Abstract
Purpose
Vaccination is a cost-efficient intervention to slow the spread of the coronavirus disease 2019 (COVID-19) pandemic. This study aims to assess the population's willingness to take the COVID-19 vaccine in Jordan and investigate potential determinants of their acceptance
Materials and Methods
This study used an online survey distributed in November 2020, before introducing the vaccine, with items investigating socio-demographic characteristics, seasonal flu vaccination history, COVID-19 vaccine acceptance once available, and factors affecting their decision-making. Also, “COVID-19 risk perception” and beliefs toward COVID-19 vaccine benefits and barriers were assessed.
Results
A total of 2,208 participants completed the survey with a participation rate of 13.1%. The mean±standard deviation age was 33.2±13.5, and 55.7% were females. Study participants were almost equally distributed between willingness, unwillingness, and indecision to take the COVID-19 vaccine (30.4%, 36.4%, and 31.5%, respectively). Younger adults, males, and those who were not married, do not have children, have a bachelor or higher education, employees or being students, healthcare workers, and those who reported receiving flu vaccine had higher rates of COVID-19 vaccine acceptance compared to their counterparts (p<0.001 for each category). COVID-19 risk perception, and perceived vaccine benefits, and barriers were significant predictors of intention. Among those undecided or unwilling to take the COVID-19 vaccine, its safety and side effects were the most common concerns.
Conclusion
The low rate of COVID-19 vaccine acceptance in a developing country is alarming, and a significant proportion are indecisive. Interventions to elevate vaccine acceptance by addressing its safety and efficacy and targeting vulnerable groups are recommended.
Keywords: COVID-19, Coronavirus, Vaccine, Acceptance, Risk perception, Benefits, Barriers, Jordan, Developing country
Introduction
While the coronavirus disease 2019 (COVID-19) pandemic has spread quickly worldwide, and imposed an unprecedented global disease burden, and caused more than 2 million deaths by late-January of 2021 [1,2], there are many potential COVID-19 vaccine candidates currently in development [3,4]. Until today, the best method to stem the spread of COVID-19 is by applying strict outbreak response measures include national lockdown and adopting precautionary measures such as social distancing, mask-wearing, and frequent handwashing [5,6,7]. With COVID-19-related socio-economic burden, along with strict measures, and as vaccination is one of the most cost-efficient and successful health interventions to prevent infectious diseases, a vaccine against COVID-19 is, perhaps, the best hope for ending this burden [3].
Providing an acquired immunity against COVID-19 by an approved vaccine is required to decrease disease mortality, severity, and complications as much as possible, to open-up societies worldwide on a more permanent basis, and to preserve societies functional state [8]. Once vaccines are proven effective and safe, they must be approved by national regulators, manufactured to exacting standards, and distributed. As of January 2021, more than 320 vaccine candidates were in clinical research, with several in phase III clinical trials [9,10]. However, on December 11, 2020, the U.S. Food and Drug Administration issued the first emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine to be distributed in the United States for the prevention of COVID-19 infection in individuals 16 years of age and older [11].
As misinformation and infodemic about COVID-19 vaccines have spread rapidly across media, public health officials, and politicians, especially in developing countries, need to begin planning for effective messaging and policies while the vaccine is introducing. Previous reports on the acceptance of the H1N1 vaccine in 2009, during the H1N1 flu pandemic, have shown unsatisfying results as the willingness to get the 2009 H1N1 pandemic vaccine among the general population ranged from 17% to 67% across studies from the United States, Australia, France, Greece, and the United Kingdom [12,13,14,15,16]. To be successful, vaccination programs require high acceptance and coverage rates and may be mandatory in some situations [17]. This study aims to assess the Jordanian population's willingness to take the COVID-19 vaccine before it was available in the country and investigate potential determinants of their acceptance. Moreover, this study provides public health officials with the data to develop appropriate vaccination strategies and immunization programs against COVID-19.
Materials and Methods
Study design, population, and ethical approval
In November 2020, an anonymous cross-sectional electronic survey was conducted online using the Google Form tool. The researchers shared the e-survey form via social media, e.g., Facebook groups, WhatsApp, and messenger, during the month of November 2020. Our sampling goal was to represent the general Jordanian population based on age, gender, and residence area. Participants were eligible if they were Jordanian, 16 years of age or older, and living in Jordan. Participants did not receive any compensation or rewards for their participation in the study.
Institutional Review Board of Jordan University of Science and Technology's Clinical Research Ethics committee approved the study (IRB no., 19/138/2021). This study was conducted following the 1975 Helsinki declaration, as revised in 2008 and its later amendments or comparable ethical standards. Informed consent for participation was obtained at the beginning of the questionnaire from all participants. Participants could terminate the survey at any time desired. The survey was anonymous, and information confidentiality was assured.
Sample size
The population of Jordan is approximately 11 million. The country was affected by the COVID-19 pandemic since March 2, 2020 [18,19]. Assuming the Jordanian population (16 years and older) to be 7,250,000 at the time of conducting this study with a vaccine acceptance of 50% and margin of error of 4% (99% confidence interval, 46%–54%) [18], we calculated a sample size of 1,040 individuals. To increase the power of our study, we aimed to include +2,000 participants.
Measures
The questionnaire was designed based on previous studies' frameworks to assess a new vaccine acceptance [20,21,22,23,24,25,26,27]. All questions were close-ended, with tick boxes provided for responses except for age. The contents of the questionnaire included: (1) an introducing statement providing a piece of information about the aim of this study and a consent to participate; (2) demographic characteristics, including age, gender, area of living in Jordan, marital status, having children, education level, employment status, and health status as well as a previous diagnosis of COVID-19; (3) seasonal flu vaccination history in the past 8 months; (4) the importance of identified impact factors on the respondents' COVID-19 vaccination decision-making, such as the national origin of vaccine, age, health status, vaccine price, potential side effects, convenience, doctors' recommendations, and so forth. (5) For those who did not intend or did not decide to accept COVID-19 vaccination, the reasons of no such intention were asked in a multiple-choice question and included a choice of “others, please specify” with an open-ended question.
Participants responded to each of the items described below using a 5-point Likert scale that was ranging from “1=strongly disagree” to “5=strongly agree” except for two questions about the worriedness about COVID-19, and the perceived likelihood of catching the virus in the “COVID-19 risk perception” domain, which 1=not at all worried/not at all likely, and 5=very worried/very likely. (6) The code was also reversed in the question “The coronavirus/COVID-19 will not affect very many people in the country I am currently living in” acceptance of taking the COVID-19 vaccine for themselves, their children, and the elderly once available [20]. (7) The “COVID-19 risk perception” scale included six items capturing participants' perceived seriousness of the COVID-19 pandemic, perceived likelihood of catching the virus themselves and their family and friends over the next 6 months, and their present level of worry about the virus [21]. The scores of the six items were summed as an overall measure of COVID-19 risk perception. The greater the score a participant receives on this scale, the greater their perceived risk of COVID-19. This scale was previously conducted on national samples of 6,991 participants from ten countries across Europe, North America, Australia, and Asia [21] and designed following previous studies on risk perception [22,23,24]. (8) Perceived benefits of a COVID-19 vaccine were measured using three items focused on the benefits of a COVID-19 vaccine, which included [25,26]: “Vaccination will decrease my chance of getting COVID-19 or its complications”; “Vaccination is a good idea because I feel less worried about catching COVID-19”; “if I get vaccinated for COVID-19, I will decrease the frequency of having to consult my doctor.” The mean of the sum scores of three items was calculated as an overall measure of perceived benefits. (9) Perceived clinical barriers to the COVID-19 vaccine were measured using four items focused on having side effects, getting sick or dying from the COVID-19 vaccine, and “the COVID-19 vaccine will be painful” as possible barriers for taking the COVID-19 vaccine [27]. The sum of these four items' scores was calculated for each participant, and the mean was reported. (10) Finally, participants were asked about their reliability and confidence in information sources about the COVID-19.
Statistical analysis
The IBM SPSS software for Windows ver. 25.0 (IBM Corp., Armonk, NY, USA) was used for data processing and analysis. The sample demographic characteristics, their COVID-19 vaccine acceptance for themselves, their children, and the elderly, the reported seasonal flu vaccination status, the factors associated with vaccination decision-making, and the reasons for no such intention were described using descriptive statistics (frequencies and percentages). Continuous variables were presented as mean±standard deviation (SD). A chi-square test or Fisher's exact test was used to assess the categorical variables, whereas continuous variables were analyzed via the Student t-test or one-way analysis of variance. Reliability analyses were conducted for each of the three belief scales, and Cronbach's α was reported.
To assess the predictors of COVID-19 vaccine acceptance, a binary logistic regression analysis was used. The dependent variable was the participants' willingness to take the COVID-19 vaccine [20], which was collapsed into “0=strongly disagree/disagree/neutral” and “1=agree/strongly agree.” Model selection using stepwise backward selection with a cutoff p-value of 0.2 was used to select the final, most parsimonious model where age, gender, residence area, social status, having children, education, employment status, working in medical fields, having chronic diseases, history of COVID-19 infection, seasonal flu vaccination status, COVID-19 risk perception, and perceived benefits and barriers to vaccination were included as explanatory variables. Adjusted odds ratios (OR) and their 95% confidence intervals (95% CI) were reported. Statistical significance was considered at a p-value of 0.05.
Results
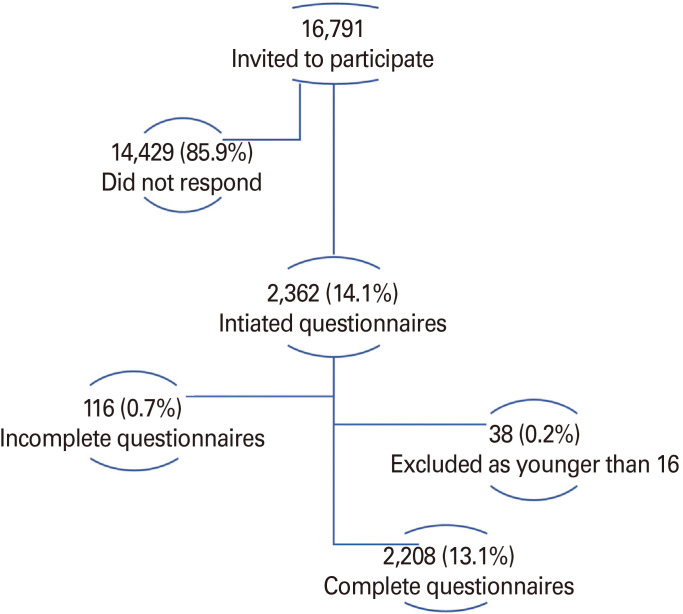
A total of 2,208 participants completed the survey and were included in this study with a participation rate of 13.1% (Fig. 1). The mean±SD age was 33.2±13.5 years, ranging between 16 and 89 years, and 1,230 (55.7%) were females. About half of the participants live in Amman (capital of Jordan), and most participants (85%) have bachelor's degrees or higher, and 14.5% reported a previous infection with COVID-19. Table 1 shows the study participants' demographics characteristics.
Fig. 1. Study participants.
Table 1. COVID-19 vaccine acceptance rates and risk perception across demographic characteristics.
| Characteristic | Total (n=2,208) | COVID-19 vaccine acceptance ratea) | p-value | Risk perception score (out of 30) | p-value | |
|---|---|---|---|---|---|---|
| Age (yr) | <0.001 | <0.001 | ||||
| ≤24 | 856 (38.8) | 307 (35.9) | 20.34±4.078 | |||
| 25–34 | 475 (21.5) | 170 (35.8) | 20.30±4.365 | |||
| 35–44 | 363 (16.4) | 80 (22.0) | 19.48±4.151 | |||
| 45–54 | 328 (14.9) | 73 (22.3) | 20.25±4.223 | |||
| 55–64 | 132 (6.0) | 31 (23.5) | 18.77±4.584 | |||
| ≥65 | 54 (2.4) | 11 (20.4) | 18.83±4.343 | |||
| Gender | <0.001 | 0.080 | ||||
| Male | 978 (44.3) | 391 (40.0) | 19.87±4.54 | |||
| Female | 1,230 (55.7) | 281 (22.8) | 20.19±3.98 | |||
| Residence area | 0.062 | 0.072 | ||||
| Amman (Jordan capital) | 1,219 (55.2) | 380 (31.2) | 20.11±3.96 | |||
| North | 798 (36.1) | 236 (29.6) | 20.10±4.56 | |||
| Middle | 131 (5.9) | 46 (35.1) | 19.74±4.40 | |||
| South | 60 (2.7) | 10 (16.7) | 18.72±4.63 | |||
| Social status | <0.001 | 0.003 | ||||
| Single | 1,171 (53.0) | 421 (36.0) | 20.30±4.12 | |||
| Married | 1,037 (47.0) | 251 (24.2) | 19.76±4.35 | |||
| Having children | <0.001 | 0.227 | ||||
| Yes | 825 (37.4) | 196 (23.8) | 19.90±4.39 | |||
| No | 1,383 (62.6) | 476 (34.4) | 20.13±4.14 | |||
| Educational level | <0.001 | <0.001 | ||||
| High school or lower | 331 (15.0) | 64 (19.3) | 19.01±4.62 | |||
| Bachelor's student or degree | 1,561 (70.7) | 498 (31.9) | 20.15±4.03 | |||
| Master or doctoral degree | 316 (14.3) | 110 (34.8) | 20.63±4.64 | |||
| Employment status | <0.001 | <0.001 | ||||
| Unemployed or retired | 618 (28.0) | 124 (20.1) | 19.39±4.19 | |||
| Employed | 871 (39.4) | 277 (31.8) | 20.18±4.40 | |||
| Student | 719 (32.6) | 271 (37.7) | 20.45±4.01 | |||
| Currently or previously working in medical fields | <0.001 | <0.001 | ||||
| Yes | 649 (29.4) | 326 (50.2) | 21.04±4.13 | |||
| No | 1,559 (70.6) | 346 (22.2) | 19.63±4.21 | |||
| Having chronic diseases | 0.096 | 0.784 | ||||
| Yes | 307 (13.2) | 81 (26.4) | 19.98±4.60 | |||
| No | 1,901 (81.8) | 591 (31.1) | 20.06±4.18 | |||
| Previously infected with COVID-19 | 0.401 | 0.011 | ||||
| Yes | 320 (14.5) | 91 (28.4) | 19.49±4.60 | |||
| No | 1,888 (85.5) | 581 (30.8) | 20.14±4.17 | |||
Values are presented as number (%) or mean±standard deviation, unless otherwise stated. Statistical test used was chi-square test. Bold type is considered statistically significant.
COVID-19, coronavirus disease 2019.
a)Acceptance includes those who agreed and strongly agreed to take a COVID-19 vaccine versus those disagreed or strongly disagreed to take a such vaccine versus those undecided.
Out of the 2,208 participants surveyed, 672 (30.4%) reported that they would take a COVID-19 vaccine if available and recommended for them (42.1% strongly agreed and 57.9% agreed). On the other hand, 803 (36.4%) reported that they would not take the COVID-19 vaccine (51.3% strongly disagreed 48.7% disagreed). About one-third of the participants, 733 (31.5%), were not decided yet if they will take the COVID-19 vaccine or not.
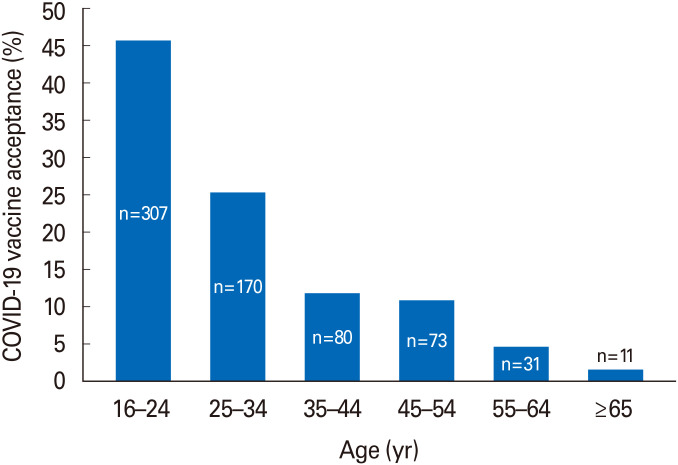
The vaccine acceptance rate significantly differed by the demographic characteristics with younger adults (p<0.001), males (p<0.001), not married (p<0.001), who do not have children (p<0.001), those with a bachelor or higher educational level (p<0.001), employees or being students (p<0.001), and workers in medical fields (p<0.001) being more likely to accept the COVID-19 vaccine if available and recommended for them compared to their counterparts in each category (Table 1). The mean±SD age of those willing to take the COVID-19 vaccine was 30.6±12.6 as compared to 35.4±13.8 for ones who were undecided and 41.1±13.7 for the unwilling group (p<0.001). Fig. 2 represents the COVID-19 acceptance rates by age groups. There was no significant difference in COVID-19 acceptance rates according to having previous COVID-19 infection or the presence of chronic diseases (p>0.05 for comparison).
Fig. 2. Comparison of coronavirus disease 2019 (COVID-19) acceptance by age groups for individuals who reported acceptance of COVID-19 vaccine (p<0.001).
The mean±SD of the “COVID-19 risk perception” score was 20.1±4.2 with internal consistency reliability of 0.723. The mean COVID-19 risk perception score amongst those who would accept the vaccine was 21.7±3.9 compared to 18.5±4.6 amongst those who would not accept the vaccine (p<0.001). Those who reported intentions to take the COVID-19 vaccine had higher perceived benefits of the vaccine and scored lower on clinical barriers to the vaccine scale than those who were undecided or unwilling to take the COVID-19 vaccine (p<0.001) (Table 2).
Table 2. COVID-19 risk perception, perceived benefits, and barriers to vaccination and their associations with the willingness to get COVID-19 vaccine.
| Cronbach's α | Range | Total sample | Willingness to get COVID-19 vaccine | ||||
|---|---|---|---|---|---|---|---|
| Yes | Undecided | No | p-value | ||||
| COVID-19 risk perception scalea) | 0.723 | 6–30 | 20.05±4.24 | 21.68±3.85 | 20.20±3.59 | 18.54±4.55 | <0.001 |
| Perceived benefits of a COVID-19 vaccine scaleb) | 0.888 | 3–15 | 8.81±3.04 | 11.58±2.16 | 8.85± 1.94 | 6.46±2.50 | <0.001 |
| Perceived clinical barriers to COVID-19 vaccine scalec) | 0.777 | 4–20 | 11.55±2.70 | 10.06±2.38 | 11.53±1.94 | 12.83±2.89 | <0.001 |
Values are presented as range or mean±standard deviation, unless otherwise stated.
COVID-19, coronavirus disease 2019.
a)How worried are you personally about coronavirus/COVID-19 at present? (1=not at all worried, 5=very worried); How likely do you think it is that you will catch the coronavirus/COVID-19 in the next 6 months? (1=not at all likely, 5=very likely); How likely do you think it is that your friends and family in the country you are currently living in will catch the coronavirus/COVID-19 in the next 6 months? (1=not at all likely, 5=very likely); How much do you agree or disagree with “The coronavirus/COVID-19 will NOT affect very many people in the country I am currently living in?” (1=strongly agree, 5=strongly disagree); How much do you agree or disagree with “I will probably get sick with the coronavirus/COVID-19?” (1=strongly disagree, 5=strongly agree); How much do you agree or disagree with “Getting sick with the coronavirus/COVID-19 can be serious?” (1=strongly disagree, 5=strongly agree). b)How much do you agree or disagree with the following statements? (1=strongly disagree, 5=strongly agree): “Vaccination will decrease my chance of getting COVID-19 or its complications.”; “Vaccination is a good idea because I feel less worried about catching COVID-19.”; “if I get vaccinated for COVID-19, I will decrease the frequency of having to consult my doctor.” c)How much do you agree or disagree with the following statements? (1=strongly disagree, 5=strongly agree): “I will have side effects from the COVID-19 vaccine.”; “I will get sick from the COVID-19 vaccine.”; “I will die from the COVID-19 vaccine.”; “The COVID-19 vaccine will be painful.”
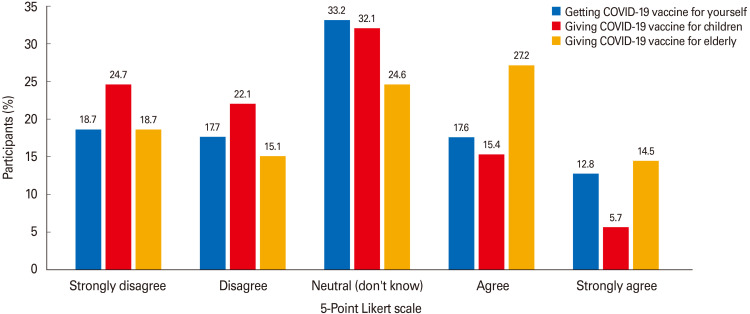
A total of 920 participants (41.7%) agreed/strongly agreed to encourage the elderly (≥64 years old) to receive the COVID-19 vaccine, and 467 (20.1%) agreed/strongly agreed to vaccinate their children against COVID-19 (Fig. 3). Half of the participating health care workers (50.2%) were willing to take the COVID-19 vaccine, and 62.4% would encourage other people to do so, although only 32% had received the influenza vaccine. Moreover, most healthcare workers who intend to take the COVID-19 vaccine for themselves would encourage others to receive it (313/326, 96%).
Fig. 3. Coronavirus disease 2019 (COVID-19) acceptance rates (%) for self, children, and the elderly.
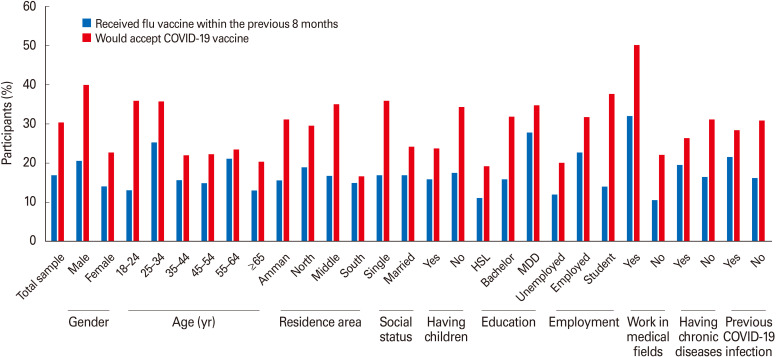
COVID-19 vaccine acceptance rates also differed compared to the seasonal flu vaccination rates. Among 374 (16.9%) of the participants who reported receiving the seasonal flu vaccine in the last 8 months, 243 (65%) were willing to take a COVID-19 vaccine (p<0.001). Notable demographic differences present when comparing reported seasonal flu vaccine uptake to reported COVID-19 vaccine acceptance rates (Fig. 4). For example, males who would accept the COVID-19 vaccine (40%) are two-folds of those who received the flu vaccine (20.6%). Another demographic difference finding is that participants in the young age group (18–24 years old) had a low seasonal flu vaccine uptake (n=113, 13.2%), but of that same group, 35.9% (n=307) reported acceptance of the COVID-19 vaccine. In comparison, those who were ≥65 years old had low seasonal flu vaccine rates and low COVID-19 vaccine acceptance rates (13%, 20.4%, respectively). Additionally, individuals who live in the south of Jordan, mostly rural areas, reported lower influenza vaccine uptake (n=9, 15%) and lower COVID-19 vaccine acceptance rates (n=10, 16.7%) than nearly all other Jordanian areas. Participants who had high school education or lower reported a lower influenza vaccination uptake and a lower COVID-19 vaccine acceptance rate than those with higher educational levels. A similar finding was observed among unemployed or retired participants in comparison to employed individuals and college students. A final interesting difference is that individuals who reported previous COVID-19 infection reported higher influenza uptake (n=69, 21.6%) but lower COVID-19 vaccine acceptance rate (n=91, 28.4%) than those who reported no previous COVID-19 history (n=305, 16.2%; n=581, 30.8%, respectively).
Fig. 4. Percentages of participants who reported receiving the influenza vaccine and who reported acceptance of coronavirus disease 2019 (COVID-19) vaccine by demographic characteristics. HSL, high school or lower; MDD, master or doctoral degree.
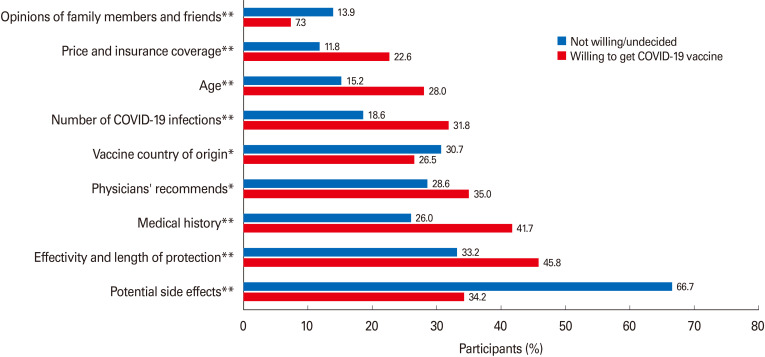
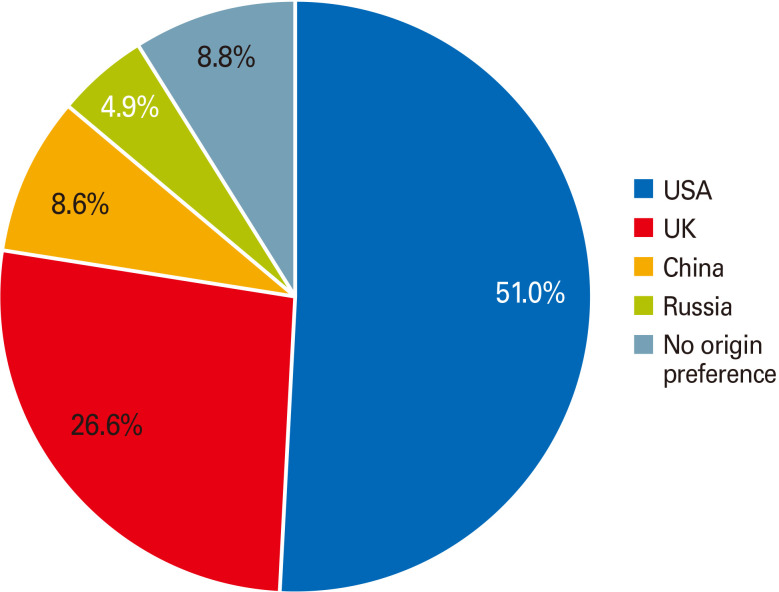
The 2,208 surveyed participants indicated that the following factors would matter in their vaccination decision-making: potential side effects of the vaccine (n=1,255, 56.8%) as the most essential factor, the effectiveness of the vaccine and length of protection it provides (n=818, 37%), their health history (e.g., presence of an underlying medical condition) (n=679, 30.8%), if a physician recommends me to get the vaccine (n=675, 30.6%), the national origin of vaccine (n=650, 29.4%), the number of people getting infected with COVID-19 (n=500, 22.6%), their age (n=421, 19.1%), vaccine price and health insurance coverage for the vaccine (n=333, 15.1%), and the least important factor in their vaccination decision-making was the opinions of family members and friends (n=262, 11.9%). Fig. 5 represents factors influencing vaccination decision-making among participants willing to take the COVID-19 vaccine and those not willing or undecided to do such yet (all p<0.05). Regarding the national origin of the vaccine, most study participants who were willing to take the COVID-19 vaccine preferred the vaccine originating in the United States or United Kingdom (77.6%) over other countries (Fig. 6).
Fig. 5. Factors influencing coronavirus disease 2019 (COVID-19) vaccination decision-making. *Significant comparison with p<0.05; **Significant comparison with p<0.001, based on chi-square tests.
Fig. 6. The preferred national origin of coronavirus disease 2019 (COVID-19) vaccine among those who are willing to get COVID-19 vaccine (n=672).
It is also essential to understand the reasons why people do not want to get vaccinated. Among those who reported no intention or had no decision to accept COVID-19 vaccination, the most common reasons were “I do not think it can be reliable as it will be a new vaccine,” “There is a lack of information about COVID-19 vaccine, and we need to know more about it,” “I am afraid of the side effects of COVID-19 vaccine,” and “COVID-19 vaccine may cause long-term health problems for me.” The reasons for indecision and rejection of the COVID 19 vaccine are presented in Table 3. The differences in the reasons were further explored between those who did not intend to accept the COVID-19 vaccination and those who did not decide on the vaccination acceptance. Those who did not decide to accept the COVID-19 vaccination were more likely to have suspicion on the safety of the vaccine, such as its possible side effects, long-term health problems, and the lack of sufficient information about the vaccine. In contrast, they were less likely to believe that the COVID-19 vaccine contains harmful substances or that COVID-19 infection is a biological weapon.
Table 3. The reasons for indecision and rejection of COVID-19 vaccine.
| Totala) | Intentions to accept COVID-19 vaccination when it is available | p-value | ||
|---|---|---|---|---|
| Undecided (n=733) | Not accept (n=803) | |||
| I do not think it can be reliable as it will be a new vaccine. | 1,074 (69.9) | 505 (68.9) | 569 (70.9) | 0.402 |
| There is a lack of information about the COVID-19 vaccine, and we need to know more about it. | 919 (59.8) | 467 (63.7) | 452 (56.3) | 0.003 |
| I am afraid of the side effects of the COVID-19 vaccine. | 917 (59.7) | 483 (65.9) | 434 (54.0) | <0.001 |
| COVID-19 vaccine may cause long-term health problems for me. | 737 (48.0) | 411 (56.1) | 326 (40.6) | 0.009 |
| COVID-19 vaccine contains harmful substances. | 460 (29.9) | 142 (19.4) | 318 (39.6) | <0.001 |
| I do not trust vaccine companies in general. | 409 (26.6) | 133 (18.1) | 276 (34.4) | <0.001 |
| COVID 19 infection is a biological weapon, and I think that the vaccine will serve those who produce this virus. | 352 (22.9) | 95 (13.0) | 257 (32.0) | <0.001 |
| COVID-19 vaccine is not an effective way to prevent the disease. | 227 (14.8) | 58 (7.9) | 169 (21.0) | <0.001 |
| COVID-19 vaccine will be very expensive. | 73 (4.8) | 45 (6.1) | 28 (3.5) | 0.015 |
| Religious reasons | 15 (1.0) | 3 (0.4) | 12 (1.5) | 0.037 |
Values are presented as number (%), unless otherwise stated. Bold type is considered statistically significant.
COVID-19, coronavirus disease 2019.
a)Out of 1,536 participants who did not intend or not decide to accept COVID-19 vaccination for themselves.
Using binary logistic regression analysis, male gender (OR, 1.55; 95% CI, 1.19–2.01; p=0.001), living in the capital (OR, 2.54; 95% CI, 1.04–6.19; p=0.041), or in middle of Jordan (OR, 3.55; 95% CI, 1.27–9.91; p=0.016), being employee (OR, 1.62; 95% CI, 1.23–2.18; p=0.038), being a college student (OR, 1.41; 95% CI, 1.00–1.98; p=0.048), working in a medical field (OR, 2.37; 95% CI, 1.77–3.15; p<0.001), having history of seasonal influenza vaccine (OR, 1.66; 95% CI, 1.20–2.28; p=0.002), having higher COVID-19 risk perception scores (OR, 1.07; 95% CI, 1.03–1.11; p<0.001), having higher perceived vaccine benefits (OR, 1.93; 95% CI, 1.80–2.07; p<0.001), and lower perceived vaccine barriers (OR, 0.82; 95% CI, 0.77–0.87; p<0.001) were independent predictors for intentions to accept COVID-19 vaccination, rather than indecision or unwillingness to accept the COVID-19 vaccine (Table 4).
Table 4. The predictors of COVID-19 vaccine acceptance.
| Variable | Adjusted odds ratios (95% confidence intervals) | p-value | |
|---|---|---|---|
| Gender (male) | 1.545 (1.191–2.005) | 0.001 | |
| Residence area | |||
| Amman (Jordan capital) | 2.537 (1.040–6.187) | 0.041 | |
| North | 2.431 (0.991–5.964) | 0.052 | |
| Middle | 3.545 (1.269–9.906) | 0.016 | |
| South | Ref | Ref | |
| Employment status | |||
| Unemployed or retired | Ref | Ref | |
| Employed | 1.620 (1.230–2.182) | 0.038 | |
| College students | 1.410 (1.003–1.981) | 0.048 | |
| Working in medical fields | 2.365 (1.774–3.151) | <0.001 | |
| History of COVID-19 infection | 0.730 (0.502–1.062) | 0.099 | |
| Influenza vaccine uptake during the past 8 months | 1.655 (1.201–2.281) | 0.002 | |
| COVID-19 risk perception score | 1.069 (1.032–1.107) | <0.001 | |
| Perceived benefits of the vaccine score | 1.933 (1.803–2.072) | <0.001 | |
| Perceived clinical barriers to vaccine score | 0.815 (0.766–0.868) | <0.001 | |
Bold type is considered statistically significant.
COVID-19, coronavirus disease 2019; Ref, reference.
Participants reported that their own physician (63.1%) and healthcare professionals (51.3%) were the most reliable sources of COVID-19 information. Other reported reliable sources of information were world health institutions (34.3%) and governmental health institutions (29%). Comparatively, social media was reported as the least reliable source of information (5.7%).
Discussion
This study is one of the first to investigate both the willingness to take the COVID-19 vaccine as well as the predictors and determinants of its acceptance rate in a developing country. Overall, study participants were almost equally distributed between willingness, unwillingness, and indecision to take the COVID-19 vaccination when available. Participants reported more COVID-19 acceptance for the elderly than themselves than for their children. The COVID-19 vaccine acceptance rate significantly differed by socio-demographic characteristics, seasonal flu vaccination status, COVID-19 risk perception, and perceived benefits and clinical barriers of the COVID-19 vaccine. Willingness type and predictors of willingness are critical for public health decision making and vaccination campaign implementation.
Our findings indicated that COVID-19 vaccine acceptance could be predicted with relatively high accuracy by readily available demographic characteristics. One of the alarming findings that older-adults and elderly were less likely to be willing to take the COVID-19 vaccine; this represents an area of concern because this group is at higher risk for COVID-19 infection morbidity and mortality and will likely be a priority group to receive the COVID-19 vaccine [28,29]. Targeting this age group with proper interventions is crucial in reducing the burden of COVID-19 infection.
A staggering gender gap in COVID-19 vaccine willingness was noted as females were less likely than males to receive COVID-19 vaccination. This finding is concordant with other reports from China and the United States [30,31,32]. This finding could be attributed to the fact that women usually tend to be more worried and careful about themselves and their families' health.
Our study also found that the COVID-19 vaccine acceptance rate differs by education, employment, and residence. Educational level is proportional to the COVID-19 vaccine acceptance rate. On the other hand, unemployed participants and those who live in rural areas reported lower COVID-19 vaccine acceptance rates. This was previously reported by Malik et al. [20] that found as years of education increase, so does COVID-19 vaccine acceptance rate, and unemployed participants reported a lower COVID-19 vaccine acceptance rate. Our study found that working in the healthcare field independently predicted COVID-19 acceptance as half of the healthcare workers were willing to take the COVID-19 vaccine. Similar findings were reported among nurses, where 40.0% of them were willing to take a COVID-19 vaccine [33].
Our study demonstrated a significant correlation between several beliefs and the COVID-19 vaccine acceptance rate. As the COVID-19 risk perception and the vaccine's perceived benefits increased, so did the reported COVID-19 vaccine acceptance rate. Also, perceived clinical barriers to the COVID-19 vaccine were associated with a lower COVID-19 acceptance rate. Importantly, future interventions could target these modifiable beliefs to encourage COIVD-19 vaccine uptake. Past interventions that have included components targeting such beliefs were successful in improving knowledge, attitudes/beliefs, and uptake of other vaccines [34,35,36,37].
Overall, the potential side effects of the COVID-19 vaccine, its effectiveness, and the duration of protection it provides were the most critical factors in vaccination decision-making. Therefore, planning for a COVID-19 vaccination should be comprehensive, focusing on its efficacy and safety. Moreover, our study highlights that certain factors in vaccination decision-making may differ depending on a person's willingness to get vaccinated and demonstrates the common reasons for no intentions or no decisions to accept COVID-19 vaccination. Therefore, we suggest that future communications about a COVID-19 vaccine should be individualized based on the person's readiness to get vaccinated. Among those undecided or unwilling to take the COVID-19 vaccine, communications should focus more on the safety of the COVID-19 vaccine and reduce concerns about its side effects. In comparison, communications for people who were willing to take the COVID-19 vaccine may need to focus more on other issues such as COVID-19 vaccine efficacy and its protection duration against COVID-19 infection, their medical health status, and physicians' recommendation. While assurance of safety is critical for the first group, the latter groups should be addressed with efficacy evidence. Such distinction is vital for a proper vaccination campaign.
Our study shows a low COVID-19 vaccine acceptance rate among the general population in a developing country. Based on some estimates, the herd immunity threshold for SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) was estimated between 55% and 82% [38]. Hence, this low level of acceptance absolutely may not be sufficient to achieve COVID-19 herd immunity. With many COVID-19 vaccines under development and substantial vaccination levels needed to achieve herd immunity, evidenced-based interventions and messages are needed to build confidence, encourage trust in a COVID-19 vaccine and minimize misinformation, especially among vulnerable groups. Also, how these messages are made available to the public should be considered. Our study found that healthcare professionals were the most reliable sources of information on COVID-19 infection, and 30.6% reported the physicians' vaccine recommendation as an essential factor in their vaccination decision-making. Hence, strong healthcare professionals' vaccine recommendations will be critical to promoting vaccine uptake, and they should be engaged in community vaccination messaging to enhance trust in a COVID-19 vaccine and encourage its uptake. Also, messaging and education should target the general population and focus on those who are undecided or unwilling to take a COVID-19 vaccine, such as females, elderly, uneducated, and unemployed individuals, and those who live in underdeveloped areas and low-income communities.
Our study's strengths include timeliness prior to introducing any COVID-19 vaccines in Jordan and a large sample size from different areas throughout the country. The study also examined a wide range of demographic factors and health beliefs to predict COVID-19 vaccine acceptance. Most vitally, this is one of the first studies that examined detailed COVID-19 vaccine acceptance. However, several limitations should be mentioned. A selection bias may influence our study because participants needed access to a smartphone/computer to participate, limiting our sample's generalizability. This may have also excluded poor people and the elderly, which are groups vulnerable to COVID-19. Besides, approximately 13% of all invited individuals completed the e-survey and were included in the analysis; however, a previous study has shown that the average response rate of internet-based surveys is low but is better than paper questionnaires in terms of data completeness [39]. The lack of available data on non-respondents and the cross-sectional design of the study could also affect the interpretation of results. Additionally, because this is an online survey-based study, we could not check if participants' responses were real (e.g., whether the participants had really received an influenza vaccine is unknown). Given that data collection occurred during the early stages of developing a COVID-19 vaccine and before its distribution, we could not provide participants with detailed information about the COVID-19 vaccine that could affect their acceptance rate, such as the dosing schedule. Lastly, our survey assessed COVID-19 vaccine acceptance rates under the condition that the vaccine was free, and acceptability might be lower if there would be out-of-pocket costs associated with the vaccine.
In conclusion, this study sets the stage for public health stakeholders to develop tailored COVID-19 vaccination interventions. While the COVID-19 vaccine acceptance rate was low, a significant proportion of participants reported being indecisive. This necessitates swift interventions to elevate vaccine acceptance by addressing its safety and efficacy. Targeting vulnerable groups is also crucial for implementing these interventions. The influence of socio-demographics on acceptance rate, along with perceived risks, benefits, and barriers should be used to fine-tune the intervention campaign. A one-size fits all national campaign may not suffice. Utilizing healthcare professionals as gatekeepers and social norms is crucial in any public health campaign for immunization.
Footnotes
No potential conflict of interest relevant to this article was reported.
The datasets generated and analyzed during the current study are available with the corresponding author.
References
- 1.Our World in Data. Coronavirus (COVID-19) deaths [Internet] Oxford: Our World in Data; 2020. [cited 2021 Jan 10]. Available from: https://ourworldindata.org/covid-deaths. [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard [Internet] Geneva: World Health Organization; 2021. [cited 2021 Jan 10]. Available from: https://covid19.who.int/?gclid=EAIaIQobChMI2_CM6e-DZ6gIVghh9Ch3nDQm1EAAYASAAEgLqwPD_BwE. [Google Scholar]
- 3.Lurie N, Saville M, Hatchett R, Halton J. Developing covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. DRAFT Landscape of COVID-19 candidate vaccines [Internet] Geneva: World Health Organization; 2021. [cited 2021 Jan 10]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. [Google Scholar]
- 5.Samrah SM, Al-Mistarehi AW, Ibnian AM, et al. COVID-19 outbreak in Jordan: epidemiological features, clinical characteristics, and laboratory findings. Ann Med Surg (Lond) 2020;57:103–108. doi: 10.1016/j.amsu.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kheirallah KA, Alsinglawi B, Alzoubi A, et al. The effect of strict state measures on the epidemiologic curve of COVID-19 infection in the context of a developing country: a simulation from Jordan. Int J Environ Res Public Health. 2020;17:6530. doi: 10.3390/ijerph17186530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bults M, Beaujean DJ, Richardus JH, Voeten HA. Perceptions and behavioral responses of the general public during the 2009 influenza A (H1N1) pandemic: a systematic review. Disaster Med Public Health Prep. 2015;9:207–219. doi: 10.1017/dmp.2014.160. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Benefits of Getting a COVID-19 Vaccine [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2021. [cited 2021 Jan 10]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html#:~:text=Based%20on%20what%20we%20know,severe%20illness%20from%20COVID%2D19. [Google Scholar]
- 9.Statistica. Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of January 7, 2021, by phase [Internet] New York (NY): Statista Inc.; 2021. [cited 2021 Jan 10]. Available from: https://www.statista.com/statistics/1119060/coronavirus-drugs-in-development-by-phase-worldwide/ [Google Scholar]
- 10.Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine [Internet] Silver Spring (MD): U.S. Food and Drug Administration; 2020. [cited 2021 Jan 10]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. [Google Scholar]
- 12.Quinn SC, Kumar S, Freimuth VS, Kidwell K, Musa D. Public willingness to take a vaccine or drug under Emergency Use Authorization during the 2009 H1N1 pandemic. Biosecur Bioterror. 2009;7:275–290. doi: 10.1089/bsp.2009.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastwood K, Durrheim DN, Jones A, Butler M. Acceptance of pandemic (H1N1) 2009 influenza vaccination by the Australian public. Med J Aust. 2010;192:33–36. doi: 10.5694/j.1326-5377.2010.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwarzinger M, Flicoteaux R, Cortarenoda S, Obadia Y, Moatti JP. Low acceptability of A/H1N1 pandemic vaccination in French adult population: did public health policy fuel public dissonance. PLoS One. 2010;5:e10199. doi: 10.1371/journal.pone.0010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sypsa V, Livanios T, Psichogiou M, et al. Public perceptions in relation to intention to receive pandemic influenza vaccination in a random population sample: evidence from a cross-sectional telephone survey. Euro Surveill. 2009;14:19437. [PubMed] [Google Scholar]
- 16.Rubin GJ, Potts HW, Michie S. The impact of communications about swine flu (influenza A H1N1v) on public responses to the outbreak: results from 36 national telephone surveys in the UK. Health Technol Assess. 2010;14:183–266. doi: 10.3310/hta14340-03. [DOI] [PubMed] [Google Scholar]
- 17.Holzmann H, Wiedermann U. Mandatory vaccination: suited to enhance vaccination coverage in Europe. Euro Surveill. 2019;24:1900376. doi: 10.2807/1560-7917.ES.2019.24.26.1900376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IndexMundi. Jordan Demographics Profile [Internet] [place unknown]: IndexMundi; 2020. [cited 2021 Jan 10]. Available from: https://www.indexmundi.com/jordan/demographics_profile.html. [Google Scholar]
- 19.Khassawneh AH, Alrabadi N, Al-Mistarehi AH, Obeidat N, Kheirallah KA. The role of non-state actors in combating COVID-19 spread in Northern Jordan. Ann Med Surg (Lond) 2020;60:484–486. doi: 10.1016/j.amsu.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dryhurst S, Schneider CR, Kerr J, et al. Risk perceptions of COVID-19 around the world. J Risk Res. 2020;23:994–1006. [Google Scholar]
- 22.Leiserowitz A. Climate change risk perception and policy preferences: the role of affect, imagery, and values. Clim Chang. 2006;77:45–72. [Google Scholar]
- 23.Van der Linden S. The social-psychological determinants of climate change risk perceptions: towards a comprehensive model. J Environ Psychol. 2015;41:112–124. [Google Scholar]
- 24.Xie B, Brewer MB, Hayes BK, McDonald RI, Newell BR. Predicting climate change risk perception and willingness to act. J Environ Psychol. 2019;65:101331 [Google Scholar]
- 25.Guidry JP, Laestadius LI, Vraga EK, et al. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2021;49:137–142. [Google Scholar]
- 26.Myers LB, Goodwin R. Determinants of adults' intention to vaccinate against pandemic swine flu. BMC Public Health. 2011;11:15. doi: 10.1186/1471-2458-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coe AB, Gatewood SB, Moczygemba LR, Goode JV, Beckner JO. The use of the health belief model to assess predictors of intent to receive the novel (2009) H1N1 influenza vaccine. Innov Pharm. 2012;3:1–11. doi: 10.24926/iip.v3i2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker M. ACIP Mulls Priority Groups for COVID-19 vaccines [Internet] New York (NY): MedPage Today; 2020. [cited 2021 Jan 10]. Available from: https://www.medpagetoday.com/meetingcoverage/acip/88760. [Google Scholar]
- 30.Wang J, Jing R, Lai X, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines (Basel) 2020;8:482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreps S, Prasad S, Brownstein JS, et al. Factors associated with US adults' likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3:e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villarreal D. Women 20% less likely to take COVID-19 vaccine if one's available in 2020 [Internet] New York (NY): Newsweek; 2020. [cited 2021 Jan 10]. Available from: https://www.newsweek.com/women-20-less-likely-take-covid-19-vaccine-if-ones-available-2020-1532469. [Google Scholar]
- 33.Wang K, Wong EL, Ho KF, et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38:7049–7056. doi: 10.1016/j.vaccine.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter PL, Katz ML, Bauermeister JA, Shoben AB, Paskett ED, McRee AL. Increasing human papillomavirus vaccination among young gay and bisexual men: a randomized pilot trial of the outsmart HPV intervention. LGBT Health. 2018;5:325–329. doi: 10.1089/lgbt.2018.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McRee AL, Shoben A, Bauermeister JA, Katz ML, Paskett ED, Reiter PL. Outsmart HPV: acceptability and short-term effects of a web-based HPV vaccination intervention for young adult gay and bisexual men. Vaccine. 2018;36:8158–8164. doi: 10.1016/j.vaccine.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gargano LM, Herbert NL, Painter JE, et al. Development, theoretical framework, and evaluation of a parent and teacher-delivered intervention on adolescent vaccination. Health Promot Pract. 2014;15:556–567. doi: 10.1177/1524839913518222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta P, Sharma M, Lee RC. Designing and evaluating a health belief model-based intervention to increase intent of HPV vaccination among college males. Int Q Community Health Educ. 2013-2014;34:101–117. doi: 10.2190/IQ.34.1.h. [DOI] [PubMed] [Google Scholar]
- 38.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of Internet versus paper-and-pencil versions. J Med Internet Res. 2007;9:e25. doi: 10.2196/jmir.9.3.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]