Abstract
Extant research is mixed regarding the relations between lifetime exposure to stressors, adrenocortical activity, and executive function (EF), particularly in children. Aggregate measures of adrenocortical activity like hair cortisol concentration (HCC), timing of stress exposure, and age at assessment may clarify these associations. This cross-sectional study examined the association between parent-reported exposure to stressors, hair cortisol concentration (HCC), and children’s executive function via a tablet task in a community sample (n = 318, 52.5% female) of children across a wide age range (4–13 years, M = 9.4, SD = 2.3). Path analyses revealed that parent-reported child lifetime exposure to stressors, but not past-year stressful life events, negatively predicted HCC. There was also a marginally significant moderation by age such that HCC was associated negatively with EF for younger children (age < 9.7 years) but not older children. HCC did not significantly mediate the association between lifetime exposure to stressors and EF. Findings are consistent with the proposition that chronically high cortisol production has a neurotoxic effect on brain regions supporting EF. However, lifetime exposure to stressors predicted relatively lower cumulative cortisol production, consistent with a stress inoculation effect in this normative-risk sample.
Keywords: hair cortisol, stress, executive function, development
Introduction
The hypothalamic-pituitary-adrenocortical (HPA) axis and its end-product, cortisol, are key components of the stress response. While stress increases cortisol production, this glucocorticoid also performs essential physiological and psychosocial functions (Gunnar & Vazquez, 2006). Acute, brief elevations in cortisol upon awakening and in response to stress are considered evidence of a well-regulated HPA axis (McEwen, 2019). However, chronic cortisol elevations that do not promptly return to baseline levels can be harmful, altering HPA axis setpoints (McEwen, 2006) that impact downstream biological and psychological functioning (Juster, McEwen, & Lupien, 2010; Strüber, Strüber, & Roth, 2014).
Chronically high cortisol production due to stress can harm cognitive and self-regulatory building blocks like executive function (EF; Shields, Sazma, & Yonelinas, 2016). Cortisol, like other steroid hormones, can easily cross the blood-brain barrier (Banks, 2012), raising concerns that excessive cortisol could have a neurotoxic effect on sensitive brain regions implicated in EF (e.g., prefrontal cortex and hippocampus; Merz et al., 2019; Porcelli et al., 2008; Vogel, Fernández, Joëls, & Schwabe, 2016). This neurotoxicity could be particularly detrimental for young children, when both their prefrontal cortex and EF skills are developing rapidly (Barrasso-Catanzaro & Eslinger, 2016). Indeed, higher morning cortisol (Wagner et al., 2016) and lower basal cortisol (Blair et al., 2011; Cutuli, 2011) both predict better EF in young children. Moderate cortisol reactivity followed by adequate recovery is also associated with better EF and self-regulation and less aggressive behavior (Blair, Granger, & Razza, 2005). However, the impact of stress exposure on HPA activity may vary by age (Ursache, Noble, & Blair, 2015).
To determine the cumulative level of cortisol that has crossed the blood-brain barrier over time and understand its relation to children’s EF, aggregate rather than acute measures of HPA activity are needed. Hair cortisol concentration (HCC) is a biomarker of cumulative HPA activity over several months. As hair grows, cortisol is incorporated into the hair at the scalp proportional to the amount in the bloodstream at that time (Stalder & Kirschbaum, 2012). HCC is moderately associated with other aspects of HPA activity including diurnal slope, cortisol awakening response, and 24-hour urinary cortisol (Stalder & Kirschbaum, 2012), yet many view HCC as an indicator of more global levels of activation (Bates, Salsberry, & Ford, 2017; Stalder et al., 2012a). Thus, HCC may represent the average level of cortisol in circulation at a given time and may be an important predictor of children’s EF. Unfortunately, there is little to no research on this association.
In adults, studies show positive (Pulopulos et al., 2014), negative (Assayag et al., 2017), and null (McLennan, Ihle, Steudte-Schmiedgen, Kirschbaum, & Kliegel, 2016) associations between HCC and cognitive functioning. In children, HCC is associated with related constructs; higher HCC predicts fewer ADHD symptoms (Pauli-Pott, Schloβ, Skoluda, Nater, & Becker, 2019; Schloβ et al., 2018) but also more behavior problems (Golub et al., 2019). A recent study of Pakistani preschoolers found a negative association between HCC and cognitive skills including EF, but only for girls with higher family wealth (Armstrong-Carter, Finch, Siyal, Yousafzai, & Obradović, 2020). Generally, lower socioeconomic status and exposure to more poverty-related stressors like household chaos, maternal distress, and overall perceived stress are associated with increased HCC in adults (O’Brien, Tronick, & Moore, 2013) and children (Andrews, 2020; Vliegenthart et al., 2016), although some results have been mixed (Gray et al., 2018). Childhood trauma predicts both higher (Karlén et al., 2015; Palmer et al., 2013; Simmons et al., 2016; Slopen et al., 2018) and lower (Grunau et al., 2013) levels of later HCC. Studies in late childhood and adolescence show a negative association between maltreatment exposure and HCC (White et al., 2017) but no significant association with perceived stress (Prado-Gascó et al., 2019). These mixed results may be due to differences in the nature of the stressor, the timing of stress exposure, and the child’s age at the time of HCC measurement. Specifically, acute, circumscribed, and recent stressful events vs. more global, chronic, and persistent stressors may show different relations with, or at least explain different portions of the variance in, HCC. Thus, cumulative cortisol production could be a key mechanism through which childhood stress affects EF. However, little to no research has examined associations between HCC and EF in childhood, which may limit service providers’ ability to support children’s healthy development (Barnes et al., 2020). Due to the inconsistency of previous findings, no directional hypotheses were posited regarding the association between stress exposure and HCC or HCC and EF.
Methods
Participants.
Participants were 318 children (52.5% female) aged 4–13 years old (M = 9.4, SD = 2.3) and their primary caregivers (68.3% biological mothers, 26.3% biological fathers, 5.4% other relatives). Caregivers ranged in age from 24–66 years (M = 41.3, SD = 6.6) and reported a median education level of a four-year college degree. Most children (88.3%) and caregivers (92.1%) were White. Most caregivers were married (83.5%) and employed (89.1%). Median annual household income was $100,000–124,999. See Table 1 for more detailed demographic information.
Table 1.
Sample demographic information (N = 318).
| M (SD) or n (%) | |
|---|---|
| Child age (years) | 9.4 (2.3) |
| Child race | |
| American Indian/Alaskan Native | — |
| Asian/Pacific Islander | 15 (4.8%) |
| Black | 2 (0.6%) |
| White | 256 (82.1%) |
| Multiracial/Other | 39 (12.5%) |
| Caregiver age (years) | 41.3 (6.6) |
| Caregiver race | |
| American Indian/Alaskan Native | 1 (0.3%) |
| Asian/Pacific Islander | 17 (5.4%) |
| Black | — |
| White | 286 (90.8%) |
| Multiracial/Other | 11 (3.5%) |
| Caregiver marital status | |
| Married | 266 (84.4%) |
| Never married | 29 (9.2%) |
| Separated/Divorced | 18 (5.7%) |
| Widowed | 2 (0.6%) |
| Caregiver education level | |
| High School diploma, GED, or less | 16 (5.1%) |
| Some college | 74 (23.7%) |
| Bachelor’s degree | 112 (35.9%) |
| Some Graduate/Professional school | 20 (6.4%) |
| Graduate/Professional degree | 90 (28.8%) |
| Annual household income | |
| Less than $25,000 | 18 (6.3%) |
| $25,000–49,999 | 27 (9.4%) |
| $50,000–99,999 | 84 (29.2%) |
| $100,000–149,999 | 78 (27.1%) |
| $150,000–199,999 | 43 (14.9%) |
| $200,000 or more | 38 (13.2%) |
Prior to participation, individuals were excluded if they were not sufficiently fluent in English to complete the tasks or had a developmental delay that interfered with study completion. Caregivers provided written informed consent and children provided verbal assent. Child participants 8 years of age or older also provided written assent. All study procedures were approved by the Institutional Review Board (IRB) at the participating University.
Procedure.
Participants were recruited at a Midwestern U.S. State Fair research booth. Caregivers completed questionnaires and children completed an EF task on tablet computers. A trained researcher collected a hair sample from each child at the base of the scalp to be assayed for cortisol.
Measures.
Hair Cortisol Concentration (HCC).
A 3-cm, approximately 7.5-mg segment of hair (2–3 small bundles) was cut from the occipital ridge. Samples were stored in foil at room temperature before being sent to the University of Trier, Germany for assay (Stalder et al., 2012b). Cortisol was extracted from all samples on the same assay. Given an average growth rate of approximately 1 cm per month (Stalder & Kirschbaum, 2012), 3 cm represents cumulative cortisol production over the 3 months preceding sample collection. HCC values were log10-transformed prior to analysis to resolve positive skew. HCC was not significantly associated with child body mass index, p = .78, or hair washing frequency, p = .14, though females showed significantly lower HCC than males, r = −.22, p = .01. HCC data were available for 172 (54.1%) children in this sample. Missingness is largely due to individuals declining to participate in this portion of the study or having hair that was too short. Besides age, r = −.18, p < .01, sex, r = −.45, p < .01, and EF, r = −.18, p < .01, missing analyses revealed no significant differences in study variables for children with and without HCC data. Younger children, males, and children with lower EF scores were more likely to have missing HCC data.
Stressful Life Events.
Caregivers reported on their children’s general lifetime exposure to stressors (Child Life Challenges Scale; CLCS; Merrick et al., 2020) and the number of potentially stressful life events children encountered over the past year (Life Events Questionnaire; LEQ; Masten, Neeman, & Andenas, 1994). Caregivers also reported the number of life events over the past three months to parallel the approximate amount of time represented by the hair sample, but the base rate of events experienced was too low to be used for analyses (66% reported no events in the past three months).
The CLCS is a one-item continuous measure where caregivers report their child’s global lifetime exposure to stressors on a sliding scale from 0 (Mildly challenging experiences) to 100 (Extremely challenging experiences). This measure was validated with a long-form caregiver-report of the number of lifetime stressors to which the child has been exposed (Merrick et al., 2020). Unlike other cumulative risk approaches, this measure incorporates both number and severity of stressors in one global metric. Therefore, the CLCS shows potential as a brief, low-burden measure of chronic exposure to stressors that could be easily implemented in health care and other community settings. Two parents declined to complete this measure.
The LEQ is a checklist of life events that a child may have experienced over the past year, including a mixture of acute and chronic events that vary in valence and whether or not they likely were influenced by the youth (e.g., winning an award versus a parent’s death; Masten et al., 1994). We shortened the LEQ to include acute and chronic but only independent negative events (e.g., “A close family member died”; “There were many arguments between adults living in the house”) and ambiguous events (e.g., “My child has a new brother or sister”) that are typically included in composite LEQ scores, plus the positive items (e.g., “My child received a special award…”) to balance the tone of the measure. At the request of the IRB, we removed potentially distressing items (e.g., child abuse, suicide) for the State Fair context. Negative and ambiguous items were summed as an index of past-year exposure to potential stressors (maximum score = 22). Twenty-four parents declined to complete this measure.
Executive Function (EF).
Using a “planned missingness” approach to reduce participant burden (Little & Rhemtulla, 2013), children randomly completed one of two tasks designed to assess EF: the Dimensional Change Card Sort (DCCS) or the Flanker task from the National Institutes of Health (NIH) Toolbox, both of which are computer-adaptive tasks validated for assessing EF in this age group (Weintraub et al., 2013). With the developmental extension (DEXT) that is designed to lower the floor of the tasks (Anderson et al., 2015; Carlson et al., 2015), possible scores ranged from −5 to 10, with higher scores indicating better EF performance. The score from either the DCCS (n = 155, 49.5%) or Flanker (n = 158, 50.5%) was used as an index of EF ability. Only 17 participants (5.4%) required the easier DEXT levels for either task. Five children declined to complete this assessment.
The DCCS and Flanker tasks assess different components of EF, which gradually differentiate across childhood (Akshoomoff, Brown, Bakeman, & Hagler, 2018; Best & Miller, 2010). However, task performance is highly correlated across tasks throughout development and there is little evidence to suggest that the different components of EF should have differing associations with life stress or HCC, especially in early childhood. Thus, the planned missingness approach should not affect the goals of the current analysis. The specific task completed was not significantly associated with child age, sex, race, family income, lifetime exposure to stressors, or past-year life events, p’s > .32.
Data Analytic Plan.
Analyses were completed in Mplus 7.4 (Muthén & Muthén, 1998–2012) with full information maximum likelihood to use all available data. First, descriptives and correlations were computed for all variables. Second, a mediated path model evaluated the indirect effects of lifetime exposure to stressors and past-year stressful life events on EF via HCC. Given our interest in the timing of stress exposure, both lifetime exposure to stressors and past-year life events were included in the model but were allowed to covary. Child gender, age, and household income were included as covariates for HCC and EF. Although previous studies have found that BMI was positively associated with HCC (Stalder et al., 2012b), BMI was excluded from our analysis because it was not significantly correlated with HCC. As a sensitivity analysis, EF task (Flanker vs. DCCS) was investigated as a potential covariate but did not substantively change the results, and so was not included in analyses.
Good model fit was evaluated using multiple indices: a nonsignificant chi-square statistic (Satorra, 2000) and several practical fit indices, including the Tucker Lewis Index (TLI > .95; Tucker & Lewis, 1973), comparative fit index (CFI > .95; Bentler, 1990), root mean square error of approximation (RMSEA < .05; MacCallum, Browne, & Sugawara, 1996), and standardized root mean square residual (SRMR < .08; Hu & Bentler, 1999). A bootstrapping procedure with 5000 resamples (Preacher & Hayes, 2008; Shrout & Bolger, 2002) was used to estimate the 95% confidence intervals for the indirect effect of HCC through the pathways from lifetime exposure to stressors and past-year life events to EF.
Finally, multigroup analyses examined whether these associations were moderated by age, split at the median age of the sample. Unconstrained models that allow the associations to differ across age groups were compared to constrained models that fixed all or specific associations to be equal across age groups. For our analyses, we compared a fully unconstrained model to a constrained model that fixed all paths to be equal across age groups as well as a constrained model that tested whether the specific path from HC to EF was similar across age groups given theoretical evidence to support developmental differences. The chi-square likelihood ratio difference test (Satorra, 2000) was used to evaluate the comparative fit across these nested models. A significant chi square indicates that there are differences between models and provides support for the unconstrained model, while a nonsignificant chi square supports the constrained model.
Results
Descriptives and correlations for all variables are provided in Table 2. HCC was associated negatively with child’s age, male sex, lifetime exposure to stressors, and EF, but was not associated significantly with household income or past-year life events. EF was associated positively with age and female sex, but was not associated significantly with household income, lifetime exposure to stressors, and past-year life events. Lifetime exposure to stressors and past-year life events were associated positively. Finally, household income was associated negatively with both lifetime exposure to stressors and past-year life events.
Table 2.
Descriptive statistics and intercorrelations among focal child variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Age (years) | — | ||||||
| 2. Sex | .08 | — | |||||
| 3. Income | .06 | .11ϯ | — | ||||
| 4. Past-year potentially stressful life events (LEQ) | −.02 | −.03 | −.37** | — | |||
| 5. Lifetime exposure to stressors (CLCS) | .05 | −.03 | −.19** | .43** | — | ||
| 6. Hair cortisol (pg/mg) | −.14ϯ | −.22** | −.01 | −.10 | −.20** | — | |
| 7. Executive function score | .69** | .11* | .09 | −.02 | −.00 | −.21* | — |
|
| |||||||
| Mean (SD) | 9.4 (2.3) | — | — | 1.1 (1.3) | 26.9 (24.5) | 18.0 (40.6) | 7.7 (2.2) |
|
| |||||||
| Range | 4.2–12.9 | — | — | 0–5 | 0–97 | 0.5–366.4 | −1.5–10 |
Note. N = 318 for the full sample. Sex: 1 = female, 0 = male.
p < .10;
p < .05;
p < .01
A mediated path model evaluated the indirect effects of lifetime stressors and past-year life events on EF via HCC (see Figure 1). This model fit the data well, χ2(7) = 7.84, p = .35; RMSEA = .02, 95% CI [.00, .07]; CFI = 1.00; TLI = .99; SRMR = .03. Results indicated that lifetime stressors and female sex were associated negatively with HCC. Child’s age was associated positively with EF. HCC was associated negatively, though marginally, with EF, p = .068. Neither household income nor past-year life events were associated significantly with HCC or EF. Finally, a test of the indirect effect of HCC via the pathways from lifetime stressors, β = .02, p = .16; [−.00, .06] and past-year life events, β = .00, p = .75; [−.02, .03] to EF revealed no significant mediation.
Figure 1.
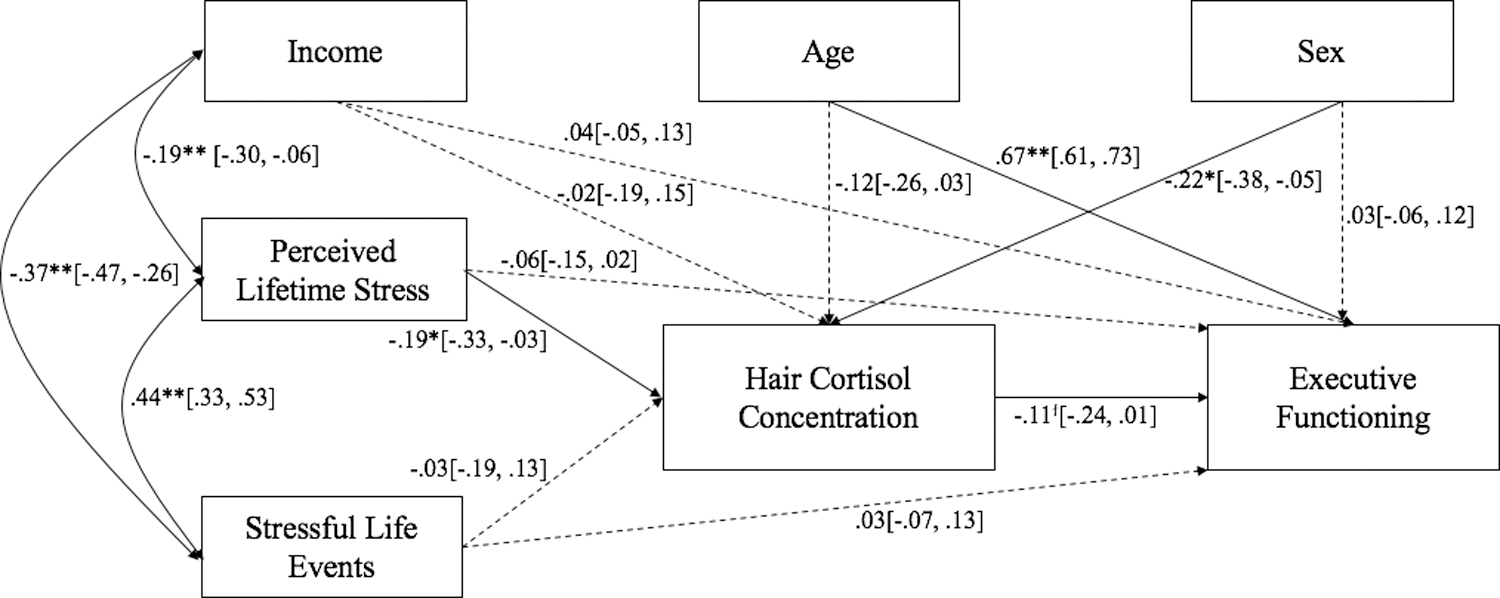
Standardized path coefficients from lifetime exposure to stressors and potentially stressful life events experienced in the past year to executive function via hair cortisol concentration. 95% confidence intervals are provided in brackets. Sex: 1 = female, 0 = male. ϯp < .10; *p < .05; **p < .01
Multigroup analyses tested whether these associations varied by child’s age. Chi-square tests and t-tests indicated that the children in each age group did not differ by sex, income category, which EF task they completed, or either measure of stress exposure (p’s > .22). A chi square difference test that compared the fully unconstrained model with the fully constrained model was not significant, Δχ2(17) = 16.38, p = .50. Although the chi square test was not significant, the chi square value indicate some degree of misfit. Given theoretical evidence indicating that HCC may have differential impact on children, the unconstrained model was then compared to a partially-constrained model that fixed the path from HCC to EF to be equal across age groups. Isolation of this path lead to a marginally significant difference, Δχ2(1) = 3.64, p = .056. Results indicated that HCC was associated negatively with EF in younger children, β = −.30, p = .04; [−.59, −.02], but not among older children, β = −.08, p = .49; [−.31, .15].
Given that past-year life events and household income were not correlated significantly with HCC or EF, a mediated path model that excluded these variables was evaluated (Figure 2). Results from this model remained consistent with the initial model. Similar findings emerged with only the 172 participants that provided HCC data (see Supplementary Material, Figures S1 and S2).
Figure 2.
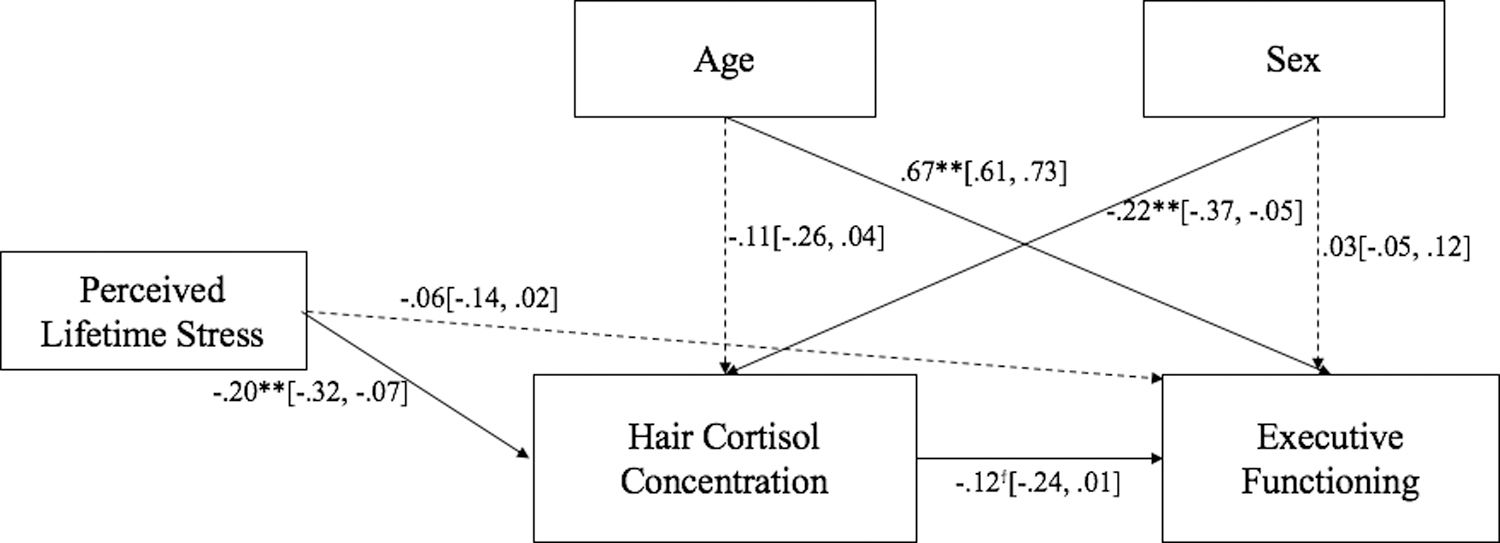
Standardized path coefficients from lifetime exposure to stressors to executive function via hair cortisol concentration. 95% confidence intervals are provided in brackets. Sex: 1 = female, 0 = male. ϯp < .10; *p < .05; **p < .01
Discussion
This study investigated associations between children’s exposure to stressors (lifetime and in the past year), HCC, and EF in a Midwestern U.S. community sample. EF was measured in a real-world setting as opposed to a controlled laboratory setting, increasing the ecological validity of this assessment. Overall, parents’ ratings of children’s lifetime exposure to stressors, but not past-year life events, was associated negatively with HCC, and HCC in turn was associated negatively, but marginally, with EF (Figure 2). Neither measure of stress exposure was directly associated with EF. HCC did not mediate the association between lifetime stressors and EF. This study is one of the first to demonstrate an association between children’s HCC and EF, in a sample of participants ranging from early to middle childhood (4–13 years). Though only marginally significant, this association varied by age: HCC was associated negatively with EF in younger, but not older, children.
The negative association between child lifetime stressors and HCC is not surprising and may clarify inconsistencies in the literature regarding associations between stress exposure and cumulative HPA activity (Grunau et al., 2013; Karlén et al., 2015; Palmer et al., 2013; Prado-Gascó et al., 2019; Slopen et al., 2018; White et al., 2017). For children who have experienced high levels of chronic stress or trauma, stress exposure may predict an initial increase in HPA activity, followed by a gradual downregulation that ultimately results in a negative association between stress exposure and HPA activity over time (McEwen, 2006; Rich & Romero, 2005). However, the current sample reported relatively high socioeconomic status and, on average, did not report high levels of lifetime exposure to stressors or past-year life events (Table 2). For this normative-risk sample, a negative association between lifetime exposure to stressors and HCC might be indicative of stress inoculation, where exposure to mild stress promotes “resistance” to subsequent stressors (Lyons, Parker, Katz, & Schatzberg, 2009; Romeo, 2015).
Still, others have found a positive association between chronic stress and children’s HCC (Slopen et al., 2018; Vliegenthart et al., 2016) as well as short-term changes in HCC in response to acute trauma/stressors (Dajani, Hadfield, Uum, Greff, & Panter-Brick, 2018; Etwel, Russell, Rieder, Van Uum, & Koren, 2014; Groeneveld et al., 2013). These studies typically, though not always, used samples exposed to more severe stress and trauma. More nuanced research is needed to examine the impact of timing, duration, and intensity of stress exposure on adrenocortical activity, EF, and other domains negatively affected by stress and trauma. The current study begins to address these open questions by demonstrating that parents’ reports of children’s lifetime stress exposure were more strongly associated with children’s HCC than the number of potentially-stressful life events in the past year. It is possible that differences between the measures of stressor exposure influenced this discrepancy. For example, recall bias may differentially influence reports of global stress exposure over the lifetime and more explicit stressors over a shorter time period. Alternatively, it may be that chronic lifetime stress is truly a better predictor of children’s HCC than more recent, specific stressors. However, measures of chronic lifetime stress are associated with measures of more recent stress, so these two influences are difficult to tease apart.
Surprisingly, neither measure of stress exposure was directly related to EF, and therefore HCC was not a significant mediator of this association. This is inconsistent with prior research suggesting that increased exposure to stressors like poverty, parental stress, and less supportive parenting behaviors all negatively impact developing EF skills, especially in early childhood (Blair & Raver, 2016; Finegood & Blair, 2017; Hackman & Farah, 2009). Further, the effects of stressors on EF have been shown to be mediated by salivary cortisol levels (Blair et al., 2011). However, the association between stress exposure and EF has been based primarily on studies that measure EF in controlled settings that minimize distractions. Even in-home assessments, as were used in the Family Life Project (e.g., Blair et al., 2011), are conducted in an environment that is familiar to the child where the parent and experimenter have reasonable control over the context in which EF is measured. In contrast, the state fair setting of the current study is novel, exciting, and possibly overstimulating in ways the parent and experimenter have less control over.
A growing body of evidence that contradicts the deficit-based approach to stress and EF suggests that both rodents and humans raised in unpredictable or stressful environments may actually perform better on learning and EF tasks in these less-controlled environments (Champagne et al., 2008; Ellis & Del Guidice, 2019; Mittal, Griskevicius, Simpson, Sung, & Young, 2015). In the present study, youth who experienced more life stressors may have performed better on the EF task in the state fair context while youth who experienced fewer stressors performed worse than they would have in a more controlled setting. If so, the direct association between stressor exposure and EF may be masked by youths’ differing contextual adaptations. This may also explain the lack of mediation by HCC; it is possible that HCC mediates the association between stress and EF for some individuals and not others, or only in relation to certain types of stressors (e.g., chronic poverty-related stressors vs. acute trauma). Future studies should examine child HCC in relation to a more detailed characterization of the type, timing, and severity of stressor exposure to better understand these processes.
HCC, however, was significantly negatively related to EF, consistent with studies of adults where acute and cumulative measures of cortisol were negatively related to EF (Assayag et al., 2017; Shields et al., 2016). In children, the direction of associations between HCC and EF-related constructs like ADHD symptoms, behavior problems, and cognitive skills have been mixed (e.g., Armstrong-Carter et al., 2020; Golub et al., 2019; Pauli-Pott et al., 2019; Schloβ et al., 2018). Here, we found a marginal difference by age group such that higher HCC predicted worse EF for younger (< 9.7 years) but not older children. Thus, at least for younger children, stress inoculation may predict better self-regulation during a time of increased brain plasticity and dynamic self-regulation development (Barrasso-Catanzaro & Eslinger, 2016). These data come with the added strength that they were collected in an unstructured, real-world setting. It is possible that previous mixed findings reflect the lack of generalizability of EF measured in controlled laboratory settings.
Alternatively, parents may know less about older children’s stress exposure, making parents’ reports at older ages less accurate. Younger children spend more time in the home and under their parents’ supervision, while older children have an expanded environment that includes their school, peers, and extracurricular teams/clubs, which are frequently encountered without parents. This possibility highlights the importance of multiple informants when assessing older children’s stress exposure, in particular child self-report, to strengthen the quality of this measure. It is also possible that the nonsignificant association between HCC and EF in later childhood reflects a ceiling effect of EF, with reduced variability to be explained at this age. If these participants were followed longitudinally, those with higher HCC may have lower EF initially, but eventually catch up to their lower HCC peers later in childhood. Because few studies have examined HCC/EF associations in childhood, and because this study found only a marginal difference in the strength of this association by age, these findings will need to be replicated and extended in future longitudinal research.
Future research should also examine whether this age-dependent association represents a transient disadvantage in EF for young children with high HCC, or if the effects of high HCC in early childhood persist over time in other ways. If high HCC indicates risk for a neurotoxic effect on brain development during early childhood (Barrasso-Catanzaro & Eslinger, 2016; Porcelli et al., 2008; Vogel et al., 2016), there may be downstream effects on other functional outcomes like general cognitive processing and mental health beyond early childhood (Vogel et al., 2016). Future research should examine longitudinal associations between HCC and EF across childhood, as well as their relations with brain development and other domains of functioning over time, to more fully understand the mechanisms through which these associations arise and persist (Vogel et al., 2016). Family and parental influences should also be considered, particularly for younger children where the home environment is more central to their daily experiences. Parental EF and possible genetic contributions to child self-regulation (Polderman et al., 2007) as well as parenting behaviors, household chaos, emotion expression, and conflict management (e.g., Bernier, Carlson, Deschênes, & Matte-Gagné, 2012; Sarsour et al., 2011) among other factors likely influence child HCC, EF, and the relations between the two.
This study’s findings have implications for clinical practice, highlighting the potential utility (and limitations) of assessments of childhood stress exposure and HCC for supporting child developmental health. For example, the CLCS may be a low-burden, less intrusive, and clinically-useful measure of overall childhood stress exposure (compared to a count of recent stressful life events) that is associated with HCC. However, more research is needed on normative and atypical levels of childhood HCC, and how they relate to the developmental trajectory of regulatory outcomes like EF (or its subcomponents), before it can be used as a biomarker of childhood stress. The sex differences in HCC and EF found in this study highlight the need to better understand sex-specific processes and whether this impacts clinical applications for males and females. More studies are also needed that include both subjective reports of stress and physiological indicators of stress to identify areas where they converge or diverge in predicting child developmental functioning. Nevertheless, clinicians and other service providers may ultimately be able to use the CLCS and HCC as part of a panel of psychosocial risk factors and biomarkers that informs treatment and prevention of cognitive and behavioral problems (Barnes et al., 2020).
An important limitation of this study is its lack of racial and economic diversity. These findings may not generalize to populations of color and/or lower socioeconomic status. Also, using parent-report of children’s lifetime stress exposure may not reflect the true extent of children’s experiences, due to systematic under- or over-reporting based on certain family and contextual factors. Even though the most distressing items were removed at the request of the IRB, 24 parents declined to complete the LEQ. The families who declined may have experienced the highest levels of stress exposure, limiting the generalizability of the findings to trauma-exposed and otherwise high-risk children. However, given the paucity of research on child lifetime stress, HCC, and EF, a homogeneous, normative-risk sample may be a good starting point, illustrating how exposure to stressors impacts children’s HCC and EF independent of confounding socioeconomic factors like poverty. Also, the tablet-based EF tasks were completed at a state fair research booth, a potentially overstimulating environment that may have negatively affected children’s performance. Alternatively, this setting could increase the ecological validity of the measure. Similarly, the study design was not conducive to completing an entire EF battery, which might have increased the reliability of EF scores obtained (Willoughby & Blair, 2011). Finally, HCC data were only available for 54% of participants. Younger children, males, and children with lower EF scores were more likely to have missing HCC data, which may have impeded our ability to detect associations with other study variables.
Conclusions
Findings from this cross-sectional study indicate potential age-dependent associations between children’s hair cortisol concentration and executive function. A measure of chronic lifetime stress exposure, but not past-year stressful life events, was significantly related to lower hair cortisol concentration. Thus, HCC holds promise as a measure of children’s biological responses to cumulative stress. Future longitudinal research is needed to examine the role of timing, duration, and intensity of exposure to stress, as well as its impact on adrenocortical regulation, executive function, and other long-term outcomes of early life stress.
Supplementary Material
Acknowledgments:
Thank you to the many students, including Jillian Merrick, who contributed greatly to the project; Dr. Clemens Kirschbaum and his lab for technical assistance with hair cortisol sampling; and the University of Minnesota Department of Pediatrics Driven to Discover Grant. Also, thank you to all of the families who participated. University of Minnesota Department of Pediatrics Driven to Discover Grant; National Institute for Mental Health training grant [T32 MH015755] to CED and FAT.
Dedication: During the process of revising this manuscript, we experienced the devastating loss of Dr. Carrie DePasquale at the age of 26. Carrie was a brilliant scholar and one of the youngest recipients of a doctoral degree in the history of the Institute of Child Development. She had recently been awarded a prestigious F32 grant from NIH on its first submission. Carrie was passionate about her research, family, and friends, and a strong advocate for social justice. This publication is dedicated to her memory, exceptional scholarship, and friendship.
Footnotes
Declarations of interest: None.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Akshoomoff N, Brown TT, Bakeman R, & Hagler DJ (2018). Developmental differentiation of executive functions on the NIH Toolbox Cognition Battery. Neuropsychology, 32(7), 777–783. 10.1037/neu0000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Zelazo PD, Carlson SM, Kalstabakken AW, & Masten AS (2015). Technical Report for the Flanker–Developmental Extension.
- Andrews K (2020). Household chaos, maternal distress and parenting: Associations with child function across multiple domains. Unpublished dissertation. https://macsphere.mcmaster.ca/handle/11375/25351
- Armstrong-Carter E, Finch JE, Siyal S, Yousafzai AK, & Obradović J (2020). Biological sensitivity to context in Pakistani preschoolers: Hair cortisol and family wealth are interactively associated with girls’ cognitive skills. Developmental Psychobiology. 10.1002/dev.21981 [DOI] [PubMed]
- Assayag EB, Tene O, Korczyn AD, Shopin L, Auriel E, Molad J, Hallevi H, Kirschbaum C, Bornstein NM, Shenhar-Tsarfaty S, Kliper E, & Stalder T (2017). High hair cortisol concentrations predict worse cognitive outcome after stroke: Results from the TABASCO prospective cohort study. Psychoneuroendocrinology, 82, 133–139. 10.1016/j.psyneuen.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Banks WA (2012). Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology, 153(9), 4111–4119. 10.1210/en.2012-1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AJ, Anthony BJ, Karatekin C, Lingras KA, Mercado R, & Thompson LA (2020). Identifying adverse childhood experiences in pediatrics to prevent chronic health conditions. Pediatric Research, 87(2), 362–370. 10.1038/s41390-019-0613-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasso-Catanzaro C, & Eslinger PJ (2016). Neurobiological bases of executive function and social-emotional development: Typical and atypical brain changes. Family Relations, 65(1), 108–119. 10.1111/fare.12175 [DOI] [Google Scholar]
- Bates R, Salsberry P, & Ford J (2017). Measuring stress in young children using hair cortisol: The state of the science. Biological Research for Nursing, 19(5), 499–510. 10.1177/1099800417711583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246. 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, & Matte-Gagné C (2012). Social factors in the development of early executive functioning: A closer look at the caregiving environment. Developmental Science, 15(1), 12–24. 10.1111/j.1467-7687.2011.01093.x [DOI] [PubMed] [Google Scholar]
- Best JR, & Miller PH (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, & Razza R (2005). Cortisol reactivity is positively related to executive function in preschool children attending Head Start. Child Development, 76(3), 554–567. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Kivlighan KT, Fortunato CK, & FLP Investigators (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82(6), 1970–1984. 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2016). Poverty, stress, and brain development: New directions for prevention and intervention. Academic Pediatrics, 16(3 Suppl), S30–S36. 10.1016/j.acap.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Zelazo PD, Anderson JE, Kalstabakken AW & Masten AS (2015). Technical Report for the Dimensional Change Card Sort–Developmental Extension.
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joëls M, & Krugers H (2008). Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience, 28(23), 6037–6045. 10.1523/JNEUROSCI.0526-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli JJ (2011). Context, cortisol, and executive functions among children experiencing homelessness. Unpublished dissertation. https://conservancy.umn.edu/bitstream/handle/11299/116142/Cutuli_umn_0130E_12249.pdf;sequence=1
- Dajani R, Hadfield K, Uum S, Greff M, & Panter-Brick C (2018). Hair cortisol concentrations in war-affected adolescents: A prospective intervention trial. Psychoneuroendocrinology, 89, 138–146. 10.1016/j.psyneuen.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Del Giudice M (2019). Developmental adaptation to stress: An evolutionary perspective. Annual Review of Psychology, 70, 111–139. 10.1146/annurev-psych-122216-011732 [DOI] [PubMed] [Google Scholar]
- Etwel F, Russell E, Rieder MJ, Van Uum SH, & Koren G (2014). Hair cortisol as a biomarker of stress in the 2011 Libyan war. Clinical and Investigative Medicine, 37(6), E403–E408. [DOI] [PubMed] [Google Scholar]
- Finegood ED, & Blair C (2017). Poverty, parent stress, and emerging executive functions in young children. In: Deater-Deckard K, Panneton R (eds) Parental stress and early child development (pp. 181–207). Springer, Cham. 10.1007/978-3-319-55376-4_8 [DOI] [Google Scholar]
- Golub Y, Kuitunen-Paul S, Panaseth K, Stonawski V, Frey S, Steigleder R, ... & Kornhuber J (2019). Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6–9 year old children. Physiology & Behavior, 112584. 10.1016/j.physbeh.2019.112584 [DOI] [PubMed]
- Gray NA, Dhana A, Van Der Vyver L, Van Wyk J, Khumalo NP, & Stein DJ (2018). Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology, 87, 204–214. 10.1016/j.psyneuen.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Groeneveld MG, Vermeer HJ, Linting M, Noppe G, van Rossum EF, & van IJzendoorn MH (2013). Children’s hair cortisol as a biomarker of stress at school entry. Stress, 16(6), 711–5. 10.3109/10253890.2013.817553 [DOI] [PubMed] [Google Scholar]
- Grunau RE, Cepeda IL, Chau CM, Brummelte S, Weinberg J, Lavoie PM, Ladd M, Hirschfeld AF, Russell E, Koren G, Van Uum S, Brant R, & Turvey SE (2013). Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PloS one, 8(9), e73926. 10.1371/journal.pone.0073926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez D (2006). Stress neurobiology and developmental psychopathology. In Cicchetti D & Cohen DJ (Eds.), Developmental Psychopathology: Developmental Neuroscience (pp. 533–577). Hoboken, NJ, US: John Wiley & Sons Inc. [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Karlén J, Ludvigsson J, Hedmark M, Faresjö Å, Theodorsson E, & Faresjö T (2015). Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics, 135(6), e1450–1457. 10.1542/peds.2014-2561 [DOI] [PubMed] [Google Scholar]
- Little TD, & Rhemtulla M (2013). Planned missing data designs for developmental researchers. Child Development Perspectives, 7(4), 199–204. 10.1177/1049731507305394 [DOI] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, & Schatzberg AF (2009). Developmental cascades linking stress inoculation, arousal regulation, and resilience. Frontiers in Behavioral Neuroscience, 3(32), 1–6. 10.3389/neuro.08.032.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, & Sugawara HM (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1(2), 130–149. 10.1037/1082-989X.1.2.130 [DOI] [Google Scholar]
- Masten AS, Neemann J, & Andenas S (1994). Life events and adjustment in adolescents: The significance of event independence, desirability, and chronicity. Journal of Research on Adolescence, 4, 71–97. [Google Scholar]
- McLennan SN, Ihle A, Steudte-Schmiedgen S, Kirschbaum C, & Kliegel M (2016). Hair cortisol and cognitive performance in working age adults. Psychoneuroendocrinology, 67, 100–103. 10.1016/j.psyneuen.2016.01.029 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2006). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS (2019). What is the confusion with cortisol? Chronic Stress, 3, 247054701983364. 10.1177/2470547019833647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick JH, Labella MH, Narayan AJ, Desjardins CD, Barnes AJ, & Masten AS (2020). The Child Life Challenges Scale: A promising brief measure of cumulative childhood adversity. Children, 7, 33. 10.3390/children7040033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Desai PM, Maskus EA, Melvin SA, Rehman R, Torres SD, Meyer J, He X & Noble KG (2019). Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biological Psychiatry, 86(12), 921–929. 10.1016/j.biopsych.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C, Griskevicius V, Simpson JA, Sung S, & Young ES (2015). Cognitive adaptations to stressful environments: When childhood adversity enhances adult executive function. Journal of Personality and Social Psychology, 109(4), 604–621. 10.1037/pspi0000028 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén [Google Scholar]
- O’Brien KM, Tronick EZ, & Moore CL (2013). Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and Health, 29(4), 337–344. 10.1002/smi.2475 [DOI] [PubMed] [Google Scholar]
- Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Völgyi E, … Tylavsky FA (2013). Early adversity, socioemotional development, and stress in urban 1-year-old children. The Journal of Pediatrics, 163(6), 1733–1739.e1. 10.1016/j.jpeds.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Schloß S, Skoluda N, Nater UM, & Becker K (2019). Low hair cortisol concentration predicts the development of attention deficit hyperactivity disorder. Psychoneuroendocrinology, 104442. 10.1016/j.psyneuen.2019.104442 [DOI] [PubMed]
- Polderman TJ, Posthuma D, De Sonneville LM, Stins JF, Verhulst FC, & Boomsma DI (2007). Genetic analyses of the stability of executive functioning during childhood. Biological Psychology, 76(1–2), 11–20. 10.1016/j.biopsycho.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, & Rypma B (2008). The effects of acute stress on human prefrontal working memory systems. Physiology & Behavior, 95(3), 282–289. 10.1016/j.physbeh.2008.04.027 [DOI] [PubMed] [Google Scholar]
- Prado-Gascó V, de la Barrera U, Sancho-Castillo S, de la Rubia-Ortí JEE, & Montoya-Castilla I (2019). Perceived stress and reference ranges of hair cortisol in healthy adolescents. PloS one, 14(4), e0214856. 10.1371/journal.pone.0214856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Hidalgo V, Almela M, Puig-Perez S, Villada C, & Salvador A (2014). Hair cortisol and cognitive performance in healthy older people. Psychoneuroendocrinology, 44, 100–111. 10.1016/j.psyneuen.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Rich EL, & Romero LM (2005). Exposure to chronic stress downregulates corticosterone responses to acute stressors. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 288(6), R1628–R1636. [DOI] [PubMed] [Google Scholar]
- Romeo RD (2015). Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiology of Stress, 1, 128–133. 10.1016/j.ynstr.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, & Boyce WT (2011). Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society, 17(1), 120–132. 10.1017/S1355617710001335 [DOI] [PubMed] [Google Scholar]
- Satorra A (2000). Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In Heijmans RDH, Pollock DSG, & Satorra A (Eds.), Innovations in multivariate statistical analysis: A Festschrift for Heinz Neudecker (pp. 233–247). Boston, MA: Springer US [Google Scholar]
- Schloß S, Ruhl I, Müller V, Becker K, Skoluda N, Nater U, & Pauli-Pott U (2018). Low hair cortisol concentration and emerging attention-deficit/hyperactivity symptoms in preschool age. Developmental Psychobiology, 60(6), 722–729. 10.1002/dev.21627 [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, & Yonelinas AP (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7(4), 422–445. 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Simmons JG, Badcock PB, Whittle SL, Byrne ML, Mundy L, Patton GC, Olsson CA, & Allen NB (2016). The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology, 63, 276–281. 10.1016/j.psyneuen.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Slopen N, Roberts AL, LeWinn KZ, Bush NR, Rovnaghi CR, Tylavsky F, & Anand KJ (2018). Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology, 98, 168–176. 10.1016/j.psyneuen.2018.08.027 [DOI] [PubMed] [Google Scholar]
- Stalder T, & Kirschbaum C (2012). Analysis of cortisol in hair–State of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, & Kirschbaum C (2012a). Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology, 37(5), 602–610. 10.1016/j.psyneuen.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, & Kirschbaum C (2012b). Cortisol in hair, body mass index and stress-related measures. Biological Psychology, 90(3), 218–223. 10.1016/j.biopsycho.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Strüber N, Strüber D, & Roth G (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neuroscience & Biobehavioral Reviews, 38, 17–37. 10.1016/j.neubiorev.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Tucker LR, & Lewis C (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38(1), 1–10. [Google Scholar]
- Ursache A, Noble KG, & Blair C (2015). Socioeconomic status, subjective social status, and perceived stress: Associations with stress physiology and executive functioning. Behavioral Medicine, 41(3), 145–154. 10.1080/08964289.2015.1024604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, Van Rossum EFC, Koper JW, Raat H, & Van den Akker ELT (2016). Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology, 65, 9–14. 10.1016/j.psyneuen.2015.11.022 [DOI] [PubMed] [Google Scholar]
- Vogel S, Fernández G, Joëls M, & Schwabe L (2016). Cognitive adaptation under stress: A case for the mineralocorticoid receptor. Trends in Cognitive Sciences, 20(3), 192–203. 10.1016/j.tics.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Wagner SL, Cepeda I, Krieger D, Maggi S, D’Angiulli A, Weinberg J, & Grunau RE (2016). Higher cortisol is associated with poorer executive functioning in preschool children: The role of parenting stress, parent coping and quality of daycare. Child Neuropsychology, 22(7), 853–869. 10.1080/09297049.2015.1080232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, … Gershon RC (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3), S54–S64. 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LO, Ising M, von Klitzing K, Sierau S, Michel A, Klein AM, … Stalder T (2017). Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. Journal of Child Psychology and Psychiatry, 58(9), 998–1007. 10.1111/jcpp.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M, & Blair C (2011). Test-retest reliability of a new executive function battery for use in early childhood. Child Neuropsychology, 17(6), 564–579. 10.1080/09297049.2011.554390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


