Abstract
Background
Multiple aspects of sleep and Sleep Disordered Breathing (SDB) have been linked to hypertension. However, the standard measure of SDB, the Apnoea Hypopnea Index (AHI), has not identified patients likely to experience large improvements in blood pressure with SDB treatment.
Methods
To use machine learning to select sleep and pulmonary measures associated with hypertension development when considered jointly, we applied feature screening followed by Elastic Net penalized regression in association with incident hypertension using a wide array of polysomnography measures, and lung function, derived for the Sleep Heart Health Study (SHHS).
Findings
At baseline, n=860 SHHS individuals with complete data were age 61 years, on average. Of these, 291 developed hypertension ~5 years later. A combination of pulmonary function and 18 sleep phenotypes predicted incident hypertension (OR=1.43, 95% confidence interval [1.14, 1.80] per 1 standard deviation (SD) of the phenotype), while the apnoea-hypopnea index (AHI) had low evidence of association with incident hypertension (OR =1.13, 95% confidence interval [0.97, 1.33] per 1 SD). In a generalization analysis in 923 individuals from the Multi-Ethnic Study of Atherosclerosis, aged 65 on average with 615 individuals with hypertension, the new phenotype was cross-sectionally associated with hypertension (OR=1.26, 95% CI [1.10, 1.45]).
Interpretation
A unique combination of sleep and pulmonary function measures better predicts hypertension compared to the AHI. The composite measure included indices capturing apnoea and hypopnea event durations, with shorter event lengths associated with increased risk of hypertension.
Funding
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 and by National Center for Advancing Translational Sciences grants UL1-TR- 000040, UL1-TR-001079, and UL1-TR-001420. The MESA Sleep ancillary study was supported by NHLBI grant HL-56984. Pulmonary phenotyping in MESA was funded by NHLBI grants R01-HL077612 and R01-HL093081. This work was supported by NHLBI grant R35HL135818 to Susan Redline.
Keywords: Sleep disordered breathing, Hypertension, Pulmonary function, Apnoea and hypopnea event duration
Abbreviations: AHI, Apnoea–hypopnea index; AUC, Area under the curve of ROC; CPAP, Continuous positive airway pressure; cSP, Composite sleep and pulmonary; DBP, Diastolic blood pressure; EEG, Electroencephalogram; FVC, Forced vital capacity; FEV1, Forced Expiratory Volume 1; HST, Home sleep apnoea testing; MESA, Multi-ethnic study of atherosclerosis; NRI, Net reclassification index; OR, Odds ratio; OSA, Obstructive sleep apnoea; PSG, Polysomnography; SBP, Systolic blood pressure; SD, Standard deviation; SDB, Sleep disordered breathing; SHHS, Sleep heart health study; WHR, Waist-to-hip circumference ratio
Research in Context.
Evidence before this study
Sleep Disordered Breathing (SDB) has been linked to elevated blood pressure as well as hypertension in various studies with multiple pathophysiological mechanisms suggested. However, the gold standard measure, Apnoea–Hypopnea Index (AHI), for quantifying SDB and defining the case population in most of previous studies has been widely questioned for oversimplifying this complex disorder. The long-term prognosis and clinical manifestations of SDB can be better reflected by integrating other polysomnography-derived measures and sleep state traits, many of which also have been previously shown in association with hypertension. Additionally, pulmonary function featured by vital capacity measures has been reported to associate with both cross-sectional and incident hypertension. A connection between reduced lung volume and SDB can also be established via increased upper airway collapsibility and reduced oxygen storage. Despite all mentioned efforts to map the relationships among three conditions, the cardio-pulmonary domain remains under-investigated in the context of SDB.
Added value of this study
Our study considered a plethora of sleep-related traits, with further incorporation of pulmonary functions to assess SDB and the SDB-related pathogenesis of hypertension. We developed composite sleep and pulmonary function measures with stronger prognostic power compared to the AHI. Apart from the main composite phenotype requiring access to electroencephalography measurements (cSPPSG), we also develop developed a version of composite phenotype friendly to home sleep studies (cSPHST).
Implications of all the available evidence
This study will lead to improved risk stratification metrics based on overnight sleep studies. It shows that greater attention should be given to lung function and to hypopnea and apnoea event durations.
Alt-text: Unlabelled box
1. Introduction
Hypertension is a medical condition affecting more than 30% of the U.S population. The correct diagnosis, early intervention and efficient treatment of hypertension are important for reducing risk of stroke, cardiovascular disease, and renal failure [1,2]. There is a large body of evidence for the association of various sleep characteristics with blood pressure and hypertension [3,4]. Sleep disordered breathing (SDB), common in middle aged and older individuals at risk for hypertension, has been shown to be associated with increase in both nocturnal and daytime blood pressure, uncontrolled blood pressure, and prevalent and incident hypertension [5,6]. In addition, independent of SDB, reduced slow wave sleep (N3), short total sleep duration, and poor sleep quality, have been implicated in hypertension [7], [8], [9]. Several pathophysiological mechanisms are proposed to link disturbed sleep to hypertension through abnormal arterial blood gas, excessive arousals and decreased slow-wave sleep affecting the balance between sympathetic and parasympathetic nervous system activity [8,[10], [11], [12]].
Previous studies that investigated the association between hypertension and sleep disordered breathing (SDB) mainly focused on defining “cases” or exposures using the polysomnography (PSG)-derived apnoea–hypopnea index (AHI), a simple measure of average number of respiratory events (apneas and hypopneas) per hour of sleep. Nevertheless, continuous positive airway pressure (CPAP), the primary treatment of OSA, has shown overall modest effects on blood pressure when individuals are selected on the basis of cutoff values for the AHI alone [13], [14], [15], [16], [17]. The relatively modest blood pressure improvements observed in these trials possibly reflects the failure of AHI to adequately capture pathophysiology of the respiratory events or to identify individual differences in response to these events. Multiple additional metrics are available from the PSG that plausibly can improve prediction of hypertension, such as sleep depth, periodic limb movement index and reduced slow wave sleep [9,18,19]. Additionally, emerging data indicate the importance of state-specific measures of sleep disturbance; e.g., REM-dominant sleep apnoea appears to drive a large portion of sleep-apnoea associated hypertension, possibly because of associations with more severe sympathetic activation, more hypoxemia, and disruptions of REM sleep-related neuroendocrine homeostasis [20]. Considering the complicated and heterogeneous mechanisms underlying hypertension in relation to sleep, a potential alternative approach to disentangle individual sleep disturbance exposures is to combine multiple sleep-related traits ranging from sleep architecture, hypoxemia, breathing disturbance and autonomic dysregulation, along with other individual characteristics, to evaluate individual phenotype contributions while accounting for potential confounders.
Cardio-pulmonary interactions have multiple physiological and clinical implications, but are relatively under-studied in the setting of SDB. Notably, variations in lung function may influence SDB susceptibility as well as SDB subtype, including predisposition to hypertension. Decreased end-expiratory lung volume increases upper airway collapsibility and can precipitate or exacerbate apneas and hypopneas [21], [22], [23], [24]. In addition, both lower lung volumes and underlying pulmonary parenchymal disease can reduce oxygen storages and result in a greater degree of hypoxemia following SDB-related respiratory events. Decreased lung volumes also may be a marker of cardiac ventricular dysfunction. Multiple studies have demonstrated an association of reduced pulmonary function with hypertension cross-sectionally [25], [26], [27], as well as with incident hypertension [25], [26], [27], [28], although those studies did not evaluate the influences of SDB. Other studies reported the association of pulmonary function with incident cardiovascular outcomes [29], [30], [31], [32], of which hypertension is a strong risk factor. We therefore hypothesized that measures of lung function, in conjunction with quantitative measures of sleep and SDB, could provide improved physiological biomarkers predictive of hypertension risk and provide insight into SDB-related pathophysiological mechanisms that cause hypertension.
In this study, we aimed to develop composite sleep and pulmonary (cSP) phenotypes based on spirometry and overnight polysomnography (PSG) measures to predict incident hypertension in the Sleep Heart Health Study (SHHS) via penalized regression, which simultaneously performs both feature selection and effect size estimation. We then evaluated the cSP traits in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort in association with hypertension in order to study generalization to a population with different characteristics. We performed side-by-side comparison between AHI and the cSP phenotypes to demonstrate the added value of the cSP phenotypes in explaining the association between sleep and hypertension, compared to the standard AHI.
2. Methods
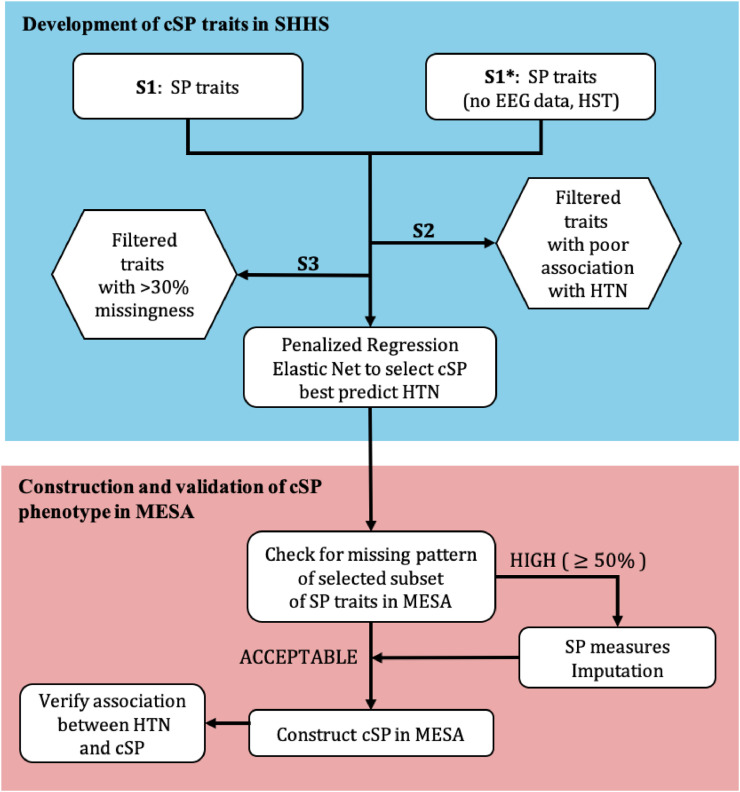
To select sleep and pulmonary phenotypes predictive of hypertension and develop a combined phenotype, we took a multi-step approach (see Figure 1). First, we used data collected in SHHS, a large prospective cohort study, to construct cSP phenotypes based on overnight PSG and spirometry measures. As the initial pool of traits for further processing, we used two different sets of sleep physiology traits: one set includes traits typically available from in-lab PSG, utilizing both respiratory and comprehensive sleep physiology measures, and the second set includes solely traits typically available in-Home Sleep Apnoea Testing (HST), utilizing respiratory measures and measures that do not require measurements of electroencephalography (EEG) signals. The first set of sleep traits is all those available from the National Sleep Research Resource [33]. We generated the second set of traits by combining together REM and NREM specific traits, and by removing sleep traits such as those related to stages and arousal events (see code in the public repository https://github.com/lijin0303/cSP-Hypertension). First, we filtered traits based on marginal association with incident hypertension and low level of missingness. Second, we performed penalized regression to select a subset of traits and computed their weights in constructing composite traits based on PSG (cSPPSG) or HST (cSPHST) that predict incident hypertension in SHHS. Finally, we studied the association of the two developed cSP traits with hypertension in MESA.
Figure 1.
Flowchart to develop cSP highly predictive of hypertension.
HST is “Home Sleep Test”, referring to information available from home sleep studies, without EEG data.
2.1. The Sleep Heart Health Study (SHHS)
The SHHS was designed to investigate the role of SDB as a risk factor for the development of hypertension and incident cardiovascular disease [34]. The NSRR sleep data included participants from four established cohort studies (The Framingham Offspring cohort, The Hagerstown, Sacramento and Pittsburgh sites of the Cardiovascular Health Study, The Hagerstown and Minneapolis/St. Paul sites of the Atherosclerosis Risk in Communities study, and a study of respiratory disease in Tucson). In total, 5,804 individuals with mixed gender and race/ethnicity participated in a first visit (1995 - 1998), and 4,080 of these individuals participated in a second visit (2001 - 2003). We excluded individuals with hypertension in visit 1, resulting in a sample size of 2,517 individuals with different levels of missingness in various baseline (visit 1) sleep and pulmonary traits.
2.2. Sleep and pulmonary phenotypes
Using the Compumedics sleep diagnostic system, overnight PSG was obtained at home for SHHS individuals. The collected data included oximetry, heart rate/ECG, chest wall and abdominal inductance plethysmography, nasal/oral airflow (thermocouple), body position, C4/A1 and C3/A2 EEG, electrooculography, and chin electromyography as described before [35]. Pulmonary function was assessed using spirometry performed according to the American Thoracic Society guidelines [36]. We calculated the % of predicted FVC and FEV1 taking into account the contribution of age, age squared, height squared and race/ethnicity [37].
We used 432 available sleep and pulmonary-based measures (both PSG and pulmonary measures). In brief, the PSG phenotypes quantify sleep architecture (sleep staging, sleep state transitions and sleep duration), hypoxemia (blood oxygen saturation patterns during sleep), breathing disturbance (numbers, duration, type, and state specificity of apneas and hypopneas), arousal-related events and heart rate measures during sleep [38]. Most PSG measures were evaluated separately by REM and non-REM sleep, by sleep position (supine/non-supine); most respiratory event subtypes were also evaluated according to the presence of arousals or a pre-set oxygen desaturation threshold.
2.3. Hypertension outcome
Blood pressure was measured using a standardized protocol [34] described in detail in [39]. In brief, blood pressure was measured on the right arm after 5 minutes rest using conventional mercury sphygmomanometer. Blood pressure was measured three times, and the average of the second and third measures was taken. Hypertension was defined as Systolic Blood Pressure (SBP) > 130 mmHg, diastolic blood pressure (DBP) > 80 mmHg, or any history of antihypertensive medication use.
2.4. Other covariates
Covariates including gender, race/ethnicity, body mass index (BMI), waist-to-hip circumference ratio (WHR), neck girth, smoking status and packs per year, collected as described before [34], were used in the penalized regression to construct the cSP phenotype as fixed (unpenalized) covariates.
2.5. Construction of HST trait set
Because EEG data often are unavailable in HST, we removed all traits related to arousal events and sleep stages, and further combined traits that were separately computed during REM and non-REM phases but that otherwise capture the same signal. For example, AHI measured during REM and non-REM phase were combined to a single AHI by weighting the REM and non-REM AHIs using sleep time spent in REM and non-REM. This resulted in a smaller sleep and pulmonary trait pool of size 122.
2.6. Construction of composite Sleep and Pulmonary (cSP) trait
In what follows, we performed the same chain of analyses with the complete (N = 432) and reduced HST set of traits (N=122), leading to the development of cSPPSG and cSPHST. To construct the cSP phenotypes, we first screened for traits marginally associated with hypertension incidence [40] requiring p-value <0.2 [linear regression t-test] and having low missingness percentage (<30%), and used these as the predictors for penalized regression, as described below.
2.7. Penalized regression
Using the selected sleep and pulmonary traits after the screening step, we constructed the cSP phenotype which best predicted incident hypertension in SHHS. The candidate pool of sleep and pulmonary traits subject to penalized regression were winsorized outlying values to their 0.5% or 99.5% quantile. Sleep and pulmonary traits undergoing winsorization were determined by visualizing the density distribution of all traits in the pool.
We applied the penalized regression method Elastic Net with the R package glmnet [41] to fit a generalized logistic regression model with covariates fixed. Regularization parameter λ was selected with 10-fold cross-validation, with selected as the one with highest cross-validated Area Under the Curve of ROC (AUC). We then re-evaluated the penalized regression model using the full data and the selected , and determined the cSP phenotype using the selected traits and coefficients. All traits were standardized before penalized regression to ensure comparable feature magnitude and thus comparable penalty. The resulting coefficients were converted back to the original scale before they were used to construct cSP phenotypes.
2.8. The Multi-Ethnic Study of Atherosclerosis (MESA) cohort
MESA is a large longitudinal cohort study that prospectively collected risk factors for cardiovascular disease from participants in six field centers across the United States. For this analysis, we considered N = 2,055 individuals who had available cross-sectional hypertension data and participated in an in-home sleep visit to collect PSG measures. Both sets of measures were ascertained in conjunction with MESA Exam 5 (2010-2013). Sleep study participants underwent single night in-home PSG (Compumedics Somte Systems, Abbotsville, Australia, AU), as described before [42]. These individuals also participated in MESA Exam 6 (2016-2018). In each of MESA exams, after 5 minutes of sitted rest, blood pressure was measured three times within 2 minutes using an automated oscillometric sphygmomanometer (Dinamap Pro 100, GE Medical Systems Information Technologies, Inc.). SBP and DBP values are the average of the second and third readings.
2.9. Sleep-pulmonary traits, hypertension, and covariates in MESA
We identified MESA traits corresponding to those selected with penalized regression in SHHS and constructed two cSP phenotypes using these subsets of traits and their weights. We used only observations with all relevant phenotypes available to construct each of the cSP phenotypes. We verified the association between hypertension and developed cSP in MESA using cross-sectional data (incident data had low sample size). Hypertension status is defined as SBP > 130 mm Hg, SBP > 80 mm Hg, or any use of hypertension medication. Other covariates including age, sex, race/ethnicity, BMI, WHR, smoking status and pack-year were collected during the clinic visit.
2.10. Model evaluation using the Net Reclassification Index
To further evaluate the informativeness of the developed cSP phenotypes, we calculated net reclassification index (NRI) by comparing the prediction model with cSP phenotypes to the model that used only the AHI as the sleep metric using R package nricens [43]. To interrogate the influence of pulmonary functions in the developed cSP phenotypes, we extended this comparison using NRI to cSP phenotype with pulmonary functions excluded (and developed the phenotype again using the same algorithm), and to each of the individual pulmonary functions. The cut-off probability for categorizing hypertension is determined when Youden index, denoted as , defined as
2.11. Ethics
All SHHS participants provided written informed consent in their recruitment site. The SHHS was approved by the institutional review boards in all participating institutions. All MESA participants signed written informed consent in their recruitment site. The MESA study and ancillary sleep study were approved by the institutional review boards in all participating institutions. This study was approved via application to the National Sleep Research Resources and by the MESA publication committee upon review of study policies. The Mass General Brigham Institutional Review board approved this research.
2.12. Role of funders
The funding source did not take any part in this work.
2.13. Statistics
All the statistical analyses involved in this study have been described in each subsection respectively, with the corresponding R packages used clearly specified. All individuals fulfilling the inclusion/exclusion criteria and with available data were included in our analyses.
3. Results
When constructing cSPPSG, we first screened out 337 traits with low association with incident hypertension (p-value 0.2; linear regression t-test), and an additional 53 traits with high missingness (>30%). Table 1 provides key characteristics of the SHHS cohort at baseline, stratified by hypertension status in the follow-up visit. A table that also includes the 42 sleep traits available after the screening step in SHHS is provided in the Supplementary Materials (Table S1). Table S1 also includes the 12 sleep and pulmonary traits that would be available (after screening step) from HST using a Type 3 portable monitor (out of 122 traits, 100 were removed due to low association, and 10 were removed due to high missingness). We applied Elastic Net to construct cSPPSG and cSPHST with respectively 860 and 1,382 complete observations.
Table 1.
Baseline Characteristics of SHHS Participants Stratified by Incident HTN Status (PSG Measures).
| Characteristics at Baseline | No incident HTN | Incident HTN | p-value |
|---|---|---|---|
| Sample Size N | 569 | 291 | |
| Follow-up days | 1920.43 ± 97.45 | 1916.22 ± 113.01 | 0.637 |
| Age | 60.66 ± 9.63 | 65.65 ± 9.53 | <0.001 |
| Sex = male (%) | 296 (52.0) | 149 (51.2) | 0.877 |
| Race/ethnicity (%) | 0.523 | ||
| Black | 21 (3.7) | 12 (4.1) | |
| other | 29 (5.1) | 10 (3.4) | |
| White | 519 (91.2) | 269 (92.4) | |
| BMI | 27.87 ± 4.32 | 28.90 ± 4.66 | 0.001 |
| WHR | 0.92 ± 0.10 | 0.94 ± 0.08 | 0.003 |
| Neck Girth | 37.74 ± 4.09 | 38.24 ± 4.10 | 0.093 |
| Packet/Year | 11.91 ± 18.97 | 12.62 ± 19.00 | 0.602 |
| Smoking Status (%) | 0.516 | ||
| Current | 61 (10.7) | 24 (8.2) | |
| Former | 254 (44.6) | 133 (45.7) | |
| Never | 254 (44.6) | 134 (46.0) | |
| FVC% | 0.95 ± 0.16 | 0.91 ± 0.16 | <0.001 |
| FEV1% | 0.95 ± 0.16 | 0.92 ± 0.16 | 0.016 |
1The format a ± b represents mean ± standard deviation while a (b) represents number (percentage).
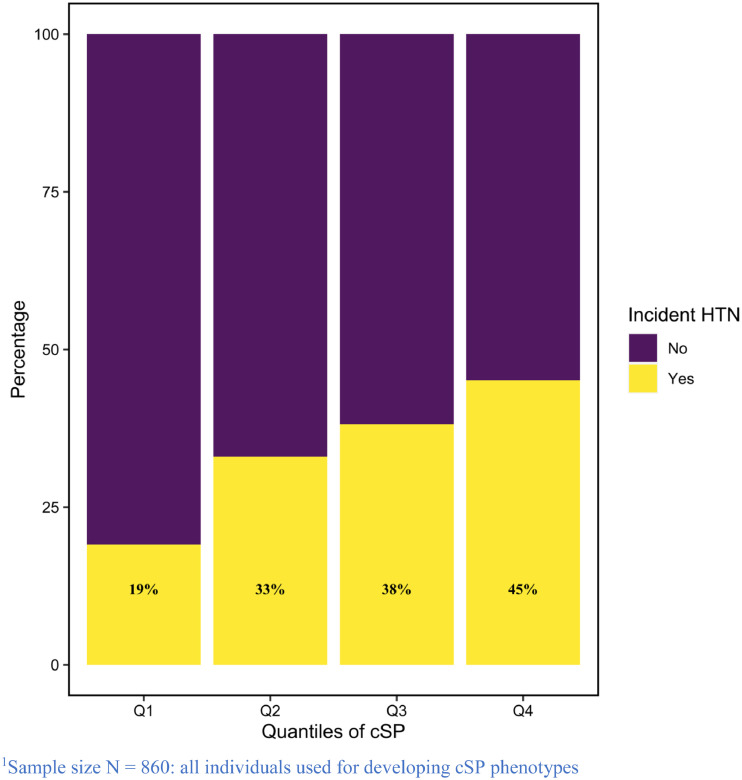
The selected 20 traits (Table 2) that comprised the cSPPSG combined information on sleep stages, sleep time, average/ minimum/ maximum hypopnea length, minimum heart rate, average/maximum oxygen desaturation, hourly obstructive apnoea events, to AHI (with events counted based on all apnoea and hypopnea, i.e., without an oxyhemoglobin desaturation minimum) and pulmonary function. The two traits with the largest weights were FVC% and percent of sleep time in stage 3 sleep. Of the remaining 18 selected PSG measures, 9 represent various measures of hypopnea length (minimum, mean, maximum, associated with events defined using different criteria and state-specificity). Additional metrics reflected cardiac autonomic function (heart rate changes), sleep architecture, and hypoxemia. Supplementary Figure 2a visualizes the correlation between the selected traits. Correspondingly, we integrated signals from pulmonary function and the selected PSG metrics to construct cSPHST (Supplementary Table 2; Supplementary Figure 2b). Results from association analyses of cSPPSG, cSPHST, as well as for a model only including the AHI as the exposure measure, are provided in Table 4. For predicting incident hypertension in SHHS, the estimated Odds Ratio (OR) of one standard deviation (SD) increase in cSPPSG was 1.72 (95% confidence interval, CI [1.45, 2.04]), and the AUC was 0.71. For cSPHST, the estimated OR was 1.47 (95% CI [1.25, 1.74]), and the AUC was 0.69. In contrast, the estimated OR for the AHI, defined as number of all apneas and hypopneas events with 3% oxygen desaturation per hour of sleep, was 1.13 per one SD (95% CI [0.97, 1.33]), and the AUC was 0.67. Considering NRI, the net proportion of individuals assigned to the more appropriate risk category compared to the AHI, using the threshold which Youden's index is maximized, is 0.12 when using cSPPSG, but lower when using cSPHST (0.009). Removal of lung function traits from the construction process of the cSP phenotypes (both PSG and HST setting) slightly reduces the estimated OR (Table 4). Figure 2 visualizes the proportions of SHHS individuals with incident hypertension by quantiles of the cSPPSG phenotype.
Table 2.
Selected Sleep and Pulmonary Phenotypes for Constructing cSP and Their Weights.
| Selected Traits | Weight | Standardized weight | Description |
|---|---|---|---|
| FVC% | -1.25 | -1.97E-1 | Percent Predicted Forced Vital Capacity (SHHS visit 1) |
| FEV1% | -3.67E-1 | -6.05E-2 | Percent Predicted Forced Expiratory Volume (SHHS visit 1) |
| TmStg34P | -3.44E-2 | -7.26E-2 | Number of rapid eye movement sleep (REM) to stage 1 shifts during sleep |
| MnHROA | -1.18E-2 | -1.28E-1 | Percent of sleep time in stage 3 sleep |
| AvHROP | -1.09E-2 | -9.30E-2 | Minimum Hypopnea length (REM, Non-supine, all oxygen desaturations, arousals) |
| AvHNOA3 | -9.28E-3 | -6.12E-2 | Average Hypopnea length (REM, Non-supine, all oxygen desaturations) |
| AvHROP3 | -7.41E-3 | -3.81E-2 | Average Hypopnea length (NREM, Non-supine, >=3% oxygen desaturation or arousal) |
| aMnBROH | -5.87E-3 | -5.66E-2 | Average Hypopnea length (REM, Non-supine, >=3% oxygen desaturation) |
| rdi0ps | 3.41E-3 | 3.83E-2 | Minimum Heart Rate (REM, Non-supine, all oxygen desaturations, arousal) |
| OARDNBA | 3.07E-3 | 8.74E-2 | Overall Apnoea Hypopnea Index (Supine, all oxygen desaturations) |
| MxHNOP | 1.72E-3 | 1.80E-2 | Obstructive Apnoea per hour (NREM, Supine, all oxygen desaturations, arousals) |
| MnHROA3 | -1.37E-3 | -2.70E-2 | Maximum Hypopnea length (NREM, Non-supine, all oxygen desaturations) |
| AvDNOP3 | -1.09E-3 | -5.07E-3 | Minimum Hypopnea length (REM, Non-supine, >=3% oxygen desaturation or arousal) |
| MxHROP | 7.28E-4 | 6.79E-4 | Average oxygen desaturation of respiratory event (NREM, Non-supine, >=3% oxygen desaturation) |
| SlpPrdP | -6.60E-4 | -1.69E-2 | Maximum Hypopnea length (REM, Non-supine, all oxygen desaturations) |
| AvHNOP | -5.37E-4 | -3.09E-2 | Sleep Time |
| TimeBedP | -4.90E-4 | -2.11E-3 | Average Hypopnea length (NREM, Non-supine, all oxygen desaturations) |
| MxDNBP | -3.45E-4 | -1.84E-2 | Time in bed |
| MnHROP3 | 2.43E-4 | 1.05E-3 | Maximum oxygen desaturation of respiratory event (NREM, Supine, all oxygen desaturations) |
| TmStg34P | -1.71E-6 | -1.15E-5 | Minimum Hypopnea length (REM, Non-supine, >=3% oxygen desaturation) |
*Standardized weight is the weight multiplied by SD of the corresponding phenotype. The standardized weight corresponds to the estimated regression coefficient for one standard deviation of the phenotype.
Table 4.
Comparison among Predictions Models on Incident Hypertension using SHHS Participants.
| Prediction model | OR per SD | 95% CI | p-value | AUC | NRI |
|---|---|---|---|---|---|
| AHI | 1.13 | [0.97, 1.33] | 0.110 | 0.67 | \ |
| cSPPSG | 1.72 | [1.45, 2.04] | <0.001 | 0.71 | 0.121 |
| cSPHST | 1.47 | [1.25, 1.74] | <0.001 | 0.69 | 0.009 |
| cSPPSG, no lung function | 1.66 | [1.41, 1.98] | <0.001 | 0.71 | 0.112 |
| cSPHST, no lung function | 1.29 | [1.11, 1.51] | 0.001 | 0.68 | 0.027 |
| FVC% | 0.78 | [0.66, 0.92] | 0.004 | 0.68 | 0.016 |
| FEV1% | 0.80 | [0.68, 0.94] | 0.007 | 0.68 | 0.002 |
The number of participants was 860.
* Within the same population, alternative AHI measures defined based on 1) respiratory (both apneas and hypopneas) events with ≥3% oxygen desaturation or arousal; 2) respiratory (both apneas and hypopneas) events with ≥4% oxygen desaturation; 3) with solely obstructive respiratory events with ≥4% oxygen desaturation are not significantly associated with incident hypertension (p values = 0.2, 0.1 and 0.1)
1All NRIs are compared to the AHI model
2The cut-off probability for categorizing hypertension is determined when Youden index is maximized
3All prediction models adjust for age, sex, race/ethnicity, BMI, waist/hip ratio, neck girth, smoking status and smoking pack years
Figure 2.
Proportion of Individuals with HTN in Different Quantiles of cSPPSG in SHHS.
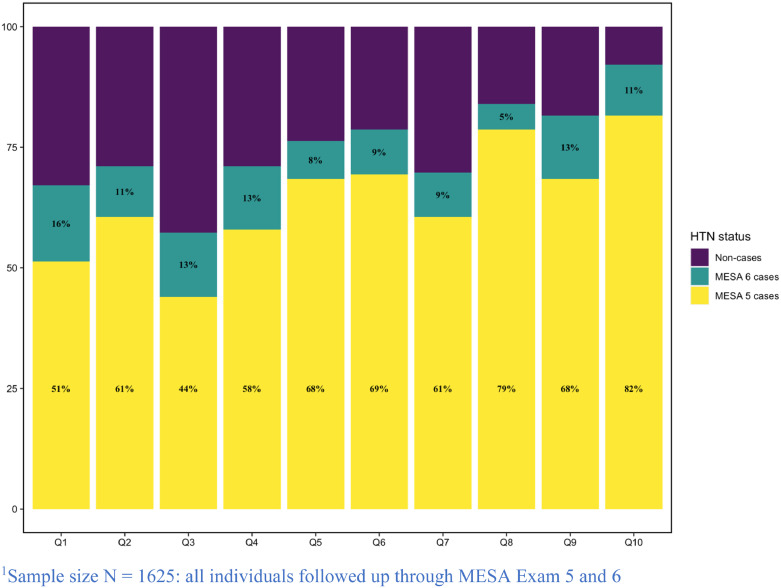
Next, we used the MESA cohort to verify the association of cSPPSG and cSPHST with hypertension. Table 3 demonstrates key characteristics of the MESA cohort at Exam 5, stratified by cross-sectional hypertension status. MESA individuals were 5 years older on average than SHHS individuals at Exam 5, and 71% had hypertension. Due to high missingness present in selected sleep and pulmonary traits, the number of complete cases for constructing two cSP phenotypes is less than 50% of original population (Supplementary Fig. 1). Results from association analyses in MESA of cSPHST, cSPHST, as well as the AHI, each using complete-case analysis, are provided in Table 5. We used the mean and SD of each of the compared measures in SHHS to standardize the traits in MESA, so that estimated effect sizes are comparable, i.e., one unit increase in the score is the same in SHHS and in MESA. In MESA cSPPSG had OR = 1.26 (95% CI [1.10, 1.45], p-value =0.001), cSPHST had OR = 1.26 (95% CI [1.12, 1.42], p-value<0.001), and the AHI had OR=1.22 (95% CI [1.05, 1.42], p-value=0.009). Thus, all these associations were statistically significant with AHI having lower estimated OR and the least significant and least precise estimate. The AUC was 0.75 for AHI and 0.77 for both cSPPSG and cSPHST. Comparing NRI relative to the AHI, cSPPSG had 0.04 and cSPHST had 0.03. The contribution of the two lung function measures towards the cSPPSG and cSPHST association with hypertension was important in MESA, and the association between the cSP measures and hypertension substantially weakened when removing these traits (Table 5). We also considered the association of the cSP measures with incident hypertension in MESA. However, because most eligible MESA individuals already developed hypertension in visit 5, and importantly, 82% of the individuals with highest decile of values of the cSPPSG trait already had hypertension at visit 5 (Figure 3), and the number of individuals with available phenotypes and free from hypertension at MESA visit 5 was low (N=272 when using cSPPSG and N=375 when using cSPHST), the power was low as well. Indeed, the estimated ORs were higher than the ones based on cross-sectional analysis, yet p-values were higher, with OR=1.47 (p-value =0.27) for cSPPSG and OR=1.40 (p-value = 0.28) for cSPHST. All p-values shown in this section were computed with t-test in linear regressions.
Table 3.
Characteristics of Participants Stratified by Cross-sectional Hypertension Status in MESA Exam 5.
| Characteristics at Baseline | No HTN | HTN | p-value |
|---|---|---|---|
| Sample Size N | 690 | 1365 | |
| Age | 64.80 ± 7.98 | 70.29 ± 9.16 | <0.001 |
| Sex = Male (%) | 324 (47.0) | 629 (46.1) | 0.742 |
| Race/ethnicity (%) | <0.001 | ||
| Asian | 106 (15.4) | 143 (10.5) | |
| Non-Hispanic Black | 107 (15.5) | 465 (34.1) | |
| Hispanic | 174 (25.2) | 317 (23.2) | |
| Non-Hispanic White | 303 (43.9) | 440 (32.2) | |
| Smoking Status (%) | 0.018 | ||
| Current | 54 (7.8) | 91 (6.7) | |
| Former | 285 (41.4) | 650 (48.0) | |
| Never | 349 (50.7) | 613 (45.3) | |
| BMI | 27.24 ± 5.05 | 29.38 ± 5.65 | <0.001 |
| WHR | 0.92 ± 0.08 | 0.95 ± 0.08 | <0.001 |
| Pack/Year | 8.96 ± 17.83 | 10.35 ± 19.09 | 0.114 |
1The format a ± b represents mean ± SD while a (b) represents number (percentage).
Table 5.
Comparison among Association Models on Cross-sectional Hypertension using MESA Participants.
| Association model | Sample Size | Case Number | OR per SD* | 95% CI | p-value | AUC | NRI |
|---|---|---|---|---|---|---|---|
| AHI | 2015 | 1365 | 1.22 | [1.05, 1.42] | 0.009 | 0.75 | \ |
| cSPPSG | 923 | 615 | 1.26 | [1.10, 1.45] | 0.001 | 0.77 | 0.04 |
| cSPHST | 1242 | 820 | 1.26 | [1.12, 1.42] | <0.001 | 0.77 | 0.03 |
| cSPPSG, no lung function | 1255 | 841 | 1.10 | [0.99, 1.21] | 0.072 | 0.75 | 0.04 |
| cSPHST, no lung function | 1697 | 1126 | 1.08 | [1.00, 1.17] | 0.042 | 0.75 | 0.01 |
| FVCpp | 3079 | 2147 | 0.80 | [0.74, 0.87] | <0.001 | 0.75 | 0.04 |
| FEV1pp | 3074 | 2142 | 0.83 | [0.77, 0.90] | <0.001 | 0.74 | 0.01 |
The number of participants varies across models due to missing data patterns. Case number is the number of individuals with hypertension in the specific association model.
1 All NRIs are calculated in regard to AHI model
2 The cut-off probability for categorizing hypertension is determined when Youden index is maximized
3 All prediction models adjust for age, sex, race/ethnicity, BMI, waist/hip ratio, smoking status and packet year
4 SD here all calculated from corresponding phenotype in SHHS.
Figure 3.
Proportion of individuals with HTN across deciles of cSPPSG in MESA Visit 5.
4. Discussion
In this study, we performed a multi-step analysis utilizing machine learning to identify a set of overnight-sleep and pulmonary phenotypes that jointly predict the development of hypertension in SHHS. We then developed two cSP phenotypes combining these measures. One phenotype was based on full PSG data that included indices of breathing disturbances, sleep architecture, overnight hypoxemia and heart rate, sleep duration, arousals, and shifts from REM to NREM sleep, and the second was based on a more limited set of variables from HSTs that did not require collection of EEG for computation. The comprehensive cSPPSG composite significantly predicted incident hypertension, with evidence of improved prediction over the use of only the traditional PSG metric, the AHI. Notably the composite included multiple metrics from the PSG not routinely considered in clinical decision making, including 9 measures of hypopnea length. Use of a simpler cSPHST derived using only 122 metrics (none requiring EEG collection) also provided improved prediction of incident hypertension over the AHI. Moreover, while FVC% and FEV1% contributed to these composite biomarkers of hypertension, excluding those traits did not substantially weaken the predicted association in SHHS. In a cross-sectional analysis in the smaller MESA sample, we confirmed that the new composite biomarkers predicted hypertension prevalence. However, in contrast to SHHS, these indices were not superior to use of the AHI and prediction was substantially influenced by the lung function variables. Our results highlight the potential for better understanding chronic disease outcomes with use of multiple physiological measures- such as from both PSG and spirometry- rather than focus on single indices, with inclusion of indices that reflect multiple physiological traits.
We used the Elastic Net penalty for the penalized regression. This penalty allows multiple correlated variables to be selected, rather than selecting one of them. The selected trait sets for both cSPHST and cSPPSG represent a combined effect of sleep and pulmonary phenotypes on hypertension from multiple physiological processes. The negative weights of the lung function traits FEV% and FVC% for cSP traits agree with previous findings associating reduced lung function with hypertension, as described in the introduction. The sleep staging variable selected into the cSPPSG phenotype describes the frequency of transitions between REM sleep to stage N1. While there is evidence that REM-specific SDB poses higher risk for adverse health effects, including hypertension, compared to non-REM SDB [20,47,48], prior research has not previously reported REM transitions as a predictor of hypertension. REM sleep is associated with relatively high levels of sympathetic nervous system activity and respiratory events in REM tend to be long and associated with the most marked hypoxemia. It is possible that individuals who more likely transition from REM to NREM sleep experience some cardio-protection related to state changes. Other sleep architecture traits negatively contributing to cSP phenotypes are all related to sleep time, in accordance with findings about relationship between duration of sleep and hypertension [13]. We also found that increased total sleep duration as well as time in N3 sleep were associated with reduced incidence of hypertension, consistent with positive effects of sleep duration and slow wave sleep on blood pressure.
There were nine traits measuring hypopnea durations in the cSPPSG trait, including traits measuring minimum, maximum, and average hypopnea durations, and three hypopnea duration traits in the cSPHST trait. The findings were consistent with shorter hypopnea duration (measured across sleep states and with different associated hypopnea definitions) increases risk of hypertension. This adverse effect of shorter event duration on blood pressure is consistent with prior findings showing that shorter event duration is associated with higher mortality as well as with a lower arousal threshold [49,50], consistent with greater autonomic nervous system activation. Minimum and average hypopnea lengths were computed over hypopnea events with different oxygen desaturation thresholds: either all oxygen desaturation levels or larger than 3% oxygen desaturation. Traits under both oxygen desaturation thresholds were selected in penalized regression, suggesting that important information is potentially lost when a specific oxygen desaturation threshold is chosen. Additionally, maximum and minimum hypopnea length used information from both REM and non-REM phases. Other selected traits reflecting minimum heart rate, hourly counts for obstructive apneas and the more general index AHI (defined as the hourly frequency of all apneas and hypopneas regardless of oxygen desaturation). High minimum heart rate is a marker of augmented cardiac sympathetic activation during sleep—a key pathway linking SDB to hypertension. A higher frequency of apneas and hypopneas may reflect the multiple pathways involved in SDB-related hypertension – frequency of oxygen desaturation/resaturation, sleep fragmentation and arousal, and airway collapsibility. Lastly, we detected contributions from both average and maximum oxygen desaturation level which can be explained by previous findings suggesting the importance of hypoxemia on activating the sympathetic nervous system and the effects of reactive oxygen species, and activation of HIF-1 pathways on vascular control mechanisms. Overall, the factors that contributed to the cSP are directionally consistent with existing background knowledge about sleep physiology, pulmonary function and hypertension.
While OSA is recognized as a cause of hypertension, and the prevalence and incidence of hypertension increase with increasing AHI in the general population [34,[51], [52], [53]], there is a growing recognition of the limitations of the AHI as the sole measure of sleep apnea severity [54,55]. This reflects not only inconsistency in the measurement of OSA and respiratory event definitions that result in unreasonably high estimates of the prevalence of OSA, but more importantly the failure of AHI to capture the physiologic diversity of apneas and hypopneas [56]. It has been argued that hypopnea and apnea events that differ by duration and degree of desaturation contribute to a different degree to symptoms such as hypertension and excessive daytime sleepiness [57,58]. Recent studies have sought to identify physiological measures that are better predictors than AHI of hypertension and cardiovascular risk. Promising measures include hypoxic burden, which is associated with both blood pressure [19] and cardiovascular mortality [59], as well as heart rate response to respiratory events [60] and respiratory event duration [61], which are associated with cardiovascular morbidity and mortality. Our work contributes to the evidence that combining multiple dimensions of sleep and related measures can provide additional information beyond that provided by the AHI alone. The predictive value of lung function in addition to direct measures of OSA suggests that multiple pathways that influence breathing, during both wake and sleep, and subsequent patterns of gas exchange and abnormalities in sympathetic activation and endothelial function may influence hypertension risk. It is also possible that the cSP phenotypes capture an OSA subtype where low lung function, associated with greater level of airway collapsibility, coupled with high AHI represents a more “collapsible” phenotype compared to high lung function coupled with high AHI. While each of these measures requires validation in independent cohorts before being employed in clinical practice, it is likely that some combination of these and other novel measures will soon be able to provide more precise OSA-related risk assessments in individual patients.
Notable strengths of our study include a disciplined analysis of multiple objectively collected sleep and the novel inclusion of lung function traits to derive cSP phenotypes. To our knowledge, this is the first study to construct composite markers of sleep and pulmonary-related hypertension. Both the SHHS and MESA cohorts are from community-samples and include diverse samples of both men and women. In both cohorts, the cSP phenotypes improved prediction of hypertension compared to AHI based on NRI improvement, and additionally had more significant associations. A study limitation was that while we attempted to replicate findings, we had limited incident data on hypertension in our independent cohort (MESA). MESA individuals were on average 5 years older than SHHS individuals at the time of the sleep exam, perhaps contributing to the difference in results, where lung function and AHI contributed relatively more to cross-sectional assessment of hypertension compared to cSPPSG. There are several factors limiting the generalizability of this study. The study participants are relatively old and cSP phenotypes utilized comprehensive PSG measures, not often available. Finally, while the models that we considered included standard measures used for hypertension risk prediction, we did not include diet and physical exercise, as they were not available in SHHS.
In conclusion, we performed variable selection to identify sets of sleep and pulmonary phenotypes associated with hypertension, and combined them to composite phenotypes to evaluate their overall, joint contribution, to hypertension development. We studied the generalization of the association of the composite phenotypes with hypertension in an independent cohort and confirmed their stronger association compared with the traditional AHI. Our findings support the use of composite measures that take advantage of the rich data routinely collected during PSG which is often under-utilized, as well as use of spirometry data, which can be easily collected in sleep centers. Potential future directions to extend our study involve applying the cSP phenotypes in cohorts with diverse age and exploring potential clinical use of the new phenotypes for better identification of at-risk population for hypertension. More precise identification of at-risk population for hypertension related to sleep physiology and pulmonary function can potentially lead to early intervention and better treatment adherence.
Contributors
Michael Rueschman and Ruitong Li verified the underlying data. Ruitong Li, Michael Rueschman, Susan Redline, and Tamar Sofer had full access to the data and reviewed the data for identifying ranges of plausible values and detection of outliers. Ruitong Li performed statistical analysis, and prepared tables and figures. Tamar Sofer supervised the analysis. Ruitong Li and Tamar Sofer drafted the manuscript. Daniel Gottlieb, Susan Redline, and Tamar Sofer interpreted the results. All authors critically reviewed the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Data sharing statement
The SHHS dataset is available from the National Sleep Research Resources sleepdata.org. The MESA dataset is available via a dbGaP application to study accession phs000209.v13.p3, or by a data use agreement following an approved paper proposal from the MESA study https://www.mesa-nhlbi.org/.
Declaration of Competing Interest
Susan Redline reports receiving NIH grant support for the present manuscript, with payments made to the institution; a contract from Jazz Inc with payments made to the institution; receiving direct consultant fees from Eisai Inc, Apnimed Inc, and Jass Inc; support from Eisai Inc to attend a meeting to discuss insomnia; an unpaid position on the patient advocacy group Alliance for Sleep Apnea Partners; and receiving equipment loans from Nox Medical and Philips Respironics for use in an NIH multi-center study. All other authors declare no conflict of interests.
Acknowledgements
The authors thank the data provided from MESA and SHHS data cohorts and the National Sleep Research Resource. This research was supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 and by National Center for Advancing Translational Sciences grants UL1-TR- 000040, UL1-TR-001079, and UL1-TR-001420. The MESA Sleep ancillary study was supported by NHLBI grant HL-56984. Pulmonary phenotyping in MESA was funded by NHLBI grants R01-HL077612 and R01-HL093081. This work was supported by NHLBI grant R35HL135818 to Susan Redline.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103433.
Appendix. Supplementary materials
References
- 1.Casey Jr DE, Thomas RJ, Bhalla V, Commodore-Mensah Y, Heidenreich PA, Kolte D. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the american college of cardiology/American heart association task force on performance measures. Circulation: Cardiovascul Qual Outcomes. 2019;12(11) doi: 10.1161/HCQ.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am College Cardiol. 2018;71(2):109–118. doi: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138(2):434–443. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pack AI. CRC Press; 2016. Sleep apnea: Pathogenesis, diagnosis and treatment. [Google Scholar]
- 5.Caples SM, Somers VK. Sleep disordered breathing and atrial fibrillation. Progr Cardiovasc Diseas. 2009;51(5):411. doi: 10.1016/j.pcad.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respirator Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Hu Y, Wang X, Yang S, Chen W, Zeng Z. The association between sleep duration and hypertension: a meta and study sequential analysis. J Hum Hypertens. 2020:1–6. doi: 10.1038/s41371-020-0372-y. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S, Redline S. Sleep, slow-wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14(5):442–448. doi: 10.1007/s11906-012-0289-0. [DOI] [PubMed] [Google Scholar]
- 9.Javaheri S, Zhao YY, Punjabi NM, Quan SF, Gottlieb DJ, Redline S. Slow-wave sleep is associated with incident hypertension: the sleep heart health study. Sleep. 2018;41(1):zsx179. doi: 10.1093/sleep/zsx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am College Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baguet J, Barone-Rochette G, Pépin J. Hypertension and obstructive sleep Apnoea syndrome: current perspectives. J Human Hypertens. 2009;23(7):431–443. doi: 10.1038/jhh.2008.147. [DOI] [PubMed] [Google Scholar]
- 12.Somers VK, Javaheri S. Cardiovascular effects of sleep-related breathing disorders. Sleep Breath Disord E-Book. 2016:270. [Google Scholar]
- 13.Wang Y, Mei H, Jiang Y-R, Sun W-Q, Song Y-J, Liu S-J. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015;11(9):1047–1056. doi: 10.5664/jcsm.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens. 2016;18(2):153–158. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varounis C, Katsi V, Kallikazaros IE, Tousoulis D, Stefanadis C, Parissis J. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: a systematic review and meta-analysis. Int J Cardiol. 2014;175(1):195–198. doi: 10.1016/j.ijcard.2014.04.240. [DOI] [PubMed] [Google Scholar]
- 16.Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144(5):1487–1494. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 17.Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. 2020;22(2):6. doi: 10.1007/s11886-020-1257-y. [DOI] [PubMed] [Google Scholar]
- 18.Dean DA, Wang R, Jacobs Jr DR, Duprez D, Punjabi NM, Zee PC. A systematic assessment of the association of polysomnographic indices with blood pressure: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(4):587–596. doi: 10.5665/sleep.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JS, Azarbarzin A, Wang R, Djonlagic IE, Punjabi NM, Zee PC. Association of novel measures of sleep disturbances with blood pressure: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2020;75(1):57–63. doi: 10.1136/thoraxjnl-2019-213533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga AW, Mokhlesi B. REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath. 2019;23(2):413–423. doi: 10.1007/s11325-018-1727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL. Effect of increased lung volume on sleep disordered breathing in patients with sleep Apnoea. Thorax. 2006;61(5):435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol (Bethesda, Md: 1985). 2010;108(2):445–451. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol (Bethesda, Md: 1985) 2010;109(4):977–985. doi: 10.1152/japplphysiol.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnabel E, Nowak D, Brasche S, Wichmann H-E, Heinrich J. Association between lung function, hypertension and blood pressure medication. Respir Med. 2011;105(5):727–733. doi: 10.1016/j.rmed.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Vollmer WM, Buist AS, Tsai R, Cen R, Wu X. Relationship between lung function and blood pressure in Chinese men and women of Beijing and Guangzhou. Int J Epidemiol. 1998;27(1):49–56. doi: 10.1093/ije/27.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Enright PL, Kronmal RA, Smith V-E, Gardin JM, Schenker MB, Manolio TA. Reduced vital capacity in elderly persons with hypertension, coronary heart disease, or left ventricular hypertrophy: the cardiovascular health study. Chest. 1995;107(1):28–35. doi: 10.1378/chest.107.1.28. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow D, Weiss ST, Vokonas PS, Cupples LA, Ekerdt DJ, Colton T. Forced vital capacity and the risk of hypertension: the normative aging study. Am J Epidemiol. 1988;127(4):734–741. doi: 10.1093/oxfordjournals.aje.a114854. [DOI] [PubMed] [Google Scholar]
- 29.Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: The Framingham study. Am Heart J. 1983;105(2):311–315. doi: 10.1016/0002-8703(83)90532-x. [DOI] [PubMed] [Google Scholar]
- 30.Marcus EB, Curb JD, Maclean CJ, Reed DM, Yano K. Pulmonary function as a predictor of coronary heart disease. Am J Epidemiol. 1989;129(1):97–104. doi: 10.1093/oxfordjournals.aje.a115128. [DOI] [PubMed] [Google Scholar]
- 31.Engström G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L. Lung function and cardiovascular risk. Circulation. 2002;106(20):2555–2560. doi: 10.1161/01.cir.0000037220.00065.0d. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD. Lung function and incident coronary heart disease: the atherosclerosis risk in communities study. Am J Epidemiol. 2003;158(12):1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G-Q, Cui L, Mueller R, Tao S, Kim M, Rueschman M. The national sleep research Resource: towards a sleep data commons. J Am Med Inf Assoc. 2018;25(10):1351–1358. doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 35.David H.Rapoport, Smith Philip M., James L.Kiley, SHHRGRSspceSMHLBKQSFICGDJBW P. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 36.Society AT. Standardization of spirometry. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 37.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 38.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73(5):472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S. Prospective study of sleep-disordered breathing and hypertension. Am J Respir Crit Care Med. 2009;179(12):1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan J, Lv J. Sure independence screening for ultrahigh dimensional feature space. J R Stat Soc: Se B (Statistical Methodology) 2008;70(5):849–911. doi: 10.1111/j.1467-9868.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman J, Hastie T, Tibshirani R. glmnet: Lasso and elastic-net regularized generalized linear models. R package version. 2009;1(4) [Google Scholar]
- 42.Dean DA, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D. Scaling up scientific discovery in sleep medicine: the national sleep research resource. Sleep. 2016;39(5):1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue E, Inoue ME. Package ‘nricens’. 2018.
- 44.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Pencina MJ, Steyerberg EW, D'Agostino Sr RB. Net reclassification index at event rate: properties and relationships. Stat Med. 2017;36(28):4455–4467. doi: 10.1002/sim.7041. [DOI] [PubMed] [Google Scholar]
- 46.Cook NR. Quantifying the added value of new biomarkers: how and how not. Diagnostic Prognostic Res. 2018;2(1):14. doi: 10.1186/s41512-018-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peter J, Grote L, Fus E, Ploch T, Stammnitz A. REM-sleep-hypertension in obstructive sleep apnea. Eur J Med Res. 1995;1(3):132. [PubMed] [Google Scholar]
- 48.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E. Obstructive sleep apnea during REM sleep and hypertension. Results of the wisconsin sleep cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A. Apnea–hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borker PV, Reid M, Sofer T, Butler MP, Azarbarzin A, Wang H. NREM apnea and hypopnea duration varies across population groups and physiologic traits. Am J Respiratory Crit Care Med. 2020 doi: 10.1164/rccm.202005-1808OC. (ja) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B. Population-based study of sleep-disordered breathing as a risk factor for hypertension. ArchInternal Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 52.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 53.Hou H, Zhao Y, Yu W, Dong H, Xue X, Ding J. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8(1) doi: 10.7189/jogh.08.010405. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T. On the rise and fall of the apnea−hypopnea index: a historical review and critical appraisal. J Sleep Res. 2020;29(4):e13066. doi: 10.1111/jsr.13066. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, McArdle N. Metrics of sleep apnea severity: beyond the AHI. Sleep. 2021 doi: 10.1093/sleep/zsab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Punjabi NM. COUNTERPOINT: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No. CHEST. 2016;149(1):16–19. doi: 10.1378/chest.14-2261. [DOI] [PubMed] [Google Scholar]
- 57.Koch H, Schneider LD, Finn LA, Leary EB, Peppard PE, Hagen E. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep Apnea. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkas A, Duce B, Leppänen T, Hukins C, Töyräs J. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath. 2017;21(4):829–835. doi: 10.1007/s11325-017-1513-6. [DOI] [PubMed] [Google Scholar]
- 59.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J. 2019;40(14):1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azarbarzin A, Sands SA, Younes M, Taranto-Montemurro L, Sofer T, Vena D. The sleep apnea-specific pulse rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med. 2021 doi: 10.1164/rccm.202010-3900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.