Abstract
Stage 4S neuroblastoma (4SNB) is associated with spontaneous tumor regression and an excellent prognosis. However, a small group of the patients have a poor prognosis. 185 4SNB cases filed at the Children’s Oncology Group Neuroblastoma Pathology Reference Laboratory were studied. MYCN oncogene status [Non-Amplified (NA) vs. Amplified (A)] determined by FISH, MYC-family (MYCN/MYC) protein expression [no-overexpression(−)/(+/−) vs. overexpression(+)] by immunohistochemistry and histopathology by International Neuroblastoma Pathology Classification [Favorable Histology (FH) vs. Unfavorable Histology (UH)] with particular attention to nucleolar hypertrophy [NH(−) vs. (+)] were assessed with patient survival. 147 (79.5%) tumors were MYCN-NA, FH, MYC-family protein(−)/(+/−), and NH(−) with a good prognosis [88.5+3.1% 5-year Event-free survival (EFS); 94.1+2.3% 5-year Overall survival (OS)]. Among MYCN-NA tumors, 11 demonstrated MYCN protein(+) with a moderate and uniform (M/U) staining pattern: they were FH(10/11), NH(−), one showed MYC protein(+) simultaneously, and all patients are alive. Also found were 5 MYC protein(+) and MYCN(−)/(+/−) tumors; they were FH without NH(4/5), and all patients are alive. Among MYCN-A tumors, 18 had MYCN protein(+) with a strong and heterogeneous (S/H) staining pattern, 9 had UH (44.4+23.4% EFS/OS) and 9 had FH (68.6+19.2% EFS/OS), and 15 showed NH(+). Two tumors had MYCN protein(−)/(+/−) despite MYCN-A; both were FH and NH(−), and one patient died. S/H staining pattern of MYCN protein overexpression by immunohistochemistry was associated with MYCN amplification, NH(+) and a poor prognosis. In contrast, the M/U staining pattern was associated with MYCN non-amplification and NH(−), and had no adverse prognostic effects for the 4SNB patients.
Keywords: Neuroblastoma, Stage 4S, MYCN amplification, MYC, Nucleolar hypertrophy
INTRODUCTION
Clinical stage 4S neuroblastoma (4SNB) is an enigmatic disease of childhood with a generally good prognosis. The International Neuroblastoma Staging System (INSS) defines stage 4SNB as that occurring in children less than 12 months of age with a localized primary tumor (clinical stage 1 or 2, approximately 75% of primary tumors in the adrenal gland) and metastases confined to the liver, skin, and/or bone morrow (less than 10% of marrow nucleated cells) (1, 2). Stage 4S disease comprises 5–10% of neuroblastoma cases (3, 4) with approximately 50% undergoing spontaneous regression without treatment (5–8). Despite historical event-free and overall survival rates of approximately 90% established by international clinical trials, a subset of these patients has a poor prognosis (8, 9). Poor prognostic features include unfavorable histology (UH) as classified by the International Neuroblastoma Pathology Classification (INPC), MYCN gene amplification, diploid chromosome status (DNA index = 1) and loss of heterozygosity (LOH) of 1p or 11q (10). Apart from these biological factors, in infants less than 2 months of age, mass effect due to hepatomegaly with resultant compartment syndrome, liver failure or renal failure have also been associated with a poor outcome (5, 6, 8, 10–14).
MYCN gene amplification is a potent predictor of poor outcome independent of other factors by driving tumor progression and increasing cell turn over. We recently reported that MYC-family protein (MYCN and MYC) overexpression is a stronger predictor of outcome than MYCN gene amplification in all neuroblastomas (15–17). It is also reported that augmented expression of MYC-family protein in undifferentiated/poorly differentiated neuroblastomas is associated with nucleolar hypertrophy. In our experience, approximately 90% of MYCN amplified neuroblastoma cases show concordant MYCN protein overexpression; however, the remaining cases are discordant with MYCN amplification and no MYCN protein overexpression and vice versa (15, 18). It is unknown if genotype/phenotype concordance or discordance affects outcome in patients with 4SNB.
In this study, we analyzed the biologic and clinicopathologic characteristics of stage 4SNB with a focus on MYCN oncogene status, histopathology, MYC-family protein expression, nucleolar morphology, and other prognostic factors.
MATERIALS AND METHODS
Patient cohort
Between October 1st, 2010 and February 28th, 2019, 185 cases of 4SNB were reviewed at the Children’s Oncology Group (COG) Neuroblastoma Pathology Reference Laboratory in the Department of Pathology and Laboratory Medicine, Children’s Hospital of Los Angeles (Los Angeles, CA, USA). All cases were derived from patients prior to the administration of chemotherapy. Informed consent and approval by the Institutional Review Board was obtained at the time of enrollment in the COG trials.
Pathology review
Hematoxylin & Eosin stained (H&E) slides from 4SNB cases were reviewed by AK and HS at the COG Neuroblastoma Pathology Reference Laboratory and classified according to the INPC as Favorable Histology (FH) or UH (19, 20). The histopathologic characteristics of the chromatin quality (e.g. stippled / typical “salt and pepper”) and presence or absence of nucleolar hypertrophy (prominent nucleolar formation) was noted. Nucleolar hypertrophy was defined as, in accordance with our prior reports, conspicuous, discrete, large, eosinophilic nucleoli, with medium to large sized nuclei with vesicular or often open chromatin (15, 21).
Immunohistochemistry
Immunostaining for MYCN and MYC protein was performed using formalin-fixed, paraffin- embedded (FFPE) sections. Unstained sections were heated for 30 min in Bond™ Epitope Retrieval Solution 2 (No. AR9640; Leica Biosystems Newcastle Ltd., Benton Ln, Newcastle Upon Tyne, UK) with Leica BOND-MAX™ (Leica Microsystems Inc., Bannockburn, IL, USA). The sections were incubated with either anti-MYCN mouse monoclonal antibody, NCM II 100 (22) at a dilution of 1:50, or anti-human MYC rabbit monoclonal antibody, clone Y69 (No. 1472–1;Epitomics, Cambridge, MA, USA) (23) at a dilution of 1:200 in Bond™ Primary Antibody Diluent (No. AR9352; Vision BioSystems Inc., Norwell, MA, USA). Staining was visualized using Bond Polymer Refine Detection™ (No.DS9800; Leica Microsystems Inc., Bannockburn, IL, USA). The slides stained for MYC protein were counterstained with hematoxylin. No counterstaining was performed for the slides after MYCN protein staining. The slides were reviewed by AK and HS. Nuclear localization of MYCN or MYC staining was scored as follows: Negative (not overexpressed): (- or +/−), no or weak staining of tumor cells; Positive (overexpressed): (+) of tumor cells. Positive staining of MYCN protein was further classified according to the pattern of protein expression: a strong with heterogeneous (S/H) staining pattern or a moderate with uniform (M/U) staining pattern.
MYCN Amplification by Fluorescence in situ hybridization (FISH)
The MYCN amplification status of each case was determined by the COG Neuroblastoma Reference Laboratory, Nationwide Children’s hospital (Columbus, OH, USA) according to standard techniques by establishing the MYCN gene copy number in relation to the centromeric region of chromosome 2 (24). Testing was performed either on touch preparation slides of snap frozen tissue or on slides of formalin fixed paraffin-embedded tissue. MYCN amplification is defined as more than 4-fold increase in the MYCN signals compared with the reference centromere probe of chromosome 2. The cases were categorized as MYCN amplified or non-amplified.
Statistical analysis
Event-free survival (EFS) was calculated as the number of days from diagnosis to the occurrence of an event or, if no event, the date of last follow-up. An event is defined as death, disease relapse or progression, or secondary malignancy. Overall survival (OS) was calculated as the number of days from diagnosis to death or, if the patient did not die, the date of last follow-up. Kaplan-Meier EFS and OS estimates (25) with standard errors per the methods of Peto et al. (26) were computed for the patients in different groups defined by MYCN oncogene status, INPC, MYC-family protein expression and presence or absence of nucleolar hypertrophy. Also analyzed were prognostic effects by DNA index, age at diagnosis (using a 2 month cutoff), presence or absence of clinical symptoms, and presence or absence of 1p/11q LOH among the most common group of stage 4S patients whose tumors had typically most favorable indicators of non-amplified MYCN, FH, no MYC-family protein overexpression and no nucleolar hypertrophy. Tests with a p-value <0.05 were considered statistically significant. Survival plots and life tables were produced for each analysis and survival probabilities are presented as 3-year estimates ± standard error and written as percentages.
RESULTS
Of the 185 cases of 4SNB, 91 were male and 94 female ranging in age from 1 day to 11.9 months (median 2.7 months). The median follow-up time for patients without an event (n=162) was 4.1 years. The median follow-up time for patients who did not die (n=170) was 4.0 years. The 3-year EFS and OS estimates for all 185 patients were 86.4±3.1% and 90.9±2.6%, respectively. For all patients, no events were reported after 2 years of follow-up and there were no deaths reported after 3 years of follow-up.
All 185 cases in this study were summarized below and in Table 1. Representative histologic features, immunohistochemical staining and FISH results are shown in Figure 1, 2 and 3.
Table 1.
Summary of 185 Stage 4S cases
| MYCN non-Amplified & FH Tumors | |||||
|---|---|---|---|---|---|
| MYC-Family | protein | Nucleolar | 3-year EFS | 3-year OS | |
| Cases (%) | MYCN | MYC | Hypertrophy | % | % |
| 147 (79%) | 0 | 0 | 0 | 88.5±3.1 | 94.1±2.3 |
| 9 ( 5%) | 9 (M/U*) | 0 | 0 | 100 | 100 |
| 6 ( 3%) | 1 (M/U*) | 6 | 1 | 100 | 100 |
|
MYCN non-Amplified & UH Tumors | |||||
| MYC-Family | protein | Nucleolar | 3-year EFS | 3-year OS | |
| Cases (%) | MYCN | MYC | Hypertrophy | % | % |
| 3 ( 2%) | 1 (M/U*) | 0 | 0 | 100 | 100 |
|
MYCN Amplified & FH Tumors | |||||
| MYC-Family | protein | Nucleolar | 3-year EFS | 3-year OS | |
| Cases | MYCN | MYC | Hypertrophy | % | % |
| 11 ( 6%) | 9 (S/H**) | 0 | 8 | 68.6±19.2 | 68.6±19.2 |
| 2 (Negative) | 0 | 0 | NA | NA | |
|
MYCN Amplified & UH Tumors | |||||
| MYC-Family | protein | Nucleolar | 3-year EFS | 3-year OS | |
| Cases | MYCN | MYC | Hypertrophy | % | % |
| 9 ( 5%) | 9 (S/H**) | 0 | 7 | 44.4±23.4 | 44.4±23.4 |
FH: Favorable Histology; UH: Unfavorable Histology according to the International Neuroblastoma Pathology Classification; EFS: Event-free survival (+ Standard error); OS: Overall survival (+ standard error); M/U*: moderate with uniform staining pattern by immunohistochemistry; S/H**: strong with heterogeneous staining pattern by immunohistochemistry; NA: not available due to the sampling size.
Figure 1:
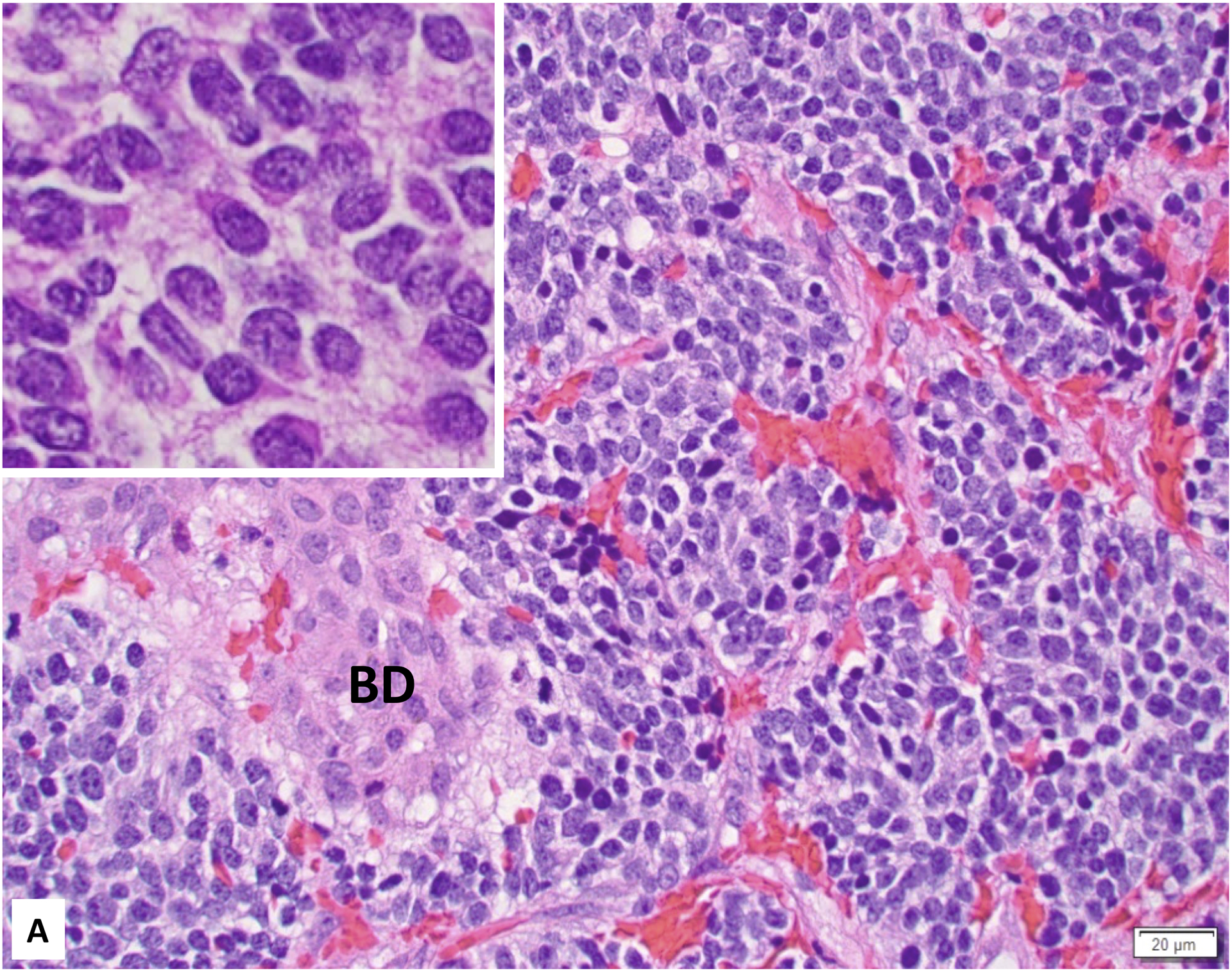
A. Favorable Histology Stage 4S Neuroblastoma (Poorly differentiated subtype with a low MKI) involving the liver (Inset: Higher magnification demonstrating “Salt-and-Pepper” Nuclei). BD: bile duct; B. Unfavorable Histology Stage 4S Neuroblastoma (Poorly differentiated subtype with a high MKI) involving the liver (Inset: Higher magnification demonstrating prominent nucleolar formation - nucleolar hypertrophy).
Figure 2:
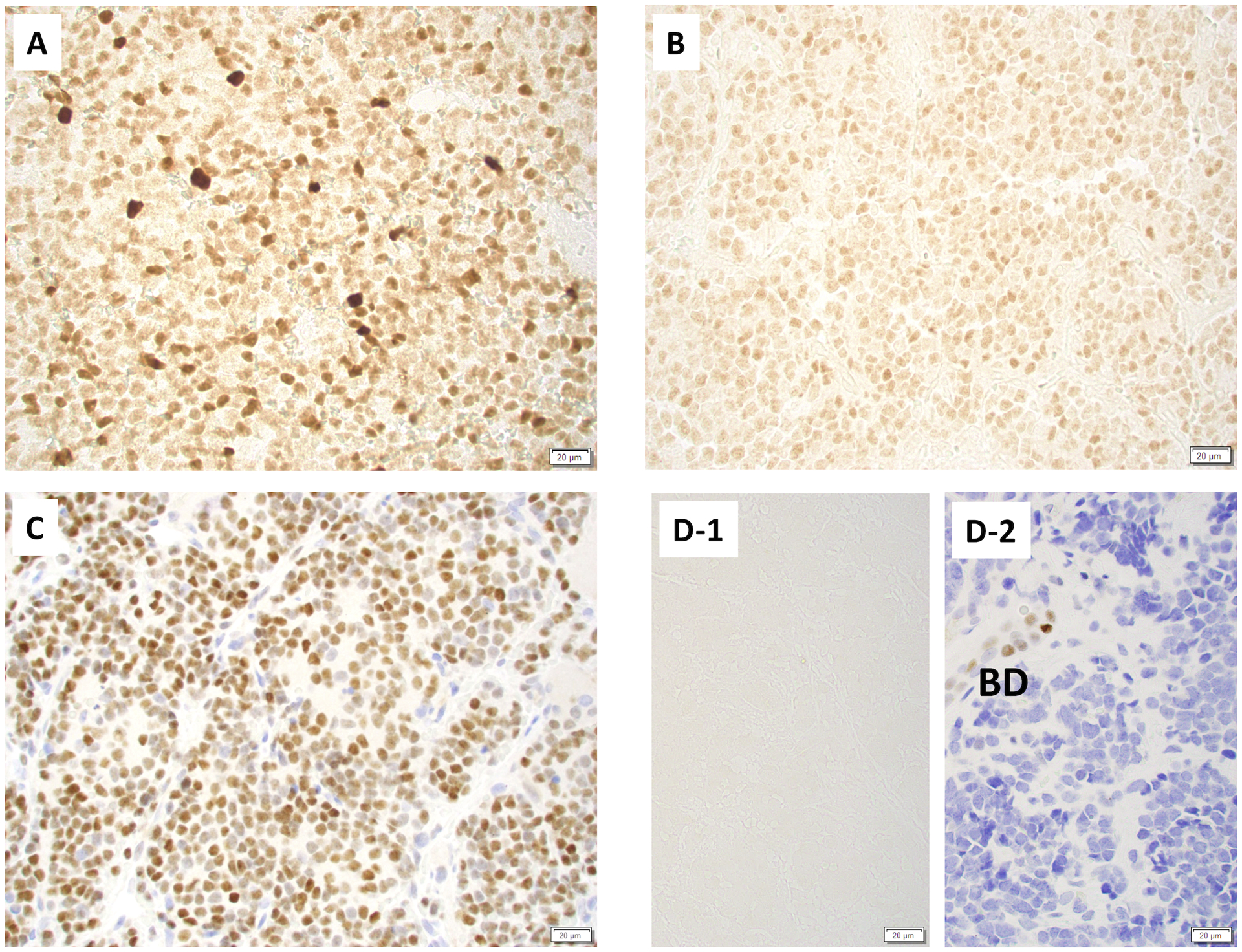
Immunohistochemical stainings on stage 4S neuroblastomas. A: Tumor expressing MYCN protein with a strong and heterogeneous staining pattern, B: Tumor expressing MYCN protein with a moderate and uniform staining pattern, C: Tumor expressing MYC protein, D: Tumor expressing no MYC-family protein expression (D-1: MYCN staining, D-2: MYC staining). BD: bile duct positively stained for this marker. Note: MYCN staining without counter staining and MYC staining with Hematoxylin staining.
Figure 3:
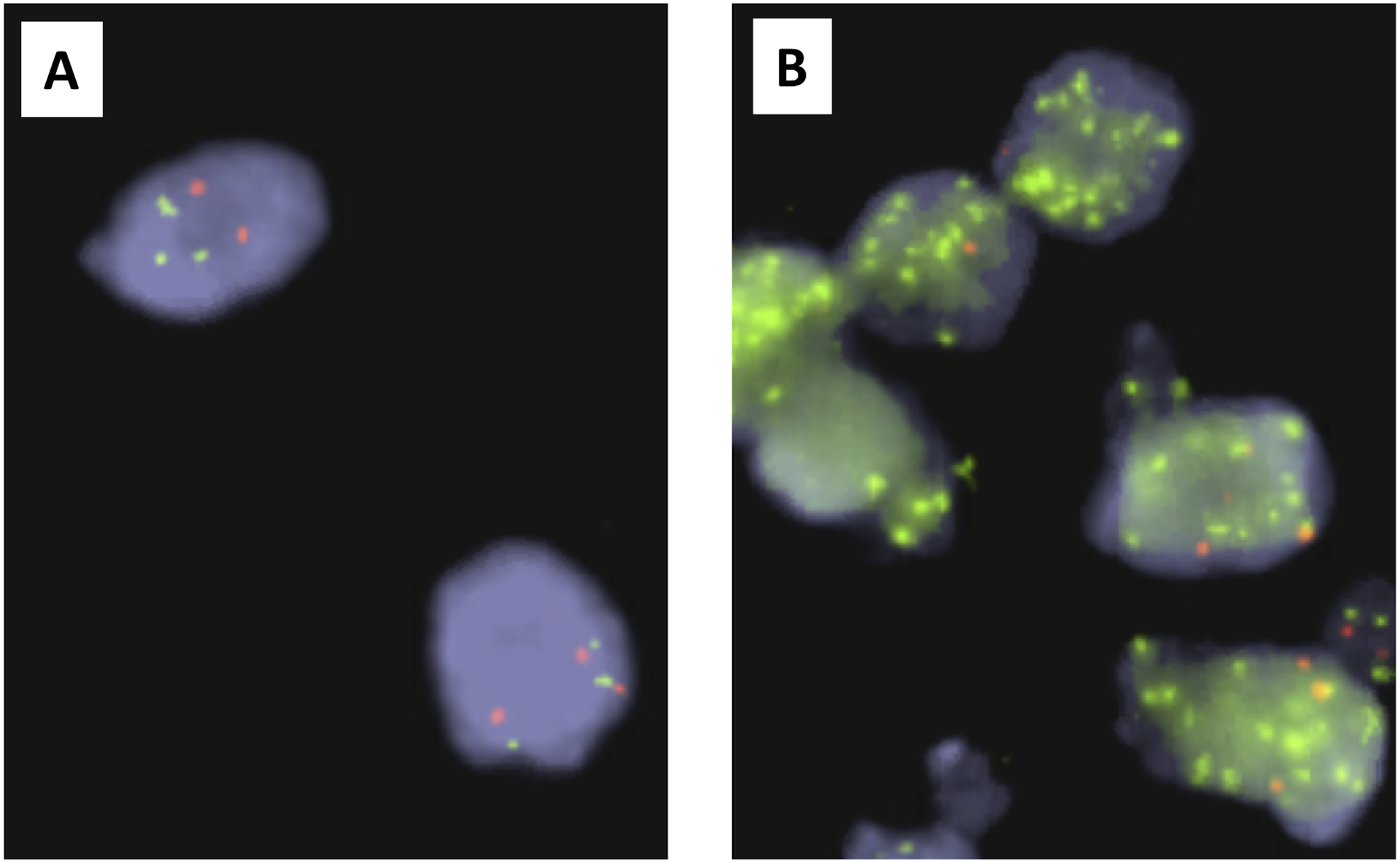
FISH test on Stage 4S Neuroblastoma. MYCN non-amplified tumor (A) and MYCN amplified tumor (B) (green: MYCN signals, red: reference signals of chromosome 2 centromere). These images were provided by Dr. Ruthann Pfau at the Children’s Oncology Group BioPath Center, Nationwide Children’s Hospital.
MYCN non-amplified and FH tumors (N=162): This is the largest group of genotype/phenotype concordant tumors comprising 87.6% of the total cohort. Among them, 147 tumors (79.5%) showed no MYC-family protein overexpression and no nucleolar hypertrophy. These patients had an excellent prognosis with 3-year EFS and OS of 88.5±3.1% and 94.1±2.3%, respectively. Fifteen tumors showed MYC-family protein overexpression (9 MYCN protein only, 5 MYC protein only, and one expressed both proteins), and 3-year EFS and OS were 100%. It was noted that MYCN protein expression in all 10 tumors in this group was the M/U staining pattern immunohistochemically and only one of 6 tumors expressing MYC protein had nucleolar hypertrophy.
MYCN non-amplified and UH tumors (N=3): This is a very rare group and 3-year EFS and OS were 100%. No tumors showed nucleolar hypertrophy and one tumor expressed MYCN protein with the M/U staining pattern.
MYCN amplified and FH tumors (N=11): This is a group composed of classic genotype-phenotype discordant tumors. Nine tumors showed MYCN protein overexpression of the S/H staining pattern and 8 of them had nucleolar hypertrophy: Prognosis of the patients in this group is poor with 3-year EFS and OS of 68.6±19. Two tumors had no MYCN or MYC protein overexpression and no nucleolar hypertrophy: One patient is alive and the other died.
MYCN amplified and UH tumors (N=9): This is a group of genotype/phenotype concordant tumors. All tumors had MYCN protein overexpression with the S/H staining pattern and 7 showed nucleolar hypertrophy: Patients in this group had a poor prognosis with 3-year EFS and OS of 44.4±23.4%.
Sorting cases by MYCN protein expression using immunohistochemistry revealed three distinct prognostic groups of 4SNB (Figure 4, EFS: p=0.0087, OS: P<0.0001).
Figure 4:
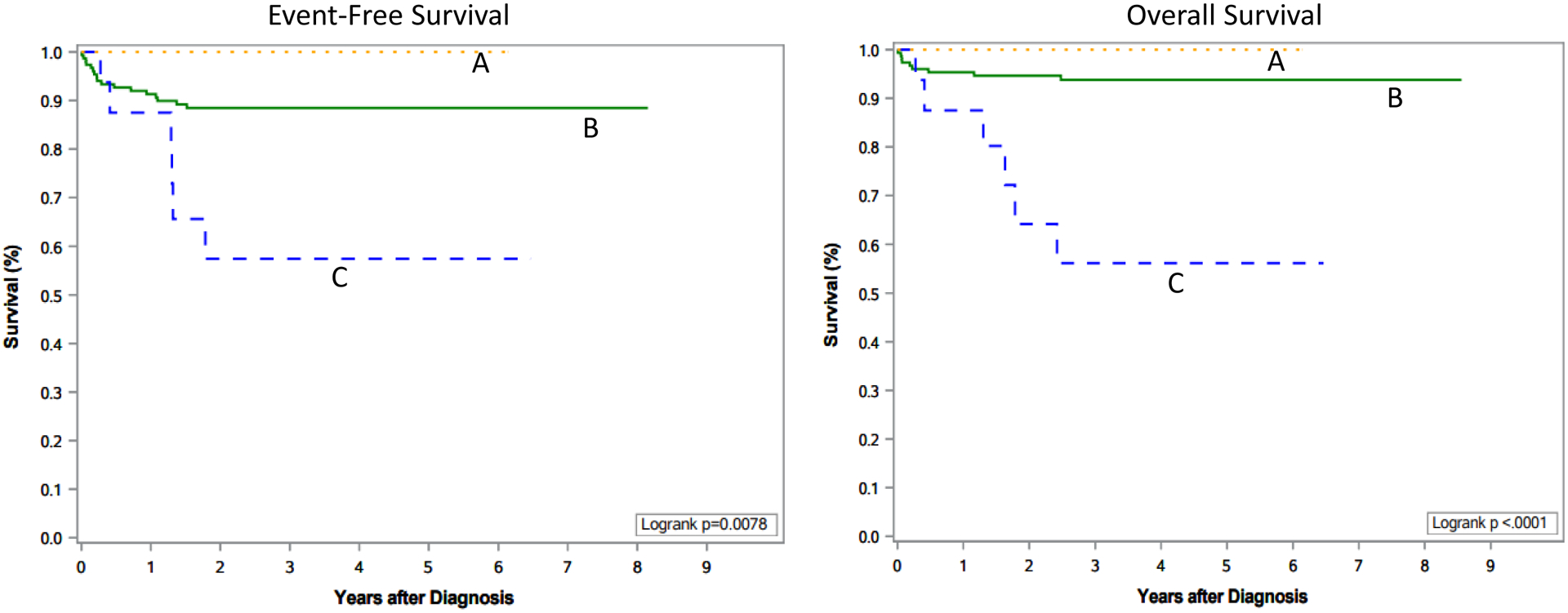
Survival Curves by MYCN Protein Expression in Stage 4S Neuroblastoma. A: Tumors expressing MYCN protein with a moderate and uniform staining pattern (N=11); B: Tumors with no MYCN protein overexpression (N=156); C: Tumors expressing MYCN protein with a strong and heterogeneous staining pattern (N=18).
Tumors with no MYCN protein overexpression (N=156): The vast majority of tumors (152, 97%) were not MYCN amplified and had FH. Only two tumors had MYCN amplification and FH, and the other two had no MYCN amplification and UH. No nucleolar hypertrophy was observed in all tumors but one. Three-year EFS and OS of the patients in this group were 88.5±3.1% and 93.8±2.3%, respectively.
Tumors with MYCN protein overexpression with the M/U staining pattern (N=11): All these tumors lacked MYCN amplification and ten of them had FH. Also all tumors had no nucleolar hypertrophy. Both 3-year EFS and OS of the patients were 100%.
Tumors with MYCN protein overexpression with the S/H staining pattern (N=18): All tumors had MYCN amplification; and 9 had FH and the other 9 had UH. Among these tumors, 15 (83%) had nucleolar hypertrophy. Three-year EFS and OS of the patients in this group were 57.4±15.3% and 56.2±15.2%, respectively.
Table 2 shows the prognostic effects by other factors in the patients with stage 4S tumors with all favorable indicators analyzed in this study (N=147 with no MYCN amplification, FH, no MYC-family protein overexpression and no nucleolar hypertrophy). Significant prognostic effects for both EFS and OS were found by DNA index (Hyperdiploid vs. Diploid) and Age (< 2 months vs. ≥ 2 months). Significant prognostic effect was found for OS by presence of clinical symptoms (yes vs. no) and for EFS by segmental chromosomal aberrations (11p/11q LOH, yes vs. no).
Table 2.
Prognostic Effects by Factors among Stage 4S Patients with Favorable “Genotype-IHC-Phenotype” Tumors* (N=147)
| Factor | N | 3-year EFS±SE(%) | 3-year OS±SE (%) | Log-rank test p-value |
|---|---|---|---|---|
| DNA Index | ||||
| Age | ||||
| Symptomatic | ||||
| 1p/11a LOH |
These tumors had no MYCN amplification, Favorable Histology, no MYC-family protein overexpression, and no nucleolar hypertrophy. IHC: Immunohistochemical; ND: Not determined; LOH: Loss of Heterozygosity; EFS: Event-free survival; OS: Overall survival; SE: standard error.
DISCUSSION
Peripheral neuroblastic tumors are biologically and clinically heterogeneous with outcomes ranging from spontaneous regression/tumor maturation to aggressive progression/resistance to multimodal therapy. Clinical stage 4SNB is a unique subtype of disease occurring in infants and predominantly composed of biologically favorable tumors. In our series of cases, the vast majority of tumors (162, 88%) were MYCN non-amplified and FH, and the patients had an excellent prognosis. However, a small subset of 4SNB patients, including those with MYCN amplified tumor (20/185, 11%) had a poor prognosis. It was previously reported that aggressive behavior of neuroblastoma tumors in general is directly and critically associated with MYC-family protein overexpression rather than MYCN oncogene amplification (15). This study disclosed the further insight of MYCN amplification, MYC-family protein expression, and their prognostic implication in a large cohort of patients with stage 4SNB.
Sorting by the MYCN protein expression using immunohistochemistry and nucleolar morphology, three distinct groups were identified. Tumors in the first group had no MYCN protein overexpression, and 155/156 had no nucleolar hypertrophy. Tumors in the second group had protein overexpression of the M/U staining pattern, and they also did not have nucleolar hypertrophy (0/11). Tumors in the third group had MYCN protein overexpression of the S/H staining pattern and 15/18 (83%) had nucleolar hypertrophy. All but 2 tumors in the first and second group were associated with MYCN non-amplification, and the patients had a good/excellent prognosis. While, tumors in the third group had MYCN amplification, and the patients had a significantly worse prognosis.
Nucleolar hypertrophy is the histologic manifestation of increased rRNA synthesis and accumulation. In addition, MYC family proteins are known to activate rRNA genes and protein translation (27). Therefore, we surmise MYCN protein overexpression due to MYCN amplification leads to nucleolar hypertrophy and the S/H staining pattern by immunohistochemistry.
In contrast, moderate level of MYCN protein expression with the M/U staining pattern is not associated with MYCN amplification, and not accompanied by nucleolar hypertrophy. Some reports suggest that certain mechanisms exist to allow overexpression or stabilization of MYCN protein without gene overexpression, however, the detailed mechanism still is remained unclear (28–31). There were also rare stage 4S tumors overexpressing MYC protein in this series. In our previous study, we reported that MYC protein overexpressing neuroblastoma, usually not associated with MYC and MYCN amplification, typically had nucleolar hypertrophy and behaved highly aggressively (18, 32). Among those stage 4S tumors overexpressing MYC protein (6 cases), however, 5 (83%) had no nucleolar hypertrophy, and no patients had events/deaths in their clinical course. It seems that MYC-family protein expression without nucleolar hypertrophy has no adverse prognostic significance and its expression might even be ceased during the clinical course of stage 4SNB.
Finally, as described in neuroblastomas in general, factors other than MYCN oncogene status and INPC had statistical significance on prognosis among stage 4SNB patients with tumors with non-amplified MYCN, FH, no MYC-family protein overexpression and no nucleolar hypertrophy. Two prognostic groups were distinguished by DNA index in both EFS and OS, and by presence or absence of segmental chromosomal aberrations of 11p/11q LOH in EFS. As defined, stage 4S patients were diagnosed before 12 months of age, and other factors associated with difficulties in clinical management of infants (8), such as very young age at diagnosis (<2 months) and presence of clinical symptoms especially due to liver enlargement causing secondary organ dysfunction (compromise of respiratory, cardiovascular, gastrointestinal and renal function and disseminated intravascular coagulation (DIC) due to tumor progression) also had significant adverse effects on both EFS and OS or in OS only, respectively, among the same group of patients.
In conclusion, clinical stage 4SNB is a heterogeneous disease with a generally good prognosis. In this study, we report the importance of MYC-family protein overexpression by immunohistochemistry. The biological and prognostic significance of MYCN protein overexpression depends on MYCN oncogene status and presence or absence of nucleolar hypertrophy. MYCN protein overexpression, driven by MYCN amplification and observed in aggressive tumors, is associated with nucleolar hypertrophy and shows the strong and heterogeneous immunohistochemical staining pattern. In contrast, MYCN protein overexpression, observed in non-aggressive tumors and not driven by the oncogene amplification, is not associated with nucleolar hypertrophy and shows the moderate and uniform staining pattern.
Acknowledgments
Conflicts of Interest and Source of Funding: Nothing to disclose. A part of this study was supported by National Institutes of Health (NIH) U10CA98543.
REFERENCES
- 1.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6(12):1874–81. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77. [DOI] [PubMed] [Google Scholar]
- 3.Schleiermacher G, Rubie H, Hartmann O, et al. Treatment of stage 4s neuroblastoma--report of 10 years’ experience of the French Society of Paediatric Oncology (SFOP). Br J Cancer. 2003;89(3):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Noesel MM, Hählen K, Hakvoort-Cammel FG, et al. Neuroblastoma 4S: a heterogeneous disease with variable risk factors and treatment strategies. Cancer. 1997;80(5):834–43. [PubMed] [Google Scholar]
- 5.Haas D, Ablin AR, Miller C, et al. Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer. 1988;62(4):818–25. [DOI] [PubMed] [Google Scholar]
- 6.Nickerson HJ, Matthay KK, Seeger RC, et al. Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: a Children’s Cancer Group study. J Clin Oncol. 2000;18(3):477–86. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur GM. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018;372(2):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twist CJ, Naranjo A, Schmidt ML, et al. Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children’s Oncology Group Study ANBL0531. J Clin Oncol. 2019;37(2):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hachitanda Y, Hata J. Stage IVS neuroblastoma: a clinical, histological, and biological analysis of 45 cases. Hum Pathol. 1996;27(11):1135–8. [DOI] [PubMed] [Google Scholar]
- 10.Matthay KK. Stage 4S neuroblastoma: what makes it special? J Clin Oncol. 1998;16(6):2003–6. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson SR, Cook BA, Mease AD, et al. The prognostic significance of age and pattern of metastases in stage IV-S neuroblastoma. Cancer. 1986;58(2):372–5. [DOI] [PubMed] [Google Scholar]
- 12.De Bernardi B, Pianca C, Boni L, et al. Disseminated neuroblastoma (stage IV and IV-S) in the first year of life. Outcome related to age and stage. Italian Cooperative Group on Neuroblastoma. Cancer. 1992;70(6):1625–33. [DOI] [PubMed] [Google Scholar]
- 13.Hsu LL, Evans AE, D’Angio GJ. Hepatomegaly in neuroblastoma stage 4s: criteria for treatment of the vulnerable neonate. Med Pediatr Oncol. 1996;27(6):521–8. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein HM, Bowman LC, Brodeur GM, et al. Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: the pediatric oncology group experience--a pediatric oncology group study. J Clin Oncol. 1998;16(6):2007–17. [DOI] [PubMed] [Google Scholar]
- 15.Suganuma R, Wang LL, Sano H, et al. Peripheral neuroblastic tumors with genotype-phenotype discordance: a report from the Children’s Oncology Group and the International Neuroblastoma Pathology Committee. Pediatr Blood Cancer. 2013;60(3):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LL, Suganuma R, Ikegaki N, et al. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: a report from the Children’s Oncology Group. Cancer. 2013;119(20):3718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LL, Teshiba R, Ikegaki N, et al. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: a Children’s Oncology Group study. Br J Cancer. 2015;113(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemas-Teshiba R, Matsuno R, Wang LL, et al. MYC-family protein overexpression and prominent nucleolar formation represent prognostic indicators and potential therapeutic targets for aggressive high-MKI neuroblastomas: a report from the children’s oncology group. Oncotarget. 2018;9(5):6416–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer. 1999;86(2):364–72. [PubMed] [Google Scholar]
- 20.Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86(2):349–63. [PubMed] [Google Scholar]
- 21.Ambros IM, Hata J, Joshi VV, et al. Morphologic features of neuroblastoma (Schwannian stroma-poor tumors) in clinically favorable and unfavorable groups. Cancer. 2002;94(5):1574–83. [DOI] [PubMed] [Google Scholar]
- 22.Ikegaki N, Bukovsky J, Kennett RH. Identification and characterization of the NMYC gene product in human neuroblastoma cells by monoclonal antibodies with defined specificities. Proc Natl Acad Sci U S A. 1986;83(16):5929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluk MJ, Chapuy B, Sinha P, et al. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7(4):e33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100(9):1471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc.1957;53:457–481. [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molenaar JJ, Domingo-Fernández R, Ebus ME, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44(11):1199–206. [DOI] [PubMed] [Google Scholar]
- 29.Beltran H The N-myc Oncogene: Maximizing its Targets, Regulation, and Therapeutic Potential. Mol Cancer Res. 2014;12(6):815–22. [DOI] [PubMed] [Google Scholar]
- 30.Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15(1):67–78. [DOI] [PubMed] [Google Scholar]
- 31.Valentijn LJ, Koster J, Haneveld F, et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci U S A. 2012;109(47):19190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuno R, Gifford AJ, Fang J, et al. Rare MYC-amplified Neuroblastoma With Large Cell Histology. Pediatr Dev Pathol. 2018;21(5):461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


