Abstract
Early life adversity can set the trajectory for later psychiatric disorders, including substance use disorders. There are a host of neurobiological factors that may play a role in the negative trajectory. The current review examines preclinical evidence suggesting that early life adversity specifically involving social factors (maternal separation, adolescent social isolation and adolescent social defeat) may influence drug abuse vulnerability by strengthening corticotropin-releasing factor (CRF) systems and weakening oxytocin (OT) systems. In adulthood, pharmacological and genetic evidence indicates that both CRF and OT systems are directly involved in drug reward processes. With early life adversity, numerous studies show an increase in drug abuse vulnerability measured in adulthood, along a concomitant strengthening of CRF systems and a weakening of OT systems. Mechanistic studies, while relatively few in number, are generally consistent with the theme that strengthened CRF systems and weakened OT systems mediate, at least in part, the link between early life adversity and drug abuse vulnerability. Establishing a direct role of CRF and OT in mediating the relation between early life social stressors and drug abuse vulnerability will inform clinical researchers and practitioners toward the development of intervention strategies to reduce risk among those suffering from early life adversities.
Keywords: Maternal separation, social isolation, social defeat, corticotropin-releasing factor, oxytocin, conditioned place preference, self-administration
Introduction
Early life events involving adverse social experiences may alter neuronal development and drive the behavioral expression of increased drug abuse vulnerability. This review focuses on preclinical models of maternal separation, social isolation, and social defeat as key models of early life adversity that have an extensive literature base. While each of these developmental models undoubtedly involve multiple neural mechanisms, we will focus on corticotropin-releasing factor (CRF) and oxytocin (OT) systems as key mediating mechanisms for driving increased risk in adulthood. CRF is chosen because early life adversity is thought to activate stress systems, while OT is chosen because of its known role in maternal behavior, pair bonding, social reward, and social recognition. Moreover, clinical literature suggests that early life problems with social attachment alter CRF and OT systems that interact with reward-relevant dopamine pathways involved in addiction (Strathearn et al., 2019).
The main theme to be advanced is that early life adversity increases drug abuse vulnerability later in life because such adversity causes, at least in part, a strengthening of CRF systems and a weakening of OT systems. The oppositional effect of early life adversity on these two neuropeptides is consistent with evidence pointing to an inverse relationship between CRF and OT systems (Neal et al., 2018), with CRF activation triggering the hypothalamic-pituitary-adrenal (HPA) stress axis and OT activation having a buffering effect on this activation (Heinrichs et al., 2003; Smith and Wang, 2014). The sections of the review are organized as follows: First, we establish in adults that CRF activation increases and OT activation decreases drug abuse vulnerability. Second, after briefly describing the normal development of CRF and OT systems, we show that early life adversity strengthens CRF systems and weakens OT systems measured in adulthood. Third, we show that these same early life adverse events increase drug abuse vulnerability assessed in adulthood. Finally, we discuss some key studies that have directly examined the specific mechanistic role of CRF and OT changes which link early life adversity to altered drug abuse vulnerability. Emphasis is placed on preclinical research, with an eye toward translation to humans, including how early life adversity alters the development of CRF and OT systems leading to exaggerated vulnerability in the expression of substance use disorders. Some notable gaps in the literature are identified as prompts for future research.
2. Role of CRF and OT in drug abuse vulnerability in adulthood
2.1. CRF
While a full review of CRF is beyond the scope of this review, we will provide a brief overview of its function [e.g., see (Heck et al., 2018)]. CRF is a 41-amino acid peptide that initiates activation of the HPA stress axis via stimulation of adrenocorticotropic hormone (ACTH) and is intimately involved in social behavior (Hostetler and Ryabinin, 2013) and drug abuse vulnerability (Zorrilla et al., 2014). CRF neurons within the paraventricular nucleus (PVN) of the hypothalamus (Hypo) control ACTH secretion from the anterior pituitary (Pit). Hypothalamic and extra-hypothalamic CRF systems also affect numerous other regions, such as the cortex (Ctx), hippocampus (Hipp), and amygdala (Amyg), as well as midbrain and brainstem structures (Figure 1). The CRF system consists of two primary receptor subtypes (CRF1 and CRF2), and at least 5 endogenous ligands (CRF, urocortin1, urocortin2, urocortin3 and CRFCRF-binding protein). The distribution of CRF receptors and ligands are heterogenous throughout the brain and there are few pharmacological tools that have high selectivity in manipulating a single ligand-system to determine their precise role in social behavior and drug reward. Among the various neuropeptides involved in drug reward, CRF has received considerable attention due to its known ability to modulate mesolimbic dopamine reward systems (Kelly and Fudge, 2018; Sinha, 2008).
Figure 1:
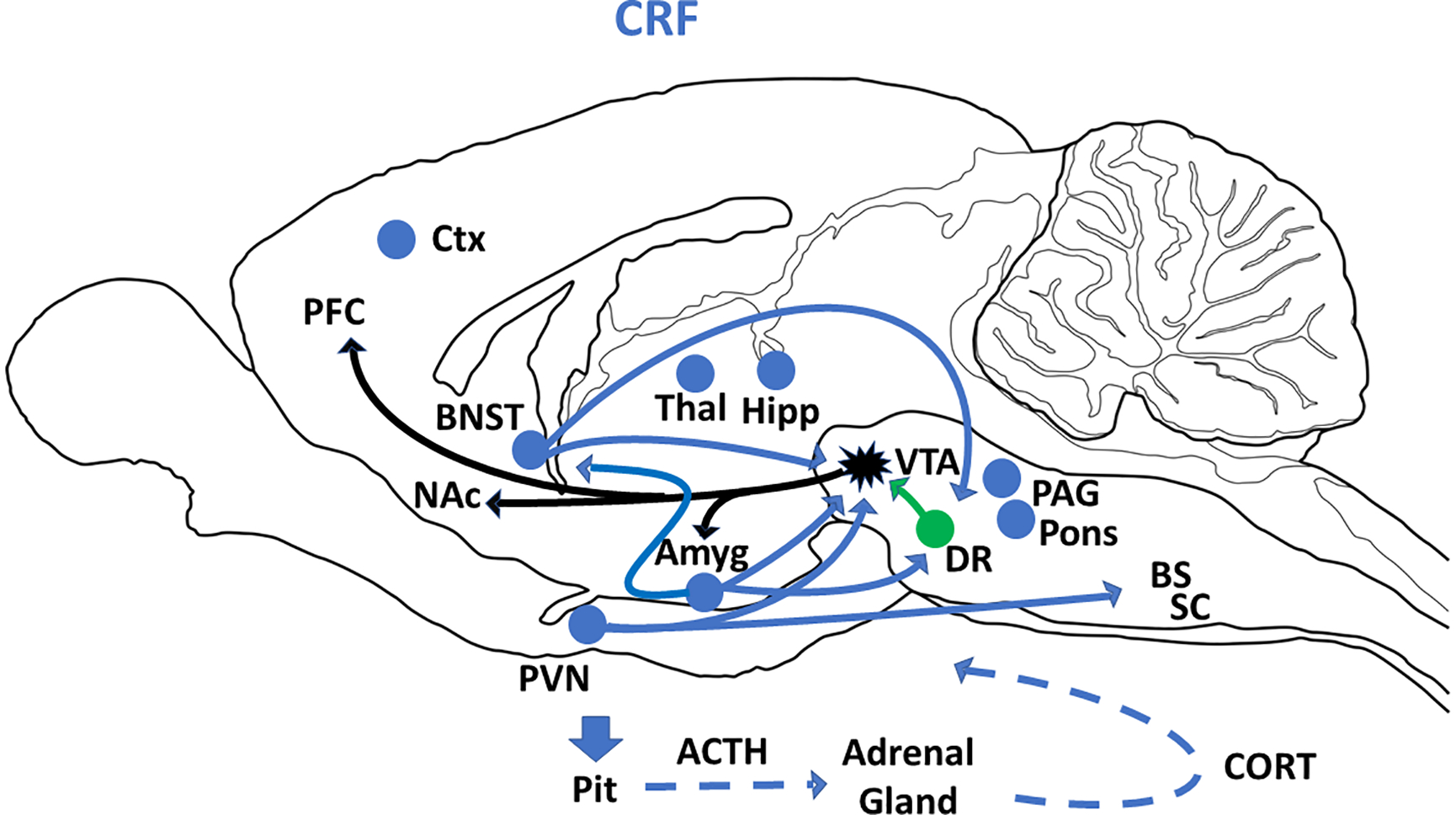
Schematic summary of CRF cell bodies (blue circles) and CRF projections (blue arrows) in sagittal plane of rat brain. Blue circles without arrows identify possible additional efferents to VTA and blue dashed arrows represent blood circulation. The black projections from VTA represent dopamine neurons and green projections from DR represent serotonergic neurons. Adapted from the following references: (Forster et al., 2018; Kelly and Fudge, 2018; Kim et al., 2017). Abbreviations: ACTH=adrenocorticotropic hormone; Amyg=amygdala; BNST=bed nucleus of the stria terminalis; BS=brainstem; CORT=corticosterone; Ctx=cortex; DR=dorsal raphe; Hipp=hippocampus; NAc=nucleus accumbens; PAG=periaqueductal gray; PFC=prefrontal cortex; Pit=pituitary gland; PVN=paraventricular nucleus of hypothalamus; SC=spinal cord; Thal=thalamus; VTA=ventral tegmental area.
There are a host of studies in adult animals indicating that activation of CRF systems enhances drug reward processes, including reward associated with stimulants, opioids and alcohol (Table 1). With stimulants, the acquisition of conditioned place preference (CPP) produced by cocaine is enhanced by pretreatment with CRF and weakened by pretreatment with the non-selective α-helical CRF antagonist (Lemos et al., 2020). Differential blockade of CRF1 and CRF2 receptors indicates that the effect on cocaine CPP involves primarily CRF1 receptors (Lu et al., 2003). Similarly, CRF1 antagonists are able to decrease cocaine self-administration (SA) in rats at doses that do not alter responding for a non-drug rewards (Nick and Glenn, 2000; Specio et al., 2008); however, this effect does not appear to generalize to monkeys (Broadbear et al., 1999; Mello et al., 2006). In adult rats, CRF1 receptor antagonists are also able to block the increase in cocaine CPP and SA that occurs following social defeat stress (Boyson et al., 2011). Similar to the effects observed with cocaine reward, CRF1 receptor antagonists are able to decrease acquisition of morphine CPP (Lasheras et al., 2015) and SA of heroin (Greenwell et al., 2009; Park et al., 2015).
Table 1:
Summary of studies examining the effect of CRF agonists and antagonists on drug reward measured by either CPP or SA.
| Reference | Species | Sex | Treatment | Route | Dose | Test | Result |
|---|---|---|---|---|---|---|---|
| Lemos et al., 2020 | Sprague Dawley rats | Male | 1) α-helical CRF 2) CRF |
i.c.v. | 1) 10 ug 2) 1 ug |
CPP Cocaine (15 mg/kg) |
1) ↓ acquisition 2) ↑ acquisition |
| Lu et al., 2003 | Sprague- Dawley rats | Male | 1) α-helical CRF 2) CP-154,526 (CRF1 antag) 3) AS-30 (CRF2 antag) |
i.c.v. | 1) 1 or 10 pg 2) 1 or 3 pg 3) 1 or 3 pg |
CPP Cocaine (10 mg/kg) |
1) ↓ acquisition 2) ↓ acquisition 3) ↔ acquisition |
| Specio et al., 2008 | Wister rats | Male | 1) Antalarmin (CRF1 antag) 2) MPZP (CRF1 antag) |
1) ip. 2) s.c. |
1) 6–25 mg/kg 2) 3–27 mg/kg |
SA Cocaine (0.6 mg/kg/inf) 1- or 6-hr sessions |
1) ↔ intake 1-hr ↓ intake 6-hr 2) ↓ intake 1-hr ↓ intake 6-hr |
| Mello et al., 2006 | Rhesus monkeys | Female and male | Antalarmin (CRF1 antag) | i.v. | 1–10 mg/kg | SA Cocaine (0.001–0.1 mg/kg/inf) |
↔ intake |
| Przegalinski et al., 2005 | Wistar rats | Male | CP 154,526 (CRF1 antag) | ip. | 10–20 mg/kg | SA Cocaine (0.5 mg/kg) |
↔ intake |
| Broadbear et al., 1999 | Rhesus monkeys | Male | Astressin (CRF1 antag) | i.v. | 0.1–1.0 mg/kg | SA Cocaine (0.03 mg/kg/inf) |
↔ intake |
| Goeders and Guerin, 2000 | Wistar rats | Male | CP 154,526 (CRF1 antag) | ip. | 10–40 mg/kg | SA Cocaine (0.125–0.5 mg/kg/inf) |
↓ intake ↔ food intake |
| Lasheras et al., 2014 | Swiss mice | Male | CP 154,526 (CRF1 antag) | ip. | 30 mg/kg | CPP Morphine (6 mg/kg) |
↓ acquisition |
| Park et al., 2015 | Wistar rats | Male | MPZP (CRF1 antag) | s.c. | 20 mg/kg | SA Heroin (0.06 mg/kg/infusion) |
↓ intake (escalation) |
| Greenwell et al., 2009 | Wistar rats | Male | 1) MJL-1–109-2 (CRF1 antag) 2) R121919 (CRF1 antag) |
1) ip. 2) s.c. |
1) 1.25–10 mg/kg 2) 5–20 mg/kg |
SA Heroin (0.06 mg/kg/inf) 1) 1- or 8-hr sessions 2) 1- or 12-hr sessions |
1) ↓ first-hr intake in 8-hr group 2) ↓ first-hr and total intake 12-hr group |
| Robinson et al., 2019 | C57Bl/6J mice | Female and male | 1) Antalarmin 2) NBI 35965 (CRF1 antag) 3) Ucn3 (CRF2 agonist) 4) K41498 (CrF 2 antag) |
Intra-mPFC | 1) 1 ug 2) 30 pmol 3) 60 pmol 4) 50 pmol |
SA Alcohol (20 %) drink in dark |
1) ↓ intake 2) ↓ intake 3) ↓ intake 4) ↔ intake 2)+4) combination reduces effect of 2) alone |
| Giardino and Ryabinin, 2013 | C57BL/6J mice | Male | 1) CP376395 (CRF1 antag) 2) NBI27914 (CRF1 antag) |
ip. | 1) 10 or 20 mg/kg 2) 10 or 30 mg/kg |
SA Alcohol (15%) 2-bottle choice w/ drink in dark |
1) ↓ intake 2) ↓ intake |
| Hwa et al., 2013 | C57BL/6J mice and Long Evans rats | Male | CP 154,526 (CRF1 antag) | 1) Intra-VTA 2) intra-DR |
0.3 and 0.6 pg | SA Alcohol (20%) Intermittent 2-bottle choice |
1) ↓ intake 2) ↓ intake, except low preferring rats |
| Lowery et al., 2010 | C57BL/6J mice | Male | 1) α-helical CRF (non selective CRF antag) 2) Ucn3 (CrF 2 agonist) 3) CP 154, 526 (CRF1 antag) |
1) i.c.v 2) i.c.v. 3) ip. |
1) 1–10 μg 2) 0.05–0.5 μg 3) 1–10 mg/kg |
SA alcohol (20 %) drink in dark |
1) ↓ intake (1 pg only) 2) ↓ intake 3) ↓ intake, also w/adrenalectomy |
Abbreviations: CPP=conditioned place preference; CRF=corticotropin-releasing factor; i.c.v.=intracerebroventricular; i.p.=intraperitoneal; i.v.=intravenous; mPFC=medial prefrontal cortex; SA=self-administration; s.c.=subcutaneous.
The pharmacological evidence suggesting CRF activation enhances stimulant reward is corroborated by other studies using direct genetic manipulations. For example, genetic deletion of CRF1 receptors in mice decreases acquisition of cocaine CPP, although this effect is only observed when a high dose of cocaine (20 mg/kg) is used (Contarino et al., 2017). In another study, overexpression of CRF by an adeno-associated virus microinjected directly into nucleus accumbens (NAc) increases nicotine SA in rats (Uribe et al., 2020). Interestingly, in this latter study, the ability of CRF overexpression to enhance the reinforcing effectiveness of nicotine was modulated by ovarian hormones, as females showed a greater effect of CRF overexpression on nicotine SA than males and this sex difference was negated by ovariectomy.
Perhaps the most compelling evidence for CRF-induced enhancement in drug reward is compiled from studies examining alcohol. Pretreatment with a CRF1 receptor antagonist reliably decreases alcohol SA in both rats and mice (Giardino and Ryabinin, 2013; Hwa et al., 2013; Lowery et al., 2010; Robinson et al., 2019). This effect occurs across different alcohol SA paradigms (drinking in the dark, 2-bottle choice, lever pressing with vapor exposure) and across different routes of administration, including systemically, intracerebroventricular (i.c.v.) or directly into medial prefrontal cortex (mPFC), central nucleus of Amyg (CeA), dorsal raphe (DR) and ventral tegmental area (VTA). In contrast, pretreatment with a CRF2 receptor antagonist has no effect on alcohol SA (Robinson et al., 2019); instead, an urocortin3 CRF2 receptor agonist decreases alcohol SA. Thus, results from pharmacological studies suggest that CRF1 receptors, rather than CRF2 receptors, mediate alcohol reward, consistent with the role of CRF1 receptors in stimulant reward.
These pharmacological results are bolstered by other studies using genetic manipulations. Genetic polymorphisms of the Crf1 gene are associated with patterns of alcohol binge drinking in human adolescents (Treutlein et al., 2006) and genetic knockout of CRF1 receptors decreases alcohol SA in mice (Giardino and Ryabinin, 2013). Alcohol SA is also reduced by optogenetic inactivation of CRF-containing neurons in CeA (Giordano de et al., 2019). Genetically-derived CRF deficient mice also show reduced sensitivity to alcohol, displayed as a reduction in alcohol CPP and a compensatory increase in alcohol SA using 2-bottle choice (Olive et al., 2003). Conversely, CRF overexpression may enhance sensitivity to alcohol, displayed as a compensatory decrease in alcohol SA using 2-bottle choice (Palmer et al., 2004).
CRF regulation of the HPA stress axis also plays a key role in withdrawal following long-term drug exposure (Kreek and Koob, 1998). With acute exposure, cortisol levels are increased by alcohol (Mendelson et al., 1971), nicotine (Kirschbaum et al., 1992), and cocaine (Heesch et al., 1995). However, the HPA axis adapts to chronic nicotine exposure by increasing basal tone and results in more intense withdrawal during early abstinence (Wemm and Sinha, 2019), likely resulting from nicotine’s ability to activate the HPA axis via release of CRF and norepinephrine in PVN (Fu et al., 1997; Matta et al., 1990; Okada et al., 2003). Similarly, opioids appear to alter the HPA axis via a change in CRF regulation. For example, former heroin users on methadone maintenance express ACTH plasma levels equal to normal male volunteers after placebo, but they express significantly higher ACTH plasma levels after the administration of high dose of CRF (Schluger et al., 2003). In rodents, i.c.v. administration of a CRF2 receptor antagonist during morphine withdrawal produces a decrease in somatic naloxone-induced withdrawal symptoms (Navarro-Zaragoza et al., 2011). Further, infusion of a CRF2 receptor antagonist also prevents cocaine-induced corticosterone (CORT) release in rodents (Sarnyai et al., 1992). Thus, while CRF1 receptors appear to mediate drug reward, there may be a role for CRF2 receptors in mediating the effects of drug withdrawal on the HPA axis.
Beyond the HPA axis, the bed nucleus of the stria terminalis (BNST) is also an important component of the stress system involved in drug reward and withdrawal. CRF neurons in the BNST receive dopamine signals from the VTA and periaqueductal gray [PAG; (Meloni et al., 2006)]. Cocaine, nicotine, morphine and alcohol all dose-dependently increase extracellular dopamine in the BNST (Carboni et al., 2000). Antagonism of BNST dopamine D1 receptors decreases both alcohol and sucrose SA (Eiler et al., 2003). The BNST sends reciprocal CRF, gamma-aminobutyric acid (GABA), and glutamate signals to VTA dopamine and GABA neurons (Dong and Swanson, 2006; Georges and Aston-Jones, 2001, 2002; Vranjkovic et al., 2017). These BNST CRF neurons play a role in drug abuse vulnerability, rather than local VTA CRF neurons (Vranjkovic et al., 2017). Withdrawal from cocaine, heroin, or alcohol can produce CRF-mediated dysregulation of BNST neurons (Francesconi et al., 2009). Similarly, intra-BNST CRF prior to alcohol exposure increases anxiety associated with alcohol withdrawal, whereas an intra-BNST CRF1 receptor antagonist prevents the increase in alcohol withdrawal-induced anxiety (Huang et al., 2010). These studies indicate that stress-induced CRF release in VTA and BNST may potentiate withdrawal, making it a novel target for pharmaceutical treatments of substance use disorders.
Additional studies suggest that CRF may potentiate stress-induced relapse. Animals with a history of extended access to cocaine reinstate to CRF when it is administered directly into VTA bilaterally (Blacktop et al., 2011). The administration of a CRF1 receptor antagonist decreases alcohol seeking following footshock-induced reinstatement (Lê et al., 2000). Similarly, CRF antagonists have been shown to attenuate stress-induced reinstatement of CPP following either cocaine or morphine (Lu et al., 2000; Lu et al., 2001). CRF1 receptor antagonists also block return to nicotine taking following a period of abstinence after nicotine escalation (George et al., 2007), further indicating that VTA CRF manipulations can alter behaviors associated with substance abuse. Beyond the VTA, microinjection of CRF into BNST also produces reinstatement of cocaine seeking, whereas microinjection of a CRF1 receptor antagonist into BNST attenuates footshock-induced reinstatement (Erb and Stewart, 1999).
Despite the preclinical evidence for a role in CRF in drug abuse vulnerability and other addictive behaviors (Roberto et al., 2017), translation into clinical treatment strategies has thus far been unsatisfactory. A review of key clinical studies examining the use of CRF1 receptor antagonists for substance use disorders have been largely negative (Shaham and de Wit, 2016). The lack of efficacy may represent inadequacies of the animal models used, as well as limitations on the pharmacological agents tested in terms of either bioavailability at brain CRF1 receptors or activity at CRF1 vs CRF2 receptors in humans (Spierling and Zorrilla, 2017). Downstream from CRF activity, however, there appears to be some promise for glucocorticoid receptor antagonists in ameliorating withdrawal, particularly with alcohol (Vendruscolo et al., 2015). It remains to be determined if pharmacotherapeutic efficacy is achieved in large-scale clinical trials.
2.2. OT
OT is a nonapeptide that has only one known G-coupled protein receptor in brain. Good drug selectivity for the OT receptor is difficult to achieve because it is only 2 amino substitutions removed from the closely related neuropeptide vasopressin. OT-producing cell bodies exist in dense clusters within PVN and supraoptic nucleus (SON) of Hypo, with dispersed cells in accessory nuclei located between PVN and SON (Baribeau and Anagnostou, 2015; Knobloch and Grinevich, 2014), as well as a sparse distribution in other regions such as BNST and Amyg in some species [(Steinman and Trainor, 2017; Wang et al., 1997); Figure 2]. Among the numerous target regions receiving OT input in rat brain (Warfvinge et al., 2020), OT neurons from PVN specifically project to the reward-relevant NAc and VTA (Knobloch et al., 2012; Ross et al., 2009; Xiao et al., 2017). Aside from its important role in milk letdown and uterine contractions in females, OT plays a vital role in various sexual and social behaviors in both males and females (Caldwell, 2017; Jurek and Neumann, 2018). OT levels are increased in NAc during social affiliative behavior (Ross et al., 2009) and OT release increases activity of dopamine neurons (Hung et al., 2017; Peris et al., 2017). While much of the seminal work has been conducted using monogamous prairie voles, evidence from studies using rats also shows that OT enhances the rewarding effect of social interaction (Ramos et al., 2015) and promotes social affiliation (Bowen and McGregor, 2014)
Figure 2:
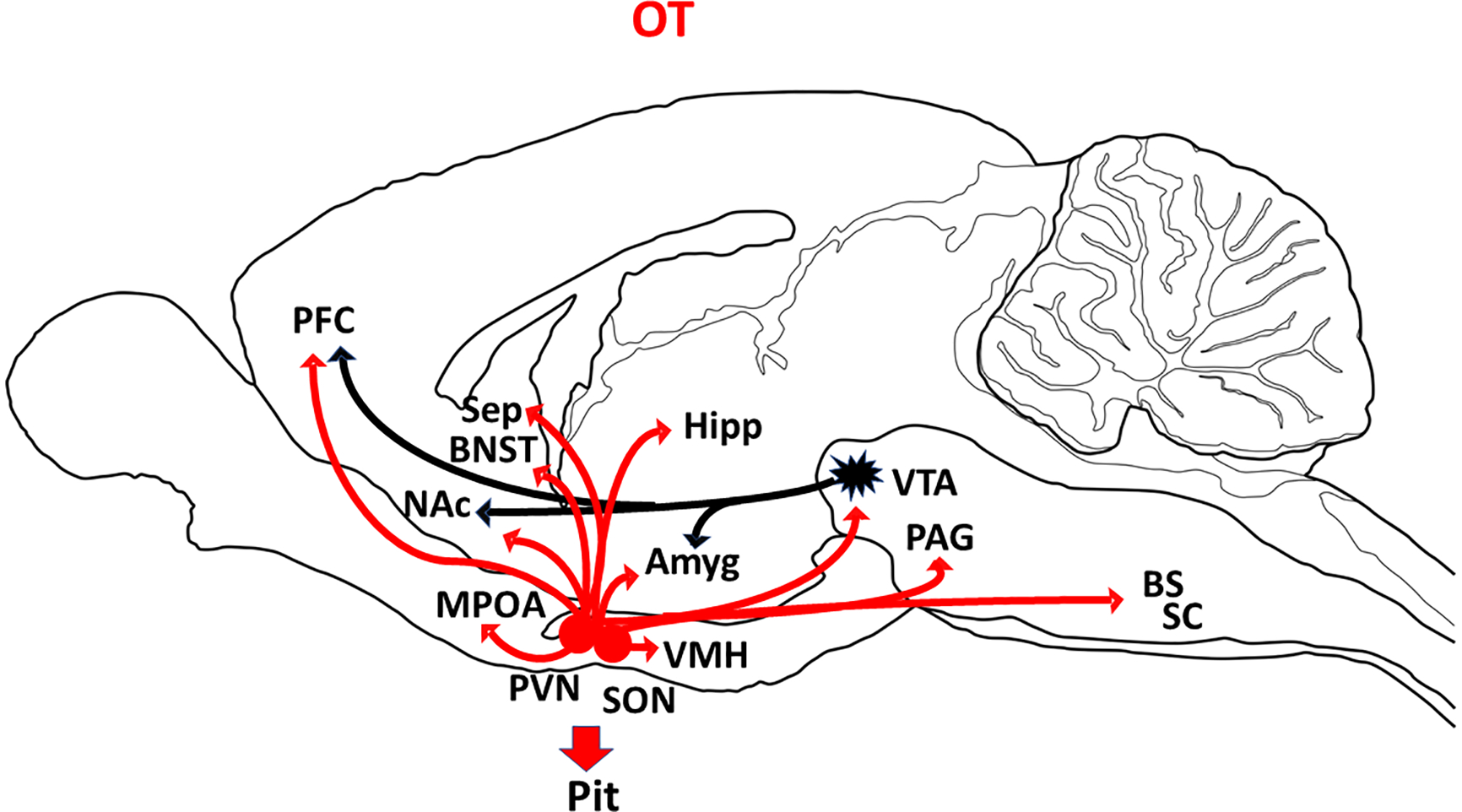
Schematic summary of OT cell bodies (red circles) and OT projections (red arrows) in sagittal plane of rat brain. The black projections from VTA represent dopamine neurons. Adapted from the following references: (Baskerville and Douglas, 2010; Grinevich et al., 2016; Kim et al., 2017). Abbreviations: Amyg=amygdala; BNST=bed nucleus of the stria terminalis; BS=brainstem; Hipp=hippocampus; Sep=septum; MPOA=medial preoptic area; NAc=nucleus accumbens; PAG=periaqueductal gray; PFC=prefrontal cortex; Pit=pituitary gland; PVN=paraventricular nucleus of hypothalamus; SC=spinal cord; SON=supraoptic nucleus of hypothalamus; VMH=ventromedial hypothalamus; VTA=ventral tegmental area.
Beyond its role in sexual and social processes, a host of studies indicate that OT activation diminishes drug reward, including reward associated with stimulants, opioids and alcohol (Table 2). With stimulants such as cocaine, methamphetamine and methylphenidate, OT injected systemically, i.c.v. or intra-NAc consistently decreases the acquisition of CPP and reduces SA in rats and mice (Baracz et al., 2012; Carson et al., 2010; Leong et al., 2016; Qi et al., 2009; Tanda et al., 2017). The OT-induced decrease in stimulant SA is reversed by OT antagonist pretreatment, indicating that OT receptors are involved. In addition, OT decreases the breakpoint on a progressive ratio schedule of methamphetamine SA, albeit only in females (Cox et al., 2013), and in both males and females it decreases the essential value of methamphetamine as expressed by an increase in elasticity (α parameter) using an economic demand analysis (Cox et al., 2017). These results indicate that activation of OT receptors in NAc diminishes the reinforcer effectiveness of stimulants. Additionally, activation of OT receptors is able to attenuate stimulant seeking triggered by a drug prime or drug-associated cue following a period of extinction (Cox et al., 2017; Cox et al., 2013; Everett et al., 2020; Weber et al., 2018).
Table 2:
Summary of studies examining the effect of OT agonists and antagonists on drug reward measured by either CPP or SA.
| Ref | Species | Sex | Treatment | Route | Dose | Measure | Result |
|---|---|---|---|---|---|---|---|
| Leong et al., 2016 | Sprague Dawley rats | Female | OT | i.p. | 0.1–3 mg/kg | SA Cocaine (0.15 mg/inf) |
↓ intake |
| Baracz et al., 2012 | Sprague Dawley rats | Male | OT | 1) i.p. 2) intra-NAc 3) intra-STh |
1) 0.6 mg/kg 2) 0.6 ng 3) 0.6 ng |
CPP Methamphetamine (1 mg/kg) |
1) ↓ acquisition 2) ↓ acquisition 3) ↓ acquisition |
| Qi et al., 2009 | Swiss mice | Male | 1) OT alone 2) OT+ atosiban (OT antag) |
1) i.c.v. 2) i.c.v + i.c.v. |
1) 0.1–2.5 μg 2) 2.5 μg + 2 μg |
CPP Methamphetamine (2 mg/kg) |
1) ↓ acquisition ↔expression 2) ↓ OT effect on acquisition |
| Cox et al., 2017 | Sprague Dawley rats | Male and female | 1) OT 2) OT 3) OT + OXA (OT antag) |
1) intra-NAc 2) i.p. 3) i.p. + intra-NAc |
1) 0.6 ug 2) 1 mg/kg 3) 1 mg/kg + 2 μg |
SA Methamphetamine (17.5 or 20 μg/inf) economic demand |
1) ↑ elasticity (α) 2) ↑ elasticity (α) 3) ↓ OT effect on elasticity |
| Cox et al., 2013 | Long Evans rats | Male and female | OT | i.p. | 0.3 or 1 mg/kg | SA Methamphetamine (17.5 or 20 μg/inf) |
↓ PR breakpoint (females only) |
| Carson et al., 2010 | Sprague Dawley rats | Male | OT | i.p. | 0.001–1 mg/kg | SA Methamphetamine (0.1 mg/kg/inf) |
↓intake |
| Lee et al., 2019 | Sprague Dawley rat | Male | OT | i.p. | 0.1–2 mg/kg | SA Methylphenidate (0.01–0.1 mg/kg/inf) |
↓intake |
| Moaddab et al., 2015 | Wistar rats | Male | 1) OT 2) OTA (OT antag) 3) OT 4) OT + OTA |
1) i.c.v. 2) i.c.v. 3) intra-NAc 3) intra-NAc |
1) 0.2 2) 0.75 μg 3) 10 ng 2) 10 + 37.5 ng |
CPP Morphine (5 mg/kg) |
1) ↔ acquisition 2) ↔ acquisition 3) ↑ expression 4) ↓ OT effect on expression |
| Ibragimov et al., 1987 | Sprague Dawley rats | Male | 1) OT 2) OT + ACME-OT (OT antag) |
1) intra-NAc or intra-Hipp 2) intra-Hipp |
1) 2 ng 2) 2 + 2 ng |
SA Heroin (20 μg/inf) tolerance induced with IP injections |
1) ↓intake 2) ↑intake |
| Kovács and Van Ree, 1985 | Wistar rats | Male | 1) OT 2) OT1–8 (fragment) 3) OT4–8 (fragment) 4) OT7–9 (fragment) |
s.c. | 1 μg | SA Heroin (30 μg/inf) tolerance induced with IP injections |
1) ↓ intake 2) ↓ intake 3) ↓ intake 4) ↔ intake |
| Kovács et al., 1985 | CFY rats | Male | OT | s.c. | 0.05–5 Mg | SA Heroin (20 μg/inf) tolerance induced with IP injections |
↓intake |
| Van Ree and de Wied, 1977 | Wistar rats | Male | 1) OT 2) tocinamide (OT frag) 3) PLG (OT frag) |
s.c. | 1) 1 μg 2) 1 μg 3) 1 μg |
SA Heroin (31 μg/inf) only 2 days acq training |
1) ↑ intake 2) ↔ intake 3) ↑ intake |
| Bahi, 2015 | C56BL/6J mice | Male | carbetocin (OT analog) | ip. | 6.4 mg/kg | CPP Alcohol (2 g/kg) |
↓acquisition |
| Stevenson et al., 2017 | Prairie voles | Male and female | OT | ip. | 1–10 mg/kg | SA Alcohol (15%) 2 bottle choice |
↓intake |
| Tunstall et al., 2019 | Wistar rats | Male | 1) OT 2) OT 3) OT 4) PF-06655075 (OT agonist) 5) OT + L-371,257 (peripheral OT antag) |
1) ip. 2) intranasal 3) i.c.v. 4) i.c.v 5) intranasal + i.p. |
1) 0.125–1 mg/kg 2) 0.25–1 mg/kg 3) 3–30 μg 4) 30 μg 5) 1 mg/kg + 5 mg/kg |
SA Alcohol (10%) lever pressing w/vapor exposure |
1) ↓ intake 2) ↓ intake 3) ↓ intake 4) ↓ intake 5) ↔ OT effect |
| King et al., 2017 | C57BL/6J mice | Male | 1) OT 2) OT + L-368,899 (OT antag) |
1) ip. 2) i.p. + i.p. |
1) 0.3–10 mg/kg 2) 1 + 10 mg/kg |
SA Alcohol (20%) drink in dark or Alcohol (12%) lever pressing |
1) ↓ intake 2) ↓ OT effect |
| Peters et al., 2017 | Wistar rats | Male | OT | i.c.v. | 1 Mg | SA Alcohol (20%) 2 bottle choice |
↓intake |
| MacFadyen et al., 2016 | Sprague Dawley rats | Male | OT | ip. | 0.05–0.5 mg/kg | SA Alcohol (10–15%) 3 bottle choice w/drink in dark or Alcohol (10% gel) lever pressing |
↓intake |
Abbreviations: CPP=conditioned place preference; Hipp=hippocampus; i.c.v.=intracerebroventricular; i.p.=intraperitoneal; mPFC=medial prefrontal cortex; NAc=nucleus accumbens; OT=oxytocin; SA=self-administration; s.c.=subcutaneous; STh=subthalamic nucleus.
OT is also involved in opioid reward processes, although the effects are somewhat mixed compared to stimulants. For example, while OT is reported to have no effect on acquisition of morphine CPP (Moaddab et al., 2015), it decreases heroin SA when administered either systemically or intra-NAc (Ibragimov et al., 1987; Kovács et al., 1985; Kovács and Van Ree, 1985). The picture is complicated by another study reporting that OT and an active OT fragment increases heroin SA (Van Ree and De Wied, 1977). However, this latter study pretreated rats with OT only during an atypical 2-day heroin SA preparation, indicating that OT may reduce opioid SA only after it is acquired over a prolonged regimen.
As for alcohol, there is robust evidence for an OT-induced blockade of reward processes measured by both CPP and SA, an effect that is reversed by OT antagonists. This evidence has accumulated using different routes of OT administration (systemic, intranasal, i.c.v. and intra-NAc), different drinking paradigms (2-bottle choice, drinking in the dark and operant lever pressing) and different species [mice, rats and prairie voles; (Bahi, 2015; King et al., 2017; Macfadyen et al., 2016; Peters et al., 2017; Stevenson et al., 2017; Tunstall et al., 2019)]. Consistent with these pharmacological results, overexpression of OT receptors via lentiviral microinjection into NAc also disrupts acquisition of alcohol CPP (Bahi, 2015) and SA (Bahi et al., 2016).
Some clinical evidence points to OT as a potential pharmacotherapy for substance use disorders, although this work is considered exploratory (Lee and Weerts, 2016). While OT does not reduce cue-induced craving in cocaine-dependent human subjects tested in a controlled laboratory setting (Lee et al., 2014), one report found that it reduces cue-elicited craving among tobacco cigarette smokers (Miller et al., 2016). There is also evidence that OT decreases the severity of withdrawal in alcohol-dependent patients undergoing in-patient detoxification (Pedersen et al., 2013), as well as decreasing cue-reactivity observed in Hipp, cingulate gyrus and associated frontal lobe regions using functional magnetic resonance imaging in heavy social drinkers (Anita et al., 2017). However, another study found that OT does not alter the cognitive and performance-impairing effects of alcohol in social drinkers (Vena et al., 2018). One limitation of these exploratory studies is that they rely primarily on acute intranasal delivery of OT and thus, it is unclear how much brain penetration is achieved via the intranasal route and how this bioavailability is affected when OT is administered under a chronic dosing regimen.
2.3. Summary
CRF1 receptor antagonists reduce drug CPP, SA and reinstatement, with evidence suggesting a critical role for CRF1 receptors in VTA, RN and mPFC, most notably for alcohol. While CRF2 receptors may be more involved in drug withdrawal, CRF1 receptors specifically in BNST have also been implicated. In contrast, OT and OT agonists reduce drug CPP, SA and reinstatement, with evidence suggesting a critical role for these receptors specifically in NAc. Taken together, these results obtained in adults indicate that drug abuse vulnerability is reduced by weakening CRF systems and/or strengthening OT systems. Unfortunately, application of these basic research findings has not yet yielded compelling clinical efficacy in treating substance use disorders.
3. Development of CRF and OT systems
Before describing how early life adversity can produce long-lasting alterations in CRF and OT systems involved in drug reward processes, this section provides a brief overview of the normal development of these systems in brain.
3.1. CRF
The human fetus has a functional HPA axis by the second trimester of pregnancy and is exposed to placental-derived CRF throughout gestation (McLean and Smith, 2001). In rats, CRF is found as early as gestational day 18 in the anterior median eminence (ME) using immunohistochemistry (Bugnon et al., 1982) and CRF mRNA is present as early as gestational day 17 in PVN (Baram and Lerner, 1991; Grino et al., 1989). CRF receptors also appear by the gestational day 17 and reach an overexpressed level by postnatal day (PND) 8. At that point, CRF receptors decrease to their adult densities by PND 21. CRF receptors are distributed widely, being more concentrated in striatum (Str) during the gestational period, while becoming more concentrated in cortex (Ctx) during postnatal development (Insel et al., 1988). While CRF mRNA expression is dense in PVN at gestational days 18–19, it decreases around the time of birth to eventually reach adult levels (Grino et al., 1989). This overexpression pattern of CRF mRNA expression appears to mimic CRF protein levels (Bugnon et al., 1982; Korosi and Baram, 2008).
The negative feedback associated with CRF synthesis in Hypo does not appear to control CRF gene expression during fetal development. Following pharmacological adrenalectomy, pregnant rats show increased CRF mRNA expression, while fetal CRF mRNA expression remains unchanged (Baram and Schultz, 1992). The absence of fetal CRF reactivity is not likely associated with a lack of glucocorticoid receptors, as glucocorticoid receptor mRNA is detected on gestational day 16 (Yi et al., 1994). Further, during postnatal development (PND 4–14), there is a continued stress hypo-responsivity period where CRF receptor numbers and baseline CORT levels are low (Graham et al., 1999), despite the fact that CRF PVN levels may be elevated (Schmidt et al., 2003). During this hypo-responsive period, mild stressors fail to produce an increase in CORT and ACTH release (Schmidt et al., 2003). After the first postnatal week, however, the glucocorticoid negative feedback system begins to exert its effect on CRF mRNA expression (Korosi and Baram, 2008). By postnatal day 16, initial onset of a matured negative feedback system is associated with a decrease in CRF expression (Schmidt et al., 2003). It is around this time that CRF begins to up-regulate in response to cold-separation stress (Baram et al., 1997). The transient hypo-responsive period during early postnatal development may represent an adaptation that protects against the negative effects of glucocorticoids and CRF in response to some stressors, although this does not appear the case for maternal separation stress (Graham et al., 1999).
Although the glucocorticoid negative feedback system becomes functional on PND 10, CRF regulation via the Amyg remains immature (Korosi and Baram, 2008). In adults, CRF levels are amplified in PVN following repeated stress (Makino et al., 1995). In contrast, even though rats at PND 10 show a heightened CRF mRNA expression in CeA following stress, this effect does not translate into increased CRF mRNA levels in PVN (Hatalski et al., 1998). Further, enhanced CeA CRF expression in adults results in dysregulation of the HPA axis (Keen-Rhinehart et al., 2009), and bilateral lesions of CeA decreases CRF expression in ME (Beaulieu et al., 1989). In contrast, neonatal Amyg lesions in male and female rhesus monkeys produces an increase in daily cortisol secretion and CRF levels in females compared to controls during the prepubertal period (Raper et al., 2014). In humans, evidence suggests that the Amyg may undergo a switch between positive and negative connectivity to the prefrontal cortex (PFC) as children approach adolescence (Gee et al., 2013). Perhaps, during development, the Amyg also shifts between positive and negative connectivity in other areas such as PVN, which may explain differential effects of CeA lesions between neonates and adults on CRF expression and regulation of stress hormones. More research is needed to understand the developmental changes associated with CeA and PVN inter-connections and the regulation of CRF in response to stress across development.
3.2. OT
Given the availability of other recent reviews covering the ontogeny of OT systems in mammalian brain (Baracz et al., 2020; Grinevich et al., 2015; Johnson and Buisman-Pijlman, 2016), only a brief overview is provided here. For humans and rodents, OT neurons are present by the second half of gestation (Altstein and Gainer, 1988; Buijs et al., 1980; Grinevich et al., 2015; Swaab, 1995). In rodents, development of OT neurons progresses rapidly from gestational day 12 to 16 (Grinevich et al., 2015). During this time, the PVN and SON develop cytoarchitecturally, the production of neurophysin-I (OT carrier protein and marker for OT pro-hormone) begins, and an intermediate form of OT becomes detectable. By birth, mature OT is detectable (Altstein and Gainer, 1988) and OT-releasing neurons are present in the accessory nucleus, PVN, and SON (Altman and Bayer, 1978; Grinevich et al., 2015). It is unclear if sex differences exist in the development of OT neurons, but males and females appear to have similar OT synthesis in PVN and SON across species (Dumais and Veenema, 2016). Human fetuses begin producing a mature form of OT relatively early during gestation, starting at 14 weeks, and they express adult-like quantities of OT neurons by approximately gestational week 26 (Swaab, 1995). Although mature OT production is achieved by birth in both rodents and humans (Grinevich et al., 2015), continued maturation of OT systems continues through adolescence.
OT receptors are widely distributed during gestation and they undergo region-specific changes from birth to adulthood, as described in recent reviews (Baracz et al., 2020; Grinevich et al., 2015). In addition, OT receptor activation can be coupled to either the excitatory Gq or the inhibitory Gi and Go signalling cascades, with the prevalence of each type of G protein being developmentally regulated. For example, by the beginning of adolescence, OT receptor coupling to Go decreases and Gi coupling begins to emerge, whereas OT receptor coupling to Gq remains ubiquitously distributed (Busnelli and Chini, 2018). Thus, experiences that induce OT release may activate different signalling cascades in an age-dependent manner. Age also moderates OT receptor expression in brain regions important for substance use disorder and social reward, such as NAc and VTA (Baracz and Cornish, 2016; Cox et al., 2017; Dolen et al., 2013; Hung et al., 2017). In NAc, for example, OT receptor binding emerges by PND 10 and peaks at PND 20, prior to the onset of adolescence (Shapiro and Insel, 1989). In VTA, OT receptor mRNA is present at gestational day 15 and peaks at PND 3. Age also affects OT receptor development in other brain regions involved in reward and social learning. For example, in Amyg, OT receptor mRNA is present at gestational day 20 and peaks in adulthood, whereas in BNST, OT receptor mRNA does not emerge until postnatal day 7 and then peaks in adulthood (Yoshimura et al., 1996). Sex differences also exist in OT receptor density during adulthood, with male rats having greater OT receptor density in many forebrain regions, including NAc, BNST, and Amyg, whereas females have greater OT receptor density in Str (Dumais et al., 2013). It is possible that these sex differences are species-specific, but additional work is required in other common laboratory species and in humans to better understand sex differences in OT receptor development (Dumais and Veenema, 2016). Thus, it is important that studies examining environment-induced alterations in OT receptor development consider the age, sex, and brain region under investigation.
Although a high concentration of OT is released within Hypo and spread to nearby regions through passive diffusion (Chini et al., 2017), OT receptor activation in NAc and VTA is likely controlled by direct axonal projections from PVN and SON neurons (Dolen et al., 2013; Grinevich et al., 2016; Hung et al., 2017; Knobloch et al., 2012; Ross et al., 2009). Projections from PVN and SON to Pit emerge during gestational development, and projections from accessory nucleus to Pit are present shortly after birth, but there has been a paucity of research into the ontogenesis of forebrain OT projections (Grinevich et al., 2015; Grinevich and Neumann, 2020). It is hypothesized, however, that development of these projections may continue throughout adolescence (Baracz et al., 2020). Given the OT receptor changes that occur during early life and the putative effects of adolescent development on OT fibers, environmental perturbation by social stressors and other life experiences likely have a lasting impact on OT system development.
3.3. Summary
While both CRF and OT can be detected in PVN and related brain structures prior to birth, there is a continued region-specific maturation of each of these systems into the late adolescent period. In rodents, the negative feedback control of the HPA axis is not expressed fully until almost weaning, and maturation of both CRF and OT neurocircuitries continue throughout adolescence. Thus, perturbations of these developing systems by early life adversity might be expected to produce long-term dysregulations that are manifested behaviorally.
4. Adverse social events during development that alter CRF and OT systems
Considerable clinical evidence indicates that early life social adversity disrupts the normal development of CRF systems, with an overall strengthening of the HPA axis. While changes in CRF systems in brain are difficult to examine directly in humans, the peripheral output signal from the HPA axis measured via salivary or plasma cortisol has been extensively studied. For example, foster children undergoing social neglect display dysregulation of the normal diurnal cortisol rhythm (Blaisdell et al., 2019). In addition, self-reported early life adversity in the pre-school years is associated with elevated CRF levels in cerebrospinal fluid (CSF) in adulthood (Carpenter et al., 2004). Among individuals with personality disorders, scores on the Childhood Trauma Questionnaire are also positively correlated with CRF levels in CSF (Lee et al., 2005). A subsequent study from that same group found that individuals with self-reported problems in parental bonding show elevated CRF levels in CSF (Lee et al., 2006). Corroborating this work in humans, more controlled laboratory studies in non-human primates reveal that adverse early rearing strengthens expression of CRF systems in adulthood (Coplan et al., 1996; Zhang, 2017).
In contrast to the strengthening of CRF systems, clinical literature indicates that early life social adversity produces long-lasting weakening of OT systems. For example, adults who experience high early life stress scores on the Childhood Trauma Questionnaire have decreased OT levels in CSF (Heim et al., 2009), as well as decreased sensitivity to the effect of OT in modulating functional activity of Amyg-PFC neurocircuitry (Fan et al., 2014). These findings are complicated somewhat because there is other evidence that peripheral OT from plasma or saliva is actually increased following early life adversity (Leslie et al., 2014; Parker et al., 2009). However, since there is a dissociation between central and peripheral OT systems (Gerald and Falk, 2001), it appears the weakening of OT systems with early life adversity is specific to central nervous system processing. Consistent with this, nursery- or peer-reared monkeys show reduced OT levels in CSF and reduced OT receptor mRNA in brain compared to normal mother-reared monkeys (Maggie et al., 2017; Winslow et al., 2003).
At least 3 major animal models have been widely used to examine the neurobiological mechanisms involved in mediating the long-term effects of early life social adversity, namely maternal separation, social isolation and social defeat (Forster et al., 2018). Importantly, cross-comparison across each of these developmental models sometimes yields conflicting results because they represent qualitatively distinct early life events. In addition, in rodents, each of these developmental models are typically applied at different periods of postnatal development, with maternal separation beginning soon after birth (PND 1), social isolation beginning at weaning (PND 21) and social defeat beginning in late adolescence (PND 28–50). Thus, differences in both how and when each of these developmental models of social adversity are applied implies that direct comparison across different developmental models should be avoided. For example, although maternal separation and social isolation might both be classified as “social deprivation” applied at different developmental periods, they can lead to opposite effects on the development of dopamine systems expressed in adulthood (Hall, 1998; Hall et al., 1999).
4.1. Maternal separation
Table 3 provides a summary of literature examining the long-term effects of maternal separation on CRF and OT systems. CRF systems are dysregulated by maternal separation models that restrict maternal interaction after the establishment of a mother-child bond (Delavari et al., 2016; O’Malley et al., 2011). Some care in reviewing this field is warranted because a variety of models of maternal separation have been used, including variations in the postnatal interval used to perform the separations, the duration of separations, and the number of separations. Maternal separation results in an immediate elevation of circulating CORT in the rat pup (Ritchey and Hennessy, 1987; Yoshida et al., 2018), as well as a long-lasting hyper-responsivity of the HPA axis to a stressor applied in adulthood, as measured by increased levels of plasma levels of ACTH and CORT (Huot et al., 2001; Roque et al., 2019). The maternal separation-induced change in HPA axis function persists into adulthood and appears resistant to change, as environment enrichment does not normalize the system after the period of maternal separation is terminated. Interestingly, however, if rats are handled during the maternal separation period, there appears to be some normalization of CORT release, at least in females (Campbell and Spear, 1999).
Table 3:
Summary of studies examining the long-lasting effect of maternal separation on development of CRF and OT systems.
| Reference | Species | Sex | Maternal Separation Duration | Maternal Separation Age | Measurement Age | Result |
|---|---|---|---|---|---|---|
| Babygirija et al., 2012 | Sprague-Dawley rats | Male | 3 hr/day | PND 2–14 | >PND 56 (following restraint stress) | ↑ CRF mRNA in PVN ↑ CRF neurons in PVN |
| Flagel et al., 2003 | Sprague-Dawley rats | Female and male | 10 min/day | Early (PND 2–8) Late (PND 8–14) | PND 70 | ↔ CRF mRNA in PVN and Amyg |
| Garcia-Gutiérrez etal., 2016 | Swiss ICR mice | Male | 12 hr/day | PND 8 and 12 | PND 28 | ↑ CRF mRNA in PVN |
| de Almeida Magalhães et al., 2018 | C57BL/6 mice | Female and male | 6 hr/day | PND 5–21 | PND 45 | ↔ CRF mRNA in Hypo ↑ CRF1 and CRF2 receptor mRNA in Hipp |
| Slotten et al., 2006 | Long-Evans rats | Male | 3 hr/day | PND 3–15 | PND 115–120 | ↔ CRF mRNA in PVN |
| Desbonnet et al., 2008 | Wistar rats | Female and male | 3 hr/day | PND 2–14 | Adulthood (stressed vs. control) | ↑ CRF neurons in PVN (stressed females only) ↓ CRF neurons in Amyg (males only) |
| Wang et al., 2014 | Sprague-Dawley rats | Female and male | 3 hr/day | PND 1–10 | PND 77 | ↑ CRF mRNA in Hipp ↑ CRF protein in Hipp |
| Murgatroyd et al., 2009 | C57BL/6N mice | Male | 3 hr/day | PND 1–10 | PND 90 | ↔ CRF mRNA in Hypo |
| Plotsky et al., 2005 | Long-Evans rats | Female and male | Short 15 min/day Long 3 hr/day |
PND 2–14 | PND 100–120 | ↑ CRF in CSF (MS Long only) ↑ CRF mRNA and protein in PVN, Amyg, BNST and LC (MS Long only) ↑ CRF total receptor binding in PVN and LC (MS Long only) ↓ CRF total receptor binding in PVN and LC (MS Short only) ↑ CRF1 receptor binding and mRNA in PVN and LC (MS Long only) ↓ CRF1 receptor binding and mRNA in Ctx (MS Long only) |
| Aisa et al., 2008 | Wistar rats | Females | 3 hr/day | PND 2–21 | PND 60–75 | ↑ CRF mRNA in PVN |
| O’Malley et al., 2011 | Sprague-Dawley rats | Male | 3 hr/day | PND 2–21 | Adulthood | ↑ CRF1 protein in Hypo ↔ CRF1 protein in PFC, Amyg or Hipp ↔ CRF2 protein in Hypo, Amyg or Hipp |
| Ladd et al., 1996 | Sprague-Dawley rats | Male | 4–6 hr/day | PND 2–20 | PND 114–115 | ↓ CRF receptor binding in Pit ↑ CRF receptor binding in RN. ↑ CRF in ME, PB and RN |
| Plotsky and Meaney, 1993 | Long-Evans rats | Female and male | 3 hr/day | PND 2–14 | PND 90–120 | ↑ CRF mRNA and content in Hypo |
| Chen et al., 2012 | Sprague-Dawley rats | Female and male | 4 hr/day | PND 2–13 | PND 60 | ↑ CRF mRNA in Hypo (females only) ↔ CRF mRNA in Amyg |
| Bravo et al., 2011 | Sprague-Dawley rats | Female and male | 3 hr/day | PND 2–14 | PND 77–91 | ↑ CRF1 mRNA in Amyg and RN ↔ CRF1 mRNA in Hipp ↓ CRF2 mRNA in Hipp, Amyg and RN |
| Hu et al., 2020 | C57BL/6J | Male | 24 hr | PND 3 | PND 70–84 | ↑ CRF neurons in BNST ↑ CRF mRNA in BNST ↑ CRF1 receptor mRNA in BNST ↔ CRF2 receptor mRNA in BNST |
| He et al., 2018 | Mandarin voles | Female | 15 min/day | PND 1–14 | Adulthood | ↔ OT cells in PVN and SON |
| Gilles and Poston, 2017 | Sprague-Dawley rats | Female and male | 4 hr/day | PND 2–21 | PND 103–107 | ↔ OT cells in PVN and SON |
| Riveros-Barrera and Duenas, 2016 | Wistar rats | Female and male | 3 hr/day | PND 1–21 | 1) PND 35 2) PND 90 |
1) ↔ OT in plasma 2) ↑ OT in plasma (females) ↓ OT in plasma (males) |
| Babygirija et al., 2012 | Sprague-Dawley rats | Male | 3 hr/day | PND 2–14 | >PND 56 (following restraint stress) | ↓ OT mRNA in PVN ↓ OT magno cells in PVN ↔ OT parvo cells in PVN |
| Tsuda et al., 2011 | C57BL/6J mice | Male | 3 hr/day | PND 1–14 | PND 28–42 | ↑ OT cells in PVN |
| Barrett et al., 2015 | Prairie voles | Female | 3 hr/day | PND 1–14 | PND 98 | ↔ OT receptors |
| Wei et al., 2020 | Sprague-Dawley rats | Male | 4 hr/day | PND 1–20 | PND 42 | ↓ OT receptor mRNA in PFC ↓ OT receptor protein in PFC |
| Holubová et al., 2019 | Wistar rats | Female and male | 3 hr/day | PND 1–21 | PND 30 | ↓ OT in plasma |
| Lukas et al., 2010 | Wistar rats | Male | 3 hr/day | PND 1–14 | 1) PND 35 2) PND 56 3) PND 112 |
1) ↓ OT receptors in Ctx 2) ↓ OT receptors in Ctx 3) ↓ OT receptors in Sep and Str ↑ OT receptors in VMH |
| Veenema et al., 2007 | C57BL/6 mice | Female and male | 3 hr/day | PND 1–14 | >PND 98 | ↓ OT parvo cells in PVN (females only) ↔ OT magno cells in PVN |
| Oreland et al., 2010 | Wistar rats | Male | Short 15 min/day Long 6 hr/day |
PND 1–21 | 1) PND 21 2) PND 70 |
1) ↓ OT in Hypo (MS Short only) ↓ OT in Amyg ↑ OT in Pit (MS Short only) 2) ↔ OT in Hypo and Pit ↓ OT Amyg (MS Short only) |
| Melchior et al., 2018 | Wistar rats | Female and male | 3 hr/day | PND 2–12 | PND 56 | ↔ OT receptor mRNA in SC |
Abbreviations: Amyg=amydala; BNST=bed nucleus of the stria terminalis; CRF=corticotropin-releasing hormone; CSF=cerebrospinal fluid; Ctx=cortex; Hipp=hippocampus; Hypo=hypothalamus; LC=locus coeruleus; ME=median eminence; mRNA=messenger ribonucleic acid; OT=oxytocin; PB=parabrachial nucleus; PFC=prefrontal cortex; Pit=pituitary; PND=postnatal day; PVN=paraventricular nucleus; RN=raphe nucleus; SC=spinal cord; Sep=septum; SON=supraoptic nucleus; Str=striatum; VMH=ventromedial hypothalamus
Neuroanatomical analyses have also revealed a host of regional brain changes in CRF-related RNA transcripts and proteins following maternal separation. When evaluated at different periods of postnatal development, the acute changes in CRF systems measured in pups immediately following maternal deprivation varies in an age- and region-specific manner, with different regions showing either increased or decreased expression of CRF and CRF receptors (Vazquez et al., 2006a).
At the genetic level, immediately after termination of the maternal separation period, expression of Crf and Crfr1 genes are increased in both Hypo and Hipp (de Almeida Magalhães et al., 2018). Consistent with this, basal CRF production is elevated in major cell body regions such as PVN, BNST, Amyg and Hipp (Aisa et al., 2008; Babygirija et al., 2012; García-Gutiérrez et al., 2016; Hu et al., 2020; Plotsky et al., 2005; Wang et al., 2014), and this elevation is more prominent when maternal separation consists of long (3 hr) rather than short (15 min) durations (Plotsky et al., 2005).
Maternal separation also alters CRF receptor levels. Total CRF receptor binding is increased in PVN (Plotsky et al., 2005), which is attributed primarily to elevated CRF1 receptor mRNA and protein (Aisa et al., 2008; O’Malley et al., 2011). In contrast to the changes in CRF receptor binding in PVN, however, changes in other regions are more complicated. For example, one study found that maternal separation increased CRF1 receptor mRNA in Amyg and DR, while CRF2 receptor mRNA in Amyg, DR and Hipp was decreased (Bravo et al., 2011). It remains to be determined to what extent, if any, these long-term changes at the cellular level are reversible.
In contrast to the strengthening in CRF systems, evidence suggests that maternal separation produces a long-lasting weakening of OT systems, although some results are mixed. Maternal separation has no effect on plasma OT (Riveros-Barrera and Duenas, 2016; Xu et al., 2018), except for a sex-specific change that does not emerge until adulthood (Riveros-Barrera and Duenas, 2016), where plasma OT decreases in separated males and increases in separated females relative to unstressed controls. In brain, while the total number of OT-containing cell bodies in PVN or SON does not appear altered by maternal separation (He et al., 2018), one report found that magnocellular OT neurons were specifically decreased (Babygirija et al., 2012). This latter finding is bolstered by other reports in rats showing diminished OT mRNA in PVN (Babygirija et al., 2012) and decreased OT in various target structures such as PFC, septum (Sep) and Amyg (Lukas et al., 2010; Oreland et al., 2010; Wei et al., 2020). In voles, there is also a decrease in the number of OT neurons, but only when the dams are exposed to gestational restraint stress (He et al., 2018). Thus, on balance, the overall results point to a diminished strength of OT systems following maternal separation.
Evidence suggests that OT can ameliorate the negative effects of maternal separation. For example, rats that undergo maternal separation have increases in brain derived neurotrophic factor that is normalized by systemic OT treatment (Mansouri et al., 2020a). Increases in pain sensitivity after maternal separation can also be attenuated by systemic (Melchior et al., 2018; Xu et al., 2018) or central (Amini-Khoei et al., 2017a) administration of OT. Administration of i.c.v. OT also reduces depression-like behavior induced by maternal separation (Amini-Khoei et al., 2017b). Guinea pigs that undergo maternal separation in the late preweaning period (PND 20–27) have lower levels of CORT and emit fewer vocalizations after i.c.v. OT (Hennessy et al., 2019). Thus, despite the lack of consistent evidence for effects of maternal stress on the developing endogenous OT system, treatment with OT appears to consistently reduce the negative impact of maternal separation stress.
4.2. Social isolation
Table 4 provides a summary of literature examining the effects of social isolation initiated in adolescence on CRF and OT systems measured in adulthood. Like maternal separation, CRF systems generally are strengthened by adolescent social isolation, which is typically defined by housing animals in single cages with no direct social interaction with conspecifics. Adolescent social isolation increases CRF levels in PVN (Pan et al., 2009; Ruscio et al., 2007) and levels of CRF1 receptor mRNA in Pit (Pinelli et al., 2017). CRF2 receptors expressed in DR are also increased, resulting in altered CRF-regulated serotonergic activity in NAc (Lukkes et al., 2009a; Lukkes et al., 2009c). Further, adolescent social isolation produces heightened anxiety-like behavior in adulthood that is decreased with infusion of a CRF2 receptor antagonist into DR (Bledsoe et al., 2011). In male prairie voles, social isolation for 6 weeks after weaning also induces anxiety-like behavior that is associated with increased CRF mRNA in PVN (Pan et al., 2009).
Table 4:
Summary of studies examining the effect of adolescent social isolation on development of CRF and OT systems.
| Reference | Species | Sex | Isolation Treatment | Isolation Age | Assessment Age | Result |
|---|---|---|---|---|---|---|
| Lukkes et al., 2009c | Sprague-Dawley rats | Male | 1 vs 3 per cage | PND 21–42 | PND 56 | ↔ CRF1 receptor protein in RN ↑ CRF2 receptor protein in dorsal RN |
| Pan et al., 2009 | Prairie voles | Male | 1 vs 2 per cage | PND 21–63 | PND 66 | ↑ CRF in PVN ↔ CRF in Amyg |
| Weintraub et al., 2010 | Sprague-Dawley rats | Female and male | 1 vs 3 or 4 per cage | PND 30–50 | PND 77 | ↔ CRF mRNA in parvocellular PVN |
| Ruscio et al., 2007 | Prairie voles | Female and male | 1 vs 2 (same or diff litter) per cage | PND 21–42 | PND 42 | ↑ CRF in PVN ↔ CRF in Amyg |
| Pinelli et al., 2017 | Sprague-Dawley rats | Female and male | 1 vs 2 per cage | PND 35-140 | PND 140 | ↔ CRF mRNA in Pit ↑ CRF1 receptor mRNA in Pit (male only) ↔ CRF2 receptor mRNA in Pit |
| Tanaka et al., 2010 | Long-Evans rats | Female and male | 1 vs 2 or 3 per cage | PND 23–37 | PND 38–48 | ↓OT cells in PVN (females only) |
| Han et al., 2018 | C57BL/6N mice | Male | single vs group cage | PND 42–77 | PND 77 | ↔ OT mRNA in PVN ↓ OT receptor mRNA in Amyg |
| Pan et al., 2009 | Prairie voles | Male | 1 vs 2 per cage | PND 21–63 | PND 66 | ↑ OT in PVN ↔ OT in SON |
| Pournajafi-Nazarloo et al., 2013 | Prairie voles | Female and male | 1 vs 2 per cage | PND 21–60 | PND 60 | ↓ OT receptor mRNA in Hypo |
| Harvey et al., 2019 | Sprague-Dawley rats | Female and male | 1 vs 3 per cage | PND 21–77 | PND 78 | ↓ OT in plasma |
| Ruscio et al., 2007 | Prairie voles | Female and male | 1 vs 2 (same or diff litter) per cage | PND 21–42 | PND 42 | ↔ OT in PVN or SON |
| Oliveira et al., 2019 | Wistar rats | Female and male | 1 vs 3 or 4 per cage | PND 21–72 | PND 74 | ↑ OT mRNA in PVN ↔ OT mRNA in SON ↓ OT receptor binding in NAc ↔ OT receptor binding in BNST, Sep, Amyg or VMH |
| Tanaka et al., 2019 | Long-Evans rats | Female and male | 1 vs 2 per cage | PND 23–87 | PND 87–94 | ↔ OT cells in PVN or SON ↓ OT cells cFos+ in PVN and SON (female only) |
| Neal et al., 2018 | Long-Evans rats | Male | 1 vs 8 per cage | PND 29–55 | >PND 55 | ↑ OT in plasma ↔ OT PVN, SON or MFB |
Abbreviations: Amyg=amygdala; BNST=bed nucleus of the stria terminalis; CRF=corticotropin-releasing hormone; Hypo=hypothalamus; MFB=medial forebrain bundle; mRNA=messenger ribonucleic acid; NAc=nucleus accumbens; OT=oxytocin; Pit=pituitary; PND=postnatal day; PVN=paraventricular nucleus; RN=raphe nucleus; Sep=septum; SON=supraoptic nucleus; VMH=ventromedial hypothalamus.
Consistent with the strengthening of CRF systems, adolescent social isolation produces a downstream enhancement of HPA activity. In particular, long-lasting increases in basal levels of circulating CORT are observed, with application of either a stressor or drug producing a CORT response that is more rapid, but of shorter duration, compared to social-housed controls (Caruso et al., 2014; Lukkes et al., 2009b; Stairs et al., 2011).
The effect of adolescent social isolation on the development of OT systems has been studied in rats, mice, and voles. In general, social isolation weakens development of OT brain systems as reflected in a decreased number of OT cell bodies in PVN, as well as a decreased number of active OT neurons measured by c-Fos immunoreactivity (Tanaka et al., 2019; Tanaka et al., 2010). OT receptor mRNA is also diminished in Hypo and CeA (Han et al., 2018; Pournajafi-Nazarloo et al., 2013), consistent with the general isolation-induced weakening of this brain system. Despite the decrease in number of OT neurons, however, the concentration of OT in PVN is increased (Oliveira et al., 2019; Pan et al., 2009), suggesting that the fewer number of OT neurons exhibit up-regulated synthesis with social isolation. It is not clear if the diminished strength of OT brain systems is reflected by concomitant changes in OT in the periphery. While one study reported decreased plasma OT with social isolation (Harvey et al., 2019), another study reported increased plasma OT (Neal et al., 2018). These conflicting results may reflect a difference in rat strains (Sprague-Dawley vs. Long-Evans); in addition, the latter study was limited to males only (Neal et al., 2018).
4.3. Social defeat
There are several different models of social defeat, including resident-intruder, witnessed social defeat and cage-within-cage resident-intruder (Verbitsky et al., 2020), each which measure hostile and stressful interactions between animals. In adulthood, social defeat is known to strengthen CRF systems (Guo et al., 2020; Han et al., 2017; Holly et al., 2016) and weaken OT systems (Hou et al., 2020; Li et al., 2019). Despite this evidence, however, few studies have confirmed that social defeat during adolescence produces similar changes that last into adulthood. A recent review compiled a list of 20 different studies that have examined adolescent social defeat, but none specifically measured the effects on either CRF or OT systems (McCormick et al., 2017). In one recent study, adolescent social defeat increased CRF2 receptors in DR, but not in other regions such as VTA or locus coeruleus [LC; (Forster et al., 2018)]. However, this finding contrasts with other work showing that CRF2 receptors in DR are decreased by maternal separation (Bravo et al., 2011), thus illustrating the differential outcomes obtained with two developmental models of adverse social events applied at different periods of development. With OT, we are aware of only one study examining the effect of adolescent social defeat on OT systems (Ferrer-Perez et al., 2019). In that study, adolescent social defeat did not alter plasma OT. More work is needed to determine if adolescent social defeat alters OT systems in brain.
4.4. Summary
There is considerable evidence that early life adversity alters both CRF and OT systems well into adulthood. With early maternal separation, CRF systems in PVN are enhanced, whereas OT systems in both PVN and Amyg are diminished. A similar pattern appears with postweaning social isolation, with CRF systems strengthened and OT systems weakened. However, even though social defeat applied during adulthood is known to impact both CRF and OT activity, there is a gap in knowledge about the effects of adolescent on CRF and OT brain systems. One study to date does show a strengthening of CRF in DR following adolescent social defeat.
5. Effects of early life social adversity on drug abuse vulnerability
The clinical literature is replete with evidence showing the long-term detrimental effects of early life adversity on long-term physical and psychological health. Regarding substance abuse specifically, physical abuse within the first 5 years of life is predictive of future substance abuse, particularly in females (Lansford et al., 2009). Others have found that stressors induce greater SA of multiple substances of abuse (Sinha, 2008). This final section covers evidence showing that early life social adversity increases drug abuse vulnerability, and describes mechanistic work, albeit limited, that demonstrates a direct role of CRF and OT systems in mediating the relation between early life social adversity and drug abuse vulnerability.
5.1. Maternal separation
Two thorough reviews of the long-term effects of maternal separation on drug reward in preclinical studies have been recently published (Baracz et al., 2020; Walters and Kosten, 2019), and these excellent compilations will not be re-presented here. These reviews provide ample evidence that maternal separation produces reliable increases in morphine reward measured by either CPP or SA, as well as greater CPP and SA using stimulants such as cocaine, amphetamine and methamphetamine; the effect also extends to nicotine CPP (Dalaveri et al., 2017). The general conclusion with opiates and stimulants is supported by results obtained in both mice and rats, as well as across different routes of administration (oral, s.c., i.p. and i.v.). However, these reviews also reveal at least 3 important caveats in the literature. First, in contrast to opioids and stimulants, maternal separation has yielded relatively mixed results on alcohol consumption, with some reports showing a reliable effect (Cruz et al., 2008; Portero-Tresserra et al., 2018) and others showing no effect (Marmendal et al., 2004; Vazquez et al., 2006b). The discrepancies in the alcohol literature may reflect procedural differences across studies, with those using longer separation protocols generally showing greater effects (Nylander and Roman, 2013). Second, the effect of maternal separation appears to be more reliable when drug reward is tested in adulthood rather than during adolescence, suggesting a period of resilience prior to the observed detrimental effect later in life. Third, most studies have been limited to males only and those using both sexes are sometimes underpowered to yield potential sex differences. As one example pointing to sex-dependent effects, maternal separation increases morphine CPP in males, but not females (Michaels and Holtzman, 2008), suggesting females may be buffered from adverse social experiences early in life. Nonetheless, females show long-term increases in excitability of VTA dopamine neurons following maternal separation (Spyrka et al., 2020).
Despite the large number of studies showing enhanced vulnerability to drug abuse following maternal separation, few studies have incorporated analyses of either CRF or OT changes into the study design, thus making it difficult to assess whether changes in these neural systems are associated with changes in CPP or SA. While the two recent reviews compiled over 30 studies examining the effect of maternal separation on drug reward (Baracz et al., 2020; Walters and Kosten, 2019), only 6 studies incorporated a measure of CRF activity and none incorporated a measure of OT activity. Among the 6 studies examining CRF activity, 4 were limited to measuring circulating CORT or ACTH levels as a correlational variable associated with changes in drug reward (Campbell and Spear, 1999; Faure et al., 2009; Huot et al., 2001; Marmendal et al., 2004), while only 2 measured CRF in brain (Faure et al., 2009; Gondré-Lewis et al., 2016). One report found that maternally separated mice showed exaggerated alcohol consumption in adolescence, which was associated with increased CRF levels in PVN, NAc, and Hipp (Faure et al., 2009); unfortunately, these results were merely correlational.
More recent mechanistic work has begun to examine the specific role of CRF systems in brain in controlling the maternal separation-induced changes in drug reward. In one study, maternal separation facilitated binge alcohol drinking and intracranial infusion of a CRF antagonist into either CeA or mPFC normalized alcohol drinking without affecting sucrose drinking (Gondré-Lewis et al., 2016). Other work has shown that either pharmacological or environment interventions can also normalize the effects of maternal separation on drug reward (Khalaji et al., 2018; Morel et al., 2009), although these latter studies did not assess a specific role for CRF. Nonetheless, these studies together indicate that the effects of maternal separation depend, at least in part, on brain CRF systems and that these effects are modifiable with an intervention.
While similar mechanistic studies have not examined a direct role for OT, there are some relevant studies to draw on. In particular, several studies show that many behavioral deficits induced by maternal separation are ameliorated by OT treatment, including depressive- and autistic-like behaviors (Amini-Khoei et al., 2017b; Ji et al., 2016; Mansouri et al., 2020b), as well as deficits in social and cognitive memory (Joushi et al., 2021). Future mechanistic work is needed to determine if this intervention also ameliorates the increase in drug abuse vulnerability induced by maternal separation.
5.2. Social Isolation
Evidence from rats and mice indicates that animals housed individually during adolescence display increased drug abuse vulnerability compared to group-housed controls. When tested in adulthood, social isolates show increased cocaine CPP (Zakharova et al., 2009), as well as increased SA of stimulants (Bardo et al., 2001; Ding et al., 2005; Schenk et al., 1987) and alcohol (Fernández et al., 2019; McCool and Chappell, 2009; Skelly et al., 2015). With cocaine or amphetamine SA, adolescent social isolation increases acquisition and escalation at low unit doses (Baarendse et al., 2013; Bardo et al., 2001; Boyle et al., 1991; Gipson et al., 2011; Howes et al., 2000; Schenk et al., 1987) and the effect is observed in both males and females (Bardo et al., 2001). While there have been some negative findings (Phillips et al., 1994; Rivera-Irizarry et al., 2020), several comprehensive reviews have concluded that most studies show increased drug SA following social isolation in adolescence, especially with stimulants and alcohol (Bardo et al., 2013; Vannan et al., 2018; Walker et al., 2019). Further, when enriching objects are introduced into the group-caged home environment, the difference between single- vs. group-housed rats is enhanced even further (Bardo et al., 2001; Stairs and Bardo, 2009), indicating that the combination of social peers and novel objects promotes greater protection against drug abuse compared to social peers alone. It is likely that social interactions in the presence of enriching objects are more complex than social interactions with peers alone because the presence of novel objects provides opportunities for hiding and naturalistic territorial behaviors that promote full maturation of species-specific social competencies.
In contrast to stimulants and alcohol, relatively less is known about the effects of adolescent social isolation on opioid reward. One study found that social isolation decreases CPP induced by s.c. morphine (Wongwitdecha and Marsden, 1996), while another found no effect on SA assessed via the oral route (Hill and Powell, 1976). In contrast, using more clinically relevant routes of administration, adolescent social isolation has been shown to increase intranasal sufentanil SA (Weinhold et al., 1993) and acquisition of i.v. remifentanil SA (Hofford et al., 2017). Thus, similar to alcohol and stimulants, evidence indicates that adolescent social isolation enhances opioid abuse vulnerability when assessed using routes of administration with rapid absorption.
In addition to the increase in drug reward with social isolation, there is a concomitant strengthening of CRF systems and weakening of OT systems as described previously (Table 4), suggesting a role for these neuropeptides in linking social isolation and drug abuse vulnerability. However, as with maternal separation, caution is needed because these behavioral and neurochemical outcome measures have been typically observed in different studies, with relatively few studies measuring both drug reward and CRF or OT systems in the same animals.
While there have been no mechanistic studies directly examining the role of CRF or OT in linking social isolation and drug reward, some relevant studies have been conducted. In one study, microinjection of a CRF receptor antagonist into DR was shown to ameliorate the anxiety-like effect induced by adolescent social isolation (Lukkes et al., 2009a). Another study found that intra-DR microinjection of a CRF2 receptor antagonist ameliorated the social isolation-induced increase in anxiety (Bledsoe et al., 2011). Further mechanistic work is needed to determine if direct inhibition of CRF activity or excitation of OT activity also ameliorates drug abuse-related behaviors following adolescent social isolation.
5.3. Social Defeat
Acute and repeated social defeat applied during adulthood has an immediate and long-lasting impact on drug abuse vulnerability, which has been demonstrated most robustly with cocaine and alcohol reward (Montagud-Romero et al., 2018; Newman et al., 2018). Much of this work in adults has strongly implicated CRF as a key mediator of the relation between social defeat and increased drug intake. Like adults, adolescents exposed to social defeat and subsequently tested in adulthood show increased CPP to amphetamine, cocaine, and alcohol (Burke et al., 2011; Montagud-Romero et al., 2017; Rodríguez-Arias et al., 2017; Whitaker et al., 2013), as well as increased SA of cocaine and alcohol (Burke and Miczek, 2015; Rodriguez-Arias et al., 2016). Despite this evidence, there is relatively little known about the specific role of CRF in mediating the effect of adolescent social defeat on drug abuse vulnerability in adulthood. One recent review compiled 20 articles that applied social defeat during the adolescent period in mice and rats (McCormick et al., 2017). Among these, 3 studies examined plasma CORT and/or ACTH in adulthood (Burke et al., 2010; Buwalda et al., 2013; Furuta et al., 2015), but none examined these peripheral markers in conjunction with drug reward. One study found that the elevation in plasma CORT elicited by experimenter-delivered amphetamine is reduced by adolescent social defeat (Burke et al., 2010) and another study found that peripheral administration of a CRF1 receptor antagonist attenuates the anhedonia associated with adolescent social defeat (Bourke et al., 2014).
A specific role of CRF systems in mediating the increase in drug reward with adolescent social defeat has been cogently advanced by others (Burke and Miczek, 2013), and is supported by literature cited in this review. Defeated rats display increased basal activity of noradrenergic neurons in LC, which is reversed by microinjection of a CRF1 antagonist into this same region (Bingham et al., 2011). Even more relevant, intra-VTA infusion of a CRF1 receptor antagonist during adolescent social defeat prevents the defeat-induced escalation of cocaine intake during adulthood (Burke et al., 2016). Similarly, while adolescent social defeat increases cocaine CPP in male mice, this effect is blunted by pretreating rats with a CRF1 receptor antagonist, but not a CRF2 receptor antagonist (Ferrer-Pérez et al., 2018). These mechanistic studies establish a direct role of CRF via activation of CRF1 receptors in mediating, at least in part, the relation between social defeat stress and drug abuse vulnerability.
There is also support for a mechanistic role of OT in mediating the relation between adolescent social defeat and drug reward. One recent study found that adolescent social defeat enhanced cocaine CPP in individually caged male mice, and that pair-housing with a familiar male or a female prevented the enhanced cocaine CPP and produced a concomitant elevation in plasma OT (Ferrer-Perez et al., 2019). In another study by this same group, pretreatment with an OT antagonist during the period of pair-housing, but not immediately prior to social defeat, abolished the protective effect of social housing on cocaine CPP (Ferrer-Pérez et al., 2020). Similarly, OT administration is able to ameliorate the increase in alcohol drinking normally induced by adolescent social defeat (Reguilón et al., 2021). These mechanistic studies indicate that the increase in drug abuse vulnerability induced by social defeat directly involves, at least in part, a weakening of OT systems and that the increased risk may be ameliorated with OT treatment.
5.4. Summary
Clear evidence indicates that early life adversity modelled by maternal separation, social isolation or social defeat increases drug abuse vulnerability across all drug classes, although the evidence is more robust with stimulants and alcohol compared to opioids. As described in the previous section (Section 4), these changes in behavioral risk are associated with a strengthening of CRF systems and weakening of OT systems. Several relevant mechanistic studies support a direct role of these neuropeptide systems in mediating the relation between drug abuse and early life adversity modelled by either maternal separation or social defeat. However, mechanistic studies are still needed to determine if these neuropeptide systems also link drug abuse and adolescent social isolation.
6. Conclusion
The overall theme presented here is that early life social adversity increases drug abuse vulnerability later in life because such adversity causes, at least in part, a strengthening of CRF systems and a weakening of OT systems. Consistent with this, ample evidence shows that drug reward processing in adults is reduced by either CRF antagonists, particularly CRF1 receptor antagonists, or OT agonists. In addition, early life adversity modelled by maternal separation, adolescent social isolation or adolescent social defeat produces an increase in drug abuse vulnerability. The adversity-induced increase in risk is generally associated with a strengthening of CRF systems and a weakening of OT systems, although some negative findings are published. Importantly, although there are relatively few mechanistic studies conducted to date, they are consistent with the theme that the adversity-induced increase in drug reward (cocaine and alcohol) is directly related, as least in part, to increased CRF activity and decreased OT activity. However, with adolescent social isolation specifically, no published mechanistic studies have examined if the increase in drug abuse vulnerability is directly due to altered CRF and/or OT systems.
Conducting preclinical mechanistic studies to establish a direct role of CRF and OT systems in linking early life social adversity and drug abuse vulnerability could have translational value for treatment interventions in humans. Clinical work strongly implicates CRF and OT systems in the relation between early life trauma and drug abuse vulnerability (Fortunata Donadon et al., 2018; Kim et al., 2017), but critical proof-of-principle mechanistic studies are difficult to implement in human populations. Further, it is likely that there are interacting effects of CRF and OT following early life stress. For example, adult male smokers who experience early childhood adversity have elevated cortisol, an effect that is ameliorated by OT treatment (Hood et al., 2020). Thus, while substance use interventions that target CRF and OT systems hold great promise, additional preclinical and clinical studies are needed to determine how early life social adversity, combined with individual genetics, may be used to identify and target those at greatest risk.
HIGHLIGHTS.
CRF and OT are involved in drug reward in adulthood.
Early life social adversity strengthens CRF and weakens OT systems.
Early life social adversity increases drug abuse vulnerability in adulthood.
Changes in CRF and OT mediate the link between adversity and drug reward.
Mechanistic studies of CRF and OT systems may be translated into clinical practice.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R21 DA041755, T32 DA16176 and T32 DA035200.
ABBREVIATIONS:
- ACTH
adrenocorticotropic hormone
- Amyg
amygdala
- BNST
bed nucleus of the stria terminalis
- BS
brainstem
- CeA
central nucleus of the amygdala
- CORT
corticosterone
- CPP
conditioned place preference
- CRF
corticotropin-releasing factor
- CSF
cerebrospinal fluid
- Ctx
cortex
- DR
dorsal raphe
- GABA
gamma-aminobutyric acid
- Hipp
hippocampus
- HPA
hypothalamic-pituitary-adrenal
- Hypo
hypothalamus
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- i.v.
intravenous
- LC
locus coeruleus
- LS
lateral septum
- ME
median eminence
- MFB
medial forebrain bundle
- mPFC
medial prefrontal cortex
- mRNA
messenger ribonucleic acid
- MPOA
medial preoptic area
- NAc
nucleus accumbens
- OT
oxytocin
- PAG
periaqueductal gray
- PB
parabrachial nucleus
- PFC
prefrontal cortex
- Pit
pituitary
- PND
postnatal day
- PVN
paraventricular nucleus
- RN
raphe nucleus
- SA
self-administration
- s.c.
subcutaneous
- SC
spinal cord
- Sep
septum
- SON
supraoptic nucleus
- STh
subthalamic nucleus
- Str
striatum
- Thal
thalamus
- VMH
ventromedial hypothalamus
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ, 2008. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience 154, 1218–1226. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA, 1978. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol 182, 995–1015. [DOI] [PubMed] [Google Scholar]
- Altstein M, Gainer H, 1988. Differential biosynthesis and posttranslational processing of vasopressin and oxytocin in rat brain during embryonic and postnatal development. J Neurosci 8, 3967–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Khoei H, Amiri S, Mohammadi-Asl A, Alijanpour S, Poursaman S, Haj-Mirzaian A, Rastegar M, Mesdaghinia A, Banafshe HR, Sadeghi E, Samiei E, Mehr SE, Dehpour AR, 2017a. Experiencing neonatal maternal separation increased pain sensitivity in adult male mice: Involvement of oxytocinergic system. Neuropeptides 61, 77–85. [DOI] [PubMed] [Google Scholar]
- Amini-Khoei H, Mohammadi-Asl A, Amiri S, Hosseini M-J, Momeny M, Hassanipour M, Rastegar M, Haj-Mirzaian A, Mirzaian AH, Sanjarimoghaddam H, Mehr SE, Dehpour AR, 2017b. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Progress in neuro-psychopharmacology & biological psychiatry 76, 169–178. [DOI] [PubMed] [Google Scholar]
- Anita CH, Anne K, Stefanie U, Sina B, Esi D, Eva K, Roberto C, Robert CF, Valery G, Falk K, Wolfgang HS, Sabine V-K, Rainer S, 2017. Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans. Neuropsychopharmacology 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJ, Limpens JH, Vanderschuren LJ, 2013. Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babygirija R, Yoshimoto S, Gribovskaja-Rupp I, Bulbul M, Ludwig K, Takahashi T, 2012. Social interaction attenuates stress responses following chronic stress in maternally separated rats. Brain Res 1469, 54–62. [DOI] [PubMed] [Google Scholar]
- Bahi A, 2015. The oxytocin receptor impairs ethanol reward in mice. Physiology & Behavior 139, 321–327. [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Maamari E, 2016. Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiology & Behavior 164, 249–258. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Cornish JL, 2016. The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Front Neuroendocrinol 43, 1–18. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, Cornish JL, 2020. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci Biobehav Rev 110, 114–132. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL, 2012. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural Brain Research 228, 185–193. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Lerner SP, 1991. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus — comparison with somatostatin. International Journal of Developmental Neuroscience 9, 473–478. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Schultz L, 1992. CRH gene expression in the fetal rat is not increased after pharmacological adrenalectomy. Neuroscience Letters 142, 215–218. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Yi S, Avishai-Eliner S, Schultz L, 1997. Development neurobiology of the stress response: multilevel regulation of corticotropin-releasing hormone function. Annals of the New York Academy of Sciences 814, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C, 2001. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 155, 278–284. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH, 2013. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev 65, 255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E, 2015. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ, 2015. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Translational psychiatry 5, e606–e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ, 2010. Dopamine and Oxytocin Interactions Underlying Behaviors: Potential Contributions to Behavioral Disorders. CNS neuroscience & therapeutics 16, e92–e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu S, Pelletier G, Vaudry H, Barden N, 1989. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence Neuroendocrinology 49, 255–261. [DOI] [PubMed] [Google Scholar]
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R, 2011. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR, 2011. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 11396–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell KN, Imhof AM, Fisher PA, 2019. Early adversity, child neglect, and stress neurobiology: From observations of impact to empirical evaluations of mechanisms. International Journal of Developmental Neuroscience 78, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe AC, Oliver KM, Scholl JL, Forster GL, 2011. Anxiety states induced by post-weaning social isolation are mediated by CRF receptors in the dorsal raphe nucleus. Brain Research Bulletin 85, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Glasper ER, Neigh GN, 2014. SSRI or CRF antagonism partially ameliorate depressive-like behavior after adolescent social defeat. Behavioural Brain Research 270, 295–299. [DOI] [PubMed] [Google Scholar]
- Bowen MT, McGregor IS, 2014. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Int J Neuropsychopharmacol 17, 1621–1633. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z, 1991. Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav 39, 269–274. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, DeBold JF, Miczek KA, 2011. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology 218, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG, Cryan JF, 2011. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol 14, 666. [DOI] [PubMed] [Google Scholar]
- Broadbear J, Winger G, Woods J, 1999. Cocaine-reinforced responding in rhesus monkeys: Pharmacological attenuation of the hypothalamic-pituitary-adrenal axis response. Journal Of Pharmacology And Experimental Therapeutics 290, 1347–1355. [PubMed] [Google Scholar]
- Bugnon C, Fellmann D, Gouget A, Cardot J, 1982. Ontogeny of the corticoliberin neurogrlandular system in rat brain. Nature 298, 159–161. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Velis DN, Swaab DF, 1980. Ontogeny of vasopressin and oxytocin in the fetal rat: early vasopressinergic innervation of the fetal brain. Peptides 1, 315–324. [DOI] [PubMed] [Google Scholar]
- Burke A, DeBold J, Miczek K, 2016. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology 233, 2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA, 2013. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology (Berlin, Germany) 231, 1557–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA, 2015. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology (Berlin, Germany) 232, 3067–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Renner KJ, Forster GL, Watt MJ, 2010. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Research 1352, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Watt MJ, Forster GL, 2011. Adolescent social defeat increases adult amphetamine conditioned place preference and alters D2 dopamine receptor expression. Neuroscience 197, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Chini B, 2018. Molecular Basis of Oxytocin Receptor Signalling in the Brain: What We Know and What We Need to Know. Curr Top Behav Neurosci 35, 3–29. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM, 2013. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: A study on the consequences of social stress in rats. Neuroscience 249, 258–270. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, 2017. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist, 1073858417708284. [DOI] [PubMed] [Google Scholar]
- Campbell J, Spear LP, 1999. Effects of early handling on amphetamine-induced locomotor activation and conditioned place preference in the adult rat. Psychopharmacology 143, 183–189. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G, 2000. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci 20, Rc102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH, 2004. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29, 777. [DOI] [PubMed] [Google Scholar]
- Carson D, Cornish J, Guastella A, Hunt G, McGregor I, 2010. Oxytocin decreases methamphetamine self-ad ministration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58, 38–43. [DOI] [PubMed] [Google Scholar]
- Caruso MJ, McClintock MK, Cavigelli SA, 2014. Temperament moderates the influence of periadolescent social experience on behavior and adrenocortical activity in adult male rats. Hormones and behavior 66, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Evans AN, Liu Y, Honda M, Saavedra JM, Aguilera G, 2012. Maternal deprivation in rats is associated with corticotropin releasing hormone (CRH) promoter hypomethylation and enhances CRH transcriptional responses to stress in adulthood. Journal of neuroendocrinology 24, 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Verhage M, Grinevich V, 2017. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior. Trends Pharmacol Sci 38, 982–991. [DOI] [PubMed] [Google Scholar]
- Contarino A, Kitchener P, Vallee M, Papaleo F, Piazza P-V, 2017. CRF1 receptor-deficiency increases cocaine reward. Neuropharmacology 117, 41–48. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB, 1996. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America 93, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G, 2017. Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand. Biological Psychiatry 81, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM, 2013. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 38, 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, da S, Planeta C, Miczek KA, 2008. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology 201, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaveri F, Nakhaee N, Esmaeilpour K, Mahani SE, Sheibani V, 2017. Effects of maternal separation on nicotine-induced conditioned place preference and subsequent learning and memory in adolescent female rats. Neuroscience Letters 639, 151–156. [DOI] [PubMed] [Google Scholar]
- de Almeida Magalhães T, Correia D, de Carvalho LM, Damasceno S, Brunialti Godard AL, 2018. Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain and Behavior 8, e00841–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavari F, Sheibani V, Esmaeili-Mahani S, Nakhaee N, 2016. Maternal Separation and the Risk of Drug Abuse in Later Life. Addict Health 8, 107–114. [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG, 2008. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. International Journal of Developmental Neuroscience 26, 259–268. [DOI] [PubMed] [Google Scholar]
- Ding Y, Kang L, Li B, Ma L, 2005. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav 82, 673–677. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC, 2013. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, 2006. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol 494, 75–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH, 2013. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav 64, 693–701. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH, 2016. Presence and Absence of Sex Differences in Structure and Function of the Brain Oxytocin System: Implications for Understanding the Regulation of Social Behavior. Sex Differences in the Central Nervous System, pp. 247–295. [Google Scholar]
- Eiler WJ 2nd, Seyoum R, Foster KL, Mailey C, June HL, 2003. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse 48, 45–56. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J, 1999. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19, Rc35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett NA, Baracz SJ, Cornish JL, 2020. The effect of chronic oxytocin treatment during abstinence from methamphetamine self-administration on incubation of craving, reinstatement, and anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 45, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Herrera-Melendez AL, Pestke K, Feeser M, Aust S, Otte C, Pruessner JC, Böker H, Bajbouj M, Grimm S, 2014. Early life stress modulates amygdala-prefrontal functional connectivity: Implications for oxytocin effects. Human brain mapping 35, 5328–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Stein DJ, Daniels W, 2009. Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metabolic Brain Disease 24, 541–559. [DOI] [PubMed] [Google Scholar]
- Fernández MS, Carrizo J, Plaza W, Haeger P, Pautassi RM, 2019. Prenatal ethanol exposure potentiates isolation-induced ethanol consumption in young adult rats. Alcohol (Fayetteville, N.Y.) 75, 39–46. [DOI] [PubMed] [Google Scholar]
- Ferrer-Pérez C, Reguilón MD, Manzanedo C, Aguilar MA, Miñarro J, Rodríguez-Arias M, 2018. Antagonism of corticotropin-releasing factor CRF1 receptors blocks the enhanced response to cocaine after social stress. European Journal of Pharmacology 823, 87–95. [DOI] [PubMed] [Google Scholar]
- Ferrer-Perez C, Reguilon MD, Manzanedo C, Minarro J, Rodriguez-Arias M, 2019. Social Housing Conditions Modulate the Long-Lasting Increase in Cocaine Reward Induced by Intermittent Social Defeat. Front Behav Neurosci 13, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Pérez C, Reguilón MD, Miñarro J, Rodríguez-Arias M, 2020. Endogenous oxytocin is essential for the buffering effects of pair housing against the increase in cocaine reward induced by social stress. Physiology & Behavior 221. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Vázquez DM, Robinson TE, 2003. Manipulations During the Second, but not the First, Week of Life Increase Susceptibility to Cocaine Self-Administration in Female Rats. Neuropsychopharmacology 28, 1741–1751. [DOI] [PubMed] [Google Scholar]
- Forster GL, Anderson EM, Scholl JL, Lukkes JL, Watt MJ, 2018. Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiology of Stress 9, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunata Donadon M, Martín-Santos Laffon R, Lima Osório F. d., 2018. The associations between oxytocin and trauma in humans: A systematic review. [DOI] [PMC free article] [PubMed]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O’Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP, 2009. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci 29, 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Valentine JD, Sharp BM, 1997. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology 138, 1935–1943. [DOI] [PubMed] [Google Scholar]
- Furuta M, Ninomiya-Baba M, Chiba S, Funabashi T, Akema T, Kunugi H, 2015. Exposure to social defeat stress in adolescence improves the working memory and anxiety-like behavior of adult female rats with intrauterine growth restriction, independently of hippocampal neurogenesis. Hormones and behavior 70, 30–37. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Navarrete F, Aracil A, Bartoll A, Martínez-Gras I, Lanciego JL, Rubio G, Manzanares J, 2016. Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addiction biology 21, 847–858. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF, 2007. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A 104, 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G, 2001. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21, Rc160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G, 2002. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22, 5173–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald G, Falk F, 2001. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol Rev 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Giardino W, Ryabinin AE, 2013. CRF1 Receptor Signaling Regulates Food and Fluid Intake in the Drinking-in-the-Dark Model of Binge Alcohol Consumption. Alcoholism-Clinical And Experimental Research 37, 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles YD, Polston EK, 2017. Effects of social deprivation on social and depressive-like behaviors and the numbers of oxytocin expressing neurons in rats. Behavioural Brain Research 328, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano de G, Marsida K, Matthew BP, Elena C, Sierra S, Paul S, George FK, Robert OM, Olivier G, 2019. Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nature communications 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT, 2011. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 214, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF, 2000. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology (New York, N.Y.) 23, 577–586. [DOI] [PubMed] [Google Scholar]
- Gondré-Lewis MC, Warnock KT, Wang H, June HL, Bell KA, Rabe H, Tiruveedhula VVNPB, Cook J, Lüddens H, Aurelian L, 2016. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA A mechanism. Stress (Amsterdam, Netherlands) 19, 235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham YP, Heim C, Goodman SH, Miller AH, Nemeroff CB, 1999. The effects of neonatal stress on brain development: implications for psychopathology. Development and Psychopathology 11, 545–565. [DOI] [PubMed] [Google Scholar]
- Greenwell T, Funk C, Cottone P, Richardson H, Chen SA, Rice K, Zorrilla E, Koob G, 2009. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addiction biology 14, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Desarmenien MG, Chini B, Tauber M, Muscatelli F, 2015. Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front Neuroanat 8, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B, 2016. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol Psychiatry 79, 155–164. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Neumann ID, 2020. Brain oxytocin: how puzzle stones from animal studies translate into psychiatry. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grino M, Young WS, Burgunder J, 1989. Ontogenyof expression of the cortico- releasing factor gene in the hypothalamic paraventricular nucleus of the proopiomelanocortin gene in rat pituitary. Endocrinology 124, 60–68. [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang L, Yuan W, Li L, Zhang J, Hou W, Yang Y, Zhang X, Cai W, Ma H, Xun Y, Jia R, He Z, Tai F, 2020. Different effects of chronic social defeat on social behavior and the brain CRF system in adult male C57 mice with different susceptibilities. Behavioural Brain Research 384. [DOI] [PubMed] [Google Scholar]
- Hall FS, 1998. Social deprivation of neonatal, adolescent and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews in Neurobiology 12, 129–162. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW, 1999. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse (New York, N.Y.) 32, 37–43. [DOI] [PubMed] [Google Scholar]
- Han RT, Kim YB, Park EH, Kim JY, Ryu C, Kim HY, Lee J, Pahk K, Shanyu C, Kim H, Back SK, Kim HJ, Kim YI, Na HS, 2018. Long-Term Isolation Elicits Depression and Anxiety-Related Behaviors by Reducing Oxytocin-Induced GABAergic Transmission in Central Amygdala. Front Mol Neurosci 11, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, DeBold J, Miczek K, 2017. Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 234, 2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Regenass W, Dreyer W, Möller M, 2019. Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: Role of corticosterone, oxytocin, and vasopressin. Journal of psychopharmacology (Oxford) 33, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ, 1998. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. J Neuroendocrinol 10, 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Wang Z, Guo G, 2018. Postnatal separation prevents the development of prenatal stress-induced anxiety in association with changes in oestrogen receptor and oxytocin immunoreactivity in female mandarin vole (Microtus mandarinus) offspring. Eur J Neurosci 47, 95–108. [DOI] [PubMed] [Google Scholar]
- Heck AL, Crestani CC, Fernández-Guasti A, Larco DO, Mayerhofer A, Roselli CE, 2018. Neuropeptide and steroid hormone mediators of neuroendocrine regulation. Journal of neuroendocrinology 30, e12599–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ, 1995. Effects of cocaine on cortisol secretion in humans. The American journal of the medical sciences 310, 61. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2009. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry 14, 954–958. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U, 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry (1969) 54, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Tai F, Carter KA, Watanasriyakul WT, Gallimore DM, Molina AL, Schiml PA, 2019. Central oxytocin alters cortisol and behavioral responses of guinea pig pups during isolation in a novel environment. Physiol Behav 212, 112710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Powell BJ, 1976. Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav 5, 701–704. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Chow JJ, Beckmann JS, Bardo MT, 2017. Effects of environmental enrichment on self-administration of the short-acting opioid remifentanil in male rats. Psychopharmacology 234, 3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA, 2016. Episodic Social Stress-Escalated Cocaine Self-Administration: Role of Phasic and Tonic Corticotropin Releasing Factor in the Anterior and Posterior Ventral Tegmental Area. J Neurosci 36, 4093–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubová A, Poništ S, Jureòvieòvá J, Šlamberová R, 2019. Different oxytocin responses to acute methamphetamine treatment in juvenile female rats perinatally exposed to stress and/or methamphetamine administration. Frontiers in physiology 10, 305–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood CO, Tomko RL, Baker NL, Tuck BM, Flanagan JC, Carpenter MJ, Gray KM, Saladin ME, McClure EA, 2020. Examining sex, adverse childhood experiences, and oxytocin on neuroendocrine reactivity in smokers. Psychoneuroendocrinology 120, 104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE, 2013. The CRF system and social behavior: a review. Front Neurosci 7, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, He Z, Yang Y, Yuan W, Wang L, Zhang J, Zhang X, Cai W, Guo Q, Tai F, 2020. The involvement of oxytocin in the effects of chronic social defeat stress on emotional behaviours in adult female mandarin voles. European Journal of Neuroscience 52, 2853–2872. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ, 2000. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 151, 55–63. [DOI] [PubMed] [Google Scholar]
- Hu P, Maita I, Phan ML, Gu E, Kwok C, Dieterich A, Gergues MM, Yohn CN, Wang Y, Zhou JN, Qi XR, Swaab DF, Pang ZP, Lucassen PJ, Roepke TA, Samuels BA, 2020. Early-life stress alters affective behaviors in adult mice through persistent activation of CRH-BDNF signaling in the oval bed nucleus of the stria terminalis. Translational psychiatry 10, 396–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR, 2010. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther 332, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dolen G, Malenka RC, 2017. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot R, K, T., Meaney M, Plotsky P, 2001. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berlin, Germany) 158, 366–373. [DOI] [PubMed] [Google Scholar]
- Hwa L, DeBold J, Miczek K, 2013. Alcohol in excess: CRF 1 receptors in the rat and mouse VTA and DRN. Psychopharmacology 225, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimov R, Kovács GL, Szabó G, Telegdy G, 1987. Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: a receptor-mediated event? Life Sciences 41, 1265–1271. [DOI] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Fairbanks DW, De Souza EB, 1988. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci 8, 4151–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Su W, Zhou R, Feng J, Lin Y, Zhang Y, Wang X, Chen X, Li J, 2016. Intranasal oxytocin administration improves depression-like behaviors in adult rats that experienced neonatal maternal deprivation. Behavioural Pharmacology 27, 689–696. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Buisman-Pijlman FT, 2016. Adversity impacting on oxytocin and behaviour: timing matters. Behav Pharmacol 27, 659–671. [DOI] [PubMed] [Google Scholar]
- Joushi S, Esmaeilpour K, Masoumi-Ardakani Y, Esmaeili-Mahani S, Sheibani V, 2021. Intranasal oxytocin administration facilitates the induction of long-term potentiation and promotes cognitive performance of maternally separated rats. Psychoneuroendocrinology 123, 105044–105044. [DOI] [PubMed] [Google Scholar]
- Jurek B, Neumann ID, 2018. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol Rev 98, 1805–1908. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME, 2009. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 14, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EA, Fudge JL, 2018. The neuroanatomic complexity of the CRF and DA systems and their interface: What we still don’t know. Neurosci Biobehav Rev 90, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaji S, Bigdeli I, Ghorbani R, Miladi-Gorji H, 2018. Research Paper: Environmental enrichment attenuates morphine-induced conditioned place preference and locomotor sensitization in maternally separated rat pups. Basic and clinical neuroscience 9, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJV, Strathearn L, 2017. Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Annals of the New York Academy of Sciences 1394, 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC, 2017. Oxytocin Reduces Ethanol Self-Administration in Mice. Alcoholism: Clinical and Experimental Research 41, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Strasburger CJ, 1992. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sciences 50, 435–442. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V, 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V, 2014. Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in behavioral neuroscience 8, 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ, 2008. The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol 583, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács GL, Borthaiser Z, Telegdy G, 1985. Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sciences 37, 17–26. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Van Ree JM, 1985. Behaviorally active oxytocin fragments simultaneously attenuate heroin self-administration and tolerance in rats. Life Sciences 37, 1895–1900. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF, 1998. Drug dependence: stress and dysregulation of brain reward pathways. Drug and Alcohol Dependence 51, 23–47. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB, 1996. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology (Philadelphia) 137, 1212–1218. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, 2009. Does physical abuse in early childhood predict substance use in adolescence and early adulthood?. Child Maltreatment 15, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasheras MC, Laorden ML, Milanés MV, Núñez C, 2015. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology 95, 168–180. [DOI] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y, 2000. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150, 317–324. [DOI] [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, Salmeron BJ, 2014. Complexity of oxytocin’s effects in a chronic cocaine dependent population. European Neuropsychopharmacology 24, 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MCH, Zanettini C, Coggiano MA, Leggio L, Tanda G, 2019. Effect of systemically administered oxytocin on dose response for methylphenidate self-administration and mesolimbic dopamine levels. Annals of the New York Academy of Sciences 1455, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Weerts EM, 2016. Oxytocin for the treatment of drug and alcohol use disorders. Behav Pharmacol 27, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Geracioti TD Jr, Kasckow JW, Coccaro EF, 2005. Childhood trauma and personality disorder: Positive correlation with adult CSF corticotropin-releasing factor concentrations. American Journal of Psychiatry 162, 995–997. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Gollan J, Kasckow J, Geracioti T, Coccaro EF, 2006. CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31,2289. [DOI] [PubMed] [Google Scholar]
- Lemos C, Salti A, Amaral IM, Fontebasso V, Singewald N, Dechant G, Hofer A, El Rawas R, 2020. Social interaction reward in rats has anti-stress effects. Addiction biology, e12878–e12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM, 2016. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol 24, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JS, Toni Z, Michael JC, Ashley RP, Seth DP, 2014. Stress-Induced Elevation of Oxytocin in Maltreated Children: Evolution, Neurodevelopment, and Social Behavior. Child development 85, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L-F, Yuan W, He Z-X, Ma H, Xun Y-F, Meng L-R, Zhu S-J, Wang L-M, Zhang J, Cai W-Q, Zhang X-N, Guo Q-Q, Lian Z-M, Jia R, Tai F-D, 2019. Reduced Consolation Behaviors in Physically Stressed Mandarin Voles: Involvement of Oxytocin, Dopamine D2, and Serotonin 1A Receptors Within the Anterior Cingulate Cortex. International Journal of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery E, Spanos M, Navarro M, Lyons A, Hodge C, Thiele T, 2010. CRF-1 Antagonist and CRF-2 Agonist Decrease Binge-Like Ethanol Drinking in C57BL/6J Mice Independent of the HPA Axis. Neuropsychopharmacology 35, 1241–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Ceng X, Huang M, 2000. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport 11, 2373–2378. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X, 2001. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol 415, 203–208. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z, 2003. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. Journal of Neurochemistry 84, 1378–1386. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH, 2010. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 58, 78–87. [DOI] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G, 2009a. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci 29, 9955–9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL, 2009b. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Hormones and behavior 55, 248–256. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL, 2009c. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience 158, 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfadyen K, Loveless R, Delucca B, Wardley K, Deogan S, Thomas C, Peris J, 2016. Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacology, Biochemistry and Behavior 140, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggie B, Stephen GL, Carlos AD, Zhifeng Z, Qiaoping Y, Melanie LS, Isaac M-C, Elizabeth AS, Annika P, Pier Francesco F, Ravi Kumar S, Muslima R, Wolfgang HS, Juan FL, Robert CT, David G, Markus H, Higley JD, Stephen JS, Christina SB, 2017. Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proceedings of the National Academy of Sciences - PNAS 114, 11769–11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW, 1995. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136, 3299–3309. [DOI] [PubMed] [Google Scholar]
- Mansouri M, Pouretemad H, Roghani M, Wegener G, Ardalan M, 2020a. Autistic-like behaviours and associated brain structural plasticity are modulated by oxytocin in maternally separated rats. Behav Brain Res 393, 112756. [DOI] [PubMed] [Google Scholar]
- Mansouri M, Pouretemad H, Roghani M, Wegener G, Ardalan M, 2020b. Autistic-like behaviours and associated brain structural plasticity are modulated by oxytocin in maternally separated rats. Behavioural Brain Research 393, 112756–112756. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJP, Nylander I, Fahlke C, 2004. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Developmental psychobiology 45, 140–152. [DOI] [PubMed] [Google Scholar]
- Matta SG, Singh J, Sharp BM, 1990. Catecholamines mediate nicotine-induced adrenocorticotropin secretion via alpha-adrenergic receptors. Endocrinology 127, 1646–1655. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM, 2009. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res 33, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Green MR, Simone JJ, 2017. Translational relevance of rodent models of hypothalamic-pituitary-adrenal function and stressors in adolescence. Neurobiology of Stress 6, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Smith R, 2001. Corticotrophin-releasing hormone and human parturition. Reproduction (Cambridge, England) 121, 493–501. [DOI] [PubMed] [Google Scholar]
- Melchior M, Juif PE, Gazzo G, Petit-Demouliere N, Chavant V, Lacaud A, Goumon Y, Charlet A, Lelievre V, Poisbeau P, 2018. Pharmacological rescue of nociceptive hypersensitivity and oxytocin analgesia impairment in a rat model of neonatal maternal separation. Pain 159, 2630–2640. [DOI] [PubMed] [Google Scholar]
- Mello N, Negus SS, Rice K, Mendelson J, 2006. Effects of the CRF1 antagonist antalarmin on cocaine self-administration and discrimination in rhesus monkeys. Pharmacology Biochemistry And Behavior 85, 744–751. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA Jr., 2006. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci 26, 3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Ogata M, Mello NK, 1971. Adrenal function and alcoholism. Psychosomatic Medicine 33, 145–158. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG, 2008. Early Postnatal Stress Alters Place Conditioning to Both μ- and κ-Opioid Agonists. Journal Of Pharmacology And Experimental Therapeutics 325, 313–318. [DOI] [PubMed] [Google Scholar]
- Miller MA, Bershad A, King A, Lee R, de Wit H, 2016. Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: a pilot study. Behavioural Pharmacology 27, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab M, Hyland BI, Brown CH, 2015. Oxytocin enhances the expression of morphine-induced conditioned place preference in rats. Psychoneuroendocrinology 53, 159–169. [DOI] [PubMed] [Google Scholar]
- Montagud-Romero S, Nuñez C, Blanco-Gandia MC, Martínez-Laorden E, Aguilar MA, Navarro-Zaragoza J, Almela P, Milanés M-V, Laorden M-L, Miñarro J, Rodríguez-Arias M, 2017. Repeated social defeat and the rewarding effects of cocaine in adult and adolescent mice: dopamine transcription factors, proBDNF signaling pathways, and the TrkB receptor in the mesolimbic system. Psychopharmacology 234, 2063–2075. [DOI] [PubMed] [Google Scholar]
- Montagud-Romero S, Blanco-Gandía MC, Reguilón MD, Ferrer-Pérez C, Ballestín R, Miñarro J, Rodríguez-Arias M, 2018. Social defeat stress: Mechanisms underlying the increase in rewarding effects of drugs of abuse. The European journal of neuroscience 48, 2948–2970. [DOI] [PubMed] [Google Scholar]
- Morel LJ, Giros B, Daugé V, 2009. Adolescent Exposure to Chronic Delta-9-Tetrahydrocannabinol Blocks Opiate Dependence in Maternally Deprived Rats. Neuropsychopharmacology (New York, N.Y.) 34, 2469–2476. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Fischer D, Holsboer F, Patchev AV, Almeida OFX, Wu Y, Micale V, Spengler D, Wotjak CT, Bockmühl Y, 2009. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience 12, 1559–1566. [DOI] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Núñez C, Ruiz-Medina J, Laorden ML, Valverde O, Milanés MV, 2011. CRF2 mediates the increased noradrenergic activity in the hypothalamic paraventricular nucleus and the negative state of morphine withdrawal in rats. British journal of pharmacology 162, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal S, Kent M, Bardi M, Lambert KG, 2018. Enriched environment exposure enhances social interactions and oxytocin responsiveness in male long-evans rats. Frontiers in behavioral neuroscience 12, 198–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EL, Leonard MZ, Arena DT, de Almeida RMM, Miczek KA, 2018. Social defeat stress and escalation of cocaine and alcohol consumption: Focus on CRF. Neurobiology of Stress 9, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick EG, Glenn FG, 2000. Effects of the CRH Receptor Antagonist CP-154,526 on Intravenous Cocaine Self-administration in Rats. Neuropsychopharmacology 23, 577. [DOI] [PubMed] [Google Scholar]
- Nylander I, Roman E, 2013. Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consumption? Psychopharmacology (Berlin, Germany) 229, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, Dinan T, Cryan J, 2011. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology 214, 221–229. [DOI] [PubMed] [Google Scholar]
- Okada S, Shimizu T, Yokotani K, 2003. Extrahypothalamic corticotropin-releasing hormone mediates (−)-nicotine-induced elevation of plasma corticosterone in rats. Eur J Pharmacol 473, 217–223. [DOI] [PubMed] [Google Scholar]
- Olive F, Mehmert K, Koenig H, Camarini R, Kim J, Nannini M, Ou C, Hodge C, 2003. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacology 165, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira V. E. d. M., Neumann ID, de Jong TR, 2019. Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology 156, 107504–107504. [DOI] [PubMed] [Google Scholar]
- Oreland S, Gustafsson-Ericson L, Nylander I, 2010. Short- and long-term consequences of different early environmental conditions on central immunoreactive oxytocin and arginine vasopressin levels in male rats. Neuropeptides (Edinburgh) 44, 391–398. [DOI] [PubMed] [Google Scholar]
- Palmer A, Sharpe A, Burkhart-Kasch S, McKinnon C, Coste S, Stenzel-Poore M, Phillips T, 2004. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology 176, 386–397. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z, 2009. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neuroscience Letters 454, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, Koob GF, 2015. Chronic CRF 1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addiction biology 20, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF, 2009. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry research 178, 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC, 2013. Intranasal Oxytocin Blocks Alcohol Withdrawal in Human Subjects. Alcoholism: Clinical and Experimental Research 37, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG, 2017. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol 525, 1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID, 2017. Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction biology 22, 702–711. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ, 1994. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berl) 115, 407–418. [DOI] [PubMed] [Google Scholar]
- Pinelli CJ, Leri F, Turner PV, 2017. Long term physiologic and behavioural effects of housing density and environmental resource provision for adult male and female sprague dawley rats. Animals (Basel) 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ, 1993. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research. Molecular brain research. 18, 195–200. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ, 2005. Long-Term Consequences of Neonatal Rearing on Central Corticotropin-Releasing Factor Systems in Adult Male Rat Offspring. Neuropsychopharmacology (New York, N.Y.) 30, 2192–2204. [DOI] [PubMed] [Google Scholar]
- Portero-Tresserra M, Gracia-Rubio I, Cantacorps L, Pozo OJ, Gómez-Gómez A, Pastor A, López-Arnau R, de la Torre R, Valverde O, 2018. Maternal separation increases alcohol-drinking behaviour and reduces endocannabinoid levels in the mouse striatum and prefrontal cortex. European Neuropsychopharmacology 28, 499–512. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, Yee J, Azizi F, Carter CS, 2013. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides 43, 20–26. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Filip M, Frankowska M, Zaniewska M, Papla I, 2005. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides (Edinburgh) 39, 525–533. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang J, Wang F, Zhao Y, Song M, Wu C, 2009. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 56, 856–865. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Caminer A, Goodwin J, McGregor IS, 2015. Oxytocin and MDMA (‘Ecstasy’) enhance social reward in rats. Psychopharmacology (Berl) 232, 2631–2641. [DOI] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Henry A, Villarreal T, Bachevalier J, Wallen K, Sanchez MM, 2014. Neonatal amygdala lesions lead to increased activity of brain CRF systems and hypothalamic-pituitary-adrenal axis of juvenile rhesus monkeys. J Neurosci 34, 11452–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguilón MD, Ferrer-Pérez C, Miñarro J, Rodríguez-Arias M, 2021. Oxytocin reverses ethanol consumption and neuroinflammation induced by social defeat in male mice. Hormones and behavior 127, 104875–104875. [DOI] [PubMed] [Google Scholar]
- Ritchey RL, Hennessy MB, 1987. Cortisol and behavioral responses to separation in mother and infant guinea pigs. Behavioral and neural biology 48, 1–12. [DOI] [PubMed] [Google Scholar]
- Rivera-Irizarry JK, Skelly MJ, Pleil KE, 2020. Social Isolation Stress in Adolescence, but not Adulthood, Produces Hypersocial Behavior in Adult Male and Female C57BL/6J Mice. Frontiers in behavioral neuroscience 14, 129–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros-Barrera I, Duenas Z, 2016. Maternal separation during nursing alters basal neuroendocrine levels in juvenile and adult rats. Biomedica 36, 67–77. [DOI] [PubMed] [Google Scholar]
- Roberto M, Spierling SR, Kirson D, Zorrilla EP, 2017. Corticotropin-Releasing Factor (CRF) and Addictive Behaviors. International review of neurobiology 136, 5–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Perez-Heydrich C, Thiele T, 2019. Corticotropin Releasing Factor Type 1 and 2 Receptor Signaling in the Medial Prefrontal Cortex Modulates Binge-Like Ethanol Consumption in C57BL/6J Mice. Brain Sciences 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Arias M, Montagud-Romero S, Rubio-Araiz A, Aguilar MA, Martín-García E, Cabrera R, Maldonado R, Porcu F, Colado MI, Miñarro J, 2017. Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood-brain barrier. Addiction biology 22, 129–141. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Navarrete F, Blanco-Gandia MC, Arenas MC, Bartoll-Andres A, Aguilar MA, Rubio G, Minarro J, Manzanares J, 2016. Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addiction biology 21, 87–97. [DOI] [PubMed] [Google Scholar]
- Roque A, Ruiz-González R, Pineda-López E, Torner L, Lajud N, 2019. Prenatal immobilization stress and postnatal maternal separation cause differential neuroendocrine responses to fasting stress in adult male rats. Developmental psychobiology 62, 737–748. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ, 2009. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162, 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C, 2007. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and behavior 51, 54–61. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró É, Penke B, Telegdy G, 1992. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Research 589, 154–156. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z, 1987. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett 81, 227–231. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ, 2003. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology 28, 985–994. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Oitzl MS, Muller MB, Ohl F, Wurst W, Holsboer F, Levine S, De Kloet ER, 2003. Regulation of the developing hypothalamic-pituitary-adrenal axis in corticotropin releasing hormone receptor 1-deficient mice. Neuroscience 119, 589–595. [DOI] [PubMed] [Google Scholar]
- Shaham Y, de Wit H, 2016. Lost in Translation: CRF1 Receptor Antagonists and Addiction Treatment. Neuropsychopharmacology 41, 2795–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Insel TR, 1989. Ontogeny of oxytocin receptors in rat forebrain: a quantitative study. Synapse 4, 259–266. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, Weiner JL, 2015. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: Possible role of disrupted noradrenergic signaling. Neuropharmacology 97, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF, 2006. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: Gender-dependent effects. Brain Research 1097, 123–132. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z, 2014. Hypothalamic Oxytocin Mediates Social Buffering of the Stress Response. Biological psychiatry (1969) 76, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio S, Wee S, O’Dell L, Boutrel B, Zorrilla E, Koob G, 2008. CRF 1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology 196, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling SR, Zorrilla EP, 2017. Don’t stress about CRF: assessing the translational failures of CRF(1)antagonists. Psychopharmacology (Berl) 234, 1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrka J, Gugula A, Rak A, Tylko G, Hess G, Blasiak A, 2020. Early life stress- induced alterations in the activity and morphology of ventral tegmental area neurons in female rats. Neurobiol Stress 13, 100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT, 2009. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav 92, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Prendergast MA, Bardo MT, 2011. Environmental-induced differences in corticosterone and glucocorticoid receptor blockade of amphetamine self-administration in rats. Psychopharmacology 218, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Trainor BC, 2017. Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Seminars in cell & developmental biology 61, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, Bohidar AE, Ndem JR, Vogel IR, O’Kane CM, 2017. Oxytocin reduces alcohol consumption in prairie voles. Physiology & Behavior 179, 411–421. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Mertens CE, Mayes L, Rutherford H, Rajhans P, Xu G, Potenza MN, Kim S, 2019. Pathways Relating the Neurobiology of Attachment to Drug Addiction. Frontiers in psychiatry 10, 737–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, 1995. Development of the human hypothalamus. Neurochem Res 20, 509–519. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, Takahashi K, Hidaka C, Tomita K, Yuri K, 2019. Effects of post-weaning social isolation on social behaviors and oxytocinergic activity in male and female rats. Heliyon 5, e01646–e01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Osako Y, Yuri K, 2010. Juvenile social experience regulates central neuropeptides relevant to emotional and social behaviors. Neuroscience 166, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Tanda G, Rohn MCH, Newman A, Coggiano M, Zanettini C, Katz J, Leggio L, Lee M, 2017. Systemic Oxytocin Attenuates Methylphenidate Self-Administration and Potentiates Its Effects on Dopamine in the Nucleus Accumbens Shell in Rats. Faseb Journal 31. [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss U, Rujescu D, Skowronek M, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G, 2006. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Molecular Psychiatry 11, 594–602. [DOI] [PubMed] [Google Scholar]
- Tsuda MC, Yamaguchi N, Ogawa S, 2011. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 22, 259–263. [DOI] [PubMed] [Google Scholar]
- Tunstall B, Kirson D, Zallar L, McConnell SA, Vendruscolo J, Ho CP, Lee M, Leggio L, Koob G, Roberto M, Vendruscolo L, 2019. CENTRALLY-MEDIATED EFFECTS OF OXYTOCIN BLOCK COMPULSIVE-LIKE ALCOHOL DRINKING IN RATS. Alcoholism-Clinical And Experimental Research 43, 274A–274A. [Google Scholar]
- Uribe KP, Correa VL, Pinales BE, Flores RJ, Cruz B, Shan Z, Bruijnzeel AW, Khan AM, O’Dell LE, 2020. Overexpression of corticotropin-releasing factor in the nucleus accumbens enhances the reinforcing effects of nicotine in intact female versus male and ovariectomized female rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 45, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree JM, De Wied D, 1977. Modulation of heroin self-administration by neurohypophyseal principles. European Journal of Pharmacology 43, 199–202. [DOI] [PubMed] [Google Scholar]
- Vannan A, Powell GL, Scott SN, Pagni BA, Neisewander JL, 2018. Animal Models of the Impact of Social Stress on Cocaine Use Disorders. International review of neurobiology 140, 131–169. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, Levine S, 2006a. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: Effect of maternal deprivation. Brain Research 1121, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Dauge V, 2006b. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behavioural Pharmacology 17, 715–724. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID, 2007. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 32, 437–450. [DOI] [PubMed] [Google Scholar]
- Vena A, King A, Lee R, Wit H, 2018. Intranasal Oxytocin Does Not Modulate Responses to Alcohol in Social Drinkers. Alcoholism: Clinical and Experimental Research 42, 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ, 2015. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125, 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitsky A, Dopfel D, Zhang N, 2020. Rodent models of post-traumatic stress disorder: behavioral assessment. Translational psychiatry 10, 132–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Pina M, Kash TL, Winder DG, 2017. The bed nucleus of the stria terminalis in drug-associated behavior and affect: A circuit-based perspective. Neuropharmacology 122, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cunningham AM, Gregory JK, Nestler EJ, 2019. Long-Term Behavioral Effects of Post-weaning Social Isolation in Males and Females. Frontiers in behavioral neuroscience 13, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters H, Kosten TA, 2019. Early life stress and the propensity to develop addictive behaviors. International Journal of Developmental Neuroscience 78, 156–169. [DOI] [PubMed] [Google Scholar]
- Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q, Sun R, 2014. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PloS one 9, e94394–e94394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR, 1997. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus). Synapse 27, 14–25. [DOI] [PubMed] [Google Scholar]
- Warfvinge K, Krause D, Edvinsson L, 2020. The distribution of oxytocin and the oxytocin receptor in rat brain: relation to regions active in migraine. Journal of headache and pain 21, 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong K-C, Peris J, Knackstedt L, Reichel CM, 2018. Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats. Int J Neuropsychopharmacol 21,677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Ma L, Ju P, Yang B, Wang Y-X, Chen J, 2020. Involvement of Oxytocin Receptor/Erk/MAPK Signaling in the mPFC in Early Life Stress-Induced Autistic-Like Behaviors. Frontiers in cell and developmental biology 8, 564485–564485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold LL, Sharpe LG, Jaffe JH, 1993. Housing conditions influence acquisition of sufentanil aerosol self-administration in rats. Pharmacol Biochem Behav 44, 141–144. [DOI] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S, 2010. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain research 1343, 83–92. [DOI] [PubMed] [Google Scholar]
- Wemm SE, Sinha R, 2019. Drug-induced stress responses and addiction risk and relapse. Neurobiology of Stress 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker Leslie R., Degoulet M, Morikawa H, 2013. Social Deprivation Enhances VTA Synaptic Plasticity and Drug-Induced Contextual Learning. Neuron (Cambridge, Mass.) 77, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Winslow JT, Noble PL, Noble PL, Lyons CK, Lyons CK, Sterk SM, Sterk SM, Insel TR, Insel TR, 2003. Rearing Effects on Cerebrospinal Fluid Oxytocin Concentration and Social Buffering in Rhesus Monkeys. Neuropsychopharmacology 28, 910–918. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA, 1996. Effect of social isolation on the reinforcing properties of morphine in the conditioned place preference test. Pharmacology, Biochemistry and Behavior 53, 531–534. [DOI] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y, 2017. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 95, 368–384.e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Qin B, Shi A, Zhao J, Guo X, Dong L, 2018. Oxytocin inhibited stress induced visceral hypersensitivity, enteric glial cells activation, and release of proinflammatory cytokines in maternal separated rats. Eur J Pharmacol 818, 578–584. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ, 1994. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Molecular and cellular neurosciences 5, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ohnishi R, Tsuneoka Y, Yamamoto-Mimura Y, Muramatsu R, Kato T, Funato H, Kuroda KO, 2018. Corticotropin-releasing factor receptor 1 in the anterior cingulate cortex mediates maternal absence-induced attenuation of transport response in mouse pups. Frontiers in Cellular Neuroscience 12, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Kimura T, Watanabe D, Kiyama H, 1996. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neuroscience Research 24, 291–304. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S, 2009. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience 163, 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, 2017. Consequences of early adverse rearing experience(EARE) on development: insights from non-human primate studies. Zoological research 38, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF, 2014. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol 35, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]


