Abstract
Novel anti-coronavirus disease 2019 mRNA vaccines are rapidly implemented worldwide. Therefore, attention should be given to potentially life-threatening adverse reactions. We report on three young male patients, who developed acute myocarditis 2 days after receiving the second dose of the BNT162b2 vaccine. Primary acute myocarditis was not previously reported in association with vaccines that do not include adjuvants. A high index of suspicion should be maintained in order to diagnose and treat patients who develop auto-inflammatory vaccine-related complications in a timely manner. Further research is required in order to explore the significance of this phenomenon and its underlying molecular mechanism.
Keywords: RNA, Messenger, Adverse effects, Inflammation
Novel anti-coronavirus disease 2019 (COVID-19) vaccines were developed and approved recently by regulatory authorities for a wide population, as part of a global effort to cope with the COVID-19 pandemic and its consequences [1]. Adverse effects of BNT162b2 as reported by the manufacturers were common but mostly mild. The first anti-COVID-19 vaccine approved by the FDA was Pfizer and BionTech m-RNA based vaccine BNT162b2 [2].
Israel was among the first states to establish a national vaccination campaign using BNT162b2, reaching more than 90% of the elderly within a couple of months, then providing free access to vaccination to every citizen over 16 years old [3].
We describe a series of three young male patients, who developed acute myocarditis 2 days after receiving the second dose of the BNT162b2 vaccine.
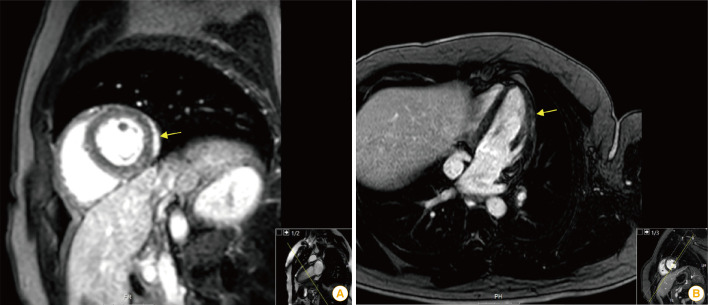
Three previously healthy men, 20, 29, and 24 years old, presented to the emergency department due to acute fever and chest pain appearing 2 days after receiving the second dose of BNT162b2. No abnormal heart sounds or evidence of heart failure were found in their physical examination. However, diffuse ST elevations were present in their electrocardiograms, and laboratory tests showed elevated inflammatory markers and myocardial enzymes. Echocardiograms were normal, but myocardial edema and gadolinium enhancement of the myocardium were evident in cardiac magnetic resonance imaging, confirming the diagnosis of myocarditis (Fig. 1).
Fig. 1. (A, B) Myocardial edema and inflammatory necrosis demonstrated with cardiac magnetic resonance imaging (arrow).
The patients were treated with Colchicine and Ibuprofen with rapid clinical and laboratory improvement.
Acute myocarditis is an inflammatory infiltration of the myocardium, most often labeled as primary or viral-induced. Although this condition may be self-limited, it may also lead to life-threatening arrhythmias, cardiomyopathy, and acute or chronic heart failure.
Autoinflammatory myocarditis had been reported as a presumed rare adverse reaction to vaccine-related adjuvants [4]. However, BNT162b2 is a novel mRNA vaccine and does not contain adjuvants. Therefore, other ingredients of BNT162b2 may be related to this adverse reaction. According to the FDA [5], in addition to a nucleoside-modified mRNA, the vaccine includes the lipids: ((4-Hydroxybutyl)azanediyl) bis(hexane-6, 1-diyl) bis(2-hexyldecanoate), 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-snglycero-3-phosphocholine, and cholesterol, potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose.
Although this limited series does not prove causation, the immediate time relation and the similarities between the cases should trigger further evaluation and systematic screening for the described phenomena in the population, as the novel anti-COVID-19 vaccines are globally implemented.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfizer-BioNTech COVID-19 vaccine [Internet] Silver Spring (MD): Food and Drug Administration; 2021. [cited 2021 Feb 2]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. [Google Scholar]
- 3.Ministry of Health [Internet] [Jerusalem]: Gov.il; 2021. [cited 2021 Feb 2]. Available from: https://www.gov.il/en/departments/news?OfficeId=104cb0f4-d65a-4692-b590-94af928c19c0&limit=10&topic=3ef9cac8-a1a9-4352-91d4-860efd3b720d&skip=0. [Google Scholar]
- 4.Cheng MP, Kozoriz MG, Ahmadi AA, Kelsall J, Paquette K, Onrot JM. Post-vaccination myositis and myocarditis in a previously healthy male. Allergy Asthma Clin Immunol. 2016;12:6. doi: 10.1186/s13223-016-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccines and Related Biological Products Advisory Committee Meeting: FDA briefing document: Pfizer-BioNTech COVID-19 Vaccine. New York (NY): Pfizer and BioNTech; 2020. [Google Scholar]