Abstract
Objectives:
Electrocochleography (ECochG), obtained prior to insertion of a cochlear implant (CI) array, provides a measure of residual cochlear function that accounts for a substantial portion of variability in postoperative speech perception outcomes in adults. It is postulated that subsequent surgical factors represent independent sources of variance in outcomes. Prior work has demonstrated a positive correlation between angular insertion depth (AID) of straight arrays and speech perception in the CI-alone condition, with an inverse relationship observed for pre-curved arrays. The purpose of the present study was to determine the combined effects of ECochG, AID, and array design on speech perception outcomes.
Design:
Participants were 50 post-lingually deafened adult CI recipients who received one of three straight arrays (MED-EL Flex24, MED-EL Flex28, and MED-EL Standard) and two pre-curved arrays (Cochlear Contour Advance and Advanced Bionics HiFocus Mid-Scala). Residual cochlear function was determined by the intraoperative ECochG total response (TR) measured prior to array insertion, which is the sum of magnitudes of spectral components in response to tones of different stimulus frequencies across the speech spectrum. The AID was then determined with postoperative imaging. Multiple linear regression was used to predict consonant-nucleus-consonant (CNC) word recognition in the CI-alone condition at 6 months post-activation based on AID, TR, and array design.
Results:
Forty-one participants received a straight array and 9 received a pre-curved array. The AID of the most apical electrode contact ranged from 341° to 696°. The TR measured by ECochG accounted for 43% of variance in speech perception outcomes (p < 0.001). A regression model predicting CNC word scores with the TR tended to underestimate performance for pre-curved arrays and deeply inserted straight arrays, and to overestimate performance for straight arrays with shallower insertions. When combined in a multivariate linear regression, the TR, AID, and array design accounted for 72% of variability in speech perception outcomes (p < 0.001).
Conclusions:
A model of speech perception outcomes that incorporates TR, AID, and array design represents an improvement over a model based on TR alone. The success of this model shows that peripheral factors including cochlear health and electrode placement may play a predominant role in speech perception with CIs.
Keywords: electrocochleography, cochlear implant, speech perception, peripheral auditory physiology, angular insertion depth, straight array, pre-curved array, ECochG
Introduction
Speech perception outcomes are highly variable among cochlear implant (CI) recipients, yet the underlying mechanisms have remained largely unexplained (Gantz et al. 1993; Blamey et al. 1996, 2013; Green et al. 2007; Lazard et al. 2012). Early work suggested that one factor influencing outcomes is duration of deafness (Rubinstein et al. 1999), but in current populations where long durations of deafness are less common, this factor accounts for less than 25% of variance (Lazard et al. 2012; Blamey et al. 2013). Recently, a single measure of residual cochlear function to auditory stimulation called the ‘Total Response (TR)’, has been shown to account for up to 40-50% of variability in CI-alone speech perception outcomes in adults (Fitzpatrick et al. 2014; McClellan et al. 2014; Fontenot et al. 2019). The TR is obtained using intraoperative electrocochleography (ECochG), which measures electrical potentials produced by hair cells and the auditory nerve during acoustic stimulation. The TR is a pre-insertion estimate of cochlear responses summed across tonal stimuli of different frequencies across the speech spectrum (Fitzpatrick et al. 2014). Other pre-insertion factors such as duration of deafness, age at implantation, and preoperative pure-tone average do not provide additional predictive power compared to the TR alone, suggesting that the causal mechanisms responsible for these effects are captured by the TR (McClellan et al. 2014).
Another factor that is significantly correlated with speech perception outcomes is the placement of the array as measured by the angular insertion depth (AID). While some work has demonstrated a decline in speech perception with deeper insertions (Finley et al. 2008; Lazard et al. 2012; Holden et al. 2013), others have shown a benefit in the CI-alone condition (Hochmair et al. 2003; Yukawa et al. 2004; Buchman et al. 2014; O’Connell et al. 2016, 2017a; Büchner et al. 2017, Chakravorti et al. 2019). In the interpretation of these discrepant findings, it is essential to highlight that studies demonstrating a benefit of deeper insertion restricted the analysis to straight arrays, while those reporting a decrement in speech perception included recipients of both pre-curved and straight arrays. After accounting for array design (i.e., straight vs. pre-curved), Chakravorti et al. (2019) demonstrated differential effects of insertion depth on speech perception outcomes.
With straight arrays, AID has been shown to account for up to about 30% of variability in speech perception outcomes (O’Connell et al. 2017a). Deep insertions achieved with a long array allow for more complete coverage of the cochlea, and less discrepancy between the electric frequency information being presented and the natural tonotopicity, known as frequency-to-place mismatch. In vocoder simulations and conventional CI recipients, a reduction in mismatch has been shown to positively affect speech perception performance (Dorman et al. 1997; Fu & Shannon 1999; Baskent & Shannon 2003, 2005; Li & Fu 2010; Canfarotta et al. 2020a, 2020b). CI recipients of longer arrays may also benefit from greater separation between neighboring contacts, which could theoretically improve spectral resolution (Canfarotta et al. 2020a). In contrast, pre-curved arrays confer benefit from closer proximity to spiral ganglion cells and are associated with a shallower range of insertion depths given their relatively short array length (Holden et al. 2013; Chakravorti et al. 2019). The negative association between AID and speech perception observed with more deeply inserted pre-curved electrodes is understood to reflect detrimental effects of over-insertion, pushing the basal contacts away from the modiolus (Wang et al. 2017; Chakravorti et al. 2019), in addition to greater likelihood of translocation into the scala vestibuli (Finley et al. 2008; Radeloff et al. 2008).
Because measurement of the TR occurs prior to insertion, subsequent factors, including AID, may represent independent sources of variance in outcomes. Assessing the total variance accounted for by the TR and AID is clinically relevant as it could indicate the relative importance of peripheral compared to central factors on speech perception outcomes in adult CI recipients. Furthermore, these results could provide preliminary evidence to inform desired insertion depths according to array design in the context of an individual patient’s cochlear physiology. While other factors including trauma to cochlear structures, inflammation, and fibrosis likely also contribute to postoperative performance, these variables are challenging to assess in-vivo with current postoperative assessments.
Our group has recently demonstrated the feasibility of determining AID by intraoperative x-ray (Giardina et al. 2020), which is routinely obtained in all CI cases at our institution. This method allows an accurate measure of insertion depth in a cohort of CI recipients in which we have previously reported a strong relationship between ECochG and speech perception outcomes (Fontenot et al. 2019). In the current study we tested the hypothesis that a model combining the TR and AID, along with array design (straight vs. pre-curved), would provide a better prediction of speech perception outcomes than either factor alone.
Materials and Methods
Participants
Participants were post-lingually deafened adult (18-79 years) CI recipients who underwent intraoperative ECochG. They were implanted with either a straight or pre-curved array. In this population, there were three straight arrays, all with 12 electrode contacts and all made by MED-EL (Innsbruck, Austria): 1) Flex24, with an “active length” of 20.9 mm between the most apical contact and the most basal contact, 2) Flex28, with an active length of 23.1 mm, and 3) Standard, with an active length of 26.4 mm. There were two pre-curved arrays: 1) Cochlear (Sydney, Australia) Contour Advance, with 22 electrode contacts and an active length of 15 mm, and Advanced Bionics (Valencia, CA, USA) HiFocus Mid-Scala, with 16 electrode contacts and an active length of 15 mm. Participants were excluded if they were non-native English speakers, had a CI revision surgery, or were listening with electric-acoustic stimulation (EAS). Those with a history of contralateral implantation or preoperative residual low-frequency acoustic hearing in the ear to be implanted were not excluded. Informed consent was obtained prior to measuring ECochG responses, and all procedures were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. Data in the current report include a subset of patients with interpretable, intraoperative radiographs.
Electrocochleography Recordings
The stimulation and recording methods, procedures for calculating TR, and participant data used in this study were previously reported (Fontenot et al. 2019). Briefly, the ECochG was done intraoperatively from the round window of participants undergoing cochlear implantation, prior to opening the round window/cochleostomy and insertion of the array. Stimulation and recording were controlled by a Biologic Navigator Pro (Natus Medical Inc., San Carlos, CA). Sound delivery was through an Etymotic speaker (ER-3B) connected by a sound tube to an in-ear foam insert. Surface electrodes (Neuroline 720, Ambu Inc, Ballerup, Denmark) on the contralateral mastoid and forehead were the reference and common electrodes, respectively. The recording electrode was a monopolar stainless-steel facial nerve monitor probe (Neurosign 3602-00- TE, Magstim Co., Wales, UK). Impedance levels on all electrodes were less than 16 kOhm before recording. Tone burst stimuli at 6 different frequencies (250, 500, 750, 1000, 2000 and 4000 Hz) were delivered at 90 dB nHL (95-114 dB peak SPL). All stimuli were presented in alternating condensation and rarefaction phases, with up to 250 repetitions to each phase (fewer repetitions were used if the signal to noise ratio was large). For recordings, the high-pass filter setting was 10 Hz, and low-pass settings were 5000 Hz (250-1000 Hz tone frequencies), 10,000 Hz (for 2000 Hz) or 15,000 Hz (for 4000 Hz tone frequency). Recording gain was 50,000x.
The spectrum of the response to each of the six tone frequencies was computed by taking a fast Fourier transform of the steady-state response to each phase of stimulation. In each spectrum, peaks to the first three harmonics were considered significant if they exceeded the noise floor by more than three standard deviations, as measured from three bins on either side of the peaks. The TR was calculated as the sum of all of the significant peaks in each spectrum (Fig. 1).
Figure 1.
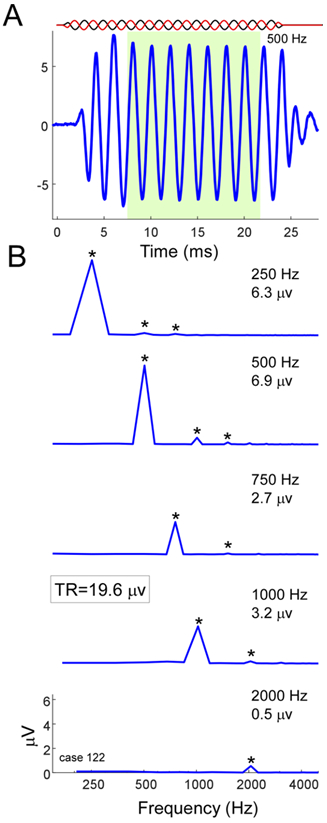
Calculation of the Total Response (TR). A) A representative response to a 500-Hz tone burst. The stimulus alternated in phase, but only the response to condensation phase is shown. The window for the steady-state response used to compute the fast Fourier transforms is shown in green (8-24 ms). B) Magnitude spectra for each stimulus frequency with significant responses (250-2000 Hz). Each spectrum is the average of the condensation and rarefaction phases that were computed separately. For each stimulus frequency, the magnitudes of the first three harmonic peaks were compared to the noise floor. Those that were significant above floor (asterisks, see text for test of significance) were added to produce the total response, in this case 19.6 μV.
Measurement of Angular Insertion Depth
The AID of the most apical electrode contact was measured with intraoperative x-ray using a rotating helical scala tympani model, which has been previously described and validated against CT imaging (Giardina et al. 2020). Briefly, this method uses a scalable, rotatable 3-dimensional model of the scala tympani to estimate AID from an x-ray obtained at an unknown acquisition angle (Fig. 2).
Figure 2.
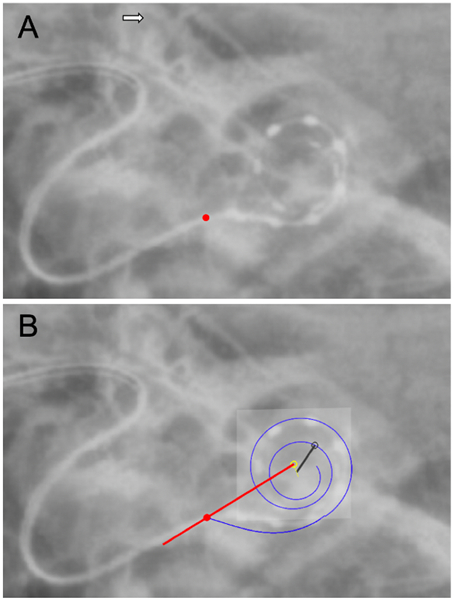
Determination of angular insertion depth (AID) with a representative radiograph of a MED-EL Standard straight electrode array, using the rotating helical scala tympani model as described by Giardina et al. (2020). A) Identification of landmarks such as the superior semicircular canal (white arrow) and round window (red circle). B) The 3-dimensional cochlear helix (blue line) is moved, scaled, and rotated to match the projection angle of the 2-dimensional radiograph. The red line from the center of the modiolus (open yellow circle) through the round window serves as the zero-degree reference point. This model then serves as a basis for estimating the AID associated with individual electrode contacts, which are marked manually (black open dot). The resultant AID of the most apical electrode contact (black open circle) in this case is 568°.
Postoperative Speech Perception
Aided speech perception testing in the familiar, CI-alone condition was conducted in a soundproof booth with the participant seated 1 meter away from the sound source. Recorded materials were presented at 60 dB SPL. Masking was presented to the contralateral ear via an insert earphone when warranted to prevent audibility. Speech perception performance was measured using the consonant-nucleus-consonant (CNC) word test (Peterson & Lehiste 1962). The CNC word score obtained at 6 months post-activation of the external audio processor was the outcome measure selected for the analysis.
Statistical Analysis
Univariate linear regression and correlation were used to determine the relationships between AID, TR, array design (straight vs. pre-curved) and speech perception outcomes. Multiple linear regression analysis was used to assess whether a combination of factors would predict CNC word scores better than TR alone. Factors incorporated into the final model were considered significant (p < 0.05) if they increased the adjusted r2 and reduced the Bayesian Information Criterion (BIC). Analyses were performed with SPSS 25 for Windows (IBM Corp, Armonk, New York).
Results
Participant Demographics and Characteristics
The characteristics of 50 adult CI recipients who underwent intraoperative ECochG are summarized in Table 1. There were more females (58%) than males, and the mean age was 60.4 years (SD = 14.7 years) at the time of surgery. The mean preoperative pure-tone average (PTA; 500, 1000, and 2000 Hz) was 84 dB HL (SD = 15 dB HL, range = 60-117 dB HL). The most prevalent etiology was sensorineural hearing loss of unknown origin. Array designs included 41 (82%) straight and 9 (18%) pre-curved arrays. Of specific devices implanted, 6 arrays were Flex24 (12%), 6 were Flex28 (12%), 29 were Standard (58%), 7 were Contour Advance (14%), and 2 were Hi-Focus Mid-Scala (4%) arrays. All participants listened with the CI-alone postoperatively.
TABLE 1.
Demographics of participants included in this study.
| Factor | n (%) |
|---|---|
| Sex | |
| Female | 29 (58) |
| Male | 21 (42) |
| Age (years) | 60.4 ± 14.7 |
| Preoperative PTA (dB HL) | 84 ± 15 |
| Etiology | |
| Unknown | 38 (76) |
| Meniere’s | 2 (4) |
| Barotrauma | 2 (4) |
| Ototoxic | 1 (2) |
| Viral | 1 (2) |
| Stroke | 1 (2) |
| Radiation | 1 (2) |
| Enlarged aqueduct | 1 (2) |
| Meningitis | 1 (2) |
| Usher’s syndrome | 1 (2) |
| Temporal bone fracture | 1 (2) |
| Device | |
| Cochlear | |
| Contour Advance | 7 (14) |
| Advanced Bionics | |
| HiFocus Mid-Scala | 2 (4) |
| MED-EL | |
| Flex24 | 6 (12) |
| Flex28 | 6 (12) |
| Standard | 29 (58) |
PTA, pure-tone average (500, 1000, and 2000 Hz)
Angular Insertion Depth
Angular insertion depth of the most apical contact is affected by array length, array design, surgical approach (i.e., round window versus cochleostomy), and cochlear morphology. The AID across the entire cohort spanned a range of 341° to 696°, with a median (M) of 572° and interquartile range (IQR) of 404-646°. The majority of participants (n = 47, 94%) had a complete insertion of the electrode array. Of those with a partial insertion, two Standard recipients had 2 extracochlear electrode contacts, and one Flex24 recipient had 1 extracochlear electrode contact. As illustrated in Figure 3, the median AID was shallowest for Contour Advance (M = 364°, IQR = 350-374°), Mid-Scala (M = 400°, IQR = 387-412°), and Flex24 (M = 400°, IQR = 394-410°) arrays, deeper for Flex28 (M = 509°, IQR = 497-553°), and deepest for Standard (M = 641°, IQR = 597-666°) arrays. Incomplete insertions resulted in reduced AID of the most apical electrode contact as indicated with open symbols (Fig. 3). Differences in AID across arrays were statistically significant when evaluated with one-way ANOVA (F(4,45) = 53.55, p < 0.001). Pairwise comparisons showed no differences in AID between the three arrays with shallowest insertions (p ≥ 0.658), significant differences between all of these arrays and Flex28 (p ≤ 0.034) and Standard arrays (p ≤ 0.001), and a significant difference between Flex28 and Standard arrays (p = 0.001).
Figure 3.
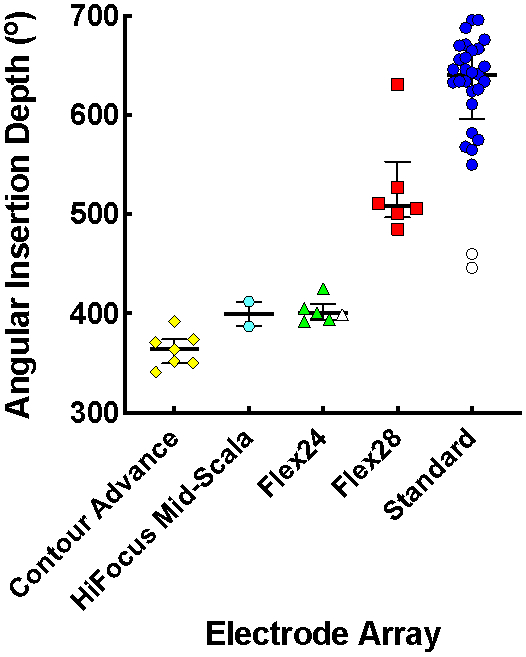
Median angular insertion depth (AID) with interquartile ranges for Contour Advance, HiFocus Mid-Scala, Flex24, Flex28 and Standard electrode arrays; each data point represents an individual participant. Open symbols signify participants with a partial insertion.
Speech Perception Performance
In contrast to differences in AID, differences in speech perception scores across array types were small (Fig. 4). The mean postoperative CNC word score across CI recipients was 58.8% (SD = 16.9 points). Differences in CNC word scores across arrays were statistically significant when evaluated with one-way ANOVA (F(4,45) = 2.85, p = 0.035). Pairwise comparisons only revealed a significant difference in scores between Flex24 and Contour Advance arrays, with better performance among Contour Advance recipients (p = 0.029).
Figure 4.
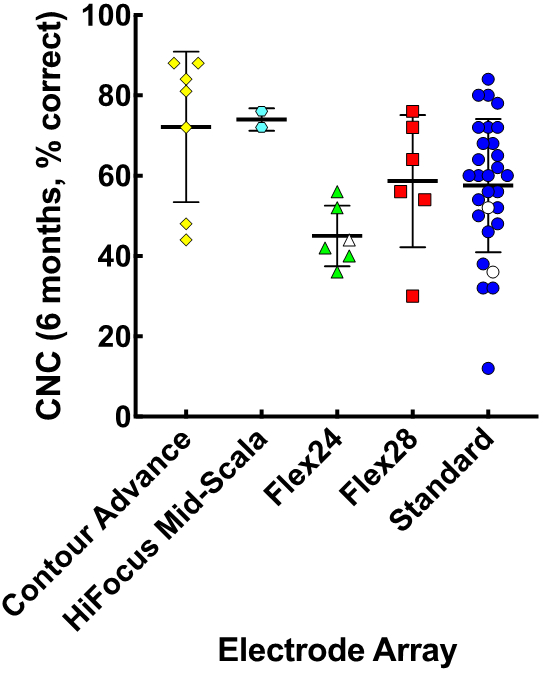
Mean consonant-nucleus-consonant (CNC) score with the CI-alone with standard deviations in relation to electrode array types; plotting conventions follow those of Figure 3.
ECochG, AID, and Array Design as Factors in Speech Perception Outcomes
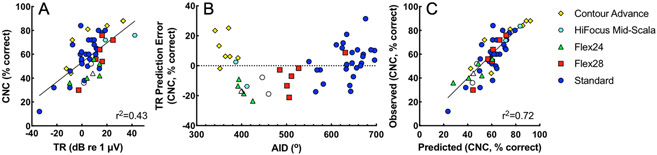
As reported previously (Fitzpatrick et al. 2014; McClellan et al. 2014; Fontenot et al. 2019), there was a positive correlation between the TR and CNC word scores in these participants. This association is illustrated in Figure 5A. In the current dataset, consisting of the subset of patients with interpretable radiographs (n = 50), the TR accounted for 43% of the variance in CNC word scores (p < 0.001). Figure 5B shows the TR prediction error (residuals) for CNC word scores from Figure 5A plotted as a function of AID. The TR alone tended to underestimate performance (e.g., positive prediction error, signifying scores above line of best fit in Fig. 5A) for pre-curved arrays and deeply inserted straight arrays (primarily Standard arrays). In contrast, predictions based on the TR tended to overestimate performance for straight arrays with shallower insertions (primarily Flex24 and Flex28 arrays).
Figure 5.
Predicting CNC word scores with the CI-alone at 6 months post-activation based on TR, AID, and array type. Different electrode arrays types are coded by color and shape, following plotting conventions of Figure 3. A) CNC word scores plotted as a function of TR. B) TR prediction error of CNC word scores by linear regression, plotted as a function of AID. Positive values signify that the TR underpredicted CNC word scores, while negative values signify that participants performed more poorly than predicted. Participants with more deeply inserted straight arrays (primarily MED-EL Standard) and pre-curved arrays (Cochlear Contour Advance and Advanced Bionics HiFocus Mid-Scala) performed better than predicted by the TR alone. C) Observed CNC word scores plotted as a function of predicted CNC word scores based a model using TR, AID, array design (straight vs. pre-curved), and the interaction of AID and array design. TR indicates total response, a summary ECochG measure; CNC, consonant-nucleus-consonant; AID, angular insertion depth.
Incorporating TR, AID, array design (straight or pre-curved) and the interaction between AID and array design into a multiple regression model accounted for 72% of the variance in CNC word scores (p < 0.001, Fig. 5C). The results of the regression are reported in Table 2. This model was constructed using forward selection stepwise regression. Each parameter in the final model significantly increased the adjusted r2 (p < 0.05) and reduced the BIC. Including the interactions between TR and AID or TR and array design did not meet these criteria and were therefore excluded from the model. Similar to prior findings (McClellan et al. 2014), the addition of preoperative PTA, age at implantation, or duration of hearing loss did not significantly increase the adjusted r2 of the model.
TABLE 2.
Model predicting percent correct CNC word scores from total response (TR), angular insertion depth (AID) of the most apical electrode contact, and array design (straight, Array = 0; pre-curved, Array = 1).
| Unstandardized Coefficients |
Standardized Coefficients |
||||
|---|---|---|---|---|---|
| B | Std. Error | Beta | t | p | |
| Intercept | 0.23 | 9.18 | 0.03 | 0.980 | |
| TR | 0.88 | 0.11 | 0.71 | 8.43 | <.001 |
| AID | 0.09 | 0.02 | 0.62 | 5.70 | <.001 |
| Array | 190.23 | 56.82 | 4.32 | 3.35 | 0.002 |
| AID*Array | −0.44 | 0.15 | −3.70 | −2.89 | 0.006 |
The AID of all subjects was not significantly related to CNC scores (r2 < 0.01, p = 0.969). However, a secondary analysis was performed on data from straight array recipients to better understand the role of TR and AID in this more homogeneous sample. With univariate linear regression, both TR and AID were associated with CNC word scores at 6 months (r2 = 0.38, p < 0.001 and r2 = 0.18, p = 0.006, respectively). A model including the TR and AID of straight arrays accounted for 68% of variance in CNC word scores (p < 0.001).
Discussion
Understanding the variability in speech perception outcomes following cochlear implantation remains a frustrating challenge to researchers and clinicians alike. Previously, the TR, which measures residual cochlear function, was shown to account for a high degree of variance in adult CI-alone users’ speech perception performance in quiet (Fitzpatrick et al. 2014; McClellan et al. 2014; Fontenot et al. 2019). The current study was primarily designed to determine whether surgical factors (i.e., insertion depth and array design), would account for additional variance in speech perception when combined with the TR. A model including TR, AID, and array design accounted for 72% of variance in CNC word scores at 6 months post-activation in this sample, a significant improvement from TR on its own, which accounts for 43% of variance.
Relationship Between TR and Speech Perception
The participants in the present study were a subset of those evaluated by Fontenot et al. (2019), including only those patients with radiographs suitable for determining AID using the method of Giardina et al. (2020). Fontenot et al. (2019) reported a positive correlation between the TR and speech perception among adult CI recipients <80 years old, excluding participants who did not achieve open-set speech perception (n = 2) and poor-performing outliers (n = 4). Adults 80 years and older (n = 10) were also found to have overall lower CNC word scores. The TR is only loosely correlated to unaided hearing detection thresholds (Fitzpatrick et al., 2014, Fontenot et al., 2019). These authors postulated that the TR is primarily a measure of hair cell activity, which is in part disconnected from the auditory nerve and is thus not reflected in the audiogram. Although the auditory nerve neurophonic contributes to the TR, this factor is typically much smaller than the cochlear microphonic (Fontenot et al., 2017). By this interpretation, hair cell activity as measured by the TR serves as a proxy for cochlear health and the underlying neural substrate available for electrical stimulation (Fontenot et al. 2019).
Relationship Between Insertion Depth, Array Design, and Speech Perception
As reported previously (Chakravorti et al. 2019), the association between AID and speech perception depends upon the array design in the present study. Both array design and the interaction of array design and AID made significant contributions to the model. There was no interaction between TR and AID, which is expected because the TR was taken prior to insertion. However, if the TR was measured from the intracochlear array we might expect to see an interaction between TR and AID.
These findings could help to reconcile the disparate results seen in the literature with respect to the correlation between speech perception and AID, which is reported to be positive in some cases and negative in others. In general, those studies reporting a detrimental effect include pre-curved arrays (Finley et al. 2008; Lazard et al. 2012; Holden et al. 2013), and those reporting a benefit are dominated by straight arrays (Hochmair et al. 2003; Yukawa et al. 2004; Buchman et al. 2014; O’Connell et al. 2016, 2017a; Büchner et al. 2017; Chakravorti et al. 2019).
The present results highlight key differences between pre-curved and straight arrays. As illustrated in Figure 5B, prediction error for a regression model with TR as the sole independent variable differed by array design. After accounting for “cochlear health” with the TR, performance with straight arrays was worse than predicted for shallow AIDs (~400-550°) and better than predicted for deeper AIDs (~600-700°). In contrast, recipients of pre-curved arrays had shallow AIDs of ~340-420° and performed better than predicted. Shallow insertions should produce similar degrees of frequency-to-place mismatch with the two array designs; however, closer proximity to neural substrate generally achieved with pre-curved arrays results in reduced spread of excitation and channel interaction (DeVries & Arenberg 2018), which may support the observed improvements in speech perception (Holden et al 2013; Chakravorti et al. 2019). The strong positive correlation between AID and TR prediction error among straight array recipients presumably reflects a reduction in frequency-to-place mismatch with deeper insertions (Canfarotta et al., 2020a).
Similar to our findings, James et al. (2019) reported optimal sentence recognition for pre-curved array recipients with insertion depths of ~360°, noting a negative correlation between AID (range = 320° to 560°) and sentence recognition scores in this cohort. These findings could indicate that any advantage gained by a reduction in frequency-to-place mismatch with a deeper insertion of a pre-curved array is offset by either loss of basal cochlear coverage with over-insertion (Finley et al. 2008; Holden et al. 2013), increased risk for apical translocation into the scala vestibuli (Finley et al. 2008; Radeloff et al. 2008), or increased distance from the modiolus (Wang et al. 2017; Chakravorti et al. 2019). While not specifically analyzed by James et al. (2019), data in their figures indicate a negative correlation between sentence recognition and AID with straight arrays. That cohort included straight array recipients of 3 different manufacturers; as such, differences in array design, signal coding strategies, and mapping may confound results. Discrepancies across studies have important clinical implications in the selection process of straight arrays; whereas the results of James et al. (2019) indicate better outcomes with a relatively short straight array (e.g., 20 to 24 mm), the present study indicates better performance with a longer straight array.
Implications for the Use of ECochG in Future Work
The main purpose of the present study was to determine whether two surgical variables (i.e., AID and electrode array design) can account for additional variance in CI-alone speech perception performance, after adjusting for “cochlear health” measured with the TR. For any given TR, the model coefficients in Table 2 demonstrate a ~20-25% increase in CNC word scores for a median Contour Advance (364°) or Standard (641°) array insertion when compared to a Flex24 (400°) array insertion. These findings suggest that a pre-curved or long straight array can provide excellent outcomes for the conventional CI recipient with severe-to-profound sensorineural hearing loss. However, array selection becomes more challenging for the growing population of patients with varying degrees of residual low-frequency acoustic hearing, where a shorter straight array increases the likelihood of hearing preservation (Gantz et al. 2016; Suhling et al. 2016; O’Connell et al. 2017a), yet results in poorer speech perception if hearing is lost (Büchner et al. 2017; O’Connell et al. 2017a). It is possible that in the future, incorporation of an intraoperative pre-insertion TR measurement could better inform array selection for patients with residual hearing. Such a process would need to model the benefit of combined EAS as compared to the CI-alone, unaided hearing detection threshold shifts as a function of insertion depth (i.e., the ability to use an EAS device), and ultimately, how the TR is related to each of these individual variables.
While this study accounts for a large portion of variance in early CI outcomes with only three variables, there are certainly limitations. Given that AID was determined with intraoperative x-ray, we were unable to integrate other surgical variables related to intracochlear array position known to impact speech perception outcomes into our model, such as scalar location and modiolar proximity, which would require higher resolution three-dimensional rendering of images with computed tomography. Prior work has consistently demonstrated a relationship between scala tympani insertions and superior speech perception when compared to electrodes translocated into the scala vestibuli (Aschendorff et al. 2007; Skinner et al. 2007; Finley et al. 2008; Holden et al. 2013; Wanna et al. 2014; O’Connell et al. 2016; O’Connell et al. 2017b). For example, translocation is associated with a 12% decrease in CNC word scores (O’Connell et al. 2016). Another consideration in the present dataset is that word recognition in quiet was assessed at the 6-month post-activation interval. Improvements in speech perception with additional listening experience has been shown for some CI recipients (Holden et al. 2013; Dillon et al. 2013; Cusumano et al. 2017), and follow-up studies should assess long-term outcomes. Speech recognition in noise is another variable to consider in the future. Lastly, the sample size of pre-curved array recipients in the current dataset is relatively small, and larger studies will be needed to confirm results observed in this subset of patients. Prospective investigations are currently under way to address these variables.
Furthermore, as this study focused on peripheral factors affecting speech perception, future work should address the degree to which central auditory processing abilities affect outcomes. Previous work has demonstrated that several measures of linguistic and neurocognitive skills correlate with speech perception abilities (Gantz et al. 1993; Lyxell et al. 1998; Heydebrand et al. 2007; Moberly et al. 2016, 2018; Pisoni et al. 2018), and current studies are incorporating cognitive factors into this model, to account for remaining variance.
Conclusions
As reported previously, measurement of the TR prior to CI insertion explained a large portion of variance (43%) in speech perception outcomes among adult CI recipients. The TR generally underestimated performance for recipients of either pre-curved or deeply inserted straight arrays, whereas performance was overestimated for recipients of shallower straight arrays. A model including the TR, AID, array design, and the interaction between AID and array design accounted for 72% of variance in postoperative CNC word scores in the CI-alone condition. These findings indicate that the relationship between insertion depth and speech perception may be dependent upon array design and highlight the relatively large role of the auditory periphery in speech perception among adult CI recipients.
Acknowledgments
Conflicts of Interest and Source of Funding
This project was funded by the NIH through NIDCD (T32 DC005360 and F30 DC015168). The authors BPO, KDB, and HCP have served on the surgical advisory board for MED-EL Corporation. HCP is a consultant for MED-EL Corporation. BPO is a consultant for Advanced Bionics. MTD and MAR are supported by a research grant from MED-EL Corporation. CAB is a surgical consultant for Advanced Bionics, Cochlear, IotaMotion, and Envoy, and has equity interest in Advanced Cochlear Diagnostics, LLC. OFA is a paid consultant for MED-EL Corporation, Advanced Bionics, Spiral Therapeutics, and AGTC Inc; receives research support from MED-EL Corporation; has ownership in Advanced Cochlear Diagnostics; and receives royalties from Advanced Bionics. OFA and DCF have a research grant from Advanced Bionics. MWC, CKG and EB declare that their involvement in research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Portions of these data were presented at the 16th Symposium on Cochlear Implants in Children in July 2019 in Hollywood, FL.
References
- Aschendorff A, Kromeier J, Klenzner T, et al. (2007). Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear, 28, 75S–79S. [DOI] [PubMed] [Google Scholar]
- Baskent D, Shannon RV (2003). Speech recognition under conditions of frequency-place compression and expansion. J Acoust Soc Am, 113, 2064–2076. [DOI] [PubMed] [Google Scholar]
- Baskent D, Shannon RV (2005). Interactions between cochlear implant electrode insertion depth and frequency-place mapping. J Acoust Soc Am, 117, 1405–1416. [DOI] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, et al. (1996). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol, 1, 293–306. [DOI] [PubMed] [Google Scholar]
- Blamey P, Artieres F, Baskent D, et al. (2013). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol, 18, 36–47. [DOI] [PubMed] [Google Scholar]
- Buchman CA, Dillon MT, King ER, et al. (2014). Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol, 35, 1773–1779. [DOI] [PubMed] [Google Scholar]
- Büchner A, Illg A, Majdani O, et al. (2017). Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One, 12, e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfarotta MW, Dillon MT, Buss E, et al. (2020a). Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear, Epub ahead of print, doi: 10.1097/AUD.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfarotta MW, O'Connell BP, Buss E, et al. (2020b). Influence of Age at Cochlear Implantation and Frequency-to-Place Mismatch on Early Speech Recognition in Adults. Otolaryngol Head Neck Surg, 194599820911707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorti S, Noble JH, Gifford RH, et al. (2019). Further Evidence of the Relationship Between Cochlear Implant Electrode Positioning and Hearing Outcomes. Otol Neurotol, 40, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusumano C, Friedmann DR, Fang Y, et al. (2017). Performance Plateau in Prelingually and Postlingually Deafened Adult Cochlear Implant Recipients. Otol Neurotol, 38, 334–338. [DOI] [PubMed] [Google Scholar]
- DeVries L & Arenberg JG (2018). Psychophysical Tuning Curves as a Correlate of Electrode Position in Cochlear Implant Listeners. J Assoc Res Otolaryngol. 19, 571–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MT, Buss E, Adunka MC, et al. (2013). Long-term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg, 139, 279–283. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Loizou PC, Rainey D (1997). Simulating the effect of cochlear-implant electrode insertion depth on speech understanding. J Acoust Soc Am, 102, 2993–2996. [DOI] [PubMed] [Google Scholar]
- Finley CC, Holden TA, Holden LK, et al. (2008). Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol, 29, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Campbell AP, Choudhury B, et al. (2014). Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults. Otol Neurotol, 35, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, Fitzpatrick DC (2017). A Model-Based Approach for Separating the Cochlear Microphonic from the Auditory Nerve Neurophonic in the Ongoing Response Using Electrocochleography. Front Neurosci, 11, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, Dillon MT, et al. (2019). Residual Cochlear Function in Adults and Children Receiving Cochlear Implants: Correlations With Speech Perception Outcomes. Ear Hear, 40, 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV (1999). Effects of electrode location and spacing on phoneme recognition with the Nucleus-22 cochlear implant. Ear Hear, 20, 321–331. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Woodworth GG, Knutson JF, et al. (1993). Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol, 102, 909–916. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Dunn C, Oleson J, et al. (2016). Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. Laryngoscope, 126:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina CK, Canfarotta MW, Thompson NT, et al. (2020). Assessing Cochlear Implant Insertion Angle from an Intraoperative X-ray Using a Rotating 3-D Helical Scala Tympani Model. Otol Neurotol, 41, e686–e694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Bhatt Y, Mawman DJ, et al. (2007). Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants Int, 8, 1–11. [DOI] [PubMed] [Google Scholar]
- Heydebrand G, Hale S, Potts L, et al. (2007). Cognitive predictors of improvements in adults' spoken word recognition six months after cochlear implant activation. Audiol Neurootol, 12, 254–264. [DOI] [PubMed] [Google Scholar]
- Hochmair I, Arnold W, Nopp P, et al. (2003). Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryngol, 123, 612–617. [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, et al. (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear, 34, 342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CJ, Karoui C, Laborde ML, et al. (2019). Early Sentence Recognition in Adult Cochlear Implant Users. Ear Hear, 40, 905–917. [DOI] [PubMed] [Google Scholar]
- Lazard DS, Vincent C, Venail F, et al. (2012). Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One, 7, e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Sonmez H, Joseph G, et al. (2012). Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngol Head Neck Surg, 147, 112–118. [DOI] [PubMed] [Google Scholar]
- Li T, Fu QJ (2010). Effects of spectral shifting on speech perception in noise. Hear Res, 270, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyxell B, Andersson J, Andersson U, et al. (1998). Phonological representation and speech understanding with cochlear implants in deafened adults. Scand J Psychol, 39, 175–179. [DOI] [PubMed] [Google Scholar]
- McClellan JH, Formeister EJ, Merwin WH 3rd, et al. (2014). Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol, 35, e245–252. [DOI] [PubMed] [Google Scholar]
- Moberly AC, Castellanos I, Vasil KJ, et al. (2018). "Product" Versus "Process" Measures in Assessing Speech Recognition Outcomes in Adults With Cochlear Implants. Otol Neurotol, 39, e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly AC, Houston DM, Castellanos I (2016). Non-auditory neurocognitive skills contribute to speech recognition in adults with cochlear implants. Laryngoscope Investig Otolaryngol, 1, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell BP, Cakir A, Hunter JB, et al. (2016). Electrode Location and Angular Insertion Depth Are Predictors of Audiologic Outcomes in Cochlear Implantation. Otol Neurotol, 37, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell BP, Hunter JB, Haynes DS, et al. (2017a). Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope, 127, 2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell BP, Holder JT, Dwyer RT, et al. (2017b). Intra- and Postoperative Electrocochleography May Be Predictive of Final Electrode Position and Postoperative Hearing Preservation. Front Neurosci, 11, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I (1962). Revised CNC lists for auditory tests. J Speech Hear Disord, 27, 62–70. [DOI] [PubMed] [Google Scholar]
- Pisoni DB, Broadstock A, Wucinich T, et al. (2018). Verbal Learning and Memory After Cochlear Implantation in Postlingually Deaf Adults: Some New Findings with the CVLT-II. Ear Hear, 39, 720–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff A, Mack M, Baghi M, et al. (2008). Variance of angular insertion depths in free-fitting and perimodiolar cochlear implant electrodes. Otol Neurotol, 29, 131–136. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Parkinson WS, Tyler RS, et al. (1999). Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol, 20, 445–452. [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Whiting BR, et al. (2007). In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl, 197, 2–24. [PubMed] [Google Scholar]
- Suhling MC, Majdani O, Salcher R, et al. (2016). The Impact of Electrode Array Length on Hearing Preservation in Cochlear Implantation. Otol Neurotol, 37:1006–1015. [DOI] [PubMed] [Google Scholar]
- Wang J, Dawant BM, Labadie RF, et al. (2017). Retrospective Evaluation of a Technique for Patient-Customized Placement of Precurved Cochlear Implant Electrode Arrays. Otolaryngol Head Neck Surg, 157, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna GB, Noble JH, Carlson ML, et al. (2014). Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope, 124 Suppl 6, S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa K, Cohen L, Blamey P, et al. (2004). Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol Neurootol, 9, 163–172. [DOI] [PubMed] [Google Scholar]