Abstract
Large bone defects are usually managed by replacing lost bone with non-biological prostheses or with bone grafts that come from the patient or a donor. Bone tissue engineering, as a field, offers the potential to regenerate bone within these large defects without the need for grafts or prosthetics. Such therapies could provide improved long- and short-term outcomes in patients with critical-sized bone defects. Bone tissue engineering has long relied on the administration of growth factors in protein form to stimulate bone regeneration, though clinical applications have shown that using such proteins as therapeutics can lead to concerning off-target effects due to the large amounts required for prolonged therapeutic action. Gene-based therapies offer an alternative to protein-based therapeutics where the genetic material encoding the desired protein is used and thus loading large doses of protein into the scaffolds is avoided. Gene- and RNAi-activated scaffolds are tissue engineering devices loaded with nucleic acids aimed at promoting local tissue repair. A variety of different approaches to formulating gene- and RNAi-activated scaffolds for bone tissue engineering have been explored, and include the activation of scaffolds with plasmid DNA, viruses, RNA transcripts, or interfering RNAs. This review will discuss recent progress in the field of bone tissue engineering, with specific focus on the different approaches employed by researchers to implement gene-activated scaffolds as a means of facilitating bone tissue repair.
1. Bone Tissue Engineering
A variety of traumas and conditions can lead to situations in which large sections of bone tissue are absent, including high energy traumas, bone cancer tumor resection, congenital malformation, and debridement of infected bone tissue1–3. Bone tissue has the ability to heal itself naturally, however, the natural healing mechanisms can be insufficient if the defect is too large (generally ≥ 2.5 cm)4. Such defects that are not expected to heal over the remainder of the patient’s lifetime are termed “critical-sized defects”, and are typically treated with an autograft, allograft, or a non-biological implant4,5. Current strategies for regeneration of bone are limited to grafts or non-biological prostheses, and while these approaches can be successful for both congenital defects and injuries, they can also have drawbacks such as limited autograft volume, risk of disease transmission (allografts), risk of immune reaction (allografts), and long-term implant failure5,6. Bone tissue engineering aims to match the therapeutic efficacy of autografts while not being limited by graft availability constraints and, when compared to allografts, also avoids the risks of rejection and disease transmission7. The goal is to create a therapeutic that can: replace the lost tissue in the defect; grow and change within pediatric patients; and does not require a secondary surgery site for donor bone collection (as is the case for autografts)8,9.
Although research groups have attempted a myriad of methods aimed at bone tissue regeneration, there are some common themes within this body of work. Collagen scaffolds are very popular within the bone tissue engineering field and have been loaded with a variety of additives, drugs, and proteins to enhance bone tissue formation10–12. Collagen is a highly biocompatible biopolymer that is naturally produced by cells, can support cell growth, and can degrade through natural mechanisms, as reviewed elsewhere13. Scaffolds made from collagen can take the form of hydrogels14, porous sponges15, and even 3D printed constructs16. Collagen is also a primary component of native bone tissue, and as such is able to provide a basis for the regeneration of new bone tissue when added as a scaffold17. Other commonly used materials include those derived from calcium phosphate (CaP), which can be used both as the base material of a scaffold or as an additive to scaffolds primarily comprised of other materials18,19. CaP-based materials can vary both in their stoichiometric ratio of calcium to phosphate and in their conformational structure, creating different mineral phases that have different physical properties20. Some prevalent CaP-based materials found in bone tissue engineering scaffolds include tri-calcium phosphate, hydroxyapatite (HA), and octacalcium phosphate (OCP)21. CaP-based materials are of particular interest for bone tissue engineering applications because the presence of CaP in biomaterial scaffolds has been shown to stimulate osteogenic differentiation in mesenchymal stem cells (MSCs), a property that could promote healing within a bone defect22. Fabricating scaffolds that mimic the natural bone environment by incorporating both collagen and CaP-based materials is a common strategy, with the goal being to create an osteogenic environment that enhances bone regeneration23. While the material properties of scaffolds are important in bone tissue engineering, growth factor additives have also been used to further enhance their bone regenerative potential.
2. Gene-Based Therapies as Alternatives to Protein Therapy
The loading of scaffolds with therapeutic proteins is a common and effective approach to promoting bone tissue repair. Growth factors such as fibroblast growth factors (FGFs), platelet-derived growth factor (PDGF), bone morphogenetic proteins (BMPs), and vascular endothelial growth factor (VEGF) all play roles in the natural healing of bone24–27. To date, BMPs are the most commonly used proteins in bone tissue engineering due to their demonstrated ability to induce bone regeneration and vascularization within bone defects (signalling pathway shown in Figure 128), though VEGF, PDGF and FGFs are also commonly explored7,24,29–32. Combinations of BMPs, VEGF, and FGFs have also been shown to enhance osteogenic differentiation synergistically33–36. However, due to the continual dilution and degradation of therapeutic protein released from scaffolds after implantation, large doses of these proteins are required to prolong their therapeutic effect37. These supraphysiological doses have been found to cause off-target effects, including inflammation, potentially increased malignancy risk, and ectopic bone formation in regions adjacent to the desired site of bone regeneration38–43. Gene-based therapies that induce expression of therapeutic proteins offer the ability to still exploit the therapeutic action of proteins while precluding the necessity for supraphysiological doses due to the protein being produced by locally transduced/transfected cells44. Alternatively, using RNA interference (RNAi) can induce similar pro-osteogenic effects to those of protein therapies through the modification of gene expression45.
Figure 1:

Schematic of BMP signalling that leads to osteogenic differentiation28. BMPs induce Smad-dependent and non-Smad-dependent signaling. In the Smad-dependent pathway, Smad (1, 5, or 8) is phosphorylated, complexes with Smad 4, then translocates to the nucleus where co-factors are recruited (including Runx2) for the regulation of osteogenic gene expression. In the non-Smad-dependent pathway, TAK1 recruits TAB1 to stimulate activation of MKK3/6 which in turn activates p38α/β. p38α/β phosphorylates Runx2, Dlx5, and Osx, all of which regulate osteogenic gene expression. BMP: Bone morphogenetic protein; BMPR: BMP receptor.
The two main subcategories of gene therapy are viral gene therapy and non-viral gene therapy. Viral gene therapy often entails the transduction of cells using attenuated viruses encoding a desired protein (usually a growth factor). Although viral-mediated transduction is an efficient process, clinical translation has been limited due to the risk of immune reaction to the virus and the risk of insertional mutagenesis46. Non-viral gene therapy uses other (non-viral) vectors and cell-penetrating techniques to transfect cells that avoid the concerns of insertional mutagenesis and immune reaction, however, they instead have the drawbacks of often being cytotoxic and exhibiting poorer transgene expression compared to viruses47.
Combining gene-based therapies with tissue engineering scaffolds often takes the form of either (1) loading scaffolds with transfection/transduction vectors (termed gene- and RNAi-activated scaffolds) that aim to transfect/transduce host cells in situ upon implantation, or (2) seeding cells (already transfected/transduced with desired gene(s)) onto scaffolds prior to implantation. This review will focus on the first of these approaches, i.e. the use of gene- and RNAi-activated scaffolds, rather than cell-seeded scaffolds (Figure 2). This technique benefits from potentially leading to an “off-the-shelf” therapeutic that does not require the more problematic and labor-intensive process of culturing and manipulating cells in the clinic. Issues currently facing cell-based therapies include, cell source variability, lack of cell handling standards, and the challenging logistics of transporting live cells48. Gene-activated scaffolds can be loaded with agents that promote up-regulation of specific proteins and these agents include: plasmid DNA (pDNA), viruses, and RNA transcripts. In addition, scaffolds can be loaded with RNAs that promote down-regulation of anti-osteogenic proteins through RNAi. Both types of scaffolds (gene-activated and RNAi-activated) will be described in greater detail below.
Figure 2:
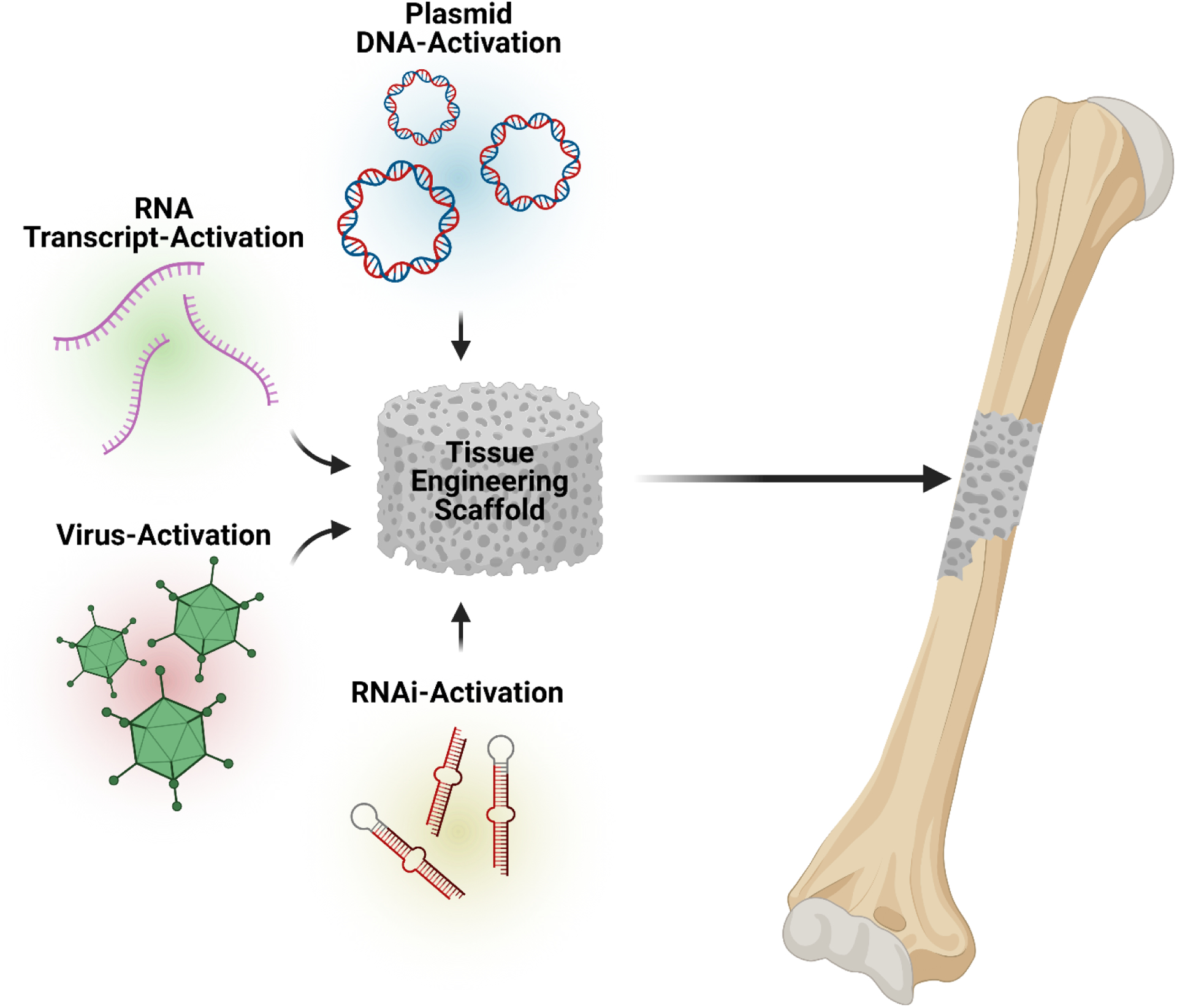
Schematic illustrating the main approaches to incorporating gene-based therapies into scaffolds for bone tissue engineering. Scaffolds can be activated with pDNA, RNA transcripts, viruses, and interfering RNAs (or RNAi) to induce gene expression changes that promote bone regeneration.
3. Plasmid DNA-Activated Scaffolds
Activation of scaffolds with pDNA for bone tissue engineering typically requires some form of delivery vehicle to protect naked pDNA from degradation and to improve its delivery into cells and therefore increase transfection efficiency49,50. This section will focus on the activation of bone tissue engineering scaffolds with pDNA using non-viral vectors. The most common non-viral approaches to transfecting cells with pDNA involve the complexation of pDNA with a cationic polymer such as polyethylenimine (PEI), chitosan, and polyamidoamine (PAMAM)49,50. The resulting nano-sized complexes (nanoplexes) can transfect cells through mechanisms that are depicted in Figure 3 (Route C), although cytotoxicity is often concomitantly observed47. Less common non-viral transfection techniques include complexation of pDNA with CaP nanoparticles or cell-penetrating peptides, though all have been shown to transfect cells successfully. These approaches will be discussed in more detail in this section, and are summarized in Table 1.
Figure 3:
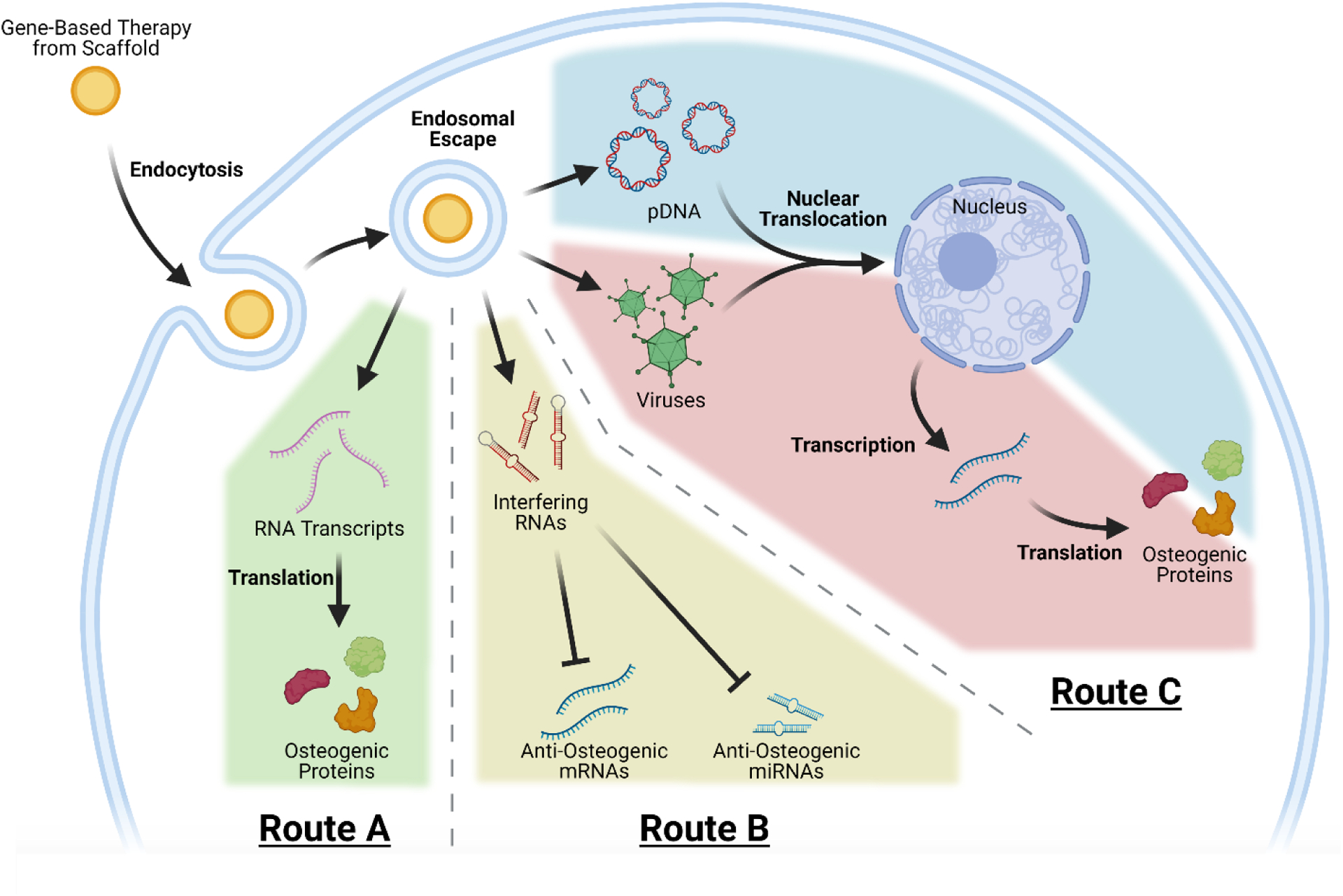
Schematic diagram illustrating the mechanisms of transfection from scaffolds activated with pDNA, viruses, interfering RNAs, and RNA transcripts. RNA transcripts have the simplest transfection mechanism (Route A), where they can be translated into osteogenic protein immediately after entering the cytosol. Interfering RNAs follow a different transfection mechanism (Route B), where after entering the cytosol they can inhibit the anti-osteogenic activity of targeted mRNAs and miRNAs. Viruses commonly used in gene-based therapy and pDNA follow similar routes within the cell (Route C), where after endosomal escape they must reach the nucleus. After entering the nucleus, the encoded genes can be transcribed into mRNA that is then translated into osteogenic protein.
Table 1:
Summary of gene-activated scaffolds utilizing pDNA
| Material | Cell Types and Animal Models Used | Method of Scaffold Loading | Results | Citation # |
|---|---|---|---|---|
| Mineralized collagen sponge loaded with PEI-pDNAs (encoding BMP-2 + FGF-2) nanoplexes | human BMSCs, rats | Nanoplexes in solution with simulated body fluid were incubated with collagen scaffolds over the course of 5 days, with the solution being replaced each day | Scaffolds mineralized with nanoplexes induced greater bone regeneration and thickness; a 5:3 ratio of BMP-2 to FGF-2 resulted in greater bone regeneration than other tested ratios | 18 |
| Porous collagen scaffolds loaded with PEI-pDNAs (encoding BMP-2 + FGF-2) nanoplexes | human BMSCs, rabbits | Nanoplex solutions were injected into collagen scaffolds then lyophilized | Scaffolds containing nanoplexes encoding both genes induced greater expression of BMP-2 in vitro and produced greater bone regeneration and union rates in vivo | 54 |
| Porous collagen scaffolds loaded with PEI-pDNAs (encoding BMP-2 + FGF-2) nanoplexes surrounding a fibrin gel core containing insulin-loaded PLGA microparticles and vitamin D3 | human BMSCs, rats | Nanoplex solutions were injected into scaffolds then lyophilized | Gene-activated scaffolds with vitamin D3 and insulin microparticles resulted in more ectopic bone formation than other experimental combinations, all gene-activated experimental groups induced more gene expression changes in local cells than non-gene-activated groups | 55 |
| Electrospun PCL scaffolds loaded with chitosan-pDNA (encoding BMP-2) nanoplexes | rat MSCs, rats | Nanoplex solutions were added to electrospun scaffolds conjugated with MMP-sensitive peptides and incubated overnight | Nanoplexes were released in response to MMP activity; nanoplex incorporation resulted in enhanced osteogenic differentiation in vitro; MMP-conjugated nanoplex-loaded scaffolds stimulated greater in vivo bone regeneration in the long term | 56 |
| Porous collagen-HA scaffolds loaded with PEI-pDNA (encoding IL-1Ra) nanoplexes | rat BMSCs | Nanoplex solutions were added to scaffolds | Gene-activated scaffolds prevented loss of mineralization caused by IL-1 treatment | 58 |
| Porous collagen-HA scaffolds loaded with chitosan-pDNA (encoding BMP-2 and VEGF) nanoplexes | rat MSCs, rats | Nanoplex solutions were added to scaffolds | Scaffolds enhanced in vitro calcium deposition and induced bone regeneration in vivo | 62 |
| Porous collagen-HA scaffolds loaded with chitosan-pDNA (encoding a truncated BMP-2) nanoplexes | rat MSCs, rats | Nanoplex solutions were added to scaffolds | Scaffolds containing chitosan-pDNA nanoplexes induced significant calcium deposition in vitro and outperformed the bone regeneration of naked plasmid-activated scaffolds in vivo | 63 |
| Porous collagen-HA scaffolds loaded with GET-pDNA (encoding BMP-2 and VEGF) nanoplexes | human MSCs, MC3T3 cells, C2C12 cells, human dermal fibroblasts, human articular chondrocytes, HUVECs, Ne4C cells, rats | Nanoplex solutions were added to scaffolds | Scaffolds implanted into bone defects were able to be infiltrated by host cells, induced transient localized transfection, and completely repaired the defect | 64 |
| Porous collagen scaffolds loaded with PEI-pDNA (encoding FGF-2) nanoplexes | human BMSCs | Nanoplex solutions were injected into scaffolds then lyophilized | Gene-activated scaffolds induced greater FGF-2 expression and had more proliferating cells | 65 |
| Porous collagen scaffolds loaded with PEI-pDNAs (encoding PDGF-B and/or VEGF) nanoplexes | human BMSCs, rats | Nanoplex solutions were injected into scaffolds then lyophilized | Scaffolds loaded with nanoplexes encoding PDGF-B induced complete bridging of the defect | 66 |
| Porous collagen scaffolds loaded with PEI-pDNA (encoding BMP-2 and FGF-2) nanoplexes | MC3T3 cells, DPSCs, tooth culture model | Nanoplex solutions were injected into scaffolds then lyophilized | Gene-activated scaffolds induced expression of loaded genes, had enhanced mineralization, and did not induce the necrotic layer observed in MTA-loaded scaffolds | 69 |
| Electrospun gelatin-PEG mats containing ALL-Fect-pAsp-pDNA (encoding BMP-2) nanoplexes | C2C12 cells, MCT3T cells | Nanoplex solutions were added to gelatin-PEG solution and electrospun | Scaffolds containing nanoplexes induced osteogenic differentiation | 72 |
| Electrospun PLGA containing PEI-pDNA (encoding BMP-2) nanoplexes | human PDLSCs | Nanoplex solutions were coaxially electrospun to create core-shell fibers with a shell of PLGA and a core of PEI-pDNA nanoplexes | Core-shell scaffolds containing nanoplexes had greater expression of BMP-2, greater osteogenic differentiation, and these effects lasted longer than standard scaffolds with nanoplexes | 73 |
| nHA-collagen scaffolds loaded with CaP nanoparticles functionalized with pDNA (encoding BMP-2) | rats | pDNA functionalized CaP nanoparticle solutions were injected into nHA-collagen scaffolds | Gene-activated scaffolds were able to transfect local cells upon implantation resulting increased ALP activity | 77 |
| Bilayer collagen-nHA scaffolds loaded with multi-shell CaP particles containing pDNAs (encoding TGF-β3 or BMP-2) | human MSCs | Multi-shell particles were loaded onto the collagen/nHA layer of the scaffold, then a collagen solution containing other multishell particles was applied onto the first layer to create a sec ond layer |
Bilayer scaffolds were able to stimulate differentiation in vitro towards the desired lineage for each layer | 78 |
| DBM scaffold loaded with PEI-pDNA (encoding BMP-2)-coated MVs | rat MSCs, rats, rabbits | MVs were coated layer by layer with PEI and pDNA (encoding BMP-2), then the coated MV solution was added to the DBM scaffolds | DBM scaffolds loaded with PEI/pDNA-coated MVs enhanced OCN and OPN expression, and collagen fiber deposition when implanted subcutaneously; the scaffolds improved bone regeneration and angiogenesis when implanted into a bone defect | 79 |
| Injectable scaffolds made from collagen solution, cell suspension, polymeric microbubble suspension, and pDNA (encoding BMP-2 and BMP-7) | C2C12 cells, mice | Solutions of collagen, cell suspension, microbubble suspension, and pDNA were mixed together | Gene-activated and ultrasound-stimulated scaffolds showed osteogenic differentiation in vitro and increased ectopic bone formation in vivo | 80 |
| 3D printed OCP scaffolds loaded with pDNA (encoding VEGFA) | rats, mice, pigs | Scaffolds were immersed in a sodium phosphate solution containing naked pDNA and incubated under agitation for 10 hours | Gene-activated scaffolds did not induce inflammation when implanted subcutaneously, integrated into mandibular and tibial bone defects, and restored stability to the defects | 81 |
3.1. Gene-activation with pDNA nanoplexes:
Complexation of pDNA with cationic polymers is a well-studied non-viral gene therapy approach, with PEI-pDNA nanoplexes being considered the gold standard50. Nanoplexes are formed by mixing cationic polymer with anionic pDNA in solution after which, at specific charge ratios, the two components spontaneously complex into nanoplexes. These nanoplexes have been shown to be endocytosed by cells and decomplex, allowing pDNA that has undergone nuclear translocation to induce expression of the gene it encodes. The exact mechanisms by which this decomplexation and nuclear translocation occur are yet to be fully understood51–53. Nonetheless, the technique is successful in inducing the expression of genes loaded within pDNA nanoplexes.
Khorsand et al. loaded PEI-pDNA (encoding BMP-2 and/or fibroblast growth factor-2 (FGF-2)) nanoplexes onto collagen scaffolds to test whether co-expression of the two growth factors can synergistically enhance bone regeneration54. In vitro experiments with human bone-marrow-derived mesenchymal stem cells (BMSCs) showed that co-delivery of nanoplexes encoding BMP-2 and FGF-2 (in the absence of collagen scaffolds) resulted in a 2-fold higher expression of BMP-2 than BMSCs treated with a single gene54. Using a diabetic rabbit long bone model Khorsand et al. showed that co-delivery of the two nanoplex formulations (loaded into collagen scaffolds) resulted in greater bone volume regeneration within the defect (133.7 mm3 new bone vs. 96.4 mm3 and 82.2 mm3 for FGF-2 alone and BMP-2 alone, respectively) and higher union rates54. In a subsequent study Khorsand et al. then applied the co-delivery of PEI-pDNA (encoding BMP-2 and FGF-2) nanoplexes to a rat diabetic model using a fibrin-collagen composite scaffold that was also loaded with vitamin D3, and insulin55. The composite scaffolds were comprised of a cylindrical outer shell consisting of porous collagen loaded with nanoplexes that surrounded a cylindrical inner core consisting of fibrin gel containing insulin-loaded poly-lactic-co-glycolic acid (PLGA) microparticles and vitamin D355. When these scaffolds were implanted into diabetic rats, they induced greater bone formation than controls as determined by micro-computed tomography (μCT) and histology. The gene-activated scaffolds also induced gene expression changes in local cells compared to controls lacking PEI-pDNA nanoplexes55.
Malek-Khatabi et al. chose to control the release of nanoplexes from scaffolds by creating a gene-activated scaffold that released nanoplexes in response to enzymatic activity from infiltrating cells56. To do this, they first modified an electrospun fibrous mat made from polycaprolactone (PCL) by conjugating peptide sequences to the surface that are sensitive to cleavage by matrix metalloproteinases (MMPs) which are involved in remodeling of the extracellular matrix57. They then fabricated nanoplexes made from chitosan and pDNA encoding BMP-2 using a microfluidic system to improve the homogeneity of the resulting nanoplexes. Finally, the resulting nanoplexes were loaded onto the modified scaffolds that were then tested for their ability to: release nanoplexes in response to MMP exposure; induce osteogenic differentiation of MSCs in vitro; and stimulate bone regeneration in an in vivo rat calvarial defect model56. Treatment with MMPs was able to induce MMP-cleavage specific release of the nanoplexes in vitro (totaling 60% of loaded nanoplexes over 24 hours); and both in vitro osteogenic differentiation and in vivo bone regeneration were superior when the modified scaffolds versus unmodified gene-activated scaffolds and controls were compared (~18 mm3 of bone was regenerated after 10 weeks for modified scaffolds vs. ~9 mm3 and ~4 mm3 for unmodified and controls, respectively)56.
Lackington et al. investigated scaffolds (made from collagen and HA) loaded with PEI-pDNA nanoplexes that encoded an interleukin 1 receptor agonist (IL-1Ra) in order to inhibit the inflammatory effects of IL-1 which can reduce osteogenic differentiation of BMSCs58–60. IL-1 is a pro-inflammatory cytokine, the inhibition of which was found to promote bone formation in vivo61. Lackington et al. showed that treating BMSCs with IL-1 reduced their osteogenic differentiation and ability to deposit calcium, however, loading nanoplexes encoding IL-1Ra into their scaffolds was able to protect seeded BMSCs from these effects58. In a series of studies, Raftery et al. explored a variety of nanoplex-based approaches to gene-activating bone tissue engineering scaffolds62–64. Initially, it was shown that chitosan-pDNA (encoding BMP-2 and VEGF) nanoplexes loaded onto scaffolds (made from collagen and HA) could induce BMP-2 and VEGF expression as well as osteogenic differentiation in rat MSCs in vitro (as evidenced by the enhanced capacity of MSCs to deposit calcium). When tested in rat calvarial defects, these scaffolds outperformed scaffolds loaded with single genes and empty scaffolds in terms of new bone volume as determined by μCT (~17% new bone volume vs ~2% and 3% for single gene and empty scaffolds, respectively) and new bone area as determined by histology62. In a subsequent study, Raftery et al. compared scaffolds (again made from collagen and HA) loaded with chitosan-pDNA nanoplexes where the pDNA encoded the standard BMP-2 or a modified sequence encoding “BMP-2-Advanced” where the codons were altered to optimize for translation63. Scaffolds loaded with chitosan-pDNA (encoding BMP-2 Advanced) nanoplexes were seeded with rat MSCs in vitro and induced greater calcium deposition compared to scaffolds loaded with chitosan-pDNA (encoding standard BMP-2) nanoplexes63. In addition, in 7 mm rat calvarial defects, the scaffolds containing the BMP-2-Advanced plasmid promoted greater bone volume regeneration than both empty scaffolds and scaffolds activated with the standard BMP-2 plasmid (~12% new bone volume for BMP-2-Advanced vs. ~2% and ~3% for empty and standard BMP-2 scaffolds, respectively)63. Finally, Raftery et al., in an attempt to avoid the typical cytotoxicity issues associated with cationic polymers47, chose to substitute chitosan with a cell penetrating peptide termed “GET” (glycosaminoglycan binding to enhance transfection). Thus pDNAs encoding BMP-2 and VEGF were complexed with GET to form nanoplexes that were subsequently loaded onto collagen-HA scaffolds (Figure 4A)64. In vitro studies with a series of cell types (MSCs, human umbilical vein endothelial cells [HUVECs], C2C12 cells, human dermal fibroblasts, human articular chondrocytes, and Ne4C cells) demonstrated transfection efficiency comparable to that of commercial reagents with minimal toxicity64. In vivo studies showed that, upon subcutaneous implantation the gene-activated scaffold induced localized transfection, and in a 7 mm rat calvarial defect the scaffold activated with both BMP-2 and VEGF could stimulate complete bridging of the defect within 4 weeks (~38% new bone volume vs. ~13% and ~22% for BMP-2 and VEGF alone, respectively, shown in Figures 4B, 4C)64.
Figure 4:

Scaffolds activated with GET-pDNA nanoplexes were shown to regenerate bone in rat calvarial defects. (A) Schematic diagram of gene-activation with GET-pDNA nanoplexes. (B) Representative μCT scans of rat calvarial defects 4 weeks post-implantation. (C) Quantitative analysis of bone regeneration. Bone volume fraction was determined using μCT to compare bone regenerated within the defect to the total volume of the defect. Area of new bone was determined by analysis of histology sections. Vessel density average was determined by fluorescence microscopy after staining for endothelial cells. Data plots represent mean ± SD (n = 8). Significance indications are as follows: * represents p < 0.05, ** represents p < 0.01 and *** represents p < 0.001. Adapted with permission from Raftery et al.64
D’Mello et al. loaded PEI-pDNA (encoding FGF-2) nanoplexes onto porous collagen scaffolds and seeded them with BMSCs65. It was found that the gene-activated scaffolds induced ~4-fold greater secretion of FGF-2 and led to more proliferating cells than scaffolds lacking the nanoplexes. D’Mello et al. also investigated the loading of PEI-pDNAs (encoding VEGF and platelet-derived growth factor B (PDGF-B)) nanoplexes onto scaffolds to enhance bone regeneration in a 5mm rat calvarial defect66. They found that the delivery of PDGF-B alone resulted in complete bridging of the defect (~53% new bone volume), while combination treatments and VEGF alone resulted in only partial regeneration in the defect (5–10% new bone volume)66.
Chakka et al. compared porous collagen scaffolds loaded with PEI-pDNAs (encoding BMP-2 and FGF-2) nanoplexes to similar scaffolds loaded with the commonly used dental pulp capping material, mineral trioxide aggregate (MTA)67,68, in in vitro cultures and an ex vivo tooth culture model69. The in vitro experiment showed that the gene-activated scaffolds induced expression of BMP-2 and FGF-2 in dental pulp stem cells (DPSCs) seeded onto the scaffolds, resulting in greater mineralization than MTA-treated scaffolds69. The ex vivo study showed that the MTA-treated scaffolds created a necrotic layer while gene-activated scaffolds did not69. Acri et al. investigated PEI-pDNA (encoding BMP-2 and FGF-2) nanoplexes that were embedded within collagen scaffolds by mineralizing the scaffolds in simulated body fluid in the presence of the nanoplexes18. This approach improved bone regeneration (~19% new bone volume for mineralized gene-activated scaffolds vs ~10% new bone volume for regular gene-activated scaffolds) and the thickness of regenerated tissue within a 5 mm rat calvarial defect. Additionally, it was found that a BMP-2 to FGF-2 ratio of 5:3 had the best regenerative outcomes in comparison to other ratios tested18.
Some groups investigated using electrospinning, a technique that has been reviewed extensively elsewhere70,71, to create scaffolds that contain nanoplexes. In brief, the technique involves using high voltages to emit jets of polymer solution that dry in flight as they move towards a grounded collection surface, creating a collection of polymer strands with diameters ranging from the microscale to nanoscale. Pankongadisak et al. loaded PEI-pDNA (encoding BMP-2) nanoplexes into electrospun gelatin-PEG mats and investigated their ability to induce osteogenic differentiation72. The nanoplexes comprised poly(aspartic acid) (pAsp), a commercial transfection reagent (ALL-Fect) and pDNA encoding BMP-2. Two pre-osteoblast cell lines, MC3T3 and C2C12, were seeded onto the mats and greater alkaline phosphatase (ALP) activity was observed for cells on mats containing the nanoplexes in comparison to untreated cells and cells treated with nanoplex solutions, indicating enhanced osteogenic differentiation72. In a separate study, Xie et al. incorporated PEI-pDNA (encoding BMP-2) nanoplexes into the core of electrospun PLGA fibers73. This was done by creating a coaxial electrospinning platform in which PLGA solution surrounded a solution of PEI-pDNA nanoplexes as the solution jetted towards the collection surface. Comparison of the gene-activated core-shell electrospun scaffolds to scaffolds produced by direct mixing of the two solutions revealed that, upon seeding of human periodontal ligament stem cells (PDLSCs), the core-shell scaffolds promoted greater BMP-2 expression, enhanced osteogenic differentiation, and prolonged BMP-2 expression73.
3.2. Gene-activation with CaP nanoparticles:
Using CaP nanoparticles complexed with pDNA has also been shown to be an effective method of non-viral transfection, as reviewed elsewhere74,75. In brief, CaP nanoparticles formed by precipitation in vitro can complex with pDNA and be taken up by cells via a variety of endocytotic pathways, as reviewed elsewhere76. Tenkumo et al. loaded CaP nanoparticles complexed with pDNA encoding BMP-2 onto scaffolds made from nanohydroxyapatite (nHA) and collagen77. When implanted subcutaneously in rats, these scaffolds were able to transfect cells with the pDNA encoding BMP-2, leading to higher levels of ALP activity than a commercial product or unloaded controls77. Lee et al. used multi-shell CaP/pDNA/PEI nanoparticles to gene-activate a bilayer scaffold intended for improving regeneration of the osteochondral interface78. This was done by crafting two layers within the scaffold; one made from pure collagen (chondrogenic layer) and the other made from a combination of collagen and nHA (osteogenic layer)78. They then made multi-shell nanoparticles by creating CaP nanoparticles, coating them with pDNA, coating these particles with more CaP, then adding a layer of PEI78. The pDNAs encoded either TGF-β3 or BMP-2 and were incorporated into the chondrogenic layer or the osteogenic layer, respectively78. Lee et al. found that when their scaffolds were seeded with human MSCs in vitro, qPCR analysis showed the cells began to differentiate toward either the chondrogenic or osteogenic lineage depending on which layer the cells were in78.
3.3. Gene-activation with other systems:
Recent research has demonstrated that other non-viral gene-therapy approaches involving bone tissue engineering scaffolds, namely the use of microvesicles (MVs), OCP, and ultrasound, have also shown promise in preclinical settings. A scaffold consisting of demineralized bone matrix (DBM) loaded with MSC-derived microvesicles (MVs) and coated with PEI and pDNA (encoding BMP-2) was developed by Liang et al79. The MVs were sequentially coated with PEI and pDNA, with the aim of reducing the cytotoxicity associated with using typical PEI-pDNA nanoplexes; and then the MVs were loaded onto DBM scaffolds79. Liang et al. implanted their MV-loaded DBM scaffolds into two in vivo models: a rat subcutaneous implantation model assaying ectopic bone formation and a rabbit femoral condyle bone defect assaying bone regeneration potential. The MV-scaffolds were compared against scaffolds loaded with standard PEI-pDNA nanoplexes and unloaded scaffolds. In the subcutaneous model increased expression of osteocalcin (OCN) and osteopontin (OPN) was observed along with an increase in collagen fiber accumulation; while the femoral condyle model showed increased bone regeneration (~15% new bone volume for MV-scaffolds vs. ~11% and ~10% for standard nanoplex scaffolds and empty scaffolds, respectively) and blood vessel formation in the defects79. Nomikou et al. investigated exerting more control over the transfection of local cells by employing an ultrasound-responsive gene-activation technique80. They did this by combining ultrasound-sensitive microbubbles, C2C12 cells, and pDNAs (encoding BMP-2 and BMP-7) in injectable collagen gels. These gene-activated and ultrasound-treated scaffolds increased osteogenic differentiation when the cell-containing gels were cultured in vitro and produced ectopic bone formation when injected into mouse hindlimb muscle80. Bozo et al. took the unique approach of combining gene-activation with 3D printed scaffolds made from OCP81. The 3D printed OCP scaffolds were immersed in a sodium phosphate solution containing naked pDNA (encoding VEGFA) for 10 hours under agitation81. The scaffolds were then implanted in 3 different animal models: a rat subcutaneous model; a mouse subcutaneous model; and pig tibial and mandibular bone defects. The subcutaneous models showed that the scaffolds could biodegrade and transfect cells upon implantation with minimal inflammation81. Implantation of these scaffolds into the pig tibial and mandibular defects led to scaffold integration and vascularized bone tissue regrowth.
4. Virus-Activated Scaffolds
The use of viruses for gene therapy is well established and is the oldest gene therapy technique available82. By exploiting the innate ability of viruses to introduce new genetic material to target cells, scientists have been able to manipulate viruses with the aim of efficiently and safely altering the genetic makeup of cells and organisms83. Specifically, modifications have been made to viral genomes to incorporate genes of interest while also curtailing their pathogenicity84–86. The result is a delivery system that uses a virus’s natural ability to penetrate cell membranes and direct genetic material to the nucleus to induce expression of the genes it carries (Figure 3, Route C). Typically, nuclear translocation after penetration of the cell membrane is vastly more efficient with viral gene therapy approaches than non-viral, pDNA-based approaches. This is due to virus’ ability to ensure that the viral genome is efficiently trafficked to its intracellular destination, while non-viral gene therapy methods often lack these advantages87,88. Adenoviruses, lentiviruses, and adeno-associated viruses (AAVs) are the most commonly used viruses for gene therapy, though a variety of viruses are used in viral gene therapy, as reviewed elsewhere83,89. However, viral gene therapy is not without its risks, namely the risk of insertional mutagenesis and the potential for immunoreactivity in response to the administration of the virus to a patient. When retroviruses insert their genetic payload into the genomes of host cells, the site of insertion can result in the dysregulation of oncogenes and thus encourage the development of cancer, making retroviral gene therapy a potentially risky avenue for gene therapy90. Research has also shown that introduction of viruses into patients can lead to toxic innate immune responses; or strong adaptive immune reactions (rendering the virus ineffective), especially when a patient has been previously exposed to the wild type version of therapeutic virus91. Despite the risks, viral gene therapy has shown encouraging results in recent literature (summarized in Table 2) and remains among the most reliable methods of transducing cells with genes of interest. The application of viruses to tissue engineering scaffolds to create gene-activated scaffolds has shown promise, with efficient production of the protein of interest being reported ubiquitously and enhancement of bone regeneration being regularly observed in in vivo studies.
Table 2:
Summary of gene-activated scaffolds utilizing viruses.
| Material | Cell Types and Animal Models Used | Method of Scaffold Loading | Results | Citation # |
|---|---|---|---|---|
| Mineralized silk fibroin scaffolds loaded with adenoviruses encoding BMP-7 | human BMSCs, mice | Solution of adenovirus was added to a silk fibroin scaffold in combination with simulated body fluid and incubated for 1 week | Scaffolds maintained expression of BMP-7 for 21 days, induced differentiation in vitro, and induced bone formation in vivo | 92 |
| MBG-silk scaffolds loaded with adenoviruses encoding PDGF-B | human MSCs, mice | MBG particles were mixed with silk solution, lyophilized, and then loaded with adenovirus solution | Virus was released for up to 3 weeks; enhanced MSC recruitment was observed; improved bone regeneration occurred in mice | 93 |
| MBG-silk scaffolds loaded with adenoviruses encoding PDGF-B and BMP-7 | human periodontal ligament cells, dogs | MBG particles were mixed with silk solution, lyophilized, and then loaded with adenovirus solution | Combining adenoviral vectors encoding PDGF-B and BMP-7 enabled synergistic wound healing in terms of gum tissue and bone growth | 94 |
| AAV vector encoding BMP-2 loaded onto a porous PLLA scaffold | human BMSCs, mice | Virus encoding BMP-2 was encased in ice-based microparticles that were then mixed with dissolved PLLA and cast into scaffolds | Cells within scaffolds underwent osteogenesis; enhanced bone formation in vivo was observed | 95 |
| Doxycycline-inducible lentiviral vectors encoding IL-1Ra, TGF-β3, or BMP-2 loaded onto cartilage-derived matrix (CDM) scaffolds | human BMSCs | Lentivirus encoding TGF-β3 and BMP-2 were loaded onto the shell and core components of the scaffolds, respectively, that had been coated in poly-L-lysine after which scaffolds were washed and seeded with cells | IL-1Ra reduced inflammation-mediated degradation of scaffolds; region-specific incorporation of BMP-2 and TGF-β3 led to regional differences in differentiation | 96 |
| Biotinylated adenovirus encoding BMP-2 linked to biotinylated gelatin sponges via biotin-avidin interaction | rats | Virus and gelatin sponges were biotinylated independently, then linked by combining the two components in an aqueous environment in the presence of avidin | Immobilization of adenovirus via biotin:avidin resulted in transgene expression being limited to the defect site in a rat calvarial defect; the immobilized virus outperformed free virus loaded into similar scaffolds in terms of both transduction and bone regeneration | 97 |
| Adenovirus encoding HIF-1α loaded onto gelatin sponge scaffolds | rats | Adenovirus solution was added to a gelatin sponge | Virus-induced expression of HIF-1α induced generation of new bone and blood vessels | 98 |
| Chitosan/HA scaffolds loaded with adenovirus encoding VEGF | human osteoblasts, rats | Chitosan and HA were mixed and lyophilized, then adenovirus solution was loaded onto the resulting sponges | Seeded osteoblasts were able to produce VEGF; scaffolds recruited host endothelial cells in vivo | 99 |
In a series of studies, Zhang et al. explored the use of adenoviral vectors loaded into porous scaffolds with the aim of enhancing bone regeneration92–94. Throughout these studies, they examined the effects of loading adenoviruses that independently encode BMP-7 and PDGF-B, both singly and in combination. In their 2012 study, Zhang et al. loaded adenoviruses encoding BMP-7 onto mineralized silk fibroin scaffolds to stimulate more efficient bone regeneration92. To create the scaffolds they lyophilized silk fibroin solutions then loaded a combination of adenovirus solution and simulated body fluid onto the scaffolds before a 7 day incubation92. The resulting gene-activated mineralized silk fibroin scaffolds were found to: induce BMP-7 expression in human BMSCs for up to 21 days, maintain normal proliferation of the seeded cells, and enhance their differentiation into osteoblasts92. These scaffolds were also implanted into a mouse 3 mm calvarial defect model and after 4 weeks they were shown to have increased the volume of regenerated bone in comparison to the empty defect and scaffolds lacking adenoviral vectors (~5.2 mm3 regenerated vs. ~1.1 mm3 and ~2.3 mm3 for empty defect and scaffold alone, respectively)92. Zhang et al. then loaded adenoviruses encoding PDGF-B into silk-mesoporous bioactive glass (MBG) scaffolds made by mixing MBG particles with silk solution and then lyophilizing the resultant product93. It was found that the viruses were released for up to 3 weeks, leading to improved in vitro MSC recruitment, and that inclusion of the viruses aided in enhancing bone regeneration in a 3 mm mouse calvarial defect when compared to scaffolds loaded with viruses encoding green fluorescent protein (scaffold control) and empty defects (~5 mm3 new bone vs. ~1.9 mm3 and ~1.1 mm3 for scaffold control and empty defect control, respectively)93. In a subsequent study, Zhang et al. (2015) loaded two adenoviral vectors, one encoding PDGF-B and the other encoding BMP-7, onto their silk-MBG scaffolds to enhance periodontal bone regeneration through recruitment of cells via PDGF-B signalling and promoting osteogenic differentiation with BMP-794. In vitro studies showed that silk-MBG scaffolds loaded with adenoviruses encoding PDGF-B alone induced more rapid recruitment of human periodontal ligament cells than those with adenoviruses encoding BMP-7 alone, while scaffolds loaded with adenoviruses encoding BMP-7 alone induced greater osteoblast differentiation than those loaded with PDGF-B alone94. Zhang et al. also tested scaffolds containing adenoviral PDGF-B alone, adenoviral BMP-7 alone, and a combination treatment in a dog periodontal defect model94. They found that combining the two adenoviral vectors in a single scaffold resulted in a synergistic healing of the defect in comparison to the scaffolds containing just one adenoviral vector94.
Some research groups have opted to load viruses into their scaffolds in specific ways to control their release or spatially control their presence. In an innovative approach, Xue et al. loaded AAV encoding BMP-2 into ice-based microparticles that were then mixed with poly-L-lactic acid (PLLA) dissolved in chloroform before being cast into scaffolds95. The AAVs were shown to be released from the scaffolds over approximately 2 weeks in vitro and maintained increased levels of BMP-2 expression in seeded BMSCs over that time. This induced expression of BMP-2 was associated with increased BMSC osteogenic differentiation as measured by osteocalcin and bone sialoprotein expression, and resulted in new bone formation in a mouse ectopic bone formation model95. Rowland et al. loaded doxycycline-inducible lentiviral vectors encoding IL-1Ra, TGF-β3, BMP-2 onto cartilage-derived matrix (CDM) scaffolds to create a biphasic osteochondral scaffold that inhibited inflammatory processes mediated by IL-1 that lead to scaffold degradation96. Specifically, they created a two-piece CDM scaffold comprised of two hemispheres, an outer shell and an inner core that were then coated with poly-L-lysine to enhance the adhesion of the lentiviral vectors. To create a biphasic scaffold that spatially controls the differentiation of seeded cells, the lentiviral vectors encoding BMP-2 and TGF-β3 were independently coated onto the inner core piece (to induce osteogenic differentiation) and the outer shell piece (to induce chondrogenic differentiation), respectively. They found that transduction with IL-1Ra reduced IL-1-mediated degradation of the scaffold; and that the inner core piece and the outer shell piece promoted osteogenic and chondrogenic differentiation, respectively.96 Hu et al. immobilized adenoviral vectors encoding BMP-2 onto gelatin sponges via biotin-avidin linkages and compared these scaffolds to scaffolds loaded with adenoviral vectors encoding BMP-2 that were not immobilized in an 8 mm diameter rat calvarial defect model97. It was found that the scaffolds containing immobilized adenovirus exhibited transduction specific to the defect site, while the scaffolds containing free adenovirus displayed transduction both within the defect site and in regions around the defect site (up to 8 mm away)97. Additionally, the scaffolds containing immobilized virus were able to induce more bone regeneration than the free virus-loaded scaffolds (~58% vs. ~22%) in the defect according to μCT analysis97.
Yang Zhang et al. (2016) opted for a simpler approach in which a solution of adenovirus encoding hypoxia-inducible factor-1α (HIF-1α) was loaded onto gelatin sponges that were subsequently implanted into bone defects within rats98. The group chose HIF-1α to stimulate osteogenesis and angiogenesis, and found that the use of virus-loaded scaffolds led to enhanced blood vessel formation and mineralization of the bone defect. Koç et al. loaded adenoviruses encoding VEGF onto porous scaffolds made from chitosan and HA to stimulate the generation of vascularized bone tissue99. In vitro studies demonstrated that their adenovirus-loaded scaffolds could successfully transduce human osteoblast cells, and maintained expression of VEGF for at least 13 days99. When these scaffolds were implanted subcutaneously in rats, they found that the scaffolds were able to recruit host endothelial cells, showing the potential for local VEGF expression to assist in vascularizing regenerated bone tissue99.
5. RNA Transcript-Activated Scaffolds
Messenger RNA (mRNA), the active component in RNA transcript-activated scaffolds, has been receiving substantial attention for its use in Moderna’s and Pfizer’s COVID-19 vaccines100. RNA transcript-activated scaffolds for bone tissue engineering employ mRNAs which encode desirable growth factors to facilitate bone growth at an injured site45. The incorporation of chemically modified bases in the mRNA transcript is a useful technique to further enhance the stability and translation efficiency of the transcript101,102. The main advantage of RNA transcript activation is that high levels of growth factor expression are possible in cells non-mitotic or slowly replicating cells compared to that of pDNA-based transfection since the RNA is functional in the cytoplasm and thus does not have to breach the nuclear envelope to be expressed (Figure 3, Route A)45. In support of this, high levels of growth factor production from a RNA transcript-activated scaffold have led to significantly increased bone growth as compared to pDNA-based systems103. However, one major drawback of using chemically modified mRNA (cmRNA) is that the modified bases used for their synthesis are expensive to produce, thus scaffolds using this technology may cost significantly more than pDNA-activated scaffolds.
CmRNA is synthesized using in vitro transcription (IVT), which involves transcribing a template strand of DNA into cmRNA using an RNA polymerase and synthetic ribonucleic bases that differ from traditional ribonucleic bases due to strategic chemical modifications. One of the synthetic ribonucleic bases used is pseudo-uridine in place of uridine, which significantly increases the translation efficiency of the cmRNA while reducing its immunostimulatory properties103. Another technique that has led to increased success with RNA transcript-based therapy is incorporating a 5’ cap, examples of which include the anti-reverse cap analog (ARCA)104, CleanCap105, and Vaccinia enzymatic capping106.
A review of the recent literature covering transcript-activated scaffolds for bone tissue engineering reveals that most studies used RNA transcripts encoding BMP-2 and that the outcomes related to bone tissue engineering were generally favorable with treatment groups incorporating cmRNA (summarized in Table 3)103,107–109. In 2015, Elangovan et al. loaded collagen sponges with PEI-based nanoplexes that contain either cmRNA encoding BMP-2 or pDNA encoding BMP-2 to directly compare the osteogenic potency of cmRNA transcript-activated scaffolds versus pDNA-activated scaffolds103. The cmRNA consisted of an ARCA 5’ cap, a 120-base pair polyadenylated tail, and either 100% substitution of uridine and cytidine with pseudouridine and 5-methyl-cytidine or 25% substitution of uridine and cytidine with 2-thiouridine and 5-methylcytidine (Figure 5A). The 100% substituted cmRNA reduced interferon-α (IFN-α) expression in vitro, so this version was used for subsequent experiments. When the scaffolds were implanted in a 5mm rat calvarial defect for four weeks, the RNA transcript-activated scaffolds were the only groups to significantly increase bone formation as determined by μCT analysis (Figures 5B, 5C). The increase in bone volume was 3.94-fold and 1.94-fold for the cmRNA transcript-activated scaffold and the pDNA activated scaffolds, respectively, as compared to the sham group103. In a similar study, Khorsand et al. (2019) compared the osteogenic potency of pDNA encoding BMP-9 to cmRNA encoding BMP-9 using BMSCs and a rat calvarial defect model110. The cmRNA was modified with 100% pseudouridine and 5-methylcytidine substitution and capped with an ARCA. The resulting cmRNA and pDNA were complexed with PEI and loaded independently into perforated collagen membrane scaffolds. ALP activity was significantly enhanced in the BMSCs treated with the cmRNA-loaded scaffolds as compared to untreated BMSCs on day 7, whereas on day 11 the ALP activity for BMSCs treated with either the cmRNA-loaded or pDNA-loaded scaffolds was significantly increased compared to untreated BMSCs110. In the rat calvarial defect model, significantly more bone volume was formed for both the cmRNA (~60%) and the pDNA treated (~54%) groups as compared to the control (15%).
Table 3:
Summary of gene-activated scaffolds utilizing RNA transcripts.
| Material | Cell Types and Animal Models Used | Method of Scaffold Loading | Results | Citation # |
|---|---|---|---|---|
| Collagen scaffold loaded with PEI-cmRNA (encoding BMP-2) nanoplexes | Human BMSCs, rats | Collagen sponge loaded with either cmRNA or pDNA encoding BMP-2 complexed with PEI and lyophilized | cmRNA increased transfection efficiency, protein secretion, and bone formation | 103 |
| Fibrin gel loaded with cmRNA nanoplexes and cmRNA-activated particles that encode BMP-2 | Rat BMSCs, rat AMSCs, rats | Cells were transfected with cmRNA encoding BMP-2 through lipofectamine or metallic particles. In vivo, cmRNA was loaded in lipoids with C12-EPE in a fibrin gel | Magnetofection outperforms lipofectamine in terms of transfection, magnetofection was slightly better than lipofectamine at inducing osteodifferentiation; bone formation significantly improved with cmRNA lipoids based compared to control | 107 |
| Collagen sponge loaded with cmRNA (encoding BMP-2) nanoplexes | MC3T3s, HEK293T, rats | Three 5’ UTR modified cmRNA constructs with lipofectamine lyophilized in sucrose solution loaded into collagen sponge | TISU 5’UTR improved BMP-2 protein secretion in vitro and bone formation in vivo | 108 |
| Perforated collagen matrix loaded with PEI-cmRNA (encoding BMP-9) nanoplexes | Human BMSCs, rats | Perforated collagen matrix loaded with either cmRNA or pDNA encoding BMP-9 complexed with PEI and lyophilized | cmRNA improved ALP expression and calcium deposition in hBMSCs in vitro, however, they were equivalent with respect to promoting bone formation | 110 |
| Collagen sponge loaded with cmRNA encoding BMP-2 or BMP-9 | Human BMSCs, rats | Collagen sponge loaded with PEI-cmRNA nanoplexes encoding BMP-2 or BMP-9 and lyophilized | cmBMP-2 improved ALP expression in hBMSCs in vitro. In vivo, the two genes were not significantly different, however, they both enhanced bone formation in vivo | 111 |
Figure 5:

Collagen sponge scaffolds activated with PEI-cmRNA (encoding BMP-2) nanoplexes were found to enhance bone regeneration in rat calvarial defects. (A) Schematic of cmRNA highlighting specific components: the ARCA 5’ cap (to enhance translation and stability), substitution of uridine and cytidine (to reduce inflammation), and the polyadenylated tail (to enhance stability). (B) Representative μCT scans of rat calvarial defects 4 weeks post-implantation. (C) Quantitative analyses of bone regeneration. Bone volume/total volume was determined by calculating new bone volume within the total volume of the defect and connective density was determined by comparing connectivity within the regenerated bone. Data plots represent mean ± SD (n = 7). Significance indications are as follows: * represents p < 0.1 and ** represents p < 0.01. Adapted with permission from Elangovan et al.103
Khorsand et al. also used cmRNAs independently encoding BMP-2 and BMP-9 to compare their ability to induce osteogenic differentiation in BMSCs in vitro and regenerate bone in 5 mm diameter rat calvarial defects111. The cmRNA was synthesized as described in the previously discussed research by Elangovan et al103. BMSCs transfected with cmRNA encoding BMP-9 showed increased calcium deposition and ALP expression based on alizarin red staining and qPCR analysis, respectively, as compared to BMSCs transfected with cmRNA encoding BMP-9. Four weeks after implanting porous collagen scaffolds activated with either of the two cmRNA species into the rat calvarial defect, analysis with μCT showed that both treatments regenerated more bone than the empty defect (~28% new bone formed for both BMP-2 and BMP-9, 4.5% for empty defect) but were not significantly different from each other111.
Balmayor et al. investigated cmRNA (encoding BMP-2) capped with ARCA and modified by 25% substitution of the cytidine and uridine bases with 2-thiouridine and 5-methyl-cytidine, respectively107. Instead of using PEI as a transfection agent, lipids and iron oxide silica core-shell nanoparticles were used for lipofection and magnetofection, respectively. The in vitro results indicated that magnetofection promoted superior levels of transfection in rat BMSCs and rat adipose-derived MSCs (AMSCs), however, both transfection methods similarly stimulated calcium deposition as indicated by alizarin red staining in the AMSCs. In a rat femoral defect model, histology analysis showed that the addition of 2.5 μg of lipid-cmRNA (encoding BMP-2) loaded in a fibrin gel significantly increased the area of bone formed in the defect as compared to gels lacking the cmRNA.
Instead of investigating other BMPs, Zhang et al. worked on improving the efficacy of cmRNA encoding BMP-2108. Specifically, they tested three different 5’ untranslated regions (UTRs): a normal 5’ UTR, minimal 5’ UTR, and a translation initiator of short 5’ UTR (TISU). The TISU is a sequence contained within the 5’ UTR region of an mRNA that enables translation without ribosomal scanning, so mRNA containing short (~12 base pairs) 5’ UTR regions can be efficiently translated112. The cmRNA possessing the TISU significantly increased BMP-2 secretion by both HEK293 and MC3T3-E1 cells as compared to cmRNAs containing the normal 5’ UTR region or the minimal 5’ UTR region. A significant increase in bone formation was found in a femoral rat defect model 8 weeks after implantation of a scaffold loaded with 15 μg of the cmRNA (encoding BMP-2) possessing the TISU 5’ UTR as compared to defects treated with noncoding cmRNA (~13% new bone volume vs. ~5% for non-coding cmRNA). This work demonstrates that research invested in optimizing cmRNA construct formation may further improve RNA transcript-activated scaffolds designed for bone tissue engineering.
6. RNAi-Activated Scaffolds
In contrast to gene-activated scaffolds, described in prior sections (Sections 3–5), which are designed to promote up-regulation of select proteins, RNAi-activated scaffolds that contain interfering RNAs are designed to modulate expression of targeted proteins at the level of transcription through RNA interference (Figure 3, Route B)45,49,113. Common types of interfering RNAs include microRNAs (miRNAs or miRs), small interfering RNAs (siRNAs), and short hairpin RNAs (shRNAs)114. siRNAs and shRNAs usually operate by targeting a specific mRNA for degradation while miRNAs can affect the expression of more than one gene through a variety of mechanisms that can be cell type dependent45,114,115. Additionally, endogenous miRs can be inhibited by treatment with oligonucleotides (termed “antagomiRs”) that are complementary to the miR targeted for inhibition116. The usual effect of RNAi treatment of cells is the down-regulation in expression of those proteins encoded by the targeted mRNAs. This can have downstream effects on the expression of a range of non-targeted proteins, resulting in phenotypic changes to the cell that also affect the local environment. Findings from recent research on RNAi-activated scaffolds for bone tissue engineering are summarized in Table 4.
Table 4:
Summary of gene-activated scaffolds utilizing interfering RNAs.
| Material | Cell Type/Animal Model | Method of Scaffold Loading | Results | Citation # |
|---|---|---|---|---|
| PLLA fibrous scaffold loaded with PLGA microspheres containing miR-26a-loaded nanoplexes | Mouse osteoblasts, mouse MSCs/mice | miRNA nanoplexes were encapsulated in PLGA microspheres, then microsphere solutions were added to the fibrous PLLA scaffolds | Scaffolds containing slow-releasing miR nanoplexes induced greater ectopic bone formation and greater calvarial bone regeneration than fast-releasing and bolus miR nanoplex treatments | 117 |
| CMCS gel containing miR-21-loaded nanocapsules | human MSCs/rats | CMCS powder was added to miRNA nanocapsule solutions, mixed, then incubated on ice | Injectable scaffolds induced osteogenic differentiation in vitro and promoted greater bone formation in vivo | 121 |
| Silk films loaded with anti-sense miR-214 | human MSCs | Anti-sense miR-214 was mixed with Xfect RNA Transfection Reagent and then added to prepared silk films | Silk scaffolds loaded with miR induced lower viability, higher osteogenic gene expression, and higher calcium deposition in seeded cells | 124 |
| Porous collagen-nHA scaffolds loaded with antagomiR-133a | human MSCs | AntagomiR-133a was complexed with nHA, then mixed with collagen solution before freeze-drying | Cells seeded on antagomiR-133a-loaded scaffolds showed enhanced osteogenic differentiation | 129 |
Zhang et al. investigated using a custom polymer to create nanoplexes with miR-26a that were then encapsulated in PLGA microspheres and loaded onto a PLLA fibrous scaffold117. They chose miR-26a because of prior work citing its osteogenic potential118–120; and they also conducted mechanistic experiments that determined its role in osteogenic differentiation by targeting glycogen synthase kinase-3β, which, upon inhibition, improves bone mass and bone mineral density117. It was found that incorporation of miR-26a nanoplexes encapsulated in slow-releasing PLGA microspheres outperformed fast-releasing scaffolds and those lacking miR-26a in a mouse ectopic bone formation model (~4.3 mm3 vs. ~1.6 mm3 and 0 mm3 for fast releasing and lacking miR-26a, respectively) and bone regeneration in a 5 mm mouse calvarial defect model (~100% vs. ~39% and ~22% for fast releasing and lacking miR26a, respectively)117. In a separate study, Meng et al. created injectable RNAi-activated scaffolds by incorporating nanocapsules containing miR-21 into carboxymethyl chitosan (CMCS)121. They chose miR-21 because of its reported promotion of osteogenic differentiation122,123. The miR-21 was loaded into cationic polymeric nanocapsules using an in situ polymerization process. After mixing the resulting solution with CMCS powder, the gene-activated injectable scaffold was tested in vitro for its ability to induce osteogenic differentiation and in vivo for its ability to regenerate bone in a rat tibial bone defect. In both cases, the scaffolds with encapsulated miR-21 significantly outperformed scaffolds with non-coding miR controls in terms of in vitro mineralization and osteogenic gene expression. In vivo studies demonstrated that the miR-21-activated scaffolds led to superior defect bridging compared to the non-coding controls after 4 weeks (~45% new bone volume vs. ~32% for non-coding controls)121.
Targeting endogenous miRs is another way for interfering RNAs to induce desired effects in targeted cells. James et al. fabricated silk films loaded with anti-sense miR-214 to target endogenous miR-214 for inhibition124. They did this because reports have shown that knocking down miR-214 enhances bone production and osteogenesis, possibly due to it targeting a key transcription factor involved in the differentiation of osteoblasts124–126. They found that seeding human MSCs onto silk scaffolds containing anti-sense miR-214 resulted in lower viability, but higher osteogenic gene expression and greater calcium deposition than the sham and negative controls124. Castaño et al. also chose to pursue inhibition of an endogenous miRNA that inhibits osteogenic differentiation127,128, miR-133a129. To do this, they complexed an inhibitor of miR-133a (antagomiR-133a) with nHA particles that were then incorporated into a collagen-nHA scaffold129. They targeted miR-133a for inhibition because it inhibits the expression of Runx2, which is a mediator of osteogenic differentiation (shown in Figure 1)28,129. Human MSCs seeded onto antagomiR-133a-activated scaffolds showed lower levels of miR-133a expression, higher Runx2 expression, and higher ALP activity, which indicated successful osteogenic differentiation129.
7. Future Directions and Conclusion
It is clear from the numerous studies described here that there are a variety of approaches to gene- and RNAi-activation of scaffolds that can lead to successful in vitro transfection and improved in vivo bone regeneration outcomes. Which approach is best, however, is yet to be determined as research in this area has thus far been focused on proving the functionality of new systems rather than comparing outcomes resulting from these different methods. Future work that directly compares these diverse approaches may lead to a consensus approach that can be used as the standard that all new gene- and RNAi-activated scaffolds can be compared to. Having such a standard would be useful for the field by allowing more accurate comparisons between studies that vary in terms of the species of animal, the type of model, and scaffold material used in these studies.
The gene-activated scaffolds described here are mainly single-gene approaches; with the occasional exceptions where 2 genes have been employed. Only a few of the approaches described here exert any temporal control over the release of the transfecting reagents loaded onto scaffolds. While these approaches have shown success, they are still far from mimicking the numerous time-dependent signals that define natural bone healing27. In vitro work has demonstrated that temporal control over growth factor exposure can lead to improved osteogenic differentiation, yet thus far there have been no attempts to replicate these findings with gene-activated scaffolds. Future work employing gene-activated scaffolds designed to exert temporal control over the expression of specific genes to more closely mimic the natural bone healing process and determine whether that mimicry enhances bone regeneration would be of great value to the field of bone tissue engineering.
Most of the research investigating activating scaffolds with gene-based therapies for bone tissue engineering has been focused on DNA-based approaches, be it with pDNA or viruses comprising DNA genomes. As discussed above (Sections 3 and 4), these approaches require nuclear translocation of the DNA payload to function (Figure 3, Route C). In contrast, the RNA-based approaches described here are active in the cytosol of cells and thus do not require nuclear translocation (Figure 3, Routes A and B). These systems have been relatively under-researched, and further work should be done to capitalize on their cytosolic activity. Research has shown that pDNA-based non-viral gene therapy with nanoplexes may rely upon the cationic polymers to help the pDNA penetrate the nuclear membrane130. An avenue of investigation that should be further explored is the use of novel or modified polymers as vectors for use in gene- and RNAi-activated scaffolds for bone tissue engineering; where such polymers maintain the therapeutic efficacy of classical non-viral vectors while also causing reduced cytotoxicity. Furthermore, the recent FDA approval and demonstration of the efficacy of RNA-based COVID-19 vaccines serves as a testament to the power of RNA transcripts as therapeutic agents. With the international acceptance and application of this technology through those vaccines, it is possible that a bone regenerative therapeutic based on activation of scaffolds with RNA transcripts could be more easily approved than therapeutics activated with viruses.
Finally, future research into scaffolds activated with gene-based therapies for bone tissue engineering should compare the bone regenerative abilities of the systems they develop to the protein-based approaches that they are trying to replace. There is a paucity of direct comparisons to currently available bone regenerative therapeutics that are used in the clinic. Gauging how close gene-activated scaffolds are to the current standard of care could be useful to investigators by providing insight on whether to guide research towards improving protein production, targeting specific cells, modifying release profiles, or combinations thereof.
The studies described here are evidence of the immense potential of gene- and RNAi-activated scaffolds for bone tissue engineering. While these scaffold systems are not yet ready to replace grafts and implants as therapies for critical-sized bone defects, progress is being made towards translating this technology into a new class of bone regenerative therapeutics. Differentiation of cells in vitro with these strategies is now the norm, and focus has shifted to in vivo results. Greater acceptance of gene-based therapeutics by the public and regulatory organizations also seems likely and will hopefully spur more progress.
Acknowledgements
NZL acknowledges support from the Graduate College Iowa Recruitment Fellowship and the Graduate College Post-Comprehensive Research Fellowship. TMA acknowledges support from the Ballard and Seashore Dissertation Fellowship. AKS acknowledges support from the Lyle and Sharon Bighley Chair of Pharmaceutical Sciences and the National Cancer Institute at the National Institutes of Health P30 CA086862 Cancer Center support grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Nauth A, Schemitsch E, Norris B, Nollin Z & Watson JT Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? Journal of Orthopaedic Trauma 32, S7 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Keating JF, Simpson AHRW & Robinson CM The management of fractures with bone loss. The Journal of Bone and Joint Surgery. British volume 87-B, 142–150 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Panetta NJ et al. Tissue Engineering in Cleft Palate and Other Congenital Malformations. Pediatric Research 63, 545–551 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Schemitsch EH Size Matters: Defining Critical in Bone Defect Size! J Orthop Trauma 31 Suppl 5, S20–S22 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Roddy E, DeBaun MR, Daoud-Gray A, Yang YP & Gardner MJ Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol 28, 351–362 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Myeroff C & Archdeacon M Autogenous Bone Graft: Donor Sites and Techniques. The Journal of Bone & Joint Surgery 93, 2227–2236 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R & García AJ Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Advanced Drug Delivery Reviews 94, 53–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw N et al. Regenerative Medicine Approaches for the Treatment of Pediatric Physeal Injuries. Tissue Eng Part B Rev 24, 85–97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanchareonsook N, Junker R, Jongpaiboonkit L & Jansen JA Tissue-Engineered Mandibular Bone Reconstruction for Continuity Defects: A Systematic Approach to the Literature. Tissue Eng Part B Rev 20, 147–162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira AM, Gentile P, Chiono V & Ciardelli G Collagen for bone tissue regeneration. Acta Biomaterialia 8, 3191–3200 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Dong C & Lv Y Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers (Basel) 8, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Du T, Ruan C & Niu X Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact Mater 6, 1491–1511 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorushanova A et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Advanced Materials 31, 1801651 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Heo DN, Hospodiuk M & Ozbolat IT Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater 95, 348–356 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Elangovan S et al. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 35, 737–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha A, Allen BN, Wiley LA, Tucker BA & Worthington KS Development of High-Resolution Three-Dimensional-Printed Extracellular Matrix Scaffolds and Their Compatibility with Pluripotent Stem Cells and Early Retinal Cells. J Ocul Pharmacol Ther 36, 42–55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fratzl P, Gupta HS, Paschalis EP & Roschger P Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem 14, 2115–2123 (2004). [Google Scholar]
- 18.Acri T et al. Non-viral gene delivery embedded in biomimetically mineralized matrices for bone tissue engineering. Tissue Eng Part A (2020) doi: 10.1089/ten.TEA.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkineni AR et al. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomaterialia 27, 264–274 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Carino A, Ludwig C, Cervellino A, Müller E & Testino A Formation and transformation of calcium phosphate phases under biologically relevant conditions: Experiments and modelling. Acta Biomaterialia 74, 478–488 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Levingstone TJ, Herbaj S & Dunne NJ Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials (Basel) 9, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih Y-RV et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. PNAS 111, 990–995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HD et al. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Advanced Healthcare Materials 6, 1700612 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Tatsuyama K, Maezawa Y, Baba H, Imamura Y & Fukuda M Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem 44, 269–278 (2000). [PubMed] [Google Scholar]
- 25.Sathyendra V & Darowish M Basic science of bone healing. Hand Clin 29, 473–481 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Einhorn TA & Gerstenfeld LC Fracture healing: mechanisms and interventions. Nature Reviews Rheumatology 11, 45–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hankenson KD, Gagne K & Shaughnessy M Extracellular signaling molecules to promote fracture healing and bone regeneration. Advanced Drug Delivery Reviews 94, 3–12 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Wu M, Chen G & Li Y-P TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research 4, 1–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detsch R et al. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J Biomed Mater Res A 102, 4055–4061 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Ong KL et al. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976) 35, 1794–1800 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi H et al. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. Journal of Bone and Mineral Research 25, 2735–2743 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Mehta M, Schmidt-Bleek K, Duda GN & Mooney DJ Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Advanced Drug Delivery Reviews 64, 1257–1276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aryal R, Chen X, Fang C & Hu Y Bone morphogenetic protein-2 and vascular endothelial growth factor in bone tissue regeneration: new insight and perspectives. Orthop Surg 6, 171–178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P et al. Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab 32, 627–635 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Bai Y et al. BMP-2, VEGF and bFGF synergistically promote the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Biotechnol. Lett 35, 301–308 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Atluri K, Seabold D, Hong L, Elangovan S & Salem AK Nanoplex-Mediated Codelivery of Fibroblast Growth Factor and Bone Morphogenetic Protein Genes Promotes Osteogenesis in Human Adipocyte-Derived Mesenchymal Stem Cells. Mol Pharm 12, 3032–3042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Silva EA & Mooney DJ Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8, 153–170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James AW et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 22, 284–297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannoury CA & An HS Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14, 552–559 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Woo EJ Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg 70, 765–767 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Carragee EJ, Hurwitz EL & Weiner BK A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11, 471–491 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Mroz TE, Wang JC, Hashimoto R & Norvell DC Complications Related to Osteobiologics Use in Spine Surgery: A Systematic Review. Spine 35, S86 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Zara JN et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng Part A 17, 1389–1399 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolk A et al. Comparative analysis of bone regeneration behavior using recombinant human BMP-2 versus plasmid DNA of BMP-2. J Biomed Mater Res A 107, 163–173 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Leng Q, Chen L & Lv Y RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics 10, 3190–3205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans CH Gene delivery to bone. Adv Drug Deliv Rev 64, 1331–1340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lv H, Zhang S, Wang B, Cui S & Yan J Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release 114, 100–109 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Ährlund-Richter L et al. Isolation and Production of Cells Suitable for Human Therapy: Challenges Ahead. Cell Stem Cell 4, 20–26 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Yin H et al. Non-viral vectors for gene-based therapy. Nature Reviews Genetics 15, 541–555 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Hall A, Lächelt U, Bartek J, Wagner E & Moghimi SM Polyplex Evolution: Understanding Biology, Optimizing Performance. Molecular Therapy 25, 1476–1490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM & Andresen TL The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Molecular Therapy 21, 149–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen RN, van der Aa MAEM, Macaraeg N, Lee AP & Szoka FC Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. Journal of Controlled Release 135, 166–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grandinetti G & Reineke TM Exploring the Mechanism of Plasmid DNA Nuclear Internalization with Polymer-based Vehicles. Mol Pharm 9, 2256–2267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khorsand B et al. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J Control Release 248, 53–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khorsand B et al. A Multi-Functional Implant Induces Bone Formation in a Diabetic Model. Adv Healthc Mater 9, e2000770 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Malek-Khatabi A et al. In situ bone tissue engineering using gene delivery nanocomplexes. Acta Biomaterialia 108, 326–336 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Lutolf MP et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. PNAS 100, 5413–5418 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lackington WA et al. Non-viral Gene Delivery of Interleukin-1 Receptor Antagonist Using Collagen-Hydroxyapatite Scaffold Protects Rat BM-MSCs From IL-1β-Mediated Inhibition of Osteogenesis. Front Bioeng Biotechnol 8, 582012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun G et al. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clinical and Experimental Pharmacology and Physiology 44, 455–462 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Claes L, Recknagel S & Ignatius A Fracture healing under healthy and inflammatory conditions. Nature Reviews Rheumatology 8, 133–143 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Martino MM et al. Inhibition of IL-1R1/MyD88 signalling promotes mesenchymal stem cell-driven tissue regeneration. Nat Commun 7, 11051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raftery RM et al. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 149, 116–127 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Raftery RM et al. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J Control Release 283, 20–31 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Raftery RM et al. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: From development to application in tissue engineering. Biomaterials 216, 119277 (2019). [DOI] [PubMed] [Google Scholar]
- 65.D’Mello S, Elangovan S & Salem AK FGF2 gene activated matrices promote proliferation of bone marrow stromal cells. Arch Oral Biol 60, 1742–1749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D’Mello SR et al. A Pilot Study Evaluating Combinatorial and Simultaneous Delivery of Polyethylenimine-Plasmid DNA Complexes Encoding for VEGF and PDGF for Bone Regeneration in Calvarial Bone Defects. Curr Pharm Biotechnol 16, 655–660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Cao L, Fan M & Xu Q Direct Pulp Capping with Calcium Hydroxide or Mineral Trioxide Aggregate: A Meta-analysis. Journal of Endodontics 41, 1412–1417 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Torabinejad M, Parirokh M & Dummer PMH Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview – part II: other clinical applications and complications. International Endodontic Journal 51, 284–317 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Chakka LRJ et al. Application of BMP-2/FGF-2 gene-activated scaffolds for dental pulp capping. Clinical Oral Investigations (2020) doi: 10.1007/s00784-020-03308-2. [DOI] [PubMed] [Google Scholar]
- 70.Pham QP, Sharma U & Mikos AG Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Engineering 12, 1197–1211 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Liang D, Hsiao BS & Chu B Functional electrospun nanofibrous scaffolds for biomedical applications. Advanced Drug Delivery Reviews 59, 1392–1412 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pankongadisak P, Tsekoura E, Suwantong O & Uludağ H Electrospun gelatin matrices with bioactive pDNA polyplexes. International Journal of Biological Macromolecules 149, 296–308 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Xie Q et al. Fabrication of Core-Shell PEI/pBMP2-PLGA Electrospun Scaffold for Gene Delivery to Periodontal Ligament Stem Cells. Stem Cells Int 2016, 5385137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sokolova V & Epple M Inorganic Nanoparticles as Carriers of Nucleic Acids into Cells. Angewandte Chemie International Edition 47, 1382–1395 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Maitra A Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Review of Molecular Diagnostics 5, 893–905 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Donahue ND, Acar H & Wilhelm S Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Advanced Drug Delivery Reviews 143, 68–96 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Tenkumo T et al. Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater Sci Eng C Mater Biol Appl 92, 172–183 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Lee Y-H et al. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater 63, 210–226 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Liang Z, Luo Y & Lv Y Mesenchymal stem cell-derived microvesicles mediate BMP2 gene delivery and enhance bone regeneration. Journal of Materials Chemistry. B 8, 6378–6389 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Nomikou N et al. Ultrasound-responsive gene-activated matrices for osteogenic gene therapy using matrix-assisted sonoporation. J Tissue Eng Regen Med 12, e250–e260 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Bozo IY et al. 3D Printed Gene-activated Octacalcium Phosphate Implants for Large Bone Defects Engineering. Int J Bioprint 6, 275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friedmann T A brief history of gene therapy. Nature Genetics 2, 93–98 (1992). [DOI] [PubMed] [Google Scholar]
- 83.Ghosh S, Brown AM, Jenkins C & Campbell K Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl Biosaf. 25, 7–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flotte TR Gene Therapy Progress and Prospects: Recombinant adeno-associated virus (rAAV) vectors. Gene Therapy 11, 805–810 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Montini E et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nature Biotechnology 24, 687–696 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Guan M et al. Increased Efficacy and Safety in the Treatment of Experimental Liver Cancer with a Novel Adenovirus-Alphavirus Hybrid Vector. Cancer Res 66, 1620–1629 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Robinson M, Schor S, Barouch-Bentov R & Einav S Viral journeys on the intracellular highways. Cell Mol Life Sci 75, 3693–3714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medina-Kauwe LK, Xie J & Hamm-Alvarez S Intracellular trafficking of nonviral vectors. Gene Therapy 12, 1734–1751 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Kotterman MA, Chalberg TW & Schaffer DV Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng 17, 63–89 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Hacein-Bey-Abina S et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 118, 3132–3142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mingozzi F & High KA Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122, 23–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y et al. Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone 51, 704–713 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Ma Y, Wu C, Miron RJ & Cheng X Platelet-derived growth factor BB gene-released scaffolds: biosynthesis and characterization. J Tissue Eng Regen Med 10, E372–E381 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y et al. Novel MesoPorous BioGlass/silk scaffold containing adPDGF-B and adBMP7 for the repair of periodontal defects in beagle dogs. J Clin Periodontol 42, 262–271 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Xue J et al. One-Step Fabrication of Bone Morphogenetic Protein-2 Gene-Activated Porous Poly-L-Lactide Scaffold for Bone Induction. Mol Ther Methods Clin Dev 7, 50–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rowland CR et al. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 177, 161–175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu W-W, Wang Z & Krebsbach PH Virus immobilization on biomaterial scaffolds through biotin-avidin interaction for improving bone regeneration. J Tissue Eng Regen Med 10, E63–72 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y et al. Application of HIF-1α by gene therapy enhances angiogenesis and osteogenesis in alveolar bone defect regeneration. J Gene Med 18, 57–64 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Koç A, Finkenzeller G, Elçin AE, Stark GB & Elçin YM Evaluation of adenoviral vascular endothelial growth factor-activated chitosan/hydroxyapatite scaffold for engineering vascularized bone tissue using human osteoblasts: In vitro and in vivo studies. J Biomater Appl 29, 748–760 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Meo SA, Bukhari IA, Akram J, Meo AS & Klonoff DC COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. European Review for Medical and Pharmacological Sciences 25, 1663–1669 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Karikó K, Buckstein M, Ni H & Weissman D Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 23, 165–175 (2005). [DOI] [PubMed] [Google Scholar]
- 102.Kormann MSD et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nature Biotechnology 29, 154–157 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Elangovan S et al. Chemically Modified RNA Activated Matrices Enhance Bone Regeneration. J Control Release 218, 22–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stepinski J, Waddell C, Stolarski R, Darzynkiewicz E & Rhoads RE Synthesis and properties of mRNAs containing the novel ‘anti-reverse’ cap analogs 7-methyl(3’-O-methyl)GpppG and 7-methyl (3’-deoxy)GpppG. RNA 7, 1486–1495 (2001). [PMC free article] [PubMed] [Google Scholar]
- 105.Vaidyanathan S et al. Uridine Depletion and Chemical Modification Increase Cas9 mRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol Ther Nucleic Acids 12, 530–542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo PX & Moss B Interaction and mutual stabilization of the two subunits of vaccinia virus mRNA capping enzyme coexpressed in Escherichia coli. Proc Natl Acad Sci U S A 87, 4023–4027 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balmayor ER et al. Chemically modified RNA induces osteogenesis of stem cells and human tissue explants as well as accelerates bone healing in rats. Biomaterials 87, 131–146 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Zhang W et al. An Improved, Chemically Modified RNA Encoding BMP-2 Enhances Osteogenesis In Vitro and In Vivo. Tissue Eng Part A 25, 131–144 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Balmayor ER et al. Modified mRNA for BMP-2 in Combination with Biomaterials Serves as a Transcript-Activated Matrix for Effectively Inducing Osteogenic Pathways in Stem Cells. Stem Cells Dev 26, 25–34 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Khorsand B, Elangovan S, Hong L, Kormann MSD & Salem AK A bioactive collagen membrane that enhances bone regeneration. J Biomed Mater Res B Appl Biomater 107, 1824–1832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khorsand B et al. A Comparative Study of the Bone Regenerative Effect of Chemically Modified RNA Encoding BMP-2 or BMP-9. AAPS J 19, 438–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elfakess R et al. Unique translation initiation of mRNAs-containing TISU element. Nucleic Acids Res 39, 7598–7609 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Curtin CM, Castaño IM & O’Brien FJ Scaffold-Based microRNA Therapies in Regenerative Medicine and Cancer. Advanced Healthcare Materials 7, 1700695 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Setten RL, Rossi JJ & Han S The current state and future directions of RNAi-based therapeutics. Nature Reviews Drug Discovery 18, 421–446 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Pritchard CC, Cheng HH & Tewari M MicroRNA profiling: approaches and considerations. Nat Rev Genet 13, 358–369 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krützfeldt J et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Li Y, Chen YE, Chen J & Ma PX Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun 7, 10376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y et al. The promotion of bone regeneration through positive regulation of angiogenic–osteogenic coupling using microRNA-26a. Biomaterials 34, 5048–5058 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Basak Icli et al. MicroRNA-26a Regulates Pathological and Physiological Angiogenesis by Targeting BMP/SMAD1 Signaling. Circulation Research 113, 1231–1241 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim K et al. MicroRNA-26a regulates RANKL-induced osteoclast formation. Mol Cells 38, 75–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meng Y et al. An injectable miRNA-activated matrix for effective bone regeneration in vivo. J Mater Chem B 4, 6942–6954 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Mei Y et al. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem 114, 1374–1384 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Meng Y-B et al. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J Orthop Res 33, 957–964 (2015). [DOI] [PubMed] [Google Scholar]
- 124.James EN, Van Doren E, Li C & Kaplan DL Silk Biomaterials-Mediated miRNA Functionalized Orthopedic Devices. Tissue Eng Part A 25, 12–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J et al. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Sci Rep 7, 40487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang X et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med 19, 93–100 (2013). [DOI] [PubMed] [Google Scholar]
- 127.Liao X-B et al. MiR-133a Modulates Osteogenic Differentiation of Vascular Smooth Muscle Cells. Endocrinology 154, 3344–3352 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Li Z et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. PNAS 105, 13906–13911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castaño IM, Curtin CM, Duffy GP & O’Brien FJ Next generation bone tissue engineering: non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis. Sci Rep 6, 27941 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grandinetti G, Smith AE & Reineke TM Membrane and nuclear permeabilization by polymeric pDNA vehicles: efficient method for gene delivery or mechanism of cytotoxicity? Mol Pharm 9, 523–538 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


