Abstract
Endothelial cells (ECs) lining all blood vessels are subjected to large mechanical stresses that regulate their structure and function in health and disease. Here, we review EC responses to substrate-derived biophysical cues, namely topography, curvature, and stiffness, as well as to flow-derived stresses, notably shear stress, pressure, and tensile stresses. Because these mechanical cues in vivo are coupled and are exerted simultaneously on ECs, we also review the effects of multiple cues and describe burgeoning in vitro approaches for elucidating how ECs integrate and interpret various mechanical stimuli. We conclude by highlighting key open questions and upcoming challenges in the field of EC mechanobiology.
Subject terms: Biophysics, Cell biology
Dessalles et al review the responses of vascular endothelial cells to mechanical forces exerted by both blood flow and physical contact with the basement membrane. Special attention is paid to how endothelial cells respond to multiple mechanical cues that are exerted simultaneously.
Introduction
Research over the past two decades has established that mechanical forces are potent regulators of cellular structure and function in both health and disease. While all cells in our tissues experience physical forces, mechanical stimulation plays a particularly prominent role in the vascular system. By virtue of its strategic location at the interface between the bloodstream and the vascular wall, the endothelium is constantly subjected to a complex set of mechanical stresses that are often highly dynamic in nature. The ability of the endothelium to sense these biomechanical stimuli and to integrate information from different types of biophysical cues is essential for regulating vascular function.
The mechanical environment of the endothelium consists of a collection of intertwined stresses, which can be broadly divided into two categories (Fig. 1): contact stresses emanating from physical features of the underlying substrate and fluid-derived stresses due to blood flow. The contact stresses act on the endothelial cell (EC) basal surface and are principally due to substrate topography, curvature, and stiffness. The fluid-derived stresses consist of the shear stress on the EC apical surface due to the flow of viscous blood, the compressive blood pressure, and the circumferential and axial tensile stresses due respectively to the transmural pressure difference and tissue movement. All these stresses are dynamic in nature, albeit with considerably different time scales. While substrate physical properties at a given vascular location remain globally constant at short time scales, shear, pressure, and tensile forces are highly dynamic and vary cyclically due to blood flow pulsatility. In addition, the nature and magnitude of all these mechanical cues vary with location in the vasculature and across organs.
Fig. 1. Summary of the biophysical forces present within the vasculature.
Endothelial cells (ECs) within blood vessels are subjected to various mechanical cues, from the substrate (orange, left panels) or from the blood flow (purple, right panels). Physiological (or pathological in parentheses) ranges of values are provided for each type of cue.
EC sensing and responsiveness to mechanical forces are critical for vascular homeostasis. How mechanical stimuli exerted on the EC surface are converted into intracellular biochemical responses has been reviewed elsewhere1–3 and will therefore not be detailed here. We can nevertheless mention that the various candidate mechanosensors in ECs can be categorized based on their cellular location. Apical mechanosensors include mechanosensitive ion channels4–7, primary cilia8,9, the glycocalyx10, GTP-binding proteins9,11, and caveole12. At cell–cell junctions, platelet endothelial adhesion molecule-1, vascular endothelial-cadherin, and vascular endothelial growth factor receptors have been shown to form an elaborate mechanosensory complex13–15. Integrins, the principal mechanosensors on the EC basal surface, provide a direct link between the actin cytoskeleton and the extracellular matrix (ECM)16,17 and are involved in the response to both substrate- and flow-derived cues15.
EC mechanobiology has been the subject of several excellent recent reviews, each with a specific focus: mechanotransduction events and governing mechanisms2,3, experimental platforms to mechanically stimulate ECs3,18, angiogenesis and vascular development19, mechanobiology of vascular diseases20, and physical/mechanical considerations21,22. Here we review EC responses to the different types of contact- and fluid-derived mechanical stresses present in the vasculature. We identify key features of each one of these biophysical cues and discuss how they affect EC morphology, intracellular organization, and overall function. We pay special attention to the interplay among these stresses and how ECs integrate multiple mechanical signals. Throughout the review, we focus on in vitro studies that have greatly advanced our understanding of EC mechanics and assess their physiological relevance and ability to mimic pathological conditions. We conclude by highlighting open questions and describing how recent technological advances promise to offer new avenues in the field of EC mechanobiology.
Substrate-derived biophysical cues
In vivo, the vascular endothelium responds to various biophysical cues derived from the underlying vascular wall structure, most notably the topography of the vascular basement membrane (BM), the curvature of the tubular vessel wall, and the stiffness arising from the mechanical properties of the BM and adjacent cellular and connective layers (Fig. 1).
Topography
Vascular ECs are anchored to a BM that is a few hundred nanometers thick and whose topography takes the form of intermingled fibers and pores23,24. The BM thus presents ECs with an isotropic topographical environment at the nanoscale, whereas at the microscale, the topography appears more anisotropic, formed by the organization of nanoscale structures and/or by tissue undulations23,25.
Influence of topography architecture: anisotropic vs. isotropic substrates
Several types of engineered substrates have been developed to mimic the anisotropy found in some native extracellular environments. Despite being highly idealized configurations that differ considerably from physiological BM topographies, unidirectional grooved substrates consisting of parallel arrays of rectangular grooves and ridges have been widely used because their layout and dimensions can be precisely controlled, thereby providing well characterized biophysical cues to cells (Fig. 2a). Studies on these ridge/groove systems have provided crucial information about fundamental EC responses to anisotropic topographies. For instance, ECs have been shown to migrate, align, and elongate in the direction of grooved substrates26–33, reproducing EC morphologies encountered in vivo. Cell alignment and elongation are reflected intracellularly by the alignment of actin filaments26,28,31–33, microtubules32, and focal adhesions (FAs)26,29,31. Additionally, anisotropic grooved surfaces lead to stronger EC adhesion, more oriented migration, and greater migration speeds compared to isotropic surfaces (such as arrays of pillars or holes)34,35.
Fig. 2. Substrate-derived cues experienced by endothelial cells and associated experimental model systems.
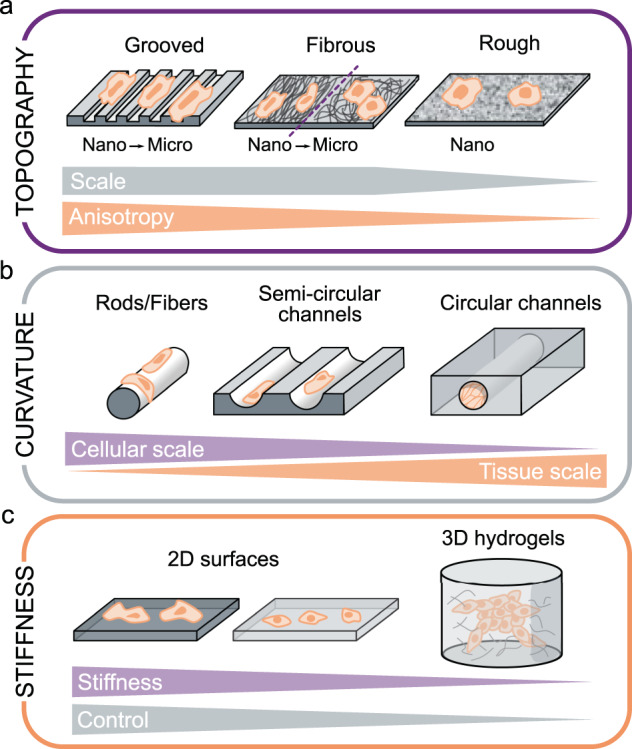
a Substrate topography can be mimicked in vitro with grooved, fibrous, or rough surfaces. Each of these systems can provide a different level of anisotropy at different scales (nano- or micro-scale). b Substrate curvature can be mimicked in vitro with microscale rods or fibers, semicircular channels, or full circular channels. These systems provide curvature ranging from the cellular scale to the tissue (monolayer) scale. c Substrate stiffness can be tuned in either 2D surfaces (where increased cell spreading is observed on stiffer substrates) or 3D hydrogels (where smaller stiffness values can be attained but at the expense of more limited control of the extracellular environment).
To assess the effect of the nanoscale BM roughness found in vivo, different types of rough substrates have been developed36–38 and have been shown to promote EC adhesion and growth relative to smooth surfaces36 (Fig. 2a). On these substrates, ECs are typically more elongated than on smooth flat surfaces but less so than on grooved substrates, and they migrate faster than on untreated surfaces37.
Fibrous networks are attractive model substrates as they resemble the physiological structure and composition of native BMs (Fig. 2a). Aligned (and thus anisotropic) fibers at both the nano- and microscale induce higher levels of cell and cytoskeletal alignment and elongation compared to random fibers39–42, as well as increased migration directionality43,44. From a more functional perspective, ECs on both aligned fibrous scaffolds39,40 and grooved substrates30,40,45,46 appear to adopt an anti-inflammatory and antiatherogenic phenotype.
Influence of topography dimensions: micro- vs. nanoscale cues
In vivo, the endothelial BM is a multi-scale topographic surface with both nanoscale fibers and microscale aligned or anisotropic topographies. Most studies suggest that decreasing groove width from the micron to the nanometer scale increases cell elongation, alignment, adhesion, proliferation, and FA size and stability28,32,33. In contrast, a similar decrease in groove depth decreases EC alignment and elongation29,31. On fibrous substrates, microscale fibers have been reported to induce both decreased41 and increased42 EC alignment and elongation relative to nanoscale fibers. This apparent contradiction may be explained by differences in pore sizes between the two studies, with cells capable of spreading over multiple fibers in the first study but not the second.
As cells usually encounter micro- and nano-cues simultaneously, combining both scales provides a more physiologically relevant environment. In a study that focused on this issue, nanofibers aligned in the same direction as microscale grooves were shown to have a synergistic effect, amplifying EC elongation and alignment, whereas an orthogonal orientation between nanofibers and microgrooves had an antagonistic effect47, demonstrating that ECs can integrate topographical cues of different scales.
Influence of EC type and cell density
ECs in vivo possess great phenotypic variability depending on the vascular bed and organs. Whether substrate topography induces different responses in different EC types remains unknown but is an important question given the differences in BM structure between arteries and veins23,25. A comparison of arterial, venous, and stem cell-derived ECs on grooves shows that while their morphological responses are similar, different phenotypic responses are observed: both primary cell lines preferentially adopt a venous phenotype, while grooves promote an arterial phenotype in stem cell-derived ECs48. The effect of groove size on the alignment and migration of ECs has also been shown to depend on EC type34, which may explain the discrepancies reported in the literature regarding the optimal topographic feature size for cell alignment.
An understudied aspect is EC response to topography in pathological settings. A recent study reveals that diabetic ECs are generally less responsive to topography than healthy ECs in terms of angiogenic capacity and monolayer integrity49. Similarly, ECs from older donors exhibit decreased cell speed but higher directionality along grooves compared to those from younger donors50.
ECs in vivo form continuous monolayers, while many of the previously mentioned in vitro studies focus on individual cells. Single ECs and EC monolayers exhibit broadly the same response to topography: they align and elongate along anisotropic substrates such as grooves or aligned fibrous scaffolds30,32,35,39,40,45,51. One study reported that EC alignment on grooved substrates decreased near confluence31, suggesting that ECs in monolayers are nevertheless less responsive to topographical cues than individual cells.
Curvature
The impact of substrate curvature on cells has only recently been investigated and is now recognized as a critical cue regulating cell behavior52. In the vasculature, ECs encounter curvature at the subcellular, cellular, and tissue (monolayer) scales.
Subcellular curvature is due to the curvature of BM fibers and is typically studied using nano- to microscale wavy surfaces (sinusoidal grooves); however, only a few studies have so far used these types of substrates on ECs. In general, ECs align and elongate in the direction of sinusoidal grooves as they do on rectangular grooves, but they exhibit lower migration speeds53. EC alignment decreases progressively with increasing wavelength (i.e., decreasing curvature) until it disappears at a wavelength of ~50 µm53–55, a dimension comparable to EC size.
The curved vascular wall subjects ECs to cell- to monolayer-scale curvature. The diameter of human blood vessels ranges from ~5 µm in capillaries to more than 1 cm in large arteries, which translates to curvatures ranging from 0.1 to 100 mm−1, spanning four orders of magnitude. Starting with simple, non-perfused systems of semicircular channels, it was observed that ECs form confluent monolayers that conform to the curved substrate56–58 (Fig. 2b), orient along the longitudinal axis, and have fewer actin stress fibers compared to adjacent flat regions56. More recently, many “vessel-on-chip” systems have been developed where ECs are cultured in closed, perfused channels of physiological diameters (Fig. 2b)59–61, but the impact of substrate curvature on the cells in these systems has not yet been investigated.
The most informative studies on the impact of curvature on ECs arise from the use of microfibers over which ECs are cultured, mimicking the high curvature found in smaller vessels such as capillaries (Fig. 2b). While endothelial colony forming cells (ECFCs) were observed to orient in the direction of fibers with diameters of 5–11 µm62, human umbilical vein ECs (HUVECs) were reported to exhibit a circumferential orientation and ring-like actin organization on 5–20 µm PCL fibers62,63 but a longitudinal orientation on 14 µm glass rods64. In this last study, human brain microvascular ECs (HBMECs) retained a random orientation regardless of rod curvature. The authors hypothesized that this resistance to curvature might be an organ-specific behavior, allowing brain ECs to minimize the length of cell–cell junctions and hence to maintain very low permeability levels64. Interestingly, the combination of cell-scale curvature with nanoscale topography reveals competing effects: while nanotopography orientation appears to drive ECFC orientation, the orientation of the secreted ECM is driven by the cell-scale curvature65.
Stiffness
An elastic material’s bulk deformability is characterized by its Young’s modulus, an intrinsic property defined as the ratio of stress to strain (SI units of Pa). Although the term “stiffness” is commonly used to indicate if a material is “soft” or “hard”, its technical definition is the ratio of an applied force to the elongation in the direction of the force (SI units of N/m). Stiffness thus depends on the material’s dimensions. In this review, the term “stiffness” corresponds to its common language usage. As such, the material stiffness values are actually Young’s moduli. It should be noted that in the literature, a clear distinction between stiffness and Young’s modulus is not always made.
Atomic force microscopy (AFM) measurements on excised adult BMs suggest that their elastic modulus is in the 1–4 MPa range25. A wide range of vessel wall stiffnesses has been reported, from ~10 kPa66,67 to 1.5 MPa68. It is important to emphasize that stiffnesses measured ex vivo in excised vessels differ from those in pressurized vessels in vivo69,70 due to the strain-stiffening behavior of extracellular matrices. Additionally, vessel wall stiffness changes with anatomical location and increases with age71,72 as well as with cardiovascular pathologies such as hypertension73 or atherosclerosis. The effective stiffness that ECs perceive in vivo is difficult to determine. Reports of the distance over which cells feel stiffness vary from a couple of microns to tens of microns74,75. By using soft gels on glass substrates, it has been shown that this distance depends both on the substrate’s Young’s modulus and the gel thickness74–76. Based on these studies, we can assume that large vessel ECs in vivo sense a combination of the stiffnesses of the BM and the underlying medial and adventitial layers, whereas ECs in small vessels might even sense the stiffness of adjacent tissues.
Influence of 2D substrate stiffness on EC morphological and functional responses
Varying substrate stiffness in vitro has a clear impact on EC morphology, with cells being more round and less spread on soft substrates (1–5 kPa) than on stiff substrates (20 kPa–2 MPa)77–81 (Fig. 2c). In terms of intracellular organization, ECs on softer substrates are associated with fewer FAs79,80,82–84 and actin stress fibers77,80,82,85–88. Furthermore, ECs on stiff substrates are more contractile and exhibit larger traction forces, both as single cells81 and in monolayers, as assessed by traction force microscopy85,88,89. The stresses within the monolayer are lower but are also more homogeneously distributed on soft substrates80.
Various studies have shown that monolayer and cell–cell junction integrity decrease with increasing substrate stiffness79,80,85,89–91, with important functional consequences for monolayer permeability and inflammation89,92. ECs in vivo can also encounter cell-scale heterogeneities in BM stiffness66. In vitro, gaps in EC monolayers were observed with increasing matrix stiffness heterogeneity, and tended to localize at matrix stiffness interfaces84, suggesting that stiffness gradients regulate EC structure and function.
Influence of EC type and cell density
Interestingly, although observed in both individual cells and monolayers, stiffness-related morphological and cytoskeletal modifications are considerably smaller in the case of monolayers78,86. This may reflect preferential force redistribution towards cell–cell junctions within monolayers. Although veins have softer walls than arteries93, it remains unknown if venous and arterial ECs respond differently to substrate stiffness. However, the switch to a venous phenotype observed for arterial ECs cultured on soft matrices (0.5 kPa) suggests that endothelial morphology and function are principally determined by the biomechanical properties of the ECM environment rather than intrinsic differences between arterial and venous ECs83.
Influence of 3D substrate stiffness on EC network formation
To better simulate the native extracellular environment, recent efforts have focused on developing 3D culture systems. Although the vascular endothelium can be viewed as an essentially 2D structure, 3D environments are particularly relevant in the context of angiogenesis, where new microvessels sprout from existing vessels in all directions (Fig. 2c). Studies using hydrogels of varying stiffnesses in the range of 0.1–10 kPa show enhanced EC vascular network formation in softer matrices87,94–98. Consequently, ECs migrate longer distances99 and are more spread in soft gels94, the opposite effect to that on 2D substrates. Importantly, tuning collagen gel stiffness by controlling the cross-linking rather than altering gel density and structure (as in the previous studies) yields the opposite effect: stiffer (500 Pa) collagen gels lead to increased spreading, number, length, and branching of angiogenic sprouts90,100. This observation highlights the importance and intrinsic difficulty of independently tuning the detailed features of the environmental cues, mainly stiffness and topography, in 3D culture systems. Using colloidal gels where stiffness and topography can be independently controlled, a recent study concluded that there is an optimal structure of tenuous strands and spacious voids for EC network formation, irrespective of matrix stiffness97. The magnitudes of the 3D substrate stiffnesses eliciting the aforementioned effects are approximately an order of magnitude lower than the 2D substrate stiffnesses, suggesting that matrix dimensionality modulates matrix stiffness effects. In any case, the apparent stiffness perceived by cells is hard to evaluate, as illustrated by the different morphologies of vascular networks seen in floating and constrained collagen gels that are otherwise identical96. In addition, while most of the different gels used in 3D studies possess purely elastic properties, the extracellular environment and its components in vivo exhibit viscoelastic and strain-stiffening behavior that are rarely reproduced in vitro, creating an additional layer of complexity for the physiological relevance of these models.
Flow-derived mechanical cues
The endothelium experiences multiple mechanical stresses due to the flow of viscous blood. These include fluid dynamic shear (or frictional) stress, compressive pressure, and tensile (or hoop) stresses due to the transmural pressure difference (Fig. 1).
Flow shear stress
Fluid shear stress is the tangential frictional force per unit area experienced by the endothelium as a result of blood flow. Blood is a complex non-Newtonian fluid composed principally of plasma and red blood cells. At sufficiently low shear rates (below ~100 s−1), blood exhibits shear-thinning behavior. Therefore, the non-Newtonian of blood strongly affects the shear stress on the EC surface within low shear regions101–103. In the microvasculature, blood can no longer be considered a homogeneous fluid but rather a suspension of deformable active particles104–106. Recent results indicate that in those vessels, the presence of blood cells can significantly alter the near-wall flow field, thereby impacting the shear stress on the EC surface106. Most of the in vitro studies discussed here document EC responses to physiological values of shear stress that are generated using more simple Newtonian fluids. Those investigations have revealed that shear stress regulates major EC functions including angiogenesis, vessel remodeling, and cell fate19. In vivo, the time-averaged wall shear stress is ~1 Pa in the aorta, ~5 Pa in small arterioles1,107, ~2 Pa in venules, and ~0.1 Pa in the vena cava107–110. Furthermore, within the same vessel, shear stress levels and profiles can vary significantly due to geometric features including vessel curvature and branching.
Steady flow: undisturbed vs. disturbed
Vascular flows can be broadly classified as either “undisturbed” or “disturbed”. Undisturbed flows are most typically uniaxial and laminar flows; whereas disturbed flows include turbulent flows and laminar flows with spatial shear stress gradients and/or secondary flows. In relatively straight vascular segments, flow streamlines are largely undisturbed and remain mostly parallel to the vascular wall111 (Fig. 3a). In contrast, flow in areas of vascular curvature, branching, and bifurcation becomes highly disturbed with regions of flow separation and recirculation (Fig. 3a). Interestingly, these zones of flow disturbance correlate with the localization of vascular diseases including atherosclerosis112,113, aortic valve calcification114,115, and inflammation and thrombosis in veins115,116.
Fig. 3. Features of flow-induced shear stress experienced by endothelial cells (ECs).

a ECs experience either undisturbed or disturbed flow, which have different effects on cytoskeletal (CSK) organization and the inflammatory state of the cells. b ECs can experience steady, pulsatile, or oscillatory flow. The flow dynamics influence cytoskeletal organization and the inflammatory state of the cells. c ECs experience flow in the luminal direction (parallel to cells, on the apical side), transmural (across the endothelium, on cell–cell junctions), or interstitial (in the vessel wall or parenchymal tissue, on the basal side). The direction of the flow influences EC quiescence and/or angiogenesis.
In vivo, ECs in undisturbed flow zones are elongated and aligned in the direction of blood flow117,118. In contrast, ECs in disturbed flow areas are more cuboidal (round) and randomly oriented119,120. In vitro, steady flow systems are able to reproduce these observations, with undisturbed shear stress elongating ECs and aligning them parallel to the flow direction4,121–125 and disturbed shear stress leading to round EC shapes and random cellular orientation121,126,127. At the cytoskeletal level, undisturbed flow leads to prominent actin stress fibers that are aligned in the direction of flow128–131, whereas ECs subjected to disturbed flow exhibit shorter and randomly oriented actin filaments4,121,123,130. Shear-induced cytoskeletal reorganization is initiated within 1 h after flow onset132, even though complete cytoskeletal remodeling and cellular shape changes require significantly longer times120.
EC morphological and alignment responses to steady flow depend not only on the type of applied shear stress but also on shear stress magnitude. Although increasing shear stress level generally increases the extent of cell elongation and alignment120,133,134, there seems to be an optimal value of shear stress above which the response decreases112. Interestingly, several recent studies have challenged the consensus of EC alignment in the direction of steady flow, with reports of perpendicular alignment at both low shear (0.3 Pa)135 and high shear (>10 Pa)136,137. The alignment response may be dynamic, with a switch from perpendicular to parallel orientation after 72 h of flow138.
In addition to its effect on EC shape and cytoskeletal organization, steady unidirectional flow elicits higher EC migration speeds than disturbed flow127,139, leading to more efficient wound healing. This effect is dependent on shear stress magnitude140 and on the direction of cell migration, with ECs along the downstream wound edge migrating more slowly than ECs along the upstream edge140. ECs also appear to retain a level of shear stress memory, with pre-sheared ECs exhibiting accelerated wound closure141,142. Moreover, steady unidirectional flow promotes an anti-inflammatory and antithrombotic EC phenotype with reduced cell apoptosis and proliferation143,144 while disturbed flow has the opposite effect126.
Two additional and important features of disturbed flow that affect EC behavior are spatial shear stress gradients and secondary flows. Shear stress gradients inhibit EC alignment in the direction of the shear in vitro138,145,146. The alignment response can be restored, however, by supra physiological levels of shear stress147 whose value depends on the gradient magnitude148. EC alignment is also restored by switching from a positive to a negative gradient, underscoring the importance of gradient direction149. In a new type of “impinging flow device”, ECs were surprisingly observed to align perpendicular to high shear stress gradients136, emphasizing our incomplete comprehension of EC response to shear gradients.
Secondary flows associated with disturbed flow zones in regions of branching and curvature arise from streamline curvature. A key consequence of secondary flows for ECs is the generation of transverse shear stresses that act orthogonal to the axial shear stress due to the primary flow150,151. Interestingly, the localization of atherosclerotic plaques has recently been correlated with transverse flow rather than with disturbed axial flow152. The response of ECs to these secondary flows remains unclear with some reports of alignment loss in orbital shakers153–155. However, the transverse shear stresses in most of these systems are also accompanied by shear stress gradients and in some cases oscillatory shear forces156, thus complicating the isolation of individual effects and underscoring the need for the development of novel in vitro platforms that allow decoupling of these various phenomena. Recently, a unique system of spiral microvessels was developed in which physiological torsion and curvature enable the generation of physiological secondary flows157. This new generation of systems will certainly enhance our understanding of complex flow patterns and their influence on ECs.
Pulsatile flow: non-reversing vs. reversing vs. oscillatory
A key feature of blood flow in larger vessels is its pulsatility due to the rhythmic heartbeat. Within the microvasculature, this pulsatility is significantly dampened, and blood flow becomes quasi-steady. Thus, the shear stress profile on the EC surface depends on the vascular location. In undisturbed flow regions in medium and large vessels, the endothelium experiences non-reversing pulsatile flow, whereas in disturbed flow zones, the shear stress exhibits periodic directional reversal and oscillation.
ECs in vitro are able to discriminate among steady, non-reversing pulsatile, reversing pulsatile, and oscillatory (zero net) flow (Fig. 3b)158–160. For instance, while both steady and non-reversing pulsatile flow induce EC elongation and alignment in the flow direction161,162 as already mentioned, they do so with different dynamics158. The recruitment of apical stress fibers and the high migration persistence observed under steady flow disappear under non-reversing pulsatile flow161. Prolonged exposure to either reversing pulsatile flow or oscillatory flow fails to elicit EC elongation, orientation, and cytoskeletal remodeling158,159,163. Contrary to steady flow, oscillatory flow disrupts cell–cell junctions125 and elicits a pro-inflammatory and atherogenic EC phenotype164–166. These results underscore the need for understanding the links between the exact flow waveform and the resulting EC responses.
Luminal vs. transmural vs. interstitial flow
ECs in vivo are subjected to a combination of luminal, transmural, and interstitial flow (Fig. 3c). Luminal blood flow exerts a shear stress on the apical EC surface. The pressure difference across the vascular wall generates a transmural flow, particularly prominent in the microvasculature with typical flow velocities of ~1 µm/sec167,168. Transmural shear forces are exerted most directly on endothelial cell–cell junctions. Interstitial flow arises from fluid movement within the tissue surrounding the ECs, shearing ECs on their basal side. For large vessels, interstitial flow can originate from transmural flow currents as well as from other sources such as fluid leakage from the vaso vasora. In the microvasculature, interstitial flow stems from porous medium flow in the surrounding parenchyma.
In vitro, physiological levels of transmural flow increase endothelial sprouting169 both in the cases of outward170,171 and inward flow172,173, with more filopodial protrusions under inward flow169. Transmural flow is also necessary for sustained sprout elongation170. In a microfluidic branching model, transmural flow was shown to restore sprouting after inhibition by luminal shear stress174. Interestingly, the shear stress threshold for triggering angiogenesis is conserved between luminal and transmural flow, at ~1 Pa170.
Because interstitial flow can have multiple sources, its intensity is highly variable across the vasculature and is difficult to measure in vivo, with the few reported velocities varying between 0.1 and 4 μm/sec168,175. In vitro, interstitial flow around ECs embedded within a 3D matrix stimulates network formation176–178. Vascular tubes align in the flow direction179, with vascular sprouts elongating against the flow direction176,180.
Influence of EC type and cell density
Arterial and venous ECs exhibit different responses to flow-induced shear stress. For instance, arterial ECs become more polarized181 and exhibit more prominent actin stress fibers in the direction of flow than venous ECs131,182,183. It is also notable that certain types of ECs such as aortic valve ECs or lymphatic ECs behave differently with alignment orthogonal to the direction of flow184,185. Similarly, brain microvascular ECs subjected to a steady shear stress of 1.6 Pa do not elongate or align and continue to exhibit a randomly oriented cytoskeleton186,187.
Although EC responses to flow have been investigated at both the single cell and monolayer levels, very few studies have specifically tackled the influence of cell density on EC flow responses. One study reported that only confluent ECs aligned in response to a 2 Pa shear stress188. Cell density also influences EC migration: while low density ECs migrate in the direction of the flow, ECs in dense monolayers move against the flow direction136.
Compressive stresses from blood pressure
Blood pressure exerts a normal force that compresses the EC apical surface. Blood pressure varies drastically along the vasculature, from ~1.3 kPa (10 mmHg) in veins up to ~16 kPa (120 mmHg) in the human aorta during systole. Severe hypertension may lead to pressures as high as ~27 kPa (200 mmHg). Blood pressure is highly pulsatile on the arterial side, but these oscillations are progressively dampened by the vessel elasticity until they virtually disappear in capillaries.
Hydrostatic pressure applied to ECs in vitro influences cell shape, cytoskeletal organization, and various aspects of vascular function. Bovine aortic ECs (BAECs) under both physiological (5–20 kPa) and low pressure (0.1–1 kPa)189,190 elongate while maintaining a random orientation. They also have a smaller area and less smooth cell contours191–194. However, some studies applying similar pressures reported no elongation in BAECs163,195,196. Interestingly, HUVECs under physiological pressure also have a reduced cell area but do not exhibit the elongation and tortuosity responses197,198, suggesting that pressure-induced changes in cell shape are specific to arterial ECs. The morphological changes are mirrored by cytoskeletal reorganization, with the formation of central stress fibers and remodeling of FAs under both physiological163,192,194 and low pressure values189,190,199. Pressure-induced cytoskeletal reorganization follows a two-step dynamic: increased cytoskeletal tension through actomyosin-mediated contraction after 1 h followed by increased cortical actin density through actin polymerization and assembly of stress fibers198,200.
Hydrostatic pressure at both physiological197 and sub-physiological levels199 increases EC proliferation, consistent with reports of increased angiogenesis and tubulogenesis in these situations198,201. For low and physiological ranges of pressure, the rate of proliferation correlates with the magnitude of the applied pressure189,193, while pathological pressures (20–25 kPa) induce EC degeneration and apoptosis202,203.
One hypothesis put forward to explain pressure-induced EC proliferation is the disruption of adherens junctions192,204, leading to a multilayer EC structure in vitro189–192. Indeed, the formation of VE-cadherin-based junctions normally inhibits proliferation within confluent monolayers205,206. The multilayer structure is not observed in subconfluent ECs under pressure193, which confirms that it originates from an excessive proliferation of confluent ECs. Physiological pressure further alters endothelial function by increasing intercellular gaps197, inducing a reversible loss of monolayer integrity191,207, loss of barrier function200, and increased permeability204. In contrast, low pressure was shown to protect pulmonary endothelial monolayer integrity against inflammatory agents208.
Unlike constant pressure, the effect of pulsatile pressure on ECs in vitro has received little attention. Similar to constant pressure, a magnitude-dependent effect of pulsatile pressure on proliferation was reported, with physiological pressure values increasing proliferation and pathological values decreasing it196,201. Application of pulsatile pressure also leads to a magnitude-dependent decrease in peripheral actin and relocalization of ZO-1 junctions associated with decreased permeability209.
Tensile stresses
Tensile stresses in the vasculature can be axial, due to tissue growth or movement, or circumferential (hoop), due to the transmural pressure difference which dilates the vessels cyclically. In vivo measurements report a wide range of axial strain magnitudes across the vasculature: ~20% in the lungs210, 5% in coronary arteries211, and 5–15% in leg arteries212–214. Hoop strains due to diameter changes during the cardiac cycle are in the range of 0–15%213,215–218. In most in vitro studies, 5–10% strains are considered physiological while 15–20% strains are viewed as pathological.
General cell response: alignment, elongation, and activation
In vitro studies that have examined the effect of stretch on ECs all report EC elongation and alignment orthogonal to the strain direction219–221. This result is in line with the longitudinal alignment of ECs in vivo, orthogonal to the direction of circumferential strain. This behavior is highly dynamic: when the direction of the strain is changed during the experiment, ECs reorient orthogonal to the new strain direction220. The cell morphological changes are reflected intracellularly by orthogonal alignment of actin filaments and an increased number of stress fibers222,223. At the onset of stretch, stress fibers disassemble224,225 with vanishing traction forces226, then stress fibers reassemble in the transverse direction within minutes222,224,227,228 with recovery of the transverse traction forces, and finally the entire cell reorients within hours221,226. Stretching the substrate upon which cells adhere increases tension in the actin cytoskeleton229, increasing cell stiffness230,231.
Another major effect of strain is EC activation: low strains (5–10%) inhibit apoptosis and increase proliferation, while large strains (15–20%) have the opposite effect232–235. The stretch-induced proliferation requires cell–cell junctions236–239. An intermediate level of strain (10%) is also able to increase endothelial motility and migration240,241, tubulogenesis, and endothelial sprouting235,242 and aligns the newly formed sprouts orthogonal to the strain243,244. The stimulation of angiogenesis is observed for both static and cyclic tensile strains.
Stretch direction: free uniaxial, pure uniaxial, and biaxial
Beyond the general observation of EC alignment orthogonal to strain, variations in the exact orientation angle have been reported. These apparent discrepancies are reconciled when viewed through the lens that ECs align in the direction of minimal strain221,223. Three major forms of stretching can be applied to cells: free uniaxial, pure uniaxial, and biaxial (Fig. 4a). In free uniaxial stretching, the substrate on which the cells are cultured is elongated actively along one axis but undergoes slight retraction in the orthogonal direction due to the positive Poisson’s ratio exhibited by most materials. In pure uniaxial stretching, the sample is constrained orthogonal to the uniaxial stretch in order to prevent the orthogonal (transverse) retraction strain. In biaxial stretching, the sample is stretched in two orthogonal directions via two unidirectional stretches of a rectangular sample, radial stretching of a circular sample, or by inflation of a circular membrane.
Fig. 4. Features of tensile stresses experienced by endothelial cells.

a Strain direction can be purely uniaxial, free uniaxial (with an additional lateral compression), or biaxial (isotropic). Purple dotted lines indicate minimum strain direction. Strain direction influences cytoskeletal (CSK) organization. b Strain amplitude influences cytoskeletal organization and cell proliferation, which are maximized for intermediate strain amplitudes. c Strain frequency and strain rate influence cytoskeletal organization and cell alignment.
The first experiments with pure uniaxial stretching were conducted in the late 1990s221, with a follow-up study that specifically explored the variation of cell orientation among the three types of stretchers223. Cells were found to orient in the minimal strain direction (90° for pure uniaxial stretching220,223,245 and ~70° for free uniaxial stretching223,246), which is determined by the balance between the longitudinal tensile strain and the transverse compressive strain228. ECs were also found to avoid pure compressive strains, albeit with slower dynamics247.
In the biaxial stretch configuration, cells are subjected to two different minimum strain directions, leading to a cuboidal morphology223,245,248. These platforms are mostly used to investigate functional responses to stretch, such as cell proliferation or changes in gene expression. Inflation of circular membranes generates equiaxial strain at the center, where cells are seen to be randomly oriented, and a gradient of anisotropic biaxial strain in the peripheral areas which complicates interpretation of cell orientation responses249,250. In contrast, radial stretching of circular membranes leads to uniform equiaxial strains248.
Stretch pattern: amplitude, frequency, and waveform
EC response to cyclic stretch loading is modulated by the stretch pattern, most notably its frequency, amplitude, and precise waveform (Fig. 4b, c). Increasing the frequency towards the physiological value of 1 Hz amplifies cell response, leading to more elongated cells with an orientation angle closer to the minimum strain direction251 and with a faster response252. A minimum frequency of 0.1 Hz for cyclic strain is necessary to induce a response251,253,254. Similarly, the stretch amplitude has a strong influence on EC mechano-response. Large physiological strains (~10%), induce a final orientation that is closer to the “minimum strain direction”228,247,255, reduced orientation dispersion220, and accelerated dynamics252 relative to smaller strains (~5%). For pathological strains, above 15%, the results are less clear, with reports of both orthogonal252,256 and longitudinal alignment250. Possible explanations are differences in cell type (venous vs. arterial ECs) or in stretching profiles (free uniaxial vs. biaxial). Finally, physiological waveforms, i.e. faster extension and slower relaxation, lead to more intense responses with faster dynamics252,257, as ECs are more responsive to tensile strain rate than to compressive strain rate258.
Influence of EC type and cell density
Single cells align in the direction of a 2% compressive strain while confluent monolayers align in the direction of the true minimum strain259. Cell confluency also modulates the response to strain amplitudes, suggesting an important role for cell–cell junctions259. The previously described switch of endothelial orientation from orthogonal to parallel to the stretch direction under pathological strain levels is lost when cell–cell junction formation is inhibited via blocking antibodies250. This collective orientation switch is hypothesized to be key for preserving monolayer integrity, which can be damaged under pathological strains260.
An additional layer of complexity is mechanical preconditioning. EC confluent monolayers grown under cyclic strain withstand deformation while their static counterparts are less compliant and detach from the substrate under strain224. Interestingly, the denudation zones correlate with areas of initial higher density, which is known to modulate cytoskeletal tension and substrate adhesion205, two key components of the strain sensing mechanism. Lastly, as strain levels vary along the vascular tree, EC type (arterial, capillary, or venous) is likely to modulate its mechano-response. One study tackled this question and found that only venous ECs reorient while only arterial ECs exhibit increased proliferation in response to stretch222. The dependence of the stretch response on EC type merits further investigation in light of the fact that most of the other studies reviewed in this section have reported orthogonal alignment despite using arterial ECs, and a number of studies using venous ECs have reported increased proliferation235,236,244.
Combined cues
While the study of individual substrate- and flow-derived mechanical cues on ECs has greatly improved our understanding of the fundamental mechanobiology of these cells, ECs in vivo experience a complex combination of all these cues. The recent development of experimental platforms integrating multiple mechanical stimuli has begun to address this issue. Depending on their respective magnitudes, waveforms, directionality, and the cellular processes they regulate, multiple cues may have synergistic or antagonistic effects when they regulate the same cellular process(es), or one cue may modulate the effect of another on a cellular response that it does not directly regulate on its own.
Flow and substrate cues
Flow and topography
How apical flow-derived shear stress and basal contact stresses due to BM topography cooperate or compete to modulate EC responses is a central question, for optimizing the design and surface properties of endovascular devices. When flow and anisotropic topographies in the form of ridges/grooves are oriented in the same direction, synergistic effects on ECs are observed. For instance, EC orientation and elongation are more pronounced for the combination of both cues than for either one alone137,261. ECs on grooves under flow also exhibit increased adhesion forces51,262, leading to higher resistance to detachment41 and a protective effect on monolayer integrity51,137. They also show enhanced counterflow migration263,264 and thus more efficient wound healing263.
When the flow and anisotropic topography cues are oriented orthogonal to one another, most studies report that ECs align and elongate in the direction of the topography rather than the flow261,265. However, this conclusion depends on both the groove dimensions, with micron-scale grooves counteracting the effect of flow more effectively than nanoscale grooves261, and the magnitude of shear stress, with ECs aligning perpendicular to the grooves for sufficiently high shear stress levels266.
In vivo, ECs that are elongated and aligned in the direction of blood flow exhibit an anti-inflammatory, atheroprotective phenotype whereas ECs in disturbed flow zones are largely cuboidal and have a pro-inflammatory and atheroprone profile267. An interesting question is whether the differences in inflammation phenotype are driven directly by the differences in cell morphology which can also be modulated by the underlying topography. Studies on ECs on grooved substrates in the absence of flow30,39,268 suggest that cell morphology regulates phenotype independently of flow. Nevertheless, a cooperative effect of the two types of cues has also been reported, with aligned collagen fibers reducing EC inflammation under disturbed flow265.
Flow and substrate stiffness
How vascular wall stiffening with age or with vascular disease affects EC responses to blood flow has motivated studies of the combination of different substrate stiffnesses with flow-induced shear stress. As an isotropic biophysical cue, substrate stiffness appears to modulate other directional cues. For instance, higher shear stresses (2.2 vs. 0.6 Pa) are required to align ECs on very soft substrates (elastic modulus of 100 Pa) compared to stiffer substrates (10 kPa)269. Kohn et al. nicely demonstrated that stiffness values characteristic of healthy vessel walls (2.5 kPa) promote the atheroprotective signals induced by fluid shear stress compared to stiffer substrates270. Thus, it appears that both exceedingly soft and pathologically stiff substrates decrease EC sensitivity to flow.
Stretch and substrate cues
The interaction between stretch and substrate cues has mostly been investigated on cell types other than ECs such as fibroblasts or cells derived from mesenchymal stem cells. As these studies reveal interesting combined effects, we review the broad findings that should guide future studies on ECs.
Stretch and substrate topography
For adhesion-based substrate cues, cell elongation and orientation are driven by the pattern anisotropy, despite the competing strain271–273. When strain competes with anisotropic substrate topography cues, the dominant cue depends on the characteristic size of the topography: for microgrooves, cells align with the topography274,275, whereas for nanogrooves276 or cell-scale micropillars277, the strain dictates cellular orientation. Interestingly, it appears that while the basal actin and cell shape follow the contact guidance of elliptical micropillars, the apical actin and nucleus orientation are dictated by the strain278, underscoring the complexity of the competition between topography and stretch. When topography and stretch are configured to reinforce one another, they have a synergistic effect as illustrated by the amplification of fibroblast alignment on grooved substrates in the presence of orthogonal stretch279.
Stretch and substrate stiffness
Substrate stiffness alters cellular responses to stretch, with soft substrates either attenuating cell alignment280 or even changing the alignment direction from orthogonal to parallel to the stretch direction281. Conversely, stretch rescues the impaired cell spreading observed on soft substrates280,282. Both substrate stiffness and stretch modify cytoskeletal tension, suggesting the following possible mechanism to explain these results: stretch restores cytoskeletal tension, allowing cell spreading on soft substrates, and soft substrates decrease cytoskeletal tension allowing cells to withstand the additional tension due to alignment parallel to stretch. One study cultured lung ECs on a stretchable membrane of physiological stiffness to mimic the native soft lung matrix and respiration-induced strains283. In this system, a synergistic effect of stiffness and stretch was observed, protecting the cells against inflammatory thrombin.
Finally, stretch and substrate stiffness are tightly coupled, with stiffness determining the level of strain for a given stress and substrate stiffness being affected by stretch. In fibrous matrices (such as collagen) that exhibit strain-stiffening behavior284, uniaxial stretching leads to anisotropic stiffening which aligns cells in the stiffer direction, i.e., the strain direction281,285. Interestingly, cells already aligned by anisotropic stiffness exhibit enhanced alignment in response to cyclic stretch, indicative of a synergistic effect of stiffness anisotropy and stretch281.
Shear stress and stretch
Shear stress and circumferential stretch both arise from blood hemodynamics and are therefore naturally coupled both in vivo and in vitro. As already described, ECs align parallel to shear stress and perpendicular to stretch; thus, axial shear stress and circumferential strain are expected to reinforce one another while shear stress and axial stretch would be expected to counteract one another. ECs subjected to strain orthogonal to an applied shear stress (either steady or pulsatile) indeed exhibit more pronounced286,287 and faster288 alignment as well as more prominent actin stress fibers289. In contrast, stretch parallel to shear stress leads to competition between the two stimuli, with the aggregate effect depending on the relative magnitudes of the cues. More specifically, a shear stress of 0.5 Pa dominates the effect of strain (even for strains of 15%), whereas a shear stress of 0.08 Pa is dominated by the strain245. Owatverot et al. introduced the notion of “equipotent stimuli”: the levels of stresses needed to elicit similar responses in terms of amplitude and dynamics288. They showed that equipotent shear and cyclic stretch cancel one another when applied in counteracting configurations. Interestingly, if the angle between the shear stress and the strain is intermediate (30°–60°), both stimuli influence the response245.
In vivo, pulsatile shear stress and cyclic hoop stretch are not synchronized, exhibiting a phase shift whose magnitude varies across the vascular tree. This phase shift was demonstrated to attenuate the synergistic effect, with an altered production of vasodilators290 and an increased expression of atherogenic genes when both stimuli are perfectly out of phase291.
Conclusions and future directions
In this review, we have described how different forms of mechanical stimulation regulate vascular EC structure and function. Engineered in vitro systems have shed light onto fundamental EC responses to individual mechanical cues, such as cell alignment in the direction of either an applied shear stress or an anisotropic substrate topography and orthogonal to an applied strain. We have also touched upon the need to better characterize possible mechanobiological differences among ECs derived from different vascular beds as well as between single cells and cellular monolayers.
To design and implement systems and experiments that address physiologically relevant questions in mechanobiology, it is essential to carefully characterize the detailed nature of the forces at play. This includes the direction of the forces (isotropic or anisotropic for topography, axial or radial for flow or strain), the dimension/magnitude of the forces (scale of topography, values of strain, shear stress, or stiffness), and, when applicable, the time-dependent pattern of the force (waveform and frequency of strain or flow profile, for instance). Much work is needed to better understand the effect of multiple mechanical cues on ECs and to elucidate the mechanisms by which ECs integrate and decipher multiple environmental signals, as discussed in the last section. In this regard, the notion of “equipotent stimuli” provides an attractive framework for translating the effects of different types of cues into a common “language” that the cell uses to integrate multiple mechanical cues exerted simultaneously. Applying this framework to both physiological and pathological conditions would define the normal “equilibrium” values of the individual stimuli and how pathologies that alter one or more of the biophysical cues would perturb this equilibrium. Synergistic effects are also reported for almost all combinations of cues, which highlights the robustness of the vascular system, as well as potential compensatory mechanisms to maintain homeostasis to the extent possible in pathological settings.
It should be noted that the different mechanical cues are often interdependent, underscoring the need for careful decoupling of their respective contributions. For instance, the topography of the substrate and the waviness of the EC surface modify the shear stress that the cells perceive292–294. Similarly, substrate stiffness changes the effective strain applied on the cells281. In a pathological context, BM thickening (up to twofold) in pathologies such as diabetes295,296 or atherosclerosis297,298 is expected to change both the topography and stiffness sensed by ECs and consequently the strain that ECs undergo. All of these aspects should therefore be carefully taken into account in the design of experimental systems and in the interpretation of the obtained results.
Cellular mechanobiology is an active field of research that nicely complements the more traditional biochemically-centered view of most biological processes. As we have highlighted throughout this review, mechanical forces are particularly diverse, dynamic, and multifaceted in the vascular system, and these forces play a critical role in regulating vascular physiology and pathology. In light of the fact that many of these forces are borne most directly by the endothelium, elucidating the mechanisms governing EC mechanobiology will enhance our understanding of the etiology of various vascular diseases including atherosclerosis, thrombosis, aneurysm formation, and diabetes. Furthermore, understanding how substrate- and flow-derived stresses regulate EC structure and function promises to inform the design and development of next generation implantable endovascular devices including stents, valves, and grafts.
One of the principal challenges in mechanobiology in the coming years is certainly technological. Two recent developments provide unique opportunities for devising novel in vitro systems that promise to greatly enhance our understanding of endothelial mechanobiology. The first development is the democratization of previously complex and expensive techniques, leading to better integration of mechanical cues. An example is the field of microfabrication where techniques such as micropatterning and microfluidics have become much more widely available in the past decade. Another example is the advent of DIY technologies such as Lego-based stretchers299 or paper-based compression-flow devices300. As a result, mechanical platforms can be more readily adapted to investigate particular diseases, as illustrated by a recent study modeling atherosclerosis on a chip60. The second development entails technological advances that allow the fabrication of innovative in vitro platforms, thus enabling new combinations of mechanical cues301. For instance, the topographical patterning of hydrogels302–304, or hydrogel laser carving, recently enabled the production of perfusable endothelialized capillaries inside a soft hydrogel305. Future systems promise to better recapitulate the complexity of the native vascular environment and to provide finer control over the detailed magnitudes, directions, and waveforms of the applied biophysical stimuli, thereby furthering our understanding of EC responses to mechanical forces.
Acknowledgements
This work was funded in part by an endowment in Cardiovascular Bioengineering from the AXA Research Fund (to A.I.B.), a doctoral fellowship from Ecole Polytechnique (to C.A.D.), a postdoctoral fellowship from the Lefoulon-Dellalande Foundation (to C.L.). A.C. was supported by a research grant from the LaSIPS Laboratory of Excellence (to A.I.B.).
Author contributions
C.A.D., C.L., A.C., and A.I.B. wrote, reviewed, and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Primary Handling Editors: Marco Fritzsche and Christina Karlsson Rosenthal.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Claire A. Dessalles, Claire Leclech.
References
- 1.Givens C, Tzima E. Endothelial mechanosignaling: does one sensor fit all? Antioxid. Redox Signal. 2016;25:373–388. doi: 10.1089/ars.2015.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charbonier, F. W., Zamani, M. & Huang, N. F. Endothelial cell mechanotransduction in the dynamic vascular environment. Adv. Biosyst.3, 1800252 (2019). [DOI] [PMC free article] [PubMed]
- 3.Gordon E, Schimmel L, Frye M. The importance of mechanical forces for in vitro endothelial cell biology. Front. Physiol. 2020;11:684. doi: 10.3389/fphys.2020.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ. Res. 1999;85:820–828. doi: 10.1161/01.RES.85.9.820. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto, K., Korenaga, R., Kamiya, A. & Ando, J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 87, 385–391 (2000). [DOI] [PubMed]
- 6.Wang, S. et al. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 126, 4527–4536 (2016). [DOI] [PMC free article] [PubMed]
- 7.Ranade, S. S. et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl Acad. Sci. USA111, 10347–10352 (2014). [DOI] [PMC free article] [PubMed]
- 8.Hierck, B. P. et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 237, 725–735 (2008). [DOI] [PubMed]
- 9.Iomini, C., Tejada, K., Mo, W., Vaananen, H. & Piperno, G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811–817 (2004). [DOI] [PMC free article] [PubMed]
- 10.Weinbaum, S., Zhang, X., Han, Y., Vink, H. & Cowin, S. C. Mechanotransduction and flow across the endothelial glycocalyx. Proc. Natl Acad. Sci. USA100, 7988–7995 (2003). [DOI] [PMC free article] [PubMed]
- 11.Gudi, S. et al. Rapid activation of Ras by fluid flow is mediated by Gα q and Gβγ subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 23, 994–1000 (2003). [DOI] [PubMed]
- 12.Boyd, N. L. et al. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am. J. Physiol. Circ. Physiol. 285, H1113–H1122 (2003). [DOI] [PubMed]
- 13.Osawa, M., Masuda, M., Kusano, K. & Fujiwara, K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction. J. Cell Biol. 158, 773–785 (2002). [DOI] [PMC free article] [PubMed]
- 14.Chiu, Y.-J., McBeath, E. & Fujiwara, K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J. Cell Biol. 182, 753–763 (2008). [DOI] [PMC free article] [PubMed]
- 15.Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature437, 426–431 (2005). [DOI] [PubMed]
- 16.Eyckmans, J., Boudou, T., Yu, X. & Chen, C. S. A hitchhiker’s guide to mechanobiology. Dev. Cell21, 35–47 (2011). [DOI] [PMC free article] [PubMed]
- 17.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 18.Gray KM, Stroka KM. Vascular endothelial cell mechanosensing: new insights gained from biomimetic microfluidic models. Semin. Cell Dev. Biol. 2017;71:106–117. doi: 10.1016/j.semcdb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Campinho P, Vilfan A, Vermot J. Blood flow forces in shaping the vascular system: a focus on endothelial cell behavior. Front. Physiol. 2020;11:552. doi: 10.3389/fphys.2020.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jufri, N. F., Mohamedali, A., Avolio, A. & Baker, M. S. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc. Cell7, 8 (2015). [DOI] [PMC free article] [PubMed]
- 21.Kaunas R. Good advice for endothelial cells: get in line, relax tension, and go with the flow. APL Bioeng. 2020;4:010905. doi: 10.1063/1.5129812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux, E., Bougaran, P., Dufourcq, P. & Couffinhal, T. Fluid shear stress sensing by the endothelial layer. Front. Physiol.11, 861 (2020). [DOI] [PMC free article] [PubMed]
- 23.Liliensiek SJ, Nealey P, Murphy CJ. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng. Part A. 2009;15:2643–2651. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brody S, et al. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12:413–421. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leclech, C., Natale, C. F. & Barakat, A. I. The basement membrane as a structured surface - role in vascular health and disease. J. Cell Sci. 133, jcs239889 (2020). [DOI] [PubMed]
- 26.Natale CF, Lafaurie-Janvore J, Ventre M, Babataheri A, Barakat AI. Focal adhesion clustering drives endothelial cell morphology on patterned surfaces. J. R. Soc. Interface. 2019;16:20190263. doi: 10.1098/rsif.2019.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biela SA, Su Y, Spatz JP, Kemkemer R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano-micro range. Acta Biomater. 2009;5:2460–2466. doi: 10.1016/j.actbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Sales A, Holle AW, Kemkemer R. Initial contact guidance during cell spreading is contractility-independent. Soft Matter. 2017;13:5158–5167. doi: 10.1039/C6SM02685K. [DOI] [PubMed] [Google Scholar]
- 29.Franco D, et al. Control of initial endothelial spreading by topographic activation of focal adhesion kinase. Soft Matter. 2011;7:7313–7324. doi: 10.1039/c1sm05191a. [DOI] [Google Scholar]
- 30.Song, K. H., Kwon, K. W., Song, S., Suh, K. Y. & Doh, J. Dynamics of T cells on endothelial layers aligned by nanostructured surfaces. Biomaterials10.1016/j.brat.2013.05.014 (2012). [DOI] [PubMed]
- 31.Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: orientation of actin filaments and focal adhesions. J. Biomed. Mater. Res. Part A. 2005;75:668–680. doi: 10.1002/jbm.a.30478. [DOI] [PubMed] [Google Scholar]
- 32.Antonini, S. et al. Sub-micron lateral topography affects endothelial migration by modulation of focal adhesion dynamics. Biomed. Mater. 10.1088/1748-6041/10/3/035010 (2015). [DOI] [PubMed]
- 33.Vandrangi, P., Gott, S. C., Kozaka, R., Rodgers, V. G. J. & Rao, M. P. Comparative endothelial cell response on topographically patterned titanium and silicon substrates with micrometer to sub-micrometer feature sizes. PLoS ONE9, e111465 (2014). [DOI] [PMC free article] [PubMed]
- 34.Liliensiek SJ, et al. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–5426. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukumberg, M. et al. Evaluation of the topographical influence on the cellular behavior of human umbilical vein endothelial cells. Adv. Biosyst. 10.1002/adbi.201700217 (2018). [DOI] [PMC free article] [PubMed]
- 36.Chung TW, Liu DZ, Wang SY, Wang SS. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24:4655–4661. doi: 10.1016/S0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 37.Brammer KS, Oh S, Gallagher JO, Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8:786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]
- 38.Ranjan, A. & Webster, T. J. Increased endothelial cell adhesion and elongation on micron-patterned nano-rough poly(dimethylsiloxane) films. Nanotechnology10.1088/0957-4484/20/30/305102 (2009). [DOI] [PubMed]
- 39.Huang, N. F. et al. The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds. Biomaterials10.1016/j.biomaterials.2013.02.036 (2013). [DOI] [PMC free article] [PubMed]
- 40.Huang NF, et al. Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature. Biomaterials. 2013;34:2928–2937. doi: 10.1016/j.biomaterials.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whited BM, Rylander MN. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2014;111:184–195. doi: 10.1002/bit.24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, et al. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B Biointerfaces. 2018;171:461–467. doi: 10.1016/j.colsurfb.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Bouta EM, et al. Biomaterial guides for lymphatic endothelial cell alignment and migration. Acta Biomater. 2011;7:1104–1113. doi: 10.1016/j.actbio.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai ES, Huang NF, Cooke JP, Fuller GG. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen. Med. 2012;7:649–661. doi: 10.2217/rme.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon H, et al. Combined effects of substrate topography and stiffness on endothelial cytokine and chemokine secretion. ACS Appl. Mater. Interfaces. 2015;7:4525–4532. doi: 10.1021/acsami.5b00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Rienzo, C. et al. Unveiling LOX-1 receptor interplay with nanotopography: mechanotransduction and atherosclerosis onset. Sci. Rep. 10.1038/srep01141 (2013). [DOI] [PMC free article] [PubMed]
- 47.Moffa M, Sciancalepore AG, Passione LG, Pisignano D. Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and imprinted micro-patterns. Small. 2014;10:2439–2450. doi: 10.1002/smll.201303179. [DOI] [PubMed] [Google Scholar]
- 48.Arora, S., Lin, S., Cheung, C., Yim, E. K. F. & Toh, Y. C. Topography elicits distinct phenotypes and functions in human primary and stem cell derived endothelial cells. Biomaterials234, 119747 (2020). [DOI] [PubMed]
- 49.Cutiongco MFA, Chua BMX, Neo DJH, Rizwan M, Yim EKF. Functional differences between healthy and diabetic endothelial cells on topographical cues. Biomaterials. 2018;153:70–84. doi: 10.1016/j.biomaterials.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sales, A., Picart, C. & Kemkemer, R. Age-dependent migratory behavior of human endothelial cells revealed by substrate microtopography. Exp. Cell Res. 10.1016/j.yexcr.2018.10.008 (2019). [DOI] [PubMed]
- 51.Stefopoulos G, Giampietro C, Falk V, Poulikakos D, Ferrari A. Facile endothelium protection from TNF-α inflammatory insult with surface topography. Biomaterials. 2017;138:131–141. doi: 10.1016/j.biomaterials.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 52.Baptista D, Teixeira L, van Blitterswijk C, Giselbrecht S, Truckenmüller R. Overlooked? Underestimated? Effects of substrate curvature on cell behavior. Trends Biotechnol. 2019;37:838–854. doi: 10.1016/j.tibtech.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Cheng D, et al. Studies of 3D directed cell migration enabled by direct laser writing of curved wave topography. Biofabrication. 2019;11:021001. doi: 10.1088/1758-5090/ab047f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bettinger, C. J., Orrick, B., Misra, A., Langer, R. & Borenstein, J. T. Microfabrication of poly (glycerol-sebacate) for contact guidance applications. Biomaterials10.1016/j.biomaterials.2005.11.029 (2006). [DOI] [PubMed]
- 55.Sun, B., Xie, K., Chen, T.-H. & Lam, R. H. W. Preferred cell alignment along concave microgrooves. RSC Adv. 10.1039/c6ra26545f (2017).
- 56.Frame, M. D. & Sarelius, I. H. Flow-induced cytoskeletal changes in endothelial cells growing on curved surfaces. Microcirculation7, 419–427 (2000). [PubMed]
- 57.Esch MB, Post DJ, Shuler ML, Stokol T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A. 2011;17:2965–2971. doi: 10.1089/ten.tea.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi JS, Piao Y, Seo TS. Fabrication of a circular PDMS microchannel for constructing a three-dimensional endothelial cell layer. Bioprocess Biosyst. Eng. 2013;36:1871–1878. doi: 10.1007/s00449-013-0961-z. [DOI] [PubMed] [Google Scholar]
- 59.Polacheck, W. J., Kutys, M. L., Tefft, J. B. & Chen, C. S. Microfabricated blood vessels for modeling the vascular transport barrier. Nat. Protoc.14, 1425–1454 (2019). [DOI] [PMC free article] [PubMed]
- 60.Zhang, X. et al. Modeling early stage atherosclerosis in a primary human vascular microphysiological system. Nat. Commun. 11, 5426 (2020). [DOI] [PMC free article] [PubMed]
- 61.Alimperti S, et al. Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell–endothelial cell-regulated barrier function. Proc. Natl Acad. Sci. USA. 2017;114:8758–8763. doi: 10.1073/pnas.1618333114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fioretta ES, Simonet M, Smits AIPM, Baaijens FPT, Bouten CVC. Differential response of endothelial and endothelial colony forming cells on electrospun scaffolds with distinct microfiber diameters. Biomacromolecules. 2014;15:821–829. doi: 10.1021/bm4016418. [DOI] [PubMed] [Google Scholar]
- 63.Jones D, et al. Actin grips: circular actin-rich cytoskeletal structures that mediate the wrapping of polymeric microfibers by endothelial cells. Biomaterials. 2015;52:395–406. doi: 10.1016/j.biomaterials.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye, M. et al. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 4, 4681 (2014). [DOI] [PMC free article] [PubMed]
- 65.Barreto-Ortiz SF, Zhang S, Davenport M, Fradkin J, Ginn B. A novel in vitro model for microvasculature reveals regulation of circumferential ECM organization by curvature. PLoS ONE. 2013;8:81061. doi: 10.1371/journal.pone.0081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peloquin J, Huynh J, Williams RM, Reinhart-King CA. Indentation measurements of the subendothelial matrix in bovine carotid arteries. J. Biomech. 2011;44:815–821. doi: 10.1016/j.jbiomech.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Engler, A. J., Richert, L., Wong, J. Y., Picart, C. & Discher, D. E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: Correlations between substrate stiffness and cell adhesion. Surf. Sci.570, 142–154 (2004).
- 68.Khanafer K, et al. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J. Thorac. Cardiovasc. Surg. 2011;142:682–686. doi: 10.1016/j.jtcvs.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 69.Jansen, K. A. et al. The role of network architecture in collagen mechanics. Biophys. J. 114, 2665–2678 (2018). [DOI] [PMC free article] [PubMed]
- 70.Humphrey, J. D. Cardiovascular Solid Mechanics (Springer 2002).10.1007/978-0-387-21576-1
- 71.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: aa community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 72.Haskett D, Johnson G, Zhou A, Utzinger U, Vande Geest J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech. Model. Mechanobiol. 2010;9:725–736. doi: 10.1007/s10237-010-0209-7. [DOI] [PubMed] [Google Scholar]
- 73.Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206. doi: 10.1161/HYPERTENSIONAHA.106.076166. [DOI] [PubMed] [Google Scholar]
- 74.Sen, S., Engler, A. J. & Discher, D. E. Matrix strains induced by cells: computing how far cells can feel. Cell. Mol. Bioeng. 2, 39–48 (2009). [DOI] [PMC free article] [PubMed]
- 75.Tusan CG, et al. Collective cell behavior in mechanosensing of substrate thickness. Biophys. J. 2018;114:2743–2755. doi: 10.1016/j.bpj.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banerjee, S. & Marchetti, M. C. Contractile stresses in cohesive cell layers on finite-thickness substrates. Phys. Rev. Lett. 109, 108101 (2012). [DOI] [PubMed]
- 77.Yeh Y-T, et al. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS ONE. 2012;7:e46889. doi: 10.1371/journal.pone.0046889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 79.Casillo SM, Peredo AP, Perry SJ, Chung HH, Gaborski TR. Membrane pore spacing can modulate endothelial cell-substrate and cell-cell interactions. ACS Biomater. Sci. Eng. 2017;3:243–248. doi: 10.1021/acsbiomaterials.7b00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andresen Eguiluz RC, Kaylan KB, Underhill GH, Leckband DE. Substrate stiffness and VE-cadherin mechano-transduction coordinate to regulate endothelial monolayer integrity. Biomaterials. 2017;140:45–57. doi: 10.1016/j.biomaterials.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell. Mol. Bioeng. 2010;3:68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fioretta ES, Fledderus JO, Baaijens FPT, Bouten CVC. Influence of substrate stiffness on circulating progenitor cell fate. J. Biomech. 2012;45:736–744. doi: 10.1016/j.jbiomech.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Van Geemen D, et al. F-actin-anchored focal adhesions distinguish endothelial phenotypes of human arteries and veins. Arterioscler. Thromb. Vasc. Biol. 2014;34:2059–2067. doi: 10.1161/ATVBAHA.114.304180. [DOI] [PubMed] [Google Scholar]
- 84.Lampi MC, Guvendiren M, Burdick JA, Reinhart-King CA. Photopatterned hydrogels to investigate the endothelial cell response to matrix stiffness heterogeneity. ACS Biomater. Sci. Eng. 2017;3:3007–3016. doi: 10.1021/acsbiomaterials.6b00633. [DOI] [PubMed] [Google Scholar]
- 85.Krishnan, R. et al. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am. J. Physiol. Cell Physiol. 300, C146–C154 (2011). [DOI] [PMC free article] [PubMed]
- 86.Birukova AA, et al. Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc. Res. 2013;87:50–57. doi: 10.1016/j.mvr.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech. 2009;42:1114–1119. doi: 10.1016/j.jbiomech.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bastounis, E. E., Yeh, Y. T. & Theriot, J. A. Subendothelial stiffness alters endothelial cell traction force generation while exerting a minimal effect on the transcriptome. Sci. Rep. 9, 18209 (2019). [DOI] [PMC free article] [PubMed]
- 89.Huynh J, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bordeleau F, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl Acad. Sci. USA. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Canver AC, Ngo O, Urbano RL, Clyne AM. Endothelial directed collective migration depends on substrate stiffness via localized myosin contractility and cell-matrix interactions. J. Biomech. 2016;49:1369–1380. doi: 10.1016/j.jbiomech.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 92.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011;118:1632–1640. doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gauvin R, et al. Mechanical properties of tissue-engineered vascular constructs produced using arterial or venous cells. Tissue Eng. Part A. 2011;17:2049–2059. doi: 10.1089/ten.tea.2010.0613. [DOI] [PubMed] [Google Scholar]
- 94.Schweller RM, West JL. Encoding hydrogel mechanics via network cross-linking structure. ACS Biomater. Sci. Eng. 2015;1:335–344. doi: 10.1021/acsbiomaterials.5b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chwalek K, Tsurkan MV, Freudenberg U, Werner C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep04414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 97.Nair SK, et al. Colloidal gels with tunable mechanomorphology regulate endothelial morphogenesis. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-018-37788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sieminski AL, Was AS, Kim G, Gong H, Kamm RD. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem. Biophys. 2007;49:73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- 99.Duan Y, et al. Migration of endothelial cells and mesenchymal stem cells into hyaluronic acid hydrogels with different moduli under induction of pro-inflammatory macrophages. J. Mater. Chem. B. 2019;7:5478–5489. doi: 10.1039/C9TB01126A. [DOI] [PubMed] [Google Scholar]
- 100.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013;9:4635–4644. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu B, Tang D. Influence of non-Newtonian properties of blood on the wall shear stress in human atherosclerotic right coronary arteries. MCB. 2011;8:73–90. doi: 10.3970/mcb.2011.008.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sriram, K., Intaglietta, M. & Tartakovsky, D. M. Non-Newtonian flow of blood in arterioles: consequences for wall shear stress measurements. Microcirculation21, 628–639 (2014). [DOI] [PMC free article] [PubMed]
- 103.Cherry, E. M. & Eaton, J. K. Shear thinning effects on blood flow in straight and curved tubes. Phys. Fluids25, 073104 (2013).
- 104.Secomb, T. W., Hsu, R. & Pries, A. R. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am. J. Physiol. Circ. Physiol. 281, H629–H636 (2001). [DOI] [PubMed]
- 105.Freund, J. B. & Vermot, J. The wall-stress footprint of blood cells flowing in microvessels. Biophys. J. 106, 752–762 (2014). [DOI] [PMC free article] [PubMed]
- 106.Hogan, B., Shen, Z., Zhang, H., Misbah, C. & Barakat, A. I. Shear stress in the microvasculature: influence of red blood cell morphology and endothelial wall undulation. Biomech. Model. Mechanobiol. 18, 1095–1109 (2019). [DOI] [PubMed]
- 107.Papaioannou T. Vascular wall shear stress: basic principles and methods. Hell. J. Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 108.Lipowsky, H. H., Kovalcheck, S. & Zweifach, B. W. The distribution of blood rheological parameters in the microvasculature of cat mesentery. 43, 738–749 (1978). [DOI] [PubMed]
- 109.Natarajan, M., Aravindan, N., Sprague, A. & Mohan, S. Hemodynamic flow-induced mechanotransduction signaling influences the radiation response of the vascular endothelium. 186, 175–188 (2016). [DOI] [PubMed]
- 110.Chatterjee S. Endothelial mechanotransduction, redox signaling and the regulation of vascular inflammatory. Pathways. 2018;9:1–16. doi: 10.3389/fphys.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol.10.1038/nrm2596 (2009). [DOI] [PMC free article] [PubMed]
- 112.Baeyens, N. et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. eLife10.7554/eLife.04645 (2015). [DOI] [PMC free article] [PubMed]
- 113.Baratchi S, et al. Molecular sensors of blood flow in endothelial cells. Trends Mol. Med. 2017;23:850–868. doi: 10.1016/j.molmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Davies, P. F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 6, 16–26 (2009). [DOI] [PMC free article] [PubMed]
- 115.Chiu, J.-J. & Chien, S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 (2011). [DOI] [PMC free article] [PubMed]
- 116.Bergan JJ, et al. Chronic venous disease. N. Engl. J. Med. 2006;355:488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 117.Flaherty JT, et al. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 1972;30:23–33. doi: 10.1161/01.RES.30.1.23. [DOI] [PubMed] [Google Scholar]
- 118.Silkworth, J. B., Stehbens, W. E. & Phil, D. The shape of endothelial cells in en face preparations of rabbit blood vessels. Angiology26, 474–487 (1975).
- 119.Langille BL, Adamson SLEE. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 1981;48:481–488. doi: 10.1161/01.RES.48.4.481. [DOI] [PubMed] [Google Scholar]
- 120.Levesque M, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 121.Estrada R, Giridharan GA, Nguyen M. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics. 2011;5:32006–3200611. doi: 10.1063/1.3608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malek, A. M. & Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. 726, 713–726 (1996). [DOI] [PubMed]
- 123.Chiu, J.-J., Wang, D. L., Chien, S., Skalak, R. & Usami, S. Effects of disturbed flow on endothelial cells. J. Biomech. Eng. 120, 2–8 (1998). [DOI] [PubMed]
- 124.Dewey, C. F., Bussolari, S. R., Gimbrone, M. A. & Davies, P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103, 177–185 (1981). [DOI] [PubMed]
- 125.Miao, H. et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J. Vasc. Res. 42, 77–89 (2005). [DOI] [PubMed]
- 126.Davies, P. F., Remuzzi, A., Gordon, E. J., Dewey, C. F. & Gimbrone, M. A. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl. Acad. Sci. USA10.1073/pnas.83.7.2114 (1986). [DOI] [PMC free article] [PubMed]
- 127.Li S, Huang NF, Hsu S. Mechanotransduction in endothelial cell migration. J. Cell Biochem. 2005;96:1110–1126. doi: 10.1002/jcb.20614. [DOI] [PubMed] [Google Scholar]
- 128.Inglebert, M. et al. The effect of shear stress reduction on endothelial cells: A microfluidic study of the actin cytoskeleton. Biomicrofluidics 14, 024115 (2020). [DOI] [PMC free article] [PubMed]
- 129.Noria S, et al. Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am. J. Pathol. 2004;164:1211–1223. doi: 10.1016/S0002-9440(10)63209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell. Cytoskeleton. 1998;330:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 131.Franke, R.-P. et al. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature307, 648–649 (1984). [DOI] [PubMed]
- 132.Steward R, et al. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol. Cell Physiol. 2015;308:C657–C664. doi: 10.1152/ajpcell.00363.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hur, S. S. et al. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proc. Natl Acad. Sci. USA109, 11110–11115 (2012). [DOI] [PMC free article] [PubMed]
- 134.Metaxa E, et al. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am. J. Physiol. Hear. Circ. Physiol. 2008;295:736–742. doi: 10.1152/ajpheart.01156.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tovar-lopez F, Thurgood P, Gilliam C, Nguyen N. A microfluidic system for studying the effects of disturbed flow on endothelial cells. Front. Bioeng. Biotechnol. 2019;7:1–7. doi: 10.3389/fbioe.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ostrowski MA, et al. Microvascular endothelial cells migrate upstream and align against the shear stress field created by impinging flow. Biophys. J. 2014;106:366–374. doi: 10.1016/j.bpj.2013.11.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robotti, F. et al. The influence of surface micro-structure on endothelialization under supraphysiological wall shear stress. Biomaterials10.1016/j.biomaterials.2014.06.046 (2014). [DOI] [PubMed]
- 138.Sato, M., Saito, N., Sakamoto, N. & Ohashi, T. High wall shear stress gradient suppress morphological responses of endothelial cells to fluid flow. In World Congress on Medical Physics and Biomedical Engineering, September 7 - 12, 2009, Munich, Germany. IFMBE Proceedings (eds. Dössel, O. & Schlegel W. C.)312–313 (Springer, Berlin, Heidelberg, 2009). 10.1007/978-3-642-03882-2_82.
- 139.Hsu P, et al. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem. Biophys. Res Commun. 2001;285:751–759. doi: 10.1006/bbrc.2001.5221. [DOI] [PubMed] [Google Scholar]
- 140.Gojova, A., Barakat, A. I. & Barakat, A. I. Vascular endothelial wound closure under shear stress: role of membrane fluidity and flow-sensitive ion channels. 95616, 2355–2362 (2005). [DOI] [PubMed]
- 141.Albuquerque MLC, Flozak AS. Lamellipodial motility in wounded endothelial cells exposed to physiologic flow is associated with different patterns of b 1 -integrin and vinculin localization. J. Cell. Physiol. 2003;60:50–60. doi: 10.1002/jcp.10228. [DOI] [PubMed] [Google Scholar]
- 142.Albuquerque, M. L. C., Waters, C. M., Savla, U., Schnaper, H. W. & Flozak, A. S. Shear stress enhances human endothelial cell wound closure in vitro. Am. J. Physiol. Circ. Physiol. 279, H293–302 (2000). [DOI] [PubMed]
- 143.Ciechanowska, A., Ladyzynski, P., Hoser, G. & Sabalinska, S. Human endothelial cells hollow fiber membrane bioreactor as a model of the blood vessel for in vitro studies. J. Artif. Organs10.1007/s10047-016-0902-0 (2016). [DOI] [PubMed]
- 144.Akimoto, S., Mitsumata, M., Sasaguri, T. & Yoshida, Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21. Circ. Res. 86, 185–190 (2000). [DOI] [PubMed]
- 145.DePaola N, Gimbrone MA, Davies PF, Dewey CF. Vascular endothelium responds to fluid shear stress gradients. Arterioscler. Thromb. 1992;12:1254–1257. doi: 10.1161/01.ATV.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 146.Sakamoto N, Saito N, Han X, Ohashi T, Sato M. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem. Biophys. Res. Commun. 2010;395:264–269. doi: 10.1016/j.bbrc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 147.Szymanski MP, Metaxa E, Meng H, Kolega J. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann. Biomed. Eng. 2008;36:1681–1689. doi: 10.1007/s10439-008-9540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoshino, D., Sakamoto, N. & Sato, M. Integrative biology fluid shear stress combined with shear stress spatial gradients regulates vascular endothelial. Integr. Biol. 10.1039/c7ib00065k (2017). [DOI] [PubMed]
- 149.Dolan JM, Meng H, Singh S, Paluch R, Kolega J. High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment. Ann. Biomed. Eng. 2011;39:1620–1631. doi: 10.1007/s10439-011-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peiffer V, Sherwin SJ, Weinberg PD. Computation in the rabbit aorta of a new metric - the transverse wall shear stress - to quantify the multidirectional character of disturbed blood flow. J. Biomech. 2013;46:2651–2658. doi: 10.1016/j.jbiomech.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frydrychowicz, A. et al. Interdependencies of aortic arch secondary flow patterns, geometry, and age analysed by 4-dimensional phase contrast magnetic resonance imaging at 3 Tesla. Eur. Radiol. 10.1007/s00330-011-2353-6 (2012). [DOI] [PubMed]
- 152.Mohamied Y, Sherwin SJ, Weinberg PD. Understanding the fluid mechanics behind transverse wall shear stress. J. Biomech. 2017;50:102–109. doi: 10.1016/j.jbiomech.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chakraborty A, et al. Impact of bi-axial shear on atherogenic gene expression by endothelial cells. Ann. Biomed. Eng. 2016;44:3032–3045. doi: 10.1007/s10439-016-1626-2. [DOI] [PubMed] [Google Scholar]
- 154.Potter CMF, et al. Role of shear stress in endothelial cell morphology and expression of cyclooxygenase isoforms. Arter. Thromb. Vasc. Biol. 2011;31:384–391. doi: 10.1161/ATVBAHA.110.214031. [DOI] [PubMed] [Google Scholar]
- 155.Dardik A, et al. Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 2005;41:869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 156.Warboys CM, Ghim M, Weinberg PD. Understanding mechanobiology in cultured endothelium: a review of the orbital shaker method. Atherosclerosis. 2019;285:170–177. doi: 10.1016/j.atherosclerosis.2019.04.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mandrycky C, Hadland B, Zheng Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci. Adv. 2020;6:3629–3645. doi: 10.1126/sciadv.abb3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Helmlinger, G., Geiger, R. V., Schreck, S. & Nerem, R. M. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J. Biomech. Eng. 113, 123–131 (1991). [DOI] [PubMed]
- 159.Lum, R. M., Wiley, L. M. & Barakat, A. I. Influence of different forms of fluid shear stress on vascular endothelial TGF-beta1 mRNA expression. Int. J. Mol. Med. 10.1088/1755-1315/117/1/012035 (2000). [DOI] [PubMed]
- 160.Helmlinger, G., Berk, B. C. & Nerem, R. M. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am. J. Physiol. Physiol. 269, C367–C375 (1995). [DOI] [PubMed]
- 161.Blackman BR, Gimbrone MA. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 162.Uzarski JS, Scott EW, Mcfetridge PS. Adaptation of endothelial cells to physiologically- modeled, variable shear. Stress. 2013;8:17–19. doi: 10.1371/journal.pone.0057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thoumine, O., Nerem, R. M. & Girard, F. R. Oscillatory shear stress and hydrostatic pressure modulate cell-matrix attachment proteins in cultured endothelial cells. Vitr. Cell. Dev. Biol. Anim. 10.1007/BF02631337 (1995). [DOI] [PubMed]
- 164.Sampath, R. et al. Shear stress-mediated changes in the expression of leukocyte adhesion receptors on human umbilical vein endothelial cells in vitro. 23, 247–256 (1995). [DOI] [PubMed]
- 165.Ohno, M., Cooke, J. P., Dzau, V. J. & Gibbons, G. H. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production modulation by potassium channel blockade. 95, 1363–1369 (1995). [DOI] [PMC free article] [PubMed]
- 166.Jiang Z, Berceli SA, Pfahnl CL, Wu L. Wall shear modulation of cytokines in early vein grafts. J. Vasc. Surg. 2004;40:345–350. doi: 10.1016/j.jvs.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 167.Chang, C., Seibel, A. J. & Song, J. W. Application of microscale culture technologies for studying lymphatic vessel biology. Microcirculation26, e12547 (2019). [DOI] [PMC free article] [PubMed]
- 168.Chary, S. R. & Jain, R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA86, 5385–5389 (1989). [DOI] [PMC free article] [PubMed]
- 169.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc. Natl Acad. Sci. USA. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Galie PA, et al. Fluid shear stress threshold regulates angiogenic sprouting. Proc. Natl Acad. Sci. USA. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hernández Vera R, et al. Interstitial fluid flow intensity modulates endothelial sprouting in restricted Src-activated cell clusters during capillary morphogenesis. Tissue Eng. Part A. 2009;15:175–185. doi: 10.1089/ten.tea.2007.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abe Y, et al. Balance of interstitial flow magnitude and vascular endothelial growth factor concentration modulates three-dimensional microvascular network formation. APL Bioeng. 2019;3:36102. doi: 10.1063/1.5094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vickerman V, Kamm RD. Mechanism of a flow-gated angiogenesis switch: early signaling events at cell-matrix and cell-cell junctions. Integr. Biol. 2012;4:863–874. doi: 10.1039/c2ib00184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akbari, E., Spychalski, G. B., Rangharajan, K. K., Prakash, S. & Song, J. W. Competing fluid forces control endothelial sprouting in a 3-D microfluidic vessel bifurcation model. Micromachines10, 451 (2019). [DOI] [PMC free article] [PubMed]
- 175.Dafni H, Israely T. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 2002;62:6731–6739. [PubMed] [Google Scholar]
- 176.Kim S, Chung M, Ahn J, Lee S, Jeon NL. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip. 2016;16:4189–4199. doi: 10.1039/C6LC00910G. [DOI] [PubMed] [Google Scholar]
- 177.Figarol, A. et al. Biochemical and Biophysical Research Communications Interstitial flow regulates in vitro three-dimensional self-organized brain micro-vessels. Biochem. Biophys. Res. Commun. 10.1016/j.bbrc.2020.09.061 (2020). [DOI] [PubMed]
- 178.Ng CP, Helm CLE, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res. 2004;68:258–264. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 179.Hsu YH, et al. Full range physiological mass transport control in 3D tissue cultures. Lab Chip. 2013;13:81–89. doi: 10.1039/C2LC40787F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shirure VS, Lezia A, Tao A, Alonzo LF, George SC. Low levels of physiological interstitial flow eliminate morphogen gradients and guide angiogenesis. Angiogenesis. 2017;20:493–504. doi: 10.1007/s10456-017-9559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kwon, H. et al. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat. Commun. 10.1038/ncomms11805 (2016). [DOI] [PMC free article] [PubMed]
- 182.Krüger-genge A, Blocki A, Franke R, Jung F. Vascular endothelial cell biology: an update. Int J. Mol. Sci. 2019;20:4411. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drenckhahn D, Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix and contractility. J. Cell Biol. 1986;102:1738–1747. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Butcher JT, Penrod AM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arter. Thromb. Vasc. Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 185.Michalaki E, Surya VN, Fuller GG, Dunn AR. Perpendicular alignment of lymphatic endothelial cells in response to spatial gradients in wall shear stress. Commun. Biol. 2020;3:57. doi: 10.1038/s42003-019-0732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reinitz, A., DeStefano, J., Ye, M., Wong, A. D. & Searson, P. C. Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc. Res. 10.1016/j.mvr.2015.02.008 (2015). [DOI] [PMC free article] [PubMed]
- 187.Destefano, J. G., Xu, Z. S., Williams, A. J., Yimam, N. & Searson, P. C. Effect of shear stress on iPSC ‑ derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS10.1186/s12987-017-0068-z (2017). [DOI] [PMC free article] [PubMed]
- 188.Ohta S, Inasawa S, Yamaguchi Y. Alignment of vascular endothelial cells as a collective response to shear flow. J. Phys. D. Appl. Phys. 2015;48:245401. doi: 10.1088/0022-3727/48/24/245401. [DOI] [Google Scholar]
- 189.Acevedo, A. D., Bowser, S. S., Gerritsen, M. E. & Bizios, R. Morphological and proliferative responses of endothelial cells to hydrostatic pressure: role of fibroblast growth factor. J. Cell. Physiol. 157, 603–614 (1993). [DOI] [PubMed]
- 190.Salwen, S. A., Szarowski, D. H., Turner, J. N. & Bizios, R. Three-dimensional changes of the cytoskeleton of vascular endothelial cells exposed to sustained hydrostatic pressure. Med. Biol. Eng. Comput. 36, 520–527 (1998). [DOI] [PubMed]
- 191.Ohashi, T., Sugaya, Y., Sakamoto, N. & Sato, M. Relative contribution of physiological hydrostatic pressure and fluid shear stress to endothelial monolayer integrity. Biomed. Eng. Lett. 6, 31–38 (2016).
- 192.Ohashi, T., Sugaya, Y., Sakamoto, N. & Sato, M. Hydrostatic pressure influences morphology and expression of VE-cadherin of vascular endothelial cells. J. Biomech. 40, 2399–2405 (2007). [DOI] [PubMed]
- 193.Sumpio, B. E., Widmann, M. D., Ricotta, J., Awolesi, M. A. & Watase, M. Increased ambient pressure stimulates proliferation and morphologic changes in cultured endothelial cells. J. Cell. Physiol. 158, 133–139 (1994). [DOI] [PubMed]
- 194.Sugaya, Y., Sakamoto, N., Ohashi, T. & Sato, M. Elongation and random orientation of bovine endothelial cells in response to hydrostatic pressure: comparison with response to shear stress. JSME Int. J. Ser. C46, 1248–1255 (2003).
- 195.Tworkoski, E., Glucksberg, M. R. & Johnson, M. The effect of the rate of hydrostatic pressure depressurization on cells in culture. PLoS ONE13, e0189890 (2018). [DOI] [PMC free article] [PubMed]
- 196.Vouyouka, A. G. et al. Ambient pulsatile pressure modulates endothelial cell proliferation. J. Mol. Cell. Cardiol. 30, 609–615 (1998). [DOI] [PubMed]
- 197.Yoshino, D., Sato, K. & Sato, M. Endothelial cell response under hydrostatic pressure condition mimicking pressure therapy. Cell. Mol. Bioeng. 8, 296–303 (2015).
- 198.Yoshino, D. & Sato, M. Early-stage dynamics in vascular endothelial cells exposed to hydrostatic pressure. J. Biomech. Eng. 141, 091006 (2019). [DOI] [PubMed]
- 199.Schwartz, E. A., Bizios, R., Medow, M. S. & Gerritsen, M. E. Exposure of human vascular endothelial cells to sustained hydrostatic pressure stimulates proliferation. Circ. Res. 84, 315–322 (1999). [DOI] [PubMed]
- 200.Prystopiuk, V. et al. A two-phase response of endothelial cells to hydrostatic pressure. J. Cell Sci. 131, jcs206920 (2018). [DOI] [PubMed]
- 201.Shin, H. Y., Gerritsen, M. E. & Bizios, R. Regulation of endothelial cell proliferation and apoptosis by cyclic pressure. Ann. Biomed. Eng. 30, 297–304 (2002). [DOI] [PubMed]
- 202.Hasel, C. et al. Pathologically elevated cyclic hydrostatic pressure induces CD95-mediated apoptotic cell death in vascular endothelial cells. Am. J. Physiol. Physiol. 289, C312–C322 (2005). [DOI] [PubMed]
- 203.Tokunaga, O. & Watanabe, T. Properties of endothelial cell and smooth muscle cell cultured in ambient pressure. In Vitr. Cell. Dev. Biol. 23, 528–534 (1987). [DOI] [PubMed]
- 204.Friedrich, E. E. et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl Acad. Sci. USA116, 12980–12985 (2019). [DOI] [PMC free article] [PubMed]
- 205.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol. Biol. Cell. 2004;15:2943–2953. doi: 10.1091/mbc.e03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Giannotta, M., Trani, M. & Dejana, E. VE-Cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell26, 441–454 (2013). [DOI] [PubMed]
- 207.Kiyoumarsioskouei, A., Saidi, M. S., Mosadegh, B. & Firoozabadi, B. A portable culture chamber for studying the effects of hydrostatic pressure on cellular monolayers. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 233, 807–816 (2019).
- 208.Müller-Marschhausen, K., Waschke, J. & Drenckhahn, D. Physiological hydrostatic pressure protects endothelial monolayer integrity. Am. J. Physiol. Physiol. 294, C324–C332 (2008). [DOI] [PubMed]
- 209.Shin, H. Y., Bizios, R. & Gerritsen, M. E. Cyclic pressure modulates endothelial barrier function. Endothelium10, 179–187 (2003). [DOI] [PubMed]
- 210.Tschumperlin DJ, Oswari J, Margulies SS. Deformation-induced injury of alveolar epithelial cells: effect of frequency, duration, and amplitude. Am. J. Respir. Crit. Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 211.Ding Z, Friedman MH. Quantification of 3-D coronary arterial motion using clinical biplane cineangiograms. Int. J. Card. Imaging. 2000;16:331–346. doi: 10.1023/A:1026590417177. [DOI] [PubMed] [Google Scholar]
- 212.Cheng CP, Wilson NM, Hallett RL, Herfkens RJ, Taylor CA. In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion. J. Vasc. Interv. Radiol. 2006;17:979–987. doi: 10.1097/01.RVI.0000220367.62137.E8. [DOI] [PubMed] [Google Scholar]
- 213.Choi G, Shin LK, Taylor CA, Cheng CP. In vivo deformation of the human abdominal aorta and common iliac arteries with hip and knee flexion: implications for the design of stent-grafts. J. Endovasc. Ther. 2009;16:531–538. doi: 10.1583/09-2806.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klein AJ, et al. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter. Cardiovasc. Interv. 2009;74:787–798. doi: 10.1002/ccd.22124. [DOI] [PubMed] [Google Scholar]
- 215.Wedding KL, et al. Measurement of vessel wall strain using cine phase contrast MRI. J. Magn. Reson. Imaging. 2002;15:418–428. doi: 10.1002/jmri.10077. [DOI] [PubMed] [Google Scholar]
- 216.Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: age-related changes. J. Vasc. Surg. 2009;49:1029–1036. doi: 10.1016/j.jvs.2008.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wittek A, et al. Cyclic three-dimensional wall motion of the human ascending and abdominal aorta characterized by time-resolved three-dimensional ultrasound speckle tracking. Biomech. Model. Mechanobiol. 2016;15:1375–1388. doi: 10.1007/s10237-016-0769-2. [DOI] [PubMed] [Google Scholar]
- 218.Dobrin PB. Mechanical properties of arteries. Physiol. Rev. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- 219.Ives CL, Eskin SG, McIntire LV. Mechanical effects on endothelial cell morphology: In vitro assessment. Vitr. Cell. Dev. Biol. 1986;22:500–507. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- 220.Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell. Signal. 2006;18:1924–1931. doi: 10.1016/j.cellsig.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 221.Wang, J. H.-C., Goldschmidt-Clermont, P. & Yin, F. C.-P. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann. Biomed. Eng. 28, 1165–1171 (2000). [DOI] [PubMed]
- 222.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc. Res. 1991;42:245–254. doi: 10.1016/0026-2862(91)90059-K. [DOI] [PubMed] [Google Scholar]
- 223.Wang, J. H. C., Goldschmidt-Clermont, P., Wille, J. & Yin, F. C. P. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 10.1016/S0021-9290(01)00150-6 (2001). [DOI] [PubMed]
- 224.Shirinsky VP, et al. Mechano-chemical control of human endothelium orientation and size. J. Cell Biol. 1989;109:331–339. doi: 10.1083/jcb.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hayakawa, K., Sato, N. & Obinata, T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 268, 104–114 (2001). [DOI] [PubMed]
- 226.Krishnan, R. et al. Fluidization, resolidification, and reorientation of the endothelial cell in response to slow tidal stretches. Am. J. Physiol. Cell Physiol. 303, C368–C375 (2012). [DOI] [PMC free article] [PubMed]
- 227.Sokabe, M. et al. Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels Suppl. 12, 191–193 (1997). [PubMed]
- 228.Takemasa T, Sugimoto K, Yamashita K. Amplitude-dependent stress fiber reorientation in early response to cyclic strain. Exp. Cell Res. 1997;230:407–410. doi: 10.1006/excr.1996.3428. [DOI] [PubMed] [Google Scholar]
- 229.Pourati, J. et al. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am. J. Physiol.274, C1283–C1289 (1998). [DOI] [PubMed]
- 230.Hatami, J., Tafazzoli-Shadpour, M., Haghighipour, N., Shokrgozar, M. A. & Janmaleki, M. Influence of cyclic stretch on mechanical properties of endothelial cells. Exp. Mech. 53, 1291–1298 (2013).
- 231.Wang, N. et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 282, C606–C616 (2002). [DOI] [PubMed]
- 232.Sumpio BE, Banes AJ, Levin LG, Johnson G. Mechanical stress stimulates aortic endothelial cells to proliferate. J. Vasc. Surg. 1987;6:252–256. doi: 10.1016/0741-5214(87)90037-1. [DOI] [PubMed] [Google Scholar]
- 233.Liu X, Ensenat D, Wang H, Schafer AI, Durante W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 2003;541:52–56. doi: 10.1016/S0014-5793(03)00285-0. [DOI] [PubMed] [Google Scholar]
- 234.Li, W. & Sumpio, B. E. Strain-induced vascular endothelial cell proliferation requires PI3K-dependent mTOR-4E-BP1 signal pathway. Am. J. Physiol. Heart Circ. Physiol. 288, H1591–H1597 (2005). [DOI] [PubMed]
- 235.Kou B, Zhang J, Singer DRJ. Effects of cyclic strain on endothelial cell apoptosis and tubulogenesis are dependent on ROS production via NAD(P)H subunit p22phox. Microvasc. Res. 2009;77:125–133. doi: 10.1016/j.mvr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 236.Neto, F. et al. YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. Elife7, e31037 (2018). [DOI] [PMC free article] [PubMed]
- 237.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-f-actin interface. J. Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 238.Abiko H, et al. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J. Cell Sci. 2015;128:1683–1695. doi: 10.1242/jcs.157503. [DOI] [PubMed] [Google Scholar]
- 239.Liu, W. F., Nelson, C. M., Tan, J. L. & Chen, C. S. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ. Res. 101, e44–e52 (2007). [DOI] [PubMed]
- 240.Von Offenberg Sweeney N, et al. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem. Biophys. Res. Commun. 2005;329:573–582. doi: 10.1016/j.bbrc.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 241.Yano, Y., Geibel, J. & Sumpio, B. E. Tyrosine phosphorylation of pp125(FAK) and paxillin in aortic endothelial cells induced by mechanical strain. Am. J. Physiol.271, C635–C649 (1996). [DOI] [PubMed]
- 242.Zeiger AS, et al. Static mechanical strain induces capillary endothelial cell cycle re-entry and sprouting. Phys. Biol. 2016;13:46006. doi: 10.1088/1478-3975/13/4/046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Joung IS, Iwamoto MN, Shiu YT, Quam CT. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc. Res. 2006;71:1–11. doi: 10.1016/j.mvr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 244.Matsumoto, T. et al. Mechanical strain regulates endothelial cell patterning in vitro. Tissue Eng.13, 207–217 (2007). [DOI] [PubMed]
- 245.Sinha, R. et al. Endothelial cell alignment as a result of anisotropic strain and flow induced shear stress combinations. Sci. Rep. 6, 29510 (2016). [DOI] [PMC free article] [PubMed]
- 246.Moretti M, Prina-Mello A, Reid AJ, Barron V, Prendergast PJ. Endothelial cell alignment on cyclically-stretched silicone surfaces. J. Mater. Sci. Mater. Med. 2004;15:1159–1164. doi: 10.1023/B:JMSM.0000046400.18607.72. [DOI] [PubMed] [Google Scholar]
- 247.Wille JJ, Ambrosi CM, Yin FCP. Comparison of the effects of cyclic stretching and compression on endothelial cell morphological responses. J. Biomech. Eng. 2004;126:545–551. doi: 10.1115/1.1798053. [DOI] [PubMed] [Google Scholar]
- 248.Meza, D., Abejar, L., Rubenstein, D. A. & Yin, W. A Shearing-stretching device that can apply physiological fluid shear stress and cyclic stretch concurrently to endothelial cells. J. Biomech. Eng. 138, 4032550 (2016). [DOI] [PubMed]
- 249.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J. Clin. Invest. 1995;96:1449–1454. doi: 10.1172/JCI118181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bernardi L, et al. Adaptive reorientation of endothelial collectives in response to strain. Integr. Biol. 2018;10:527–538. doi: 10.1039/C8IB00092A. [DOI] [PubMed] [Google Scholar]
- 251.Hsu, H.-J., Lee, C.-F. & Kaunas, R. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS ONE4, e4853 (2009). [DOI] [PMC free article] [PubMed]
- 252.Haghighipour, N. et al. Topological remodeling of cultured endothelial cells by characterized cyclic strains. Mol. Cell Biomech.4, 189–199 (2007). [PubMed]
- 253.Greiner AM, Biela SA, Chen H, Spatz JP, Kemkemer R. Featured article: temporal responses of human endothelial and smooth muscle cells exposed to uniaxial cyclic tensile strain. Exp. Biol. Med. 2015;240:1298–1309. doi: 10.1177/1535370215570191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee CF, Haase C, Deguchi S, Kaunas R. Cyclic stretch-induced stress fiber dynamics - Dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 2010;401:344–349. doi: 10.1016/j.bbrc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 255.Takemasa T, Yamaguchi T, Yamamoto Y, Sugimoto K, Yamashita K. Oblique alignment of stress fibers in cells reduces the mechanical stress in cyclically deforming fields. Eur. J. Cell Biol. 1998;77:91–99. doi: 10.1016/S0171-9335(98)80076-9. [DOI] [PubMed] [Google Scholar]
- 256.Birukov, K. G. et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L785–L797(2003). [DOI] [PubMed]
- 257.Haghighipour N, Tafazzoli-Shadpour M, Shokrgozar MA, Amini S. Effects of cyclic stretch waveform on endothelial cell morphology using fractal analysis. Artif. Organs. 2010;34:481–490. doi: 10.1111/j.1525-1594.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 258.Tondon A, Hsu HJ, Kaunas R. Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J. Biomech. 2012;45:728–735. doi: 10.1016/j.jbiomech.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 259.Yamada H, Ando H. Orientation of apical and basal actin stress fibers in isolated and subconfluent endothelial cells as an early response to cyclic stretching. Mol. Cell. Biomech. 2007;4:1–12. [PubMed] [Google Scholar]
- 260.Birukova AA, et al. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am. J. Pathol. 2006;168:1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Morgan JT, et al. Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials. 2012;33:4126–4135. doi: 10.1016/j.biomaterials.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hwang, S. Y. et al. Adhesion assays of endothelial cells on nanopatterned surfaces within a microfluidic channel. Anal. Chem. 10.1021/ac100107z (2010). [DOI] [PubMed]
- 263.Franco D, et al. Accelerated endothelial wound healing on microstructured substrates under flow. Biomaterials. 2012;34:1488–1497. doi: 10.1016/j.biomaterials.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 264.Potthoff E, et al. Toward a rational design of surface textures promoting endothelialization. Nano Lett. 2014;14:1069–1079. doi: 10.1021/nl4047398. [DOI] [PubMed] [Google Scholar]
- 265.Nakayama KH, et al. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Lett. 2016;16:410–419. doi: 10.1021/acs.nanolett.5b04028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Uttayarat P, et al. Microtopography and flow modulate the direction of endothelial cell migration. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1027–H1035. doi: 10.1152/ajpheart.00816.2007. [DOI] [PubMed] [Google Scholar]
- 267.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vartanian KB, Berny MA, Mccarty OJT, Hanson SR, Hinds MT. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am. J. Physiol. Cell Physiol. 2010;298:333–341. doi: 10.1152/ajpcell.00340.2009. [DOI] [PubMed] [Google Scholar]
- 269.Galie PA, Van Oosten A, Chen CS, Janmey PA. Application of multiple levels of fluid shear stress to endothelial cells plated on polyacrylamide gels. Lab Chip. 2015;15:1205–1212. doi: 10.1039/C4LC01236D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kohn JC, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 2015;108:471–478. doi: 10.1016/j.bpj.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Balachandran K, et al. Cyclic strain induces dual-mode endothelialmesenchymal transformation of the cardiac valve. Proc. Natl Acad. Sci. USA. 2011;108:19943–19948. doi: 10.1073/pnas.1106954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Greiner AM, et al. Stable biochemically micro-patterned hydrogel layers control specific cell adhesion and allow long term cyclic tensile strain experiments. Macromol. Biosci. 2014;14:1547–1555. doi: 10.1002/mabi.201400261. [DOI] [PubMed] [Google Scholar]
- 273.Ahmed WW, et al. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials. 2010;31:250–258. doi: 10.1016/j.biomaterials.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 274.Wang JHC, Grood ES, Florer J, Wenstrup R. Alignment and proliferation of MC3T3-E1 osteoblasts in microgrooved silicone substrata subjected to cyclic stretching. J. Biomech. 2000;33:729–735. doi: 10.1016/S0021-9290(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 275.Wang JHC, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect. Tissue Res. 2000;41:29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- 276.Prodanov L, et al. The interaction between nanoscale surface features and mechanical loading and its effect on osteoblast-like cells behavior. Biomaterials. 2010;31:7758–7765. doi: 10.1016/j.biomaterials.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 277.Doroudian G, Curtis MW, Gang A, Russell B. Cyclic strain dominates over microtopography in regulating cytoskeletal and focal adhesion remodeling of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013;430:1040–1046. doi: 10.1016/j.bbrc.2012.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tamiello C, et al. Cellular strain avoidance is mediated by a functional actin cap - observations in an Lmna-deficient cell model. J. Cell Sci. 2017;130:779–790. doi: 10.1242/jcs.184838. [DOI] [PubMed] [Google Scholar]
- 279.Loesberg WA, et al. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28:3944–3951. doi: 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 280.Quinlan AMT, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS ONE. 2011;6:e23272. doi: 10.1371/journal.pone.0023272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tondon, A. & Kaunas, R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS One9, e89592 (2014). [DOI] [PMC free article] [PubMed]
- 282.Cui Y, et al. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Dan A, Huang RB, Leckband DE. Dynamic imaging reveals coordinate effects of cyclic stretch and substrate stiffness on endothelial integrity. Ann. Biomed. Eng. 2016;44:3655–3667. doi: 10.1007/s10439-016-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gavara N, Roca-Cusachs P, Sunyer R, Farré R, Navajas D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys. J. 2008;95:464–471. doi: 10.1529/biophysj.107.124180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Korff T, Augustin H. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 1999;112:3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 286.Moore JE, et al. A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann. Biomed. Eng. 1994;22:416–422. doi: 10.1007/BF02368248. [DOI] [PubMed] [Google Scholar]
- 287.Zheng, W. et al. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip12, 3441–3450 (2012). [DOI] [PubMed]
- 288.Owatverot TB, Oswald SJ, Chen Y, Wille JJ, Yin FCP. Effect of combined cyclic stretch and fluid shear stress on endothelial cell morphological responses. J. Biomech. Eng. 2005;127:374–382. doi: 10.1115/1.1894180. [DOI] [PubMed] [Google Scholar]
- 289.Zhao S, et al. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler. Thromb. Vasc. Biol. 1995;15:1781–1786. doi: 10.1161/01.ATV.15.10.1781. [DOI] [PubMed] [Google Scholar]
- 290.Qiu Y, Tarbell JM. Interaction between wall shear stress and circumferential strain affects endothelial cell biochemical production. J. Vasc. Res. 2000;37:147–157. doi: 10.1159/000025726. [DOI] [PubMed] [Google Scholar]
- 291.Amaya, R., Pierides, A. & Tarbell, J. M. The interaction between fluid wall shear stress and solid circumferential strain affects endothelial gene expression. PLoS ONE10, e0129952 (2015). [DOI] [PMC free article] [PubMed]
- 292.Brown A, Burke G, Meenan BJ. Modeling of shear stress experienced by endothelial cells cultured on microstructured polymer substrates in a parallel plate flow chamber. Biotechnol. Bioeng. 2011;108:1148–1158. doi: 10.1002/bit.23022. [DOI] [PubMed] [Google Scholar]
- 293.Barbee, K. A., Mundel, T., Lal, R. & Davies, P. F. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am. J. Physiol.268, H1765–H1772 (1995). [DOI] [PubMed]
- 294.Lafaurie-Janvore, J., Antoine, E. E., Perkins, S. J., Babataheri, A. & Barakat, A. I. A simple microfluidic device to study cell-scale endothelial mechanotransduction. Biomed. Microdevices10.1007/s10544-016-0090-y (2016). [DOI] [PubMed]
- 295.Siperstein MD, Unger RH, Madison LL. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J. Clin. Invest. 1968;47:1973–1999. doi: 10.1172/JCI105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Feingoldf KR, Browner WS, Siperstein MD. Prospective studies of muscle capillary basement membrane width in prediabetics. J. Clin. Endocrinol. Metab. 1989;69:784–789. doi: 10.1210/jcem-69-4-784. [DOI] [PubMed] [Google Scholar]
- 297.Begieneman MPV, et al. The basement membrane of intramyocardial capillaries is thickened in patients with acute myocardial infarction. J. Vasc. Res. 2009;47:54–60. doi: 10.1159/000231721. [DOI] [PubMed] [Google Scholar]
- 298.Nielsen, S. H. et al. Markers of basement membrane remodeling are associated with higher mortality in patients with known atherosclerosis. J. Am. Heart Assoc. 7, e009193 (2018). [DOI] [PMC free article] [PubMed]
- 299.Boulter, E., Tissot, F. S., Dilly, J., Pisano, S. & Féral, C. C. Cyclic uniaxial mechanical stretching of cells using a LEGO®parts-based mechanical stretcher system. J. Cell Sci. 133, jcs234666 (2020). [DOI] [PubMed]
- 300.Kaarj, K., Madias, M., Akarapipad, P., Cho, S. & Yoon, J. Y. Paper-based in vitro tissue chip for delivering programmed mechanical stimuli of local compression and shear flow. J. Biol. Eng. 14, 20 (2020). [DOI] [PMC free article] [PubMed]
- 301.Shemesh J, et al. Flow-induced stress on adherent cells in microfluidic devices. Lab a Chip. 2015;15:4114–4127. doi: 10.1039/C5LC00633C. [DOI] [PubMed] [Google Scholar]
- 302.Cutiongco, M. F. A. et al. Planar and tubular patterning of micro and nano-topographies on poly(vinyl alcohol) hydrogel for improved endothelial cell responses. Biomaterials10.1016/j.biomaterials.2016.01.036 (2016). [DOI] [PubMed]
- 303.Munoz‐Robles BG, Kopyeva I, DeForest CA. Surface patterning of hydrogel biomaterials to probe and direct cell–matrix interactions. Adv. Mater. Interfaces. 2020;7:2001198. doi: 10.1002/admi.202001198. [DOI] [Google Scholar]
- 304.Rizwan M, et al. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials. 2017;120:139–154. doi: 10.1016/j.biomaterials.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 305.Arakawa, C. et al. Biophysical and biomolecular interactions of malaria-infected erythrocytes in engineered human capillaries. Sci. Adv. 6, eaay7243 (2020). [DOI] [PMC free article] [PubMed]



