Abstract
Background:
Abiotic environmental stresses, especially drought stress, is one of the most important problems in arid and semi-arid regions. Like other major crops, Brassica napus is vulnerable to drought stress.
Objective:
The present study was conducted to evaluate efficacy of Sargassum angustifolium extract on mitigating adverse effects of drought stress on B. napus seedlings during vegetative growth under greenhouse conditions.
Materials and Methods:
Seedlings were periodically sprayed with the seaweed extract until they reached 7-leaf stage. Then water deficit stress was imposed and measurements were performed at morphological, biochemical and molecular levels on three phases: 80% field capacity for 20 days (Phase I), 60% field capacity for 20 days (Phase II) and 40% field capacity for 20 days (Phase III). Real-Time PCR assay was carried out to monitor the changes in expression of the genes involved in proline biosynthesis.
Results:
Morphological measurements revealed that seaweed treatment improved shoot height and dry weight compared to control (p<0.05). Biochemical analyses indicated that foliar application of seaweed extract significantly enhanced the photosynthetic pigments’ content, free radical scavenging and superoxide dismutase activity (p<0.05). Moreover, proline content was significantly increased in plant tissues treated with seaweed extract (p<0.05). The results of Real-Time PCR assay showed that the increase in proline content is due to enhanced expression of P5CS which is involved in biosynthesis of proline, and to decreased expression of PRODH which catalyzes proline degradation.
Conclusions:
Overall, the results obtained in this research suggest that application of S. angustifolium extract as a biostimulant is able to protect canola seedlings against deteriorating effects of drought stress.
Keywords: Drought Stress, Proline, Sargassum Angustifolium, Seaweed Extract
1. Background
Existence of abiotic environmental stresses, especially drought stress, is one of the most important problems in arid and semi-arid regions of the world ( 1 ). Stress is the presence of an organism under the influence of the severity of an environmental factor that causes loss of appearance, efficiency or value ( 2 ). Drought stress occurs when plant receives less water than its losses ( 3 ). Drought is one of the most important environmental stresses that negatively affects the crops and hence has major adverse effects on agricultural production ( 4 , 5 ). The drought stress is regarded as the most important and common stress in the environment, causing huge damage to crop products in the world ( 6 ). In most parts of the world, the drought stress that results from the shortage of rainfall, especially in those stages where the water requirement of plants and its evapotranspiration potential is increased, negatively affects sensitive phases of crop growth, even in irrigated areas ( 7 ). When growth is restricted, it’s difficult to achieve high performance. According to the estimates, about 40% of the earth’s land is located in semi-arid regions which shows the importance of drought and calls for remedies to protect the crop against this stress ( 8 ).
Canola (Brassica napus L.) is a major crop widely cultivated as a source for production of edible oil. According to FAO reports, canola cultivation area had increased to 68.9 million metric tons, and the harvested area had expanded to 33.7 million hectares ( 9 ). Like other major crops, B. napus is vulnerable to environmental stress combinations, such as heat and drought. Plants are equipped with a polygenic defense mechanism against drought and other stresses composing of interrelated dynamic processes ( 10 ). Plant adaptation to drought stress is accompanied by expression of stress-specific genes, accumulation of metabolites, extension of root network and reduced leaf area ( 11 - 14 ). Application of plant biostimulants has been proved to be an efficient and cost-effective approach for improvement of drought tolerance among crops. This approach has gained much attention in both scientific societies and marketplace ( 15 , 16 ).
Seaweed extracts represent a major category of products accepted as a biostimulants. Extracts of brown seaweeds are increasingly used in agri-horticultural crop productions ( 14 , 17 , 18 ). Soil or foliar application of seaweed extract has been shown to enhance chlorophyll content ( 19 ), water retention capacity and generally ameliorating biotic and abiotic stresses ( 20 , 21 ). Positive effects of the extract of Sargassum species on plant tolerance against stresses has been reported by a number of researchers ( 22 - 24 ). Although positive and considerable effect of seaweed extracts in improving crops’ tolerance to both biotic and abiotic stresses has been reported in many studies ( 25 - 27 ), the mode of action of seaweed extract in improving stress tolerance is not fully understood and various genes and metabolite pathways have been proposed as possible mechanisms justifying this effect ( 15 , 28 ). To this end, the present study was conducted to evaluate the impact of S. angustifolium extract on drought tolerance of canola (B. napus) and to unravel the physiological, biochemical and molecular mechanisms involved in this process. The novelty of this research lies in the fact that together with investigating morphological and physiological changes caused by seaweed extract treatment, molecular basis of these changes, at least for proline which is a prominent agent in endowing drought tolerance, is also studied.
2. Objective
The present study was conducted to evaluate efficacy of Sargassum angustifolium extract on mitigating adverse effects of drought stress on Brassica napus seedlings during vegetative growth under greenhouse conditions.
3. Materials and Methods
3.1. Algae Source
The brown algae S. angustifolium was collected from Chabahar shores and extraction procedure was followed by the method developed before ( 29).
3.2. Plant Cultivation and Induction of Drought Stress
Canola seeds were sown under controlled conditions. When canola seedlings reached 3-leaf stage, control group was sprayed with distilled deionized water while the experimental groups were sprayed with 7.0 mL of 1:1000 (w/v) concentration of the algae extract. When the seedlings reached 7-leaf stage (70 days after spraying), water stress was started. Measurements were made in three phases: 80% field capacity for 20 days (Phase I), 60% field capacity for 20 days (Phase II) and 40% field capacity for 20 days (Phase III).
3.3. Plant Height and Dry Weight
Plant height was measured in cm in all phases of the experiment. The canola seedlings were collected and transferred to the laboratory. Their fresh weight was carefully weighed. For measurement of dry weight, above-ground parts of the canola seedlings were dried at 80 °C until constant dry weight was obtained.
3.4. Photosynthetic Pigments
The amount of photosynthetic pigments of leaves including chlorophyll a, b, total chlorophyll and carotenoids was measured using Lichthen Thaler 1987 method ( 30).
3.5. Antioxidative Capacity
Free radical scavenging activity was measured according to the method proposed by Shukla et al. ( 28).
3.6. Proline Measurement
The proline concentration in plant tissues was determined according to the method of Bates et al. ( 31).
3.7. Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was assayed according to Mansori et al. ( 32).
3.8. Real- Time PCR analysis
Expression of two genes involved in proline metabolism viz. P5CS (delta-1-pyrroline-5-carboxylate synthetase A), PRODH (proline dehydrogenase) was evaluated in transcription level by Real-Time PCR assay and Elongation factor 1-alpha was applied as housekeeping gene (Table 1). Quantitative Real-Time PCR experiments were performed in duplicate for each sample the relative-fold expression was determined using 2-DDCT method as described by Livak and Schmittgen ( 33). Elongation factor 1-alpha gene was used as a housekeeping gene.
Table1.
Primer details of proline biosynthesis and degradation genes (P5CS and PRODH, respectively) and a housekeeping gene (Elongation factor 1-alpha) used in this study.
| Gene | Primer* | Tm (°C) |
|---|---|---|
| Elongation factor 1-alpha | F: 5′ ACAAAATCCCATTCGTCCCCATC 3′ | 55.2 |
| R: 5′ ACTGGCACCGTTCCAATACCAC 3′ | 57.2 | |
| P5CS | F: 5′ GCTACAGCACAAGAAGCTGGAC 3′ | 56 |
| R: 5′ TCCAAAACAAGACCATCTGCCAC 3′ | 55.6 | |
| PRODH | F: 5′ CTGAAGACACAATCCTCCAACCC 3′ | 55.2 |
| R: 5′ CACCTCTCACCAACTTAAACCCC 3′ | 55.1 |
F: forward, R: reverse.
3.9. Statistical Analysis
The research was conducted as a factorial experiment in the form of completely randomized design (three levels of drought and two levels of seaweed extract with three replications). Duncan test was used for mean comparison. Statistical analyses were performed at 95% confidence interval (p<0.05). All of the statistical analyses were performed with SPSS software version 16.0. REST 2009 V2.0.13 software was used for Real-Time PCR analysis.
4. Results
4.1. Morphological Traits
The results of measurement of plant height and dry weight are presented in Table 2. According to the results, application of algae extract (AE) mitigated the negative impact of drought stress on plant height. The same results were observed for dry weight in all three phases which was greater in AE-treated plants compared to control (p<0.05).
Table2.
Effect of algae extract (AE) treatment on morphological traits (plant height and dry weight) of canola seedling: 80% field capacity for 20 days (Phase I), 60% field capacity for 20 days (Phase II) and 40% field capacity for 20 days (Phase III).
| Traits | Plant height | Dry weight | ||||
|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | Phase I | Phase II | Phase III | |
| Control | 49.01±1.32c | 47.56±2.22d | 44.33±1.07e | 12.98±1.76c | 12.45±0.34cd | 11.21±1.16d |
| AE | 61.64±1.19a | 59.48±1.13a | 56.22±2.18b | 20.27±1.81a | 19.06±1.45a | 16.13±1.28b |
4.2. Photosynthetic Pigments
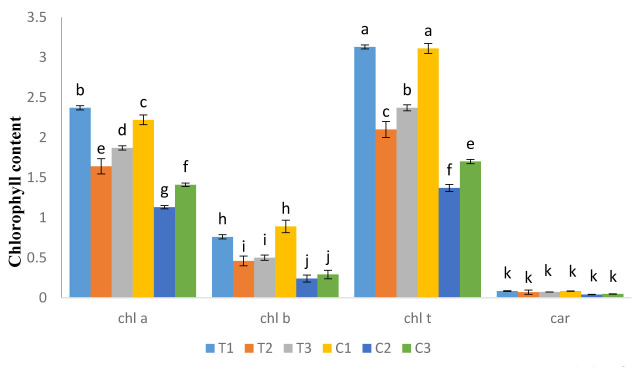
Figure 1 represents the impact of AE on photosynthetic pigment contents of canola seedlings. According to the results, the highest content of chlorophyll a was observed in AE-treated plants and control group during Phase I. Chlorophyll a content during drought stress (in both Phase II and III) was significantly higher in AE-treated plants compared to control group (p<0.05).
Figure 1.
Increase in photosynthetic pigments by AE treatment during three phases of drought stress. (T1, T2 and T3: AE treatment; C1, C2 and C3: control condition during Phase I, II, and III; respectively. chl a: chlorophyll a; chl b: chlorophyll b: chl t: total chlorophyll, car: carotenoid).
4.3. Free Radical Scavenging
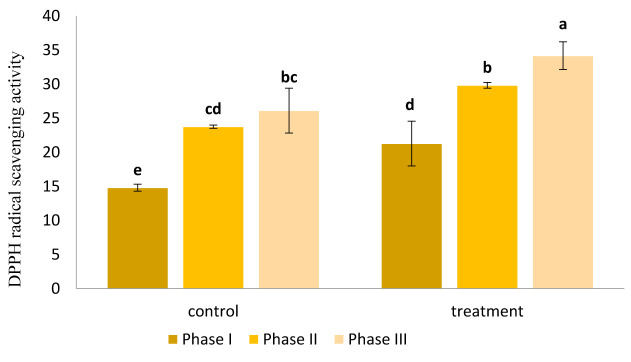
Free radical scavenging activity of AE-treated and control seedlings was investigated by common DPPH assay and the results are presented in Figure 2. Accordingly, AE treatment significantly improved reactive oxygen species (ROS) scavenging capacity of the canola plants compared to control group (p<0.05). An ascending trend was found in ROS scavenging activity of the plants through different phases of the experiment.
Figure 2.
Impact of AE on ROS scavenging activity of canola seedlings during three phases of drought tolerance. Free radical scavenging activity followed an ascending trend in both control and treatment groups. The values represented in the graph are the mean of three replications.
4.4. SOD Activity
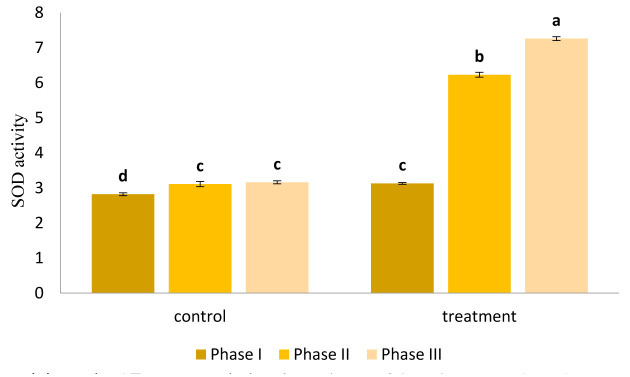
Activity of SOD as an antioxidant mechanism during occurrence of environmental stress was measured and the results are presented in Figure 3. Increased SOD activity was observed in both control and AE treatment groups, however, the increase in SOD activity was significantly higher in AE-treated group compared to control (p<0.05).
Figure 3.
Variation in SOD activity under AE treatment during three phases of drought stress. The values represented in the graph are the mean of three replications.
4.5. Proline Measurement
Proline content was measured during Phases I, II and III and the results are presented in Figure 4. Comparison of AE treatment and control groups revealed that seaweed treatment significantly increased proline content in canola seedlings (p<0.05) as proline content of AE-treated plants even during Phase II was higher than that observed during Phase III of control group (Fig. 4).
Figure 4.
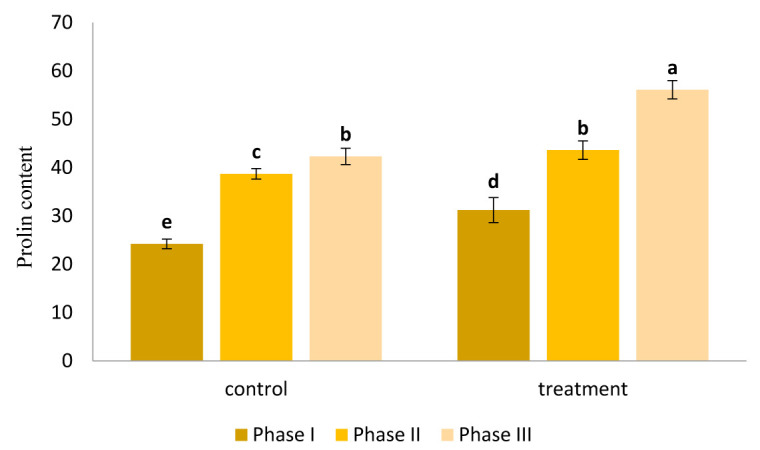
Variation in proline content of canola plants affected by water deficiency. AE treatment significantly improved proline content of the plants compared to control (p<0.05)
4.6. Proline Biosynthesis-related Gene Expression
Figure 5 shows the results of Real-Time PCR assay as fold change in gene expression. According to the result, expression of P5CS gene was enhanced by induction of drought stress in both AE and control groups. In contrast, PD expression was decreased through stress period. The main finding of Real-Time PCR assay was that P5CS expression was significantly higher in AE-treated seedlings than in control plants (p<0.05), whereas PD expression was significantly lower in AE plants than in control plants (p<0.05).
Figure 5.
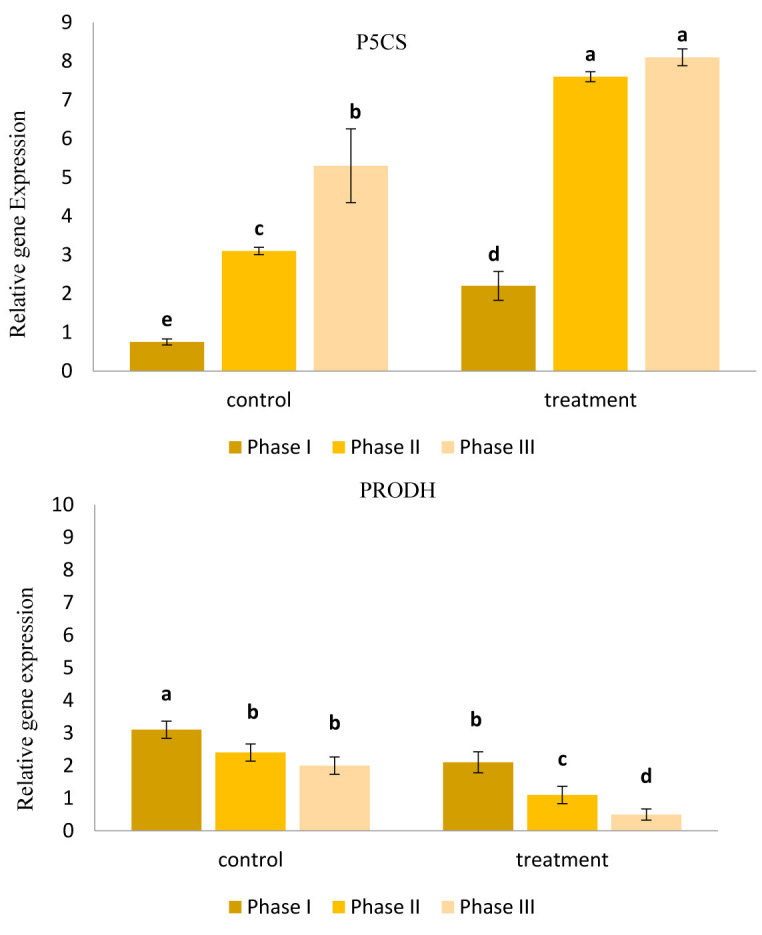
The results of Real-Time PCR assay on expression of genes including P5CS (top) and PRODH (bottom) involved in biosynthesis of proline.Conclusion
5. Discussion
The results obtained from this experiment revealed that foliar spray of AE significantly improved both shoot dry weight and seedling height compared to control group. This positive impact is consistent with those reported for application of seaweed on other crops ( 16 , 31 , 34 , 35 ). The promoting impact of seaweed on growth parameters might be attributed to the presence of auxin ( 32 , 36 ), cytokinin ( 10 ) and other growth promoting agents ( 28 ) as well as both micro- and macronutrients ( 37 ) in algae extract. Moreover, bioactive compounds contained in seaweed extract may exhibit synergic effect on plant growth ( 15 ). The results obtained in this research also revealed positive effect of AE on photosynthetic pigments. Promoting photosynthesis machinery may justify enhanced shoot dry weight and plant growth as seen in this research. The positive effect of AE on photosynthetic pigments has been reported by other authors as well ( 19 , 38 ). It has been speculated that promoting effect of seaweed extract on photosynthetic pigments is primarily due to reduction of chlorophyll degradation during water shortage ( 39 ). Betaines is a notable biological agent in seaweed extract that enhances chlorophyll content of plants ( 40 ), thus improved chlorophyll content of canola plants as treated with AE may be due to betaines content of the seaweed extract.
Due to their ability for ROS scavenging, seaweed extracts have gained much attention as novel source of natural antioxidants ( 41 ). The results obtained in this study indicated that application of AE promoted ROS scavenging capacity (1.31 fold compared to contorl) of canola seedling so that the highest rate of ROS scavenging was obtained on Phase III of the experiment in the seedlings treated with AE. This positive effect of AE on ROS scavenging capacity has been reported in other studies ( 42 , 43). It was shown here that SOD activity was enhanced by foliar application of S. angustifolium on canola plants. SOD catalyzes the dismutation of the superoxide radical into either ordinary molecular oxygen or hydrogen peroxide. Uncontrolled formation of superoxide is responsible for many types of cell damages during abiotic stresses ( 2 ), therefore, activity of SOD is a major mechanism to attenuate adverse effects of drought stress on crops’ growth and yield ( 44 ). Increased SOD activity of AE-treated plants has been reported by many studies ( 26 , 32, 45). Aaccording to the results, it can be concluded that increased activity of SOD (82 % compared to control) and promoted ROS scavenging capacity in canola plants promotes plant vigor during drought stress which results in better growth of the seedlings compared to control.
A notable finding of the present study was increased proline content of canola seedling as a result of AE application. Proline is major component of plant defense mechanism during water shortage and drought stress and is known as the most accumulated osmolyte under various stressful conditions. Many researchers have reported positive effect of proline on plant tolerance to abiotic stresses ( 1 , 9 , 46 , 47 ). Accumulation of proline in control group shows that increase of this amino acid during drought stress is a natural mechanism of plants to cope with water shortage. However, proline content was significantly higher in AE-treated seedlings (p<0.05) which shows another positive effect of seaweed extract on plant tolerance to water deficit. This is consistent with previous reports on the impact of seaweed extract on proline content of crops ( 48 , 49 ).
Real-Time PCR assay revealed that enhanced accumulation of proline in foliar tissues of canola was the result of a combination of up-regulation of proline biosynthesis gene (P5CS) and down-regulation of proline degradation gene (PRODH). P5CS is a key gene through biosynthesis pathway of proline which is overexpressed under stressful conditions. The enzyme encoded by P5CS is composed of two domains acting as kinase and dehydrogenase and is accumulated in cytosol and plastids ( 50 ). This finding is of great importance because it implies a sophisticated mechanism for accumulation of proline during water stress. This finding accords with previous results suggesting that proline accumulation in plants is the result of both increased proline biosynthesis and decreased proline degradation ( 51). As a whole, these findings support early evidences that seaweed extract act as an elicitor the boost growth and yield of crops under normal and stress conditions ( 12, 15 , 27, 28 , 38, 49).
6. Conclusion
The results obtained in this study reconfirmed early evidence suggesting high potential of algae as efficient bioresources for enhancing crop tolerance to biotic and abiotic stresses. Considering the magnitude of drought stress in many areas across the world, together with the growing demand for natural substance as alternatives to chemicals for promotion of crop yield, our results may have practical implications for farmland managers and agriculture policymakers. Particularly, regarding economic importance of canola, the results of this research indicate that application of the brown algae S. angustifolium may be an eco-friendly, available and cost-effective approach for mitigating adverse effect of drought stress on canola plant. Further research in the field of algae-based fertilizers and biostimulants production will pave the road for extension of practical application of marine algae.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci. 2015;72(4):673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu JK. Salt and drought stress signal transduction in plants. Ann Rev Plant Biol. 2002;53(1):247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects mechanisms and management. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C, editors. Sustainable Agriculture. Dordrecht: Springer ; 2009. pp. 153–188. [DOI] [Google Scholar]
- 4.Wang W, Vinocur B, Altman A. Plant responses to drought salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- 5.Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell & Environ. 2017;40(1):4–10. doi: 10.1111/pce.12800. [DOI] [PubMed] [Google Scholar]
- 6.Golbashy M, Ebrahimi M, Khorasani SK, Choukan R. Evaluation of drought tolerance of some corn (Zea mays L.) hybrids in Iran. African J Agric Res. 2010;5(19):2714–2719. [Google Scholar]
- 7.Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi MHS, Etemadi N, Arab MM, Aalifar M, Arab M, Pessarakli M. Molecular and physiological responses of Iranian Perennial ryegrass as affected by Trinexapac ethyl Paclobutrazol and Abscisic acid under drought stress. Plant Physiol Biochem. 2017;111(129-143) doi: 10.1016/j.plaphy.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Elferjani R, Soolanayakanahally R. Canola responses to drought heat and combined stress: shared and specific effects on carbon assimilation seed yield and oil composition. Frontiers Plant Sci. 2018;9:1224. doi: 10.3389/fpls.2018.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Ervin EH. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004;44(5):1737–1745. doi: 10.2135/cropsci2004.1737. [DOI] [Google Scholar]
- 11.Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol. 2007;10(3):296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Martynenko A, Shotton K, Astatkie T, Petrash G, Fowler C, Neily W, et al. Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. Springerplus. 2016;5(1):1393. doi: 10.1186/s40064-016-3019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Frontiers Plant Sci. 2015;6:84. doi: 10.3389/fpls.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla PS, Shotton K, Norman E, Neily W, Critchley AT, Prithiviraj B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants. 2017;10(1):plx051. doi: 10.1093/aobpla/plx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regulation. 2009;28(4):386–399. doi: 10.1007/s00344-009-9103-x. [DOI] [Google Scholar]
- 16.Jithesh MN, Wally OS, Manfield I, Critchley AT, Hiltz D, Prithiviraj B. Analysis of seaweed extract-induced transcriptome leads to identification of a negative regulator of salt tolerance in Arabidopsis. HortScience. 2012;47(6):704–709. doi: 10.21273/hortsci.47.6.704. [DOI] [Google Scholar]
- 17.Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Scientia Horticulturae. 2015;196:39–48. doi: 10.1016/j.scienta.2015.09.012. [DOI] [Google Scholar]
- 18.Tandon S, Dubey A. Effects of Biozyme (Ascophyllum nodosum) biostimulant on growth and development of soybean [Glycine max (L.) Merill] Communications soil Sci Plant Analysis. 2015;46(7):845–858. doi: 10.1080/00103624.2015.1011749. [DOI] [Google Scholar]
- 19.Thirumaran G, Arumugam M, Arumugam R, Anantharaman P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (l) medikus. Am-Eurasian J Agron. 2009;2(2):57–66. [Google Scholar]
- 20.Sangha JS, Ravichandran S, Prithiviraj K, Critchley AT, Prithiviraj B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol Mol Plant Pathol. 2010;75(1-2):38–45. doi: 10.1016/j.pmpp.2010.08.003. [DOI] [Google Scholar]
- 21.Subramanian S, Sangha JS, Gray BA, Singh RP, Hiltz D, Critchley AT, et al. Extracts of the marine brown macroalga Ascophyllum nodosum induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv, tomato DC3000 and Sclerotinia sclerotiorum. Europ J Plant Pathol. 2011;131(2):237–248. doi: 10.1007/s10658-011-9802-6. [DOI] [Google Scholar]
- 22.Erulan V, Soundarapandian P, Thirumaran G, Ananthan G. Studies on the effect of Sargassum polycystum (C, Agardh 1824) extract on the growth and biochemical composition of Cajanus cajan (L,) Mill sp. Am-Eurasian J Agric Environ Sci. 2009;6(4):392–399. [Google Scholar]
- 23.Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. 2011;23(3):371–393. [Google Scholar]
- 24.Kumari R, Kaur I, Bhatnagar AK. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth yield and quality of Lycopersicon esculentum Mill. J Appl Phycol. 2011;23(3):623–633. doi: 10.1007/s10811-011-9651-x. [DOI] [Google Scholar]
- 25.Mancuso S, Briand X, Mugnai S, Azzarello E. Marine Bioactive Substances (IPA Extract) Improve Foliar Ion Uptake and Water Stress Tolerance in Potted Vitis vinifera Plants. Adv Hort Sci [Rivista dell’ortofloroftutticoltura Italiana] 2006;20(2):1000–1006. [Google Scholar]
- 26.Goñi O, Quille P, O’Connell S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol Biochem. 2018;126:63–73. doi: 10.1016/j.plaphy.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Wang K, Ervin EH. Optimizing dosages of seaweed extract-based cytokinins and zeatin riboside for improving creeping bentgrass heat tolerance. Crop Sci. 2010;50(1):316–320. doi: 10.2135/cropsci2009.02.0090. [DOI] [Google Scholar]
- 28.Shukla PS, Borza T, Critchley AT, Hiltz D, Norrie J, Prithiviraj B. Ascophyllum nodosum extract mitigates salinity stress in Arabidopsis thaliana by modulating the expression of miRNA involved in stress tolerance and nutrient acquisition. PloS One. 2018;13(10):e0206221. doi: 10.1371/journal.pone.0206221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifi-sirchi G. Effect of macroalgae extract on growth characteristics of tomato seedlings. J Aquatic Ecology. 2016;6(1):53–61. [Google Scholar]
- 30.Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents . Biochem Soc Trans. 1983;11(5):591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- 31.Bates L, Waldre R, Teare I. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. doi: 10.1007/bf00018060. [DOI] [Google Scholar]
- 32.Mansori M, Chernane H, Latique S, Benaliat A, Hsissou D, Kaoua ME. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.) J Appl Phycol. 2015;27(4):1689–1698. doi: 10.1007/s10811-014-0455-7. [DOI] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2(-delta delta c(t)) method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.El-Kaoaua M, Chernane H, Benaliat A, Neamallah L. Seaweed liquid extracts effect on Salvia officinalis growth biochemical compounds and water deficit tolerance. African J Biotechnol. 2013;12(28):4481–4489. doi: 10.5897/ajb2013.12807. [DOI] [Google Scholar]
- 35.Hernández-Herrera RM, Santacruz-Ruvalcaba F, Briceño-Domínguez DR, Filippo-Herrera DAD, Hernández-Carmona G. Seaweed as potential plant growth stimulants for agriculture in Mexico. Hidrobiológica. 2018;28(1):129–140. doi: 10.24275/uam/izt/dcbs/hidro/2018v28n1/hernandezc. [DOI] [Google Scholar]
- 36.Vinoth S, Gurusaravanan P, Sivakumar S, Jayabalan N. Influence of seaweed extracts and plant growth regulators on in vitro regeneration of Lycopersicon esculentum from leaf explant. J Appl Phycol. 2019;31(3):2039–2052. doi: 10.1007/s10811-018-1703-z. [DOI] [Google Scholar]
- 37.Pise NM, Sabale AB. Effect of seaweed concentrates on the growth and biochemical constituents of Trigonella Foenum-Graecum L. J Phytol. 2010;2(4):50–56. [Google Scholar]
- 38.Uthirapandi V, Suriya S, Boomibalagan P, Eswaran S, Ramya SS, Vijayanand N, et al. Bio-fertilizer potential of seaweed liquid extracts of marine macro algae on growth and biochemical parameters of Ocimum sanctum. J Pharmacognosy Phytochem. 2018;7(3):3528–3532. doi: 10.20546/ijcmas.2018.706.312. [DOI] [Google Scholar]
- 39.Whapham CA, Blunden G, Jenkins T, Hankins SD. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J Appl Phycol. 1993;5(2):231. doi: 10.1007/bf00004023. [DOI] [Google Scholar]
- 40.Blunden G, Jenkins T, Liu YW. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J Appl Phycol. 1996;8(6):535–543. doi: 10.1007/bf02186333. [DOI] [Google Scholar]
- 41.Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47(1):94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heo SJ, Park EJ, Lee KW, Jeon YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Biores Technol. 2005;96(14):1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Park PJ, Heo SJ, Park EJ, Kim SK, Byun HG, Jeon BT, et al. Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. J Agric Food Chem. 2005;53(17):6666–6672. doi: 10.1021/jf050582+. [DOI] [PubMed] [Google Scholar]
- 44.Arabzadeh N, Khavari-Nejad RA. Effect of drought stress on superoxide dismutase activity in two species of Haloxylon aphyllum and Haloxylon persicum. Pak J Biol Sci. 2013;16:351–361. doi: 10.3923/pjbs.2013.351.361. [DOI] [PubMed] [Google Scholar]
- 45.Fike JH, Allen VG, Schmidt RE, Zhang X, Fontenot JP, Bagley CP, et al. Tasco-Forage: I, Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J Animal Sci. 2001;79(4):1011–1021. doi: 10.2527/2001.7941011x. [DOI] [PubMed] [Google Scholar]
- 46.Aksouh-Harradj N, Campbell L, Mailer R. Canola response to high and moderately high temperature stresses during seed maturation. Canad J Plant Sci. 2006;86(4):967–980. doi: 10.4141/p05-130. [DOI] [Google Scholar]
- 47.Ashraf MF, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Experimental Botany. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 48.Butler T, Hunter A. Impact of seaweed extract on turfgrass growth and nutrition on a Golf green to USGA specification. XXVII International Horticultural Congress-IHC2006: International Symposium on Horticultural Plants in Urban and Peri-Urban. 2006 doi: 10.17660/actahortic.2007.762. [DOI] [Google Scholar]
- 49.Carvalho MEA, Camargo PRd, Gaziola SA, Azevedo RA. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comunicata Scientiae. 2018;9(2):292–297. doi: 10.14295/cs.v9i2.2134. [DOI] [Google Scholar]
- 50.Amini S, Ghobadi C, Yamchi A. Proline accumulation and osmotic stress: an overview of P5CS gene in plants. J Plant Mol Breed. 2015;3(2):44–55. doi: 10.22058/jpmb.2015.17022. [DOI] [Google Scholar]
- 51.Maghsoudi K, Emam Y, Niazi A, Pessarakli M, Arvin MJ. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J Plant Interactions. 2018;13(1):461–471. doi: 10.1080/17429145.2018.1506516. [DOI] [Google Scholar]