Abstract
Background:
Organophosphate esters (OPEs) are a class of flame retardants in common use. OPEs can easily leach from materials, resulting in human exposure. Increasing concentrations have been reported in human populations over the past decade. Recent studies have linked prenatal OPE exposure to hyperactivity and attention problems in children. Such behaviors are often found among children with attention-deficit hyperactivity disorder (ADHD), however, no study has investigated OPEs in relation to clinically assessed ADHD.
Objective:
To evaluate prenatal exposure to OPEs as risk factors for clinically assessed ADHD using a case-cohort study nested within the Norwegian Mother, Father, and Child Cohort Study (MoBa).
Methods:
We included in the case group 295 ADHD cases obtained via linkage with the Norwegian Patient Registry, and the sub-cohort group 555 children sampled at baseline, irrespective of their ADHD case status. Prenatal concentrations of OPE metabolites were measured in maternal urine collected at 17 weeks of gestation, and included diphenyl phosphate (DPHP), di-n-butyl phosphate (DNBP), bis(2-butoxyethyl) hydrogen phosphate (BBOEP), and bis(1,3-dichloro-2-propyl) phosphate (BDCIPP). We estimated risk ratios and the corresponding 95% confidence intervals [95% CI] using logistic regression, adjusting for season of urine collection, child sex, birth year, and maternal depression, education, and sum of urinary di(2-ethylhexyl) phthalate metabolites (DEHP) concentration during pregnancy. To assess the overall impact of simultaneously decreasing exposure to all chemical constituents of an OPE-phthalate mixture, quantile based g-computation was implemented. The mixture constituents included OPE and phthalate metabolites commonly detected in our study. In all models, we considered effect measure modification by child sex and polymorphisms in genes encoding paraoxonase 1 (PON1) and cytochrome P450 (P450) enzymes. Mediation analysis was conducted using thyroid function biomarkers estimated from maternal blood collected at 17 weeks of gestation.
Results:
DPHP was detected in nearly all samples (97.2%), with a higher geometric mean among the case group (0.70 μg/L) as compared to the sub-cohort (0.52 μg/L). DNBP was commonly detected as well (93.8%), while BBOEP (52.9%) and BDCIPP (22.9%) were detected less frequently. A higher risk of ADHD was observed in children with greater than median exposure to DPHP during pregnancy (risk ratio: 1.38 [95% CI: 0.96, 1.99]), which was slightly higher among girls (2.04 [1.03, 4.02]) and children of mothers with PON1 Q192R genotype QR (1.69 [0.89, 3.19]) or PON1 Q192R genotype RR (4.59 [1.38, 15.29]). The relationship between DPHP and ADHD (total risk ratio: 1.34 [0.90, 2.02]) was partially mediated through total triiodothyronine to total thyroxine ratio (natural direct effect: 1.29 [0.87, 1.94]; natural indirect effect: 1.04 [1.00, 1.10]; 12.48% mediated). We also observed an elevated risk of ADHD in relation to BDCIPP detection during pregnancy (1.50 [0.98, 2.28]). We did not observe notable differences in ADHD by DNBP (0.88 [0.62, 1.26]) or BBOEP (1.03 [0.73, 1.46]) during pregnancy. Simultaneously decreasing all constituents of common-detect OPE-phthalate mixture, specifically DPHP, DNBP, and 6 phthalate metabolites, by a quartile resulted in an ADHD risk ratio of 0.68 [0.64, 0.72].
Conclusion:
Prenatal exposure to DPHP and BDCIPP may increase the risk of ADHD. For DPHP, we observed potential modification by child sex and maternal PON1 Q192R genotype and partial mediation through maternal thyroid hormone imbalance at 17 weeks gestation.
Keywords: Diphenyl phosphate; Bis(1,3-dichloro-2-propyl) phosphate; PON1 Q192R; ADHD; MoBa
1. Introduction
Concurrent with the decline in usage of polybrominated diphenyl ethers (EPA, 2013; EU, 2003), a class of persistent flame retardants, was an increase in the production and usage of organophosphate esters (OPEs) as flame retardants or plasticizers. Although OPEs do not bioaccumulate in the environment, they tend to easily leach and volatilize from the materials to which they are added, resulting in widespread human exposure (van der Veen and de Boer 2012). Several OPE metabolites are commonly detected, such as diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), di-n-butyl phosphate (DNBP), and bis(2-butoxyethyl) hydrogen phosphate (BBOEP) (Ospina et al. 2018; Wang et al. 2019); however, their impacts on human health and disease are not well understood.
An increasing number of studies suggest that exposure to OPEs may affect child neurobehavioral development. Neurotoxic (Dishaw et al. 2011; Jarema et al. 2015) and endocrine disruptive properties (Wang et al. 2013; Zhang et al. 2016) have been identified in experimental settings. Further, recent epidemiological studies link OPE exposure measured from prenatal urine (Castorina et al. 2017; Doherty et al. 2019) or concurrent dust (Lipscomb et al. 2017) samples with externalizing behaviors, attention problems, and/or hyperactivity, which are often found among children with attention-deficit hyperactivity disorder (ADHD) (Charach et al. 2011). To our knowledge, no study has examined OPE exposure with clinically confirmed neurodevelopmental outcomes, such as ADHD. The prevalence of ADHD diagnosis in children has more than doubled over the last decade (Danielson et al. 2018), surpassing 9% in the United States (Danielson et al. 2018) and 5% worldwide (Polanczyk et al. 2007). Despite the high prevalence, few non-genetic risk factors have been established as causal, other than lead (Eubig et al. 2010; Ji et al. 2018). Although causality has not been fully established, an increasing number of studies point to environmental agents as potential risk factors (Engel et al. 2018; Froehlich et al. 2011).
Epidemiological investigations on the modifiers of the potential relationships between OPEs and ADHD are even more sparse. The existence of sex-specific associations has been inconsistent (Castorina et al. 2017; Doherty et al. 2019) and vulnerability by OPE metabolism has not been previously examined. OPE metabolism may involve enzymes such as cytochrome P450 (P450) and paraoxonase 1 (PON1) (Bigley et al. 2016; Van den Eede et al. 2015; Van den Eede et al. 2013; Wei et al. 2018), whose concentrations and activity have been well-characterized with single nucleotide polymorphisms (SNPs) in genes (Davies et al. 1996; Furlong et al. 1988; Humbert et al. 1993; Mackness et al. 1997). PON1 functional polymorphisms have been particularly well-described, and multiple studies report that individuals with slow-metabolizing genes are more susceptible to neurodevelopmental impacts of organophosphate pesticide exposure as compared to individuals with fast-metabolizing genes (Davies et al. 1996; Engel et al. 2007; Furlong et al. 1988; Humbert et al. 1993; Mackness et al. 1997). Although paraoxonases are also candidate enzymes involved in the metabolism of OPEs, gene-environment interaction remains unclear for OPEs. A better understanding of susceptible sub-populations will help in the characterization of OPE-related health effects.
The potential relationships between OPEs and ADHD may involve mechanisms such as endocrine disruption, including maternal thyroid dysfunction. Failure to maintain normal maternal thyroid function during pregnancy is a potential risk factor for ADHD (Drover et al. 2018; Pakkil¨ a et al. 2014¨ ), and mid-pregnancy thyroid disruption has been previously linked with DPHP exposure in MoBa (Choi et al., 2021). Therefore, we additionally explored whether the potential relationship between urinary DPHP in 17 weeks’ gestation and children’s ADHD was partially mediated through maternal thyroid function measured from blood biomarkers at 17 weeks’ gestation.
It is also important to consider the fact that environmental exposures often occur in mixtures, which has rarely been accounted for in relation to ADHD. Human exposure to some OPEs may co-occur with phthalates (Bergh et al. 2011; Bi et al. 2018; Bornehag et al. 2005; Ionas et al. 2014; Liang and Xu 2014; Yang et al. 2020), which have also been associated with ADHD (Engel et al. 2018). However, due to the potential for these chemicals to co-occur, targeting a reduction in a specific chemical may not always be feasible. Rather, it may be useful to quantify the impact of a hypothetical intervention whereby exposure to multiple chemicals is jointly reduced.
We undertook an investigation of OPE exposure during pregnancy and the risk of offspring ADHD, using a case-cohort study nested within the Norwegian Mother, Father, and Child Cohort (MoBa). We explored mediation through pregnancy maternal thyroid function and effect measure modification by child sex and polymorphisms in enzymes that are suspected to play a role in the metabolism of OPEs. Further, we quantified the risk of ADHD with reduced exposure to OPE-phthalate mixtures.
2. Material and methods
2.1. Study population
MoBa is an ongoing prospective population-based pregnancy cohort study of Norwegian-speaking women, conducted by the Norwegian Institute of Public Health (Magnus et al. 2016; Magnus et al. 2006). Pregnant women were recruited at their routine prenatal ultrasound visit (≈17 gestational weeks) from all over Norway between 1999 and 2008. Upon enrollment, women were asked for a urine and a blood sample after providing written consent (Rønningen et al. 2006). At birth, cord blood samples were also collected. The cohort now includes 114,500 children, 95,200 mothers, and 75,200 fathers.
For the current study, we nested a case-cohort study within MoBa (Engel et al. 2018). Eligibility criteria included singleton births without Down’s syndrome or cerebral palsy who were born between 2003 and 2008, and whose mothers donated a urine sample during pregnancy, completed the standard MoBa questionnaire when the child was 36 months old, and resided within proximity to Oslo, Norway (Engel et al. 2018). Among the eligible population, we selected participants into the case group and sub-cohort. In a case-cohort study, the standard estimation of odds ratios can be interpreted as risk ratios if the sub-cohort is selected at baseline irrespective of their case status (Pearce 1993). Further, restricting the case group only to cases that are not already sampled into the sub-cohort allows the estimation of variance using the standard formula for odds ratios (Keogh and Cox 2014). The sub-cohort children were selected at baseline from the entire base population that met the eligibility criteria for the current study, irrespective of their ADHD case status (n = 555).
2.2. Case group definition
ADHD cases were identified from all MoBa enrollees eligible for the current study, by data linkage with the Norwegian Patient Registry (NPR). The NPR collects diagnostic codes from all hospitals and outpatient clinics in Norway, including registrations of hyperkinetic disorders by specialist health services (ICD-10 code: F90 (WHO 1992). Hyperkinetic disorder is mandatorily reported in Norway (Suren et al. 2012), and therefore individuals first diagnosed with or continuing to seek care/treatment can be identified. To exclude any erroneous or false diagnosis, ADHD cases were defined as having at least two registrations of hyperkinetic disorder. The case group was defined as ADHD cases who were not simultaneously sampled as part of the sub-cohort. A total of 295 identified ADHD cases were included in our case group, which excludes 2 children who had already been selected into the sub-cohort (n = 2).
2.3. Exposure assessment
Maternal urine samples were collected at 17 weeks gestation and shipped unrefrigerated to a central ISO-certified lab in Oslo (Biobank), where the samples were stored at −80 °C (Paltiel et al. 2014). Urinary concentrations of DPHP, DNBP, BBOEP, and BDCIPP were measured using ultra-performance liquid chromatography (UPLC) coupled with quadrupole-time-of-flight (QTOF) at the Norwegian Institute of Public Health, by a modified method published by Cequier et al. (Cequier et al. 2014; Choi et al., 2021). The modification was done in the sample preparation procedure and was adapted from an earlier published method by Cequier et al. (Cequier et al. 2016). In brief, labeled internal standards, 300 μL of water, and 40 μL of formic acid were added to 300 μL of the urine sample. The OPE metabolites were extracted using Strata-X-AW 96-well plates (Phenomenenx, U.S.A.), which were conditioned first with 0.5 mL of MeOH and subsequently with 0.5 mL of H2O, both containing 1% formic acid. Samples were loaded, eluted by gravity, and washed with 0.5 mL of MeOH to remove neutral interferences. The OPE metabolites were then eluted with 0.5 mL of acetone containing 5% triethylamine. Fifty microliters of water were added and the samples were evaporated with a gentle stream of nitrogen (10 L/min) for 1 h. Ten microliters were injected into the UPLC system as described elsewhere (Cequier et al. 2014). UPLC was performed on Acquity® C18 BEH column (50 mm × 2.1 mm × 1.7 μm) from Waters Corp. (Milford, MA, U.S. A.). The OPE metabolites were identified and quantified with tandem mass spectrometry using a Xevo® G2-S QTOF from Waters Corp. (Milford, MA, U.S.A.). All assays were conducted in randomized batches, each of which included laboratory in-house as well as laboratory-blinded pooled urine quality control samples. Specific gravity, as defined by the ratio of the density of urine to water, was measured in each urine sample using a pocket refractometer (PAL-10S), Atago.
2.4. Genotyping of maternal and fetal DNA
Maternal blood samples were collected in EDTA tubes at enrollment (17 weeks gestation). Immediately after birth, an umbilical cord blood sample was collected. Maternal and fetal DNA was extracted from whole blood samples using the FlexiGene kit (Qiagen, Hilden, Germany) and stored at −20 °C in the MoBa Biobank (Rønningen et al. 2006). For genotyping, we selected three SNPs with established functional polymorphisms on candidate enzymes that may metabolize OPEs: PON1 Q192R, PON1 L55M, and CYP1A2 − 1545C/T. Genotyping was conducted at the Norwegian University of Life Sciences (NMBU) using the Sequenom MassARRAY IPLEX® platform (Gabriel et al. 2009).
We included the two most common coding region polymorphisms in PON1 protein, which codes for amino acids 192 and 55 (Costa et al. 2003). Q192R refers to A >G mutation at codon 192, which results in an exchange of the amino acids glutamine (Q) to arginine (R) (Gln192Arg; rs662). This variant influences substrate-specific enzymatic activities of PON1, for example, carriers of R192 have stronger PON1 enzymatic activity for oxon substrates such as paraoxon (Costa et al. 2003; Mackness et al. 1997). L55M refers to T > A mutation at codon 55, which results in a leucine (L)-to-methionine (M) substitution (Leu55Met; rs854560). L55M is in linkage disequilibrium with rs705379 (−108C/T), and consequently, the carriers of M55 have lower PON1 enzyme concentrations in plasma (Costa et al. 2005).
A coding region polymorphism in CYP1A2 was also examined, specifically − 1545C/T (rs2470890). Although this is a synonymous variant that does not result in altered protein, it is in linkage disequilibrium with CYP1A2*1F (rs762551; (Veatch et al. 2015)), which has been associated with higher enzyme inducibility. In the carriers of C allele, CYP1A2 can be induced even at low exposure to a substrate, resulting in a quicker clearance, as has been reported for smoking (Ghotbi et al. 2007), melatonin (Braam et al. 2013), and caffeine (Palatini et al. 2009).
2.5. Covariate assessment
Given that phthalates may share common source products as OPEs and are considered independent risk factors for ADHD, we considered phthalates as 1) confounders when evaluating individual OPEs as risk factors, and 2) part of an exposure mixture to quantify the impact of reduced joint exposures. Specifically, we considered metabolites of 6 phthalates previously measured (Engel et al. 2018; Villanger et al. 2020): monoethyl phthalate (MEP), mono-iso-butyl phthalate (MiBP), mono-n-butyl phthalate (MnBP), monobenzyl phthalate (MBzP), the molar sum of di(2-ethylhexyl)phthalate metabolites (DEHP), and the molar sum of di-iso-nonyl phthalate metabolites (DiNP).
We also utilized covariate data from MoBa questionnaires and via linkage with the Medical Birth Registry of Norway (MBRN). We obtained from the first MoBa questionnaire, received in pregnancy weeks 13 to 17, maternal education, any depression self-reported before or up to the completion of the questionnaire, and smoking and alcohol intake self-reported up to the completion of the questionnaire. We also obtained from a food frequency questionnaire that mothers completed at their 22 weeks’ gestation, maternal dietary intakes of fish, selenium, and iodine (Brantsæter et al. 2008). Briefly, pregnant women responded to a semi-quantitative questionnaire that was intended to characterize diet during the first four months of pregnancy via 255 food items and additional dietary supplements. Total fish intake was estimated by summing the daily intake of all fish types (g/day) and dietary intakes of iodine and selenium were calculated in grams per day. From the questionnaire that mothers completed at 36-months postpartum, we obtained maternal ADHD symptoms that were measured using the World Health Organization Adult ADHD Self-Report Scale (Kessler et al. 2005). Finally, maternal age at delivery, parity, birth year, and child sex were obtained from MBRN, which is a national health registry containing information about all births in Norway (Irgens 2000).
Since pregnancy DPHP has been previously linked with maternal thyroid disruption (Choi et al., 2021), a potential risk factor for ADHD (Drover et al. 2018; Päkkilä et al. 2014), we additionally explored pregnancy maternal thyroid function as a potential mediator. Specifically, we included total thyroxine (TT4), total triiodothyronine (TT3), thyroid stimulating hormones (TSH), and TT3:TT4 ratio estimated from blood samples that were collected at the same date as the urine samples, approximately 17 weeks’ gestation (Choi et al., 2021).
2.6. Ethics
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is now based on regulations related to the Norwegian Health Registry Act. The current study was approved by the Norwegian Data Inspectorate and the Norwegian Committee for Medical and Health Research Ethics (REC), and the Institutional Review Board at the University of North Carolina Chapel Hill.
2.7. Analytic approach
We used descriptive statistics to characterize the distribution of exposures and covariates according to case-cohort status. Exposure concentrations below the limit of detection (LOD) were multiply imputed (m = 20), assuming a log-normal distribution truncated at the LOD. The following missing covariate data were imputed assuming missing at random, conditional on all other covariates, outcome, and exposure: sex (n = 2 missing), fish consumption (n = 48), maternal ADHD symptoms (n = 172), and maternal education (n = 36). Imputation was conducted using the R package mice.
We used directed acyclic graphs to identify minimally sufficient adjustment sets in order to appropriately account for confounders, which included birth year, season of urine collection, maternal fish consumption during pregnancy, maternal depression before or during pregnancy, maternal ADHD symptoms, maternal education, and prenatal maternal phthalate concentrations. Specifically for phthalates, we adjusted for DEHP due to its association with ADHD in the current study population identified in a previous study (Engel et al. 2018). In addition to the minimally sufficient adjustment sets identified above, we considered child sex, a strong risk factor for ADHD. We further evaluated bias-variance tradeoffs and identified a final adjustment set that we applied to all analyses: season of urine collection, child sex, birth year, maternal depression, maternal education, and log DEHP.
For frequently detected OPE metabolites (i.e., DPHP and DNBP), we explored the potential for non-linear trend of associations using quartiles of exposure (Supplementary Fig. 1). Considering the non-linear dose–response observed for DPHP, we present our primary analysis using a binary indicator with a cut-off of sub-cohort median. Since we did not observe dose–response for DNBP, a metabolite that had moderate detection of blank quality control samples, we alternatively used a binary indicator with a cut-off of the limit of quantification (LOQ). Infrequently detected metabolites were coded with binary indicators using LOD. We estimated risk ratios and 95% confidence intervals (CI) of ADHD using logistic regression in each imputed dataset and pooled the estimates using Rubin’s rule (Rubin 2004).
We investigated effect measure modification by child sex (alpha: 0.2), since the underlying mechanism of OPEs may be sex-specific as has been reported in experimental settings (Li et al. 2020; Liu et al. 2019). We applied the augmented product-term approach, including product terms between child sex and all covariates, which is roughly equivalent to conducting a fully-stratified analysis (Buckley et al. 2017). Using the same approach, we also investigated effect measure modification by maternal and fetal genotypes. Genotypes were coded as −1 (most common homozygote), 0 (heterozygote), and 1 (least common homozygote), and modeled assuming additivity across strata. We also report results treating the genotype variable as nominal categorical.
Mediation analysis was conducted to decompose the total effect of DPHP on ADHD into a natural direct effect and a natural indirect effect through maternal thyroid function during pregnancy, using a previously published method (VanderWeele 2016; Vanderweele and Vansteelandt 2010). Briefly, the natural direct effect of DPHP on ADHD was defined as the risk ratio associated with elevated DPHP while adjusting for a thyroid function biomarker and confounders [eq. (1); Model 1]. The natural indirect effect of DPHP on ADHD via a thyroid function biomarker was estimated by multiplying two estimates and then taking its exponential [eq. (2)]. These two estimates included: 1) log(risk ratio) of ADHD associated with elevated thyroid function biomarker while adjusting for DPHP and confounders in the case-cohort [Model 1], and 2) the beta coefficient from a linear regression model fitted in the sub-cohort with DPHP and confounders as the independent variables and the thyroid function biomarker as the dependent variable [Model 2]. The total effect was defined as the product of the natural direct and indirect effect [eq. (3)] and percent mediated was calculated using eq. (4). We included in each model, all confounders identified for DPHP–ADHD, DPHP–maternal pregnancy thyroid function, and maternal pregnancy thyroid function–ADHD relationships: maternal self-reported depression during and before pregnancy, education, age, dietary intakes of selenium and iodine during pregnancy, and urinary log(DEHP) during pregnancy, and season of bio-sample collection, parity, birth year, and child sex. Estimates were pooled across multiply imputed datasets using Rubin’s rules and confidence intervals were bootstrapped (Schomaker and Heumann 2018).
| (1) |
| (2) |
| (3) |
| (4) |
Y: binary indicator for being in ADHD case group
e: binary indicator for DPHP exposure
Mi: thyroid function indicator (TT3, TT4, TSH, TT3:TT4 ratio)
c: all confounders for DPHP-ADHD, DPHP-maternal pregnancy thyroid function, and maternal pregnancy thyroid function-ADHD relationships (maternal self-reported depression during and before pregnancy, education, age, dietary intakes of selenium and iodine during pregnancy, and urinary log(DEHP) during pregnancy, and season of bio-sample collection, parity, birth year, and child sex).
Since study participants are likely exposed to a mixture of chemicals, whereby reducing exposure to a single metabolite is not always feasible, we quantified the impact of a hypothetical intervention that can jointly reduce exposures to common-detect OPE-phthalate mixtures. Specifically, the marginal risk ratios of ADHD with a simultaneous 1-quartile decrease of DPHP, DNBP, and 6 phthalate metabolites were estimated using quantile-based g computation in R using package qgcomp (Keil et al. 2020).
The current study is based on version 9 of the quality-assured data files.
3. Results
A total of 555 children were sampled into the sub-cohort, inclusive of 2 children with ADHD (Table 1). The case group consisted of 295 children with ADHD, who were not already selected into the sub-cohort. The ADHD case group, as compared to the sub-cohort, had a higher percentage of boys, births in 2003–2004, maternal urine collected during summer months, mothers with symptoms indicative of ADHD, mothers with education less than college, mothers with depression, and mothers who smoked during pregnancy.
Table 1.
Descriptive characteristics of the study population by ADHD case-cohort status.
| Case group (N = 295) N (%) | Sub-cohort (N = 555) N (%) | ||
|---|---|---|---|
| Maternal age at birth | Mean ± sd (N missing) | 29.2 ± 5.03 (2) | 30.9 ± 4.22 (2) |
| ADHD status | Identified as an ADHD case from NPR | 295 (100%) | 2 (<0.1%) |
| Not identified as an ADHD case from NPR | 0 (0%) | 553 (100%) | |
| Sex | Boy | 211 (72.0%) | 275 (49.7%) |
| Girl | 82 (28.0%) | 278 (50.3%) | |
| Birth year | 2003–2004 | 130 (44.1%) | 56 (10.1%) |
| 2005 | 87 (29.5%) | 130 (23.4%) | |
| 2006 | 44 (14.9%) | 194 (35.0%) | |
| 2007–2008 | 34 (11.5%) | 175 (31.5%) | |
| Parity | Nulliparous | 140 (47.8%) | 271 (49.0%) |
| Multiparous | 153 (52.2%) | 282 (51.0%) | |
| Maternal education | > College completed | 25 (9.4%) | 169 (30.8%) |
| College completed | 74 (27.8%) | 238 (43.4%) | |
| < College completed | 158 (59.4%) | 125 (22.8%) | |
| Other | 9 (3.4%) | 16 (2.9%) | |
| Maternal ADHD symptoms | Indicative of ADHD (SRS ≥ 4) | 11 (8.1%) | 21 (3.9%) |
| (Self-Report Scale, SRS) | Not indicative of ADHD (SRS < 3) | 125 (91.9%) | 522 (96.1%) |
| Maternal depression (any before/during pregnancy) | Reported any | 44 (14.9%) | 34 (6.1%) |
| Did not report any | 251 (85.1%) | 521 (93.9%) | |
| Fish intake during pregnancy reported at 22 weeks gestation (recommended by USFDA, 2020) | ≥340 g per week | 33 (13.0%) | 60 (11.0%) |
| 227–340 g per week (recommended) | 43 (16.9%) | 109 (19.9%) | |
| <227 g per week | 178 (70.1%) | 379 (69.2%) | |
| Prenatal smoking | Yes | 93 (34.7%) | 79 (14.4%) |
| No | 175 (65.3%) | 470 (85.6%) | |
| Prenatal alcohol intake | Yes | 26 (10.9%) | 66 (12.9%) |
| No | 212 (89.1%) | 445 (87.1%) | |
| Month of urine | May – August | 121 (41.0%) | 183 (33.0%) |
| sample collection | September – November | 56 (19.0%) | 121 (21.8%) |
| December – April | 118 (40.0%) | 251 (45.2%) | |
Abbreviations: ADHD, attention deficit hyperactivity disorder; NPR, Norwegian patient registry; DPHP, diphenyl phosphate; DNBP, di-n-butyl phosphate; BBOEP, bis(2-butoxyethyl) hydrogen phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; GM, geometric mean; SE, geometric standard error; LOD, limit of detection; DEHP, di(2-ethylhexyl) phthalate.
DPHP and DNBP were commonly detected in our study population (>90% above the LOD), while BBOEP and BDCIPP were detected less frequently (Table 2). The geometric mean of urinary DPHP concentrations in the case group mothers was higher than that in the sub-cohort mothers (2 sample t-test p-value < 0.0001). Similar trends were observed for DEHP, which have been previously reported in detail (Engel et al. 2018).
Table 2.
Descriptive characteristics of environmental exposures in the current study, by case-cohort status.
| LOD | LOQ | Case group (N = 295) | Sub-cohort (N = 555) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >LOD | >LOQ | GM ± SD | Percentiles | >LOD | >LOQ | GM ± SD | Percentiles | |||||||
| 25th | 50th | 75th | 25th | 50th | 75th | |||||||||
| DPHP (μg/L) | 0.03 | 0.1 | 98.0% | 93.2% | 0.70 ± 2.80 | 0.40 | 0.71 | 1.25 | 96.8% | 90.5% | 0.52 ± 2.77 | 0.29 | 0.52 | 0.97 |
| DNBP (μg/L) | 0.07 | 0.2 | 93.6% | 54.2% | 0.26 ± 2.06 | 0.16 | 0.24 | 0.40 | 93.9% | 54.9% | 0.28 ± 2.27 | 0.17 | 0.25 | 0.42 |
| BBOEP (μg/L) | 0.07 | 0.2 | 55.9% | 11.9% | 0.08 ± 2.44 | <LOD | 0.08 | 0.15 | 51.8% | 8.6% | 0.09 ± 2.38 | <LOD | 0.08 | 0.15 |
| BDCIPP (μg/L) | 0.17 | 0.5 | 26.1% | 10.8% | 0.20 ± 2.48 | <LOD | <LOD | 0.30 | 21.2% | 12.6% | 0.23 ±2.83 | <LOD | <LOD | 0.30 |
Note: All OPEs standardized for specific gravity and concentrations < LOD substituted for LOD/ for the calculation of GM and SD.Abbreviations: DPHP, diphenyl phosphate; DNBP, di-n-butyl phosphate; BBOEP, bis(2-butoxyethyl) hydrogen phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; GM, geometric mean; SE, geometric standard error; DEHP, di(2-ethylhexyl)phthalate; LOQ, limit of quantification; LOD, limit of detection; CI, confidence interval.
We observed an elevated risk of ADHD in relation to higher DPHP and BDCIPP exposure during pregnancy (Table 3). The risk of ADHD in children with maternal prenatal DPHP concentrations above the sub-cohort median was 1.38 times [95% CI: 0.96, 1.99] that of children with maternal pregnancy DPHP concentrations below the sub-cohort median. Similarly, detectable concentrations of BDCIPP during pregnancy were associated with a risk ratio of 1.50 [0.98, 2.28]. Alternative exposure classification using quartiles did not substantially alter the interpretation (Supplementary Fig. 1). Associations between DPHP and ADHD appeared to be somewhat stronger among girls (2.04 [1.03, 4.02]) than boys (1.15 [0.74, 1.80], p-interaction 0.17; Table 3).
Table 3.
Risk ratios of ADHD from a case-cohort study using binary exposure, pooled across multiply imputed datasets (m = 20).
| OPE | Cutoff (μg/L) | %≥ cutoff | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Girls | Boys | Exposure-sex interaction term | |||||||||
| Case group (N = 295) | Sub-cohort (N = 555) | Risk ratio | 95% CI | Risk ratio | 95% CI | Risk ratio | 95% CI | p-value | ||||
| DPHP | Sub-cohort median (0.52) | 63% | 49% | 1.38 | (0.96, 1.99) | 2.04 | (1.03, 4.02) | 1.15 | (0.74, 1.80) | 0.17 | ||
| DNBP | LOQ (0.20) | 54% | 55% | 0.88 | (0.62, 1.26) | 0.77 | (0.42, 1.44) | 0.99 | (0.64, 1.53) | 0.52 | ||
| BBOEP | LOD (0.07) | 55% | 52% | 1.03 | (0.73, 1.46) | 1.15 | (0.62, 2.13) | 1.01 | (0.65, 1.55) | 0.74 | ||
| BDCIPP | LOD (0.17) | 26% | 21% | 1.50 | (0.98, 2.28) | 1.52 | (0.77, 3.02) | 1.55 | (0.90, 2.65) | 0.97 | ||
Note: Model1 adjusted for birth year, child sex, maternal education, maternal depression, maternal urinary log(DEHP), and the season of urine collection. Model2 additionally included sex interaction terms between all covariates. DPHP, diphenyl phosphate; DNBP, di-n-butyl phosphate; BBOEP, bis(2-butoxyethyl) hydrogen phosphate; BDCIPP, bis(1,3-dichloro-2-propyl) phosphate; DEHP, di(2-ethylhexyl)phthalate; LOQ, limit of quantification; LOD, limit of detection; CI, confidence interval.
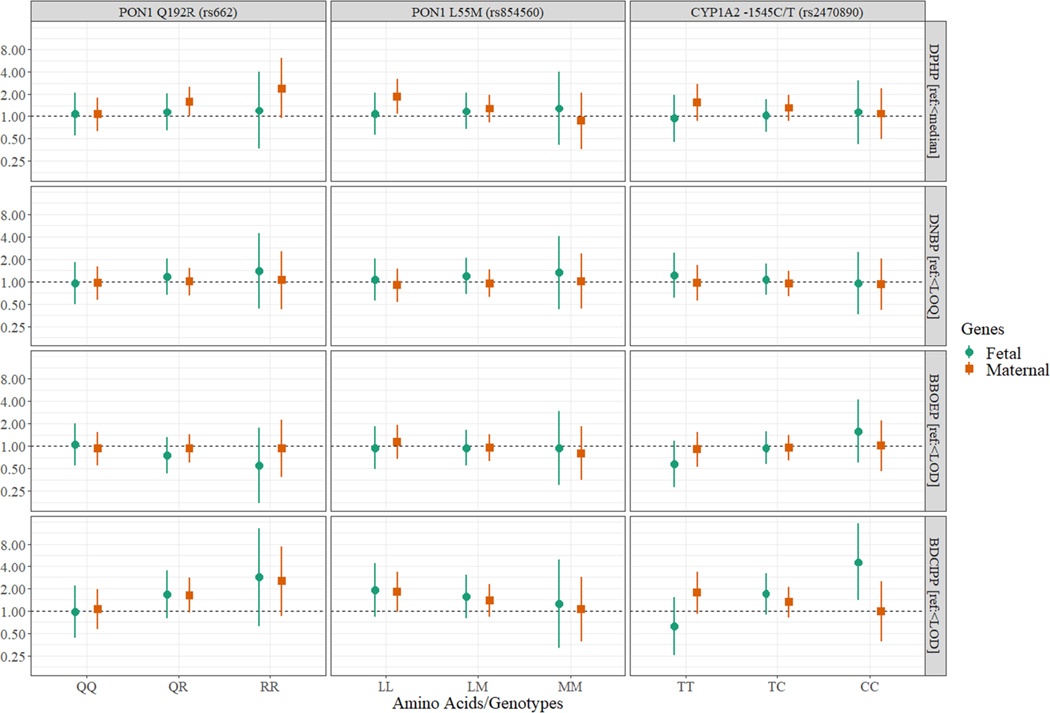
We also observed suggestive modification by SNPs (Fig. 1). When interaction terms with maternal PON1 Q192R were included in the model, the risk ratios of ADHD associated with prenatal DPHP exposure was higher among the carriers of PON1 Q192R QR (1.60 [1.01, 2.54]) and PON1 Q192R RR (2.41 [0.94, 6.23]), with the interaction p-value of 0.19. Child’s CYP1A2 − 1545C/T polymorphism also modified the association, with stronger associations among the carriers of CYP1A2 − 1545C/T CC (4.59 [1.38, 15.29]) and CYP1A2 − 1545C/T CT (1.69 [0.89, 3.19]), with an interaction p-value of 0.02. However, the detection of BDCIPP and genotype CC was uncommon in the current study population (Supplementary Table 1), thus strata were sparse. Alternative genotype coding resulted in a similar relationship (Supplementary Figure 2).
Fig. 1.
Risk ratios of ADHD using binary OPE exposure, stratified by genotypes. This figure shows the risk ratios and corresponding 95% confidence intervals of ADHD stratified by maternal (square) or fetal (circle) genotypes of three different genes (PON1 Q192R, PON1 L55M, and CYP1A2 – 1545C/T), using additive gene model.
Given the potential for DPHP to disrupt maternal thyroid function during pregnancy, a risk factor for ADHD (Drover et al. 2018; Päkkilä et al. 2014), we conducted an additional mediation analysis. The total effect of ADHD associated with above the sub-cohort median DPHP during pregnancy (risk ratio: 1.34 [0.90, 2.02]) was decomposed into a natural direct risk ratio of 1.29 [0.87, 1.94] and a natural indirect risk ratio of 1.04 [1.00, 1.10] through maternal TT3:TT4 ratio (12.48% mediated; Table 4).
Table 4.
Decomposition of the total effect of DPHP on ADHD into a natural direct effect of DPHP and a natural indirect effect of DPHP on ADHD through maternal thyroid function during pregnancy.
| Thyroid function biomarker | Total Effect | Natural Direct Effect | Natural Indirect Effect | % Mediated |
|---|---|---|---|---|
| TT3:TT4 ratio | 1.34 (0.90, 2.02) | 1.29 (0.87, 1.94) | 1.04 (1.00, 1.10) | 12.48 |
| TT3 | 1.31 (0.88, 1.99) | 1.29 (0.86, 1.96) | 1.02 (0.98, 1.07) | 6.75 |
| TT4 | 1.30 (0.87, 1.96) | 1.31 (0.88, 1.97) | 0.99 (0.96, 1.01) | − 2.57 |
| TSH | 1.32 (0.90, 2.01) | 1.33 (0.90, 2.01) | 1.00 (0.98, 1.02) | − 0.46 |
Note: All models adjusted for maternal depression before or during pregnancy, education, age, dietary selenium and iodine intake during pregnancy, smoking during pregnancy, and log(di(2-ethylhexyl)phthalate) during pregnancy, parity, birth year, season of biosample collection, and child sex.
Abbreviations: total triiodothyronine, TT3; total thyroxine, TT4; Thyroid stimulating hormone, TSH; diphenyl phosphate, DPHP.
Since study participants are likely exposed to a mixture of OPEs and phthalates, which are suspected risk factors for ADHD and ADHD-like behavior, we estimated the impact of simultaneously reducing all constituents of a common-detect OPE-phthalate mixture. A simultaneous 1-quantile reduction of DPHP, DNBP, MEP, MiBP, MnBP, MBzP, DEHP, and DiNP was associated with an ADHD risk ratio of 0.68 [0.64, 0.72].
4. Discussion
In this large, nested case-cohort study of MoBa, we observed elevated concentrations of DPHP and DNBP during pregnancy, that were oftentimes comparable to the levels reported worldwide (Supplementary Table 2 (Butt et al., 2014, 2016; Carignan et al., 2017; Castorina et al., 2017; Cequier et al., 2015; Feng et al., 2016; Hoffman et al., 2017b; Kosarac et al., 2016; Ospina et al., 2018; Romano et al., 2017)). We demonstrated that children born to mothers with elevated urinary DPHP during mid-gestation had a higher risk of ADHD. The risk ratio for ADHD associated with greater than median DPHP concentrations during pregnancy was greater among female offspring than among male offspring. Part of this association was mediated through maternal thyroid function measured at 17 weeks gestation, specifically TT3:TT4 ratio. We also observed weak modification by maternal SNPs; specifically, slightly stronger associations were observed among children born to mothers with PON1 Q192R genotypes RR or QR than among mothers with PON1 Q192R genotype QQ. For BDCIPP, we observed that detection, though rare, was associated with an elevated risk of ADHD in both sexes. We also observed modification by the child’s CYP1A2 − 1545C/T genotype, where stronger associations were observed among children with CC and CT. However, we caution over-interpretation given the small number of children with CC and low detection of BDCIPP. We further quantified the impact of simultaneously decreasing all constituents of an OPE-phthalate mixture using a quartile-based g computation, which demonstrated that a quartile decrease in all exposure constituents may reduce the risk of ADHD (risk ratio: 0.68 [0.64, 0.72]). This study adds to the growing literature linking prenatal exposure to organophosphate esters with ADHD or ADHD-like behaviors in children.
4.1. Prenatal DPHP and BDCIPP exposure as potential risk factors for ADHD
We estimated elevated risk ratios of ADHD in children with higher than median DPHP (1.38 [0.96, 1.99]) and detection of BDCIPP (1.50 [0.98, 2.28]). Our findings are in line with the only two prospective investigations of pregnancy OPE exposure on abnormal child behaviors conducted to date. The Center for the Health Assessment of Mothers and Children of Salinas, a pregnancy cohort of a Mexican-American farmworker community in California, found that higher DPHP and BDCIPP was associated with slightly more hyperactivity and attention problems reported by teachers and/or mothers at age 7 (Castorina et al. 2017). Similarly in the Pregnancy, Infection and Nutrition cohort, DPHP and BDCIPP were associated with monotonically increasing hyperactivity, attention problems, externalizing behaviors, and overall more problematic behaviors in 3-year-olds (Doherty et al. 2019). The strongest association was observed for isopropyl-phenyl phenyl phosphate, which was not measured in our study. Although estimates were imprecise in both studies, in part due to small sample sizes (n = 227 and 310), the implicated behaviors are often found in children with ADHD and therefore are highly relevant to our study. The similarity in findings across these three studies is notable in light of their heterogeneous characteristics regarding exposure measurement (mid-vs late-gestation), study population (farming community vs general population), exposure concentrations (Supplementary Table 2), years of study (1999–2008), child age at assessment (2.5–10 years), and outcome assessment (maternal-/parent-reported behaviors using standardized inventories vs clinically diagnosed ADHD).
The existence of sex-specific effects, however, remains unclear. Castorina and colleagues (Castorina et al. 2017) did not observe statistically significant sex-interactions (significance level and stratum-specific estimates not provided). On the other hand, Doherty and colleagues (Doherty et al. 2019) reported slightly stronger associations among girls, for DPHP and BDCIPP exposure, although they were cautious in interpretation due to small sample sizes and imprecise estimates (interaction term p-values not provided). Our findings support the hypothesis that girls are more vulnerable to ADHD from DPHP exposure, which is in line with findings in experimental settings as well: heightened anxiety (Gillera et al. 2020) and hyperactivity (Baldwin et al. 2017) have been reported in female rats perinatally dosed with Firemaster 550, which contain parent OPEs of DPHP. We, however, did not observe sexually dimorphic relationships for BDCIPP exposure.
Several mechanisms may underlie the relationship between prenatal OPE exposure and fetal neurodevelopment, some of which could be sex-specific or involve maternal thyroid disruption during pregnancy. One of the parent compounds of DPHP, triphenyl phosphate (TPHP), is estrogenic (Kojima et al. 2016) and therefore could significantly impact the developing brain through interfering with synaptic organizations (Rebuli et al. 2016), particularly in the hypothalamus (Rebuli and Patisaul 2016). TPHP is additionally a potential thyroid hormone disruptor (Liu et al. 2019). Maternal thyroid dysfunction could critically impact fetal development (Allan et al. 2000; Andersen et al. 2014; Karakosta et al. 2012; Päkkilä et al. 2014), particularly during early pregnancy, when the fetus is heavily reliant on the maternal supply (Burrow et al. 1994). We also observed in mediation analysis, that the relationship between pregnancy DPHP exposure and offspring ADHD can be partly decomposed into a natural indirect effect that involved maternal TT3:TT4 ratio at 17 weeks gestation. The percent mediated, however, was small; which suggests that a larger portion is mediated through a lagged effect on maternal thyroid function or through mechanisms other than maternal thyroid disruption during pregnancy. Alternatively, OPEs or their metabolites may cross the placenta (Ding et al. 2016; Zhao et al. 2017) and directly affect fetal brain development by influencing neuronal cell replication and differentiation (Dishaw et al. 2011; Li et al. 2020).
4.2. Modification by maternal and fetal genotypes
The relationship between prenatal DPHP exposure and childhood ADHD was weakly modified by maternal PON1 Q192R in our study. Since Q192R is known to affect substrate-specific PON1 activity (Richter et al. 2009), it is possible that DPHP and/or some of its parent compounds are substrates for PON1. We did not observe evidence of modification by PON1 L55M, which is known to affect the concentration of PON1 in peripheral blood (Davies et al. 1996; Furlong et al. 1988; Humbert et al. 1993; Mackness et al. 1997). PON1 L55M, however, is an imperfect proxy for PON1 concentrations in peripheral blood (Richter et al. 2010)) and therefore measurement of PON1 enzyme itself in blood could yield different results. The interpretation of gene-environment interaction is further complicated by the fact that DPHP can derive from multiple parent compounds such as resorcinol bis-diphenyl phosphate (Ballesteros-Gomez et al. 2015), ethylhexyl diphenyl phosphate (Van den Eede et al. 2016), and TPHP (Su et al. 2015; Van den Eede et al. 2016). Further epidemiological and experimental investigations using various biomarkers for DPHP parent compounds and additional measures of candidate enzymes will help elucidate the metabolism of OPEs and identify susceptible subpopulations. We also did not observe evidence of modification by corresponding fetal genotypes, possibly due to 1) immature fetal xenobiotic-metabolizing system or 2) limited power since DNA was available from fewer children.
While we observed substantial BDCIPP effect measure modification by fetal CYP1A2 − 1545C/T, we caution over-interpretation of findings and acknowledge that further research is needed to confirm this relationship. A chance finding could arise from sparse strata, given the infrequent detection of BDCIPP, rarity of CC genotype, and limited power from utilizing fetal DNA. Additionally, CYP1A2 is involved in the metabolism of numerous exogenous factors (Anzenbacher and Anzenbacherova 2001), which can be correlated with prenatal BDCIPP exposure and thus bias the relationship. It is also possible that the modification by fetal CYP1A2 observed in this study, is instead reflecting modification of postnatal BDCIPP exposure. Although some studies report the existence of enzyme in fetal liver, hepatic clearance is immature in fetuses compared to adults and CYP1A2 is hardly detected until after infancy (Alcorn and McNamara 2002; Mahmood et al. 2016). Also, BDCIPP has been reported to have relatively moderate to high intra-class correlation coefficients (ICC; 0.48–0.70; (Carignan et al., 2017; Cequier et al. 2015; Hoffman et al. 2014; Meeker et al. 2013b; Romano et al. 2017; Wang et al. 2019)), which suggest suggests stability of exposure over time. Therefore, our measurement of prenatal concentrations could be correlated with postnatal concentrations through continued exposure to source products that are common to mothers and babies.
4.3. Prenatal exposure to OPE-phthalate mixture and offspring ADHD
Since we are likely exposed to multiple environmental exposures simultaneously, increased attention has been placed on exposure mixtures (Gibson et al. 2019; Hamra and Buckley 2018). Multiple statistical approaches to environmental mixtures have been developed to address different aspects of a complex exposure, and therefore it is critical to specify the question and purpose of analyzing exposure mixtures in order to identify the optimal analytic tool. We sought to answer a public health-oriented question: what is the overall impact of increased exposure to multiple correlated environmental triggers? This question is distinct from identifying a specific metabolite or synergistic/antagonistic relationships across metabolites, and the answers can be intuitively interpreted and often directly mapped onto the effects of potential public health interventions (Keil et al. 2020). In this study, we utilized quantile based g-computation (Keil et al. 2020) to examine the public health impact of simultaneous decrease in the OPE-phthalate mixture and found a substantial decrease in the risk of ADHD in relation to a 1-quartile decrease in exposure to all OPE-phthalate mixture constituents (DPHP, DNBP, and 6 phthalate metabolites mixture risk ratio: 0.68 [0.64, 0.72]). Quantile based g-computation was applied given its computational efficiency compared to BKMR and reduced bias for joint exposure–response relative to weighted quantile sums approach; furthermore, it is not subject to issues of exposure transformation given the preservation of rank-orders in quantized exposure. The estimates from this approach could help hypothesize the impact of behavioral or regulatory interventions that are targeted to jointly reduce multiple exposures, for example, cleaning more frequently to reduce exposure to contaminated house dust or intervening on another common consumer source of exposure. Further investigation of various intervention scenarios could be particularly useful since targeting a reduction in a specific chemical may not always be feasible
We note that the impact of simultaneously increasing OPE-phthalate mixture could be larger in other populations, since our study population had low DPHP exposure (median: 0.51 ng/mL) compared to the previous studies of pregnant women (0.77–2.94 ng/mL; Supplementary Table 2). Additionally, BDCIPP, another potential neurotoxicant, was not included in the quantile based g-computation due to limited detection (74–79%<LOD). Overall, the high risk ratios of ADHD observed with increasing exposure to OPE-phthalate mixtures in this population-based study, is in line with and summarizes the neurodevelopmental impacts of individual OPE and phthalate metabolites reported previously (Castorina et al. 2017; Doherty et al. 2019; Engel et al. 2010; Engel et al. 2018; Gascon et al. 2015; Kobrosly et al. 2014; Lien et al. 2015; Philippat et al. 2015; Whyatt et al. 2012), calling for a timely need to take action to reduce exposure at a population level.
4.4. Strengths and limitations
Our investigation was uniquely positioned to evaluate prenatal OPE exposure as a risk factor for clinical ADHD, leveraging an existing study that was nested in a large prospective study. As such, we were able to utilize the existing measures of phthalate exposure and well-characterized, prospective covariate data. Nesting our case-cohort study within a population-based pregnancy cohort and obtaining clinical diagnoses through linkage with a population-based registry enabled us to examine associations with a clinically diagnosed developmental disability, ADHD. Further, we took advantage of the large sample size and explored important potential modifiers, such as child sex and polymorphisms in genes suspected to play a role in the metabolism of OPEs. We also provide evidence for OPE metabolites infrequently studied in epidemiological investigations, i.e., DNBP and BBOEP. Another advantage of our study is in the consideration of phthalates to estimate 1) the individual association of OPE metabolites as well as 2) the impact of combined exposure to phthalates and OPEs. Lastly, this is the first study to be conducted outside of the United States, where we evaluated our hypothesis in a low-level-exposure environment.
Our investigation was limited to a single spot urine sample collected at 17 weeks’ gestation, which may not be reflective of pregnancy-wide exposures due to the short half-lives of OPEs. However, OPEs are included in consumer products that people use on a daily basis. Ubiquitous exposure to OPE sources is expected to result in relatively stable concentrations, at least in the short term. For repeat-samples collected over several hours to weeks, moderate to high ICCs ranging from 0.43 to 0.70 have been reported for DPHP (Carignan et al., 2017; Cequier et al. 2015; Hoffman et al. 2014; Wang et al. 2019); although the ICCs across studies were more variable in the long term (Carignan et al., 2017; Hoffman et al. 2014; Ingle et al., 2019; Meeker et al. 2013a; Preston et al. 2017; Romano et al. 2017). Further, we considered the season of urine sample collection to adjust for seasonal variability in prenatal exposure to OPEs (Preston et al. 2017) that can arise from seasonal variability in human behaviors (e.g., ventilation and time-activity patterns) and exposures from point sources (e.g., rate of vaporization). The season of urine sample collection may also be a proxy of birth month, which may be a predictor for ADHD symptoms, although the underlying causes are not established (Zhang et al. 2018).
Another limitation is in the potential for inaccuracies in ADHD case diagnoses registered in the NPR. Although the NPR captures around 90% of ADHD cases in Norway (Suren et al. 2012), false-positive cases may also be included (Surén et al. 2018) due to the absence of a gold standard clinical assessment for ADHD diagnosis. The ADHD diagnostic criteria are based on a constellation of behavioral symptoms evaluated by clinical providers (Taylor et al. 2004), and therefore diagnosis and treatment vary by the clinical practice and the experience of the provider (Hinshaw et al. 2011). We attempted to remove erroneous or false registrations by limiting our cases to those that had more than one registration of ADHD in the NPR. Moreover, the relationships between NPR ADHD diagnosis status and parental reported ADHD symptoms at ages 3 and 5 using the Child Behavior Checklist (CBCL) and Conners Parent Rating Scale have been previously investigated (Oerbeck et al. 2017). Oerbeck and colleagues found that ADHD symptoms substantially predicted later NPR ADHD diagnosis (hazard ratio for CBCL at age 5 = 10.30, 95% CI: 7.44, 14.26). These data support the congruence of parent-reported symptoms of ADHD in MoBa questionnaires with later registration of ADHD within the NPR. In addition, any outcome misclassification as a result of inaccuracies in diagnosis in childhood would not be expected to be differential by maternal OPE exposure during pregnancy.
Lastly, DPHP is a biomarker that lacks specificity and is reflective of exposures to multiple parent compounds such as resorcinol bis-diphenyl phosphate (Ballesteros-Gomez et al. 2015), ethylhexyl diphenyl phosphate (Van den Eede et al. 2016), and TPHP (Su et al. 2015; Van den Eede et al. 2016). If only a subset of DPHP parent compounds exhibits thyroid disrupting properties, the associations estimated with DPHP may not accurately reflect that of the thyroid active compound. However, toxic properties have also been reported for DPHP (Hill et al. 2018), in which case the utilization of DPHP as an integrated marker will be beneficial to quantify the adverse impact of DPHP-related exposures.
4.5. Public health implications
Although ADHD is one of the most common psychiatric disorders in children (Avenevoli et al. 2013; Lensing et al. 2015), few modifiable risk factors for ADHD have been identified. We found that prenatal exposure to DPHP and BDCIPP may be associated with an increased risk of ADHD, and that girls may be particularly vulnerable to DPHP exposure. OPEs, though prevalent, are modifiable through the implementation of population-wide regulatory policies, voluntary removal of these chemicals from consumer products, or behavioral interventions. Since population exposure to OPEs has been shown to be steadily increasing (Hoffman et al. 2017), there is an urgent need to take action to reduce subsequent health effects of exposure.
5. Conclusion
DPHP and BDCIPP exposure during pregnancy may increase the risk of ADHD in offspring. Combined exposure to common-detect OPE and phthalates resulted in a substantial increase in ADHD risk. For DPHP, the relationship was stronger among girls and varied by maternal PON1 Q192R polymorphisms.
Supplementary Material
Acknowledgments
We thank all families who participated in this ongoing cohort study. GC was supported in part by a training grant from the National Institute of Environmental Health Sciences [T32ES007018]. This research was funded in part by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) R01ES021777, P30 ES010126, and by the Intramural Research Program of the NIH/NIEHS. The Norwegian Mother, Father and Child (MoBa) Controls Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (no. N01-ES-75538), NIH/National Institute of Neurological Disorders and Stroke (NINDS) (no. 1 UO1 NS 047537-01 and no. 2 UO1 NS 047537-06A1).
We thank the Norwegian Institute of Public Health (NIPH) for generating high-quality genomic data. This research is part of the HARVEST collaboration, supported by the Research Council of Norway (#229624). We also thank the NORMENT Centre for providing genotype data, funded by the Research Council of Norway (#223273), South East Norway Health Authority and KG Jebsen Stiftelsen. We further thank the Center for Diabetes Research, the University of Bergen for providing genotype data and performing quality control and imputation of the data funded by the ERC AdG project SELECTionPREDISPOSED, Stiftelsen Kristian Gerhard Jebsen, Trond Mohn Foundation, the Research Council of Norway, the Novo Nordisk Foundation, the University of Bergen, and the Western Norway Health Authorities (Helse Vest).
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106530.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alcorn J, McNamara PJ, 2002. Ontogeny of hepatic and renal systemic clearance pathways in infants part I. Clin. Pharmacokinet 41, 959–998. [DOI] [PubMed] [Google Scholar]
- Allan W, Haddow J, Palomaki G, Williams J, Mitchell M, Hermos R, Faix J, Klein R, 2000. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J. Med. Screen 7, 127–130. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Laurberg P, Wu C, Olsen J, 2014. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a D anish nationwide cohort study. BJOG: An International Journal of Obstetrics & Gynaecology 121, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Anzenbacher P, Anzenbacherova E, 2001. Cytochromes P450 and metabolism of xenobiotics. Cellular and Molecular Life Sciences CMLS 58, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenevoli S; Baio J; Bitsko RH; Blumberg SJ; Brody DJ; Crosby A; Gfroerer J; Ghandour RM; Hall JE; Hedden SL Mental health surveillance among children–United States, 2005–2011. 2013. [PubMed] [Google Scholar]
- Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, Patisaul HB, 2017. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci. Rep 7, 7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Gomez A, Van den Eede N, Covaci A, 2015. In vitro human metabolism of the flame retardant resorcinol bis(diphenylphosphate) (RDP). Environ. Sci. Technol 49, 3897–3904. [DOI] [PubMed] [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Ostman C, 2011. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air 21, 67–76. [DOI] [PubMed] [Google Scholar]
- Bi C, Maestre JP, Li H, Zhang G, Givehchi R, Mahdavi A, Kinney KA, Siegel J, Horner SD, Xu Y, 2018. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: Association with season, building characteristics, and childhood asthma. Environ. Int 121, 916–930. [DOI] [PubMed] [Google Scholar]
- Bigley AN, Xiang DF, Ren Z, Xue H, Hull KG, Romo D, Raushel FM, 2016. Chemical Mechanism of the Phosphotriesterase from Sphingobium sp. Strain TCM1, an Enzyme Capable of Hydrolyzing Organophosphate Flame Retardants. J. Am. Chem. Soc 138, 2921–2924. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J, 2005. Phthalates in indoor dust and their association with building characteristics. Environ. Health Perspect 113, 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam W, Keijzer H, Struijker Boudier H, Didden R, Smits M, Curfs L, 2013. CYP1A2 polymorphisms in slow melatonin metabolisers: a possible relationship with autism spectrum disorder? J. Intellect. Disabil. Res 57, 993–1000. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Haugen M, Alexander J, Meltzer HM, 2008. Validity of a new food frequency questionnaire for pregnant women in the Norwegian Mother and Child Cohort Study (MoBa). Maternal & child nutrition 4, 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM, 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ. Health Perspect 125, 067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrow GN, Fisher DA, Larsen PR, 1994. Maternal and fetal thyroid function. N. Engl. J. Med 331, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM, 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM, 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int 94, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Minguez-Alarcon L, Butt CM, Williams PL, Meeker JD, Stapleton HM, Toth TL, Ford JB, Hauser Team R, 2017. E.S. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ. Health Perspect 125, 087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Stapleton HM, Butt C, Avery D, Harley KG, Gunier RB, Holland N, Eskenazi B, 2017. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere 189, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Marce RM, Becher G, Thomsen C, 2014. A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography-high resolution mass spectrometry. Anal. Chim. Acta 845, 98–104. [DOI] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Haug LS, Thomsen C, 2016. Development of an ion-pair liquid chromatography–high resolution mass spectrometry method for determination of organophosphate pesticide metabolites in large-scale biomonitoring studies. J. Chromatogr. A 1454, 32–41. [DOI] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C, 2015. Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ. Int 75, 159–165. [DOI] [PubMed] [Google Scholar]
- Charach A; Dashti B; Carson P; Booker L; Lim CG; Lillie E; Yeung E; Ma J; Raina P; Schachar R. Attention deficit hyperactivity disorder. 2011. [PubMed] [Google Scholar]
- Choi G, Keil AP, Villanger GD, Richardson DB, Daniels JL, Hoffman K, Sakhi AK, Thomsen C, Herring AH, Drover SSM, Nethery RC, Aase H, Engel SM, 2021. Pregnancy exposure to common-detect organophosphate esters and phthalates and maternal thyroid function. Sci. Total Environ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Vitalone A, Furlong CE, 2005. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin. Chim. Acta 352, 37–47. [DOI] [PubMed] [Google Scholar]
- Costa LG, Richter RJ, Li W-F, Cole T, Guizzetti M, Furlong CE, 2003. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers 8, 1–12. [DOI] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ, 2018. Prevalence of parent-reported ADHD diagnosis and associated treatment among US children and adolescents, 2016. J. Clinical Child & Adolescent Psychology 47, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE, 1996. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet 14, 334–336. [DOI] [PubMed] [Google Scholar]
- Ding J, Xu Z, Huang W, Feng L, Yang F, 2016. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci. Total Environ 554–555, 211–217. [DOI] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM, 2011. Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol 256, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, Olshan AF, Daniels JL, 2019. Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. NeuroToxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover SS, Villanger GD, Aase H, Skogheim TS, Longnecker MP, Zoeller RT, Reichborn-Kjennerud T, Knudsen GP, Zeiner P, Engel SM, 2018. Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: A systematic review. Epidemiology (Cambridge, Mass). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS, 2007. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol 165, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS, 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect 118, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SS, Hoppin JA, Zeiner P, Knudsen GP, Reichborn-Kjennerud T, 2018. Prenatal Phthalates, Maternal Thyroid Function, and Risk of Attention-Deficit Hyperactivity Disorder in the Norwegian Mother and Child Cohort. Environ. Health Perspect 126, 057004 057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Polybrominated diphenyl ethers (PBDEs) action plan. 2013. [Google Scholar]
- EU. Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Official Journal of the European Union 2003;13:L37. [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL, 2010. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 118, 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Ouyang F, Liu L, Wang X, Wang X, Li YJ, Murtha A, Shen H, Zhang J, Zhang JJ, 2016. Levels of Urinary Metabolites of Organophosphate Flame Retardants, TDCIPP, and TPHP, in Pregnant Women in Shanghai. J Environ Public Health 2016, 9416054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC, 2011. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 13, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Seidel SL, Motulsky A, 1988. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum. Genet 43, 230. [PMC free article] [PubMed] [Google Scholar]
- Gabriel S; Ziaugra L; Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Current protocols in human genetics 2009;60:2.12. 11–12.12. 18. [DOI] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, Monfort N, Ventura R, Sunyer J, Vrijheid M, 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int. J. Hyg. Environ. Health 218, 550–558. [DOI] [PubMed] [Google Scholar]
- Ghotbi R, Christensen M, Roh HK, Ingelman-Sundberg M, Aklillu E, Bertilsson L, 2007. Comparisons of CYP1A2 genetic polymorphisms, enzyme activity and the genotype-phenotype relationship in Swedes and Koreans. Eur. J. Clin. Pharmacol 63, 537–546. [DOI] [PubMed] [Google Scholar]
- Gibson EA, Goldsmith J, Kioumourtzoglou M-A, 2019. Complex mixtures, complex analyses: an emphasis on interpretable results. Current Environmental Health Reports 6, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillera SEA, Marinello WP, Horman BM, Phillips AL, Ruis MT, Stapleton HM, Reif DM, Patisaul HB, 2020. Sex-specific effects of perinatal FireMaster(R) 550 (FM 550) exposure on socioemotional behavior in prairie voles. Neurotoxicol. Teratol 79, 106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, Buckley JP, 2018. Environmental exposure mixtures: questions and methods to address them. Current epidemiology reports 5, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Hamers T, Kamstra JH, Willmore WG, Letcher RJ, 2018. Organophosphate triesters and selected metabolites enhance binding of thyroxine to human transthyretin in vitro. Toxicol. Lett 285, 87–93. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Scheffler RM, Fulton BD, Aase H, Banaschewski T, Cheng W, Mattos P, Holte A, Levy F, Sadeh A, 2011. International variation in treatment procedures for ADHD: social context and recent trends. Psychiatric Services 62, 459–464. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Butt CM, Webster TF, Preston EV, Hammel SC, Makey C, Lorenzo AM, Cooper EM, Carignan C, Meeker JD, 2017. a. Temporal trends in exposure to organophosphate flame retardants in the United States. Environ. Sci. Technol. Lett 4, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Daniels JL, Stapleton HM, 2014. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int 63, 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL, 2017b. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int 98, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE, 1993. The molecular basis of the human serum paraoxonase activity polymorphism. Nat. Genet 3, 73–76. [DOI] [PubMed] [Google Scholar]
- Ingle ME; Watkins D; Rosario Z; Velez Vega CM; Huerta-Montanez G; Calafat AM; Ospina M; Cordero JF; Alshawabkeh A; Meeker JD The association of urinary organophosphate ester metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico. Environ Res 2019; 179:108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionas AC, Dirtu AC, Anthonissen T, Neels H, Covaci A, 2014. Downsides of the recycling process: harmful organic chemicals in children’s toys. Environ. Int 65, 54–62. [DOI] [PubMed] [Google Scholar]
- Irgens LM, 2000. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet. Gynecol. Scand 79, 435–439. [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S, 2015. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hong X, Wang G, Chatterjee N, Riley AW, Lee LC, Surkan PJ, Bartell TR, Zuckerman B, Wang X, 2018. A Prospective Birth Cohort Study on Early Childhood Lead Levels and Attention Deficit Hyperactivity Disorder: New Insight on Sex Differences. J. Pediatr 199 (124–131), e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakosta P, Alegakis D, Georgiou V, Roumeliotaki T, Fthenou E, Vassilaki M, Boumpas D, Castanas E, Kogevinas M, Chatzi L, 2012. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J. Clin. Endocrinol. Metab 97, 4464–4472. [DOI] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect 128, 047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh RH, Cox DR, 2014. Case-control studies edêds. Cambridge University Press. [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, 2005. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol. Med 35, 245–256. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, Swan SH, 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ. Health Perspect 122, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Van den Eede N, Covaci A, 2016. Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors. Toxicol. Lett 245, 31–39. [DOI] [PubMed] [Google Scholar]
- Kosarac I, Kubwabo C, Foster WG, 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J. Chromatogr. B 1014, 24–30. [DOI] [PubMed] [Google Scholar]
- Lensing MB, Zeiner P, Sandvik L, Opjordsmoen S, 2015. Quality of life in adults aged 50+ with ADHD. J Atten Disord 19, 405–413. [DOI] [PubMed] [Google Scholar]
- Li R, Guo W, Lei L, Zhang L, Liu Y, Han J, Chen L, Zhou B, 2020. Early-life exposure to the organophosphorus flame-retardant tris (1,3-dichloro-2-propyl) phosphate induces delayed neurotoxicity associated with DNA methylation in adult zebrafish. Environ. Int 134, 105293. [DOI] [PubMed] [Google Scholar]
- Liang Y, Xu Y, 2014. Improved method for measuring and characterizing phthalate emissions from building materials and its application to exposure assessment. Environ. Sci. Technol 48, 4475–4484. [DOI] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, Chen WJ, Wang SL, 2015. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect 123, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML, 2017. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environmental health 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cai Y, Wang Y, Xu S, Ji K, Choi K, 2019. Effects of tris(1,3-dichloro-2- propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol. Environ. Saf 170, 25–32. [DOI] [PubMed] [Google Scholar]
- Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN, 1997. Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br. J. Pharmacol 122, 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, Handal M, Haugen M, Høiseth G, Knudsen GP, 2016. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int. J. Epidemiol 45, 382–388. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C, 2006. Cohort profile: the Norwegian mother and child cohort study (MoBa). Int. J. Epidemiol 35, 1146–1150. [DOI] [PubMed] [Google Scholar]
- Mahmood I; Burckart GJ; Ward RM Perinatal pharmacology and maternal/fetal dosing. Fundamentals of pediatric drug dosing: Springer; 2016. [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013a. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocr Disruptors (Austin) 1, e26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cooper EM, Stapleton HM, Hauser R, 2013b. Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ. Health Perspect 121, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerbeck B; Overgaard KR; Pripp AH; Reichborn-Kjennerud T; Aase H; Zeiner P. Early Predictors of ADHD: Evidence from a Prospective Birth Cohort. J Atten Disord 2017:1087054717696765. [DOI] [PubMed] [Google Scholar]
- Ospina M, Jayatilaka NK, Wong L-Y, Restrepo P, Calafat AM, 2018. Exposure to organophosphate flame retardant chemicals in the US general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int 110, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päkkilä F, Männistö T, Pouta A, Hartikainen A-L, Ruokonen A, Surcel H-M, Bloigu A, Vääräsmäki M, Järvelin M-R, Moilanen I, 2014. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J. Clinical Endocrinology Metabolism 99, E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Ceolotto G, Ragazzo F, Dorigatti F, Saladini F, Papparella I, Mos L, Zanata G, Santonastaso M, 2009. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J. Hypertens 27, 1594–1601. [DOI] [PubMed] [Google Scholar]
- Paltiel L, Anita H, Skjerden T, Harbak K, Bækken S, Kristin SN, Knudsen GP, Magnus P, 2014. The biobank of the Norwegian Mother and Child Cohort Study–present status. Norsk epidemiologi 24. [Google Scholar]
- Pearce N, 1993. What does the odds ratio estimate in a case-control study? Int. J. Epidemiol 22, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Philippat C, Bennett DH, Krakowiak P, Rose M, Hwang HM, Hertz-Picciotto I, 2015. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ Health 14, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, De Lima MS, Horta BL, Biederman J, Rohde LA, 2007. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry 164, 942–948. [DOI] [PubMed] [Google Scholar]
- Preston EV, McClean MD, Henn BC, Stapleton HM, Braverman LE, Pearce EN, Makey CM, Webster TF, 2017. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int 101, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Gibson P, Rhodes CL, Cushing BS, Patisaul HB, 2016. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen. Comp. Endocrinol 238, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Patisaul HB, 2016. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J. Steroid Biochem. Mol. Biol 160, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RJ, Jarvik GP, Furlong CE, 2009. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol. Appl. Pharmacol 235, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RJ, Jarvik GP, Furlong CE, 2010. Paraoxonase 1 status as a risk factor for disease or exposure. Paraoxonases Inflammation Infection Toxicology: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Hawley NL, Eliot M, Calafat AM, Jayatilaka NK, Kelsey K, McGarvey S, Phipps MG, Savitz DA, Werner EF, Braun JM, 2017. Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA, 2006. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur. J. Epidemiol 21, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys edêds. John Wiley & Sons. [Google Scholar]
- Schomaker M, Heumann C, 2018. Bootstrap inference when using multiple imputation. Stat. Med 37, 2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Letcher RJ, Crump D, Gooden DM, Stapleton HM, 2015. In vitro metabolism of the flame retardant triphenyl phosphate in chicken embryonic hepatocytes and the importance of the hydroxylation pathway. Environ. Sci. Technol. Lett 2, 100–104. [Google Scholar]
- Suren P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, Magnus P, Reichborn-Kjennerud T, Schjolberg S, Oyen AS, Stoltenberg C, 2012. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics 130, e152–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P; Thorstensen AG; Tørstad M; Emhjellen PE; Furu K; Biele G; Aase H; Stoltenberg C; Zeiner P; Bakken IJ Diagnosis of hyperkinetic disorder among children in Norway. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke 2018. [DOI] [PubMed] [Google Scholar]
- Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Rothenberger A, Sonuga-Barke E, Steinhausen HC, Zuddas A, 2004. European clinical guidelines for hyperkinetic disorder – first upgrade. Eur. Child Adolesc. Psychiatry 13 (Suppl 1), I7–I30. [DOI] [PubMed] [Google Scholar]
- USFDA, 2020. Advice about eating fish for women who are or might become pregnant, breastfeeding mothers, and young children. https://www.fda.gov/food/consumers/advice-about-eating-fish (Accessed 30, December 2020).
- Van den Eede N, Ballesteros-Gomez A, Neels H, Covaci A, 2016. Does Biotransformation of Aryl Phosphate Flame Retardants in Blood Cast a New Perspective on Their Debated Biomarkers? Environ. Sci. Technol 50, 12439–12445. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Erratico C, Exarchou V, Maho W, Neels H, Covaci A, 2015. In vitro biotransformation of tris(2-butoxyethyl) phosphate (TBOEP) in human liver and serum. Toxicol. Appl. Pharmacol 284, 246–253. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Maho W, Erratico C, Neels H, Covaci A, 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett 223, 9–15. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J, 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, 2016. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 37, 17–32. [DOI] [PubMed] [Google Scholar]
- Vanderweele TJ, Vansteelandt S, 2010. Odds ratios for mediation analysis for a dichotomous outcome. Am. J. Epidemiol 172, 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch OJ, Pendergast JS, Allen MJ, Leu RM, Johnson CH, Elsea SH, Malow BA, 2015. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J. Autism Dev. Disord 45, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanger GD, Drover SSM, Nethery RC, Thomsen C, Sakhi AK, Overgaard KR, Zeiner P, Hoppin JA, Reichborn-Kjennerud T, Aase H, Engel SM, 2020. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ. Int 137, 105509. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liang K, Liu J, Yang L, Guo Y, Liu C, Zhou B, 2013. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat. Toxicol 126, 207–213. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li W, Martinez-Moral MP, Sun H, Kannan K, 2019. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environ. Int 122, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Yin H, Peng H, Lu G, Dang Z, 2018. Bioremediation of triphenyl phosphate by Brevibacillus brevis : Degradation characteristics and role of cytochrome P450 monooxygenase. Sci. Total Environ 627, 1389–1395. [DOI] [PubMed] [Google Scholar]
- WHO. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines edêds: World Health Organization; 1992. [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP, Factor-Litvak P, 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect 120, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Harris SA, Jantunen LM, Kvasnicka J, Nguyen LV, Diamond ML, 2020. Phthalates: Relationships between Air, Dust, Electronic Devices, and Hands with Implications for Exposure. Environ. Sci. Technol 54, 8186–8197. [DOI] [PubMed] [Google Scholar]
- Zhang C, Brook JS, Leukefeld CG, Rosa M, Brook DW, 2018. Season of birth: A predictor of ADHD symptoms in early midlife. Psychiatry Res. 267, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Ji C, Yin X, Yan L, Lu M, Zhao M, 2016. Thyroid hormone-disrupting activity and ecological risk assessment of phosphorus-containing flame retardants by in vitro, in vivo and in silico approaches. Environ. Pollut 210, 27–33. [DOI] [PubMed] [Google Scholar]
- Zhao F, Chen M, Gao F, Shen H, Hu J, 2017. Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environ. Sci. Technol 51, 6489–6497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.