Abstract
Extrahepatic recurrence (EHR) after curative hepatectomy for hepatocellular carcinoma (HCC) is associated with a poor prognosis. We investigated the features of EHR and identified its predictive factors. This retrospective study included 398 treatment-naive patients who underwent curative hepatectomy for HCC at two tertiary hospitals. Multivariate Cox-regression analysis was performed to identify the variables associated with EHR. EHR was diagnosed in 94 patients (23.6%) over a median follow-up period of 5.92 years, most commonly in the lungs (42.6%). The 5-/10-year cumulative rates of HCC recurrence and EHR were 63.0%/75.6% and 18.1%/35.0%, respectively. The median time to EHR was 2.06 years. Intrahepatic HCC recurrence was not observed in 38.3% of patients on EHR diagnosis. On multivariate analysis, pathologic modified Union for International Cancer Control stage (III, IVa), surgical margin involvement, tumor necrosis, sum of tumor size > 7 cm, and macrovascular invasion were predictive factors of EHR. Four risk levels and their respective EHR rates were defined as follows: very low risk, 1-/5-year, 3.1%/11.6%; low risk, 1-/5-year, 12.0%/27.7%; intermediate risk, 1-/5-year, 36.3%/60.9%; and high risk, 1-year, 100.0%. Our predictive model clarifies the clinical course of EHR and could improve the follow-up strategy to improve outcomes.
Subject terms: Cancer, Gastroenterology, Risk factors
Introduction
Despite recent advances in the diagnosis and treatment of hepatocellular carcinoma (HCC), HCC continues to be associated with poor prognosis, presenting the third highest cancer-related mortality rate worldwide1,2. Curative hepatectomy remains the treatment choice for such cases, especially in settings where liver transplantation is not feasible3. However, the long-term prognosis after curative hepatectomy remains unsatisfactory, with the 5-year rate of HCC recurrence ranging between 60 and 70%4,5. Therefore, identifying risk factors of HCC recurrence and standardizing the perioperative management protocol could be important to improve long-term prognosis after curative hepatectomy for HCC.
According to the current practice, curative hepatectomy is indicated over other local therapies, such as radiofrequency ablation (RFA), for patients with advanced HCC who have larger size tumors and/or presence of microvascular tumor invasion. The more advanced disease status of patients who undergo curative hepatectomy could explain the comparatively higher risk of HCC recurrence after curative hepatectomy than RFA. Current treatment guidelines recommend surveillance after treatment, with curative hepatectomy or RFA, including abdominal computed tomography (CT) and measurement of serum alpha fetoprotein (AFP) levels6,7. However, this recommendation does not consider the differences in the risk of recurrence between patients treated using curative hepatectomy and those treated with RFA8. Moreover, although intrahepatic recurrence (IHR) is the most common type of recurrence, extra-hepatic recurrence (EHR) is possible, with the most common sites of EHR being the lungs, lymph nodes, and bones, which could be difficulty evaluated using conventional abdominal CT imaging9–11. Considering the aggressive nature of metastatic hepatic tumors and the limited treatment options for recurrent HCC, the prognosis for patients with EHR is generally worse than that for those with IHR. Despite the dismal prognosis of EHR, few studies have showed improved outcomes with mestastasectomy in selected patients12,13. Thus, early identification would be important to improve the oncological outcomes and survival. However, at present, there are insufficient data on the clinical course and pathological progression after curative hepatectomy for HCC to identify the predictive factors of EHR. Accordingly, we aimed to determine the risk factors of EHR among patients who had undergone curative hepatectomy as the initial treatment for HCC and to use these risk factors to construct a simple parametric model to predict EHR.
Results
Baseline characteristics of enrolled patients
EHR was identified in 94 (23.6%) out of 398 enrolled patients. The 10-year cumulative rate of HCC recurrence was 75.6%, with a rate of 35.0% for EHR (Fig. 1). Compared to those without EHR, those with EHR were younger and had a higher serum alkaline phosphatase level, a lower serum albumin level, absence of fatty change in the liver, and a more advanced HCC stage (Table 1). The serum AFP level at the time of first recurrence of HCC after curative hepatectomy was higher and the time interval to the first recurrence was also significantly shorter in the EHR group.
Figure 1.
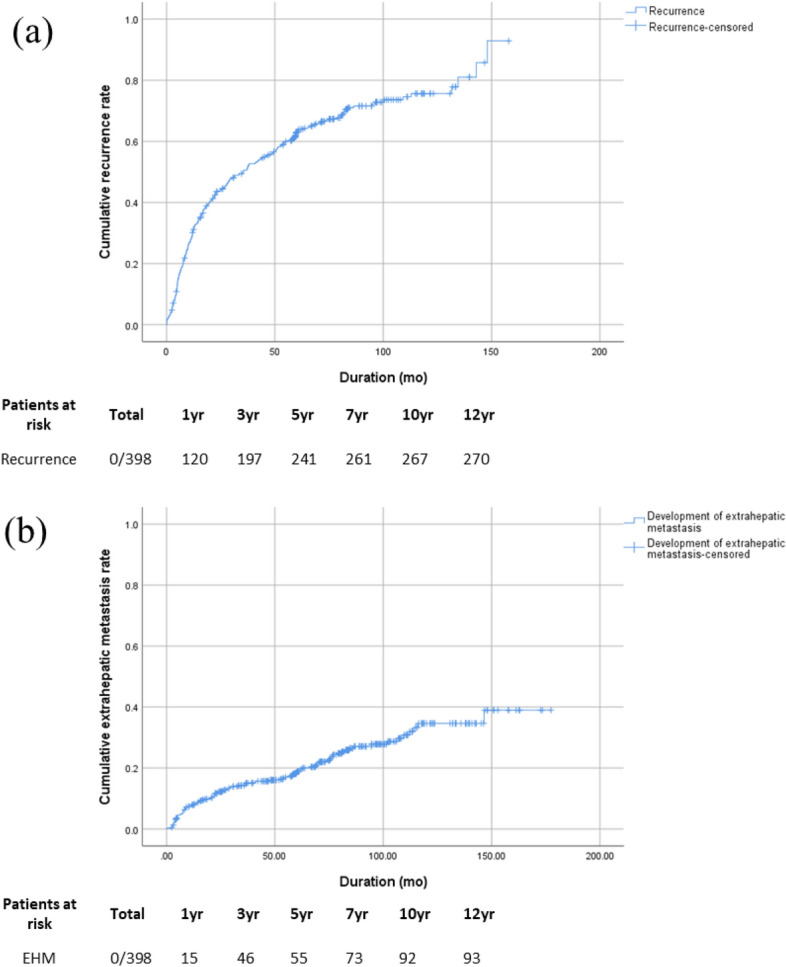
Recurrence curves of hepatocellular carcinoma (HCC) after surgical resection (a): Cumulative rates of HCC recurrence; the 1-, 3-, 5-, 7-, and 10-year cumulative rates of recurrence were 30.4%, 50.5%, 63.0%, 71.0%, and 75.6%, respectively; (b): Cumulative rates of extrahepatic recurrence (EHR); the 1-, 3-, 5-, 7-, and 10-year cumulative rates of EHR were 7.9%, 14.2%, 18.1%, 25.5%, and 35.0%, respectively.
Table 1.
Baseline characteristics of the enrolled patients.
| Patients without extrahepatic recurrence (n = 304) | Patients with extrahepatic recurrence (n = 94) | p-value | ||
|---|---|---|---|---|
| Age (years) | 59.11 ± 10.02 | 56.01 ± 10.45 | 0.010 | |
| Male (n, %) | 259 (85.2) | 85 (90.4) | 0.196 | |
| Etiology of liver cirrhosis, n (%) | 0.897 | |||
|
HBV*/HCV Alcohol/combined NASH/unknown |
176 (63.1)/21 (7.5) 24 (8.6)/17 (6.1) 1 (0.4)/40(14.4) |
56 (65.9)/6 (7.1) 10 (11.8)/4 (4.7) 0 (0.0)/9 (10.6) |
||
| ALP (U/L) | 86.88 ± 28.21 | 99.25 ± 49.72 | 0.023 | |
| Albumin (mg/dL) | 4.35 ± 0.48 | 4.13 ± 0.45 | < 0.001 | |
| ALBI grade ≥ 2, n (%) | 47 (15.5) | 23 (24.7) | 0.041 | |
| ICG R15 | 10.33 ± 7.62 | 10.99 ± 8.08 | 0.496 | |
| Preoperative serum AFP (IU/mL) | 0.363 | |||
|
≤ 400 > 400 |
235 (80.5%) 57 (19.5%) |
70 (76.1%) 22 (23.9%) |
||
| Tumor size | 4.18 ± 2.41 | 5.16 ± 3.69 | < 0.001 | |
| Tumor numbers | 1.26 ± 0.83 | 1.32 ± 0.79 | 0.525 | |
| BCLC stage, n (%) | 0.045 | |||
| 0/A / ≥ B | 34 (11.2)/234 (77.2)/35 (11.6) | 6 (6.4)/68 (72.3)/20 (21.3) | ||
| Pathological mUICC stage, n (%) | 0.012 | |||
| I/II / ≥ III | 43 (14.5)/183 (61.6)/71 (23.9) | 7 (8.0)/49 (56.3)/31 (35.6) | ||
| Radiological mUICC stage, n (%) | 0.001 | |||
| I/II / ≥ III | 48 (15.8)/205 (67.7)/50 (16.5) | 10 (10.6)/59 (62.8)/212 (26.6) | ||
| Beyond Milan criteria, n (%) | 75 (24.8) | 45 (47.9) | < 0.001 | |
| Metastatic lymph nodes, n (%) | 0 (0.0) | 2 (2.1) | 0.011 | |
| Macrovascular invasion, n (%) | 6 (2.0) | 8 (8.5) | 0.003 | |
| mUICC T stage at 1st recurrence, n (%) | < 0.001 | |||
|
0/1 2 /3 4 |
0 (0.0)/81 (45.8) 67 (37.9)/25 (14.1) 4 (2.3) |
6 (6.5)/19 (20.7) 32 (34.8)/22 (23.9) 13 (14.1) |
||
| Serum AFP at 1st recurrence | 209.12 ± 1 098.83 | 1,225.05 ± 5 775.70 | 0.045 | |
| Hospital stay, days (median, range) | 13 (5–69) | 13 (4–60) | 0.015 | |
| Time to first recurrence, months (median, range) | 42.54 (0.16–157.91) | 10.14 (0.23–100.34) | < 0.001 | |
| Follow-up duration, months (median, range) | 75.21 (2–177) | 38.93 (3–150) | 0.857 | |
Values are presented as mean ± SD.
SD standard deviation, HBV hepatitis B virus, HCV hepatitis C virus, AST aspartate transaminase, ALT alanine transaminase, ALP alkaline phosphatase, AFP alpha-fetoprotein, BCLC Barcelona Clinic Liver Cancer, mUICC modified Union for International Cancer Control.
*Patients with suppressed HBV DNA (HBV DNA < 200 IU/mL)42 at pre-operative state had lower rates of EHR (11.1% vs. 28.8%, p = 0.047).
Clinical features of patients with EHR
The most common site of the first HCC recurrence in the EHR group was intrahepatic (66.0%), with the most common initial site of EHR being the lungs (42.6%), followed by the lymph nodes (19.1%), peritoneum (18.1%), and bones (14.9%) (Supplementary Table S1). In half of the cases, EHR was confined to the abdominal cavity, identified by abdominal imaging, while in the other 48.9% of cases, EHRs were identified within the thoracic cavity, including the lungs and the bony structures of the thoracic spine. The median time to EHR was 2.06 years. At the time of EHR diagnosis, 36 patients (38.3%) had no IHR. At the time of first HCC recurrence, EHR was identified in 32 patients (34.0%).
Comparison of surgical findings between patients with and without EHR
Microvascular and serosal invasion were more prevalent among patients with EHR than in those without (16.9 versus 29.8% and 2.1 versus 6.5%, respectively) (Table 2). Moreover, the presence of satellite nodules and tumor necrosis in resected specimens was more prominent in patients with EHR than in those without (13.0 versus 31.2% and 46.9 versus 68.8%, respectively).
Table 2.
Comparison of surgical findings in patient groups with and without extrahepatic recurrence.
| Patients without extrahepatic recurrence (n = 304) | Patients with extrahepatic recurrence (n = 94) | p-value | |
|---|---|---|---|
| Margin involvement, n (%) | 9 (3.0) | 6 (6.5) | 0.134 |
| Microvascular invasion, n (%) | 51 (16.9) | 28 (29.8) | 0.006 |
| Serosal invasion, n (%) | 6 (2.1) | 6 (6.5) | 0.034 |
| Bile duct invasion, n (%) | 1 (0.3) | 2 (2.25) | 0.085 |
| Capsule formation, n (%) | 211 (73.3) | 64 (68.8) | 0.405 |
| Multicentricity, n (%) | 34 (11.7) | 9 (9.7) | 0.593 |
| Satellite nodule, n (%) | 38 (13.0) | 29 (31.2) | < 0.001 |
| Underlying liver cirrhosis, n (%) | 201 (66.1) | 61 (64.9) | 0.827 |
| Intrahepatic metastasis, n (%) | 3 (1.0) | 2 (2.1) | 0.830 |
| Necrosis, n (%) | 136 (46.9) | 64 (68.8) | < 0.001 |
| Haemorrhage, n (%) | 139 (47.9) | 53 (57.0) | 0.121 |
| Fatty change, n (%) | 109 (38.0) | 29 (31.9) | 0.291 |
| Cell type, n (%) | 0.213 | ||
|
Clear type Hepatic type Classic type |
53 (17.5) 271 (89.4) 240 (79.2) |
22 (23.4) 89 (94.6) 70 (74.5) |
|
| Major Edmondson Steiner grade, n (%) | 0.336 | ||
|
1/2 3/4 |
14 (4.8)/152 (52.2) 116 (39.9)/9 (3.1) |
4 (4.3)/39 (41.9) 46 (49.5)/4 (4.3) |
|
| Worst Edmondson Steiner grade, n (%) | 0.665 | ||
|
1/2 3/4 |
2 (0.7)/46 (15.8) 178 (61.2)/65 (22.3) |
2 (2.2)/14 (15.1) 55 (59.1)/22 (23.7) |
Comparison of characteristics between patients with early and non-early EHR
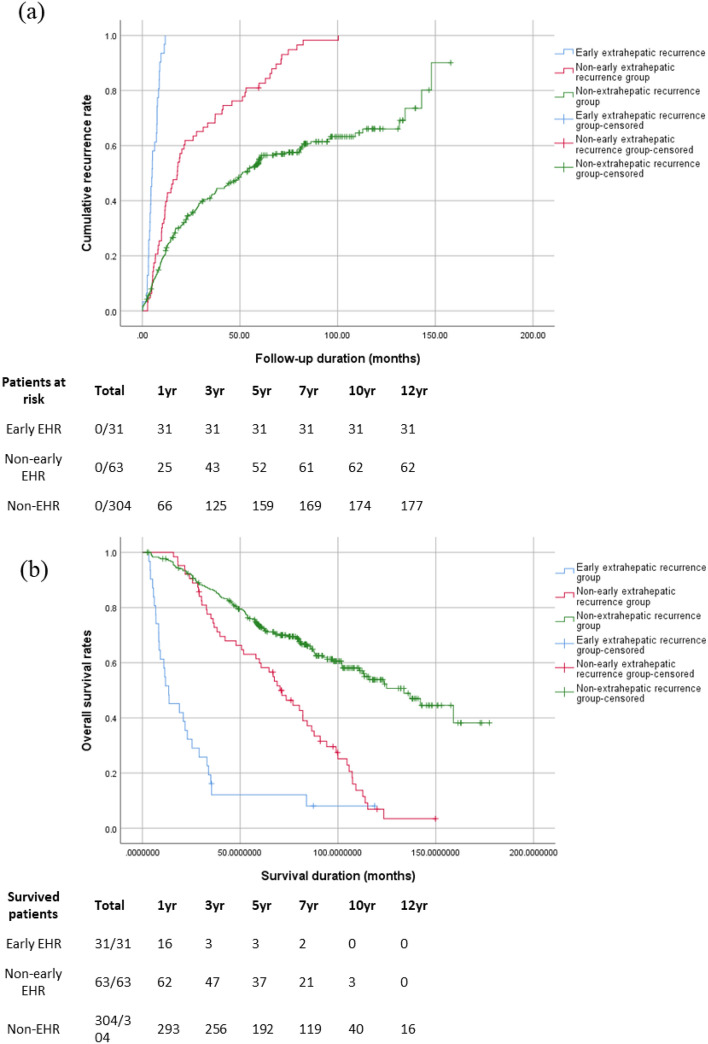
Both the radiologic and pathologic mUICC stages were more advanced in the early EHR than in the non-early EHR group (Supplementary Table S2). The early EHR group also had a markedly shorter recurrence-free-survival (RFS) and survival rates compared to the non-early EHR and non-EHR groups (Fig. 2). Moreover, the proportion of tumors with a mUICC stage ≥ III at the time of first recurrence was larger in the early EHR than in the non-early EHR group. Especially, in 54.8% of patients in the early EHR group, EHR was the first presenting recurrence after curative hepatectomy.
Figure 2.
Comparison of the (a) intra- and/or extra-hepatic recurrence rate of hepatocellular carcinoma (HCC) and (b) overall survival rates between the early extrahepatic recurrence (EHR), non-early EHR, and non-EHR groups. The 6-month cumulative recurrence rates among the early EHR, non-early EHR, and non-EHR groups were 77.7%, 39.7%, and 21.9%, respectively. The 1-year, 5-year, and 10-year overall survival rates were 51.6%, 9.7%, and 0% in the early EHR group; 98.4%, 58.7%, and 4.8% in the non-early EHR group; and 96.4%, 63.2%, and 13.2% in the non-EHR group, respectively (p < 0.001).
Analysis of factors associated with EHR
In the multivariate analysis, the following factors were retained as independent predictors of EHR: pathologic mUICC stage (III, IVa) (Hazard ratio [HR]: 2.664, p = 0.013), surgical margin involvement (HR: 3.040, p = 0.040); tumor necrosis on pathological assessment of resected specimens (HR: 1.797, p = 0.037); sum of tumor size > 7 cm (HR: 2.481, p = 0.014); macrovascular invasion on imaging studies (HR: 3.295, p = 0.011 (Table 3). Regarding the factors associated with early EHR, serum alkaline phosphatase (ALP) > 120 U/L, a pathological mUICC stage III or IVa, surgical margin involvement, absence of fatty change in the liver, and macrovascular invasion were found to be closely associated on multivariate analysis (Table 4).
Table 3.
Univariate and multivariate analyses of factors associated with extrahepatic metastasis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Serum alkaline phosphatase > 130 U/L | 2.800 (1.222–6.416) | 0.015 | ||
| ALBI grade ≥ 2 | 1.889 (1.175–3.036) | 0.009 | ||
| Pathologic mUICC stage (III, IVa) | 2.398 (1.586–3.626) | < 0.001 | 2.664 (1.232–5.763) | 0.013 |
| Multiple tumorsa | 1.980 (1.183–3.313) | 0.009 | ||
| Major Edmondson Steiner grade ≥ 3 | 1.529 (1.016–2.299) | 0.042 | ||
| Surgical margin involvement | 2.749 (1.200–6.298) | 0.017 | 3.040 (1.050–8.804) | 0.040 |
| Venous/lymphatic involvement | 2.043 (1.312–3.180) | 0.002 | ||
| Serosa invasion | 3.167 (1.380–7.271) | 0.007 | ||
| Bile duct invasion | 5.720 (1.401–23.350) | 0.015 | ||
| Satellite nodule | 2.632 (1.694–4.090) | < 0.001 | ||
| Tumor necrosis | 2.333 (1.504–3.620) | < 0.001 | 1.797 (1.035–3.118) | 0.037 |
| Sum of tumor size > 7 cm | 3.792 (2.394–6.004) | < 0.001 | 2.481 (1.198–5.135) | 0.014 |
| Beyond Milan criteria | 2.832 (1.881–4.263) | < 0.001 | ||
| Macrovascular invasion | 4.005 (1.934–8.295) | < 0.001 | 3.295 (1.310–8.292) | 0.011 |
| BCLC stage C | 2.271 (1.382–3.731) | 0.001 | ||
| Serum AFP ≥ 50,000 IU/mL | 5.519 (1.347–22.621) | 0.018 | ||
HR hazards ratio, CI confidence interval, mUICC modified Union for International Cancer Control, CT computed tomography, MRI magnetic resonance imaging, BCLC Barcelona Clinic Liver Cancer, AFP alpha-fetoprotein.
aNumber of tumors examined at pathologic findings.
Table 4.
Univariate and multivariate analyses of factors associated with early extrahepatic recurrence.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Serum ALP > 120 U/L | 2.790 (1.279–6.084) | 0.010 | 2.362 (1.018–5.478) | 0.045 |
| Pathologic mUICC stage (III, IVa) | 3.418 (1.634–7.151) | 0.001 | 2.610 (1.154–5.901) | 0.021 |
| Worst Edmonson Steiner grade ≥ 4 | 2.221 (1.063–4.643) | 0.034 | ||
| Surgical margin involvement | 2.991 (1.043–8.576) | 0.041 | 4.035 (1.280–12.725) | 0.017 |
| Venous/lymphatic involvement | 1.890 (0.925–3.860) | 0.081 | ||
| Absence of fatty change | 3.582 (1.249–10.276) | 0.018 | 3.246 (1.114–9.461) | 0.031 |
| Sum of tumor size > 7 cm | 2.142 (1.055–4.347) | 0.035 | ||
| Macrovascular invasion | 3.818 (1.559–9.351) | 0.003 | 3.207 (1.169–8.799) | 0.024 |
HR hazards ratio, CI confidence interval, ALP alkaline phosphatase, mUICC modified Union for International Cancer Control, AFP alpha-fetoprotein.
Prediction of EHR
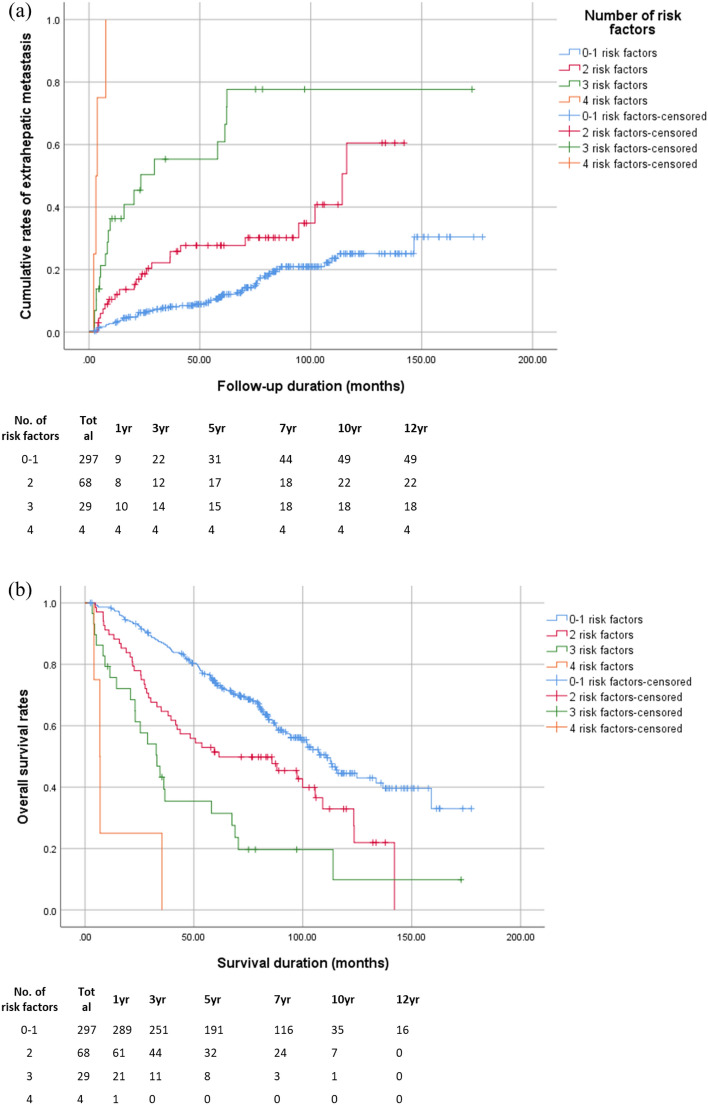
Based on our multivariate analyses, the following five variables were used to build a parametric model to predict EHR. Then, the risk of EHR was stratified into four levels based on the number of predictive factors present, as follows: very low risk, 0–1 risk factors; low risk, 2 risk factors; intermediate, 3 risk factors; and high, ≥ 4 risk factors. Then, cumulative rate of EHR was calculated using the Kaplan–Meier survival curve analysis for each risk level (Fig. 3a). The 1-, 3-, 5-, and 10- year cumulative rates of EHR were significantly related to the numbers of risk factors present: very low risk: 3.1%, 7.3%, 11.6%, and 25.1%, respectively; low risk: 12.0%, 22.2%, 27.7%, and 60.5%, respectively; intermediate risk: 36.3%, 55.3%, 60.9%, and 73.7%, respectively; and high risk: 100.0% at 1st year. Furthermore, the overall survival rates showed a significant correlation with the risk level of EHR (Fig. 3b). The 1-, 3-, 5-, and 10- year overall survival rates were as follows: very low risk: 97.3%, 84.5%, 64.3%, and 11.8%, respectively; low risk: 89.7%, 64.7%, 47.1%, and 10.3%, respectively; intermediate risk: 72.4%, 37.9%, 27.6%, and 3.4%, respectively; and high risk: 25.0% at 1st year and no survived patient from 35 months. We assessed the discriminative ability of the model with Harrell’s C index, which showed a value of 0.685 (95% confidence interval [CI], 0.624–0.745)14,15. The median times to EHR development were 71.41, 50.01, 14.47, and 3.44 months for the very-low, low, intermediate, and high-risk levels, respectively.
Figure 3.
(a) Cumulative rate of extrahepatic recurrence and (b) overall survival rates after curative hepatectomy, stratified by the number of risk factors present.
Analysis of factors associated with the overall survival rates
To analyze the association with EHR with the overall survival rate, we conducted Cox-regression analysis including many potential risk factors, such as EHR (Table 5). On multivariate analysis, pathologic modified Union for International Cancer Control (mUICC) stages III and IVa (HR: 3.118, p < 0.001), surgical margin involvement (HR: 4.847, p < 0.001), initial tumor stage beyond Milan criteria (HR: 2.242, p = 0.012), and presence of EHR (HR: 4.723, p < 0.001) were found to be associated with the overall survival rates.
Table 5.
Univariate and multivariate analyses of factors associated with overall survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Serum albumin < 3.7 mg/dL | 3.012 (1.674–5.419) | < 0.001 | ||
| ALBI grade ≥ 2 | 2.216 (1.328–3.699) | 0.002 | ||
| Pathologic mUICC stage (III, IVa) | 3.167 (1.980–5.066) | < 0.001 | 3.118 (1.682–5.782) | < 0.001 |
| Multiple tumorsa | 4.004 (2.181–7.352) | < 0.001 | ||
| Surgical margin involvement | 6.013 (2.980–12.135) | < 0.001 | 4.847 (1.875–12.532) | 0.001 |
| Venous/lymphatic involvement | 2.442 (1.490–4.002) | < 0.001 | ||
| Serosa invasion | 5.083 (2.321–11.132) | < 0.001 | ||
| Bile duct invasion | 7.365 (1.799–30.152) | 0.005 | ||
| Multicentricity | 2.391 (1.305–1.380) | 0.005 | ||
| Satellite nodule | 2.243 (1.329–3.786) | 0.002 | ||
| Tumor necrosis | 2.173 (1.307–3.612) | 0.003 | ||
| Sum of tumor size > 7 cm | 3.224 (1.881–5.526) | < 0.001 | ||
| Beyond Milan criteria | 2.486 (1.548–3.991) | < 0.001 | 2.242 (1.191–4.219) | 0.012 |
| Presence of EHR | 3.994 (2.495–6.395) | < 0.001 | 4.723 (2.512–8.882) | < 0.001 |
HR hazards ratio, CI confidence interval, mUICC modified Union for International Cancer Control, EHR extrahepatic recurrence.
aNumber of tumors examined at pathologic findings.
Discussion
Based on the data of many patients who underwent curative hepatectomy for HCC, we proposed a simple parametric model predicting the risk of EHR development. This model is straightforward and easy-to-use, and consists of five easy-to-obtain variables that constitute the essentials of pre- and post-operative clinical parameters and the postoperative pathologic findings. Because of the lack of current consensus on follow-up strategies for the detection of EHR after resection, our prediction models may aid in monitoring patients for individual risk and in appropriately assigning patients for participation in clinical trials for postoperative adjuvant therapy (e.g., patients would be categorized as intermediate or high risk, if they present a predicted 5-year EHR rate of 50.5% or 100%, respectively).
Recent studies have reported improved prognosis for recurrent HCC based on the pattern of IHR5,16. However, these studies did not clarify the clinical features and pathological course of EHR, with the absence of models to predict EHR after curative treatment for HCC, thus, limiting the early detection of EHR. In our study, we identified the risk factors of EHR after curative hepatectomy for HCC and used these factors to stratify the risk for EHR into four levels. Our findings highlighted the necessity for the development of a predictive score based on risk stratification to inform optimal surveillance for prompt detection of EHR to improve patient outcomes.
The current results of HCC recurrence and EHR development rates suggested some distinction from our previous study of HCC patients who underwent RFA17. In that study, the median times to first HCC recurrence and EHR after RFA were 1.75 and 2.68 years, respectively. Moreover, the 1-, 3-, 5-, 8-, and 10-year rates of EHR development were 1.0%, 2.9%, 8.1%, 15.7%, and 33.7%, respectively. These rates were comparably lower than those reported after curative hepatectomy.
Regarding the pattern of HCC recurrence, the most common initial site of recurrence was within the intrahepatic area, which was consistent with a previous report18. Another study identified IHR as the most common initial site of recurrence, with EHR developing after several treatments for IHR19. Uchino et al. reported that 82.2% of patients with HCC with EHR presented with IHR, a finding comparable to those of our previous study17. Therefore, multiple IHRs may indicate EHR risk in patients with HCC20.
In our study cohort, the lungs were the most common site of EHR (42.6%). Thoracic metastases, which included the lungs and the vertebrae of the thoracic spine, were, thus, relatively common as previously reported21. Thoracic metastases reflect a systemic involvement with poor prognosis, as they are largely not curable. Of further concern is the fact that thoracic metastases may not be detected using conventional surveillance methods that rely on abdominal imaging. Therefore, the use of chest CT images should be included in the surveillance strategy for patients at risk for EHR after curative hepatectomy for HCC for the early detection of thoracic metastases. In addition, the rate of EHR at the time of the initial recurrence of HCC after hepatectomy was 34%, with 38.3% of these patients having no sign of IHR at the time of EHR diagnosis. Therefore, even if intrahepatic HCC lesions are stable, close surveillance for possible development of EHR may be necessary.
Regarding the risk factors for EHR, macrovascular invasion, pathologic mUICC stage (III, IVa), large tumor size (sum > 7 cm), surgical margin involvement, and necrotic HCC were associated with EHR after curative hepatectomy. Vascular invasion is a well-known prognostic indicator of HCC. Natsuizaka et al. showed that vascular invasion was more frequently observed in patients with EHR at the first diagnosis of HCC1. Senthilnathan et al. also reported a two-fold increase in EHR in the presence compared to the absence of portal vein invasion (24% versus 12%)22. Yang et al. reported that EHR was more common among patients with vascular invasion, intrahepatic metastasis, and more advanced HCC stages11. A recent study revealed that the presence of tumor necrosis was associated with an advanced tumor stage, HCC recurrence, and patient survival after curative hepatectomy for HCC23. In agreement with these findings, we also identified that necrotic HCC was associated with a high rate of EHR. HCC tumors > 6 cm in size were also predictive of EHR after curative resection for HCC, exhibiting similar results to those of a previous study24. We identified involvement of the margin of resection as a significant risk factor of EHR (HR: 4.035, 95% CI: 1.28–12.725), which was consistent with the findings of a previous study25.
In addition to the aforementioned risk factors for EHR, the first recurrence free survival of < 12 months (HR: 5.748, 95% CI 3.787–8.722, p < 0.001) and the serum AFP level > 400 IU/mL at the time of first recurrence during follow-up after curative hepatectomy (HR: 3.127, 95% CI 1.935–5.057, p < 0.001) were significantly associated with EHR according to the Kaplan–Meier analysis results. These findings were consistent with those reported by Kim et al. who reported that EHR developed more frequently in patients with early HCC recurrence26. They suggested that aggressive tumor pathology was, therefore, a risk factor of early HCC recurrence. Recent studies have shown that high AFP levels were independent risk factors of HCC invasiveness27–30. Similarly, our previous study on EHR in RFA for HCC also demonstrated an association between the AFP level and HCC recurrence when the AFP level was > 400 IU/mL, in line with our findings17.
After performing multivariate analysis, we found that the presence of EHR was significantly associated with the overall survival rates. Furthermore, pathologic mUICC stage (III, IVa) and surgical margin involvement, which were risk factors of EHR, were also related to the overall survival rate. This finding suggested a close correlation of the EHR with the overall survival rates. Moreover, HCC beyond Milan criteria was associated with the risk of developing HER, as previously reported31,32.
Regarding the early EHR, apart from the mentioned risk factors (i.e., advanced pathologic mUICC stage, surgical margin involvement, and macrovascular invasion), the serum ALP levels and absence of fatty change were also significantly associated with early EHR. Although there is still controversy concerning the association between the ALP level and HCC prognosis, a recent study showed that the serum ALP levels increase in liver disease and may reflect liver injury33. A recent meta-analysis showed that high pre-treatment ALP levels were significantly associated with poor overall survival rate (HR: 1.14, 95% CI: 1.10–1.18) and RFS (HR: 1.78, 95% CI: 1.37–2.31)34. This finding was concordant with our present result presenting an association of higher ALP level with early EHR. The frequency of diffuse steatosis in early HCCs peaks at a diameter of approximately 1.5 cm, decreasing as a function of increasing tumor size and grade35. Thus, diffuse fatty change is uncommon in HCCs > 3 cm and with progressing HCC disease status, and is also not usually observed in patients with poorly differentiated HCC35,36. Therefore, the absence of fatty change in the liver with HCC is associated with the tumor aggressiveness. In our study, the proportion of patients with the absence of fatty change in the liver was higher for the early EHR than for the non-early EHR group.
We stratified the risk for EHR into four levels based on the number of predictive factors present for EHR. We demonstrated that the cumulative rates of EHR and the median time to her were correlated with these four risk levels. Moreover, we showed that the overall survival rates were correlated with risk levels representing dismal prognosis in patients with EHR. Further, to assess the calibration of our model, the Brier score was calculated (Supplementary Table S3) and showed fine correspondence. Our novel parametric model, albeit simple, could assist clinicians in identifying patients at high risk for EHR before surgery.
We finally noted that 54.8% of the participants in the early EHR group exhibited EHR at the time of first recurrence. This underlines the importance of identifying patients who are at high risk for early EHR, which may be informative concerning the best strategy for surveillance after surgery for the rapid identification of EHR development.
The limitations of our study should be noted in the interpretation of results. First, the diagnosis of EHR was mostly based on medical imaging and, thus, the possibility of other primary cancers from an origin other than the liver could not be completely ruled out. However, in circumstances when the origin of the tumor was not certain, pathologic confirmation was performed at the discretion of the treating physician. Second, this was a retrospective study based on medical records from patients enrolled from two tertiary hospitals. Therefore, the effect of bias on results cannot be denied. Moreover, as this was a retrospective study, there was no concrete treatment algorithm strategy and criteria for resection. Third, there was no uniform post-treatment or surveillance schedule, and the surveillance modality used for each patient was at the discretion of the treating physician. Moreover, patients underwent different treatment modalities for local HCC recurrence depending on the tumor and patient status. There may be diverse conditions concerning the tumor stage, liver reserve function, and patients’ physical performance status. However, we tried to overcome these limitations by using a considerable number of patients with a long-term follow-up duration in multiple tertiary centers. Finally, it might have been insufficient to predict extrahepatic recurrence of HCC using only our model in all patients with HCC. There is a need to conduct a well-organized prospective study to validate more effective methods of EHR prediction and management.
In conclusion, we present a simple parametric model to predict EHR after curative hepatectomy for HCC. This tailored approach may be useful for the early detection of EHR and permit a more refined estimation of risk on an individual basis.
Methods
Patients
Between January 2004 and December 2013, 493 consecutive patients who underwent surgical resection for HCC at two tertiary hospitals were evaluated for study enrolment (Supplementary table S4). The inclusion criteria were as follows: age ≥ 18 years; and HCC diagnosis. Surgical resection of HCC was determined by physician’s decision regarding tumor stage and patient’s physical status. There had been no changes in the keynote of surgical resection during study period in both centers. Patients with hepatic tumors other than HCC and those with follow-up duration shorter than 90 days were excluded. After screening, 398 patients were enrolled (Supplementary Fig. S1). Baseline clinical and tumor characteristics, resection method, pathological findings, status of recurrence, and RFS were assessed retrospectively.
Statement of ethics
The design of our cohort study was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. CNUH-2019-203). Owing to the retrospective design of our study and the use of de-identified data, the requirement for informed consent was waived under approval of the Institutional Review Board of Chonnam National University Hospital. The study was performed in compliance with the tenets of the Helsinki Declaration of 1975.
Data collection
The following information was extracted from patients’ medical records for analysis: age, sex, underlying diseases (including hepatitis infection status and alcohol use), blood chemistry [including the Child–Pugh and model for end stage liver disease scores], serum AFP level, pathological findings (tumor size, histological grade, micro-vascular invasion, and presence of satellite lesions), and abdominal imaging for tumor staging at the time of HCC diagnosis. Data on blood chemistry, serum tumor markers, and abdominal imaging obtained at each follow-up visit were also obtained for analysis. Measured data at the time of first recurrence were also evaluated. Survival was defined as the time interval between the date of HCC diagnosis and that of death or last follow-up examination.
Baseline HCC staging, curative hepatectomy, and follow-up
HCC was diagnosed according to the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center37. HCC staging at the time of diagnosis was determined using the mUICC staging system38 and the Barcelona Clinic Liver Cancer (BCLC) classification system39. Milan criteria were based on radiologic findings at initial diagnosis of HCC40. Abdominal CT or magnetic resonance imaging (MRI) and assessment of serological tumor markers were routinely performed at 1 month after surgical resection and at each 3–6-month follow-up visit.
The tumor size was measured by radiologic modalities with CT or MRI. The histological differentiation of HCC was graded according to the criteria of Edmondson and Steiner41. The presence of macrovascular invasion was detected on CT imaging or MRI, while microvascular invasion was defined as invasion of vascular structures on microscopic analysis of resected tumor specimens. Bile duct invasion and tumor necrosis were confirmed from pathological findings of resected tumor specimens.
Post-operative complications after surgical resection were analyzed (Supplementary Table S5) and treatment strategies after first intra-and/or extra-hepatic recurrence and extrahepatic recurrence were also assessed (Supplementary Fig. S2). Overall survival days were defined as the time interval between the date diagnosis of HCC and the date of death or last follow-up examination. The cause of death of each patient is presented in Supplementary Table S6.
Diagnosis of EHR
Most cases of EHR were diagnosed during routine follow-up studies, with few cases diagnosed during evaluation of new onset symptoms or based on significant elevation in AFP or serial AFP without definite intra-hepatic lesions. The diagnosis of EHR was confirmed by contrast-enhanced CT imaging or MRI, and by pathological examination in some patients. In addition, bone scintigraphy, positron emission tomography-CT, and brain MR or CT imaging were also performed at the discretion of the treating physician. Chest radiographs were obtained routinely at the time of admission and when pulmonary symptoms were present. Early EHR was defined as EHR developing within the 1st year after initial curative hepatectomy.
Statistical analysis
The data are presented as means ± standard deviations or as medians and ranges, as appropriate for the data type and distribution. Univariate analyses were performed using chi-squared test or student’s t-test, as appropriate. Variables with a p-value < 0.05 on univariate analyses were included in a multivariate logistic regression analysis to identify factors predictive of EHR. The multivariable Cox regression model was built using stepwise backward selection of variables, with variables having a p-value < 0.05 retained as predictive factors. The risk levels of EHR were defined based on the number of risk factors present, with Kaplan–Meier survival curves constructed for each risk level. An equivalent of the concordance statistic for use with Cox regression was calculated by Harrell’s C-index.
All statistical analyses were performed using SPSS software (version 25.0, IBM Corp., Armonk, NY, USA).
Supplementary Information
Author contributions
J.H.Y. and C.H.J. wrote the manuscript. S.K.C. and C.H.J. designed the concept of the study. W.J.L., S.M.K., K.T.K., H.J.K., Y.S.K., and H.Y.K. collected and analyzed data of baseline patient characteristics and recurrence of hepatocellular carcinoma. B.S.K., S.Y.C., and H.Y. collected data regarding tumor pathology, survival duration and cause of death. Y.L., S.S. gathered and analyzed data regarding viral hepatitis and treatment modalities after recurrence. J.H.Y. interpreted the data and S.K.C., S.B.C., and C.H.J. supervised the project.
Funding
This study was supported by a grant (BCRI121007) of Chonnam National University Hospital Biomedical Research Institute and the Research Supporting Program of The Korean Association for the Study of the Liver and The Korean Liver Foundation (KASLKLF2019-06).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chung Hwan Jun, Email: estevanj@naver.com.
Sung Kyu Choi, Email: choisk@jnu.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92503-6.
References
- 1.Natsuizaka M, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura H, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 5.Wen T, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg. Nutr. 2018;7:353–371. doi: 10.21037/hbsn.2018.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix, J., Sherman, M. & American Association for the Study of Liver, D. Management of hepatocellular carcinoma: An update. Hepatology53, 1020–1022. 10.1002/hep.24199 (2011). [DOI] [PMC free article] [PubMed]
- 7.Korean Liver Cancer, A. & National Cancer Center, G. K. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the management of hepatocellular carcinoma. Korean J. Radiol.20, 1042–1113. 10.3348/kjr.2019.0140 (2019). [DOI] [PMC free article] [PubMed]
- 8.Lee CH, et al. Nomogram predicting extrahepatic metastasis of hepatocellular carcinoma based on commonly available clinical data. JGH Open. 2019;3:38–45. doi: 10.1002/jgh3.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katyal S, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 10.Sneag DB, et al. Extrahepatic spread of hepatocellular carcinoma: Spectrum of imaging findings. AJR Am. J. Roentgenol. 2011;197:W658–664. doi: 10.2214/AJR.10.6402. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, et al. Clinical factors predictive of a better prognosis of pulmonary metastasectomy for hepatocellular carcinoma. Ann. Thorac. Surg. 2019;108:1685–1691. doi: 10.1016/j.athoracsur.2019.06.086. [DOI] [PubMed] [Google Scholar]
- 13.Invenizzi F, et al. Pulmonary resection for metastasis of hepatocellular carcinoma recurring after liver transplant: An Italian multicenter experience. Front. Oncol. 2020;10:381. doi: 10.3389/fonc.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid M, Wright MN, Ziegler A. On the use of Harrell's C for clinical risk prediction via random survival forests. Expert Syst. Appl. 2016;63:450–459. doi: 10.1016/j.eswa.2016.07.018. [DOI] [Google Scholar]
- 15.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(Sici)1097-0258(19960229)15:4<361::Aid-Sim168>3.0.Co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: According to the recurrence pattern. Eur. J. Gastroenterol. Hepatol. 2015;27:933–940. doi: 10.1097/MEG.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JH, et al. Features of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol. 2020;26:4833–4845. doi: 10.3748/wjg.v26.i32.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchino K, et al. Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: Analysis of prognostic factors. J. Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Shah SA, et al. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Hong SS, et al. Extrahepatic spread of hepatocellular carcinoma: A pictorial review. Eur. Radiol. 2003;13:874–882. doi: 10.1007/s00330-002-1519-7. [DOI] [PubMed] [Google Scholar]
- 22.Senthilnathan S, et al. Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: Analyzing patterns of progression in 285 patients. Hepatology. 2012;55:1432–1442. doi: 10.1002/hep.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atanasov G, et al. Angiogenic inflammation and formation of necrosis in the tumor microenvironment influence patient survival after radical surgery for de novo hepatocellular carcinoma in non-cirrhosis. World J. Surg. Oncol. 2019;17:217. doi: 10.1186/s12957-019-1756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochiai T, et al. Clinicopathologic features and risk factors for extrahepatic recurrences of hepatocellular carcinoma after curative resection. World J. Surg. 2012;36:136–143. doi: 10.1007/s00268-011-1317-y. [DOI] [PubMed] [Google Scholar]
- 25.Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann. Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WJ, Lim TW, Park PJ, Choi SB, Kim WB. Prognostic markers affecting the early recurrence of hepatocellular carcinoma with liver cirrhosis after curative resection. Int. J. Biol. Markers. 2019;34:123–131. doi: 10.1177/1724600819834306. [DOI] [PubMed] [Google Scholar]
- 27.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transplant. 2013;19:1318–1323. doi: 10.1002/lt.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transplant. 2014;20:945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 30.Dumitra TC, et al. Pretransplantation alpha-fetoprotein slope and milan criteria: Strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation. 2013;95:228–233. doi: 10.1097/TP.0b013e31827743d7. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto J, et al. Effectiveness of hepatic resection for early-stage hepatocellular carcinoma in cirrhotic patients: Subgroup analysis according to Milan criteria. Jpn. J. Clin. Oncol. 2007;37:287–295. doi: 10.1093/jjco/hym025. [DOI] [PubMed] [Google Scholar]
- 32.Zaydfudim VM, et al. Liver resection and transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann. Surg. 2016;264:650–658. doi: 10.1097/SLA.0000000000001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 34.Sun P, Chen S, Li Y. The association between pretreatment serum alkaline phosphatase and prognosis in hepatocellular carcinoma: A meta-analysis. Medicine. 2020;99:e19438. doi: 10.1097/MD.0000000000019438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J. Hepatol. 2000;33:282–289. doi: 10.1016/s0168-8278(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 36.Choi, J. Y., Lee, J. M. & Sirlin, C. B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology272, 635–654. 10.1148/radiol.14132361 (2014). [DOI] [PMC free article] [PubMed]
- 37.Llovet JM, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 38.Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: The outstanding achievements of the Liver Cancer Study Group of Japan. Dig. Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 39.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 40.Mazzaferro V, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 41.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Kim HN, et al. Factors associated with delayed hepatitis B viral suppression on tenofovir among patients coinfected with HBV-HIV in the CNICS cohort. J. Acquir. Immune Defic. Syndr. 2014;66:96–101. doi: 10.1097/QAI.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.