Abstract
Coronavirus disease 2019 [COVID-19] is a global health threat caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV2] that requires two proteins for entry: angiotensin-converting enzyme 2 [ACE2] and -membrane protease serine 2 [TMPRSS2]. Many patients complain from pneumonia, cough, fever, and gastrointestinal (GI) problems. Notably, different TRP channels are expressed in various tissues infected by SARS-CoV-2. TRP channels are cation channels that show a common architecture with high permeability to calcium [Ca2+] in most sub-families. Literature review shed light on the possible role of TRP channels in COVID-19 disease. TRP channels may take part in inflammation, pain, fever, anosmia, ageusia, respiratory, cardiovascular, GI and neurological complications related to COVID-19. Also, TRP channels could be the targets for many active compounds that showed effectiveness against SARS-CoV-2. Desensitization or blocking TRP channels by antibodies, aptamers, small molecules or venoms can be an option for COVID-19 prevention and future treatment. This review provides insights into the involvement of TRP channels in different symptoms and mechanisms of SARS-CoV-2 , potential treatments targeting these channels and highlights missing gaps in literature.
Keywords: COVID-19, Treatment, Prevention, Symptoms, SARS-CoV-2, TRP channels
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- ARDS
Acute respiratory distress syndrome
- ATP
Adenosine triphosphate
- Ca2+
Calcium
- CBD
Cannabidiol
- COVID-19
Coronavirus disease 2019
- CTX
Ciguatoxin
- 3D
Three dimensional
- DRG
Dorsal root ganglia
- EC
Enterochromaffin cells
- EECs
Enteroendocrine cells
- ER
Endoplasmic reticulum
- GI
Gastrointestinal
- HSP70
Heat shock protein 70
- HSR
Heat shock response
- ICU
Intensive care unit
- IFNγ
Interferon gamma
- IL
Interleukin
- IL-1β
Interleukin 1 beta
- Nrf2
Nuclear factor erythroid 2–related factor 2
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NTS
Nucleus tractus solitarius
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- ROS
Reactive oxygen species
- RTX
Resiniferatoxin
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SP
Substance P
- S protein
Spike protein
- TG
Trigeminal ganglia
- TMPRSS2
Trans-membrane protease serine 2
- TNFα
Tumor necrosis factor alpha
- TRP
Transient receptor potential
- TRPA
Transient receptor potential ankyrin
- TRPC
Transient receptor potential canonical or classical
- TRPM
Transient receptor potential melastatin
- TRPML
Transient receptor potential mucolipin
- TRPP
Transient receptor potential polycystin
- TRPV
Transient receptor potential vanilloid
- WHO
World Health Organization
1. Introduction
Coronavirus disease 2019 [COVID-19] is one of the worst pandemics in the world. According to new estimates from the World Health Organization [WHO] published in May 25, 2021, over 4.1 million new cases and 84,000 new deaths were globally reported in a week [1]. Although in some cases COVID-19 patients were asymptomatic, many people complained from pneumonia, cough, fever, and gastrointestinal [GI] problems [2,3]. Recently, the long haul COVID-19 illness started to gain recognition whereby some patients suffer from symptoms in the long term even when their viral tests were confirmed negative [4].
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 [SARS-CoV2], which acts via a spike protein [S protein] that binds to host cell receptors and regulates viral infection [5]. It is widely accepted that two key proteins are required for SARS-CoV2 entry: angiotensin-converting enzyme 2 [ACE2] and trans-membrane protease serine 2 [TMPRSS2] [6]. Accumulating evidence showed that calcium [Ca2+] channel blockers inhibited SARS-CoV-2 infectivity in epithelial lung cells proving that Ca2+ ions are required for the viral entry into host cells [7]. Since transient receptor potential [TRP] channels are cation channels with high permeability to Ca2+ in most sub-families [8], they could be involved in certain steps of SARS-CoV-2 life cycle.
TRP channels are divided according to the amino acid sequences into different subgroups including TRPM [melastatin], TRPML [mucolipin], TRPA [ankyrin], TRPV [vanilloid], TRPP [polycystin] and TRPC [canonical or classical] channels [8]. In fact, most TRP channels are widely expressed in tissues that are infected by SARS-CoV2 virus. Therefore, TRP channels can be valuable targets interfering with COVID-19 life cycle. It was noted that uncoating of the viral envelope is a crucial step for the entry of enveloped viruses, including SARS-CoV-2 virus that enters host cells through endocytosis. In order for this step to occur, different receptors are needed to trigger fusion of the viral envelope with the endolysosomal membrane [9,10]. Importantly, a TRP channel, TRPML2 channel, plays a key role in this process. It enhances the efficiency of viral trafficking in the endosomal system, thus affecting the viral entry [9,10]. Therefore, TRP channels can be valuable targets interfering with COVID-19 life cycle.
Despite the surge of papers published about COVID-19, the mechanisms that underpin this disease are still inconclusive. In fact, many COVID-19 symptoms result from the activation of different TRP channels. It has been suggested that TRPV1 desensitization [e.g. by resiniferatoxin (RTX)] in the afferent neurons can decrease complications associated with the standard therapy in severely compromised COVID-19 patients; thus can improve their immune and inflammatory responses [11]. Notably, headache and myalgia are common symptoms in COVID-19 disease [12,13]. Unfortunately, the use of non-steroidal anti-inflammatory drugs [NSAIDs] to alleviate these symptoms may increase the susceptibility to coronavirus by upregulating ACE2 receptors therefore enhancing viral entry [14]. In this context, it is well established that several TRP channels are involved in pain sensation such as TRPV1 channel [15]. Therefore, these channels represent promising targets for pain relief in COVID-19.
Worthy to mention, around 53% of COVID-19 mortalities resulted from acute respiratory distress syndrome [ARDS] caused by edema in the lungs [16,17]. It has been demonstrated that TRPV4 and TRPC6 channels are implicated in pulmonary edema [18,19] while TRPV4 and TRPM7 channels are regulators for pulmonary fibrosis [20,21]. Thus, TRPV4 channel was recently emerged as a therapeutic target in SARS-CoV-2 infection [22]. Taken together, the aim of this review is to explore the possible involvement of TRP channels in different COVID-19 symptoms in line with potential trends in the prevention and treatment of this disease.
2. Methods
Literature search was done for articles using the following keywords: angiotensin-converting enzyme 2, ageusia, anosmia, cardiovascular, cough, coronavirus disease 2019, desensitization, diet, edema, fever, gastrointestinal, headache, hearing, inflammation, medicinal plants, myalgia, natural products, pain, pulmonary, respiratory, severe acute respiratory syndrome coronavirus 2, tachyphylaxis, transient receptor potential channels, and venom. PubMed and Google scholar were used to search for the articles.
3. Results
3.1. TRP channels
There are different types of TRP channels being involved in many physiological and pathological responses. TRPV1 (capsaicin receptor) is a non-selective cation channel correlated with several inflammatory conditions such as airway obstruction [23]. Compelling lines of evidences revealed that TRPA1, a Ca2+-permeable channel, is co-localized with TRPV1 channel in neuronal and non-neuronal cells [15]. Importantly, TRPV2 and TRPV3 channels are selective to Ca2+ and take part in Ca2+ homeostasis [24]. TRPV6 is another channel that exhibits higher selectivity to Ca2+ [100:1 ratio] compared to 3:1 ratio in TRPV2 channel [24]. Additionally, TRPM2 channel has the ability to respond to oxidative stress and is permeable to Ca2+ as well as to other ions [25,26]. This channel is considered a suicidal channel that compromises the cell by overloading it with Ca2+ under stress conditions [27]. In more detail, TRPM2 channel is activated by reactive oxygen species [ROS] and is involved in the production of pro-inflammatory chemokines [25]. On the other hand, TRPM5 channel has an unusual characteristic compared to other TRP channels [15]. It is a Ca2+ impermeable channel with high selectivity to Na+ that leads to depolarization, action potential and release of adenosine triphosphate [ATP] transmitter in the afferent neurons [15]. Further, it was revealed that TRPM8 channel has Ca2+ and Na+ dual selectivity [28].
TRPC channels are non-selective cation channels that have 1.1:9 Ca2+:Na+ selectivity ratio and are involved in several responses [29]. A point of interest is that the existence of multiple TRP channels in a tissue can form heterodimers that trigger different responses compared to monomers and this fact adds a complexity to validating the mechanism of action of TRP channels in a response [30]. Importantly, TRPML channels are non-selective cation channel expressed in the endosomal vesicles [31]. TRPML2 channel is expressed in immune cells, thymus and spleen compared to the ubiquitous expression of TRPML1 channel [31].
3.2. TRP channels and different symptoms of COVID-19
3.2.1. TRP channels and inflammatory response in COVID-19
Several studies demonstrated the existence of a hyperinflammatory state [cytokine storm] in COVID-19 cases due to the upregulation of several pro-inflammatory mediators [32]. In more detail, multiple reports showed that COVID-19 patients had high levels of interferon gamma [IFNγ], interleukin 1 beta [IL-1β], IL-6 and IL-10 [33,34]. Additionally, it was noted that COVID-19 patients admitted to the intensive care unit [ICU] in Wuhan had higher amounts of tumor necrosis factor alpha [TNFα] and other cytokines compared to healthy individuals [34].
Several studies shed light on the role of different TRP channels in immune and inflammatory responses suggesting that there is an interplay between these channels and the production of inflammatory mediators in COVID-19 patients. For instance, it was reported that TRPV4 channel is a regulator for pulmonary inflammation due to its expression in alveolar macrophages [35]. Particularly, TRPV4 channel is involved in the recruitment of neutrophils and macrophages during lung injury [20].
The possible role of TRPA1 channel in inflammation in COVID-19 was also highlighted [36]. Several natural compounds and diets reduced inflammatory symptoms in COVID-19 patients in a TRPA1 dependent mechanism [36]. Examples include broccoli, black pepper, ginger, green tea and curcuma whereby their consumption improved nasal obstruction and cough in COVID-19 patients [36].
TRPM2 is another channel that takes part in stress-related inflammatory and immune processes [37]. Also, the communication between immune cells and TRPV1-expressing fibers was found to be critical in mediating airway inflammation caused by inhaled allergens or viral infection [38]. Collectively, all the aforementioned TRP channels can be positive targets in alleviating COVID-19 symptoms that are related to immune and inflammatory processes.
3.2.1.1. TRP channels and pain in COVID-19
It is widely accepted that COVID-19 patients suffer from several types of pain such as referred pain, myalgia, hyperalgesia and headache [12,13]. Caution must be observed when using the currently available drugs for pain relief in COVID-19 patients [14]. Unfortunately, inconclusive reports indicated that the use of NSAIDs may increase the susceptibility to Coronavirus by upregulating viral entry through ACE2 receptors [14]. These facts urge the need to explore new therapeutics that can alleviate rather than aggravate COVID-19 symptoms. In this regard, accumulating knowledge showed that several TRP channels (e.g. TRPV1 channel) are promising targets for pain. Besides, the involvement of TRPA1 channel in mechanical hyperalgesia and inflammatory pain is an indication for its role in pain [39]. Of the receptors belonging to TRPM subfamily, it was found that TRPM2, TRPM3 and TRPM8 channels are associated with the processing of noxious stimuli; thus pain sensation [39]. Other TRP receptors are also considered transducers of pain such as TRPV2, TRPV3, TRPV4, TRPC1 and TRPC6 channels [39]. Noteworthy to mention, several interventional procedures (e.g. dextrose injections) that were used to reduce pain in COVID-19 patients interact with TRPV1 channel [40]. Similarly, Bonvini et al. reported the activation of TRPM3 and TRPV4 channels by hypoosmolar solutions that were used to challenge cough [28]. Taken together, TRP channels are promising targets for alleviating pain in COVID-19 patients.
3.2.2. TRP channels and fever in COVID-19
Fever is one of the symptoms reported in 98% of COVID-19 patients [34]. It was demonstrated that a continuous fever that lasts for long term is responsible for heat shock protein 70 [HSP70] depletion, ARDS and death [16]. Also, it is widely accepted that the expression of thermosensitive ion channels (e.g. some TRP channels) plays a key role in thermoregulation conducted by hypothalamus [41]. These channels allow hypothalamic neurons to respond to minor thermal fluctuations in the body in addition to other functions [41]. Particularly, high expression of TRPV1, TRPV2, TRPV3 and TRPV4 channels compared to lower expression for TRPA1 and TRPM8 channels was revealed in the hypothalamus [41]. Of note , TRPV1, TRPV2, TRPV3, TRPV4 and TRPM3 channels are gated by warm temperatures [16].
When body temperature progressively increases, the heat shock response [HSR] is activated leading to plasma membrane fluidization and a transient opening of TRPV1 channel [42,43]. It was revealed that using TRPV1 agonists such as capsaicin and RTX up-regulated the accumulation of several HSP proteins (e.g. HSP70, HSP27 and HSP90) in epithelial cells [44] while the use of TRPV1 siRNA and TRPV1 antagonists [e.g. capsazepine, or AMG-9810] attenuated HSPs [44]. Also, it was documented that TRPV1 trafficking can be modulated by HSP70 proteins [44]. Interestingly, Bromberg and Weiss proved the co-precipitation of TRPV1 and HSP70 from the lungs of septic rats treated with HSP70-expressing adenovirus [42].
TRPV4 immunoreactivity was found in the anterior hypothalamic structures that are involved in the integration of thermal and osmotic signals [45]. Importantly, it was reported that TRPM2 channel is a heat sensor in the hypothalamus contributing to hypothermia during fever [46]. Another aspect that can link TRP channels and fever is their sensitization by phosphatidylinositol 4,5-bisphosphate [PIP2] which is highly abundant in the membrane [42]. All these data suggest that TRP channels can play a crucial role in fever in COVID-19 patients and can be promising targets in developing antipyretic drugs.
3.2.3. TRP channels and respiratory complications in COVID-19
Pursuant to many published researches, Ca2+ is needed for the entry, morphogenesis, replication, assembly, release and immune evasion of SARS-CoV viruses [47]. Due to the high selectivity of many TRP channels to Ca2+, it is expected that several TRP channels contribute to the respiratory complications associated with COVID-19. In this context, it was noted that the upper and lower respiratory tracts are densely populated by sensory afferent neurons in dorsal root ganglia [DRG] and nodose vagal ganglia that express high levels of TRPV1 channel [38]. Additionally, several reports showed that TRPV1 channel is expressed in the epithelial cells of lungs [20].
3.2.3.1. Pulmonary edema
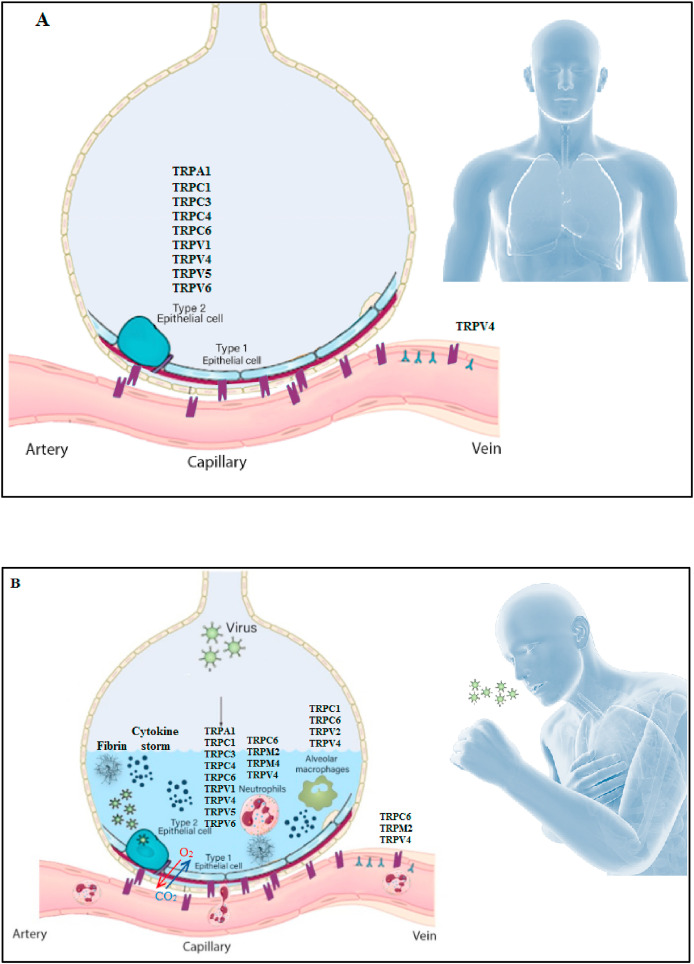
Around 53% of COVID-19 mortalities resulted from ARDS caused by the accumulation of protein-rich inflammatory edema leading to cell death of alveolar cells in the lungs [16,17]. Notably, pulmonary edema and disruption of the alveolo-capillary barrier are key drivers to the critical stages of COVID-19 infection [48]. Further, it was mentioned that there is link between the increase in intracellular Ca2+ and ROS levels in mitochondria, an important matter that contributes to the functions of many viruses including members of Coronaviridae family [47]. In this line, earlier reports showed that several TRP channels [such as TRPM2, TRPV4, TRPV1, TRPC1, TRPC4 and TRPC6 channels] are involved in enhancing the vascular permeability in lung endothelium [19] (Fig. 1 ). In fact, many TRP channels are expressed in the lung, including TRPV2, TRPV5 and TRPV6 channels [49]. In addition, in primary afferent neurons, TRPV1 channel can be activated by ROS or heat produced during tissue injury and inflammation [43,50]. Besides, several studies pointed to TRPA1 channel as a molecular target for ROS and other oxidative stress byproducts [28].
Fig. 1.
Alveolus in lung A] Normal state B] Pulmonary edema in COVID-19 disease.
TRPV4 channel was recently emerged as a therapeutic target in many respiratory diseases including SARS-CoV-2 infection [22]. TRPV4 can be activated by osmotic gradients that occur during edema [18]. TRPV4 activation also contributes to the formation of nitric oxide, ROS in alveolar macrophages and mediates vasoconstriction in pulmonary artery smooth muscle cells [49]. Thus, the use of TRPV4 inhibitors in a model of acute lung injury prevented edema, reduced arterial pressure and hyperactivity in the airways [20]. Besides, Balakrishna et al. reported that blocking TRPV4 is correlated with inhibiting inflammation and edema [18].
Worthy to mention, the depletion of Ca2+ stores from the endoplasmic reticulum (ER) can activate other channels such as TRPC1 and TRPC4 channels [51]. This activation increases Ca2+ influx and endothelial contraction in lung endothelium [52]. Also, it was reported that platelet-activating factor causes recruitment of TRPC6 channel into lung endothelial cells and increases Ca2+ influx and endothelial permeability [53]. Of note, George and colleagues depicted that there could be fibrotic consequences after SARS-CoV-2 infection [54].
Using proteomics and metabolomics of data obtained from 85 confirmed COVID-19 cases in Guangxi, China, it was revealed that the interplay between TRP channels and inflammatory pathways is a crucial factor for pulmonary fibrosis; in terms of occurrence and development [55]. In this context, previous studies showed that TRPV4 and TRPM7 channels are regulators for pulmonary fibrosis [20,21]. More research is needed to investigate the involvement of TRP channels in COVID-19- associated fibrosis. Furthermore, Jian and co-workers affirmed the link between TRPV4-induced Ca+2 uptake and lung congestion, dyspnea as well as the increase in pulmonary vascular pressure [56]. With respect to dyspnea, previous studies published that restoring serotonin levels can convert silent hypoxemia into symptomatic hypoxemia at an early stage in SARS-CoV-2 patients, thus can help them to seek early treatment [57]. Importantly, several researches highlighted the relation between serotonin release and TRP channels suggesting that TRP channels can play a vital role in hypoxemia in COVID-19 patients [[58], [59], [60], [61], [62], [63]]. Consistent with the previously mentioned information, TRP channels can be therapeutic targets for alleviating pulmonary edema in COVID-19 patients.
3.2.3.2. Cough
Cough was documented in 76% of COVID-19 patients [34]. Several TRP channels are involved in respiratory complications associated with COVID-19 infection [36]. In general, the activation of TRP channels in sensory nerves of the airways causes Ca2+ influx, depolarization and action potential that collectively lead to cough [27]. Additionally, previous works affirmed that the respiratory viral infection up-regulates TRPA1 and TRPV1 channels in airway cells [64]. Importantly, the administration of TRPA1, TRPV4 and TRPV1 agonists evoked cough in a dose dependent manner in preclinical studies [65]. Also, the use of gabapentin neuromodulator was effective in patients suffering from chronic cough in a mechanism that involved blocking subtypes of TRP channels [65]. Interestingly, it was documented that pulmonary viral exposure can convert the low-threshold cough mechanoreceptors into nociceptors due to the expression of TRPV1 and substance P [SP] [65]. In fact, the role of TRPV1 channel in cough was highlighted in several studies whereby one study drew attention to the finding that capsaicin oral administration improved cough through desensitization of TRPV1 channel [66]. Moreover, it was demonstrated that using a TRPV4 antagonist reversed cough induced by a TRPV4 agonist in a mechanism that involves P2X3 receptor [67]. Also, it was hypothesized that black pepper, ginger, green tea and curcuma improved nasal obstruction and cough in a TRPA1 dependent mechanism [36]. All of these studies display the possible involvement of TRP channels in cough reflexes in COVID-19.
3.2.4. TRP channels and neurological alterations in COVID-19
Accumulating lines of evidences showed that several neurological alterations are associated with COVID-19 including headache, and symptoms of muscle damage (e.g. soreness and weakness of limbs) [14,68]. SARS-CoV-2 virus is one of the neuroinvasive viruses detected in the brain parenchyma and cerebrospinal fluid in human and animal models [69]. Thus, the virus contributes to neurological alterations that are associated with COVID-19 [69].
3.2.4.1. Headache
Headache is a frequent symptom observed in COVID-19 patients in the emergency rooms [14]. It was documented that viral proliferation in lung tissues can disrupt alveolar exchange and can cause hypoxia in CNS, obstruction of cerebral blood flow and accordingly headache due to ischemia [69]. Cerebral edema and coma may also develop if hypoxia persisted [69].
It is accepted that many TRP channels play crucial roles in headache and migraine symptoms since these channels (e.g. TRPV1 channel) are expressed in trigeminal ganglia [TG] neurons and dural nerve fibers [70]. One of the drugs that proved effectiveness in alleviating headache is sumatriptan that inhibits TRPV1 channel [71]. Also, it was found that the use of civamide [an intranasal TRPV1 agonist] reduced the frequency of headache attacks in patients [72]. Furthermore, TRPV4 mRNA is expressed in TG neurons compared to the minimal expression of TRPM8 mRNA [70,73]. According to Wei et al., TRPV4 activation contributed to headache-like behavior in rats [74]. Similarly, TRPA1 channel is expressed in peripheral sensory neurons and contributes to the emergence of headache [70]. The importance of TRP channels in the occurrence of headache can be manifested by the fact that these channels are activated by many stimuli that cause headache such as low pH [70].
3.2.4.2. Myalgia
Myalgia is a common symptom in 44% of COVID-19 patients [34]. Earlier reports showed that SARS-CoV-2 can affect skeletal muscle cells by direct binding to ACE2 or via indirect routes [75]. Also, several hypotheses were proposed about the mechanisms that underlie myofascial pain syndrome [13]. One of them is the muscle contracture and energy crisis hypothesis in which Ca2+ leakage from the sarcoplasmic reticulum in injured muscles can cause taut bands, energy crisis and production of different sensitizers that lead to pain [13]. The involvement of Ca2+ raises the possibility of TRP channels' involvement in muscular disorders. In this regard, previous studies reported that TRPV channels have a role in delayed onset muscle soreness that occurs after strenuous exercise [13]. Moreover, the interaction between TRPV1 channel and other receptors (e.g. acid sensing ion channels) is widely accepted to take part in ischemic muscle [76].
3.2.5. TRP channels anosmia, ageusia and hearing loss in COVID-19
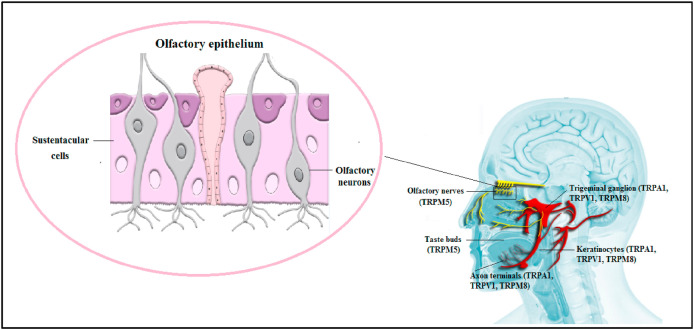
Worldwide, it was emerged that COVID-19 infection causes a reduction or loss of taste and smell [77]. Notably, ACE2 and TMPRSS2 proteins are expressed in the olfactory epithelial support cells [sustentacular cells] but not olfactory sensory neurons [6]. Also, it was found that taste buds don't express ACE2 while epithelial cells of the tongue do [78].
TRP channels are abundant in oral mucosal cells, epithelial cells of the tongue and taste buds [32]. For instance, TRPV1 channel is expressed in the neurons that innervate the oral cavity [15]. Additionally, TRPM4 channel is expressed in type I and II cells [79] while TRPM5 channel is expressed in type II cells [15]. Therefore, a loss of either TRPM4 or TRPM5 channels may significantly impair taste [79]. In more detail, the tastants interact with their G-protein coupled receptors leading to an increase in intracellular Ca2+ and TRPM5 activation; hence, taste recognition [15]. Importantly, Dnate’ Baxter and co-workers documented that mouse TRPM5-expressing cells showed expression of different transcripts that take part in immunity, inflammation and viral infection such as TMPRSS2 transcript [80]. This fact points to the potential role of TRPM5 channel in smell loss [80], albeit not directly. Noteworthy, the nasal and oral TG nerve endings are activated by irritant chemicals that can be recognized by TRP channels such as menthol, capsaicin, thymol and allicin resulting in different sensations [81] suggesting that TRP channels can contribute to anosmia and ageusia in COVID-19 patients. Interestingly, many of the taste chemicals (e.g. eugenol, menthol and linalool) can activate TRPV1 and TRPM8 channels [82]. Others (e.g. eugenol and allicin) can activate TRPV1 and TRPA1 channels [82]. Collectively, this information highlights the possible involvement of TRP channels in anosmia and ageusia in COVID-19 disease as summarized in Fig. 2 .
Fig. 2.
TRP channels, anosmia and ageusia in COVID-19.
The authors of this review need to point to hearing loss/impairment symptom in patients infected with the new strain of SARS-CoV-2 (B.1.617 variant) in addition to other symptoms of COVID-19 (nausea, vomiting, diarrhea and abdominal pain) [83]. There is relationship between one type of the TRP channels (TRPV4) and hearing impairment as reported by Tabuchi and collaborators who found that there was a hearing impairment in TRPV4-knockout mice [84]. Future studies may indicate the involvement of TRP channels in the complications of the new strain of SARS-CoV-2.
3.2.6. TRP channels and GI complications in COVID-19
Nausea, anorexia, vomiting and diarrhea are reported symptoms in many patients infected with SARS-CoV-2 [3]. In addition, it became well-recognized that appetite loss is a common and may be severe symptom in COVID-19 patients especially in people infected with the SARS-CoV-2 variant B.1.617 [[83], [85]].
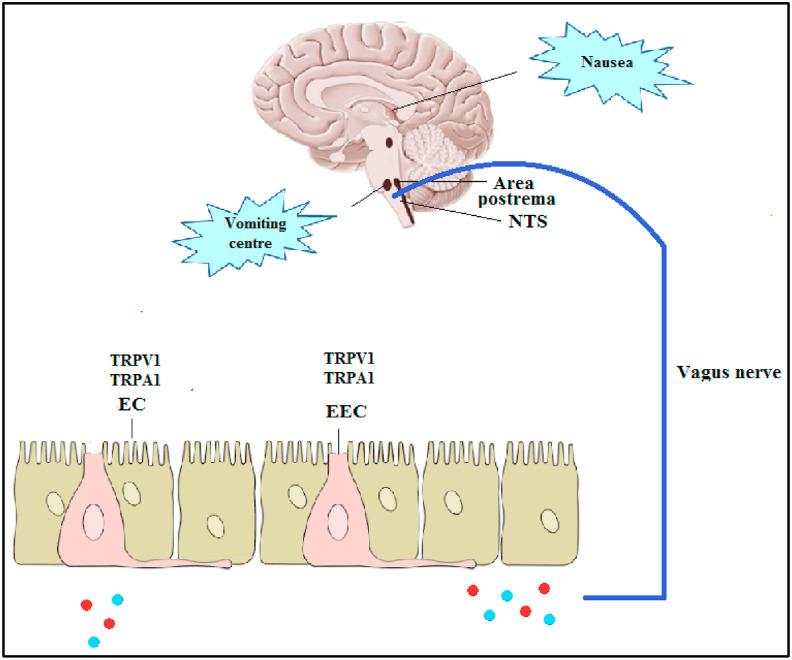
There are several mechanisms hypothesized to be involved in the viral induced symptoms in the GI system (e.g. nausea and vomiting) (Fig. 3 ). In the first days post-infection, the virus can induce the release of hormones and neuroactive agents [e.g. serotonin and SP] from the enteroendocrine cells [EECs] in the upper GI tract or cytokines from inflamed GI epithelia [3]. These mediators act systemically by binding to their receptors in the area postrema or locally by sensitizing the abdominal vagal afferent neurons leading to nucleus tractus solitarius [NTS] stimulation [86]. This stimulation evokes motor pathways responsible for the vomiting reflex and sends signals to the higher brain regions to generate the sensation of nausea [86]. In delayed onset symptoms, the viral attack to the area postrema in the dorsal brainstem that regulates the digestive system can be another mechanism for SARS-CoV-2 in inducing these complications [3].
Fig. 3.
Possible involvement of TRP channels in nausea and vomiting in COVID-19.
Importantly, previous studies highlighted the broad expression of several TRP channels in the GI tract and their role in response to noxious irritants [87]. TRPV1 expression had been well described in esophageal sensory neurons, stomach-labeled vagal nodose neurons and colon labeled afferent neurons [58,88,89]. In this line, Hammer and Vogelsang reported that capsaicin infusion into the proximal small intestine of human volunteers evoked sensations of pain, cramps, pressure, warmth and nausea [59]. Likewise, it was reported that TRPA1 channel was co-expressed with TRPV1 channel in the esophagus, stomach, intestine and colon [[60], [61], [62]]. Importantly, the in vitro activation of TRPA1 channel with allyl isothiocyanates evoked serotonin release from enterochromaffin cells [EC], leading to the stimulation of vomiting [90]. Also, TRPM8 channel was expressed in colonic afferent neurons, esophageal vagal jugular, stomach-labeled nodose and jugular neurons [63,89]. Additionally, there is broad expression of TRPV4 channel in the GI tract including the primary sensory neurons [20]. On the other hand, it was documented that there is close interplay between some TRP channels and food intake suggesting the possible role of TRP channels in appetite loss in COVID-19 patients [91,92]. For instance, TRPA1 channel has a role in satiety and food intake [91]. Moreover, TRPV1 channel plays a fundamental role in the control of appetite via influencing key hormones or modulating the signaling of GI vagal afferent nerve [92]. The involvement of TRP channels in COVID-19-associated GI symptoms needs further consideration in future studies.
3.2.7. TRP channels and cardiovascular complications in COVID-19
Cardiovascular complications are one of the risks that are associated with COVID-19 particularly in elderly and individuals suffering from hypertension [93]. Also, arrhythmias, cardiac fibrosis and myocyte hypertrophy were reported in many COVID-19 patients [94,95]. Importantly, several studies exemplified that multiple TRP channels are expressed in the heart and contribute to several cardiac complications [21,96]. For instance, TRPC channels is involved in the development of cardiac hypertrophy and heart failure while TRPM4 channel is implicated in cardiac arrhythmias [96]. Also, it was reported that TRPM7 channel contributes to the pathogenesis of cardiac fibrosis [21].
In fact, TRPC3, TRPC4 and TRPM2 channels act as endothelial redox sensors meanwhile TRPC1, TRPC4, TRPC6, TRPV4 and TRPM2 channels have been implicated in endothelial barrier dysfunction [97]. TRPM2 channel is involved in facilitating Ca2+ entry in response to oxidative stress and regulating endothelial barrier integrity [97]. Also, Xu and collaborators reported that TRPV4 activation limited atherosclerosis and vascular inflammation [98]. In the endothelial cells of carotid artery, TRPV4 loss of function caused problems in blood pressure and vascular tone [99]. In another study, it was demonstrated that TRPV4 loss of function induced Ca2+ release from internal stores, thus affecting vascular homeostasis [72]. The relation between TRP channels and Ca2+ ions that are needed for the viral activities suggests that TRP channels can be targeted in COVID-19 disease. Table 1 summarizes the possible involvement of TRP channels in COVID-19 symptoms.
Table 1.
TRP channels and COVID-19 symptoms.
| Symptom | TRP channel(s) involved in the symptom | Reference |
|---|---|---|
| Inflammation | TRPV4 | [20] |
| Inflammation | TRPA1 | [36] |
| Stress-related inflammatory processes | TRPM2 | [37] |
| Airway inflammation induced by viral infection | TRPV1 | [38, 40] |
| Pain | TRPV1 | [15] |
| Pain | TRPM2, TRPM3,TRPM8 | [39] |
| Pain | TRPV2, TRPV3, TRPV4, TRPC1, TRPC6 | [39] |
| Fever | TRPM2 | [46] |
| Pulmonary edema | TRPM2, TRPV4, TRPV1, TRPC1, TRPC4, TRPC6 | [19] |
| Ventilator-induced lung injury | TRPV4 | [48] |
| Oxidative stress | TRPV1 | [43] |
| Oxidative stress | TRPA1 | [28] |
| Nitric oxide and ROS production in alveolar macrophages | TRPV4 | [49] |
| Increase in the endothelial permeability of pulmonary blood vessels | TRPC6 | [53] |
| Pulmonary fibrosis | TRPV4, TRPM7 | [20, 21] |
| Cough | TRPA1, TRPV4, TRPV1 | [65] |
| Headache | TRPV1 | [71] |
| Headache | TRPV4 | [74] |
| Headache | TRPA1 | [70] |
| Myalgia | TRPV channels | [13] |
| Ischemic muscle Myalgia | TRPV1 | [76] |
| Loss of taste | TRPM4, TRPM5 | [79] |
| Loss of smell | TRPM5 | [80] |
| Hearing impairment | TRPV4 | [84] |
| Vomiting | TRPA1 | [90] |
| Nausea | TRPV1 | [59] |
| Loss of appetite | TRPA1 | [91] |
| Loss of appetite | TRPV1 | [92] |
| Cardiac arrhythmia | TRPM4 | [96] |
| Cardiac fibrosis | TRPM7 | [21] |
| Endothelial redox sensors | TRPC3, TRPC4, TRPM2 | [97] |
| Endothelial barrier dysfunction | TRPC1, TRPC4, TRPC6, TRPV4, TRPM2 | [97] |
3.3. Potential trends for the prevention and treatment of COVID-19
3.3.1. Blocking/desensitization of TRP channels
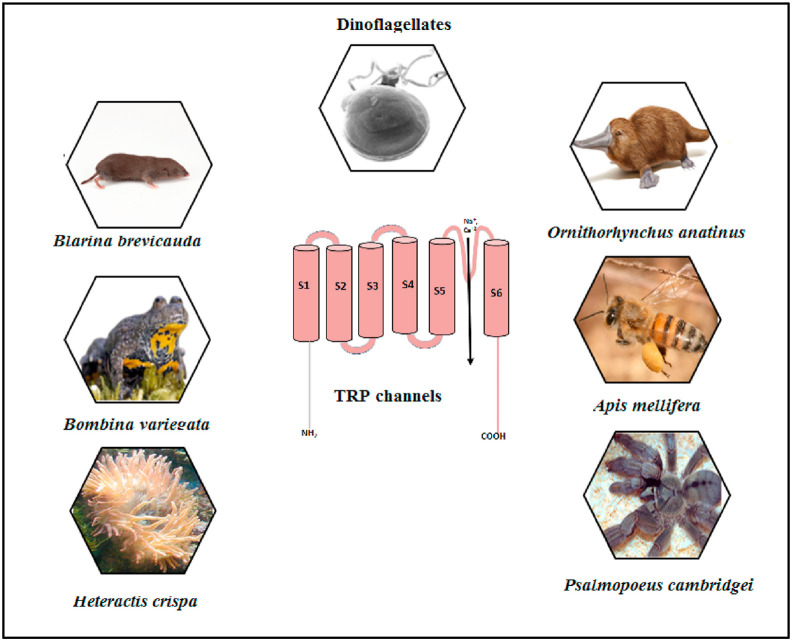
Finding optimal therapies for the relief of symptoms in COVID-19 patients relies on understanding the pathways and signaling mechanisms that are activated by the viral entry. The complexity lies in the multi-organ systems that are affected by the infectious virus. However, Ca2+ signaling was reported to be an important contributor [47]. As shown in previous studies, TRP channels provide a favorable environment for viruses and contribute significantly to the increase in cellular Ca2+ levels and viral infection [64,100]. Accordingly, blocking TRP channels can be an effective measure in controlling different viruses including SARS-CoV-2. In more detail, it was found that blocking the function of TRP channels using antagonists, antibodies or aptamers is a well-known strategy for treating certain diseases. Some TRP antagonists are already in clinical trials for this purpose. For example, a TRPV4 antagonist (GSK2798745) was developed as a novel therapeutic intervention for the treatment of pulmonary edema associated with heart failure [101]. A TRPC4 and TRPC5 inhibitor [Hydra/Boehringer Ingelheim] is currently in clinical trial phase I for treating anxiety disorder and depression [102]. Noteworthy, there is trend towards using concentrations of compounds that cause desensitization of TRP channels rather than blocking them [103]. Tachyphylaxis, defined as a reduction in the response after repeated applications of agonists, is another form of desensitization and can be considered in targeting TRP channels in this context [104]. The importance of the agonistic approach relies on the fact that using agonists inhibits the function in the entire nociceptor compared to using antagonists that only blocks the channel's activation [103]. Other reason is that the intake of many antagonists produces undesirable effects [105]. For instance, the use of TRPV1 antagonists causes hyperthermia and block of thermosensation [105]. Several researches showed the benefits of using TRP agonists in this context. It appeared that silencing TRPV1-expressing pulmonary sensory neurons using RTX improved survival in a mouse model of cytokine storm lethal pneumonia induced by Staphylococcus aureus [106]. Therefore, TRPV1 desensitization [e.g. by using RTX] in afferent pathways can decrease the severity of ARDS syndrome in COVID-19 patients [11]. Additionally, TRPV1 desensitization by the non-pungent synthetic analogue of capsaicin (olvanil) inhibited nociceptive processing in DRG neurons and decreased capsaicin-induce thermal hyperalgesia [107]. Also, it was proposed that rapid desensitization of TRPA1 channel could reduce complications of COVID-19 symptoms [36]. Importantly, it is well-known that TRP channels are targets for venomous toxins (Fig. 4 ) [108]. The use of toxins (e.g. RTX) for COVID-19 treatment has been suggested [11]. Previous researches showed that the spider toxins from Psalmopoeus cambdridgei [e.g. vanillotoxins 1–3] and Ornithoctonus huwena [Earth Tiger] [e.g. double-knot toxin] can specifically activate TRPV1 channel [109]. Bv8 is another toxin (obtained from the skin of Bombina variegata frog) that causes hyperalgesia in a TRPV1 dependent mechanism [110]. Besides, it was documented that polycyclic ether toxins [e.g. brevetoxin and gambierol] extracted from marine dinoflagellates act as allosteric modulators for TRPV1 channel [111]. Furthermore, previous reports showed that multiple toxins inhibit TRPV1 channel including the venom of the tropical sea anemone Heteractis crispa, the analgesic polypeptide toxin and two toxins extracted from Agelenopsis aperta spider, named as AG505 and AG489 [112,113].
Fig. 4.
Examples of venoms that modulate the functions of TRP channels.
Other TRP channels can be also targeted by toxins. For example, it was reported that soricidin (extracted from the salivary glands of Blarina brevicauda shrew) inhibits TRPV6-induced Ca2+ uptake [114]. Also, melittin venom from bee [Apis mellifera] can target TRPC and TRPV1 channels while the venoms from Ornithorhynchus anatinus platypus and fish-containing ciguatoxin [CTX] affect TRPA1 channel [108,115]. Besides, TRPC6 channel can be blocked by the tarantula peptide, GsMTx-4 [116]. All of this information suggests that venoms can be used to target TRP channels to alleviate COVID-19 symptoms .
3.3.2. Potential use of medicinal plants and phytochemicals interacting with TRP channels for the prevention and treatment of COVID-19
Medicinal plants are considered rich sources for active ingredients that proved to be valuable in treating different diseases including viral diseases. In a randomized, open label, placebo-controlled, multi-center clinical trial, the administration of Nigella sativa L. with honey for thirteen days caused viral clearance and a reduction in the severity of COVID-19 symptoms [117]. To add, it was shown that thymoquinone, the active compound in N. sativa, caused 98% inhibition of SARS-CoV-2 [118]. Another study provided a solid evidence for the effectiveness of N. sativa in decreasing coronavirus load in HeLa-infected cells as well as its effect on the expression of several TRP genes [119].
In a study conducted by Wang et al., the researchers cultivated 800 types of Cannabis sativa L. containing high amount of the non-psychotropic phytocannabinoid cannabidiol (CBD) which shows anti-inflammatory properties [120]. The authors identified 13 C. sativa extracts that are high in CBD and low in 9-tetrahydrocannabinolic acid and showed that these extracts can decrease ACE2 and TMPRSS2 protein levels as proved in artificial three dimensional (3D) human models of oral, airway and intestinal tissues [120]. Noteworthy, TRP channels (e.g. TRPV1-4, TRPA1, and TRPM8 channels) are putative cannabinoid receptors [121]. Thus, CBD might decrease the viral load through direct or indirect interaction with TRP channels.
In addition, several phytochemicals that interact with TRP channels can be potential treatments for COVID-19 [[122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143]]. However, the implication of the involvement of TRP channels in their mechanism of action had not been investigated yet.
Berbamine is a bis-benzylisoquinoline alkaloid used in traditional Chinese medicine, Berberis [122]. This alkaloid potently inhibited the infection of various coronaviruses (e.g., SARS-CoV-2), inhibited TRPML channels and compromised the endolysosomal tracking of viral receptors, such as ACE2 [122]. As mentioned previously, TRPML channels are Ca2+ permeable, non-selective cation channel present in the endosomes and lysosomes [31] suggesting that targeting TRML channels to inhibit SARS-CoV-2 entry to the host cells is a useful strategy that can be involved in the antiviral effect of this alkaloid. . In fact, this information opens a door for future in silico and in vitro studies to discover lead compounds that can inhibit TRML channels and SARS-CoV-2 entry to host cells.
To add, some natural compounds have the potential for inhibiting SARS-CoV-2 as well as treating at least some symptoms of COVID-19. Examples include, but not limited to, resveratrol, spermidine, spermine, naringenin, quercetin, curcumin and baicalin.
Resveratrol is a polyphenol present in peanuts, berries, and red grapes 122]. This polyphenol is a ligand for TRPV1, TRPA1, TRPM2, and TRPC5 channels [[124], [125], [126], [127]]. In Vero E6 cells, resveratrol and its structurally related compound (pterostilbene) inhibited SARS-CoV-2 infection effectively by interfering with the post-entry steps of viral replication cycle [128]. Resveratrol is currently in several clinical trials for COVID-19 treatment [123]. Meanwhile, its exact mechanism of action against SARS-CoV-2 and its interaction with various types of TRP channels to alleviate COVID-19 symptoms needs further investigation.
Spermidine and spermine are potent TRPV1 ligands. It was documented that these polyamines inhibited SARS-CoV-2 infection possibly by inducing viral degradation in the endolysosomes [129,130]. Furthermore, spermidine attenuated bleomycin-induced lung fibrosis and inhibited ER stress-induced cell death in mice [131].
Naringenin targets several TRP channels producing analgesic effects [132]. In more detail, it reduced TRPV1 activation and blocked TRPM3 channel [132]. Also, naringenin inhibited human coronaviruses infection effectively [133] suggesting that this inhibition can be mediated by interaction with one or more of TRP channels.
Quercetin is a well known ligand for TRPM7, TRPV1 and TRPA1 channels [[134], [135], [136]]. In a prospective, randomized, controlled, and open-label study, a daily dose of 1000 mg of quercetin was given for 30 days to 152 COVID-19 outpatients to study its adjuvant effect in treating the early symptoms and in preventing the severe consequences of the disease. Quercetin was effective in improving COVID-19 early symptoms as well as preventing the severity of the disease [137].
Curcumin is a ligand for TRPM8, TRPV1 and TRPA1 channels while piperine is a TRPV1 ligand [138,139]. It was revealed that COVID-19 patients with mild, moderate, and severe symptoms who received curcumin/piperine treatment showed recovery from early symptoms [fever, cough, sore throat, and breathlessness] accompanied with better ability to maintain oxygen saturation above 94% and better clinical outcomes [140]. Moreover, in silico drug discovery suggested that curcumin acts as SARS-CoV-2 main protease inhibitor [141].
Finally, baicalin exhibited potent antiviral activities and was identified as the first non-covalent, non-peptidomimetic inhibitor of SARS-CoV-2 3CLpro [142]. Notably, earlier reports showed that baicalin caused down-regulation of TRPV1 mRNA expression levels in DRG neurons [143]. Taken together, all of the aforementioned studies suggest that TRP channels contribute to several symptoms of COVID-19 and can be seriously considered as targets for the treatment of this disease.
It is out of space to mention all the researches that were conducted about the effects of medicinal plants and active compounds on SARS-CoV-2. We suggest reading reviews that can be helpful in this context and that demonstrate the broad spectrum of TRP activation by active compounds [[144], [145], [146], [147]]. TRP channels are targets for many of the active compounds that exhibited effectiveness against SARS-CoV-2 [148] and can be valuable targets for future subjective studies.
3.3.3. Use of dietary constituents and vitamins interacting with TRP channels for the prevention and treatment of COVID-19
There is substantial evidence revealing that variations in COVID-19 death rates between countries can be, partly, related to differences in dietary habits [149]. Notably, several diet varieties interact with TRP channels in different contexts. For example, many spices and fermented vegetables [e.g. allicin, capsaicin, curcumin, gingerol, piperine and Wasabi] interact with TRPA1 and TRPV1 channels [36]. Further, it was hypothesized that some diets can activate the nuclear factor erythroid 2–related factor 2 [Nrf2] and desensitize TRP channels, thus can alleviate several symptoms of COVID-19 [150]. For example, the consumption of spicy food caused desensitization of TRP channels in synergy with Nrf2 [149]. Of note, Nrf2 is a potent antioxidant that can inhibit SARS-CoV-2 induced oxidative stress [150]. Moreover, in a trial conducted during the first 2 phases of COVID-19 infection, it was found that there was a reduction in cough, fatigue, nasal and GI symptoms in patients who consumed either broccoli and paracetamol or broccoli with the agonists of TRPA1/TRPV1 and paracetamol [36]. Of note, broccoli capsules are considered potent agonists for Nrf2 and weak TRPA1 agonists [36]. In addition, open-labeled induced cough challenges were carried out in a COVID-19 patient using different treatments [137]. The results of this study suggested that there could be fast desensitization of TRPA1 and TRPV1 channels as well as a crosstalk with Nrf2 [137]. In more detail, the study was performed using nutrients that have different agonistic activities for Nrf2, TRPA1 and TRPV1. It was depicted that the use of red pepper [has high TRPV1 agonistic activity] or curcumin and black pepper [both are potent TRPA1 agonists] were effective in decreasing cough with a 3-h persistent effect. The use of broccoli [has strong Nrf2 agonistic activity] reduced cough with an effect that lasted for 6 h. The combination of broccoli, curcumin and black pepper was more effective in cough reduction and this effect persisted for more than 9 h.
In another study, the authors referred to a Korean traditional food [Kimchi] as a food that may be associated with low COVID-19 mortalities in Korea [151]. Kimchi is prepared by fermenting baechu cabbage with cruciferous vegetables and other ingredients such as ginger, red pepper and garlic [151]. These ingredients are known to be activators for Nrf2 and certain TRP channels [e.g. TRPA1, TRPV1] [150].
Worthy to emphasize on, it was speculated that vitamin D deficiency is associated with severe symptoms and a high fatality rate in COVID-19 patients [152]. Recently, it was shown that 25-hydroxyvitamin D is a partial agonist for TRPV1 channel contributing to a decrease in T-cell activation and Ca2+ signaling mediated by TRPV1 channel in TG neurons [153]. This fact suggests that there could be link between several symptoms of COVID-19, vitamin D and TRP channels.
4. Conclusions
Several studies provided foundations for the possible involvement of TRP channels in different pathophysiological mechanisms in COVID-19. Since TRP channels contribute to several symptoms of COVID-19, they can be seriously considered as targets for the prevention and treatment of this disease. However, the specific role of TRP channels in COVID-19 symptoms needs further investigation.
The authors of this review suggest that using TRP channel- targeted therapy (e.g. venoms and agonists) to desensitize TRP channels can alleviate COVID-19 symptoms. Particularly, the desensitization of TRPV1 channel using RTX or venoms may decrease the severity of ARDS syndrome in COVID-19 patients. To add, the inhibition of TRPML2 channel can be used as a strategy to inhibit SARS-CoV-2 entry into the host cells. Further investigation is recommended. New insights will undoubtedly arise in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the architect Maram Jaffal for her professional creation of the illustrations in this review. The authors thank the University of Jordan, Amman, Jordan. This work was published with the support of Al-Ahliyya Amman University, Amman, Jordan.
References
- 1.Weekly epidemiological update on COVID-19 - 25 May 2021. (n.d.). WHO World Health Organization. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19 [2021]. .
- 2.El-Tallawy S.N., Nalamasu R., Pergolizzi J.V., Gharibo C. 2020. Pain Management during the COVID-19 Pandemic, Pain and Therapy; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P.L., Cai W., Rudd J.A., Sanger G.J. COVID‐19, nausea, and vomiting. J. Gastroenterol. Hepatol. 2020;36(3):646–656. doi: 10.1111/jgh.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Outhoff K. Sick and tired of COVID-19: long haulers and post viral (fatigue) syndromes. South Afr. General Pract. 2020:132–133. [Google Scholar]
- 5.Huang Y., Yang C., Xu X., Xu W., Liu S. Structural and functional properties of SARS-Cov-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brann D., Tsukahara T., Weinreb C., Logan D.W., Datta S.R. BioRxiv; 2020. Non-neural Expression of SARS-CoV-2 Entry Genes in the Olfactory Epithelium Suggests Mechanisms Underlying Anosmia in COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straus M.R., Bidon M., Tang T., Whittaker G., Daniel S. bioRxiv; 2020. FDA Approved Calcium Channel Blockers Inhibit SARS-CoV-2 Infectivity in Epithelial Lung Cells. [Google Scholar]
- 8.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 9.Rinkenberger N., Schoggins J.W. Mucolipin-2 cation channel increases trafficking efficiency of endocytosed viruses. mBio. 2018;9 doi: 10.1128/mBio.02314-17. e02314-02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z., Qin P., Huang Y.-W. Cell Calcium; 2021. Lysosomal Ion Channels Involved in Cellular Entry and Uncoating of Enveloped Viruses: Implications for Therapeutic Strategies against SARS-CoV-2; p. 102360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahama A., Ramachandran R., Cisternas A.F., Ji H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: a review. Med. Drug Discov. 2020;5:100033. doi: 10.1016/j.medidd.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caronna E., Ballvé A., Llauradó A., Gallardo V.J., Ariton D.M., Lallana S., López Maza S., Olivé Gadea M., Quibus L., Restrepo J.L., Headache A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40:1410–1421. doi: 10.1177/0333102420965157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumura K. Peripheral mechanism of muscle pain: an update. Curr. Anaesth. Crit. Care. 2009;20:183–187. [Google Scholar]
- 14.González-Ramírez R., Chen Y., Liedtke W.B., Morales-Lázaro S.L. TRP channels and pain. Neurobiol. TRP Channels. 2017;2 9781315152837-9781315152838. [PubMed] [Google Scholar]
- 15.Fernandes E., Fernandes M., Keeble J. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guihur A., Rebeaud M.E., Fauvet B., Tiwari S., Weiss Y.G., Goloubinoff P. Moderate fever cycles as a potential mechanism to protect the respiratory system in COVID-19 patients. Front. Med. 2020;7:583. doi: 10.3389/fmed.2020.564170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balakrishna S., Song W., Achanta S., Doran S.F., Liu B., Kaelberer M.M., Yu Z., Sui A., Cheung M., Leishman E. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L158–L172. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons S., Erfinanda L., Bartz C., Kuebler W.M. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J. Physiol. 2018;597(4):997–1021. doi: 10.1113/JP276245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum T., Benítez-Angeles M., Sánchez-Hernández R., Morales-Lázaro S.L., Hiriart M., Morales-Buenrostro L.E., Torres-Quiroz F. TRPV4: a physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 2020;21:3837. doi: 10.3390/ijms21113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T., Wu B.M., Yao H.W., Meng X.M., Huang C., Ni M.M., Li J. Novel insights into TRPM7 function in fibrotic diseases: a potential therapeutic target. J. Cell. Physiol. 2015;230:1163–1169. doi: 10.1002/jcp.24801. [DOI] [PubMed] [Google Scholar]
- 22.Mazzotta S., Carullo G., Schiano Moriello A., Amodeo P., Di Marzo V., Vega-Holm M., Vitale R.M., Aiello F., Brizzi A., De Petrocellis L. Design, synthesis and in vitro experimental validation of novel TRPV4 antagonists inspired by labdane diterpenes. Mar. Drugs. 2020;18:519. doi: 10.3390/md18100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Hashim A.Z., Jaffal S.M. Nerve growth factor enhances cough and airway obstruction via TrkA receptor-and TRPV1-dependent mechanisms. Thorax. 2009;64:791–797. doi: 10.1136/thx.2009.113183. [DOI] [PubMed] [Google Scholar]
- 24.Haustrate A., Prevarskaya N. Role of the TRPV channels in the endoplasmic reticulum calcium homeostasis. Cells. 2020;9:317. doi: 10.3390/cells9020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau C., Kirchberger T., Swarbrick J.M., Bartlett S.J., Fliegert R., Yorgan T., Bauche A., Harneit A., Guse A.H., Potter B.V. Structure–activity relationship of adenosine 5′-diphosphoribose at the Transient Receptor Potential Melastatin 2 [TRPM2] channel: rational design of antagonists. J. Med. Chem. 2013;56:10079–10102. doi: 10.1021/jm401497a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dastjerdeh M.S., Kouhpayeh S., Sabzehei F., Khanahmad H., Salehi M., Mohammadi Z., Shariati L., Hejazi Z., Rabiei P., Manian M. Zinc finger nuclease: a new approach to overcome beta-lactam antibiotic resistance. Jundishapur J. Microbiol. 2016;9 doi: 10.5812/jjm.29384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill K., McNulty S., Randall A. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. N. Schmied. Arch. Pharmacol. 2004;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- 28.Bonvini S.J., Belvisi M.G. Cough and airway disease: the role of ion channels. Pulm. Pharmacol. Therapeut. 2017;47:21–28. doi: 10.1016/j.pupt.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen S.F., Owsianik G., Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Ma X., Cao J., Luo J., Nilius B., Huang Y., Ambudkar I.S., Yao X. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler. Thromb. Vasc. Biol. 2010;30:2249–2255. doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosato A.S., Tang R., Grimm C. Two-pore and TRPML cation channels: regulators of phagocytosis, autophagy and lysosomal exocytosis. Pharmacol. Therapeut. 2021;220:107713. doi: 10.1016/j.pharmthera.2020.107713. [DOI] [PubMed] [Google Scholar]
- 32.Wang B., Danjo A., Kajiya H., Okabe K., Kido M. Oral epithelial cells are activated via TRP channels. J. Dent. Res. 2011;90:163–167. doi: 10.1177/0022034510385459. [DOI] [PubMed] [Google Scholar]
- 33.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheraga R.G., Southern B.D., Grove L.M., Olman M.A. The role of TRPV4 in regulating innate immune cell function in lung inflammation. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bousquet J., Czarlewski W., Zuberbier T. Clin Transl Allergy; 2020. Potential Control of COVID-19 Symptoms by Nrf2-Interacting Nutrients with TRPA1 [transient Receptor Potential Ankyrin 1] Agonist Activity. [Google Scholar]
- 37.Chen G.L., Zeng B., Eastmond S., Elsenussi S.E., Boa A.N., Xu S.Z. Pharmacological comparison of novel synthetic fenamate analogues with econazole and 2‐APB on the inhibition of TRPM2 channels. Br. J. Pharmacol. 2012;167:1232–1243. doi: 10.1111/j.1476-5381.2012.02058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talbot S., Abdulnour R.-E.E., Burkett P.R., Lee S., Cronin S.J., Pascal M.A., Laedermann C., Foster S.L., Tran J.V., Lai N. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dey S., Usmani H., Hussain A. Pain practice during the COVID-19 pandemic: transitioning to a new normal. Ind. J. Pain. 2020;34 61-61. [Google Scholar]
- 40.Maniquis-Smigel L., Reeves K.D., Rosen H.J., Lyftogt J., Graham-Coleman C., Cheng A.-L., Rabago D. Short term analgesic effects of 5% dextrose epidural injections for chronic low back pain: a randomized controlled trial. Anesthesiol. Pain Med. 2017;7 doi: 10.5812/aapm.42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voronova I., Tuzhikova A., Kozyreva T. Thermosensitive TRP channels gene expression in hypothalamus of normal rats and rats adapted to cold. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova. 2012;98:1101–1110. [PubMed] [Google Scholar]
- 42.Bromberg Z., Weiss Y. The role of the membrane-initiated heat shock response in cancer. Front. Mol. Biosci. 2016;3:12. doi: 10.3389/fmolb.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iftinca M., Flynn R., Basso L., Melo H., Aboushousha R., Taylor L., Altier C. The stress protein heat shock cognate 70 [Hsc70] inhibits the Transient Receptor Potential Vanilloid type 1 [TRPV1] channel. Mol. Pain. 2016;12 doi: 10.1177/1744806916663945. 1744806916663945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromberg Z., Goloubinoff P., Saidi Y., Weiss Y.G. The membrane-associated transient receptor potential vanilloid channel is the central heat shock receptor controlling the cellular heat shock response in epithelial cells. PloS One. 2013;8 doi: 10.1371/journal.pone.0057149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Güler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song K., Wang H., Kamm G.B., Pohle J., de Castro Reis F., Heppenstall P., Wende H., Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353:1393–1398. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyser J.M., Estes M.K. Pathophysiological consequences of calcium-conducting viroporins. Annu. Rev. Virol. 2015;2:473–496. doi: 10.1146/annurev-virology-100114-054846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalick L., Liedtke W., Kuebler W.M. A novel actor in mechanotransduction: transient receptor potential cation channel vanilloid [TRPV] 1 in ventilator-induced lung injury [VILI] Faseb. J. 2017;31 1074.1076-1074.1076. [Google Scholar]
- 49.Morty R.E., Kuebler W.M. TRPV4: an exciting new target to promote alveolocapillary barrier function. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L817–L821. doi: 10.1152/ajplung.00254.2014. [DOI] [PubMed] [Google Scholar]
- 50.Dib M., Zsengeller Z., Mitsialis A., Lu B., Craig S., Gerard C., Gerard N.P. A paradoxical protective role for the proinflammatory peptide substance P receptor [NK1R] in acute hyperoxic lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L687–L697. doi: 10.1152/ajplung.90509.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwingshackl A. The role of stretch-activated ion channels in acute respiratory distress syndrome: finally a new target? Am. J. Physiol. Lung Cell Mol. Physiol. 2016;311:L639–L652. doi: 10.1152/ajplung.00458.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uhlig S., Yang Y., Waade J., Wittenberg C., Babendreyer A., Kuebler W.M. Differential regulation of lung endothelial permeability in vitro and in situ. Cell. Physiol. Biochem. 2014;34:1–19. doi: 10.1159/000362980. [DOI] [PubMed] [Google Scholar]
- 53.Samapati R., Yang Y., Yin J., Stoerger C., Arenz C., Dietrich A., Gudermann T., Adam D., Wu S., Freichel M. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am. J. Respir. Crit. Care Med. 2012;185:160–170. doi: 10.1164/rccm.201104-0717OC. [DOI] [PubMed] [Google Scholar]
- 54.George P.M., Wells A.U., Jenkins R.G. The Lancet Respiratory Medicine; 2020. Pulmonary Fibrosis and COVID-19: the Potential Role for Antifibrotic Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J., Chen C., Chen W., Huang L., Fu Z., Ye K., Lv L., Nong Z., Zhou X., Lu W. 2020. Proteomics and Metabonomics Analyses of Covid-19 Complications in Patients with Pulmonary Fibrosis. Research Square preprint, version 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jian M.-Y., King J.A., Al-Mehdi A.-B., Liedtke W., Townsley M.I. High vascular pressure–induced lung injury requires P450 epoxygenase–dependent activation of TRPV4. Am. J. Respir. Cell Mol. Biol. 2008;38:386–392. doi: 10.1165/rcmb.2007-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med. Hypotheses. 2021:110627. doi: 10.1016/j.mehy.2021.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan L., Bornstein J., Anderson C. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 59.Hammer J., Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neuro Gastroenterol. Motil. 2007;19:279–287. doi: 10.1111/j.1365-2982.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 60.Yu S., Gao G., Peterson B.Z., Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in Guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G34–G42. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- 61.Zhao H., Sprunger L.K., Simasko S.M. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G212–G221. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitrovic M., Shahbazian A., Bock E., Pabst M.A., Holzer P. Chemo‐nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br. J. Pharmacol. 2010;160:1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrington A.M., Hughes P.A., Martin C.M., Yang J., Castro J., Isaacs N.J., Blackshaw L.A., Brierley S.M. A novel role for TRPM8 in visceral afferent function. Pain®. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 64.Omar S., Clarke R., Abdullah H., Brady C., Corry J., Winter H., Touzelet O., Power U.F., Lundy F., McGarvey L.P. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PloS One. 2017;12 doi: 10.1371/journal.pone.0171681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keller J.A., McGovern A.E., Mazzone S.B. Translating cough mechanisms into better cough suppressants. Chest. 2017;152:833–841. doi: 10.1016/j.chest.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 66.Ternesten-Hasséus E., Johansson E.-L., Millqvist E. Cough reduction using capsaicin. Respir. Med. 2015;109:27–37. doi: 10.1016/j.rmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Bonvini S.J., Birrell M.A., Grace M.S., Maher S.A., Adcock J.J., Wortley M.A., Dubuis E., Ching Y.-M., Ford A.P., Shala F. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: role of adenosine triphosphate. J. Allergy Clin. Immunol. 2016;138:249–261. doi: 10.1016/j.jaci.2015.10.044. e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan H., Tang X., Song Y., Liu P., Chen Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatric Dis. Treat. 2020;16:1359. doi: 10.2147/NDT.S251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dussor G., Yan J., Xie J.Y., Ossipov M.H., Dodick D.W., Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem. Neurosci. 2014;5:1085–1096. doi: 10.1021/cn500083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans M.S., Cheng X., Jeffry J.A., Disney K.E., Premkumar L.S. Sumatriptan inhibits TRPV1 channels in trigeminal neurons, Headache. J. Head Face Pain. 2012;52:773–784. doi: 10.1111/j.1526-4610.2011.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McFarland S.J., Weber D.S., Choi C.-s., Lin M.T., Taylor M.S. Ablation of endothelial TRPV4 channels alters the dynamic Ca2+ signaling profile in mouse carotid arteries. Int. J. Mol. Sci. 2020;21:2179. doi: 10.3390/ijms21062179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitahara T., Li H.-S., Balaban C.D. Changes in transient receptor potential cation channel superfamily V [TRPV] mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear. Res. 2005;201:132–144. doi: 10.1016/j.heares.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Wei X., Edelmayer R.M., Yan J., Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31:1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Can N.U., Özgecan K., Kotan D. Myalgia frequency in patients with COVID-19 and its relationship with creatine kinase levels. Düzce Tıp Fakültesi Dergisi. 2020;22:34–38. [Google Scholar]
- 76.Queme L.F., Ross J.L., Jankowski M.P. Peripheral mechanisms of ischemic myalgia. Front. Cell. Neurosci. 2017;11:419. doi: 10.3389/fncel.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butowt R., von Bartheld C.S. The Neuroscientist; 2020. <? Covid19?> Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. 1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banik D.D., Martin L.E., Freichel M., Torregrossa A.-M., Medler K.F. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115:E772–E781. doi: 10.1073/pnas.1718802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dnate'Baxter B., Larson E.D., Feinstein P., Polese A.G., Bubak A.N., Niemeyer C.S., Merle L., Shepherd D., Ramakrishnan V.R., Nagel M.A. bioRxiv; 2020. Transcriptional Profiling Reveals TRPM5-Expressing Cells Involved in Viral Infection in the Olfactory Epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viana F. Chemosensory properties of the trigeminal system. ACS Chem. Neurosci. 2011;2:38–50. doi: 10.1021/cn100102c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nilius B., Appendino G. Spices: the savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 2013;164:1–76. doi: 10.1007/112_2013_11. [DOI] [PubMed] [Google Scholar]
- 83.Williams H., Hutchinson D., Stone H. Watching Brief: the evolution and impact of COVID-19 variants B. 1.1. 7, B. 1.351, P. 1 and B. 1.617. Global Biosecur. 2021;3 [Google Scholar]
- 84.Tabuchi K., Suzuki M., Mizuno A., Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci. Lett. 2005;382:304–308. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 85.Mao R., Qiu Y., He J.-S., Tan J.-Y., Li X.-H., Liang J., Shen J., Zhu L.-R., Chen Y., Iacucci M. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol. & Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koch K.L., Hasler W.L. Springer; 2016. Nausea and Vomiting: Diagnosis and Treatment. [Google Scholar]
- 87.Yu X., Yu M., Liu Y., Yu S. Springer; 2016. TRP Channel Functions in the Gastrointestinal Tract, Seminars in Immunopathology; pp. 385–396. [DOI] [PubMed] [Google Scholar]
- 88.Matthews P.J., Aziz Q., Facer P., Davis J.B., Thompson D.G., Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur. J. Gastroenterol. Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Zhang L., Jones S., Brody K., Costa M., Brookes S.J. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G983–G991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]
- 90.Doihara H., Nozawa K., Kawabata-Shoda E., Kojima R., Yokoyama T., Ito H. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur. J. Pharmacol. 2009;617:124–129. doi: 10.1016/j.ejphar.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 91.Wu W., Zhou H.R., Pestka J.J. Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1 (TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin) Arch. Toxicol. 2017;91:495–507. doi: 10.1007/s00204-016-1687-x. [DOI] [PubMed] [Google Scholar]
- 92.Christie S., Wittert G.A., Li H., Page A.J. Involvement of TRPV1 channels in energy homeostasis. Front. Endocrinol. 2018;9:420. doi: 10.3389/fendo.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 [COVID-19] outbreak–an update on the status. Milit. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watanabe H., Murakami M., Ohba T., Takahashi Y., Ito H. TRP channel and cardiovascular disease. Pharmacol. Therapeut. 2008;118:337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 97.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel: evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 98.Xu S., Liu B., Yin M., Koroleva M., Mastrangelo M., Ture S., Morrell C.N., Zhang D.X., Fisher E.A., Jin Z.G. A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget. 2016;7:37622. doi: 10.18632/oncotarget.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartmannsgruber V., Heyken W.-T., Kacik M., Kaistha A., Grgic I., Harteneck C., Liedtke W., Hoyer J., Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PloS One. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen X., Cao R., Zhong W. Host calcium channels and pumps in viral infections. Cells. 2020;9:94. doi: 10.3390/cells9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goyal N., Skrdla P., Schroyer R., Kumar S., Fernando D., Oughton A., Norton N., Sprecher D.L., Cheriyan J. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects. Am. J. Cardiovasc. Drugs. 2019;19:335–342. doi: 10.1007/s40256-018-00320-6. [DOI] [PubMed] [Google Scholar]
- 102.Wulff H., Christophersen P., Colussi P., Chandy K.G., Yarov-Yarovoy V. Antibodies and venom peptides: new modalities for ion channels. Nat. Rev. Drug Discov. 2019;18:339–357. doi: 10.1038/s41573-019-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brederson J.-D., Kym P.R., Szallasi A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Vyklický L., Novakova-Tousova K., Benedikt J., Samad A., Touska F., Vlachová V. Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol. Res. 2008;57:S59–S68. doi: 10.33549/physiolres.931478. [DOI] [PubMed] [Google Scholar]
- 105.Rowbotham M.C., Nothaft W., Duan W.R., Wang Y., Faltynek C., McGaraughty S., Chu K.L., Svensson P. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 106.Baral P., Umans B.D., Li L., Wallrapp A., Bist M., Kirschbaum T., Wei Y., Zhou Y., Kuchroo V.K., Burkett P.R. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018;24:417. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alsalem, Millns P., Altarifi A., El-Salem K., Chapman V., Kendall D.A. Anti-nociceptive and desensitizing effects of olvanil on capsaicin-induced thermal hyperalgesia in the rat. BMC Pharmacology and Toxicology. 2016;17(1):31–42. doi: 10.1186/s40360-016-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siemens J., Hanack C. 2014. Modulation of TRP Ion Channels by Venomous Toxins, Mammalian Transient Receptor Potential [TRP] Cation Channels; pp. 1119–1142. [DOI] [PubMed] [Google Scholar]
- 109.Siemens J., Zhou S., Piskorowski R., Nikai T., Lumpkin E.A., Basbaum A.I., King D., Julius D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 110.Vellani V., Colucci M., Lattanzi R., Giannini E., Negri L., Melchiorri P., McNaughton P.A. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J. Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cuypers E., Yanagihara A., Rainier J.D., Tytgat J. TRPV1 as a key determinant in ciguatera and neurotoxic shellfish poisoning. Biochem. Biophys. Res. Commun. 2007;361:214–217. doi: 10.1016/j.bbrc.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andreev Y.A., Kozlov S.A., Koshelev S.G., Ivanova E.A., Monastyrnaya M.M., Kozlovskaya E.P., Grishin E.V. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 [TRPV1] J. Biol. Chem. 2008;283:23914–23921. doi: 10.1074/jbc.M800776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitaguchi T., Swartz K.J. An inhibitor of TRPV1 channels isolated from funnel Web spider venom. Biochemistry. 2005;44:15544–15549. doi: 10.1021/bi051494l. [DOI] [PubMed] [Google Scholar]
- 114.Peters A.A., Simpson P.T., Bassett J.J., Lee J.M., Da Silva L., Reid L.E., Song S., Parat M.-O., Lakhani S.R., Kenny P.A. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor–negative breast cancer. Mol. Canc. Therapeut. 2012;11:2158–2168. doi: 10.1158/1535-7163.MCT-11-0965. [DOI] [PubMed] [Google Scholar]
- 115.Ding J., Xiao Y., Lu D., Du Y.-R., Cui X.-Y., Chen J. Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca 2+ rise in primary sensory cells. Neurosci. Bull. 2011;27:135–142. doi: 10.1007/s12264-011-1018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spassova M.A., Hewavitharana T., Xu W., Soboloff J., Gill D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ashraf S., Ashraf S., Ashraf M., Imran M.A., Kalsoom L., Siddiqui U.N., Ghufran M., Majeed N., Farooq I., Habib Z. medRxiv; 2020. Therapeutic Efficacy of Honey and Nigella Sativa against COVID-19: A Multi-Center Randomized Controlled Clinical Trial [HNS-COVID-PK] [Google Scholar]
- 118.Seadawy M.G., Gad A.F., Shamel M., Elharty B., Mohamed M.F., Elfiky A.A., Ahmed A., Zekri A.R.N. 2020. In Vitro: Natural Compounds [Thymol, Carvacrol, Hesperidine, and Thymoquinone] against SARS-CoV2 Strain Isolated from Egyptian Patients. [Google Scholar]
- 119.Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., Igci Y.Z., Cakmak E.A., Arslan A. The effects of Nigella sativa [Ns], Anthemis hyalina [Ah] and Citrus sinensis [Cs] extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014;41:1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang B., Kovalchuk A., Li D., Ilnytskyy Y., Kovalchuk I., Kovalchuk O. In search of preventative strategies: novel anti-inflammatory high-CBD cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging (N Y) 2020;12(22):22425–22444. doi: 10.18632/aging.202225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Storozhuk M.V., Zholos A.V. TRP channels as novel targets for endogenous ligands: focus on endocannabinoids and nociceptive signalling. Curr. Neuropharmacol. 2018;16:137–150. doi: 10.2174/1570159X15666170424120802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang L., Li H., Yuen T.T.-T., Ye Z., Fu Q., Sun W., Xu Q., Yang Y., Chan J.F.-W., Zhang G. 2020. Berbamine Inhibits the Infection of SARS-CoV-2 and Flaviviruses by Compromising TPRMLs-Mediated Endolysosomal Trafficking of Viral Receptors. [Google Scholar]
- 123.Khan N., Chen X., Geiger J.D. Possible therapeutic use of natural compounds against COVID-19. J. Cell. Signal. 2021;2:63. [PMC free article] [PubMed] [Google Scholar]
- 124.Akyuva Y., Nazıroğlu M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-63577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nalli M., Ortar G., Moriello A.S., Morera E., Di Marzo V., De Petrocellis L. TRPA1 channels as targets for resveratrol and related stilbenoids. Bioorg. Med. Chem. Lett. 2016;26:899–902. doi: 10.1016/j.bmcl.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 126.Naylor J., Eman A.-S., McKeown L., Manna P.T., Porter K.E., O'Regan D., Muraki K., Beech D.J. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J. Biol. Chem. 2011;286:5078–5086. doi: 10.1074/jbc.M110.196956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y., Huang F., Xu Y., Xiang W., Xie C. TRPV1 is involved in the antinociceptive effects of resveratrol in paclitaxel-induced neuropathic pain. Life. 2021;14:66–74. [Google Scholar]
- 128.ter Ellen B.M., Kumar N.D., Bouma E.M., Troost B., van de Pol D.P., van der Ende-Metselaar H.H., Apperloo L., van Gosliga D., van den Berge M., Nawijn M.C. bioRxiv; 2020. Resveratrol and Pterostilbene Potently Inhibit SARS-CoV-2 Infection in Vitro. [Google Scholar]
- 129.Ahern G.P., Wang X., Miyares R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- 130.Gassen N.C., Papies J., Bajaj T., Dethloff F., Emanuel J., Weckmann K., Heinz D.E., Heinemann N., Lennarz M., Richter A. BioRxiv; 2020. Analysis of SARS-CoV-2-Controlled Autophagy Reveals Spermidine, MK-2206, and Niclosamide as Putative Antiviral Therapeutics. [Google Scholar]
- 131.Baek A.R., Hong J., Song K.S., Jang A.S., Chin S.S., Park S.W. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp. Mol. Med. 2020:1–12. doi: 10.1038/s12276-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Straub I., Mohr F., Stab J., Konrad M., Philipp S., Oberwinkler J., Schaefer M. Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM 3. Br. J. Pharmacol. 2013;168:1835–1850. doi: 10.1111/bph.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Clementi N., Scagnolari C., D'Amore A., Palombi F., Criscuolo E., Frasca F., Pierangeli A., Mancini N., Antonelli G., Clementi M. Pharmacological research; 2020. Naringenin Is a Powerful Inhibitor of SARS-CoV-2 Infection in Vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim M.C., Lee H.J., Lim B., Ha K.-T., Kim S.Y., So I., Kim B.J. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int. J. Mol. Med. 2014;33:1657–1663. doi: 10.3892/ijmm.2014.1704. [DOI] [PubMed] [Google Scholar]
- 135.Gao W., Zan Y., Wang Z.-j.J., Hu X.-y., Huang F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016;37:1166–1177. doi: 10.1038/aps.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nakamura T., Miyoshi N., Ishii T., Nishikawa M., Ikushiro S., Watanabe T. Activation of transient receptor potential ankyrin 1 by quercetin and its analogs. Biosci. Biotechnol. Biochem. 2016;80:949–954. doi: 10.1080/09168451.2015.1132148. [DOI] [PubMed] [Google Scholar]
- 137.Di Pierro F., Derosa G., Maffioli P., Bertuccioli A., Togni S., Riva A., Allegrini P., Khan A., Khan S., Khan B.A. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 2021;14:2359–2366. doi: 10.2147/IJGM.S318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nalli M., Ortar G., Moriello A.S., Di Marzo V., De Petrocellis L. Effects of curcumin and curcumin analogues on TRP channels. Fitoterapia. 2017;122:126–131. doi: 10.1016/j.fitote.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 139.McNamara F.N., Randall A., Gunthorpe M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor [TRPV1] Br. J. Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pawar K.S., Mastud R.N., Pawar S.K., Pawar S.S., Bhoite R.R., Bhoite R.R., Kulkarni M.V., Deshpande A.R. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front. Pharmacol. 2021;12:1056. doi: 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ibrahim M.A., Abdelrahman A.H., Hussien T.A., Badr E.A., Mohamed T.A., El-Seedi H.R., Pare P.W., Efferth T., Hegazy M.-E.F. In silico drug discovery of major metabolites from spices as SARS-CoV-2 main protease inhibitors. Comput. Biol. Med. 2020;126:104046. doi: 10.1016/j.compbiomed.2020.104046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su H., Yao S., Zhao W., Li M., Liu J., Shang W., Xie H., Ke C., Gao M., Yu K. BioRxiv; 2020. Discovery of Baicalin and Baicalein as Novel, Natural Product Inhibitors of SARS-CoV-2 3CL Protease in Vitro. [Google Scholar]
- 143.Sui F., Zhang C.-B., Yang N., Li L.-F., Guo S.-Y., Huo H.-R., Jiang T.-L. Anti-nociceptive mechanism of baicalin involved in intervention of TRPV1 in DRG neurons in vitro. J. Ethnopharmacol. 2010;129:361–366. doi: 10.1016/j.jep.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 144.Vetter I., Lewis R.J. 2011. Natural Product Ligands of TRP Channels, Transient Receptor Potential Channels; pp. 41–85. [DOI] [PubMed] [Google Scholar]
- 145.da Silva Antonio A., Wiedemann L.S.M., Veiga-Junior V.F. Natural products' role against COVID-19. RSC Adv. 2020;10:23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Adhikari B., Marasini B.P., Rayamajhee B., Bhattarai B.R., Lamichhane G., Khadayat K., Adhikari A., Khanal S., Parajuli N. Phytotherapy Research; 2020. Potential Roles of Medicinal Plants for the Treatment of Viral Diseases Focusing on COVID‐19: A Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abbas M.A. Chemico-Biological Interactions; 2020. Modulation of TRPV1 Channel Function by Natural Products in the Treatment of Pain; p. 109178. [DOI] [PubMed] [Google Scholar]
- 148.Vennekens R., Vriens J., Nilius B. Herbal compounds and toxins modulating TRP channels. Curr. Neuropharmacol. 2008;6:79–96. doi: 10.2174/157015908783769644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bousquet J., Czarlewski W., Zuberbier T., Mullol J., Blain H., Cristol J.-P., De La Torre R., Le Moing V., Lozano N.P., Bedbrook A. International archives of allergy and immunology; 2020. Spices to Control COVID-19 Symptoms: Yes, but Not Only…; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bousquet J., Czarlewski W., Zuberbier T., Bedbrook A., Blain H., Anto J.M. Kimchi, a possible food prototype for the control of COVID-19 by nutrients due to an interplay between Nrf2, TRPA1 and TRPV1, Nitric oxide. Int. Arch. Allergy Immunol. 2021;99:100. doi: 10.1159/000514204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bousquet J., Czarlewski W., Zuberbier T., Mullol J., Blain H., de la Torre R., Anto J.M. TRPA1 and TRPV1 agonist; 2021. Induced Cough Challenges in a Single Patient with COVID-19 Showing an Interplay between Nrf2. [Google Scholar]
- 152.Jain A., Chaurasia R., Sengar N.S., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Long W., Fatehi M., Soni S., Panigrahi R., Philippaert K., Yu Y., Kelly R., Boonen B., Barr A., Golec D. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020;598:4321–4338. doi: 10.1113/JP279961. [DOI] [PMC free article] [PubMed] [Google Scholar]