Abstract
Aggressive behavior in middle childhood can contribute to peer rejection, subsequently increasing risk for substance use in adolescence. However, the quality of peer relationships a child experiences can be associated with his or her genetic predisposition, a genotype-environment correlation (rGE). Additionally, recent evidence indicates that psychosocial preventive interventions can buffer genetic predispositions for negative behavior. The current study examined associations between polygenic risk for aggression, aggressive behavior, and peer rejection from 8.5 to 10.5 years, and the subsequent influence of peer rejection on marijuana use in adolescence (n = 515; 256 control, 259 intervention). Associations were examined separately in control and intervention groups for children of families who participated in a randomized controlled trial of the family-based preventive intervention, the Family Check-Up. Using time-varying effect modeling (TVEM), polygenic risk for aggression was associated with peer rejection from approximately age 8.50 to 9.50 in the control group but no associations were present in the intervention group. Subsequent analyses showed peer rejection mediated the association between polygenic risk for aggression and adolescent marijuana use in the control group. The role of rGEs in middle childhood peer processes and implications for preventive intervention programs for adolescent substance use are discussed.
Keywords: Peer rejection, Marijuana use, Gene-environment correlation, Middle childhood, Time varying effect modeling
Behavioral Disinhibition models propose that aggressive behavior can contribute to experiencing negative peer relationships, which increases risk for adolescent substance use (Iacono, Malone, & McGue, 2008; King et al., 2009; Zucker, Heitzeg, & Nigg, 2011). Identifying developmental pathways to adolescent marijuana use is important based on increases in marijuana use with legalization and evidence that early chronic marijuana use can adversely affect cognitive functioning across the lifespan (Meier et al., 2012; Volkow et al., 2016). In particular, aggression in middle childhood (ages 6–11 years) is associated with peer rejection (Chen, Drabick, & Burgers, 2015), which can contribute to marijuana use in adolescence (Prinstein & La Greca, 2004). Importantly, one’s underlying genetic predisposition for behaviors such as aggression, can influence peer relationships (e.g., peers a child affiliates with, quality of peer relationships), a phenomenon known as a gene-environment correlation (rGE; Plomin, DeFries, & Loehlin, 1977). Thus, in addition to being influenced by social context, peer relationships may provide risk for adolescent substance use through genetic processes.
Emerging evidence indicates that genetic influences on risk for psychopathology and substance use may be moderated by psychosocial interventions. For example, a meta-analysis found that genetic effects were detected in control groups but muted in intervention groups (see van Ijzendoorn & Bakermans-Kranenburg, 2015 for a meta-analysis). Accordingly, psychosocial programs are thought to provide parent and/or child level supports for behavior that may prevent the expression of underlying genetic predispositions. However, in the absence of such positive supports, aggression and negative peer relationships can increase risk for substance use. Thus, it is important to identify etiological risks for negative peer processes originating from genetic predispositions and test whether psychosocial interventions may be able to prevent their expression.
To address these gaps, we formulated two aims as part of our conceptual model (see Figure 1). The first aim focuses on polygenic risk for aggression and aggressive behavior as two risk factors for peer rejection across middle childhood. The second aim leverages findings from the first aim to examine a mediated model in which polygenic risk for aggression predicts adolescent marijuana use via age-specific peer rejection. Because the design of the current study included a randomized controlled trial examining the efficacy of a family-based intervention, the Family Check-Up, all models were tested separately in intervention and control groups. For the second aim this provided an opportunity to test whether the strength of paths from genetic risk for aggression to peer relations to substance use differed between intervention and control groups.
Figure 1.
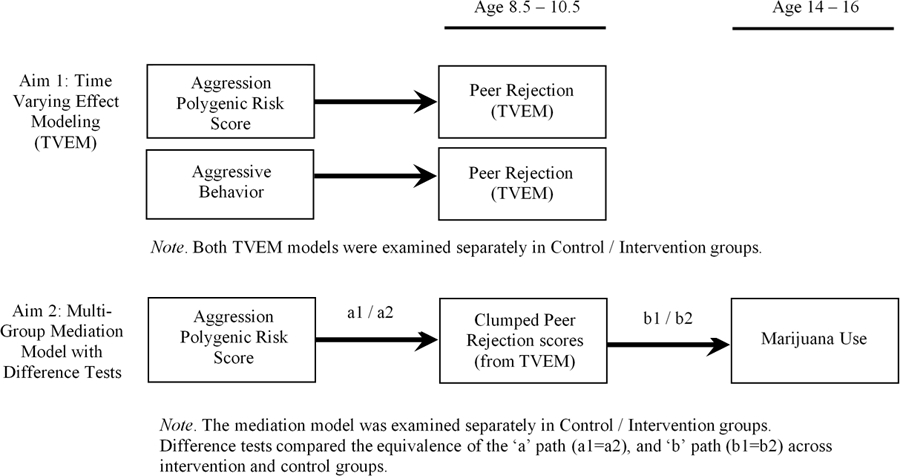
Conceptual Model
This preventive intervention was administered beginning at age 2 years and was designed to prevent children’s problem behavior by incorporating motivational interviewing and family management practices to motivate parents and provide training to improve parenting skills. Thus, two-group models were tested to examine possible buffering effects of the intervention on pathways leading to adolescent substance use.
In support of the first aim we provide background literature on the role of aggression and genetic effects underlying peer rejection. In support of our second aim we build on the prevention literature to illustrate cascading associations between genetic predispositions for aggression, peer rejection, and marijuana use. Finally, we conclude by illustrating how a novel approach, time-varying effect modelling, can be used to examine developmental genetic effects.
Aggression and Peer Rejection
Middle childhood is a particularly important time for the development of peer relationships as children develop key social skills, and failure to develop such skills can lead to long-term social difficulties (Chen et al., 2015). Behavioral difficulties during middle childhood are particularly disruptive for peer relationships. In particular, a robust literature has found aggression to elicit peer rejection (Brendgen, 2012; Brendgen & Boivin, 2015; Chen et al., 2015). The association of behavioral difficulties and adolescence is proposed to occur as normative peers do not endorse, accept, or tolerate aggressive behavior, typically reacting by excluding aggressive peers from social interactions and activities (Chen et al., 2015; Brendgen & Boivin, 2015). This body of research finds there are concurrent and longitudinal reciprocal effects between aggression and peer rejection during middle childhood. For some children, a genetic predisposition for aggression may, in part, underlie peer difficulties (Boivin et al., 2013; Brendgen & Boivin, 2015; Brendgen et al., 2011).
Genetic Predisposition and Peer Rejection
An evocative rGE occurs when children evoke a reaction from their environment, such as from peers, based on heritable traits or behaviors (Plomin et al., 1977). Twin studies provide evidence of rGE in peer relationships (see Brendgen & Boivin, 2015 for a review) by estimating the extent to which an individual’s genetic predisposition accounts for variation in peers’ behaviors and whether covariation between children’s behavior and their peers’ behavior is genetically influenced (DiLalla, Mullineaux, & Elam, 2008). Using twin designs, significant genetic variance has been found to underlie the link between peer rejection and victimization (e.g., Ball et al., 2008; Boivin et al., 2013; Brendgen & Boivin, 2015), indicating an rGE. Based on evidence that aggression is a heritable trait and that genetic effects on aggression vary with age (Lubke, McArtor, Boomsma, & Bartels, 2018; Pappa et al., 2016; Porsch et al., 2016), twin studies have investigated concurrent and longitudinal associations between aggression and peer rejection. Findings indicate that genetic factors, in part, account for the association between aggression and peer rejection/victimization, providing further evidence that a child’s genetic predisposition for aggression, operating through rGE, can evoke peer rejection (Boivin et al., 2013; Brendgen & Boivin, 2015; Brendgen et al., 2011).
Whereas twin studies provide consistent evidence that rGE may underlie peer rejection, very few studies have examined molecular genetic variants in rGE with peer processes or how rGE may vary as a function of age. A handful of studies have examined peer processes using candidate genes across developmentally diverse periods. In one study, DAT1 variants were found to be associated with affiliation with substance using peers in young adult males (Yun, Cheong, & Walsh, 2011). DAT1 also was linked to affiliation with substance using peers for white adolescent males from high risk families (Beaver, Wright, & DeLisi, 2008). In another study, adult male friends were found to have similar variation in the DRD2 gene, which may indicate peer selection effects based on genotype (Fowler, Settle, & Christaki, 2011). Finally, in late adolescent boys, a polymorphism within the 5HT 2A serotonin receptor gene (-G1438A) was associated with peer likability, and the association of 5HT 2A serotonin and peer likability was mediated by rule-breaking behavior (Burt & Mikolajewski, 2008).
The existing studies only examine single or a few genetic variants that are unable to capture much of the genetic variance relevant to aggression. Polygenic risk scores (PRS) aggregate across multiple genetic variants; using such scores to study peer processes serves to replicate and extend findings from twin studies and capture greater genetic variance than single candidate gene studies. To our knowledge, there are no studies that examine rGE in peer rejection using PRS. Additionally, previous molecular studies of rGE in peer processes have not investigated developmentally specific genetic effects. Genetic effects on aggression are known to vary across the life course but also be transitory during specific developmental periods (Lacourse et al., 2014; Porsch et al., 2016). Yet no research has examined age-varying evocative genetic effects (rGE). In support of our first aim (see Figure 1), we examined the association between an aggression PRS and peer rejection using Time Varying Effect Modeling (TVEM), which estimates age-varying associations. The TVEM method has the ability to reveal specific ages when evocative rGE underlies peer rejection. We also examined associations between aggressive behavior and peer rejection to confirm findings with past literature. Further, peer rejection in middle childhood can contribute to continued aggression, peer rejection, and deviant behavior, all of which increased risk for substance use in adolescence (see Chen et al., 2015 for a review; Ettekal & Ladd, 2015). Thus, in support of our second aim we examined whether genetic predisposition for aggression may contribute to adolescent marijuana use via peer rejection. To note, the association between genetic predisposition for aggression and aggressive behavior was previously examined using TVEM as part of Elam, Clifford, Shaw, Wilson, & Lemery-Chalfant (2019) which we extend as part of the current study.
Gene-Environment Interplay, Peer Rejection, and Substance Use
Building on literature that demonstrates evocative rGE may contribute to negative peer interactions in middle childhood, peer rejection in middle childhood may subsequently contribute to substance use in adolescence. Whereas extant literature demonstrates concurrent links between adolescent peer influences and substance use (e.g., Dishion & Loeber, 2009), there is less evidence for longitudinal links between peer rejection in middle childhood and substance use in adolescence. However, exceptions do exist. In one early study of boys in middle childhood, low social preference was associated with alcohol, marijuana, and tobacco use in univariate models of substance use, but not in multivariate models that simultaneously examined parent, family, and individual level characteristics, and their interactions (Dishion, Capaldi, & Yoeger, 1999). More recently, Ettekal and Ladd (2015) found high peer rejection in middle childhood to mediate the association between early aggression and adolescent rule breaking, which included substance use. Also, aggression and greater peer rejection for girls in middle childhood were related to greater marijuana use, hard drug use, and drinking in adolescence (Prinstein & La Grecca, 2004).
Genetic predisposition is also implicated in associations between peer processes and substance use. A substantial body of genetics research, consolidated in multiple reviews (e.g., Dick & Kendler, 2012; Milaniak, Watson, & Jaffee, 2015; Young-Wolff, Enoch, & Prescott, 2011), has predicted substance use from interactions between peer processes and single candidate genes thought to reflect predispositions for disinhibited behaviors and substance use. An important extension is to examine rGEs underlying peer processes, which suggests that peer processes may mediate the association between genetic predisposition and substance use. Based on the reviewed literature, genetic predisposition for aggression may contribute to substance use by evoking peer rejection. This process has been termed the social mediation pathway (Reiss & Leve, 2007), in which the social environment partially mediates the influence of genetic predisposition on psychopathology. To our knowledge no study has investigated rGEs underlying peer processes in pathways to adolescent marijuana using a PRS. Related studies have found that polygenic risk for impulsivity evoked poorer parental monitoring via impulsive behavior, which predicted affiliation with deviant peers (Elam et al., 2017), and that polygenic risk for aggression evoked less family cohesion in predicting alcohol use in young adulthood (Elam, Chassin, & Pandika, 2018). Elam’s previous studies provide evidence that harmful developmental cascades to adolescent substance use may originate from early genetic predisposition for aggression and behavioral disinhibition. These findings also highlight the importance of identifying genetic risk factors for behavioral precursors of substance use, such as aggression, and how rGEs may be implicated in peer processes earlier in life and contribute to subsequent substance use to help inform preventive interventions.
Preventive Family Interventions Attenuating Pathways to Substance Use
Social mediation pathways may be attenuated by psychosocial interventions. Robust evidence indicates that negative peer relationships and risk for substance use can be reduced by preventive family-based interventions (Connell, Dishion, & Deater-Deckard, 2006; Gifford, Dodge, Dishion, & McCord, 2005). There is accumulating evidence that such interventions can buffer genetic predisposition for aggression, disinhibited behavior, and/or substance use (e.g., Schlomer et al., 2017; Shaw et al., 2019; van Izjendoorn & Bakermans-Kranenburg, 2015) such that genetic associations with youth problem behavior are detected in the control group but not in the intervention group, a genotype-intervention effect (GxI). Buffering effects between peer relationships and adolescent substance use may occur when psychosocial preventive interventions provide parent and/or child level supports and skills that over time lead to more prosocial adolescent behavior that may not reflect underlying genetic predispositions. However, with the exception of Shaw et al. (2019), genetic studies have examined single candidate genes, with few examining cumulative measures of genetic risk.
One recent study examined the interaction between polygenic risk for alcohol dependence and a family-based intervention in predicting lifetime alcohol dependence diagnosis at ages 26–27 (Kuo et al., 2019). Within the control condition, the PRS was associated with alcohol dependence diagnosis, but effects were absent in the intervention condition. Using TVEM, Russell et al. (2018) examined the interactive effect between the GABRA2 gene and a preventive intervention on alcohol misuse across ages 11 to 20. Russell and associates found unique developmental effects in which the intervention reduced alcohol misuse for those with the risk genotype, but only from ages 12.5 to 17 (Russell et al., 2018). However, of the very few TVEM studies that exist, most examine single candidate genes or single nucleotide polymorphisms (SNPs) that account for very little genetic variance. The only study to date to examine polygenic risk in conjunction with TVEM examined the association between a PRS for aggression and aggressive behavior across early and middle childhood in the same data set as the current study (Elam et al., 2019), finding that genetic main effects on aggression varied non-linearly with age. As both genetic and peer processes are inherently developmental, it is important to examine the specificity of developmental effects by age. Age specificity has important implications for prevention efforts in light of emerging evidence that preventive interventions can buffer genetic risk factors. Taking a developmental approach also has important implications for identifying early risk factors for adolescent substance use. Thus, the following section highlights one approach for examining age-specific genetic effects.
A Developmental Approach to Examining Genetic Effects
One limitation of past polygenic approaches is that very often polygenic risk scores are tested in samples that do not developmentally align with the discovery genome wide association study (GWAS). That is, polygenic scores are examined in childhood samples but SNPs were derived from a GWAS of adults. An additional limitation is that SNPs from GWAS may not have any functional effects. An innovative extension of the polygenic approach is to further refine the SNPs in a PRS to only include functional variants. In the current context, ‘functional’ indicates that a SNP has a known biological function or is located in a coding region, which is an important distinction as many SNPs have no functionality, acting simply as placeholders. The present study provides further confidence that biological mechanisms underlie the genetic association. A recent study addressed these developmental and functional limitations by creating a functional polygenic risk score for aggression and testing it in a developmentally aligned sample (Elam et al., 2019).
Based on a meta-GWAS on aggression in childhood, Pappa et al. (2016) identified SNPs associated with aggression separately in early and middle childhood. Leveraging summary statistics from Pappa et al. (2016), Elam et al. (2019) filtered SNPs from the meta-GWAS using Gene-Set Enrichment Analysis (Mooney & Wilmot, 2015; Zhang, Chang, Guo, & Wang, 2015) to identify functional SNPs, which were subsequently formed into a functional PRS for aggression in middle childhood. Age varying associations between the functional PRS and aggression in middle childhood were tested using TVEM in the current sample. TVEMs are unique in that they model the strength of associations as a varying function of age with no assumption of the shape of the TVEMS associations (Tan et al., 2012). The functional PRS was associated with aggression from approximately 8.75 to 10 years of age (see Elam et al. 2019 for details). Using a functional PRS extends past genetic and longitudinal research.
The functional approach offers some advantages over the standard approach by including SNPs with known biological functions. The functional strategy offers a more stringent approach, increasing confidence that associations represent true genetic effects. Whereas less stringent criteria may explain greater variance in a phenotype, it also likely includes SNPs that are spuriously associated in the original meta-GWAS or those that have less biological relevance. Using functional PRSs as predictors in TVEM advances the credibility of findings by identifying age-specific genetic effects. The current study uses the functional PRS for middle childhood aggression created in Elam et al. (2019) to study variation in rGE by child age. We extend these findings through the following aims all of which were tested separately in control and intervention groups. It is not currently possible to examine mediation using TVEM or to compare estimates across groups; thus, our first aim was to identify age-specific genetic effects on peer rejection. Secondly, where peer rejection was genetically influenced, we examined it as a mediator of polygenic risk for aggression and marijuana use in adolescence and tested whether the magnitude of the aforementioned pathways varied as a function of family’s random assignment to the Family Check-Up.
The Present Study
Collectively, research demonstrates the importance of aggression in the emergence of peer rejection and subsequent associations with substance use (Chen et al., 2015; Leung et al., 2014). Twin studies provide evidence of genetic covariance between aggressive behavior and peer rejection (Brendgen & Boivin, 2015), consistent with evocative rGE. Peer rejection is a known risk factor for psychopathology and substance use in adolescence. Although implicated in theory, empirical examination of the rGE processes in middle childhood and associations with marijuana use in adolescence is sparse. The current study examined the association between a functional polygenic risk score for aggression (from Elam et al., 2019) with peer rejection from 8.5 to 10.5 years of age, and subsequent associations with marijuana use from age 14 to 16. TVEM was first used to examine age varying longitudinal associations between the PRS for aggression and peer rejection across time. As half of the participants in the current study had been randomly assigned to the family-based Family Check-Up intervention as part of a multisite randomized controlled trial (the Early Steps Multisite study), associations were examined separately in intervention and control groups. Subsequent to this, multi-group model was fit to examine associations between the PRS and adolescent marijuana use via peer rejection, also separately in intervention and control groups. We first hypothesized that the Family Check-Up would mitigate the effect of genetic risk for aggression on peer rejection, and aggressive behavior on peer rejection. Thus, we expected to see associations between aggressive behavior and peer rejection in the control group but not the intervention group. Similarly, we hypothesized that where associations were found between genetic risk for aggression and peer rejection, that the peer rejection scores would mediate the association between genetic risk for aggression and adolescent marijuana use but only in the control group.
Methods
Participants
Seven hundred and thirty-one ethnically and racially diverse, low-income families with 2-year-old children were recruited between 2002 and 2003 from Women, Infants, and Children Nutritional Supplement Programs (WIC) at three sites in metropolitan Pittsburgh, Pennsylvania (urban), Eugene, Oregon (suburban), and within and outside Charlottesville, Virginia (rural). Screening procedures were used to recruit families of toddlers at high risk for conduct problems, based on socio-demographic risk, primary caregiver risk, and toddler behavior problems. Participation rates of those families invited to participate who qualified by risk status were high across the three sites [83.2% total (49% female); 84% in Eugene (n = 271), 76% in Charlottesville (n =188), 88% in Pittsburgh (n = 272)]. More than two thirds of the families reported an annual income of less than $20,000, with 24% of primary caregivers having less than a high school education, 41% having a high school diploma or general education diploma, and an additional 32% having 1–2 years of more than high school education. Primary caregivers (96% mothers) self-identified as belonging to the following ethnic groups: 11% Latino, 28% African American, 54% European American, 4% biracial, and 3% other groups (e.g., Native American, Asian American, Pacific Islander). For more information about sample characteristics, see Dishion, Shaw, Connell, Gardner, Weaver, & Wilson (2008).
Families were randomly assigned to control or intervention conditions after the baseline assessment at child age 2 years. Those in the control condition received WIC services as usual. Those in the intervention condition had the opportunity to receive the Family Check-Up following each of the assessments from ages 2 to 10.5. Incorporating motivational interviewing, the Family Check-Up includes a formal assessment of youth and family factors that have been found to predict youth problem behavior, then sharing with the family the revealed concerns and family strengths during a feedback session to both motivate change but also demarcate assets the family can build on to address youth risk for problem behavior. At the end of the feedback session, parents identify goals they have for their child and family in the coming year and are given the option to work with the family coach on the family issues using evidence-based family management practices. In clinical practice, an initial interview precedes the assessment component, but to ensure that control and intervention families had comparable levels of contact with research staff, the assessment was convened for families in both the control and intervention groups, but only intervention group families were offered the initial interview, feedback, and follow-up parent management sessions. All families were re-contacted at child ages 3, 4, 5, 7.5, 8.5, 9.5, 10.5, 14, and 16 years (81% of the sample participated at age 16) for home-based assessments. In terms of engagement in the intervention among intervention families, 76% of families engaged at age 2, with over 90% of the families engaging in at least one session of the Family Check-Up by child age 5. Of the adolescents who participated at 14 years, 515 were genotyped (86.7% of the sample who participated in home visits at age 14), and a subsample of the genotyped adolescents with data available on peer rejection and marijuana use (n = 485) comprise the sample for the current study. Selective attrition analyses revealed no significant differences between members of the initial sample with no genetic data and those who were genotyped with respect to parental education, race, gender, study site, child problem behaviors at age 2, temperament, or parental depression.
Procedures
All assessments were conducted in the home at ages 2 to 16 with primary caregivers (96% biological mothers at age 2) and children. Primary caregivers completed questionnaires regarding the physical and socio-cultural environment and children’s behavior. All study protocols were approved by the university Institutional Review Board, parental written consent was obtained for all families (with assent obtained from children beginning at age 14), and families were compensated for their time at each age.
Participants provided saliva samples with Oragene kits for genotyping during the age 14 home visit. RUCDR Infinite Biologics at Rutgers University extracted and normalized the DNA, and then genotyped the samples using the Affymetrix Axiom Biobank1 Array. SNPs that did not meet the criteria of Hardy-Weinberg equilibrium at p <10−6 and SNPs with a minor allele frequency less than 1% were removed. Also, any SNP or individual with a missing data rate greater than or equal to 5% was removed (no participants met the Hardy-Weinberg criteria).
Measures
Polygenic risk score (PRS).
The functional PRS used in this study was created as part of previous work in the same sample. Elam et al. (2019) created the PRS based on summary statistics from a meta-GWAS of aggression in early childhood and middle childhood (Pappa et al., 2016). Briefly, PRSs for aggression in middle childhood were created at the p < .05 threshold using both the standard unit-weighted score approach and a functional approach. Gene set enrichment analysis using iGSEA4GWASv2 (Zhang et al., 2015) was used to identify functional SNPs (e.g., annotated, regulatory, eQTLs) from all SNPs in the original meta-GWAS, and these functional SNPs (n = 66) were formed into a functional PRS (see Elam et al. (2019) for greater detail). Briefly, the functional PRS, but not the standard PRS, was associated with aggression in both early and middle childhood and the functional PRS explained greater variance than the standard PRS. The current study uses the functional PRS created for middle childhood.
Population genetic admixture.
We conducted a Principal Components Analysis of all autosomal SNPs to represent population admixture using PLINK (see Price, Patterson, Plenge, Weiblatt, Shadick, & Reich, 2006). Prior to extracting principle components, we screened out regions of long-range linkage disequilibrium (LD; correlation among the SNPs), and used PLINK’s sliding window procedure to prune local LD. We extracted the first 20 components. The first component (PC1) had an eigenvalue of 28.84 and differentiated European-American and Latino groups from African-American groups, with most biracial participants falling in the middle. The second component (PC2) had an eigenvalue of 5.62 and differentiated non-Latino participants (European and African American) from Latino participants. The first two principal components were controlled for in all genetic analyses. The remaining components had eigenvalues ranging from 1.45 to 1.21 and were excluded from the analyses.
Peer Rejection.
Primary caregivers reported on their child’s peer rejection using the Peer Acceptance and Social Affiliation scale (ASA; Dishion & Kavanagh, 2003) at ages 8.5, 9.5, and 10.5. The ASA measure assesses the percentage of the child’s peers (1 = very few – less than 25% to 5 = almost all – more than 75%), who exhibit dislike or rejection toward him/her based on three items (e.g., what percentage of your child’s peers at school disliked or rejected him?). The three peer rejection items were mean composited and had good internal consistency at each age (αs ranging from .72 to .83).
Aggression.
Primary caregivers completed the Child Behavioral Checklist 6–18 (Achenbach & Rescorla, 2001) at the age 8.5, 9.5, and 10.5 assessments. Parents rated each item on a 3-point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). The aggression subscale was used in the current analyses, which assesses children’s aggressive behavior in middle childhood (e.g., “Cruel, bullying, or mean to others”). Internal consistency was good across middle childhood (αs range .91 to .92). Aggression T-scores were computed and used in analyses.
Marijuana Use.
Adolescents reported on their own marijuana use at the age 16 assessment using one self-report question (Child and Family Center (CFC), 2000), ‘Have you ever used marijuana?’ (0 = no, 1 = yes). It should be noted that the CFC measure of marijuana use did not specify a response period, so to ensure the temporal precedence of peer rejection (maximum age 11.58) we excluded cases that reported marijuana use at age 13 or younger (n = 58). Thus, the CFC measure only represents marijuana use for those age 14 to 16 years old.
Covariates.
Covariates for all analyses included gender (females = 2, males = 1), study site location (1 = rural, 2 = suburban, 3 = urban), child race, primary caregiver education, and the first two genetic ancestry principal components. The first two ancestry principal components were controlled for to account for population admixture.
Statistical Analyses
We examined all relevant statistical assumptions (e.g., multivariate normality) and affirmed prior to analyses. All variables were normally distributed (West, Finch, & Curran, 1995). As a preliminary test, an ANOVA was conducted in SPSS v26 to determine whether study variables differed by intervention vs. control condition. TVEM analyses were examined using a time-varying effects model macro in SAS v9.4 (Li et al., 2015). TVEMs estimate the association between a predictor and longitudinal outcome allowing the predictor’s effect to vary as a function of time. TVEMs are an extension of linear regression but makes no parametric assumptions about the shape (e.g., linear, quadratic) or rate of change over time in associations (Tan et al., 2012). Rather, using a regression framework, TVEMs allow the effect of predictor(s) to vary across time as a function of continuous time or age, with the shape of change modeled as the time-varying unstandardized regression coefficients and 95% confidence intervals. Significant effects are indicated when the 95% confidence interval around a regression coefficient does not include zero.
In the current models we included the standardized functional PRS as a time-varying predictor and covariates as time-invariant effects in-line with TVEM recommendations (Li et al., 2015; Tan et al., 2012). The PRS was included as a time-varying predictor of peer rejection from ages 8.5 to 10.5 years old (actual ages ranged from 100–139 months). The TVEM framework allows the strength of the association between the PRS and peer rejection to vary over time. Separate models were run for the intervention (n = 238) and control (n = 247) groups. Within the time-varying effects models, a normal distribution for a continuous outcome (peer rejection) was specified using the penalized truncated power-spline method. Currently, it is not possible to examine variables as outcomes of TVEM functions, so secondary analyses were performed to examine peer rejection scores as mediators between the PRS and adolescent marijuana use.
Following TVEM, peer rejection scores were clumped based on patterns of associations observed in TVEM models. That is, where significant associations were detected, peer rejection scores for those age periods were clumped into a single measure and peer rejection scores for the age periods prior to and after significant associations were clumped, respectively. A mediation model was then tested between the PRS, the clumped peer rejection scores, and marijuana use, separately for the control and intervention groups using multi-group modelling via logistic regression with a weighted least squares estimator in Mplus 8.3 (Muthén & Muthén, 2005). Aggression was also included as a mediator from the PRS to marijuana use to account for its influence. Relevant covariates were examined and retained where significant. Chi-square difference tests were used to test the equivalence of the association between the PRS and peer rejection and peer rejection and marijuana use across the two groups. Model constraints were used to test the equivalence of the indirect effects across the two groups. Indirect effects were estimated using RMediation (Tofighi & MacKinnon, 2011).
Results
Means, standard deviations, and correlations among primary study variables can be found in Table 1. Across middle childhood, there was no evidence of change in mean levels of peer rejection in either the control or intervention group. The PRS was associated with age 8 and 9 peer rejection in the control group and with age 10 peer rejection in the intervention group. Peer rejection was correlated across ages and was correlated with aggression within and across age in both groups. In the control group peer rejection at age 9 was associated with marijuana use assessed at age 16. Finally, mean levels of study variables did not differ by intervention vs. control condition (ps > .33).
Table 1.
Correlations, Means, and Standard Deviations of Study Variables for the Intervention Group (above the diagonal) and the Control Group (below the diagonal)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PRS | 1 | .10 | .04 | .09 | .05 | .20** | .08 | .01 | .36*** | .03 | .02 (1.01) |
| 2. Peer Rejection 8yo | .16* | 1 | .27*** | .30*** | .32*** | .32*** | .25*** | .02 | −.05 | .15* | 1.39 (.62) |
| 3. Aggression 8yo | −.02 | .28*** | 1 | .16* | .76*** | .25*** | .67*** | .03 | .13 | .10 | 58.40 (9.54) |
| 4. Peer Rejection 9yo | .16* | .32*** | .28*** | 1 | .26*** | .32*** | .23*** | −.11 | .14 | −.02 | 1.41 (.68) |
| 5. Aggression 9yo | .07 | .26*** | .77*** | .28*** | 1 | .26*** | .77*** | .06 | .08 | .16* | 58.27 (9.43) |
| 6. Peer Rejection 10yo | .08 | .31*** | .19** | .37*** | .24** | 1 | .35*** | −.05 | .17* | .07 | 1.42 (.73) |
| 7. Aggression 10yo | −.02 | .19** | .73*** | .19** | .77*** | .27*** | 1 | −.02 | .09 | .13 | 58.29 (9.65) |
| 8. Marijuana Use | .07 | .03 | .05 | .24** | .08 | .05 | .10 | 1 | .08 | .12 | .22 (.41) |
| 9. PC 1 | .43*** | .14* | −.01 | .01 | .06 | .04 | .01 | −.06 | 1 | .01 | −.001 (.04) |
| 10. PC 2 | .02 | .05 | .08 | .01 | .10 | .01 | .06 | .00 | −.00 | 1 | −.001 (.05) |
| Mean (SD) | .002 (1.01) | 1.40 (.61) | 58.90 (9.70) | 1.40 (.68) | 58.99 (9.56) | 1.42 (.64) | 57.86 (9.43) | .23 (.42) | .003 (.05) | .00 (.04) |
Note. Intervention group n = 238, control group n = 247. Peer rejection reflects scale scores. PRS = aggression polygenic risk score, PC = ancestry principal component.
p < .05,
p < .01,
p < .001.
TVEM results for associations between the PRS and peer rejection can be found in Figure 2. In the control group, the PRS was associated with peer rejection from approximately age 8.50 to 9.50 (association from age 8.56 to 9.44). The glowing portion of the line illustrates the significant effect, where the lower bound of the confidence interval departs from zero. In the intervention group, there were no associations between the PRS and peer rejection. TVEM results for associations between aggression and peer rejection can be found in Figure 3. Aggression was associated with peer rejection from approximately age 8.50 to 11.00 in the control group (association from age 8.50 to 11.06). Aggression was also associated with peer rejection from approximately age 9.00 to 11.00 in the intervention group (association from age 9.02 to 10.91).
Figure 2.
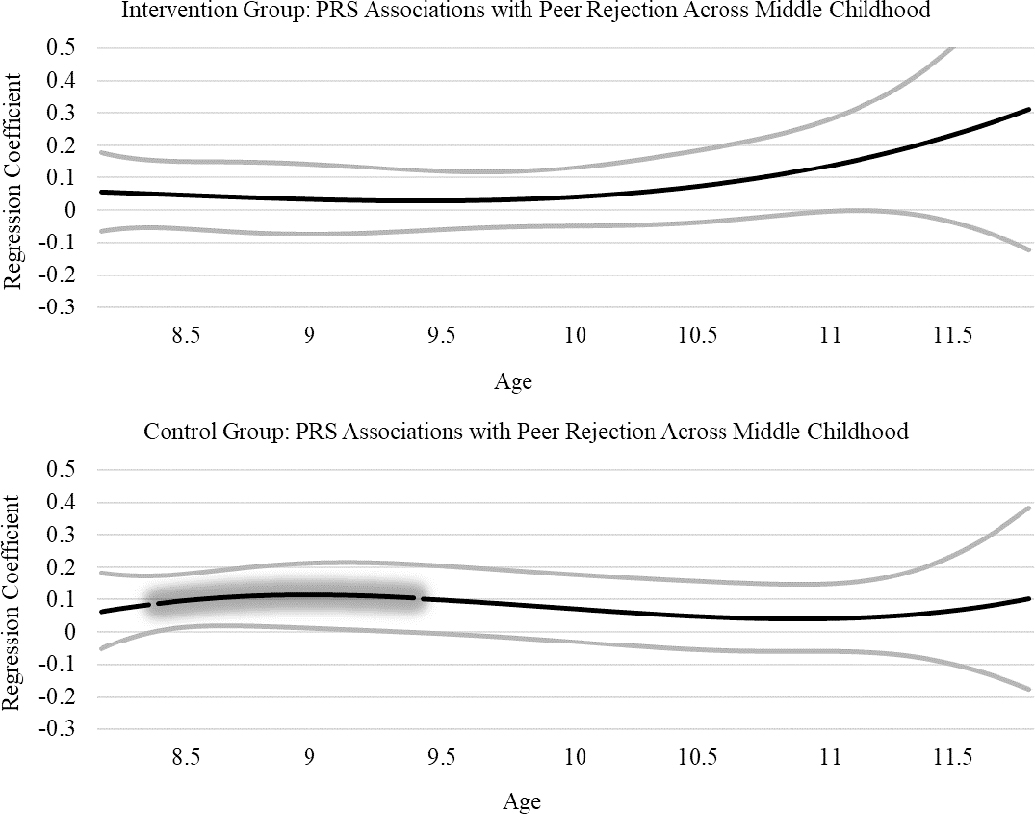
PRS Associations with Peer Rejection in Intervention and Control Groups.
Figure 3.
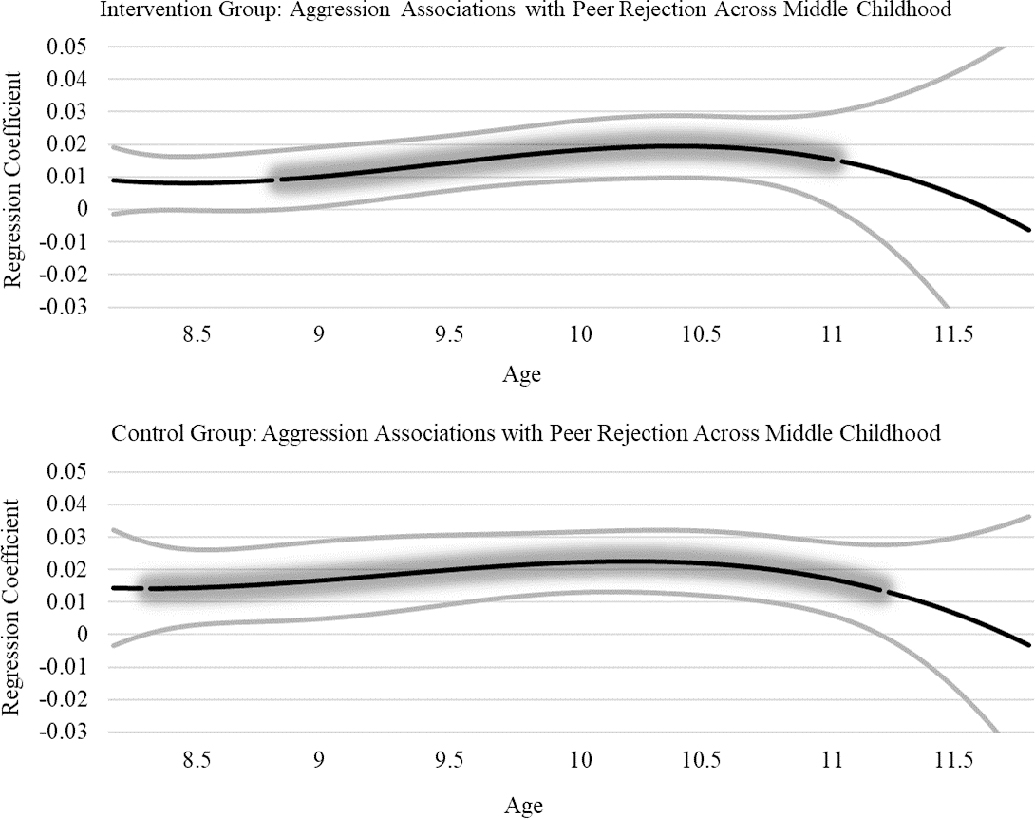
Aggression Associations with Peer Rejection in Intervention and Control Groups.
Following the TVEM analyses, peer rejection clumps were created by grouping individual scores into the following ages: (1) prior to 8.50 years old, (2) 8.50 to 9.50 years old, (3) 9.5 to 10.50 years old, and (4), 10.50 to 11.50 years old. Respectively, peer rejection clumps represented the period prior to the genetic effect, the period during the genetic effect, and two periods following the genetic effect. In preliminary tests only the peer rejection clump for 8.50 to 9.50 years old, representative of the span when a genetic influence on peer rejection was observed, was associated with marijuana use in the control group (β = .25, SE β = .10, p = .014) but not the intervention group (β = −.17, SE β = .14, p = .23) and these estimates were found to be significantly different (Wald X2(1) = 5.62, p =.018). No other peer rejection clumps were associated with marijuana use in either group so were not retained for the mediation analyses.
In the multi-group mediation model (X2 (3) = .934, RMSEA = 0.0, CFI = 1.0, TLI = 1.0), a significant indirect effect was found in the control group (β = .051, SE β = .026, 95% CI [.006, .11]) whereby the PRS predicted peer rejection (β = .22, SE β = .06, p < .001), and peer rejection predicted adolescent marijuana use (β = .23, SE β = .10, p = .024). The PRS was not associated with aggression and aggression was not associated with adolescent marijuana use. No associations were found in the intervention group. Comparing estimates in the intervention and control groups, a trend level difference was detected for the path between the PRS and peer rejection (Wald X2 (1) = 3.26, p = .07), and a significant difference for the path between peer rejection and adolescent marijuana use (Wald X2 (1) = 4.93, p = .026). The comparison of the indirect effect across intervention and control groups yielded a significant difference (β = .07, SE β = .03, p = .047).
In post hoc sensitivity analyses, significant effects in the larger sample were examined separately for European American (EA) and African American (AA) subgroups. However, based on the small sample sizes represented in the subgroups, these results bear replication. The shape of the EA control and AA control TVEM results were the same as the overall control sample with significant effects in both subgroups. In the control group the PRS was positively associated with peer rejection from approximately 9.15 to 9.65 in the EA subgroup and from 8.5 to 9.0 in the AA subgroup. Aggression was associated with peer rejection from 8.65 to 11 in the EA subgroup and 9.5 to 11 in the AA subgroup. In the intervention group there were no significant associations between the PRS and peer rejection in either the EA or AA subgroup. Aggression was associated with peer rejection from 9.5 to 10.9 in the EA subgroup and 9.7 to 10.6 in the AA subgroup. For the mediation model, significant indirect effects were found from the PRS to adolescent marijuana use via peer rejection in both the EA control subgroup (β = .07, SE β = .04, 95% CI [.001, .16]) and AA control subgroup (β = .14, SE β = .07, 95% CI [.02, .32]). In the intervention group, no significant effects were detected from the PRS to peer rejection, or peer rejection to adolescent marijuana use for EA (ps > .63) or AA (ps > .22) subgroups.
Discussion
Collectively, results indicate that genetic predisposition for aggression and aggressive behavior are associated with peer rejection during specific developmental periods in middle childhood. In turn, peer rejection during middle childhood mediated the association between genetic predisposition for aggression and marijuana use in adolescence. The effects were not apparent for children whose family were offered the Family-Check Up, but only in children of families in the control group.
We hypothesized that the PRS for aggression and aggressive behavior would be associated with peer rejection during middle childhood in the control group but not the intervention group. In support of this hypothesis, the PRS was associated with peer rejection in the control group from approximately 8.50 to 9.50 years, but no associations were present in the intervention group. Complementary to the genetic association, aggression was found to be associated with peer rejection during the 8.5–9.5 age period in the control group. However, aggression was also associated with peer rejection in the intervention group. These findings indicate that when aggression is present, despite intervention effects, it is still associated with peer rejection. However, the genetically influenced path to aggression was only present in the control group.
In line with Brendgen and Boivin (2015), the presence of PRS and aggressive behavior associations with peer rejection likely represent an evocative rGE in which genetic predisposition for aggressive behavior evokes or elicits greater rejection from peers via aggression. It is interesting to note that genetic influences on aggression were found using the same PRS and sample from approximately age 8.75 to 10.5 (Elam et al., 2019). Thus, genetic predisposition for aggression appears to be both contributing to aggression and evoking peer rejection during a similar period. That is, genetic risk for aggression contributes to aggression, which evokes peer rejection, an evocative rGE.
The presence of an evocative rGE between genetic predisposition for aggression and peer rejection from 8.50 to 9.50 years of age may be due to a number of factors. Aggression in middle childhood is heritable with some evidence that new genetic effects emerge during the period of middle childhood (van Beijsterveldt, Bartels, Hudziak, & Boomsma, 2003). Concurrently, during middle childhood children are encountering new social experiences and pressures, such as peer pressure, social conformity, and gender identity, making peer rejection during the middle childhood period especially salient (Chen et al., 2015). The presence of aggressive and other externalizing behavior is a primary risk factor for poor social interactions, including rejection in middle childhood (Boivin et al., 2015; Chen et al., 2015; Ettekal & Ladd, 2015). The effect of poor social interactions likely fades with age; as individuals enter pre-adolescence, internalizing problems, such as anxiety and social withdrawal, are more negatively perceived by peers, and aggressive behavior becomes less associated with rejection and victimization and more associated with popularity (Boivin et al., 2010; Brendgen et al., 2016; Cillessen & Mayeux, 2004).
In addition, the genetic association was only present in the control group, indicating that the Family Check-Up buffered the association between genetic predisposition for aggression and peer rejection. As the Family Check-Up was initially offered to families when the child was 2 years old, the effects may be evident because of long-term changes in the family environment, which have been documented in this sample previously for such factors as parenting (Dishion et al., 2008), maternal depression (Shaw, Connell, Dishion, Wilson, & Gardner, 2009), and social support satisfaction (McEachern, Fosco, Dishion, Shaw, Wilson & Gardner, 2013), all during early childhood. The novel finding of Family Check-Up effects on the association between genetic predisposition for aggression and peer rejection adds to a growing literature showing that family-based preventive interventions can moderate genetic predisposition for disinhibited behaviors and risk for substance use (Chhangur et al., 2016; Glenn et al., 2018; Shaw et al., 2019). Sensitivity analyses with the major racial subgroups indicated that although the PRS was calculated based on a GWAS of European Americans, effects held in the control group for both the EA and AA subgroups.
Second, we hypothesized that in the control group peer rejection would mediate the association between genetic predisposition for aggression and subsequent marijuana use in adolescence. An indirect effect was found between the PRS and adolescent marijuana use via the peer rejection clump representing the genetic effect, again only in the control group. This cascading effect is consistent with Behavioral Disinhibition models of substance use (Iacono et al., 2008; King et al., 2009; Zucker et al., 2011), a developmental cascade framework (Masten & Cicchetti, 2010), and a social mediation pathway (Reiss & Leve, 2007). Genetic predisposition for aggression in middle childhood can lead to greater rejection by peers, driven by an evocative rGE, and those rejected children are at greater risk for marijuana use in adolescence. The longitudinal prediction of marijuana use from peer rejection across an approximate 4-year span illustrates how important peer processes are in middle childhood. Theoretical models propose that middle childhood factors associated with increased risk for adolescent substance use may occur via continued aggressive behavior, chronic peer rejection, greater affiliation with deviant peers, or a combination of the adolescent developmental processes (Chen & Drabick, 2015; Ettekal & Ladd, 2015). It may be that genetic predisposition for aggression may initiate a developmental cascade by evoking peer rejection (evocative rGE), which increases risk for marijuana use in adolescence. This may preferentially occur in the absence of positive supports, such as psychosocial interventions (Schlomer et al., 2017; Shaw et al., 2019; van Izjendoorn & Bakermans-Kranenburg, 2015), leading to other maladaptive processes, such as affiliation with deviant peers, which also increase risk for marijuana use during adolescence. Relatedly, genetic and peer rejection effects were present only in the control group, illustrating that the Family Check-Up buffered both genetic and social risk factors across childhood. The genetic and peer rejection finding highlights the utility of early preventive interventions in mitigating psychosocial and genetic risk early in life. Of note, the PRS was not associated with aggression in the control or intervention group. This is in-line with findings from Elam et al. (2019), which illustrated that associations between the PRS and aggressive behavior only emerged using TVEM models to identify developmentally specific effects.
In addition to the novel genetic and peer rejection findings, this study advances developmental genetic research in a number of ways. Broadly, this study addresses a recent call that integrating genetic measures into longitudinal studies has the ability identify developmental risky pathways to psychopathology (Dick et al., 2019). The current study considered developmentally targeted environmental risks (peer rejection), the role of intervention effects (the Family Check-Up) and functional genetic indices to better identify such risk mechanisms. This study is the first to use a PRS to examine rGE underlying peer processes in developmental pathways to marijuana use, and our findings support and extend evidence from twin studies that peer rejection is partially explained by genetic influences on children’s aggression. Further, the PRS was based on a large meta-GWAS of children in middle childhood, and only included functional genetic variants. Developmentally aligning the discovery GWAS used to create the PRS with that of the subsequent sample is crucial, as these and other results indicate variable genetic effects with age (e.g., Russell et al., 2018). The focus on functional SNPs increases confidence that associations are meaningful rather than due to randomly associated genetic variants. More specifically, SNPs included in the current PRS represent serotonin and glutamate systems among others, which have been associated with neurodevelopmental deficits and aggression in childhood (Moretto, Murru, Martano, Sassone, & Passafaro, 2018; Narvaes & Martins de Almeida, 2014). Finally, we were able to investigate these genetic (rGE) and behavioral effects using TVEM, a developmentally sensitive method. The TVEM method allowed for detection of age varying associations rather than testing concurrent associations, longitudinal associations at single time points, or being constrained to trajectories of behavior. Indeed, in some cases, estimating influences on longitudinal trajectories of behavior may not reveal developmentally specific associations (Sher et al., 2011). Given the importance of friendship during the developmental period of middle childhood, negative peer processes can emerge and stabilize, and subsequently have long-term impacts on adolescent substance use (Cambron et al., 2017; Otten et al., 2019).
Limitations should also be considered. Many of the effect sizes in the current study were small, particularly for the PRS associations. The small effect sizes are similar to past studies using PRS (e.g., Elam et al., 2017), and indicate PRSs that include a smaller number of meaningful SNPs have utility. The current PRS only had 66 SNPs, which can be expanded as more is learned about SNP functionality. The use of a functional PRS increases confidence that the present findings, albeit modest, represent a true genetic association. Relatedly, the GWAS used to create the PRS was only for EA participants which may not generalize to AA participants. However, in the larger sample we controlled for the first two genetic ancestry principal components, and when performing sensitivity analyses we observed similar associations in the EA and AA subgroups. Second, the current sample is high-risk and results may therefore not generalize to lower-risk community-based samples. Finally, the peer measures were parent-reported, which may be a weakness because of parent’s lack of awareness of their child’s peer relationships. However, adolescents reported on their marijuana use, and parents and children have both been found to be valid reporters of peer behaviors (Fergusson & Horwood, 1999; Tilton-Weaver, Burk, Kerr, & Stattin, 2013).
Despite these limitations, the current study advances research on the interplay between genetic and social processes in substance use. First, it provides evidence that genetic influences can act via social pathways, which are in turn susceptible to intervention. It also illustrates that improving children’s overall family environment is protective across a number of domains. Finally, this study provides evidence of specific developmental periods connecting evocative rGE, peer rejection, and adolescent marijuana use. This approach highlights the importance of using GWAS that are developmentally aligned with the samples in which PRS are subsequently created. As GWAS data grow in diversity it is similarly important to align racial/ethnic groups in GWAS and PRS samples, one approach being the use of multi-ethnic PRS (Marquez-Luna et al., 2017; Peterson et al., 2019). Collectively the findings of this study can help to inform prevention and intervention strategies regarding developmental risk for aggression and peer rejection, and in the presence of peer rejection strategies to decrease risk for substance use (Dishion & Tipsord, 2011; Fite, Colder, & O’Connor, 2006).
Acknowledgments:
The research reported in this paper was supported by grants DA25630 and DA26222 to Daniel Shaw, Thomas Dishion, and Melvin Wilson from the National Institute on Drug Abuse and DA042828 to Kit Elam from the National Institute on Drug Abuse and National Institutes of Health: Office of the Director, and Office of Behavioral and Social Sciences Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We gratefully acknowledge the research staff for their assistance, and all the participating families.
Funding Statement: The research reported in this paper was supported by the National Institute on Drug Abuse (DS, TD, MW, grant numbers DA25630 and DA26222; KE, grant number DA042828) and the National Institutes of Health: Office of the Director, and Office of Behavioral and Social Sciences Research (KE, grant number DA042828).
Footnotes
Conflict of Interest: None.
References
- Achenbach TM, & Rescorla LA (2001). Manual for the Achenbach system of empirically based assessment school-age forms profiles. Burlington, VT: Aseba. [Google Scholar]
- Ball HA, Arseneault L, Taylor A, Maughan B, Caspi A, & Moffitt TE (2008). Genetic and environmental influences on victims, bullies and bully-victims in childhood. Journal of Child Psychology and Psychiatry, 49(1), 104–112. doi: 10.1111/j.1469-7610.2007.01821.x [DOI] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, & DeLisi M (2008). Delinquent peer group formation: Evidence of a gene X environment correlation. The Journal of Genetic Psychology, 169(3), 227–244. [DOI] [PubMed] [Google Scholar]
- Boivin M, Brendgen M, Vitaro F, Dionne G, Girard A, Pérusse D, & Tremblay RE (2013). Strong genetic contribution to peer relationship difficulties at school entry: Findings from a longitudinal twin study. Child Development, 84(3), 1098–1114. doi: 10.1111/cdev.12019 [DOI] [PubMed] [Google Scholar]
- Boivin M, Brendgen M, Vitaro F, ForGEt-Dubois N, Feng B, Tremblay RE, & Dionne G (2013). Evidence of gene–environment correlation for peer difficulties: Disruptive behaviors predict early peer relation difficulties in school through genetic effects. Development and Psychopathology, 25(1), 79–92. doi: 10.1017/S0954579412000910 [DOI] [PubMed] [Google Scholar]
- Brendgen M, Boivin M, Dionne G, Barker ED, Vitaro F, Girard A, … Pérusse D (2011). Gene–environment processes linking aggression, peer victimization, and the teacher–child relationship. Child Development, 82(6), 2021–2036. doi: 10.1111/j.1467-8624.2011.01644.x [DOI] [PubMed] [Google Scholar]
- Brendgen M, & Boivin M (2015). Gene-environment transactions in childhood and adolescence: Problematic peer relationships. In Gene-Environment Interplay in Interpersonal Relationships across the Lifespan (pp. 97–129). Springer, New York, NY. doi: 10.1007/978-1-4939-2923-8_5 [DOI] [Google Scholar]
- Brendgen M, Vitaro F, Barker ED, Girard A, Dionne G, Tremblay RE, & Boivin M (2013). Do other people’s plights matter? A genetically informed twin study of the role of social context in the link between peer victimization and children’s aggression and depression symptoms. Developmental Psychology, 49(2), 327–340. doi: 10.1037/a0025665 [DOI] [PubMed] [Google Scholar]
- Brendgen M, Boivin M, Vitaro F, Bukowski WM, Dionne G, Tremblay RE, & Pérusse D (2008). Linkages between children’s and their friends’ social and physical aggression: evidence for a gene–environment interaction? Child Development, 79(1), 13–29. doi: 10.1111/j.1467-8624.2007.01108.x [DOI] [PubMed] [Google Scholar]
- Burt SA, & Mikolajewski AJ (2008). Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggressive Behavior: Official Journal of the International Society for Research on Aggression, 34(4), 437–445. 10.1002/ab.20251 [DOI] [PubMed] [Google Scholar]
- Button TM, Corley RP, Rhee SH, Hewitt JK, Young SE, & Stallings MC (2007). Delinquent peer affiliation and conduct problems: A twin study. Journal of Abnormal Psychology, 116(3), 554–564. 10.1037/0021-843X.116.3.554 [DOI] [PubMed] [Google Scholar]
- Button TM, Stallings MC, Rhee SH, Corley RP, Boardman JD, & Hewitt JK (2009). Perceived peer delinquency and the genetic predisposition for substance dependence vulnerability. Drug and Alcohol Dependence, 100(1), 1–8. doi: 10.1016/j.drugalcdep.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambron C, Guttmannova K, & Fleming CB (2017). State and national contexts in evaluating cannabis laws: A case study of Washington State. Journal of Drug Issues, 47(1), 74–90. doi: 10.1177/0022042616678607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Drabick DA, & Burgers DE (2015). A developmental perspective on peer rejection, deviant peer affiliation, and conduct problems among youth. Child Psychiatry & Human Development, 46(6), 823–838. doi: 10.1007/s10578-014-0522-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhangur RR, Weeland J, Overbeek G, & Matthys W (2016). Orobio de Castro B, van der Giessen D, & Belsky J(2016). Genetic moderation of intervention efficacy: Dopaminergic genes, the incredible years, and externalizing behavior in children. Child Development, 88, 796–811. doi: 10.1111/cdev.12612 [DOI] [PubMed] [Google Scholar]
- Choukas-Bradley S, Giletta M, Neblett EW, & Prinstein MJ (2015). Ethnic differences in associations among popularity, likability, and trajectories of adolescents’ alcohol use and frequency. Child Development, 86(2), 519–535. doi: 10.1111/cdev.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillessen AH, & Mayeux L (2004). From censure to reinforcement: Developmental changes in the association between aggression and social status. Child development, 75(1), 147–163. [DOI] [PubMed] [Google Scholar]
- Connell AM, Dishion TJ, & Deater-Deckard K (2006). Variable-and person-centered approaches to the analysis of early adolescent substance use: Linking peer, family, and intervention effects with developmental trajectories. Merrill-Palmer Quarterly (1982-), 421–448.
- Côté S, Vaillancourt T, LeBlanc JC, Nagin DS, & Tremblay RE (2006). The development of physical aggression from toddlerhood to pre-adolescence: A nation wide longitudinal study of Canadian children. Journal of abnormal child psychology, 34(1), 68–82. doi: 10.1007/s10802-005-9001-z [DOI] [PubMed] [Google Scholar]
- Dick DM, & Kendler KS (2012). The impact of gene–environment interaction on alcohol use disorders. Alcohol Research: Current Reviews, 34(3), 318. [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, & Rose RJ (2007). Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology, 116(1), 213. doi: 10.1037/0021-843X.116.1.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLalla LF, Bersted K, & John SG (2015). Peer victimization and DRD4 genotype influence problem behaviors in young children. Journal of Youth and Adolescence, 44(8), 1478–1493. doi: 10.1007/s10964-015-0282-4 [DOI] [PubMed] [Google Scholar]
- DiLalla LF, Mullineaux PY, & Elam K (2008). Twins. In Encyclopedia of infant and early childhood development (pp. 369–379). Elsevier Inc. [Google Scholar]
- DiLalla LF, & DiLalla DL (2018). Gene–Environment Correlations Affecting Children’s Early Rule-Breaking and Aggressive Play Behaviors. Twin Research and Human Genetics, 21(4), 285–288. doi: 10.1017/thg.2018.30 [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Capaldi DM, & Yoerger K (1999). Middle childhood antecedents to progressions in male adolescent substance use: An ecological analysis of risk and protection. Journal of Adolescent Research, 14(2), 175–205. [Google Scholar]
- Dishion TJ, & Kavanagh K (2003). Intervening in adolescent problem behavior: A family-centered approach. New York: Guilford. [Google Scholar]
- Dishion TJ, & Loeber R (1985). Adolescent marijuana and alcohol use: The role of parents and peers revisited. The American Journal of Drug and Alcohol Abuse, 11(1–2), 11–25. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Shaw D, Connell A, Gardner F, Weaver C, & Wilson M (2008). The family check-up with high-risk indigent families: Preventing problem behavior by increasing parents’ positive behavior support in early childhood. Child Development, 79(5), 1395–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, & Tipsord JM (2011). Peer contagion in child and adolescent social and emotional development. Annual Review of Psychology, 62, 189–214. doi: 10.1146/annurev.psych.093008.100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam KK, Chassin L, Lemery-Chalfant K, Pandika D, Wang FL, Bountress K, … & Agrawal A (2017). Affiliation with substance-using peers: Examining gene-environment correlations among parent monitoring, polygenic risk, and children’s impulsivity. Developmental Psychobiology, 59(5), 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam KK, Chassin L, & Pandika D (2018). Polygenic risk, family cohesion, and adolescent aggression in Mexican American and European American families: Developmental pathways to alcohol use. Development and Psychopathology, 30(5), 1715–1728. doi: 10.1017/S0954579418000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettekal I, & Ladd GW (2015). Developmental pathways from childhood aggression–disruptiveness, chronic peer rejection, and deviant friendships to early-adolescent rule breaking. Child Development, 86(2), 614–631. 10.1111/cdev.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, & Horwood LJ (1999). Prospective childhood predictors of deviant peer affiliations in adolescence. Journal of Child Psychology and Psychiatry, 40(4), 581–592. doi: 10.1111/1469-7610.00475 [DOI] [PubMed] [Google Scholar]
- Fite PJ, Colder CR, & O’Connor RM (2006). Childhood behavior problems and peer selection and socialization: Risk for adolescent alcohol use. Addictive Behaviors, 31, 1454–1459. doi: 10.1016/j.addbeh.2005.09.015 [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, … & Van Den Bree MB (2007). Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction, 102(3), 413–422. doi: 10.1111/j.1360-0443.2006.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JH, Settle JE, & Christakis NA (2011). Correlated genotypes in friendship networks. Proceedings of the National Academy of Sciences, 108(5), 1993–1997. doi: 10.1073/pnas.1011687108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford-Smith M, Dodge KA, Dishion TJ, & McCord J (2005). Peer influence in children and adolescents: Crossing the bridge from developmental to intervention science. Journal of abnormal child psychology, 33(3), 255–265. doi: 10.1007/s10802-005-3563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Lochman JE, Dishion T, Powell NP, Boxmeyer C, & Qu L (2018). Oxytocin receptor gene variant interacts with intervention delivery format in predicting intervention outcomes for youth with conduct problems. Prevention Science, 19(1), 38–48. doi 10.1007/s11121-017-0777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G (2006). Genetic similarity shared by best friends among adolescents. Twin Research and Human Genetics, 9(1), 113–121. doi: 10.1375/twin.9.1.113 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, & McGue M (2008). Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review Clinical Psychology, 4, 325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157 [DOI] [PubMed] [Google Scholar]
- King SM, Keyes M, Malone SM, Elkins I, Legrand LN, Iacono WG, & McGue M (2009). Parental alcohol dependence and the transmission of adolescent behavioral disinhibition: A study of adoptive and non-adoptive families. Addiction, 104(4), 578–586. doi: 10.1111/j.1360-0443.2008.02469.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SI, Salvatore JE, Aliev F, Ha T, Dishion TJ, & Dick DM (2019). The Family Check-up Intervention Moderates Polygenic Influences on Long-Term Alcohol Outcomes: Results from a Randomized Intervention Trial. Prevention Science, 20(7), 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse E, Boivin M, Brendgen M, Petitclerc A, Girard A, Vitaro F, … & Tremblay RE (2014). A longitudinal twin study of physical aggression during early childhood: evidence for a developmentally dynamic genome. Psychological Medicine, 44(12), 2617–2627. [DOI] [PubMed] [Google Scholar]
- Leung RK, Toumbourou JW, & Hemphill SA (2014). The effect of peer influence and selection processes on adolescent alcohol use: a systematic review of longitudinal studies. Health Psychology Review, 8(4), 426–457. 10.1080/17437199.2011.587961 [DOI] [PubMed] [Google Scholar]
- Li R, Dziak JD, Tan X, Huang L, Wagner AT, Yang J (2015). TVEM (time-varying effect modeling) SAS macro users’ guide (version 3.1.0). University Park (PA): The Methodology Center, Penn State; updated 2015. [Google Scholar]
- Lubke GH, McArtor DB, Boomsma DI, & Bartels M (2018). Genetic and environmental contributions to the development of childhood aggression. Developmental Psychology, 54(1), 3–12. 10.1037/dev0000403 [DOI] [PubMed] [Google Scholar]
- Marschall-Lévesque S, Castellanos-Ryan N, Parent S, Renaud J, Vitaro F, Boivin M, … Séguin JR (2017). Victimization, suicidal ideation, and alcohol use from age 13 to 15 years: Support for the self-medication model. Journal of Adolescent Health, 60(4), 380–387. doi: 10.1016/j.jadohealth.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez-Luna C, Loh PR, South Asian Type 2 Diabetes (SAT2D) Consortium, SIGMA Type 2 Diabetes Consortium, & Price AL (2017). Multiethnic polygenic risk scores improve risk prediction in diverse populations. Genetic epidemiology, 41(8), 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22, 491–495. doi: 10.1017/S0954579410000222 [DOI] [PubMed] [Google Scholar]
- McEachern A, Dishion TJ, Shaw DS, & Wilson MN, Gardner F (2012). Parenting Young Children (PARYC): Validation of a self-report parenting measure. Journal of Child and Family Studies, 21, 498–511. 10.1007/s10826-011-9503-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern AD, Fosco GM, Dishion TJ, Shaw DS, Wilson MN, & Gardner F (2013). Collateral benefits of the family check-up in early childhood: Primary caregivers’ social support and relationship satisfaction. Journal of Family Psychology, 27(2), 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … & Moffitt TE (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences, 109(40), E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaniak I, Watson B, & Jaffee SR (2015). Gene-environment interplay and substance use: A review of recent findings. Current Addiction Reports, 2(4), 364–371. [Google Scholar]
- Mooney MA, & Wilmot B (2015). Gene set analysis: A step-by-step guide. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 168(7), 517–527. doi: 10.1002/ajmg.b.32328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto E, Murru L, Martano G, Sassone J, & Passafaro M (2018). Glutamatergic synapses in neurodevelopmental disorders. Progress in Neuropsychopharmacology & Biological Psychiatry, 84, 328–342. doi: 10.1016/j.pnpbp.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2005). Mplus: Statistical analysis with latent variables: User’s guide (pp. 1998–2012). Los Angeles: Muthén & Muthén. [Google Scholar]
- National Institute of Drug Abuse. (2014). Principles of adolescent substance use disorder treatment: A research-based guide (NIH Publication No. 14–7953). Retrieved July 22, 2015, from https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/podata_1_17_14.pdf
- Narvaes R, & Martins de Almeida RM (2014). Aggressive behavior and three neurotransmitters: Dopamine, GABA, and serotonin – A review of the last 10 years. Psychology & Neuroscience, 7(4), 601–607. doi: 10.3922/j.psns.2014.4.20 [DOI] [Google Scholar]
- Niño MD, Cai T, & Ignatow G (2016). Social isolation, drunkenness, and cigarette use among adolescents. Addictive Behaviors, 53, 94–100. 10.1016/j.addbeh.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Otten R, Mun CJ, Shaw DS, Wilson MN, & Dishion TJ (2019). A developmental cascade model for early adolescent-onset substance use: the role of early childhood stress. Addiction, 114(2), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa I, St Pourcain B, Benke K, Cavadino A, Hakulinen C, Nivard MG, … Evans DM (2016). A genome-wide approach to children’s aggressive behavior: The EAGLE consortium. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(5), 562–572. 10.1002/ajmg.b.32333 [DOI] [PubMed] [Google Scholar]
- Patterson GR, DeBaryshe BD, & Ramsey E (1989). A developmental perspective on antisocial behavior. American Psychologist, 44(2), 329–335. doi: 10.1037/0003-066X.44.2.329 [DOI] [PubMed] [Google Scholar]
- Patterson GR, Dishion TJ, & Yoerger K (2000). Adolescent growth in new forms of problem behavior: Macro-and micro-peer dynamics. Prevention Science, 1(1), 3–13. doi: 10.1023/A:1010019915400 [DOI] [PubMed] [Google Scholar]
- Peterson RE, Kuchenbaecker K, Walters RK, Chen CY, Popejoy AB, Periyasamy S, … & Carey CE(2019). Genome-wide association studies in ancestrally diverse populations: Opportunities, methods, pitfalls, and recommendations. Cell, 179, 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, & Loehlin JC (1977). Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin, 84(2), 309–322. doi: 10.1037/0033-2909.84.2.309 [DOI] [PubMed] [Google Scholar]
- Porsch RM, Middeldorp CM, Cherny SS, Krapohl E, Van Beijsterveldt CE, Loukola A, … Kaprio J (2016). Longitudinal heritability of childhood aggression. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(5), 697–707. doi: 10.1002/ajmg.b.32420 [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, & Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38(8), 904–909. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, & La Greca AM (2004). Childhood peer rejection and aggression as predictors of adolescent girls’ externalizing and health risk behaviors: a 6-year longitudinal study. Journal of consulting and clinical psychology, 72(1), 103–112. doi: 10.1037/0022-006X.72.1.103 [DOI] [PubMed] [Google Scholar]
- Provençal N, Booij L, & Tremblay RE (2015). The developmental origins of chronic physical aggression: biological pathways triggered by early life adversity. Journal of Experimental Biology, 218(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, … Sham PC (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics, 81(3), 559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, & Leve LD (2007). Genetic expression outside the skin: Clues to mechanisms of Genotype× Environment interaction. Development and Psychopathology, 19(4), 1005–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ (1998). A developmental behavior-genetic perspective on alcoholism risk. Alcohol Health Res World, 22(2), 131–143. [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Schlomer GL, Cleveland HH, Feinberg ME, Greenberg MT, Spoth RL, … & Vandenbergh DJ (2018). PROSPER intervention effects on adolescents’ alcohol misuse vary by GABRA2 genotype and age. Prevention Science, 19(1), 27–37. doi 10.1007/s11121-017-0751-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Hesselbrock M, … Edenberg HJ (2015). Polygenic risk for externalizing disorders: Gene-by-development and gene-by-environment effects in adolescents and young adults. Clinical Psychological Science, 3(2), 189–201. doi: 10.1177/2167702614534211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Edwards AC, Evans DM, Macleod J, Hickman M, … Latvala A (2014). Polygenic scores predict alcohol problems in an independent sample and show moderation by the environment. Genes, 5(2), 330–346. doi: 10.3390/genes5020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomer GL, Cleveland HH, Feinberg ME, Wolf PS, Greenberg MT, Spoth RL, … & Vandenbergh DJ (2017). Extending Previous cG× I Findings on 5-HTTLPR’s Moderation of Intervention Effects on Adolescent Substance Misuse Initiation. Child development, 88(6), 2001–2012. doi: 10.1111/cdev.12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Connell A, Dishion TJ, Wilson MN, & Gardner F (2009). Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Development and Psychopathology, 21(2), 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Galán CA, Lemery-Chalfant K, Dishion TJ, Elam KK, Wilson MN, & Gardner F (2019). Trajectories and Predictors of Children’s Early-Starting Conduct Problems: Child, Family, Genetic, and Intervention Effects. Development and Psychopathology, 31, 1911–1921. doi: 10.1017/S0954579419000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, & Williams NA (2005). The development of alcohol use disorders. Annual Review of Clinical Psychology, 1, 493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107 [DOI] [PubMed] [Google Scholar]
- Sher KJ, Jackson KM, & Steinley D (2011). “Alcohol use trajectories and the ubiquitous cat’s cradle: Cause for concern”: Correction to sher et al. (2011).Journal of Abnormal Psychology, 120(2), 510. doi: 10.1037/a0023342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Shiyko MP, Li R, Li Y, & Dierker L (2012). A time-varying effect model for intensive longitudinal data. Psychological methods, 17(1), 61–77. doi: 10.1037/a0025814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton-Weaver LC, Burk WJ, Kerr M, & Stattin H (2013). Can parental monitoring and peer management reduce the selection or influence of delinquent peers? Testing the question using a dynamic social network approach. Developmental Psychology, 49(11), 2057–2070. doi: 10.1037/a0031854 [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Masse LC, Vitaro F, & Dobkin PL (1995). The impact of friends’ deviant behavior on early onset of delinquency: Longitudinal data from 6 to 13 years of age. Developmental Processes in Peer Relations and Psychopathology, 7(4), 649–667. doi: 10.1017/S0954579400006763 [DOI] [Google Scholar]
- Trucco EM, Colder CR, Wieczorek WF, Lengua LJ, & Hawk LW (2014). Early adolescent alcohol use in context: How neighborhoods, parents, and peers impact youth. Development and Psychopathology, 26(2), 425–436. doi: 10.1017/S0954579414000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lier P, Boivin M, Dionne G, Vitaro F, Brendgen M, Koot H, … Perusse D (2007). Kindergarten children’s genetic vulnerabilities interact with friends’ aggression to promote children’s own aggression. Journal of the American Academy of Child & Adolescent Psychiatry, 46(8), 1080–1087. doi: 10.1097/CHI.0b013e318067733e [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, & Bakermans-Kranenburg MJ (2015). Genetic differential susceptibility on trial: Meta-analytic support from randomized controlled experiments. Development and Psychopathology, 27(1), 151–162. [DOI] [PubMed] [Google Scholar]
- Van Ryzin MJ, DeLay D, & Dishion TJ (2016). Being well-liked predicts increased use of alcohol but not tobacco in early adolescence. Addictive Behaviors, 53, 168–174. doi: 10.1016/j.addbeh.2015.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryzin MJ, & Dishion TJ (2014). Adolescent deviant peer clustering as an amplifying mechanism underlying the progression from early substance use to late adolescent dependence. Journal of Child Psychology and Psychiatry, 55(10), 1153–1161. doi: 10.1111/jcpp.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaro F, Pedersen S, & Brendgen M (2007). Children’s disruptiveness, peer rejection, friends’ deviancy, and delinquent behaviors: A process-oriented approach. Development and Psychopathology, 19, 433–453. doi: 10.10170S0954579407070216 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, … & Baler R (2016). Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry, 73(3), 292–297. [DOI] [PubMed] [Google Scholar]
- West SG, Finch JF, & Curran PJ (1995). Structural equation models with nonnormal variables: Problems and remedies. In Hoyle RH (Ed.), Structural equation modeling: Concepts, issues, and applications (pp. 56–75). Thousand Oaks, CA, US: Sage Publications. [Google Scholar]
- Wolchik SA, Wilcox KL, Tein J-Y, & Sandler IN (2000). Maternal Acceptance and Consistency of Discipline as Buffers of Divorce Stressors on Children’s Psychological Adjustment Problems. Journal of Abnormal Child Psychology, 28(1), 87–102. [DOI] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, & Middeldorp CM (2014). Research review: polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, 55(10), 1068–1087. [DOI] [PubMed] [Google Scholar]
- Young R, Sweeting H, & West P (2008). A longitudinal study of alcohol use and antisocial behaviour in young people. Alcohol & Alcoholism, 43(2), 204–214. doi: 10.1093/alcalc/agm147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, & Prescott CA (2011). The influence of gene–environment interactions on alcohol consumption and alcohol use disorders: A comprehensive review. Clinical Psychology Review, 31(5), 800–816. doi: 10.1016/j.cpr.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun I, Cheong J, & Walsh A (2011). Genetic and environmental influences in delinquent peer affiliation: From the peer network approach. Youth Violence and Juvenile Justice, 9(3), 241–258. doi: 10.1177/1541204010388527 [DOI] [Google Scholar]
- Zhang K, Chang S, Guo L, & Wang J (2015). I-GSEA4GWAS v2: A web server for functional analysis of SNPs in trait-associated pathways identified from genome-wide association study. Protein Cell, 6(3), 221–224. doi: 10.1007/s13238-014-0114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Cui S, Chang S, Zhang L, & Wang J (2010). i-GSEA4GWAS: A web server for identification of pathways/gene sets associated with traits by applying an improved gene set enrichment analysis to genome-wide association study. Nucleic Acids Research, 38, W90–W95. doi: 10.1093/nar/gkq324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, & Nigg JT (2011). Parsing the undercontrol–disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Development Perspectives, 5(4), 248–255. doi: 10.1111/j.1750-8606.2011.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


