Abstract
Background:
Cipangopaludina cahayensis contains active fibrinolytic proteins and has been considered a potential anti-cancer agent. However, its anti-cancer characteristics and functions have yet to be elucidated
Objectives:
To study the fibrinolytic activity and anticancer activity of crude protein extracts from Cipangopaludina cahayensis.
Materials and Methods:
Crude proteases were separated and extracted from the Cipangopaludina cahayensis through homogenization, desalting, ammonium sulfate fractionation, dialysis, and ion exchange chromatography. The fibrinolytic activity of extracted proteins was assessed using the fiber plate method. Total protein concentrations of the crude proteases were determined via BCA assay. Molecular weights (MWs) were determined through SDS-PAGE electrophoresis.
Results:
The crude extract had a MW of ~ 50 kDa, and the highest protein concentration was 3.026 mg.mL-1. The optimum pH for fibrinolytic activity was 7.0. Cell culture assays demonstrated that the addition of the crude enzyme extracts to the human ovary cancer cell line Ovcar-3 resulted in significant growth defects.
Conclusions:
Our data showed that crude proteins purified from Cipangopaludina cahayensis are novel fibrinolytic proteases and have potential anti-cancer propertie
Keywords: Cipangopaludina cahayensis, Fibrinolytic activity, Isolation, Purification, Tumor suppression
1. Background
Both cancer and cardiovascular diseases are a serious threat to human health, and their incidence continues to rise. Despite advances in cancer therapeutics, the vast majority of cancer related deaths arise from cancer metastasis or recurrence. Tumor growth and metastasis are closely related to the formation of new blood vessels and changes in the function of fibrinolytic system ( 1, 2). The accumulation of clots reduces blood flow leading to the development of thrombosis related cardiovascular disease ( 3, 4). Current anticancer therapeutics include alkylating agents, antimetabolites, antitumor antibiotics, plant antitumor drugs, hormones, metal platinum, auxiliary antitumor drugs, immunomodulators and mTOR inhibitors ( 5, 6, 7). Anti-clotting therapies include anti-platelet drugs, anticoagulants, and thrombolytic agents ( 3, 4). Fibrinolytic enzymes and their activators display anti-thrombotic effects including the inhibition of neovascularization and the prevention of cancer cell metastasis.
As a traditional Chinese medical material, Cipangopaludina cahayensis is distributed across various provinces and regions in the South of China and North America. Its meat is of nutritional value due to its high protein and low fat content, and contains phosphorus, calcium, iron, vitamin B, thiamine, and glucose ( 8).
2. Objectives
So far, only few studies have focused on Cipangopaludina cahayensis. Given that Cipangopaludina cahayensis contains active fibrinolytic proteins, it has been considered a potential anti-cancer agent. In this study, we assessed the fibrinolytic activity, physicochemical properties, and anti-cancer activity of crude protein extracts that were derived from Cipangopaludina cahayensis.
3. Materials and Methods
3.1. Instruments and Reagents
Bovine fibrinogen was obtained from Beijing Solarbio Science & Technology Co., Ltd. Urokinase and thrombin were obtained from the China National Institute for the Control of Pharmaceutical and Biological Products. Protein columns (10.0 mm × 25 cm) were obtained from GE, USA. BCA kits were purchased from Beijing ComWin Biotech Co., Ltd. RPMI 1640 media was purchased from Beijing Solarbio Science & Technology Co., Ltd. Newborn bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
3.2. Materials
Cipangopaludina cahayensis was purchased from the Nanchang Aquatic Products Wholesale Market. The human ovarian cancer cell line OVCAR-3 was obtained from the laboratory of Xiong Xiangyuan.
3.3. Preparation of Fibrinolytic Protein
Cipangopaludina cahayensis was homogenized in a high-speed tissue masher with leaching at 4 °C for 24 h in PBS. Extracts were obtained by centrifuging at 10,000 rpm for 15 min at 4 °C. Precipitates were discarded and supernatants were collected. Crude extracts were subjected to fractional desalting through ammonium sulfate saturation ( 9). Proteins were collected in a minimum unit of 60%. Precipitates of each desalting condition were dissolved in Tris-HCl and stored at 4 °C. Samples were dialyzed, desalted, and concentrated with polyethylene glycol 10000. The fibrinolytic activity of purified proteins was detected using the fibrin plate method as previously described ( 10). Urokinase was included as a positive control. Sterile Tris-HCl was used as a negative control. Prior to staining, plates were incubated at 37 °C for 24 h.
A gradient elution solution was prepared using DEAE-32 cellulose as the chromatographic medium in 0.2, 0.4, 0.6 and 0.8 moL.L-1 NaCl (pH 7.4) ( 11). Following chromatography, protein absorption peaks were imaged and the fibrinolytic activity of each peak was measured and plotted.
3.4. Molecular Weight Determination
Separation gel (12%, 0.001% bovine fiber), concentrated gel (5%) and 5 x loading buffer were prepared. Samples were electrophoresed at 80 V for 30 min, and 120 V for 1 h ( 12, 13). Gels were washed with tap water, rinsed with distilled water, and treated with washing buffer (50 mM Tris, pH 8.0; 2.5% Triton X-100) for 60 min. Gels were then washed and immersed in reaction buffer (50 mM PBS, pH 7.2; 0.15 M NaCI) at 37 °C for 18 hours. Gels were stained with Coomassie blue, destained until protein bands were visible, and imaged.
3.5. Determination of Total Pprotein Content
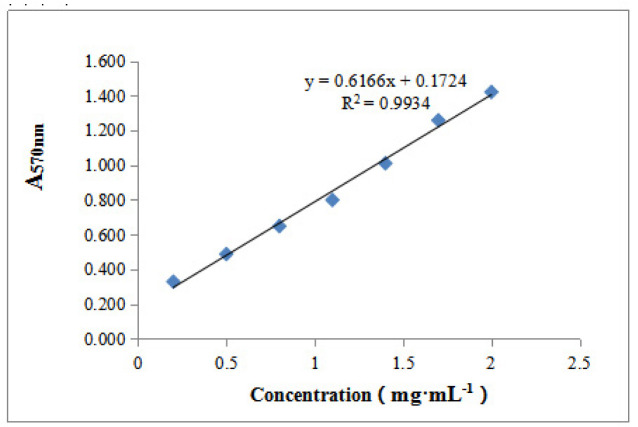
Bicinchoninic acid (BCA) assays were used to determine total protein content ( 14). Standard curves were produced using BCA standards (2, 1.7, 1.4, 1.1, 0.8, 0.5, and 0.2 μg·μL-1). Absorbances were read at 570 nm. Crude protein concentrations were determined from the standard curves.
3.6. Effects of pH on the Activity of Crude Cipangopaludina cahayensis Extracts
Samples were dissolved in an equal volume of sample solution at a range of pH conditions. pH 6-8 samples were prepared with phosphate buffers, pH 8-8.5 buffers were prepared with Tris-HCl, and pH 9-10 buffers were prepared through NaOH addition to physiological saline. Buffers were allowed to stand at 4 °C for 30 min. Enzyme activity was measured using the fibrin plate method.
3.7. OVCAR-3 Cell Viability Assays
OVCAR-3 cells were passaged in the logarithmic growth phase and plated into 96 well plates at a density of 6.5×104 cells. mL-1. Cells were treated with the target protein or PBS (controls) in serum-free medium for 24 h. For cell viability assessments, 20 µL of MTT solution (5 mg·mL-1) was added for 4 h in the dark. Supernatants were discarded and 150 μL of DMSO was added to each well to dissolve the formazan crystals. Absorbances were read at 490 nm on a microplate reader.
Cell inhibition rates were calculated using the following formula
4. Results
4.1. Fibrinolytic Activity
Fibrin plate analyses were performed to assess the fibrinolytic activity of crude protein extracts (Fig.1). The assay employs colorimetric qualitative methods to assess fibrin gel degradation. Fibrin degradation halos were evident after 20 h of urokinase or crude extract treatment (0-60, 60-80, and 80-100). Halos were absent in 0.05 M Tris-HCl treated controls. Positive controls (Fig.1, NO. 4) and samples with different concentration gradients (Fig.1, NO.1 and 6 60% CFP, 2 and 7 80% CFP, 3 and 8 100% CFP, ) showed fiber activity except for the negative control (Fig.1, NO. 5). This confirmed the fibrolytic activity of Cipangopaludina cahayensis extracts.
Figure 1.

Cipangopaludina cahayensis fibrinolytic protease assessments. A total of 8 labeled halos are shown representing the crude fibrolytic proteins obtained through desalting in different concentrations of ammonium sulfate: NO.1 and 6 60% CFP, 2 and 7 80% CFP, 3 and 8 100% CFP, 4 80U·mL-1 UK (positive control), 5 0.05M Tris-HCl (negative control).
4.2. Ion Exchange Chromatography, MW Assessment, and Optimal pH of Fibrolytic Activity of the Cipangopaludina cahayensis Extracts
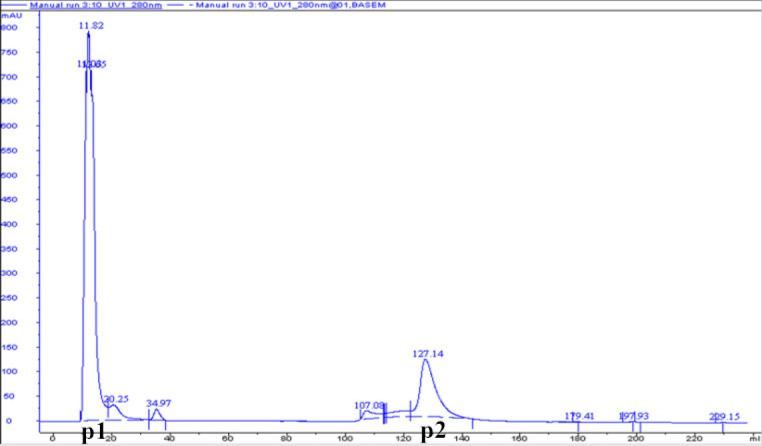
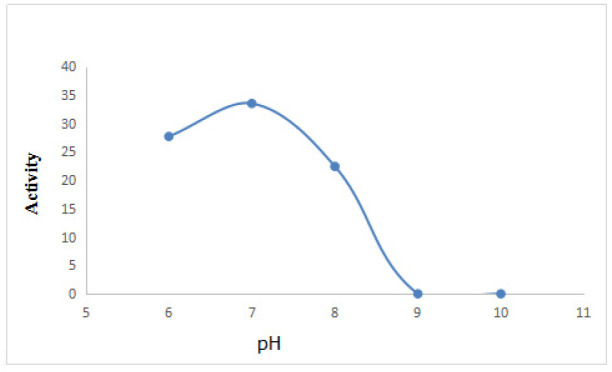
Figure. 2 shows that DEAE-32 cellulose ion exchange chromatography of Cipangopaludina cahayensis extracts led to two distinct peaks at 280 nm. This suggested the presence of two protein species (p1 and p2). SDS-PAGE electrophoresis (Fig. 3) revealed that p1 and p2 were ~50 kDa in size. The crude extracts were 3.026 mg·mL-1 as assessed via BCA assays ( Table 1, Fig. 4). The optimum pH of the fibrolytic activity of the extracts was 7.0. Fibrolytic activity was inhibited at higher pH conditions, and completely inactivated at pH 9.0 (Fig. 5).
Figure 2.
Chromatographic separation maps of CFP. Samples were injected into DEAE-32 cellulose columns connected to the AKTA protein purification system. Each peak represents possible fibrinolytic proteins.
Figure 3.

SDS-PAGE electrophoresis. Following ion exchange chromatography, the molecular mass of the fibrinolytic proteins were determined via SDS-PAGE (12%).
Table 1.
Protein concentration determination.
| Absorb | Average | Tough Concentration (mg·mL-1) | Salting out gradient | ||
|---|---|---|---|---|---|
| 2.316 | 2.220 | 2.060 | 2.199 | 1.513 | 100% |
| 0.026 | 0.024 | 0.025 | 0.025 | blank | |
Figure 4.
Protein concentrations and standard curves.
Figure 5.
Effects of PH on fibrinolytic activity. Five pH conditions were assessed (6.0, 7.0, 8.0, 9.0, and 10.0). Optimal activity was observed at pH 7.0.
4.3. Cancer Cell Growth Inhibition Assays
Crude proteins from the Cipangopaludina cahayensis extracts had a significant inhibitory effect on OVCAR-3 cell growth. The inhibition of cell growth increased with increasing protein concentrations. At a concentration of 500 mg.mL-1, the inhibitory rates were as high as 103.66%, indicating high fibrinolysis activity ( Table 2) .
Table 2.
Cancer cell proliferation inhibition assays.
| 100% protein | Blank | |||||
|---|---|---|---|---|---|---|
| Protein concentration | 1μg·mL-1 | 25μg·mL-1 | 125μg·mL-1 | 250μg·mL-1 | 500μg·mL-1 | |
| Absorb | 0.87 | 0.78 | 0.749 | 0.761 | 0.154 | 0.788 |
| 0.881 | 0.881 | 0.783 | 0.762 | 0.186 | 0.823 | |
| 0.841 | 0.898 | 0.823 | 0.808 | 0.197 | 0.765 | |
| Average | 0.864 | 0.853 | 0.785 | 0.777 | 0.179 | 0.792 |
| Survival rate(%) | 109.09 | 107.70 | 99.12 | 98.11 | 22.60 | - |
| Inhibition rate(%) | 17.17 | 18.56 | 27.15 | 28.17 | 103.66 | - |
5. Discussion
The use of traditional Chinese medicines for the treatment of human disease continues to attract intense research interest. However, the complex composition of traditional Chinese medicine and the lack of clarity of the major active ingredients continues to restrict its development. Traditional Chinese medicinal materials including Cipangopaludina cahayensis, are not only nutritious, but display favorable pharmacodynamic effects, including anti-cancer activity. However, the anti-cancer mechanisms of these compounds remain poorly understood.
In this study, we combined traditional Chinese medicine with modern biotechnology, and extracted and purified the fibrinolytic proteins of Cipangopaludina cahayensis, which inhibited the proliferation of OVCAR-3 cells in vitro. At the cellular level, systematic and in-depth molecular studies are required to characterize the anti-cancer mechanisms of these fibrinolytic proteins. This will enhance our understanding of Cipangopaludina cahayensis and provide important impetus for the development and utilization of traditional Chinese medicinal materials. SDS-PAGE revealed that the MW of the fibrinolytic protein was 50 kDa, which was different from that of other natural sources of fibrinolytic enzymes, such as the fibrinolytic enzyme from Porcellio scaber Latreille with a MW of 38.5 kDa( 15), the MW of fibrinolytic enzyme from Pheretima posthumousis is 29.5 kDa( 16), the MW of the fibrinolytic enzyme from Whitmania pigra Whitman is 26.7 kDa( 17), and the molecular weight of fibrinolytic enzyme from Lumbrineris nipponica( 18) is 28 kDa. This indicates that the fibrinolytic enzyme is a novel fibrinolytic enzyme. Moreover, the results showed that the optimum pH of the fibrinolytic protein of Cipangopaludina cahayensis is 7.0, as was pH of the medicinal animal Porcellio scaber Latreille( 15), but it was lower than that of Pheretima posthumous( 16) and Whitmania pigra Whitman ( 17), which had an optimum pH of 8.0 and 8.5 respectively. Thus, these findings indicated that the fibrinolytic enzyme that was extracted from Cipangopaludina cahayensis was closer to the pH of humans.
Fibrinolytic enzymes and their active products degrade the tumor extracellular matrix, leading to a loss of tumor cell attachment, survival, metastasis, and invasion ( 19, 20). Thus, the discovery of fibrinolytic proteases in Cipangopaludina cahayensis highlights its potential as a thrombolytic or anti-cancer therapy. Fibrinolytic proteins have similarly been extracted from Pheretima posthumous ( 16), Bothrops colombiesis ( 21), Neanthes japonica ( 22), and animal fibers. Drugs in which these active proteins are the major ingredient have been developed and are currently available for clinical use, including Dilong thrombolytic capsules.
In this study, proteins were obtained from crude extracts, but were not purified. Contaminants could therefore influence the reproducibly of the experimental data, but our findings were consistent across individual experimental replicates. In subsequent experiments, fibrolytic enzymes could be purified and isolated by affinity chromatography ( 18), molecular exclusion chromatography ( 23), or reverse phase chromatography ( 24). This would permit a more in-depth analysis of the anti-cancer activity of individual proteases.
To-date, crude extracts of fibrinolytic proteins have been isolated from Cipangopaludina cahayensis and have been shown to inhibit the proliferation of the human lung cancer cell line A549 ( 25). This provided a theoretical basis to further study these fibrinolytic enzymes. In this study, we further characterized the fibrolytic activity of Cipangopaludina cahayensis extracts and highlighted their thrombolytic and anti-cancer potential.
6. Conclusions
In this study, proteins were isolated from Cipangopaludina cahayensis through desalting in ammonium sulfate. The fibrinolytic activity of the purified proteins was determined through fibrin plate analysis. According to these analyses, 60%, 80%, and 100% of the proteins extracted from Cipangopaludina cahayensis displayed fibrinolytic activity, of which the highest activity occurred in the most concentrated sample and at an optimum pH of 7.0. The protein concentration was 3.026 mg.mL-1. Ion exchange chromatography showed that the Cipangopaludina cahayensis extracts contained p1 and p2 proteins. SDS-PAGE electrophoresis showed that these two major proteins had a molecular weight of ~ 50 kDa. MTT assays revealed that high concentrations of the active fibrinolytic proteins from Cipangopaludina cahayensis displayed significant inhibitory effects on OVCAR-3 cells.
Acknowledgement
We thank Dr. Xiangyuan Xiong (School of life Science, Jiangxi Science & Technology Normal University) for the generous gift of OVCAR-3 cells.
Conflict of Interest: None.
Funding/support: This study was supported by the Science and Technology project of Jiangxi Province Education Department (Jiangxi, China) (No. GJJ170676), the Ph.D. Start-up Fund of Natural Science Foundation of Jiangxi Science & Technology Normal University (Jiangxi, China) (No. 3000990122), the Jiangxi Province's bulk freshwater fish industry technology system, the Aquatic Product Safety and Control Engineering Research Center Development Fund Project of Jiangxi Provincial(Jiangxi, China) (No. KFGJ18021), the Undergraduate National Research Training Program of Jiangxi Science & Technology Normal University (Jiangxi, China) (No. 202011318005).
References
- 1.Falanga A, Schieppati F, Russo D. Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Semin Thromb Hemost. 2015;41(7):756–764. doi: 10.1055/s-0035-1564040. [DOI] [PubMed] [Google Scholar]
- 2.Hisano Y, Hla T. Bioactive lysolipids in cancer and angiogenesis. Pharmacol Ther. 2019;193:91–98. doi: 10.1016/j.pharmthera.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu TM, Yang FY, Zhu HM, Zhu HX, Guo LW. Rapid extraction and purification of lumbrokinase from Lumbricus rubellus using a hollow fiber membrane and size exclusion chromatography. Biotechnol Lett. 2016;38:251–258. doi: 10.1007/s10529-015-1979-x. [DOI] [PubMed] [Google Scholar]
- 4.Sun ZB, Liu PP, Cheng GY, Zhang BY, Dong WL, Sun XL, et al. A fibrinolytic protease Afe E from Streptomyces sp. CC5, with potent thrombolytic activity in a mouse model. Int J Biol Macromol. 2016;85:346–354. doi: 10.1016/j.ijbiomac.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Parveen A, Subedi L, Kim HW, Khan Z, Zahra Z, Farooqi MQ, et al. Phytochemicals Targeting VEGF and VEGF-Related Multifactors as Anticancer Therapy. J Clin Med. 2019;8(3):350. doi: 10.3390/jcm8030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian T, Li XY, Zhang JH. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;(20):1–34. doi: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffat JG, Rudolph J, Bailey D. Phenotypic screening in cancer drug discovery- past, present and future. Nat Rev Drug Discov. 2014;13(8):588–602. doi: 10.1038/nrd4366. [DOI] [PubMed] [Google Scholar]
- 8.Xiong QP, Jiao YP, Zhao XR, Chen XM, Zhang QH, Jiang CX. Purification,characterization and immunostimulatory activity of polysaccharide grom Cipangopaludina chinensis. Carbonhyd Polym. 2013;98(1):217–223. doi: 10.1039/c7fo01821e. [DOI] [PubMed] [Google Scholar]
- 9.Deng ZH, Wang SH, Li Q, Ji X, Zhang LZ, Hong M. Purification and characterization of a novel fibrinolytic enzyme from the polychaete,Neanthes japonica (Iznka) Bioresour Technol. 2010;101:1954–1960. doi: 10.1016/j.biortech.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Astrup T, Müllertz S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952;40:346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Han YL, Wu YL, Tan ZJ, Sun JP, Dai CY. Purification and Characterization of a Fibrinolytic Protein from Mylabris. Lishizhen Med and Mat Med Res. 2012;23(3):550–552. doi: 10.3969/j.issn.1008-0805.2012.03.016. [DOI] [Google Scholar]
- 12.Kim DW, Choi JH, Park SE, Kim S, Sapkota K, Kim SJ. Purification and characterization of a fibrinolytic enzyme from Petasites japonicus. Int J Biol Macromol. 2015;72:1159–1167. doi: 10.1016/j.ijbiomac.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Tian Z, Li B, Guo LW, Wu MH, Fu TM, Cheng HB, et al. Purification and biochemical characterization of a novel fibrinolytic enzyme, PSLTro01, from a medicinal animal Porcellio scaber Latreille. Int J Bio Macromol. 2015;80:536–546. doi: 10.1016/j.ijbiomac.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Verma MK, Pulicherla KK. Broad substrate affinity and catalytic diversity of fibrinolytic enzyme from Pheretima posthumous-purification and molecular characterization study. Int J Bio Macromol. 2016;95:1011–1021. doi: 10.1016/j.ijbiomac.2016.10.090. [DOI] [PubMed] [Google Scholar]
- 17.Chu FL, Wang XC, Sun QQ, Liang H, Wang SJ, An DK, et al. Purification and characterization of a novel fibrinolytic enzyme from Whitmania pigra Whitman. Clin Exp Hypertens. 2016;38(7):594–601. doi: 10.3109/10641963.2016.1174254. [DOI] [PubMed] [Google Scholar]
- 18.Yeon SJ, Chung GY, Hong JS, Hwang JH, Shin HS. Purification of serine protease from polychaeta, Lumbrineris nipponica, and assessment of its fibrinolytic activity. Int Vitro Cell Dev-An. 2017;53:494–501. doi: 10.1007/s11626-017-0137-2. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed HM, David LM, Mohammad HP. Urokinase plasminogen activator system as a potential target for cancer therapy . Future Oncol. 2016;5(9):1487–1499. doi: 10.2217/fon.09.108. [DOI] [PubMed] [Google Scholar]
- 20.Kirtane AR, Sadhukha T, Kim H, Khanna V, Koniar B, Panyam J. Fibrinolytic enzyme co-therapy improves tumor perfusion and therapeutic efficacy of anticancer nanomedicine. Cancer Res. 2017;77(6):1–11. doi: 10.1158/0008-5472.CAN-16-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girón ME, Guerrero B, Salazar AM, Sanchez EE, Alvarez M, Rodriguez-Acosta A. Functional characterization of fibrinolytic metalloproteinases (colombienases) isolated from Bothrops colombiesis venom. Toxicon. 2013;74(3):116–126. doi: 10.1016/j.toxicon.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Wang SH, Deng ZH, Li Q, Ge X, Bo QQ, Liu JK, et al. A novel alkaline serine protease with fibrinolytic activity from the polychaete, Neanthes japonica. Comp Biochem Phys B. 2011;159:18–25. doi: 10.1016/j.cbpb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento TP, Sales AE, Porto TS, Costa RMPB, Breydo L, Uversky VN, et al. Purification, biochemical, and structural characterization of a novel fibrinolytic enzyme from Mucor subtilissimus UCP 1262. Bioprocess Biosyst Eng. 2017;40:1209–1219. doi: 10.1007/s00449-017-1781-3. [DOI] [PubMed] [Google Scholar]
- 24.Vivas J, Ibarra C, Salazar AM, Neves-Ferreira AGC, Sanchez EE, Perales J, et al. Purification and characterization of tenerplasminin-1, a serine peptidase inhibitor with antiplasmin activity from the coral snake (Micrurus tener tener) venom. Comp Biochem Physiol C Toxicol Pharmacol. 2016;1(179):107–115. doi: 10.1016/j.cbpc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Wang B, Zhu H, Li YP, Liu JT. Bacteriostatic effect of antibacterial peptide from cipangopaludina cathayensis and the determination of physical and chemical properties and tumor-suppressor activity. Food Res and Develo. 2013;34(20):5–8. doi: 10.3969/j.issn.1005-6521. [DOI] [Google Scholar]