Abstract
Bone is a mechano-responsive tissue that adapts to changes in its mechanical environment. Increases in strain lead to increased bone mass acquisition, whereas decreases in strain lead to a loss of bone mass. Given that mechanical stress is a regulator of bone mass and quality, it is important to understand how bone cells sense and transduce these mechanical cues into biological changes to identify druggable targets that can be exploited to restore bone cell mechano-sensitivity or to mimic mechanical load. Many studies have identified individual cytoskeletal components – microtubules, actin, and intermediate filaments – as mechano-sensors in bone. However, given the high interconnectedness and interaction between individual cytoskeletal components, and that they can assemble into multiple discreet cellular structures, it is likely that the cytoskeleton as a whole, rather than one specific component, is necessary for proper bone cell mechano-transduction. This review will examine the role of each cytoskeletal element in bone cell mechano-transduction and will present a unified view of how these elements interact and work together to create a mechano-sensor that is necessary to control bone formation following mechanical stress.
Keywords: Cytoskeleton, Microtubules, Actin, Intermediate Filaments, Osteocyte, Mechano-transduction
I. Introduction
Bone senses and adapts to changes in its mechanical environment, settling at a homeostatic setpoint around the “typical” loading events it experiences. Significant changes to its mechanical environment, such as those that happen with heavy weightlifting, push the bone out of its homeostatic setpoint into an anabolic zone, which spurs bone formation to adapt to this increase in strain1. Conversely, a sustained lack of mechanical loading, such as during disuse, paralysis, and injury or defects in the ability to sense and transduce mechanical loading cues - as occurs in aging –pushes bone into a catabolic zone and resets the homeostatic setpoint at a lower bone mass. The resulting decrease in bone mass can lead to an increase risk of fracture. As such, understanding how bone cells sense and transduce mechanical signals into biological changes is important to develop new therapeutic targets to address conditions of low bone mass and consequent frailty.
Bone is a porous structure filled with interstitial fluid, bound by semi-permeable fibrous periosteal and endosteal membranes. Mechanical loading of bone causes pressurization of interstitial fluid, resulting in fluid shear stress experienced by mechano-sensitive bone-embedded osteocytes, surface osteoblasts, and their progenitors in the endosteal and periosteal membranes2–4. These cells sense and respond to mechanical events, activating mechano-transduction cascades that translate mechanical cues into biological signals to regulate bone formation and remodeling. In bone cells, the initiation of mechano-transduction cascades, which invariably involves intracellular calcium signaling, results in the regulation of bone effector molecules such as sclerostin, nitric oxide, prostaglandin E2 (PGE2), insulin like growth factor 1 (IGF1), parathyroid hormone related protein (PTHrP), and RANKL5–7. Identification of these effectors of bone mechano-signaling has led to therapeutic agents that improve bone mass. For example, sclerostin is a fundamentally important regulator of bone formation in response to anabolic bone loads. Sclerostin, a glycoprotein that is secreted by bone embedded osteocytes, inhibits the differentiation and activity of bone forming osteoblasts by disrupting Wnt/β-catenin signaling8. Mechanical loading decreases sclerostin, relieving the repression on osteoblast differentiation, and unleashing de novo bone formation. Targeting this mechano-transduction effector has proven to be useful strategy to improve bone mass. Antagonizing sclerostin protein using neutralizing antibodies with the drug Romosozumab is effective at increasing bone mass and has been approved by the FDA to treat osteoporosis in post-menopausal women at a high risk for fracture9,10.
While many of the downstream effectors of osteocyte mechano-transduction are known, precisely how bone cells sense mechanical cues and translate them into signaling events that lead to mechano-activated bone formation is much less clear. Understanding the mechano-sensors and the downstream pathways that control this ensemble of bone effectors represents important, untapped therapeutic opportunities for improving bone mass. While targeting a single effector, such as sclerostin with Romosozumab, is effective at improving bone mass, better understanding the elements that are mechano-responsive in osteocytes will reveal new therapeutic targets that can be exploited to expand treatment options for conditions of low bone mass and skeletal fragility. This review will focus on recent data that implicates the cellular cytoskeleton as a sensor, integrator, and transducer of mechanical loading signals in bone, revealing a structure that is integral to mechano-sensing in bone that may contain druggable targets to improve bone mass and quality.
II. Cytoskeletal Elements in Osteocyte Mechano-Signaling
The cellular cytoskeleton provides internal structure and support to the cell and its organelles. Under mechanical stress, the cytoskeleton compresses and provides an opposing outward force. Additionally, the cytoskeleton indirectly links with the extracellular matrix and other cells through adherence junctions and forms specialized cell projections, making it well-positioned to sense mechanical forces like fluid shear stress, stresses on extracellular matrix attachments, and changes in stiffness, all of which compress, pull, and tug on cells and their extracellular connections (Figure 1A–B). Therefore, the cytoskeleton has obvious potential as a mechano-sensor.
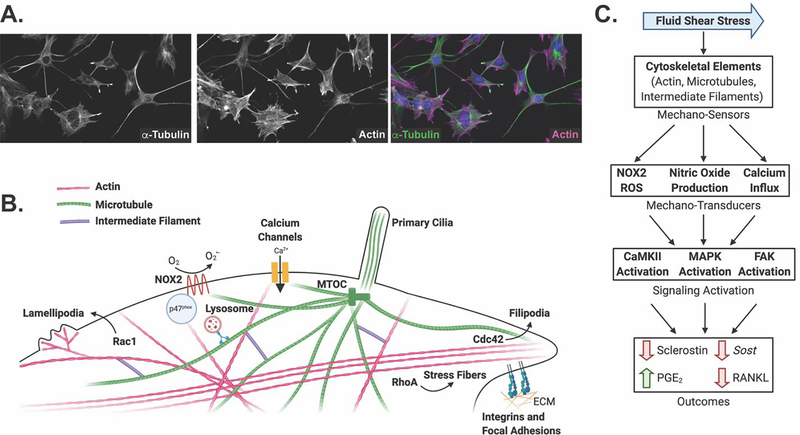
Figure 1: The cytoskeleton comprises an interconnected mechano-sensor that transduces mechanical signals into biological changes affecting bone cell function.
A. Immunofluorescent staining of α-tubulin and actin microfilaments shows their localization throughout the cell body and cell processes of Ocy454 cells. B. The three cytoskeletal elements – actin, microtubules, and intermediate filaments – form a highly interconnected network, as well as specialized structures, that sense and transduce mechanical signals in response to external forces, including fluid shear stress, substrate stiffness, and cell deformation. For example, a subset of post-translationally modified microtubules, the microtubule-based primary cilia, actin microfilaments, and actin-based structures such as lamellipodia, filipodia, stress fibers, and focal adhesions, have been implicated as bone mechano-sensors. Actin dynamics are regulated by Rac1, Cdc42, and RhoA, respectively. In addition, the cytoskeleton is linked to other mechano-sensors and transducers such as NOX2, calcium channels, integrins, and focal adhesions, which transduce changes in the extracellular matrix (ECM) to the intracellular cytoskeleton. C. Mechanical cues are sensed through multiple cytoskeletal mechano-sensors, which, in turn, activate many mechano-transducers including reactive oxygen species production, nitric oxide production, and calcium influx. These signaling molecules activate signal transduction cascades through CaMKII, MAPK, and FAK. Ultimately, these signaling pathways affect bone cell function through changes in sclerostin protein and gene expression, PGE2 release, and RANKL abundance.
The cytoskeleton is made of three main components – actin microfilaments, microtubule filaments, and intermediate filaments (Figure 1A and 1B). Septins and spectrins are additional components of the cytoskeleton, but little is known about their role in bone mechano-transduction. Each component has its own specific makeup and specialized function, although the organization of cytoskeletal proteins together creates an interacting dynamic network that can disassemble and reassemble to adapt to changing intra- and extracellular cues. As a result of this integration, specifically assessing the contribution of an individual cytoskeletal component to mechano-signaling can prove quite challenging.
The cytoskeleton is a well characterized mechano-transducer in many different cell types, serving as a signaling hub via interactions with mechanically activated calcium channels, ion channels, kinases, and GPCRs to initiate mechano-to-chemo signaling events essential to mechano-transduction11–13. In bone, microtubules, actin, primary cilia, integrins, and focal adhesions have all been linked to mechanically-induced bone responses. These cytoskeletal elements transduce mechanical signals to many different classes of mechano-transducers - such as calcium channels (i.e. Piezo14–18, transient receptor potential (TRP)19–22, and voltage-gated calcium (CaV)23,24 channels), Nitric Oxide Synthases (NOS)25,26, NADPH Oxidase 2 (NOX2)19,25,27, purinergic signaling20,28–30, and connexin4331 - to drive changes in bone cell function. These changes in cell function arise from changes in important effectors such as sclerostin, nitric oxide, PGE2, and the expression of osteoblast genes (Figure 1B–C).
Depending on the specific make up, modification, and cross-linking of the individual cytoskeletal components, the integrated cytoskeleton can vary drastically in its stiffness and in its with association with signaling proteins like ion channels, enzymes, and kinases. This property allows the cytoskeleton to be an adaptive mechano-sensor that can vary depending on the its current mechanical environment – which becomes its homeostatic setpoint. Changes from this homeostatic reference then elicit changes in osteocyte function. Additionally, sustained changes in the mechanical environment can cause a remodeling and adaptation of the cytoskeleton to establish a new homeostatic setpoint.
Here, we will expand on these cytoskeletal elements and their interactions to present a unified vision of how numerous mechano-sensors can integrate through the cytoskeleton to regulate bone cell homeostasis. Given the interconnectedness of and crosstalk between individual elements of the cytoskeleton, the cytoskeleton serves as a central hub of mechano-signaling in bone. As such, understanding how cytoskeletal components sense and transduce mechanical signals, individually and in unison, may ultimately reveal therapeutic targets that can be exploited to mimic mechanical stress and modulate bone formation.
i. Microtubule Structure and Dynamics
Microtubules are dynamic cytoskeletal filaments composed of α-tubulin and β-tubulin dimers that provide structural support to cells, work in conjunction with dynein and kinesin motors to facilitate vesicular and organellar transport, and are critical to cell division. Globular α-tubulin and β-tubulin dimers bound to GTP are incorporated into growing 25 nm diameter hollow filaments that originate at microtubule organizing centers (MTOCs) and extend to the periphery of the cell. Hydrolysis of bound GTP to GDP lowers the affinity for adjacent dimers, favoring depolymerization of microtubules (Figure 2A). It is this intrinsic dynamic instability and constant turn over that allows microtubules to readily adapt to changes in cellular dynamics32.
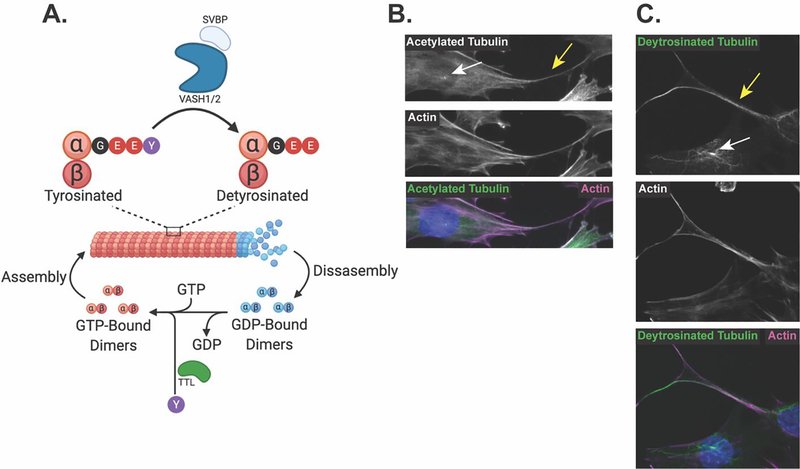
Figure 2: Microtubules are a dynamic structure that become post-translationally modified and are found in osteocytic cell bodies and cell processes.
A. GTP-bound microtubule α/β-tubulin dimers are incorporated into growing filaments. Intrinsic GTP-ase activity of the tubulin dimers cleaves GTP to GDP, promoting disassembly of microtubule filaments, while GTP exchange factors facilitate the transition from GDP- to GTP-bound dimers to promote reassembly of microtubules. VASH1/2 in complex with SVBP removes the terminal tyrosine on α-tubulin in stable microtubules, producing detyrosinated microtubules. This detyrosination can be reversed by TTL, which acts on released α/β-tubulin dimers to ligate the terminal tyrosine onto the α-tubulin tail. B. Immunofluorescent staining of acetylated tubulin and actin microfilaments shows their localization to both the cell body, primary cilia (white arrow) and the dendrite-like osteocyte cell processes (yellow arrow) of Ocy454 cells. C. Immunofluorescent staining of detyrosinated tubulin and actin microfilaments shows their localization to the cell body, primary cilia (white arrow) and the dendrite-like osteocyte cell processes (yellow arrow) of Ocy454 cells.
Specific post-translational modifications to the α/β-tubulin dimers can alter the stability, biophysical properties, protein-protein interactions, and functions of microtubules33. These diverse tubulin post-translational modifications, such as detyrosination, acetylation, methylation, polyglutamylation, glycylation, polyamination, phosphorylation, ubiquitinylation, sumoylation, and palmitoylation, are referred to as the tubulin code33,34. This tubulin code is critical as it confers specific functions to a subset of microtubules, allowing for the same base protein (α/β-tubulin) to carry out multiple functions depending on how they are modified.
With respect to mechano-transduction, two of these microtubule post-translational modifications – acetylation and detyrosination – are particularly important. Acetylation occurs within the lumen of the hollow microtubule filament where α-tubulin acetyl transferase (αTAT) transfers acetyl groups from acetyl-CoA to the lysine 40 residue on α-tubulin35. Acetylated microtubules are more stable and flexible, which makes them more resistant to snapping under mechanical stress36,37 (Figure 2B). This enhanced stability then allows for the opportunity for an additional post-translational modification to occur: detyrosination. Microtubule filaments become detyrosinated when the terminal tyrosine residue of the C-terminal tail of α-tubulin is cleaved by vasohibin 1 (VASH1) or vasohibin 2 (VASH2) in complex with small vasohibin binding protein (SVBP), revealing a glutamate residue38–41. Following microtubule depolymerization, tubulin tyrosine ligase (TTL) then acts on the α-tubulin tail, reattaching the terminal tyrosine to reverse this detyrosination. Similar to acetylation, microtubule detyrosination enhances the microtubule‟s ability to withstand mechanical load, allowing it to bend and buckle, without breaking, under mechanical stress42 (Figure 2C). Accordingly, this subset of acetylated and detyrosinated microtubules display increased flexibility and resistance to breakage that may be ideal for mechano-sensing and, importantly, are distinct from the dynamic pool of microtubules involved in cell division and organellar trafficking. These properties make this subset of mechano-responsive microtubule filaments an intriguing druggable target. These detyrosinated and acetylated microtubules are enriched in the long, dendrite-like cellular processes that extend from osteocytes, as well as in the primary cilia that extends from the cell body19,43–47 (Figure 2B–C, arrows). This is relevant because both the osteocyte dendritic processes and primary cilia have been implicated in bone mechano-signaling48–51.
ii. Microtubules in Bone Mechano-Transduction
Interest in microtubules as mechano-transducers in osteocytes is supported by parallel analyses of the dual role of microtubules in both the cytoskeleton as a whole and in the primary cilia. Several important studies laid the groundwork for microtubules as mechano-transducers in bone. In osteocytes, microtubules rearrange and increase their density in response to fluid shear stress52. Interestingly, the rearrangement of the microtubule cytoskeleton does not seem to occur in osteoblasts, which are believed to be less mechano-responsive than osteocytes53,54. Disrupting microtubules impairs mechano-transduction in bone cells, preventing fluid shear stress-induced calcium influx, down regulation of sclerostin protein, changes in Col1a1 and Mmp1 gene expression, and blunts the phosphorylation of various signaling kinases, including calcium/calmodulin kinase II (CaMKII), extracellular signal-regulated kinases 1/2 (ERK1/2), and focal adhesion kinase (FAK)19,55,56.
Recent work from our lab has revealed a signaling cascade, which is dependent upon a subset of detyrosinated microtubules, that links the fluid shear response of osteocytes to the loss of sclerostin protein (Figure 3A). Briefly, the application of fluid shear stress to cultured osteocytes initiates the production of reactive oxygen species from NOX2, the opening of transient receptor potential cation channel subfamily V member 4 (TRPV4) cation channels, which leads to calcium influx, the activation of CaMKII, and the stimulation of lysosomes that rapidly degraded sclerostin protein19,20,25.
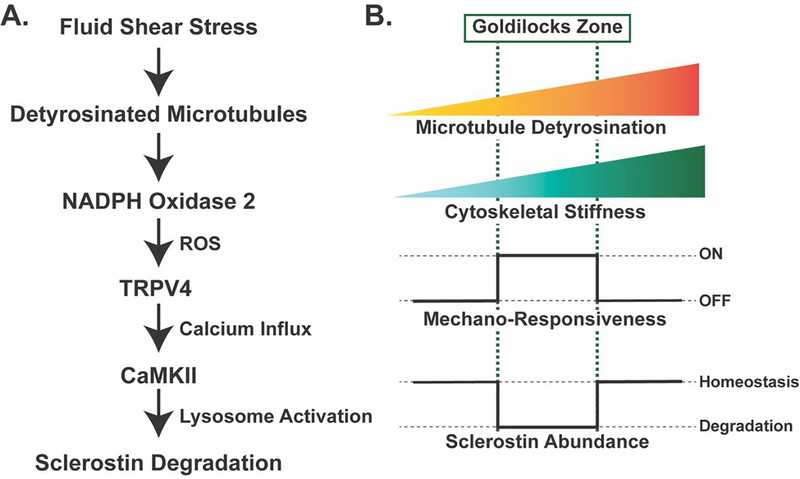
Figure 3: Mechano-sensing in Ocy454 cells through detyrosinated microtubules contributes to the regulated degradation of sclerostin protein and reveals a tunable mechano-sensor by contributing to changes in cytoskeletal stiffness.
A. Fluid shear stress is sensed through a pool of detyrosinated microtubules, which activate NOX2 to produce reactive oxygen species. This reactive oxygen species sensitizes TRPV4 calcium-permeable channels on the cell membrane to allow for calcium influx. Calcium influx activates CaMKII, which activates the rapid degradation of sclerostin protein by the lysosome19,25. B. The level of microtubule detyrosination affects the overall stiffness of the cytoskeleton. Based off in vitro studies in Ocy454 cells, there is a “Goldilocks” level of detyrosination and, in turn, cytoskeletal stiffness, that permits osteocyte mechano-responsiveness, allowing for fluid shear stress to degrade sclerostin protein to reduce its abundance. If detyrosination and cytoskeletal stiffness are above or below this Goldilocks zone, osteocytes are no longer mechano-responsive and sclerostin abundance is unchanged with fluid shear stress19.
Integral to the activation of this pathway is a subset of detyrosinated microtubules and their effect on cytoskeletal stiffness19. As osteocyte microtubule detyrosination increases, cytoskeletal stiffness also increases. The degree of detyrosination and, by extension, the degree of cytoskeletal stiffness, tunes the ability of the cells to respond to a specific fluid shear force to elicit calcium influx into the cell (Figure 3B). High levels of microtubule detyrosination make the cytoskeleton stiffer and resistant to fluid shear stress-induced calcium influx, activation of CaMKII, and loss of sclerostin protein. Low levels of microtubule detyrosination make the cytoskeleton too compliant and also prevents the mechano-response. However, a moderate level of detyrosination creates a “Goldilocks zone” of cytoskeletal stiffness that permits robust mechano-responsiveness, initiating the a cascade of NOX2-dependent reactive oxygen species, TRPV4 activation, calcium influx, CaMKII phosphorylation, and the degradation of sclerostin by the lysosome19,25. The finding that stiffness of the cytoskeleton can be tuned by the level of microtubule detyrosination parallels work in skeletal and cardiac muscle, where detyrosination affects mechano-sensitivity, NOX2 activation, and calcium signaling, indicating a conserved mechanism of mechano-transduction through detyrosinated microtubules across various cell types57.
While we have not yet shown that targeting detyrosinated microtubules in vivo alters the bone mechano-response, we have observed that the mechano-transduction pathway (Figure 3A) beginning with microtubules and ending in the degradation of sclerostin protein is necessary for the in vivo activation of bone formation in response to mechanical loading25. In vitro, microtubules are necessary for sensing fluid shear stress and subsequent activation of NOX2 that ultimately leads to the lysosomal degradation of sclerostin to permit bone formation19,25. In support of the relevance of this microtubule-dependent mechano-transduction pathway in vivo, inhibiting NOX2 pharmacologically prevents load-induced bone formation25. Similarly, blocking lysosomal function, which is responsible for the mechano-activated degradation of sclerostin protein, also prevents load-induced bone formation in vivo25. While these findings indirectly support a role for microtubules in the in vivo bone mechano-response, NOX2 activation and the lysosomal degradation of sclerostin originate at the level of mechano-sensing through the microtubule cytoskeleton.
Not only are detyrosinated microtubules involved in mechano-sensing and the activation of NOX2-ROS early in this pathway, detyrosinated microtubules may also be involved in sclerostin degradation at the terminus of this pathway. Detyrosinated microtubules are integral for the localization of lysosomes throughout the cell, with lysosomes accumulating on detyrosinated microtubules. Low detyrosination is associated with decreased lysosome abundance and the fusion of autophagosomes with lysosomes, which likely disrupts protein turnover58. This suggests the role of detyrosinated tubulin in bone cell mechano-signaling and mechano-responsiveness may be unsurprisingly multifactorial.
The issue of where these microtubules are acting to initiate these signaling events is less clear. Does fluid shear stress act through microtubules at extracellular matrix (ECM) attachment sites, where microtubules and actin interact with focal adhesions, at sites of cell-cell adhesions where the cytoskeleton links to cadherin junctions, or does the mechano-sensing occur throughout the cytoplasm? Another possibility is that mechano-sensing happens in the microtubule-based primary cilium.
There has been long-standing interest in the role of the primary cilium, a microtubule-based structure, in bone homeostasis and mechano-sensing. The non-motile primary cilium consists of nine circumferentially arranged microtubule doublets, named the axoneme, that extend outward from the MTOC into the extracellular space on the apical cell surface59. Like other microtubule mechano-sensors, microtubules of the primary cilium are heavily post-translationally modified by acetylation and detyrosination (Figure 2B–C, arrows). This microtubule-based antenna, once considered a vestigial organelle, has been implicated as a mechano-sensor in liver and kidney epithelial cells, spurring studies examining its role in osteocyte mechano-sensing60. The role of the primary cilium in osteocyte mechano-transduction has been widely reviewed49,50,61,62. Here, we highlight those findings that support the primary cilium’s role in mechanical load-induced bone formation, as studied in vitro and in vivo.
Genetic disruption of components of the primary cilium supports its role as a mechano-sensing organelle in vivo. Conditional deletion of Pkd1, which encodes polycystin-1, a putative mechano-sensor enriched in primary cilia63,64, or Kif3a, a subunit of a kinesin motor complex that is integral for intraflagellar transport of microtubule subunits and other axoneme proteins to primary cilia, in osteoblasts or osteocytes reduces load-induced bone formation and bone quality65–67. Similarly, disrupting intraflagellar transport in vitro prevents fluid shear stress-induced remodeling of microtubules and the upregulation of osteogenic genes52,68,69. The effects of targeting intraflagellar transport may also be due to alterations in the length of the primary cilium. Under fluid shear stress, the primary cilium amplifies applied strain, which is highly dependent on the length of the primary cilium, with longer cilium experiencing more tip deflection and, therefore, higher strain70–72. The opposite is also true, with shorter cilium being less mechano-responsive51,71,72. This strain amplification and deflection of the primary cilium is likely integral for activating mechano-transduction cascades in osteocytes. Mechanical stimulation of the primary cilia is associated with calcium influx through TRPV4 calcium-permeable ion channels73 and activation of adenylyl cyclase 674,75. The adenylyl cyclase 6-dependent production of cyclic-AMP is necessary for fluid shear stress-induced gene expression changes in vitro and load-induced bone formation in vivo74,75.
Whether the calcium influx through TRPV4 happens through channels actually on the primary cilium or are activated near the primary cilium is less clear51,76; studies that show calcium influx is activated by the primary cilium may be measuring calcium influx due to stress on the microtubule cytoskeleton as a whole. Indeed, it is very difficult to separate changes in the microtubule cytoskeleton as a whole and those in the primary cilia as they are functionally interconnected and difficult to independently target. Indeed, a recent study has highlighted this controversy in finding that stimulating the primary cilium directly does not cause calcium influx into the primary cilium76. This could mean that the subsequent calcium signaling typically observed after primary cilium activation is due to indirect activation of other cytoskeletal or plasma membrane-associated components or the primary cilium activates other, non-calcium effectors in response to mechanical events. Despite this controversy, interfering with cilium structure, length, or function does impact osteocyte mechano-responsiveness, supporting that this microtubule-based structure contributes to the osteocyte mechano-response directly or indirectly.
While all the studies above implicate both the microtubule cytoskeleton and the primary cilium as mechano-sensors in osteocytes, it is important to reiterate that it is challenging to discriminate the effect targeting microtubules has on the cell body microtubule cytoskeleton and on the primary cilium. Given that the primary cilium is a microtubule-based structure that originates from the same MTOC that anchors the cell body microtubule cytoskeleton and shares many of the same post-translational modifications, disrupting microtubules or the primary cilia likely has effects on the cytosolic microtubule cytoskeleton and vice versa52. Furthermore, the microtubules themselves directly and indirectly interact with other cytoskeletal components, like actin, actin binding proteins, and the large class of intermediate filaments, that each have also been linked to mechano-signaling.
iii. Actin Filament Structure and Dynamics
Actin microfilaments are polymers of individual globular actin monomers (G-actin) that assemble together, along with a number of co-factor and regulatory proteins, to form stabilized filaments (F-actin) of about 6 nm diameter. These thin and semi-flexible filaments allow for mechanical support and maintenance of cell shape but are also integral to cellular movement and locomotion77,78. Actin exists in a state of constant remodeling, where filaments grow at the positive end and depolymerize at the minus end. ATP-bound G-actin can be incorporated into the growing actin filament. Actin has intrinsic ATPase activity, which cleaves the ATP to ADP, thus favoring disassembly into G-actin monomers (Figure 4A). As the state of the cell changes, the equilibrium can be pushed to favor G-actin or F-actin depending on the co-factors and binding proteins present on each actin type.
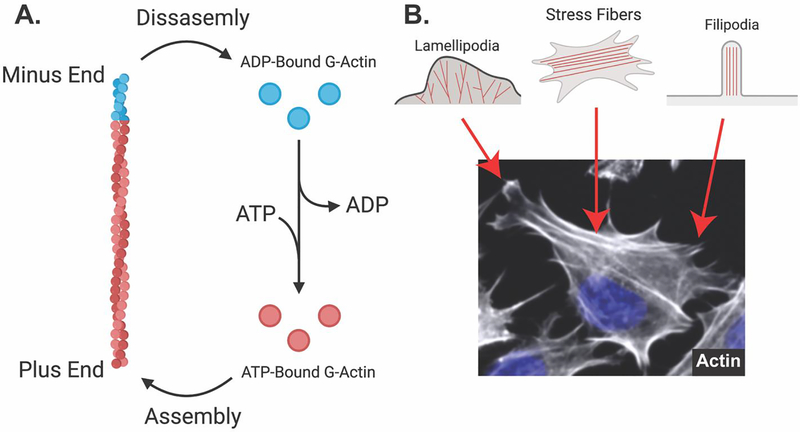
Figure 4: Actin microfilaments are dynamic and assemble into different structures in osteocytes.
A. Globular actin monomers bound to ATP are assembled into actin microfilaments (F-actin) on the plus end. Intrinsic ATP-ase activity of actin microfilaments facilitates the cleavage of ATP to ADP, which favors disassembly on the minus end. ADP-bound G-actin exchanges ADP for ATP, again promoting polymerization. B. Fluorescent staining (phalloidin) of Ocy454 cells shows different actin-based structures. Lamellipodia contain branched actin filaments and show ruffled membrane edges. Stress fibers contain bundles of actin filaments that feed into filipodia. Filipodia facilitate the formation of focal adhesions, attaching the cellular cytoskeleton to the extracellular matrix.
In non-contractile cells, actin is organized into many different structures, such as stress fibers, lamellipodia, and filipodia, which help with cell anchoring and aid with outside-in signaling (Figure 4B). The Rho family of small GTPases is integral to controlling actin dynamics to create these specialized structures. Members of the Rho family are activated downstream of G-protein coupled receptors, receptor tyrosine kinases, integrins, and other macromolecular structures that link the extracellular environment to affect intracellular changes. While there are 20 members of the Rho family79, focus has remained on three specific members in bone: RhoA, Rac1, and Cdc42 (Figure 1B and Figure 4B). RhoA stimulates actin polymerization and the formation of stress fibers through its effector proteins Rho-associated protein kinase (ROCK) and the formin mDia180–84. ROCK, through LIM domain kinase (LIMK) proteins85–87, inactivates cofilin to promote actin stability by preventing cofilin from binding to ADP-bound monomers in F-actin and severing the filaments, which would create free ends to allow for the release of actin monomers86,87. Rac1 contributes to the formation of lamellipodia, which are cell membrane ruffles containing branched actin fibers and are important for cell motility88,89. Cdc42 regulates the formation of filipodia, which contains linear actin fibers and are important for the anchoring of cells through focal adhesions88,89.
Focal adhesions contain actin stress fibers and, along with actin binding proteins such as α-actinin, talin, and vinculin, as well as integrins, form an attachment to the ECM to facilitate anchoring of the cell and its cytoskeleton to its substrate90–92. These focal adhesions are also rich with signaling molecules like FAK and Rho-family kinases, which uniquely positions focal adhesions as a signaling hub that can transduce changes in the extracellular environment, such as changes in mechanical stress, to the cytoskeleton, and to other nearby structures to influence cell function.
iv. Actin Microfilaments in Bone Mechano-Transduction
Actin filaments are enriched in the mechano-sensitive dendrite-like cell processes of osteocytes, similar to detyrosinated and acetylated microtubules30,31,79–81 (Figure 1A, 2B–C). Likewise, in response to fluid shear stress, actin stress fibers rearrange forming dense stress fibers oriented parallel to the direction of fluid shear stress54,96–105. These stress fibers can form as early as 15-minutes post-fluid shear stress55. This actin rearrangement is necessary for the activation of downstream signaling events following fluid shear stress stimulation. Disrupting actin and actin rearrangement prevents fluid shear stress-induced PGE2 production and prevents changes in Ptgs2/COX-2 mRNA and protein expression54,99,102,105,105–109. Similar to detyrosinated microtubules, actin stress fibers also increase cell stiffness, which may allow them to adapt the set point for mechano-sensitivity. In line with this concept, suppressing actin reorganization by silencing LIMK2 exacerbates the fluid shear stress-induced increase in COX-2 protein expression104, possibly due to a decrease in cellular stiffness, therefore increasing cell mechano-sensitivity110.
Studies focusing on mammalian target of rapamycin complex 2 (mTORc2) show that actin remodeling contributes to bone maintenance and the bone mechano-response. mTORc2 which contributes to cell spreading, actin polymerization, and strain-induced stress fiber formation, is a complex that is distinct from the nutrient, energy, and redox sensor mTORc1111,112. Their differential functions are associated with their composition - mTORc2 contains Rictor, whereas mTORc1 contains Raptor113,114. Conditionally deleting Rictor in osteochondroprogenitors, early or late osteoblasts, or osteocytes all produce bone phenotypes115–118. Though there are some sex-specific effects, all models generally have decreased bone mass, especially in cortical parameters, and decreased mineralization101–104. These phenotypes may be due to mesenchymal stem cells being pushed towards adipogenic differentiation, rather than osteogenic differentiation111. In strain matched loading experiments, deletion of Rictor in osteochondroprogenitors or osteocytes causes decreased periosteal mineral apposition rates (MAR) and bone formation rates (BFR) compared to control animals115,117. Rictor deletion also decreases the length and number of the mechano-sensitive osteocyte cell dendrite-like processes, which may explain the deficits in the loading response in conditional deletion animals117.
mTORc2 controls actin dynamics possibly through interacting with Rac and RhoA GTPases111,112. The small GTPases RhoA, Rac1, and Cdc42 can directly or indirectly regulate local actin filament assembly and disassembly. Fluid shear stress activates these small GTPases, which are necessary for β-catenin-dependent TCF/LEF activation to allow for the induction of Wnt target genes such as Ptgs2, Spp1, and Runx296,101,108,119. At a protein regulation level, RhoA, through its effector proteins ROCK and LIMK, allows for fluid shear stress-induced stress fiber formation and the activation of ERK1/2, p38, c-FOS, and COX-2103,104,108. Supporting a role of these small GTPases in vivo, deletion of Cdc42 in chondrocytes decreases calcification and decreases trabecular bone mineralization120. Similarly, Rac1 conditional deletion in osteoblasts and osteocytes causes a decrease in trabecular and cortical bone parameters, indicating that these small GTPases contribute to proper bone development121.
A key way that the actin cytoskeleton integrates with its mechanical environment is via focal adhesions. Focal adhesions are multi-protein complexes that create a physical linkage between the cytoskeleton and the ECM through integrins and facilitate in outside-in signaling, allowing cells to adapt to extracellular changes, including changing mechanical forces. Integrins are transmembrane, heterodimeric proteins made of an alpha subunit, of which there are 18, and a beta subunit, of which there are 8, that associate to form 24 unique heterodimeric integrins122. They are able to bind directly to components of the ECM, such as laminin, collagens, and fibronectin, through their extracellular domains. Intracellularly, integrins interact with the actin cytoskeleton, actin binding proteins like talin, α-actinin, filamin, vinculin, and the microtubule network via KANK to create a tether between the ECM and the cytoskeleton. It is through interaction with numerous cytoskeletal-binding proteins that integrins can activate many signal transduction cascades, such as FAK signaling, PI3K signaling, and integrin-linked kinase (ILK) signaling.
In situ, osteocytes have discrete structures that contain β3 integrins and resemble focal adhesions. These structures connect the osteocyte cell processes to the canalicular wall and amplify small strains, allowing for the transduction of mechanical signals into biological changes123–125. Stimulating focal adhesions in osteocytic cell processes amplifies applied strains and initiates a stronger calcium signal than stimulating the cell body or areas without firm attachment to the substrate23,48,72,125,126. Opposingly, disrupting β3 integrin attachment prevents calcium influx, blunts PGE2 release, BMP2 secretion and gene expression changes, and Alp, Ptgs2, and Spp1 gene expression changes after fluid shear stress97,105,106,127–129. Blocking αv, α2, α5, β3 or β1 integrins by preincubation with antibodies, disrupting expression with siRNA, or plating cells on a substrate that does not allow integrin attachment prevents fluid shear stress-induced increases in pERK, pJNK, and p-P38, which regulate gene expression of Ptgs2 and Spp1129,130.
In vivo, knocking out β1 integrins in mesenchymal cells with Twist2-Cre causes hypomineralization of E19.5 mouse embryos and results in poor survival after birth131. Deletion of β1 integrins in pre-osteoblasts using the Osterix-Cre mouse strain causes lagging skull mineralization and decreased cortical bone mineral density; however, deletion of β1 integrins or transgenic expression of a dominant negative β1 integrin construct in mature osteoblasts and osteocytes using the Osteocalcin-Cre mice has very little effect on bone mineralization131,132. In contrast to its role in late osteoblasts and osteocytes, knocking out β1 integrins in early osteoblasts with the Col1a1-Cre mice blunts the accrual of load-induced bone formation, indicating that, similar to in vitro results, β1 integrins are important for bone mechano-transduction in vivo.
FAK, a non-receptor tyrosine kinase, associates with the cytoskeleton through integrins, talin, and paxillin at focal adhesions. In bone cells, specifically, FAK colocalizes with integrins, whose attachment contributes to the phosphorylation and activation of FAK, and plays a role in mechano-transduction and the regulation of mechano-responsive genes and proteins133,134. Similar to targeting integrins, disrupting FAK signaling blunts Akt activation, β-catenin stabilization, and fluid shear stress-induced changes in C-fos135. FAK also regulates the expression of the sclerostin encoding gene, Sost, with FAK inhibition leading to decreased Sost gene expression and blunted fluid shear stress-induced decreases in Sost133. Deleting FAK in osteoblasts with a Col2.3-Cre blunts load-induced bone formation, but conditional knockout animals have the same relative increase in bone formation rate in loaded limbs compared to non-loaded limbs as control animals, indicating that, while FAK is important for bone mechano-transduction both in vitro and in vivo, a compensatory mechanism may exist in vivo that is absent in vitro136. In aggregate, actin and its many regulators play a large role in bone cell mechano-transduction and the development of bone with or without mechanical perturbations.
v. Intermediate Filaments in Osteoblasts and Osteocytes
Intermediate filaments, dubbed “intermediate” due to their diameter being between the small actin microfilaments and larger microtubules, comprise the third class of cytoskeletal proteins. Approximately 70 genes code for intermediate filament proteins of 6 different classes137–139. These include the keratins (types I and II); desmin, vimentin and glial fibrillar acidic protein (type III); neurofilament proteins, internexin and synemin (type IV); the nuclear lamins (type V); and other, tissue-specific filament proteins (e.g., phakinin, filensin, and nexin (type VI)). When purified, some form homopolymers (vimentin, desmin, GFAP: type III) or stoichiometric heteropolymers (keratins: types I and II)138,140–142.
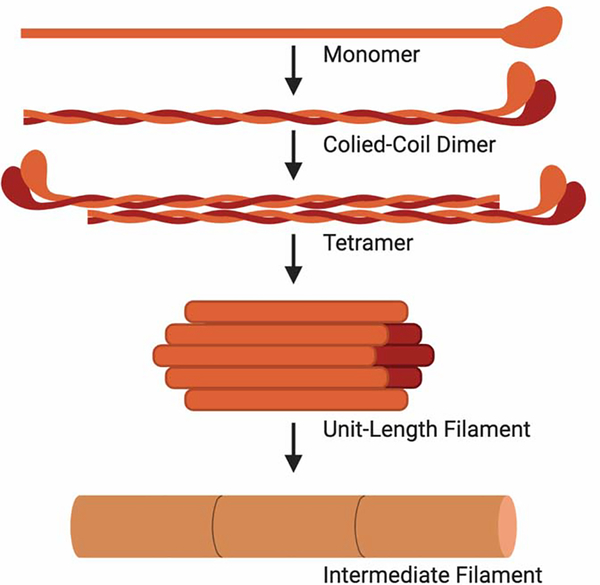
Despite the classification into one of the six subgroups, all intermediate filaments have a similar secondary structure143 (Figure 5). Two α-helical rod monomers twist around each other to form a very stable coiled-coil dimer. Two dimers then associate in an anti-parallel orientation to form a tetramer. Eight tetramers then coil around each other to form a unit-length filament (ULF) that is equivalent on both ends due to the anti-parallel nature of the dimer building blocks. This apolarity is distinct from both actin filaments and microtubules, which both have distinct positive and negative ends. ULFs can then anneal longitudinally to form long intermediate filaments. While the overall secondary structure is conserved, the N- and C-termini of intermediate filaments vary widely, likely contributing to the variance in function across the subtypes.
Figure 5: Most Intermediate filaments assemble into a similar secondary structure.
The basic unit of an intermediate filament is an α-helical monomer. Two monomers coil together to formed a coiled-coil dimer. Two coiled-coil dimers assemble in an anti-parallel fashion to form a tetramer that is apolar and is symmetrical on both ends. Eight tetramers then coil together to form a unit-length filament (ULF). ULFs can then anneal longitudinally to form long intermediate filaments.
In addition to their self-assembly, intermediate filament bundles are also made and stabilized with accessory proteins. Not only do these accessory proteins help bundle intermediate filaments, they can also facilitate the interaction of intermediate filaments with other components of the cytoskeleton. For example, plectin is integral to bundling intermediate filaments144–147 but it also links intermediate filaments to actin filaments and microtubules, creating a large, interconnected cytoskeleton148,149. Intermediate filaments also direct the assembly of microtubules150–152 thereby modulating many cellular functions, including motility, trafficking of cargo, migration, and mechano-transduction153–157. The scaffolding functions of intermediate filaments can have profound effects on cellular behavior, including changes in cellular gene expression158–165. These properties of intermediate filaments also underscore the inherent challenges of trying to ascribe mechano-transduction roles exclusively to a subset of the cytoskeleton, as these components are all interconnected and interdependent.
Though the literature examining the role of intermediate filaments in bone mechano-transduction has been relatively sparse, intermediate filaments have properties that likely place them in a position to sense and transduce mechanical signals. Due to their coiled-coil structure, intermediate filaments can convert from an α-helical structure to a β-sheet structure, allowing for a deformation of up to 300% without breaking166,167. Where type I-IV and VI intermediate filaments are cytoplasmic, type V lamins are nuclear proteins that help to support to structure of the nucleus. Similar to cytoplasmic intermediate filaments, lamins are important for the mechanical properties of the nucleus. Lamins A and B modulate the viscoelasticity and “shock absorber” properties of the nucleus, allowing for transduction of stretching and compressive forces, respectively168,169. Given that intermediate filaments are poised to act as mechano-sensors in both the cytoplasm and the nucleus, combined with their attachment to other cytoskeletal elements, intermediate filaments are likely able to resist cell deformation and transduce mechanical signals throughout the entire cytoskeleton, activating many downstream signaling cascades to influence bone cell function.
While little direct evidence demonstrates specific roles for intermediate filaments in osteocyte mechano-transduction, several studies demonstrate a role of intermediate filaments in bone development. Osteoblasts express the cytoplasmic intermediate filament proteins: vimentin, keratins, and synemin47,170–174. Global deletion of synemin results in osteopenia in male mice, with decreased trabecular bone, reduced cross-sectional thickness, and decreased osteoblast number despite an increased osteogenic capacity170. Unlike most intermediate filaments, synemin does not homopolymerize175 or form 1:1 heteropolymers with other subunits. Rather, it co-assembles into desmin or vimentin filaments176–178 and associates with keratin filaments177 as well as with other proteins179. Furthermore, synemin is not only an intermediate filament, it is also an A-kinase anchoring protein (AKAP) with the ability to regulate phosphorylation of other proteins in its vicinity180,181. AKAPs organize distinct signaling compartments by tethering Protein Kinase A (PKA) to specific sub-cellular domains. Thus, AKAPs can permit diverse biologic responses to similar cues that converge on cyclic-AMP-PKA-dependent pathways. Understanding how AKAPs spatially control signaling domains in cells has provided unique insights into how diverse biological outputs can occur in response to similar stimuli182–185. This is important in bone, where cyclic-AMP-activating hormones like prostaglandins, PTH, and PTHrP are not only potent bone anabolic agents when administered therapeutically, but also play an important role in calcium homeostasis and skeletal development. While the absence of synemin in skeletal and cardiac muscle results in subtle phenotypes186,187, the skeletal phenotype is comparatively quite severe170.
Other intermediate filaments, such as keratin and lamin A/C, are also implicated in bone development. Acidic keratins and basic keratins comprise type I and II intermediate filaments, respectively, and they heterodimerize to form keratin filaments. Deletion of keratin 14 decreases osteoblast mineralization, likely due to decrease osteoblastogenesis188. Oppositely, deletion of keratin 8 improves bone mass in a mouse model of cystic fibrosis, indicating a repressor role of keratin 8 in bone development171. Keratins regulate the activation of mTOR and Akt, important regulators of osteoblast energetics and actin dynamics, possibly indicating crosstalk between keratins and actin to control bone development and cell function136.
In addition to cytoplasmic keratins, nuclear lamin A/C also affects bone development and is implicated in bone diseases. Lamins are segregated into Type A lamins, which contains two lamins (Lamins A and C) produced due to splice variation of the Lmna gene, and Type B lamins produced from the genes Lmnb1 and Lmnb2. Lamin A/C can direct cell differentiation, with high levels of Lamin A associated with enhanced osteogenic differentiation189, expression decreases with age190, and mutations in the gene encoding Lamin A/C, Lmna, is a common genetic cause of Hutchinson Gilford Progeria Syndrome (HGPS), a disease that is grouped into “early aging” diseases. Knocking out Lamin A/C systemically, mutating residue L530P, or overexpressing the common HGPS mutations C1824T in osteoblasts all cause a progeroid phenotype with decreased body weight and decreased bone mineral density due to decreased osteoblast differentiation and activity because of decreased β-catenin activation and translocation to the nucleus191–196. Similarly, Zmpste24 is a metalloprotease that processes pro-lamin into the functional version of lamin A/C197,198. Knocking out Zmpste24 results in a similar phenotype as the HPGS moues models, resulting in decreased body weight and bone mass, resulting in the development of spontaneous rib fractures191,197. Reflecting the deficits in β-catenin activation found in vitro and in vivo, activating Wnt/β-catenin signaling in osteoblasts by targeting the osteocyte-derived Wnt antagonist sclerostin with neutralizing antibodies rescues the decreased trabecular parameters in Zmpste24 knockout animals191.
Evidence from in vitro and in vivo studies supports a role of intermediate filaments; however, the contribution of these intermediate filaments to the bone mechano-response has yet to be fully explored. It is expected that, due to the deficits in β-catenin signaling and osteoblastogenesis, intermediate filaments are necessary for the mechano-response and mutations or knockouts of these proteins will lead to decreased bone formation in response to mechanical load. However, these studies will have to be completed to fully examine this hypothesis.
vi. Spectrin and Septins: Other Cytoskeletal Elements
Despite not being categorized into a specific type of cytoskeletal element, spectrin and septins interact with other components of the cytoskeleton to help stabilize the cytoskeleton. Spectrin colocalizes along actin microfilaments in the osteocyte cell process94. GWAS studies have associated single nucleotide polymorphisms (SNPs) in the SPTBN1, the gene that encodes for spectrin B1, with increased risk of fracture147–150. Spectrin contributes to the cellular distribution of endothelial NOS (eNOS) and nitric oxide production, which contributes to fluid shear stress-induced regulation of sclerostin abundance in osteocytes25,26. Disrupting spectrin also increases ATP in osteocytes, which contributes to mechanically-induced calcium oscillations20,199. Septins, another filamentous component of the cytoskeleton, are necessary for osteoclastic bone formation200. However, they have not yet been implicated in osteocyte mechano-transduction. Interestingly, septins localize to the base of the primary cilia, placing them near a mechano-sensitive structure201. While not yet explored, both spectrins and septins aid in vesicle transport along microtubules202,203, which may have large implications in osteocyte mechano-transduction given the necessity of microtubules and lysosomal degradation in the regulation of sclerostin protein abundance following fluid shear stress and mechanical load19,25.
vii. The Cytoskeleton Links to the Nucleoskeleton
One way that the cytoskeleton influences cell behavior is through direct transmission of mechanical forces to the nucleus. Through connections with the cytoskeleton, nuclear dynamics change in response to mechanical cues, allowing for changes in gene expression and cell function204. The Linker of Nucleus and Cytoskeleton (LINC) complex on the nuclear envelope interacts with microtubules, actin, and intermediate filaments, which transduce mechanical signals to the nucleus205. For example, disrupting the LINC complex impairs intracellular force transduction, resulting in dysregulated β-catenin translocation into the nucleus206, which ultimately affects osteogenic differentiation. Indicating a reciprocal regulation between nuclear and cytoskeletal dynamics, disrupting the LINC complex affects cytoskeletal architecture and disrupting the actin cytoskeleton changes the morphology of the nucleus, partially due to disconnected LINC complexes207,208. Additionally, the stiffness of the extracellular matrix and the polymerization state of the actin cytoskeleton can influence the lineage allocation of mesenchymal stem cells111,209. Interestingly, disrupting the actin cytoskeleton causes translocation of G-actin monomers into the nucleus, where they can form actin filaments, increasing osteogenic differentiation and bone formation207,210,211. From this, it is clear that the cytoskeleton and the nucleus interact in a complex way, with one affecting the other, which will have large effects on cellular function and regulation. It is important to take nuclear dynamics into account when disrupting any aspect of the cytoskeleton, since the LINC complex can interact with all cytoskeletal components.
III. Conclusions
In all, it is clear that the cytoskeleton is central to bone cell mechano-transduction and that the role of the cytoskeleton in this process is not clear cut. All three cytoskeletal elements – actin, microtubules, and intermediate filaments – affect mechano-transduction individually; however, given the high level of interconnectedness between the three elements and other cellular structures, it raises multiple questions about these mechano-sensitive elements: do these cytoskeletal elements act independently or, due to the interconnectedness, are all studies indirectly querying the same sensor (i.e., the integrated cytoskeleton), with disruption of one element causing a collapse of the entire mechano-sensitive cytoskeleton? Alternatively, do these individual cytoskeletal elements act in series, with certain cytoskeletal elements detecting different magnitudes or types of strain to activate different downstream pathways with distinct biological effectors? While much is known about the cytoskeleton in mechano-signaling, future work should focus on continuing to tease apart these nuanced, yet critically important, molecular mechanisms.
Additionally, not only is there tremendous interconnectedness within the cytoskeleton itself, the cytoskeleton also creates discrete cellular structures. Structures such as the primary cilia and focal adhesions, distinct subpopulations of post-translationally modified components, attachments to other cellular signaling components such as calcium channels and NOX2, and the control the cytoskeleton has on lysosome localization, all affect osteocyte mechano-transduction (Figure 1B). Understanding this interconnected network is fundamental to grasping the complexities of how cells experience and respond to their environment. Regardless, many sources of evidence now point to the cytoskeleton as a mechano-sensor in bone. As we expand the molecular details of how, where, and when the cytoskeleton responds to mechanical cues, we will expand the arsenal of druggable targets to impact disease of skeletal fragility.
Highlights.
The cytoskeleton is composed of microtubules, actin, and intermediate filaments
The cytoskeleton forms a network that interacts with signaling proteins
The cytoskeletal network converts mechanical forces into biochemical signals
In bone cells, these mechano-transduction cascades regulate skeletal physiology
Acknowledgements:
Cartoon figures were created with Biorender.com. This work was supported by grants from the National Institutes of Health (AR071614, JPS; and GM008181, NRG).
Footnotes
Competing Interests: Declaration of competing interest JPS holds a patent for the targeting microtubules (part of a mechano-transduction pathway mentioned in this review) to improve bone mass (US Patent No US 2019/0351055 A1).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Storey A & Smith HK Unique aspects of competitive weightlifting: performance, training and physiology. Sports Med. Auckl. NZ 42, 769–790 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Evans SF, Chang H & Knothe Tate ML Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum? Tissue Eng. Part B Rev. 19, 147–159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang H & Knothe Tate ML Structure-function relationships in the stem cell‟s mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure. Mol. Cell. Biomech. MCB 8, 297–318 (2011). [PMC free article] [PubMed] [Google Scholar]
- 4.Song MJ et al. Mapping the mechanome of live stem cells using a novel method to measure local strain fields in situ at the fluid-cell interface. PloS One 7, e43601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein-Nulend J, van Oers RFM, Bakker AD & Bacabac RG Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 25, 1427–1437 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Thompson WR, Rubin CT & Rubin J Mechanical regulation of signaling pathways in bone. Gene 503, 179–193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uda Y, Azab E, Sun N, Shi C & Pajevic PD Osteocyte Mechanobiology. Curr. Osteoporos. Rep. 15, 318–325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Calle J, Sato AY & Bellido T Role and mechanism of action of sclerostin in bone. Bone 96, 29–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerschan-Schindl K Romosozumab: a novel bone anabolic treatment option for osteoporosis? Wien. Med. Wochenschr. 1946 170, 124–131 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman F et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 375, 1532–1543 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Ohashi K, Fujiwara S & Mizuno K Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. (Tokyo) 161, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Martino F, Perestrelo AR, Vinarský V, Pagliari S & Forte G Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 9, 824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher DA & Mullins RD Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J et al. Blocking glucocorticoid signaling in osteoblasts and osteocytes prevents mechanical unloading-induced cortical bone loss. Bone 130, 115108 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Sun W et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L et al. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 11, 282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jiang J, Yang X, Wang L & Xiao B Tethering Piezo channels to the actin cytoskeleton for mechanogating via the E-cadherin-β-catenin mechanotransduction complex. bioRxiv 2020.05.12.092148 (2020) doi: 10.1101/2020.05.12.092148. [DOI] [PubMed] [Google Scholar]
- 19.Lyons JS et al. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci. Signal. 10, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KM et al. TRPV4 calcium influx controls sclerostin protein loss independent of purinergic calcium oscillations. Bone 136, 115356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrigan MA et al. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 8, 3824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, Hirao A & Mizuno A Microtubule-associated [corrected] protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J. Biol. Chem. 278, 51448–51453 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Cabahug-Zuckerman P et al. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 36, 642–652 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson WR et al. Association of the α(2)δ(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 26, 2125–2139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould NR et al. Disparate Bone Anabolic Cues Activate Bone Formation by Regulating the Rapid Lysosomal Degradation of Sclerostin Protein. bioRxiv 2020.10.26.355800 (2020) doi: 10.1101/2020.10.26.355800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X-T et al. The potential role of spectrin network in the mechanotransduction of MLO-Y4 osteocytes. Sci. Rep. 7, 40940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J-R et al. p47phox-Nox2-dependent ROS Signaling Inhibits Early Bone Development in Mice but Protects against Skeletal Aging. J. Biol. Chem. 290, 14692–14704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Liu D, Ke HZ, Duncan RL & Turner CH The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 280, 42952–42959 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A & Gartland A P2X7 receptors: role in bone cell formation and function. J. Mol. Endocrinol. 54, R75–88 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Rumney RMH, Wang N, Agrawal A & Gartland A Purinergic signalling in bone. Front. Endocrinol. 3, 116 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkin LI, Speacht TL & Donahue HJ Cx43 and mechanotransduction in bone. Curr. Osteoporos. Rep. 13, 67–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchison T & Kirschner M Dynamic instability of microtubule growth. Nature 312, 237–242 (1984). [DOI] [PubMed] [Google Scholar]
- 33.Janke C & Magiera MM The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Gadadhar S, Bodakuntla S, Natarajan K & Janke C The tubulin code at a glance. J. Cell Sci. 130, 1347–1353 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Ly N et al. αTAT1 controls longitudinal spreading of acetylation marks from open microtubules extremities. Sci. Rep. 6, 35624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portran D, Schaedel L, Xu Z, Théry M & Nachury MV Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 19, 391–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Hu Y, Qi S, Luo X & Yu H Structural basis of tubulin detyrosination by vasohibins. Nat. Struct. Mol. Biol. 26, 583–591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwenhuis J & Brummelkamp TR The Tubulin Detyrosination Cycle: Function and Enzymes. Trends Cell Biol. 29, 80–92 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Nieuwenhuis J et al. Vasohibins encode tubulin detyrosinating activity. Science 358, 1453–1456 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Aillaud C et al. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Robison P et al. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352, aaf0659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westermann S & Weber K Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell Biol. 4, 938–947 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Wloga D, Joachimiak E, Louka P & Gaertig J Posttranslational Modifications of Tubulin and Cilia. Cold Spring Harb. Perspect. Biol. 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzbekov RE et al. Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc. Microanal. Off. J. Microsc. Soc. Am. Microbeam Anal. Soc. Microsc. Soc. Can. 18, 1430–1441 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Murshid SA et al. Actin and microtubule cytoskeletons of the processes of 3D-cultured MC3T3-E1 cells and osteocytes. J. Bone Miner. Metab. 25, 151–158 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Tanaka-Kamioka K, Kamioka H, Ris H & Lim SS Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 13, 1555–1568 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Wu D, Schaffler MB, Weinbaum S & Spray DC Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process. Proc. Natl. Acad. Sci. U. S. A. 110, 12096–12101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoey DA, Chen JC & Jacobs CR The primary cilium as a novel extracellular sensor in bone. Front. Endocrinol. 3, 75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temiyasathit S & Jacobs CR Osteocyte primary cilium and its role in bone mechanotransduction. Ann. N. Y. Acad. Sci. 1192, 422–428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malone AMD et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. U. S. A. 104, 13325–13330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espinha LC, Hoey DA, Fernandes PR, Rodrigues HC & Jacobs CR Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskelet. Hoboken NJ 71, 435–445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein-Nulend J et al. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 9, 441–445 (1995). [DOI] [PubMed] [Google Scholar]
- 54.Norvell SM, Ponik SM, Bowen DK, Gerard R & Pavalko FM Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J. Appl. Physiol. Bethesda Md 1985 96, 957–966 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Li P et al. Cytoskeletal reorganization mediates fluid shear stress-induced ERK5 activation in osteoblastic cells. Cell Biol. Int. 36, 229–236 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Myers KA, Rattner JB, Shrive NG & Hart DA Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem. Biophys. Res. Commun. 364, 214–219 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Kerr JP et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat. Commun. 6, 8526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohan N, Sorokina EM, Verdeny IV, Alvarez AS & Lakadamyali M Detyrosinated microtubules spatially constrain lysosomes facilitating lysosome-autophagosome fusion. J. Cell Biol. 218, 632–643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davenport JR & Yoder BK An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am. J. Physiol. Renal Physiol. 289, F1159–1169 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Whitfield JF Primary cilium--is it an osteocyte‟s strain-sensing flowmeter? J. Cell. Biochem. 89, 233–237 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A & Weinbaum S Mechanosensation and transduction in osteocytes. Bone 54, 182–190 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Kaku M & Komatsu Y Functional Diversity of Ciliary Proteins in Bone Development and Disease. Curr. Osteoporos. Rep. 15, 96–102 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Chauvet V et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 114, 1433–1443 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nauli SM et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Moore ER, Chen JC & Jacobs CR Prx1-Expressing Progenitor Primary Cilia Mediate Bone Formation in response to Mechanical Loading in Mice. Stem Cells Int. 2019, 3094154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Temiyasathit S et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PloS One 7, e33368 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao Z et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 25, 2418–2432 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haycraft CJ et al. Intraflagellar transport is essential for endochondral bone formation. Dev. Camb. Engl. 134, 307–316 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Hoey DA, Kelly DJ & Jacobs CR A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res. Commun. 412, 182–187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spasic M & Jacobs CR Lengthening primary cilia enhances cellular mechanosensitivity. Eur. Cell. Mater. 33, 158–168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding D et al. Pharmacological Regulation of Primary Cilium Formation Affects the Mechanosensitivity of Osteocytes. Calcif. Tissue Int. 107, 625–635 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Vaughan TJ, Mullen CA, Verbruggen SW & McNamara LM Bone cell mechanosensation of fluid flow stimulation: a fluid-structure interaction model characterising the role integrin attachments and primary cilia. Biomech. Model. Mechanobiol. 14, 703–718 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Lee KL et al. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwon RY, Temiyasathit S, Tummala P, Quah CC & Jacobs CR Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 24, 2859–2868 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee KL et al. Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 28, 1157–1165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delling M et al. Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blanchoin L, Boujemaa-Paterski R, Sykes C & Plastino J Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Dominguez R & Holmes KC Actin structure and function. Annu. Rev. Biophys. 40, 169–186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boureux A, Vignal E, Faure S & Fort P Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol. Biol. Evol. 24, 203–216 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hotulainen P & Lappalainen P Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173, 383–394 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tominaga T et al. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell 5, 13–25 (2000). [DOI] [PubMed] [Google Scholar]
- 82.Watanabe N et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 16, 3044–3056 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leung T, Chen XQ, Manser E & Lim L The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 16, 5313–5327 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tojkander S, Gateva G & Lappalainen P Actin stress fibers--assembly, dynamics and biological roles. J. Cell Sci. 125, 1855–1864 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Maekawa M et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285, 895–898 (1999). [DOI] [PubMed] [Google Scholar]
- 86.Andrianantoandro E & Pollard TD Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Cao W, Goodarzi JP & De La Cruz EM Energetics and kinetics of cooperative cofilin-actin filament interactions. J. Mol. Biol. 361, 257–267 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Kurokawa K, Itoh RE, Yoshizaki H, Nakamura YOT & Matsuda M Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell 15, 1003–1010 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nobes CD & Hall A Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995). [DOI] [PubMed] [Google Scholar]
- 90.Rafiq NBM et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat. Mater. 18, 638–649 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Seetharaman S & Etienne-Manneville S Microtubules at focal adhesions - a double-edged sword. J. Cell Sci. 132, (2019). [DOI] [PubMed] [Google Scholar]
- 92.Burridge K Focal adhesions: a personal perspective on a half century of progress. FEBS J. 284, 3355–3361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sugawara Y, Kamioka H, Honjo T, Tezuka K & Takano-Yamamoto T Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 36, 877–883 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Kamioka H, Sugawara Y, Honjo T, Yamashiro T & Takano-Yamamoto T Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 19, 471–478 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Zhang D et al. Extracellular Matrix Elasticity Regulates Osteocyte Gap Junction Elongation: Involvement of Paxillin in Intracellular Signal Transduction. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 51, 1013–1026 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Wan Q, Cho E, Yokota H & Na S RhoA GTPase interacts with beta-catenin signaling in clinorotated osteoblasts. J. Bone Miner. Metab. 31, 520–532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mai Z et al. Single bout short duration fluid shear stress induces osteogenic differentiation of MC3T3-E1 cells via integrin β1 and BMP2 signaling cross-talk. PloS One 8, e61600 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malone AMD et al. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am. J. Physiol. Cell Physiol. 292, C1830–1836 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ponik SM, Triplett JW & Pavalko FM Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J. Cell. Biochem. 100, 794–807 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Jackson WM, Jaasma MJ, Tang RY & Keaveny TM Mechanical loading by fluid shear is sufficient to alter the cytoskeletal composition of osteoblastic cells. Am. J. Physiol. Cell Physiol. 295, C1007–1015 (2008). [DOI] [PubMed] [Google Scholar]
- 101.Arnsdorf EJ, Tummala P, Kwon RY & Jacobs CR Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 122, 546–553 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen NX et al. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am. J. Physiol. Cell Physiol. 278, C989–997 (2000). [DOI] [PubMed] [Google Scholar]
- 103.Fu Q, Wu C, Shen Y, Zheng S & Chen R Effect of LIMK2 RNAi on reorganization of the actin cytoskeleton in osteoblasts induced by fluid shear stress. J. Biomech. 41, 3225–3228 (2008). [DOI] [PubMed] [Google Scholar]
- 104.Yang Z et al. Inhibition of FSS-induced actin cytoskeleton reorganization by silencing LIMK2 gene increases the mechanosensitivity of primary osteoblasts. Bone 74, 182–190 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Pavalko FM et al. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. 275, C1591–1601 (1998). [PubMed] [Google Scholar]
- 106.Ponik SM & Pavalko FM Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J. Appl. Physiol. Bethesda Md 1985 97, 135–142 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Simfia I, Schiavi J & McNamara LM ROCK-II inhibition suppresses impaired mechanobiological responses in early estrogen deficient osteoblasts. Exp. Cell Res. 396, 112264 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Hamamura K et al. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect. Tissue Res. 53, 398–406 (2012). [DOI] [PubMed] [Google Scholar]
- 109.McGarry JG, Klein-Nulend J & Prendergast PJ The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem. Biophys. Res. Commun. 330, 341–348 (2005). [DOI] [PubMed] [Google Scholar]
- 110.Jaasma MJ, Jackson WM, Tang RY & Keaveny TM Adaptation of cellular mechanical behavior to mechanical loading for osteoblastic cells. J. Biomech. 40, 1938–1945 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Sen B et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 29, 78–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jacinto E et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 (2004). [DOI] [PubMed] [Google Scholar]
- 113.Sarbassov DD et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. CB 14, 1296–1302 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Wullschleger S, Loewith R & Hall MN TOR signaling in growth and metabolism. Cell 124, 471–484 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Chen J, Holguin N, Shi Y, Silva MJ & Long F mTORC2 signaling promotes skeletal growth and bone formation in mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 30, 369–378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lai P et al. Loss of Rictor with aging in osteoblasts promotes age-related bone loss. Cell Death Dis. 7, e2408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lewis KJ et al. The mTORC2 Component Rictor Is Required for Load-Induced Bone Formation in Late-Stage Skeletal Cells. JBMR Plus 4, e10366 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu D-M et al. Rictor/mTORC2 loss in osteoblasts impairs bone mass and strength. Bone 90, 50–58 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Wan Q, Cho E, Yokota H & Na S Rac1 and Cdc42 GTPases regulate shear stress-driven β-catenin signaling in osteoblasts. Biochem. Biophys. Res. Commun. 433, 502–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suzuki W et al. Cdc42 is critical for cartilage development during endochondral ossification. Endocrinology 156, 314–322 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Lane SW et al. Rac signaling in osteoblastic cells is required for normal bone development but is dispensable for hematopoietic development. Blood 119, 736–744 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takada Y, Ye X & Simon S The integrins. Genome Biol. 8, 215 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V & Schaffler MB Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. Hoboken NJ 2007 292, 355–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, McNamara LM, Schaffler MB & Weinbaum S A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc. Natl. Acad. Sci. U. S. A. 104, 15941–15946 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thi MM, Suadicani SO, Schaffler MB, Weinbaum S & Spray DC Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proc. Natl. Acad. Sci. U. S. A. 110, 21012–21017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geoghegan IP, Hoey DA & McNamara LM Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos. Rep. 17, 195–206 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Haugh MG, Vaughan TJ & McNamara LM The role of integrin α(V)β(3) in osteocyte mechanotransduction. J. Mech. Behav. Biomed. Mater. 42, 67–75 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Litzenberger JB, Kim J-B, Tummala P & Jacobs CR Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif. Tissue Int. 86, 325–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee D-Y et al. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 23, 1140–1149 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Plotkin LI et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am. J. Physiol. Cell Physiol. 289, C633–643 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Shekaran A et al. The effect of conditional inactivation of beta 1 integrins using twist 2 Cre, Osterix Cre and osteocalcin Cre lines on skeletal phenotype. Bone 68, 131–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Globus RK et al. Skeletal phenotype of growing transgenic mice that express a function-perturbing form of beta1 integrin in osteoblasts. Calcif. Tissue Int. 76, 39–49 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Sato T et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 11, 3282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wozniak M, Fausto A, Carron CP, Meyer DM & Hruska KA Mechanically strained cells of the osteoblast lineage organize their extracellular matrix through unique sites of alphavbeta3-integrin expression. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 15, 1731–1745 (2000). [DOI] [PubMed] [Google Scholar]
- 135.Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JMA & Klein-Nulend J Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem. Biophys. Res. Commun. 391, 364–369 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Wang Y et al. Focal adhesion proteins Pinch1 and Pinch2 regulate bone homeostasis in mice. JCI Insight 4, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eriksson JE et al. Introducing intermediate filaments: from discovery to disease. J. Clin. Invest. 119, 1763–1771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hesse M, Magin TM & Weber K Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J. Cell Sci. 114, 2569–2575 (2001). [DOI] [PubMed] [Google Scholar]
- 139.Preisner H, Habicht J, Garg SG & Gould SB Intermediate filament protein evolution and protists. Cytoskelet. Hoboken NJ 75, 231–243 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Herrmann H & Aebi U Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 8, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Omary MB Intermediate filament proteins of digestive organs: physiology and pathophysiology. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G628–G634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacob JT, Coulombe PA, Kwan R & Omary MB Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godsel LM, Hobbs RP & Green KJ Intermediate filament assembly: dynamics to disease. Trends Cell Biol. 18, 28–37 (2008). [DOI] [PubMed] [Google Scholar]
- 144.Hieda Y, Nishizawa Y, Uematsu J & Owaribe K Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 116, 1497–1506 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lieska N, Yang HY & Goldman RD Purification of the 300K intermediate filament-associated protein and its in vitro recombination with intermediate filaments. J. Cell Biol. 101, 802–813 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pytela R & Wiche G High molecular weight polypeptides (270,000–340,000) from cultured cells are related to hog brain microtubule-associated proteins but copurify with intermediate filaments. Proc. Natl. Acad. Sci. U. S. A. 77, 4808–4812 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Castañón MJ, Walko G, Winter L & Wiche G Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem. Cell Biol. 140, 33–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Svitkina TM, Verkhovsky AB & Borisy GG Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 135, 991–1007 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Foisner R, Bohn W, Mannweiler K & Wiche G Distribution and ultrastructure of plectin arrays in subclones of rat glioma C6 cells differing in intermediate filament protein (vimentin) expression. J. Struct. Biol. 115, 304–317 (1995). [DOI] [PubMed] [Google Scholar]
- 150.Coulombe PA, Bousquet O, Ma L, Yamada S & Wirtz D The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol. 10, 420–428 (2000). [DOI] [PubMed] [Google Scholar]
- 151.Whipple RA et al. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 68, 5678–5688 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gan Z et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 3, 500–501 (2016). [DOI] [PubMed] [Google Scholar]
- 153.Potokar M et al. Cytoskeleton and vesicle mobility in astrocytes. Traffic Cph. Den. 8, 12–20 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Gerashchenko MV, Chernoivanenko IS, Moldaver MV & Minin AA Dynein is a motor for nuclear rotation while vimentin IFs is a „brake‟. Cell Biol. Int. 33, 1057–1064 (2009). [DOI] [PubMed] [Google Scholar]
- 155.Chang L et al. The dynamic properties of intermediate filaments during organelle transport. J. Cell Sci. 122, 2914–2923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mendez MG, Kojima S-I & Goldman RD Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 24, 1838–1851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Margiotta A & Bucci C Role of Intermediate Filaments in Vesicular Traffic. Cells 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tiwari R et al. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways regulated by site-specific phosphorylation of Keratin-8 in skin squamous cell carcinoma derived cell line. Proteomics 17, (2017). [DOI] [PubMed] [Google Scholar]
- 159.Kim HJ et al. Novel involvement of RhebL1 in sphingosylphosphorylcholine-induced keratin phosphorylation and reorganization: Binding to and activation of AKT1. Oncotarget 8, 20851–20864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seltmann K, Cheng F, Wiche G, Eriksson JE & Magin TM Keratins Stabilize Hemidesmosomes through Regulation of β4-Integrin Turnover. J. Invest. Dermatol. 135, 1609–1620 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Zhan Q, Korngold R, Lezcano C, McKeon F & Murphy GF Graft-versus-host disease-related cytokine-driven apoptosis depends on p73 in cytokeratin 15-positive target cells. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 18, 841–851 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang S et al. Piwil2 inhibits keratin 8 degradation through promoting p38-induced phosphorylation to resist Fas-mediated apoptosis. Mol. Cell. Biol. 34, 3928–3938 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khapare N et al. Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis. PloS One 7, e38561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paladini RD & Coulombe PA Directed expression of keratin 16 to the progenitor basal cells of transgenic mouse skin delays skin maturation. J. Cell Biol. 142, 1035–1051 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hobbs RP, Jacob JT & Coulombe PA Keratins Are Going Nuclear. Dev. Cell 38, 227–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qin Z, Kreplak L & Buehler MJ Nanomechanical properties of vimentin intermediate filament dimers. Nanotechnology 20, 425101 (2009). [DOI] [PubMed] [Google Scholar]
- 167.Qin Z, Kreplak L & Buehler MJ Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments. PloS One 4, e7294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dahl KN, Kahn SM, Wilson KL & Discher DE The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779–4786 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Banerjee A et al. Viscoelastic behavior of human lamin A proteins in the context of dilated cardiomyopathy. PloS One 8, e83410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moorer MC, Buo AM, Garcia-Pelagio KP, Stains JP & Bloch RJ Deficiency of the intermediate filament synemin reduces bone mass in vivo. Am. J. Physiol. Cell Physiol. 311, C839–C845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Le Henaff C et al. Genetic deletion of keratin 8 corrects the altered bone formation and osteopenia in a mouse model of cystic fibrosis. Hum. Mol. Genet. 25, 1281–1293 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Shapiro F, Cahill C, Malatantis G & Nayak RC Transmission electron microscopic demonstration of vimentin in rat osteoblast and osteocyte cell bodies and processes using the immunogold technique. Anat. Rec. 241, 39–48 (1995). [DOI] [PubMed] [Google Scholar]
- 173.Kumar A et al. Identification of kaempferol-regulated proteins in rat calvarial osteoblasts during mineralization by proteomics. Proteomics 10, 1730–1739 (2010). [DOI] [PubMed] [Google Scholar]
- 174.Lian N et al. Transforming growth factor β suppresses osteoblast differentiation via the vimentin activating transcription factor 4 (ATF4) axis. J. Biol. Chem. 287, 35975–35984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Becker B, Bellin RM, Sernett SW, Huiatt TW & Robson RM Synemin contains the rod domain of intermediate filaments. Biochem. Biophys. Res. Commun. 213, 796–802 (1995). [DOI] [PubMed] [Google Scholar]
- 176.Granger BL & Lazarides E Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell 22, 727–738 (1980). [DOI] [PubMed] [Google Scholar]
- 177.Hirako Y et al. Characterization of mammalian synemin, an intermediate filament protein present in all four classes of muscle cells and some neuroglial cells: co-localization and interaction with type III intermediate filament proteins and keratins. Cell Tissue Res. 313, 195–207 (2003). [DOI] [PubMed] [Google Scholar]
- 178.Lund LM et al. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 26, 137–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun N, Critchley DR, Paulin D, Li Z & Robson RM Identification of a repeated domain within mammalian alpha-synemin that interacts directly with talin. Exp. Cell Res. 314, 1839–1849 (2008). [DOI] [PubMed] [Google Scholar]
- 180.Li Z et al. Synemin acts as a regulator of signalling molecules during skeletal muscle hypertrophy. J. Cell Sci. 127, 4589–4601 (2014). [DOI] [PubMed] [Google Scholar]
- 181.Russell MA et al. The intermediate filament protein, synemin, is an AKAP in the heart. Arch. Biochem. Biophys. 456, 204–215 (2006). [DOI] [PubMed] [Google Scholar]
- 182.Torres-Quesada O, Mayrhofer JE & Stefan E The many faces of compartmentalized PKA signalosomes. Cell. Signal. 37, 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 183.Ercu M & Klussmann E Roles of A-Kinase Anchoring Proteins and Phosphodiesterases in the Cardiovascular System. J. Cardiovasc. Dev. Dis. 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wild AR & Dell‟Acqua ML Potential for therapeutic targeting of AKAP signaling complexes in nervous system disorders. Pharmacol. Ther. 185, 99–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reggi E & Diviani D The role of A-kinase anchoring proteins in cancer development. Cell. Signal. 40, 143–155 (2017). [DOI] [PubMed] [Google Scholar]
- 186.García-Pelagio KP et al. Myopathic changes in murine skeletal muscle lacking synemin. Am. J. Physiol. Cell Physiol. 308, C448–462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.García-Pelagio KP et al. Absence of synemin in mice causes structural and functional abnormalities in heart. J. Mol. Cell. Cardiol. 114, 354–363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khedgikar V et al. Kaempferol targets Krt-14 and induces cytoskeletal mineralization in osteoblasts: A mechanistic approach. Life Sci. 151, 207–217 (2016). [DOI] [PubMed] [Google Scholar]
- 189.Swift J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Duque G & Rivas D Age-related changes in lamin A/C expression in the osteoarticular system: laminopathies as a potential new aging mechanism. Mech. Ageing Dev. 127, 378–383 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Choi JY et al. Diminished Canonical β-Catenin Signaling During Osteoblast Differentiation Contributes to Osteopenia in Progeria. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 33, 2059–2070 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rauner M et al. Inhibition of lamin A/C attenuates osteoblast differentiation and enhances RANKL-dependent osteoclastogenesis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 24, 78–86 (2009). [DOI] [PubMed] [Google Scholar]
- 193.Akter R, Rivas D, Geneau G, Drissi H & Duque G Effect of lamin A/C knockdown on osteoblast differentiation and function. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 24, 283–293 (2009). [DOI] [PubMed] [Google Scholar]
- 194.Schmidt E et al. Expression of the Hutchinson-Gilford progeria mutation during osteoblast development results in loss of osteocytes, irregular mineralization, and poor biomechanical properties. J. Biol. Chem. 287, 33512–33522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mounkes LC, Kozlov S, Hernandez L, Sullivan T & Stewart CL A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, 298–301 (2003). [DOI] [PubMed] [Google Scholar]
- 196.Li W et al. Decreased bone formation and osteopenia in lamin a/c-deficient mice. PloS One 6, e19313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bergo MO et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc. Natl. Acad. Sci. U. S. A. 99, 13049–13054 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pendás AM et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, 94–99 (2002). [DOI] [PubMed] [Google Scholar]
- 199.Wu X-T et al. Spontaneous cellular vibratory motions of osteocytes are regulated by ATP and spectrin network. Bone 128, 112056 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Møller AMJ et al. Septins are critical regulators of osteoclastic bone resorption. Sci. Rep. 8, 13016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hu Q et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leterrier C A dual role for βII-spectrin in axons. Proc. Natl. Acad. Sci. U. S. A. 116, 15324–15326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M & Nelson WJ Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J. Cell Biol. 180, 295–303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sankaran J, Uzer G, van Wijnen AJ & Rubin J Gene regulation through dynamic actin control of nuclear structure. Exp. Biol. Med. Maywood NJ 244, 1345–1353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crisp M et al. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41–53 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Uzer G et al. Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates βcatenin nuclear access. J. Biomech. 74, 32–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sen B et al. Intranuclear Actin Regulates Osteogenesis. Stem Cells Dayt. Ohio 33, 3065–3076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lombardi ML et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286, 26743–26753 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 210.Samsonraj RM et al. Validation of Osteogenic Properties of Cytochalasin D by High-Resolution RNA-Sequencing in Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissues. Stem Cells Dev. 27, 1136–1145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Samsonraj RM et al. Osteogenic Stimulation of Human Adipose-Derived Mesenchymal Stem Cells Using a Fungal Metabolite That Suppresses the Polycomb Group Protein EZH2. Stem Cells Transl. Med. 7, 197–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]