of Δ9-THC (3 mg/kg) affected these measures of task performance, suggesting that the phytocannabinoid selectively increases impulsive behavior within a narrow range of doses, and this may also contribute to the variability observed among earlier studies.
Although systemic Δ9-THC increased impulsive responding in the 5CSRTT, and direct LHb infusion of AM251 blocked this effect, neither AM251 alone, nor local Δ9-THC infusions into the LHb altered 5CSRTT measures in the present study. Similarly, systemic Δ9-THC promoted NoGo responding for cocaine and this was blocked by intra-LHb infusion of AM251, but neither intra-LHb infusion of Δ9-THC or AM251 alone altered NoGo responding. This suggests that although LHb CB1Rs are involved in these impulsive behaviors, their activation appears to require priming by actions of Δ9-THC or cocaine outside of the LHb. Thus, exposure to these drugs may recruit brain regions that project to the LHb and engage eCB-dependent mechanisms within this brain structure. Future experiments will examine this possibility.
Our observed interaction between CB1Rs and cocaine in the Go/NoGo task is also interesting because unlike most abused drugs, the primary rewarding and motivational effects of cocaine and other psychostimulants are not strongly inhibited by antagonism or genetic deletion of brain CB1Rs (Parsons and Hurd, 2015). Rather, CB1Rs appear to be involved in modulating psychostimulant-associated conditioned reward and play a more important role in reinstatement or relapse to drug seeking. Thus, systemic exposure to CB1 agonists, like Δ9-THC or HU-210, can trigger reinstatement of cocaine seeking after extinction of this operant response, and CB1R antagonists can prevent relapse to cocaine seeking elicited by conditioned cues (Serrano and Parsons, 2011; Vries et al., 2001). There is also recent evidence supporting the idea that the LHb is involved in relapse to drug seeking, where both cocaine-primed reinstatement of conditioned place preference and cue-evoked reinstatement of heroin seeking are associated with increased activation of LHb neurons, as indicated by increased c-Fos expression (Brown et al., 2010)(Zhang et al., 2005). Moreover, chemogenetic inhibition of the LHb inhibits both cocaine-primed and cue-induced reinstatement of cocaine seeking after extinction (Nair et al., 2020). Here we show that systemic Δ9-THC increases cocaine seeking during NoGo periods, despite the fact that rats have learned that cocaine is not available at these times, and we show that this Δ9-THC-induced increase in NoGo responding is prevented by intra-LHb infusion of a CB1R antagonist. Therefore, our results suggest the intriguing possibility that Δ9-THC may trigger relapse to cocaine seeking by activating LHb CB1Rs and increasing impulsive action associated with obtaining the drug. More generally, our study and those describing a role for the LHb in reinstatement of cocaine seeking suggest that this brain region could be involved in relapse in humans and that LHB CB1Rs may be critically involved in this hallmark of psychostimulant addiction. Although our investigation did not examine a potential difference between male and female rats in the role of the eCB system in impulsivity, this merits further investigation because a study has reported sex differences in an interaction between LHB eCBs and basal corticosterone levels (Berger et al 2008). Thus, it is possible that stress-response associated changes in LHb activity and eCB activity may contribute to the effects we observed, and this may differ between males and females.
The output of the LHb to downstream brain structures is mediated predominantly by excitatory glutamatergic axons (Aizawa et al., 2013). One of these output pathways targets a collection of GABAergic neurons in a brain area known as the rostromedial tegmentum (RMTg) that provides strong inhibitory control of midbrain DA neurons (Christoph et al., 1986; Hong et al., 2011). As midbrain DA neurons are thought to play a role in impulsivity as well as in other neuropsychiatric disorders (Dalley and Roiser, 2012; Pattij and Vanderschuren, 2020, 2008), it is possible that engagement of the eCB system by Δ9-THC might increase impulsivity by increasing dopaminergic tone through this circuitry. Alternatively, substantial evidence also points to a role for 5HT in impulse control, and the LHb also projects to median and dorsal raphe nuclei where it inhibits 5HT activity (Zhao et al., 2015). Thus, decreased 5-HT tone is associated with increased impulsivity in Go/NoGo and 5CSRT tasks (Fletcher, 1993; Harrison et al., 1999, 1997; Winstanley et al., 2004), and elevated 5-HT levels decrease impulsivity in the 5CSRTT (Baarendse and Vanderschuren, 2012). Therefore, CB1R activation within LHb may alter its output to 5-HT-containing brain regions and this may be involved in controlling impulsive behavior.
In summary, our results add to a growing body of evidence supporting the idea that the LHb is involved in regulating the inhibition of impulsive action. Thus, acute Δ9-THC or cocaine caused deficits in impulse control in the 5CSRTT and Δ9-THC increased NoGo responding for cocaine self-administration, and both of these indices of impulsive behavior involved LHb CB1Rs. These results therefore suggest that the LHb may be relevant to impulsive behavior related to SUDs, and further implicate the LHb eCB system as a potential target for therapeutic strategies aimed at restoring impaired impulse regulation in addiction.
Fig. 6:
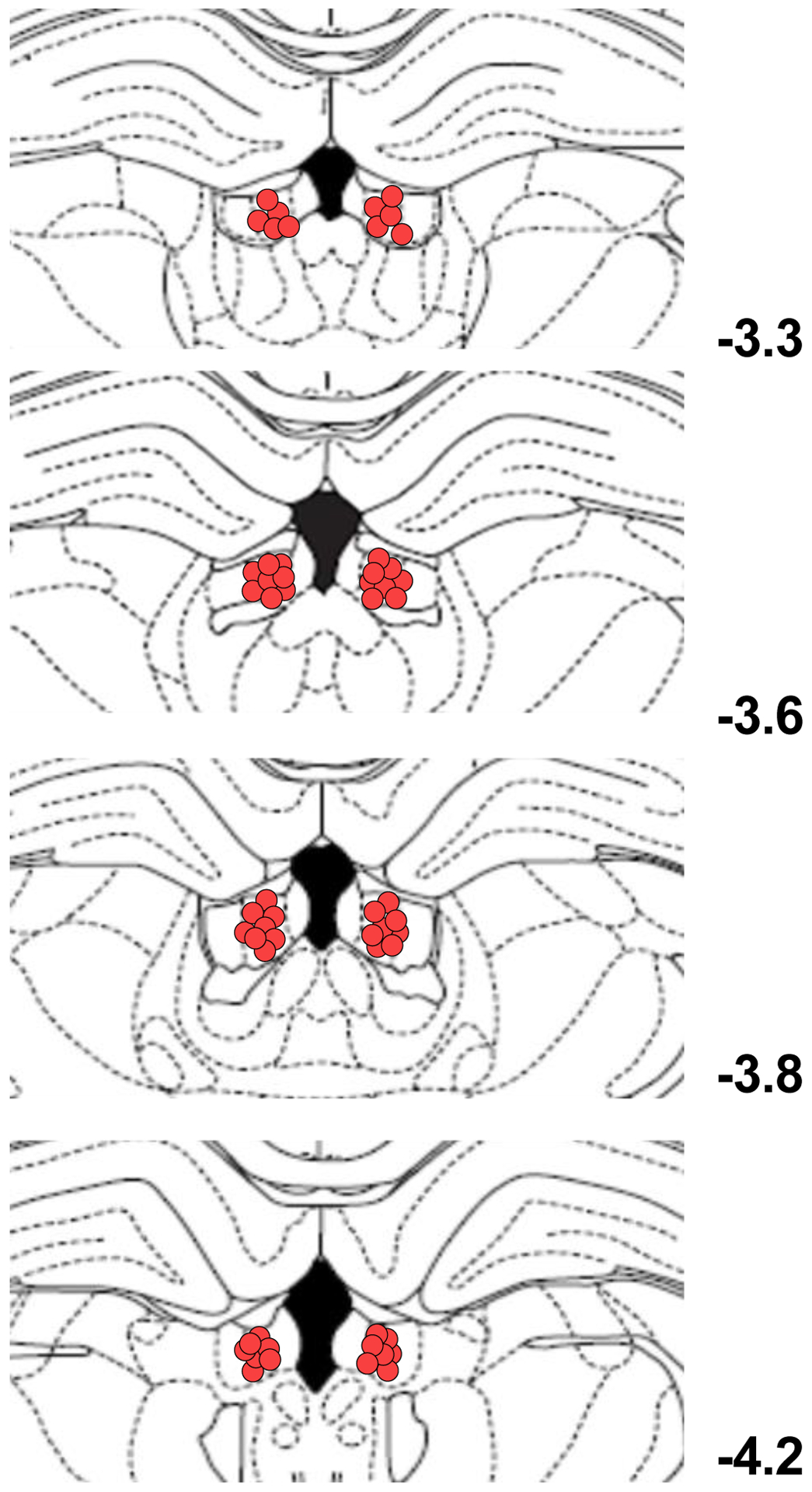
Diagrams of coronal sections displaying the histological verification of the infusion sites in the LHb. Circles show the location of the infusion sites for the rats included in the data analysis for all the experiments (numbers indicate mm from bregma).
Studies show that cannabis increases relapse to cocaine seeking following withdrawal.
Human cannabis use is associated with impulse control deficits.
Lateral Habenula endocannabinoids in are involved in impulsive behavior.
Cocaine and Δ9-tetrahydrocannabinol (Δ9-THC) increased impulsive behavior.
Δ9-THC increased impulsive cocaine seeking via effect in the lateral habenula
Funding, Disclosures, Acknowledgements
Supported by funding from the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. The authors declare no competing financial interests in relation to this work. We would like to thank Dr. Alexander F. Hoffman for helpful comments on the manuscript.
Abbreviations:
- LHb
Lateral Habenula
- eCB
endogenous cannabinoids
- 5CSRTT
5-choice serial reaction time task
- CB1R
CB1 receptors
- Δ9-THC
Δ9-tetrahydrocannabinol
- SUDs
substance use disorders
- RMTg
rostromedial tegmental nucleus
- IVSA
intravenous self-administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Yanagihara S, Kobayashi M, Niisato K, Takekawa T, Harukuni R, McHugh TJ, Fukai T, Isomura Y, Okamoto H, 2013. The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 8909–21. 10.1523/JNEUROSCI.4369-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Sonders MS, 1998. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depen 51, 87–96. 10.1016/s0376-8716(98)00068-4 [DOI] [PubMed] [Google Scholar]
- Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, Naish KR, Reed DD, McCabe RE, 2019. Delay Discounting as a Transdiagnostic Process in Psychiatric Disorders. Jama Psychiat 76, 1176–1186. 10.1001/jamapsychiatry.2019.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME, 2009. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, biochemistry, and behavior 93, 343–8. 10.1016/j.pbb.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello PA, Jentsch JD, 2004. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology 177, 141–150. 10.1007/s00213-004-1953-0 [DOI] [PubMed] [Google Scholar]
- Argyriou E, Um M, Carron C, Cyders MA, 2018. Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacol Biochem Be 164, 106–117. 10.1016/j.pbb.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JC, Dielenberg RA, McGregor IS, 2010. Cannabinoids increase conditioned ultrasonic vocalisations and cat odour avoidance in rats: strain differences in drug-induced anxiety. Life Sci 87, 572–8. 10.1016/j.lfs.2010.09.018 [DOI] [PubMed] [Google Scholar]
- Baarendse PJ, Vanderschuren LJ, 2012. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology 219, 313–326. 10.1007/s00213-011-2576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJY, Stephenson-Jones M, Vicentic A, 2016. The Lateral Habenula Circuitry: Reward Processing and Cognitive Control. J Neurosci 36, 11482–11488. 10.1523/jneurosci.2350-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW, 2008. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3, 759–67. 10.1038/nprot.2008.41 [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ, 2008. High impulsivity predicts the switch to compulsive cocaine-taking. Science (New York, N.Y.) 320, 1352–5. 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AL, Henricks AM, Lugo JM, Wright HR, Warrick CR, Sticht MA, Morena M, Bonilla I, Laredo SA, Craft RM, Parsons LH, Grandes PR, Hillard CJ, Hill MN, McLaughlin RJ, 2018. The lateral habenula directs coping styles under conditions of stress via recruitment of the endocannabinoid system. Biol Psychiat 84, 611–623. 10.1016/j.biopsych.2018.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Short JL, Lawrence AJ, 2010. Identification of Brain Nuclei Implicated in Cocaine-Primed Reinstatement of Conditioned Place Preference: A Behaviour Dissociable from Sensitization. Plos One 5, e15889. 10.1371/journal.pone.0015889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin NMWJ, de Lange JHM, Kruse CG, Herremans AH, Schoffelmeer ANM, Drimmelen M van, Vries TJD, 2011. SLV330, a cannabinoid CB1 receptor antagonist, attenuates ethanol and nicotine seeking and improves inhibitory response control in rats. Behav Brain Res 217, 408–415. 10.1016/j.bbr.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA, 2009. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33, 631–646. 10.1016/j.neubiorev.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS, 1986. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 6, 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW, 2011. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680–94. 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW, 2017. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 18, 158–171. 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP, 2012. Dopamine, serotonin and impulsivity. Neuroscience 215, 42–58. 10.1016/j.neuroscience.2012.03.065 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piazza PV, 2014. Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology 76 Pt B, 437–49. 10.1016/j.neuropharm.2013.07.014 [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ, 2009. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biological psychiatry 65, 851–6. 10.1016/j.biopsych.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET, 2011. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134, 2013–2024. 10.1093/brain/awr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland J-MN, Carr MR, Lee AM, Hoogeland ME, Winstanley CA, Pattij T, 2018. Examination of the effects of cannabinoid ligands on decision making in a rat gambling task. Pharmacol Biochem Be 170, 87–97. 10.1016/j.pbb.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Perales JC, Moreno-López L, Pérez-García M, Verdejo-García A, 2012. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology 219, 673–683. 10.1007/s00213-011-2485-z [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, 2002. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depen 66, 265–273. 10.1016/s0376-8716(01)00206-x [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, 1993. A comparison of the effects of dorsal or median raphe injections of 8-OH-DPAT in three operant tasks measuring response inhibition. Behavioural brain research 54, 187–97. [DOI] [PubMed] [Google Scholar]
- Gaalen MM van, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJMJ, 2006. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology 187, 73–85. 10.1007/s00213-006-0396-1 [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW, 1999. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res 100, 99–112. 10.1016/s0166-4328(98)00117-x [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW, 1997. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 133, 329–342. 10.1007/s002130050410 [DOI] [PubMed] [Google Scholar]
- Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, Cheer JF, 2014. Endocannabinoids Promote Cocaine-Induced Impulsivity and Its Rapid Dopaminergic Correlates. Biol Psychiat 75, 487–498. 10.1016/j.biopsych.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O, 2011. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 11457–71. 10.1523/jneurosci.1384-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci D, Posavac SS, Kardes FR, Schneider MJ, Popovich DL, 2015. Toward a more nuanced understanding of the statistical properties of a median split. J Consum Psychol 25, 652–665. 10.1016/j.jcps.2014.12.002 [DOI] [Google Scholar]
- Jentsch JD, Pennington ZT, 2014. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology 76, 479–486. 10.1016/j.neuropharm.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD, 2007. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 6923–30. 10.1523/jneurosci.0958-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H, 2003. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. J Neurosci 23, 7839–7843. 10.1523/jneurosci.23-21-07839.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH, 2007. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews 31, 658–72. 10.1016/j.neubiorev.2007.01.004 [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH, 2005. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 30, 484–96. 10.1038/sj.npp.1300595 [DOI] [PubMed] [Google Scholar]
- MacKillop J, Weafer J, Gray JC, Oshri A, Palmer A, Wit H. de, 2016. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology 233, 3361–3370. 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2009. Representation of negative motivational value in the primate lateral habenula. Nature neuroscience 12, 77–84. 10.1038/nn.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2007. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–5. 10.1038/nature05860 [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, Wit H. de, 2003. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 28, 1356–65. 10.1038/sj.npp.1300176 [DOI] [PubMed] [Google Scholar]
- Nair SG, Smirnov DS, Estabrook MM, Chisholm AD, Silva PR, Neumaier JF, 2020. Effect of chemogenetic inhibition of lateral habenula neuronal activity on cocaine- and food-seeking behaviors in the rat. Addict Biol e12865. 10.1111/adb.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Rhee J, Lee S, Chung C, 2017. Selectively Impaired Endocannabinoid-Dependent Long-Term Depression in the Lateral Habenula in an Animal Model of Depression. Cell Reports 20, 289–296. 10.1016/j.celrep.2017.06.049 [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL, 2015. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16, 579–94. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MCW, Schepers I, González-Cuevas G, Vries T.J. de, Schoffelmeer ANM, 2007. Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology 193, 85–96. 10.1007/s00213-007-0773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJMJ, 2020. Current Topics in Behavioral Neurosciences. Curr Top Behav Neurosci 1–20. 10.1007/7854_2020_143 [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJMJ, 2008. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29, 192–199. 10.1016/j.tips.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R, 2014. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci 17, 1146–1152. 10.1038/nn.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M, 2008. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23, 266–277. 10.1177/0269881108092393 [DOI] [PubMed] [Google Scholar]
- Ratano P, Everitt BJ, Milton AL, 2014. The CB1 Receptor Antagonist AM251 Impairs Reconsolidation of Pavlovian Fear Memory in the Rat Basolateral Amygdala. Neuropsychopharmacol 39, 2529–2537. 10.1038/npp.2014.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Parsons LH, 2011. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Therapeut 132, 215–241. 10.1016/j.pharmthera.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM, 2014. Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug Alcohol Depen 145, 1–33. 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Stevens L, Roeyers H, Dom G, Joos L, Vanderplasschen W, 2015. Impulsivity in Cocaine-Dependent Individuals with and without Attention-Deficit/Hyperactivity Disorder. Eur Addict Res 21, 131–143. 10.1159/000369008 [DOI] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB, 2013. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nature neuroscience. 10.1038/nn.3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, 1982. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience and biobehavioral reviews 6, 1–13. 10.1016/0149-7634(82)90003-3 [DOI] [PubMed] [Google Scholar]
- Szkudlarek HJ, Desai SJ, Renard J, Pereira B, Norris C, Jobson CEL, Rajakumar N, Allman BL, Laviolette SR, 2019. Δ−9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacol 44, 817–825. 10.1038/s41386-018-0282-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR, 2011. Cannabinoid Transmission in the Basolateral Amygdala Modulates Fear Memory Formation via Functional Inputs to the Prelimbic Cortex. J Neurosci 31, 5300–5312. 10.1523/jneurosci.4718-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen EL, Kauert GF, Toennes SW, Moeller MR, Sambeth A, Blanchard MM, Ramaekers JG, 2012. Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology 220, 341–50. 10.1007/s00213-011-2479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen KR, Callesen MB, Hesse M, Kvamme TL, Pedersen MM, Pedersen MU, Voon V, 2018. Impulsivity traits and addiction-related behaviors in youth. J Behav Addict 7, 317–330. 10.1556/2006.7.2018.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinova K, Mameli M, 2016. mGluR-LTD at Excitatory and Inhibitory Synapses in the Lateral Habenula Tunes Neuronal Output. Cell Reports 16, 2298–2307. 10.1016/j.celrep.2016.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vries TJD, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJMJ, Schoffelmeer ANM, 2001. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7, 1151–1154. 10.1038/nm1001-1151 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW, 2004. Fractionating Impulsivity: Contrasting Effects of Central 5-HT Depletion on Different Measures of Impulsive Behavior. Neuropsychopharmacol 29, 1331–1343. 10.1038/sj.npp.1300434 [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Mourik Y van, Schetters D, Schoffelmeer ANM, Pattij T, 2012. On the Role of Cannabinoid CB1- and μ-Opioid Receptors in Motor Impulsivity. Front Pharmacol 3, 108. 10.3389/fphar.2012.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer ANM, Pattij T, 2011. Cannabinoid CB1 Receptor Activation Mediates the Opposing Effects of Amphetamine on Impulsive Action and Impulsive Choice. Plos One 6, e25856. 10.1371/journal.pone.0025856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit H. de, 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology 14, 22–31. 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Hwang E, Neuropsychopharmacology, L.-C., 2017. Lateral habenula involvement in impulsive cocaine seeking. 10.1038/npp.2016.286 [DOI] [PMC free article] [PubMed]
- Zeeb FD, Robbins TW, Winstanley CA, 2009. Serotonergic and Dopaminergic Modulation of Gambling Behavior as Assessed Using a Novel Rat Gambling Task. Neuropsychopharmacol 34, 2329–2343. 10.1038/npp.2009.62 [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou W, Liu H, Zhu H, Tang S, Lai M, Yang G, 2005. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci Lett 386, 133–137. 10.1016/j.neulet.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang B-LL, Yang S-JJ, Rusak B, 2015. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behavioural brain research 277, 89–98. 10.1016/j.bbr.2014.09.016 [DOI] [PubMed] [Google Scholar]


