Abstract
Introduction
A clinical trial (RACAT) reported the noninferiority of triple therapy compared to biologic agents (etanercept + methotrexate), and previous studies confirmed that biologic disease-modifying antirheumatic drugs (bDMARDs) are more expensive but less beneficial than triple therapy for patients with rheumatoid arthritis (RA) in whom methotrexate (MTX) fails. However, from the perspective of the Chinese healthcare system, the cost-effectiveness of triple therapy versus bDMARD treatment sequences as a first-line therapy for patients with RA is still unclear.
Methods
An individual patient simulation model was used to extrapolate the lifetime cost and health outcomes by tracing patients from initial treatment through switches to further treatment lines in a sequence. Therapeutic efficacy and physical function were evaluated using the American College of Rheumatology (ACR) response, 28-Joint Disease Activity Score (DAS28), and Health Assessment Questionnaire score. All input parameters in the model were derived from published studies, national databases, local hospitals, and experts’ opinions. Both direct costs and indirect costs were taken into consideration. Probabilistic and one-way sensitivity analyses were performed to test the uncertainty of the model, as were multiple scenario analyses.
Results
The lifetime analysis demonstrated that triple therapy was associated with lower costs and quality-adjusted life years (QALYs) than bDMARD sequences. These resulted in incremental cost-effectiveness ratios (ICERs) ranging from $87,090/QALY to $104,032/QALY, higher than the willingness-to-pay (WTP) threshold in China ($30,950/QALY). The baseline DAS28 impacted the model outcomes the most. Scenario analyses indicated that adding triple therapy to bDMARD sequences as a first-, second-, third-, or fourth-line therapy is very cost-effective, at a WTP of $10,316/QALY.
Conclusions
From a Chinese payer perspective, triple therapy as first-line treatment in treatment sequence could be regarded as cost-effectiveness option for patients who failed MTX, compared to bDMARDs as first-line treatment, and instead of prescribing triple therapy as a substitute for bDMARDs as a first-line treatment, adding triple therapy to the bDMARD treatment sequence is likely to be very cost-effective for patients with active RA compared to bDMARD sequences.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00300-4.
Keywords: Biologic treatment sequence, Cost-effectiveness analysis, Rheumatoid arthritis, Triple therapy
Key Summary Points
| Why carry out this study? |
| Rheumatoid arthritis (RA) as a chronic autoimmune disease that can occur at any age not only causes a decline in patients' physical function, quality of life, and social participation, but also places a major economic burden on patients' families and society. |
| From the perspective of the Chinese healthcare system, the cost-effectiveness of triple therapy versus biologic disease-modifying antirheumatic drugs (bDMARD) treatment sequences as a first-line therapy for patients with RA is still unclear. |
| We hypothesize that triple therapy could likely be cost-effective compared to bDMARD sequences as a first-line treatment for patients with RA unresponsive to MTX. |
| What was learned from the study? |
| From a Chinese payer perspective, triple therapy as first-line treatment in treatment sequences is likely to be a cost-effective option comparing bDMARDs as first-line treatment for RA patients who failed MTX. |
| Instead of prescribing triple therapy as a substitute for bDMARDs as a first-line treatment, adding triple therapy to the bDMARDs treatment sequence is likely to be very cost-effective for patients with active RA compared to bDMARDs sequences. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14186810.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that can occur at any age, with a high incidence in patients ranging from 30 to 50 years old [1, 2]. The incidence of RA is 0.5–1% worldwide, while the prevalence is 0.28% in China, indicating that the total number of Chinese patients is approximately 4 million; additionally, the ratio of affected males and females is approximately 1:4 [2–4]. In China, the disability rates of RA patients with disease durations of 1–5 years, 5–10 years, 10–15 years, and ≥ 15 years are 18.6, 43.5, 48.1, and 61.3%, respectively [5]. With increasing disease duration, the incidence of disability and functional limitation increases [5]. The average direct cost per RA patient is $1917.21 ± $2559.06/year, with drug costs accounting for more than 50% of the total cost ($1283.89 ± $1898.15) [6]. Therefore, RA not only causes a decline in patients' physical function, quality of life (QoL), and social participation, but also places a major economic burden on patients' families and society [7, 8].
For patients with active RA, although methotrexate (MTX) as the conventional disease-modifying antirheumatic drug (cDMARD) prescribed most commonly, the use of MTX is limited because of poor tolerability and inadequate efficacy [9, 10]. Then, a combination of cDMARDs, such as triple therapy with MTX, sulfasalazine, and hydroxychloroquine, was considered for use in RA patients who have a suboptimal response to MTX [11]. After the failure of monotherapy or a combination of cDMARDs, biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor (TNF) and non-TNF inhibitors, are recommended for patients with active RA on the basis of the guidelines of the American College of Rheumatology (ACR), European League Against Rheumatism, and Chinese Rheumatology Association [9, 12, 13]. Consequently, TNF inhibitors (etanercept, adalimumab, infliximab, certolizumab, and golimumab) and non-TNF inhibitors (abatacept, rituximab, and tocilizumab) have been approved by the Chinese National Medical Products Administration and have become widely used [14]. Although the use of biologic agents has contributed significantly to the effective control and early treatment of active RA to prevent permanent disability, the use of biologics in early RA remains limited due to cost considerations [15–19].
According to previous studies, triple therapy is not only noninferior to but also as safe as adding a biologic to MTX [19, 20]. A systematic review and network meta-analysis demonstrated that triple therapy and the combination of most bDMARDs with MTX had clinical efficacy in controlling disease progression [21]. Moreover, a multicenter, phase III, randomized controlled trial (RCT) (RACAT) compared triple therapy with biologic treatment (etanercept + MTX) as a first-line treatment for patients with active RA in whom MTX monotherapy failed [22]. The results of this study confirmed the noninferiority of triple therapy and suggested that patients with active RA achieved neither significant clinical improvement nor favorable responses after 24 weeks of treatment with etanercept + MTX compared to triple therapy [22]. According to the evidence of triple therapy noted above, the objective of this study was to consider the cost-effectiveness of implementing triple therapy compared to bDMARD sequences as a first-line treatment for patients with RA unresponsive to MTX.
Methods
Model Structure Overview
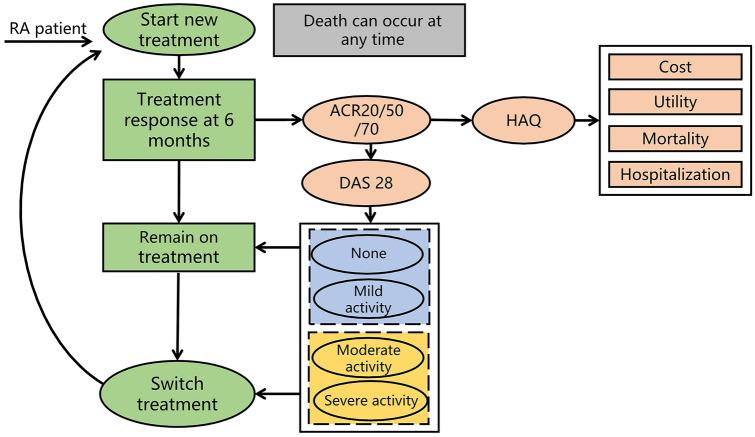
To best simulate the heterogeneity of RA patients and reflect clinical practice, an individual patient-level iviRA model in which 20,000 patients transitioned through a predefined treatment sequence was performed using R software (version 4.0.3, https://www.r-project.org/) (Fig. 1). The iviRA model (version 2.0) is an open-source project for value assessments in RA; the model was developed by the Innovation and Value Initiative (IVI) and simulates the health outcomes and costs related to all DMARDs [23]. Based on this model, we evaluated the cost-effectiveness of strategy initiation with triple therapy compared to strategy initiation with bDMARDs over lifetime horizons (50 years). In the treatment sequence, patients were evaluated in every model cycle (6 months). Patients were able to remain on the current treatment if they achieved a favorable response to the current treatment and did not experience adverse events (AEs); otherwise, the patients were switched to the next-line therapy. The main outcomes of this cost-effectiveness analysis were the total cost, life year (LY), quality-adjusted life year (QALY), and the incremental cost-effectiveness ratio (ICER). According to the economic evaluation guidelines in China, both costs and outcomes were discounted at 3% per year.
Fig. 1.
Model structure. RA rheumatoid arthritis, ACR American College of Rheumatology, HAQ Health Assessment Questionnaire, DAS 28 28-Joint Disease Activity Score
Model Inputs
All information about model input parameters, including patient characteristics and distributions of variables, is listed in Table 1. The characteristics of the patients, including age, sex, baseline Health Assessment Questionnaire (HAQ) score, and baseline 28-Joint Disease Activity Score (DAS28), were obtained from a phase III RCT (ORAL Sync) that recruited Chinese patients with RA [24, 25]. On the basis of the report on Chinese nutrition and chronic disease in 2015, the sex-specific weights for the Chinese population were also taken into consideration [26]. This is a model-based economic evaluation for which the patient data were all obtained from previous published studies. It does not contain any studies with animals or human participants.
Table 1.
Key model parameters
| Variable | Value (95% CI or range) | Distribution | Source |
|---|---|---|---|
| Population baseline characteristics | |||
| Age (year) | 48.1 | Truncated normal | 24–25 |
| Sex: female, % | 85.2 | – | 24–25 |
| Body weight (kg) | |||
| Female | 57.3 | – | 26 |
| Male | 66.2 | – | |
| HAQ score | 1.24 | Truncated normal | |
| DAS28 | 6.3 | 24–25 | |
| Response, percent of patients achieving ACR20, ACR50, ACR70 | |||
| cDMARDs | 0.291 (0.277, 0.306), 0.120 (0.111, 0.130), 0.040 (0.036, 0.044) | Multivariate normal (NMA parameters) | 23 |
| Etanercept | 0.584 (0.466, 0.690), 0.343 (0.240, 0.453), 0.165 (0.100, 0.242) | ||
| Adalimumab | 0.588 (0.495, 0.669), 0.346 (0.263, 0.426), 0.166 (0.113, 0.222) | ||
| Certolizumab pegol | 0.737 (0.639, 0.821), 0.507 (0.394, 0.616), 0.289 (0.198, 0.390) | ||
| Golimumab | 0.615 (0.482, 0.744), 0.375 (0.252, 0.513), 0.187 (0.106, 0.292) | ||
| Infliximab | 0.585 (0.481, 0.701), 0.344 (0.253, 0.460), 0.165 (0.107, 0.253) | ||
| Tofacitinib | 0.586 (0.453, 0.704), 0.346 (0.229, 0.466), 0.167 (0.093, 0.253) | ||
| Baricitinib | 0.554 (0.345, 0.760), 0.321 (0.154, 0.535), 0.153 (0.055, 0.308) | ||
| Abatacept SC | 0.632 (0.486, 0.760), 0.392 (0.258, 0.537), 0.200 (0.109, 0.311) | ||
| Rituximab | 0.560 (0.422, 0.704), 0.323 (0.205, 0.466), 0.152 (0.080, 0.252) | ||
| Tocilizumab | 0.667 (0.562, 0.761), 0.427 (0.321, 0.535), 0.224 (0.148, 0.313) | ||
| Responses for bDMARDs- and tofacitinib-experienced patients | |||
| Treatment effect factor | 0.84 (0.75, 0.92) | Uniform | 32 |
| DAS28 changed by ACR response at 6 months | |||
| ACR < 20 | 0 | Uniform | 30–31 |
| ACR 20–50 | − 1.550 | ||
| ACR 50–70 | − 1.543 | ||
| ACR > 70 | − 3.310 | ||
| Parameters of generalized gamma distribution | |||
| µ | 2.7165009 | Multivariate normal | 34 |
| Σ | 0.3839689 | ||
| Q | − 0.8940388 | ||
| Serious infection rate | |||
| bDMARDs | 0.035 (0.027, 0.046) | Normal | 37 |
| cDMARDs | 0.026 (0.018, 0.034) | ||
| HAQ-DI score change by ACR response at 6 months | |||
| Nonresponder | − 0.11 (− 0.25, 0.03) | Normal | 32 |
| ACR20 | − 0.44 (− 0.55, − 0.32) | ||
| ACR50 | − 0.76 (− 0.93, − 0.58) | ||
| ACR70 | − 1.07 (− 1.21, − 0.92) | ||
| Annual rate of HAQ progression | |||
| cDMARDs | 0.031 (0.026, 0.036) | Normal | 40–41 |
| Etanercept | − 0.005 (− 0.0115, 0.0115) | ||
| Adalimumab | − 0.003 (− 0.0175, 0.0115) | ||
| Certolizumab pegol | − 0.001 (− 0.004, 0.002) | ||
| Golimumab | − 0.001 (− 0.004, 0.002) | ||
| Infliximab | − 0.001 (− 0.004, 0.002) | ||
| Tofacitinib | − 0.001 (− 0.004, 0.002) | ||
| Baricitinib | − 0.001 (− 0.004, 0.002) | ||
| Abatacept SC | − 0.001 (− 0.004, 0.002) | ||
| Rituximab | − 0.001 (− 0.004, 0.002) | ||
| Tocilizumab | − 0.001 (− 0.004, 0.002) | ||
| NBT | 0.031 (0.026, 0.036) | ||
| Age-specific annual progression rates of HAQ progression, mean (s) | |||
| Age < 40 | − 0.020 (− 0.028, − 0.011) | Normal | 41 |
| 40 < Age < 64 | − 0.008 (− 0.01, − 0.005) | ||
| Age > = 65 | 0.017 (0.012, 0.021) | ||
| Impact of baseline HAQ on mortality | |||
| Odds ratio | 2.22(0.24) | Normal | 44 |
| Impact of 0.25-unit change in HAQ from baseline on mortality | |||
| Log HR 0–6 months | 0.13 (0.07, 0.157) | Normal | 45 |
| Log HR 6–12 months | 0.148 (0.104, 0.191) | ||
| Log HR 12–24 months | 0.148 (0.095, 0.91) | ||
| Log HR 24–36 months | 0.191 (0.131, 0.247) | ||
| Log HR > 36 months | 0.174 (0.104, 0.239) | ||
| Unit costs ($) | |||
| MTX (15 mg QW) | 0.4026/2.5 mg tablet | Fixed | 14 |
| Sulfasalazine (2 g daily) | 0.097/500 mg | ||
| Hydroxychloroquine sulfate (400 mg daily) | 0.66/200 mg | ||
| Etanercept (50 mg QW) | 67.1976/25 mg | ||
| Adalimumab (40 mg EOW) | 188.856/40 mg | ||
| Certolizumab pegol (400 mg at weeks 0, 2, 4 then 200 mg Q2W) | 359.7048/200 mg | ||
| Golimumab (50 mg QM) | 717.36/50 mg | ||
| Infliximab (3 mg/kg at 0, 2, and 6 weeks, 3 mg/kg Q8W) | 293.79552/100 mg | ||
| Tofacitinib (5 mg BID) | 5.124/5 mg tablet | ||
| Baricitinib (2 mg daily) | 20.9038704/2 mg | ||
| Abatacept SC (125 mg SC QW) | 231.312/125 mg | ||
| Rituximab (1000 mg at weeks 0, 2; repeated every 9 months) | 1151.62/500 mg | ||
| Tocilizumab (8 mg/kg every 4 weeks, for patients with weight > 100 kg, 800 mg) | 121.512/80 mg | ||
| Administration, $ | |||
| Subcutaneous injection | 0.586 | Fixed | Local charge |
| Intravenous injection | 9.004 | Fixed | Local charge |
| General management cost | 120.34 | Gamma | 47 |
| Hospital days per year by HAQ | |||
| 0–0.5 | 0.26 (0, 1.725) | Gamma | 32 |
| 0.5–1 | 0.13 (0, 1.409) | ||
| 1–1.5 | 0.51 (0.015, 1.85) | ||
| 1.5–2 | 0.72 (0.092, 1.979) | ||
| 2–2.5 | 1.86 (1.013, 2.96) | ||
| 2.5–3 | 4.16 (3.238, 5.196) | ||
| Hospitalization, $ | 226.20 (180.96, 271.44) | Gamma | 47 |
| AE cost, $ | 1761.40 (1409.1, 2113.6) | Uniform | 48 |
| Disutility due to serious infection | 0.156 (0.125, 0.187) | Uniform | 36 |
Treatment Sequence
The model simulates up to four lines of treatment, which were obtained based on the 2018 RA treatment guidelines in China and current clinical practice. In the comparator group, TNF inhibitor bDMARDs (e.g., etanercept) were used as a first-line strategy when patients were inadequately responsive to MTX [27]. After failure of the first-line treatment, patients who had experienced unresponsiveness or intolerance to one TNF inhibitor would not use another brand-named TNF inhibitor due to unfavorable Chinese reimbursement policies [27]. Then, patients were switched to a non-TNF inhibitor bDMARD (e.g., abatacept, rituximab, or tocilizumab) as second-line treatment; non-TNF inhibitor bDMARDs are another class of biologic agents with different mechanisms of action [28, 29]. After second-line treatment failure, patients were treated with a Janus kinase (JAK) or signal transducer and activator of transcription (STAT) inhibitor (e.g., tofacitinib) as a third-line treatment. Finally, after third-line treatment failure, patients were eventually switched to the nonbiologic therapy (NBT) phase, which mainly comprised cDMARDs, such as MTX, hydroxychloroquine, cyclosporine, and leflunomide, until death [27]. In the study group, aside from the administration of triple therapy instead of TNF inhibitors as a first-line treatment, the treatment sequence was the same as that in the comparator group. Three baseline analyses were simulated; the difference among them was that three non-TNF inhibitors (abatacept, rituximab, and tocilizumab), which have been approved for the market in China, were used as the second-line treatment. All target DMARDs (tDMARDs, including bDMARDs and JAK and STAT inhibitors) in the treatment sequences were administered in combination with MTX.
Therapeutic Efficacy and Treatment Switching
One crucial index of the model was the response level (therapeutic efficacy) measured using the ACR criteria, stratified as ACR 70 (ACR 70% improvement criteria), ACR 50 (ACR 50% improvement criteria), ACR 20 (ACR 20% improvement criteria), or nonresponsive (not achieving ACR 20% improvement criteria) [17]. The response level was estimated after the first 6 months of every treatment in the sequence except for in the NBT phase because the NBT would not have an associated initial ACR response level [23]. The therapeutic efficacy of DMARDs was obtained from a network meta-analysis that included 96 unique RCTs of RA treatment performed by IVI [23]. Another core parameter in the model was the RA activity measured by the DAS28, which was associated with treatment switching after the first 6 months for every treatment line. The DAS28 could be classified as severe (> 5.1), moderate (3.3–5.1), mild (2.6–3.2), or absent (< 2.6) [12]. All patients commenced with severe activity, and the relationship between the ACR response level and change in RA activity is listed in Table 1, as evaluated by Aletaha et al. [30, 31]. We supposed that the ACR response rates in patients who had received bDMARDs before dropped to 84% of that in bDMARD-naïve patients (treatment effect factor), consistent with a study by Carlson et al. [32]. According to the DAS28, discontinuation probability and AEs, patients passed through the treatment sequence and finally progressed to the NBT phase. During every model cycle, patients continued the current treatment or switched to the next-line treatment according to a previous study and international recommendations: treatment failure was diagnosed if the DAS28 was > 3.2 or less than 1.2 [9, 12, 33]. As we mentioned above, there was a certain probability of treatment discontinuation among patients who continued the current treatment, including all-cause discontinuation and the time to discontinuation. The discontinuation probability was evaluated on the basis of the survival curve obtained from the CORRONA database using a generalized gamma distribution model [34] (Supplementary Material Table 1). According to a study conducted by Zhang et al., patients with mild or no disease activity had approximately 0.52 times the odds of treatment discontinuation as patients with moderate RA activity [35]. We adjusted the curve from the CORRONA database using an odds ratio (OR = 0.52) and estimated the treatment duration for patients with mild or no disease activity since patients in the CORRONA database have nearly moderate RA activity [35]. Apart from the lack of response to the current treatment and the discontinuation of treatment, AEs may also result in treatment switching. Based on a study by Stevenson et al., we only considered serious infection (i.e., pneumonia) in the model since only severe infection significantly impacted the cost and QoL [36, 37].
Disease Progression and HAQ Score Change
The HAQ score, an instrument for measuring the physical function and disease progression of patients, ranges from 0 to 3 in multiples of 0.125 (a higher score indicates greater disease progression). The HAQ score depends on the disease activity, and both of these factors impact the utility value, mortality risk, and hospitalization cost in the model. The HAQ score changed over time in the model; however, the change was not related to treatment but was associated with the ACR response and the time spent in the NBT phase. The relationship between the ACR response level and the change in the HAQ score after the first 6 months was reported by Carlson et al. and is displayed in Table 1 [32]. After the first 6 months of each treatment line, the HAQ score decreased by subtracting the baseline HAQ score of patients to simulate improvement with treatment. After the initial 6 months, apart from the NBT phase, a constant annual rate (no disease progression according to the HAQ score) was applied for long-term treatment if patients continued the current therapy [38, 39]. In the NBT phase, an annual rate and an age-specific rate were used to model HAQ score progression, which was obtained from an observational study conducted by Wolfe et al. and a longitudinal study performed by Michaud et al., respectively [40, 41]. At the time patients switched to the next-line treatment, they the HAQ score rebounded, which means that any improvement in the HAQ score obtained from the last treatment line was lost at the time of initiation of a new treatment [42]. It was assumed that the HAQ score of patients would increase back to their baseline score at the beginning of the initial 6 months for each treatment line. All parameters are listed in Table 1.
Mortality
Death could occur at any point in time in the model. Based on the probability of death from the Chinese life table and HAQ score, a function of age- and sex-specific mortality was simulated [43]. We applied an OR (2.22) for the effect of the HAQ score on the mortality rate from the life table, which was estimated by Wolfe et al. [44]. Moreover, we also considered the impact of the change in the HAQ score on mortality; with every 0.25-unit HAQ score increase, the mortality rate of the subsequent 6 months increased to a certain degree, according to the hazard ratio reported by Michaud et al. [45] (Table 1).
Cost and Utility Estimates
In this study, both direct medical costs (including the costs of drug acquisition, AE management, administration, monitoring, and hospitalization), and indirect costs (such as the costs of productive loss), were considered in the model. All unit costs were derived from national databases, local hospitals, previously published studies, and the consensus of experts. Drug acquisition costs were obtained from the website of China Medical Bidding [46]. General management costs, including those of routine clinical tests, X-ray examinations, and outpatient follow-up visits, were based on a cost-effectiveness analysis performed by Wu et al. [47]. The annual days of hospitalization were associated with the HAQ score, so we estimated the cost of hospitalization according to a study by Carlson et al. due to the paucity of relevant information about this relationship in China [32]. The average expense of patients in the hospital per day was derived from a study by Wu et al. [47]. We assumed that the cost of AE management was the same across different tDMARDs and equal to the cost of treating pneumonia ($1761.4), according to Tian et al. [48]. All costs in this study were converted into 2019 US dollars (1 USD = 6.83 RMB), and the Chinese consumer price index was also used to adjust the costs from past sources to 2019 USD [49].
Based on the following algorithm, which was obtained from a previous cost-effectiveness study for Chinese RA patients conducted by Tian et al., the health-related QoL was estimated by mapping HAD-DI scores to EuroQol five-dimensional three-level (EQ-5D-3L) utility values [48].
In this study, we only included serious infection (i.e., pneumonia) as an AE in the model because the safety profiles among tDMARDs are similar and would not impact the results significantly [23]. The impact of pneumonia on QoL was measured by the health disutility weight, which dropped by 0.156 units of utility during the month of infection [36, 50].
Sensitivity and Scenario Analyses
We performed multiple analyses, including one-way sensitivity analyses, probability sensitivity analyses (PSAs), and a series of scenario analyses, to explore the uncertainty and robustness of the model. We changed the variables over a reasonable range and plausible distribution to determine crucial drivers in the model; for example, we varied the upper and lower limits of the drug price by 20%. PSAs were conducted for 2000 sets of 5000 patients by Monte Carlo simulation. The willingness-to-pay (WTP) threshold was set at $30,950/QALY or $10,316/QALY in China by using three times or one time the per-capita gross domestic product, as recommended by WHO guidelines as a “cost-effective” threshold or “very cost-effective” threshold, respectively [51, 52].
To understand the comprehensive cost-effectiveness of setting triple therapy as a first-line treatment for patients with RA who are unresponsive to MTX in China, six scenario analyses were performed: (1) Based on the original baseline treatment sequence, we replaced DMARDs (ETN) as the first-line treatment in the comparator group with TNF inhibitors of other brands, such as adalimumab, infliximab, certolizumab, and golimumab (Supplementary Material Table 3). (2) We varied the price of all tDMARDs to 75, 50, and 25% of their price, considering the potential implication of drug tapering and biosimilars (Supplementary Material Table 4). (3) On the basis of the rule of clinical practice we mentioned in the treatment sequence section, 36 possible strategies were formulated (Supplementary Material Table 5). All bDMARD sequences were compared to triple therapy followed by NBT. (4) According to the results of scenario 3, we selected the treatment sequence (TT-IFX-RTX-TOF-NBT) that had the lowest ICER, and in addition to the first-line triple therapy, the location of other drugs in the sequence was adjusted for comparison with the sequence without triple therapy (IFX-RTX-TOF-NBT) (Supplementary Material Table 6). (5) Based on scenario 4, another treatment sequence was chosen (RTX-TOF-IFX-NBT) (Supplementary Material Table 7). In this scenario, triple therapy was inserted at different positions in the treatment sequence as a first-, second-, third-, and fourth-line therapy to assess its impact on the cost-effectiveness analysis.
Results
Base Case Results
The results of base case analyses obtained by running 50,000 Monte Carlo simulations are presented in Table 2. Three base case analyses showed that triple therapy was associated with lower costs but also fewer LYs and QALYs than bDMARD sequences. These produced ICERs ranging from $87,090/QALY to $104,032/QALY, above the WTP threshold of $30,950/QALY.
Table 2.
Results of base case analyses
| Sequence | Total cost | LY | QALY | Incremental cost | Incremental | ICER | ||
|---|---|---|---|---|---|---|---|---|
| LY | QALY | /LY | /QALY | |||||
| TT-ABT-TOF-NBT | 52,521.01 | 15.50543 | 8.818586 | − 13,651.97 | − 0.09761 | − 0.148297 | 139,862.4116 | 92,058.30192 |
| ENT-ABT-TOF-NBT | 66,172.98 | 15.60304 | 8.966883 | |||||
| TT-RTX-TOF-NBT | 35,109.58 | 15.36617 | 8.666598 | − 12,328.12 | − 0.05785 | − 0.118503 | 213,104.9265 | 104,032.1342 |
| ENT-RTX-TOF-NBT | 47,437.70 | 15.42402 | 8.785101 | |||||
| TT-TCZ-TOF-NBT | 47,856.98 | 15.52555 | 8.884292 | − 13,902.04 | − 0.10895 | − 0.159629 | 127,600.1836 | 87,089.68922 |
| ENT-TCZ-TOF-NBT | 61,759.02 | 15.63450 | 9.043921 | |||||
LY life year, QALY quality-adjusted life year, ICER incremental cost-effectiveness ratio, TT triple therapy, ABT abatacept, TOF tofacitinib, NBT non biologic therapy, ENT etanercept, RTX rituximab, TCZ tocilizumab
Sensitivity and Scenario Analyses
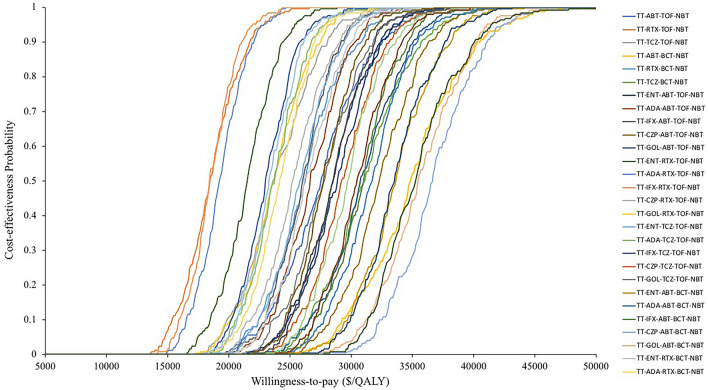
Because there were three pairs of baseline results with small differences in health outcomes, a tornado diagram was prepared to compare the TT-TCZ-TOF-NBT and ENT-TCZ-TOF-NBT strategies, which had the lowest ICERs among the base case analyses (Fig. 2). The model was highly sensitive to the baseline DAS28, the ACR response rate of tofacitinib, and the therapeutic efficacy. Other parameters, such as the baseline HAQ score, drug costs, sex proportion, and initial age, had a moderate or minor impact on the results. The PSA results showed that the three bDMARD strategies had a 0% likelihood of being cost-effective compared to the triple therapy strategy at a WTP threshold of $30,950/QALY (Fig. 2). The PSA results of scenario 3 showed that half of the strategies had probabilities of 80% or over of being considered cost-effective treatments compared to triple therapy. The results of the analyses of the first scenario were similar to the results of analyses of the base case, which revealed that bDMARDs as first-line treatment in treatment sequences is unlikely be cost-effectiveness comparing with triple therapy (Table 2, online Supplementary). In scenario 2, except for the TT-RTX-TOF-NBT and TT-TCZ-TOF-NBT treatment strategies, all strategies could be regarded as cost-effective compared to bDMARDs, and when we varied all DMARDs price to 25% of its original, TT-TCZ-TOF-NBT versus ENT-TCZ-TOF-NBT have the lower ICER at 19,501/QALYs. In scenario 3, we only compared 36 bDMARD strategies with triple therapy because there were slight differences in the QALYs among the bDMARD strategies, which may cause misunderstanding in considering the ICERs and cost-effectiveness. In scenario 3, 36 strategies were produced ICERs ranging from $20,560/QALY to $45,018/QALY, and over half of the bDMARD sequences yielded values lower than the WTP in China and could be considered cost-effective strategies compared to triple therapy monotherapy. Although the TT-IFX-RTX-TOF-NBT strategy had the lowest ICER ($20,150.6/QALY) compared to the other treatment sequences, it still could not be regarded as a “very cost-effective” strategy, since the ICER was above the WTP threshold at $10,316/QALY. Based on the results of scenario 3, scenario 4 was produced. The results showed that compared to bDMARD treatment, adding triple therapy before bDMARD treatment as a first-line therapy instead of using triple therapy to replace first-line bDMARD treatment can be recognized as a very cost-effective treatment option. The results of scenario 5 showed that inserting triple therapy into the bDMARD sequence as a first-, second-, third- or fourth-line treatment could be considered to be very cost-effective compared to no triple therapy. Among them, the RTX-TOF-TT-IFX-NBT sequence had the lowest ICER, at $894.8/QALY, compared to the comparator group.
Fig. 2.
The probability sensitivity analyses of scenario 3. TT triple therapy, ABT abatacept, TOF tofacitinib, NBT non biologic therapy, RTX rituximab, TCZ tocilizumab, BCT baricitinib, ENT etanercept, ADA adalimumab, IFX infliximab, CZP certolizumab, GOL golimumab
Discussion
Compared to bDMARDs, triple therapy is less commonly used in clinical practice as a first-line treatment after MTX failure, although triple therapy has been promoted for many years. Current guidelines, clinical practice, and reimbursement policies permit the initiation of bDMARDs after an inadequate response to MTX, but this could cause the inefficient use of medical sources. Therefore, we conducted the first study to evaluate the cost-effectiveness of triple therapy versus bDMARD treatment sequences with or without triple therapy in patients with RA who were unresponsive to MTX in China. This topic is relevant to patients, rheumatology immunologists, and policymakers. The results indicate that using triple therapy as a first-line treatment is likely to be cost-effective compared to bDMARDs. However, the implication of this study is not that triple therapy should be substituted for bDMARDs or that bDMARDs should be withheld from patients with RA in whom MTX failed. Rather, this study suggests that triple therapy should be prescribed within the bDMARD treatment sequence. The results of scenario analyses indicate that when triple therapy is inserted as a first-, second-, third-, or fourth-line therapy in the bDMARD sequence, all sequences could be regarded as “very cost-effective” compared to sequences involving bDMARDs only. Although prescribing triple therapy after bDMARDs failure is seldom in clinical practice, our study provided several economical treatment sequences option for treat-to-target strategy in future practice.
The results of our study are consistent with those of other economic analyses studying triple therapy compared to biologics, although they only compared triple therapy with single biologic agents instead of bDMARD treatment sequences. A study in the United States calculated the cost-effectiveness of triple therapy versus etanercept plus MTX as a first-line treatment and found that commencing bDMARDs without trying triple therapy first yielded a minimal incremental benefit but an increase in cost. A study in Sweden showed that the use of infliximab cost €20,916 more than triple therapy and only gained a 0.01 increase in QALYs over a duration of 21 months, leading to an ICER of €2,404,197/QALY. Our study was based on the perspective of Chinese health care system, so the conclusion of this study might not be applicable to other countries because of the differences in the costs, clinical guidelines, policies, and health systems among countries. To our knowledge, this is the first modeling study from the Chinese health care system perspective to explore the cost-effectiveness of treatment sequences for RA patients who failed MTX treatment.
As with any model, there are some limitations to this study. First, we derived the therapeutic efficacy from a network meta-analysis in which most RCTs were conducted over the short term (approximately 12–24 months). This would introduce some biases to the results since the model extrapolates lifetime efficacy and costs. However, most RCTs had this unavoidable limitation due to the resources and major expenditures required for long-term follow-up. Thus, we not only constructed a decision analytic model that reflects the heterogeneity of patients but also made plausible assumptions and conducted multiple sensitivity analyses and scenario analyses to improve the robustness of the model. Second, in the absence of reliable data, we hypothesized that the ACR response rate after bDMARD failure would be decreased to 84% in patients who had not previously received a biologic. However, the sensitivity analyses showed that the impact of the treatment effect factor on the model outcomes was marginal. Third, in this study, we assumed that patients will finally switch to the NBT phase, which is commonly and justifiably used in economic evaluations in RA [23, 27]; however, the treatment sequence in clinical practice will have more permutations and combinations than the limited treatment sequences in this study. Finally, our model considered neither other AEs nor the possibility that the risk of AEs might differ among different treatment sequences, which might underestimate the direct costs and overestimate the health outcomes, such as the efficacy of bDMARDs. However, a published study suggested that since the safety profiles of bDMARDs are similar, the outcomes of the model would not be changed when adding other AEs into the model [32].
Conclusions
From the perspective of the Chinese healthcare system, compared to bDMARD treatment sequences, triple therapy is estimated to be cost-effective for patients with active RA, at a WTP threshold of $30,950/QALY. Furthermore, a very cost-effective level was obtained regardless of the position of triple therapy within the bDMARD treatment sequence compared to sequences not including triple therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by the National Natural Science Foundation of China (No. 71874209) and Hunan Provincial Natural Science Foundation of China (No. 2019JJ40411).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Sini Li constructed the model, collected and analyzed data, and drafted the manuscript. Xiaomin Wan conceptualized the study and provided the model framework. Yamin Li contributed to the revision of the manuscript. Liubao Peng and Jianhe Li was the guarantor of the study and provided technical and material support. Thanks to Mr. Zhu Wenjie for his love and encouragement to the first author. All authors gave final approval for this version to be published.
Disclosures
Sini Li, Jianhe Li, Liubao Peng, Yamin Li, and Xiaomin Wan have nothing to disclose.
Compliance with Ethics Guidelines
This is a model-based economic evaluation for which the patient data were all obtained from previous published studies. It does not contain any studies with animal or human participants.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
YaMin Li, Email: aminny@csu.edu.cn.
XiaoMin Wan, Email: wanxiaomin@csu.edu.cn.
References
- 1.McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet (London, England). 2017;389(10086):2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (London, England) 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 4.Jin S, Li M, Fang Y, Li Q, Liu J, Duan X, et al. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):251. doi: 10.1186/s13075-017-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y-S, An Y, Li C, Xiao-ying Z, Duan T, Zhu J, Li X, Wang L. A multicenter study of deformity and disability in rheumatoid arthritis patients in China. Chin J Rheumatol. 2013;17(8):526–532. doi: 10.3760/cma.j.issn.1007-7480.2013.08.006. [DOI] [Google Scholar]
- 6.Hu H, Luan L, Yang K, Li SC. Burden of rheumatoid arthritis from a societal perspective: a prevalence-based study on cost of this illness for patients in China. Int J Rheumatic Dis. 2018;21(8):1572–1580. doi: 10.1111/1756-185X.13028. [DOI] [PubMed] [Google Scholar]
- 7.Li HB, Wu LJ, Jiang N, Yang PT, Liu SY, Shi XF, et al. Treatment satisfaction with rheumatoid arthritis in patients with different disease severity and financial burden: a subgroup analysis of a nationwide survey in China. Chin Med J (Engl) 2020;133(8):892–898. doi: 10.1097/CM9.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Mu R, Wang X, Xu C, Duan T, An Y, et al. The impact of rheumatoid arthritis on work capacity in Chinese patients: a cross-sectional study. Rheumatology (Oxford) 2015;54(8):1478–1487. doi: 10.1093/rheumatology/kev014. [DOI] [PubMed] [Google Scholar]
- 9.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 10.Agency EM. Guideline on clinical investigation of medicinal products other than NSAIDS for treatment of rheumatoid arthritis (draft); 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500187583.pdf[last accessed 24 Aug 2020].
- 11.Gaujoux-Viala C, Smolen JS, Landewe R, Dougados M, Kvien TK, Mola EM, et al. Current evidence for the management of rheumatoid arthritis with synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheumatic Dis. 2010;69(6):1004–1009. doi: 10.1136/ard.2009.127225. [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheumatic Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 13.Association CR. Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Chin J Intern Med. 2018;57(4):242–251. doi: 10.3760/cma.j.issn.0578-1426.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 14.National Medical Produces Administration. http://app1.nmpa.gov.cn/data_nmpa/face3/dir.html?type=yp&CbSlDlH0=qGcHracIvy5Ivy5IvZAUk61nPe8mAvgEOF0A6_kpmMlqqxZ. [Accessed 24 Jan 2021].
- 15.Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl 1):S1. doi: 10.1186/ar2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YM, Lu YP, Lan JL, Chen DY, Wang JD. Lifetime risks, life expectancy and healthcare expenditures for rheumatoid arthritis. A nationwide cohort followed from 2003 to 2016. Arthritis Rheumatol (Hoboken, NJ) 2003 doi: 10.1002/art.41597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 19.Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum. 2012;64(9):2824–2835. doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Vollenhoven RF, Ernestam S, Geborek P, Petersson IF, Coster L, Waltbrand E, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet (London, England). 2009;374(9688):459–466. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]
- 21.Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ (Clinical research ed) 2016;353:i1777. doi: 10.1136/bmj.i1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med. 2013;369(4):307–318. doi: 10.1056/NEJMoa1303006. [DOI] [PubMed] [Google Scholar]
- 23.Incerti D, Curtis JR, Shafrin J, Lakdawalla DN, Jansen JP. A flexible open-source decision model for value assessment of biologic treatment for rheumatoid arthritis. Pharmacoeconomics. 2019;37(6):829–843. doi: 10.1007/s40273-018-00765-2. [DOI] [PubMed] [Google Scholar]
- 24.Li ZG, Liu Y, Xu HJ, Chen ZW, Bao CD, Gu JR, et al. Efficacy and safety of tofacitinib in Chinese patients with rheumatoid arthritis. Chin Med J (Engl) 2018;131(22):2683–2692. doi: 10.4103/0366-6999.245157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 26.Withall J, Haase AM, Walsh NE, Young A, Cramp F. Physical activity engagement in early rheumatoid arthritis: a qualitative study to inform intervention development. Physiotherapy. 2016;102(3):264–271. doi: 10.1016/j.physio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Tian L, Xiong X, Guo Q, Chen Y, Wang L, Dong P, et al. Cost-effectiveness of tofacitinib for patients with moderate-to-severe rheumatoid arthritis in China. Pharmacoeconomics. 2020 doi: 10.1007/s40273-020-00961-z. [DOI] [PubMed] [Google Scholar]
- 28.Chinese Rheumatology A 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi. 2018;57(4):242–251. doi: 10.3760/cma.j.issn.0578-1426.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Lee MY, Park SK, Park SY, Byun JH, Lee SM, Ko SK, et al. Cost-effectiveness of tofacitinib in the treatment of moderate to severe rheumatoid arthritis in South Korea. Clin Therapeutics. 2015;37(8):1662–1676. doi: 10.1016/j.clinthera.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–R806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–S108. [PubMed] [Google Scholar]
- 32.Carlson JJ, Ogale S, Dejonckheere F, Sullivan SD. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health. 2015;18(2):173–179. doi: 10.1016/j.jval.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Van De Laar CJ, Oude Voshaar MAH, Fakhouri WKH, Zaremba-Pechmann L, De Leonardis F, De La Torre I, et al. Cost-effectiveness of a JAK1/JAK2 inhibitor vs a biologic disease-modifying antirheumatic drug (bDMARD) in a treat-to-target strategy for rheumatoid arthritis. ClinicoEcon Outcomes Res. 2020;12:213–222. doi: 10.2147/CEOR.S231558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand V, Miller P, Williams SA, Saunders K, Grant S, Kremer J. Discontinuation of biologic therapy in rheumatoid arthritis: analysis from the Corrona RA registry. Rheumatol Ther. 2017;4(2):489–502. doi: 10.1007/s40744-017-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Shan Y, Reed G, Kremer J, Greenberg JD, Baumgartner S, et al. Thresholds in disease activity for switching biologics in rheumatoid arthritis patients: experience from a large U.S. cohort. Arthritis Care Res (Hoboken) 2011;63(12):1672–1679. doi: 10.1002/acr.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610. doi: 10.3310/hta20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tosh J, Brennan A, Wailoo A, Bansback N. The Sheffield rheumatoid arthritis health economic model. Rheumatology (Oxford) 2011;50(Suppl 4):iv26–iv31. doi: 10.1093/rheumatology/ker243. [DOI] [PubMed] [Google Scholar]
- 39.Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum. 2008;58(4):939–946. doi: 10.1002/art.23374. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe F, Michaud K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. Arthritis Res Ther. 2010;12(2):R35. doi: 10.1186/ar2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaud K, Wallenstein G, Wolfe F. Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis: a longitudinal study of 18,485 patients. Arthritis Care Res (Hoboken). 2011;63(3):366–372. doi: 10.1002/acr.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess (Winchester, England). 2011;15(14):1–278. doi: 10.3310/hta15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Life tables for WHO member states. World Health Organization. 2016.
- 44.Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1530–1542. doi: 10.1002/art.11024. [DOI] [PubMed] [Google Scholar]
- 45.Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–59. doi: 10.3899/jrheum.110491. [DOI] [PubMed] [Google Scholar]
- 46.China Medical Bidding website. http://www.eyiyao.org.cn/. (Accessed 30 Sep 2020).
- 47.Wu B, Wilson A, Wang FF, Wang SL, Wallace DJ, Weisman MH, et al. Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in china. PLoS ONE. 2012;7(10):e47373. doi: 10.1371/journal.pone.0047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian L, Xiong X, Guo Q, Chen Y, Wang L, Dong P, et al. Cost-effectiveness of tofacitinib for patients with moderate-to-severe rheumatoid arthritis in China. Pharmacoeconomics. 2020;38(12):1345–1358. doi: 10.1007/s40273-020-00961-z. [DOI] [PubMed] [Google Scholar]
- 49.Mathews AL, Burns PB, Chung KC. How rheumatoid arthritis patients make decisions regarding hand reconstruction: a qualitative study from the silicone arthroplasty in rheumatoid arthritis project. Plastic Reconstruct Surg. 2016;137(5):1507–1514. doi: 10.1097/prs.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 50.Oppong R, Kaambwa B, Nuttall J, Hood K, Smith RD, Coast J. The impact of using different tariffs to value EQ-5D health state descriptions: an example from a study of acute cough/lower respiratory tract infections in seven countries. Eur J Health Econ. 2013;14(2):197–209. doi: 10.1007/s10198-011-0360-9. [DOI] [PubMed] [Google Scholar]
- 51.National data base.[cited 2020 27 October]. Available from: http://data.stats.gov.cn/index.htm.
- 52.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.