Abstract
Background
The Centers for Disease Control and Prevention state that a severe or immediate allergic reaction to the first dose of an mRNA COVID-19 vaccine is a contraindication for the second dose.
Objective
To assess outcomes associated with excipient skin testing after a reported allergic reaction to the first dose of mRNA COVID-19 vaccine.
Methods
We identified a consecutive sample of patients with reported allergic reactions after the first dose of mRNA COVID-19 vaccine who underwent allergy assessment with skin testing to polyethylene glycol (PEG) and, when appropriate, polysorbate 80. Skin testing results in conjunction with clinical phenotyping of the first-dose mRNA COVID-19 vaccine reaction guided second-dose vaccination recommendation. Second-dose mRNA COVID-19 vaccine reactions were assessed.
Results
Eighty patients with reported first-dose mRNA COVID-19 vaccine allergic reactions (n = 65; 81% immediate onset) underwent excipient skin testing. Of those, 14 (18%) had positive skin tests to PEG (n = 5) and/or polysorbate 80 (n = 12). Skin testing result did not affect tolerance of the second dose in patients with immediate or delayed reactions. Of the 70 patients who received the second mRNA COVID-19 vaccine dose (88%), 62 had either no reaction or a mild reaction managed with antihistamines (89%), but 2 patients required epinephrine treatment. Three patients with positive PEG-3350 intradermal (methylprednisolone) testing tolerated second-dose mRNA COVID-19 vaccination. Refresh Tears caused nonspecific skin irritation.
Conclusions
Most individuals with a reported allergic reaction to the first dose of mRNA COVID-19 vaccines, regardless of skin test result, received the second dose safely. More data are needed on the value of skin prick testing to PEG (MiraLAX) in evaluating patients with mRNA COVID-19 vaccine anaphylaxis. Refresh Tears should not be used for skin testing.
Key words: COVID-19 vaccine, Drug allergy, Vaccine allergy, COVID-19, PEG allergy, Skin testing, Polysorbate allergy, Excipient allergy, Anaphylaxis
Abbreviations used: BWH, Brigham and Women’s Hospital; CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency Use Authorization; ID, Intradermal; MGB, Mass General Brigham; MGH, Massachusetts General Hospital; NPV, Negative predictive value; PEG, Polyethylene glycol
What is already known about this topic? An expert-informed risk stratification protocol was recommended to guide clinical care after mRNA COVID-19 vaccine reactions. However, at the time, no supportive evidence was available.
What does this article add to our knowledge? Most individuals after first-dose mRNA COVID-19 vaccine reactions, regardless of excipient skin testing result, were able to receive the second mRNA COVID-19 vaccine dose safely. Refresh Tears were irritating and should not be used for skin testing to polysorbate 80.
How does this study impact current management guidelines? More data are needed on the value of skin prick testing to PEG in evaluating patients with mRNA COVID vaccine anaphylaxis, and the positive predictive value remains unknown. Most patients may be able to proceed to second vaccination without skin testing.
Introduction
One year after the World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic, more than 138 million people have been infected and every country in the world has been affected.1 With excitement and hope, millions of Americans welcomed the approval of 2 novel COVID-19 mRNA vaccines in December 2020. However, almost immediately after the Emergency Use Authorization (EUA)2 by the Food and Drug Administration of the Pfizer-BioNTech COVID-19 mRNA vaccine, initial reports of anaphylaxis and allergic reactions began. When the Moderna COVID-19 mRNA vaccine was approved soon thereafter, similar reports emerged.3 The reports of anaphylaxis prompted the Centers for Disease Control and Prevention (CDC) to develop formal guidance for administration of the Pfizer-BioNTech and the Moderna mRNA COVID-19 vaccines, stating that patients with a history of anaphylaxis to vaccine components should not receive mRNA COVID-19 vaccines,4 and patients with a history of an immediate allergic reaction to the first dose of mRNA COVID-19 vaccine should not receive the second dose.5
In December 2020, national anaphylaxis rates were initially reported by the CDC to be as high as 11.1 per million doses of the Pfizer-BioNTech COVID-19 vaccine,6 but by January 2021, CDC estimates reported an anaphylaxis incidence of 2.5 to 4.7 per million doses of the Moderna and Pfizer-BioNTech COVID-19 vaccines, respectively.7 In the first prospective real-world cohort of 60,000 employees vaccinated at a large health care system (Mass General Brigham [MGB]), an incidence of 2.5 cases of anaphylaxis per 10,000 mRNA COVID-19 vaccines administered was reported.8 The cause of allergic reactions to mRNA COVID-19 vaccines remains unknown, but the excipient polyethylene glycol (PEG)-2000, which is present in the mRNA COVID-19 vaccines,9 has been an important focus of investigation with recent limited supportive evidence.10 Furthermore, the utility of skin testing to PEG (MiraLAX, Bayer, Boca Raton, FL) and polysorbate 80 was unclear, but experts suspected excipient skin testing to have a high positive predictive value and a low negative predictive value (NPV).11 , 12 At the time of publication, 3 COVID-19 vaccines have received Food and Drug Administration EUA: 2 contain PEG-2000 (Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines) and 1 contains polysorbate 80 (Janssen adenovirus vector vaccine). Thus, it is important to understand whether there is a relationship between allergy to these excipients and allergic reactions to the COVID-19 vaccines.9
Our risk stratification algorithm13 uses clinical history with skin testing to PEG and/or polysorbate to evaluate higher-risk individuals who report a history of an immediate allergic reaction to the first dose of mRNA COVID-19 vaccine. Here, we report the outcomes of individuals referred for reported allergic reactions to the first dose of mRNA COVID-19 vaccine, who completed skin testing and followed our initial published skin testing guidance.13 We review patient and reaction characteristics with skin testing findings along with second-dose vaccine outcomes, which motivate an updated approach to individuals who present after a reported allergic reaction to the first dose of mRNA COVID-19 vaccine.
Methods
This cohort included consecutive patients referred for an in-person allergy/immunology excipient skin testing visit, which occurred at MGB between January 6 and March 3, 2021 after a first-dose mRNA COVID-19 vaccine reaction. In-person visits occurred after initial allergy/immunology telemedicine visits with clinical phenotyping and risk stratification. All included patients received the first mRNA vaccine dose at an MGB vaccination clinic; second doses were scheduled at the vaccination clinics associated with the MGB academic medical centers: Massachusetts General Hospital (MGH) or Brigham and Women’s Hospital (BWH).
We recommended excipient skin testing to all patients who reported symptoms concerning an IgE-mediated allergy within 4 hours of vaccination. Patients with symptoms consistent with an allergic reaction that was delayed (occurring more than 4 hours after vaccination but before day 3 after vaccination) may also have proceeded to excipient skin testing, especially if the symptoms were more severe or the patient preferred skin testing after using shared decision-making. For patients who reported symptoms that were potentially IgE-mediated but subjective (eg, globus sensation or itch without rash), the decision to proceed to skin testing was also based on shared decision-making considering patient preferences. Interested and eligible patients were referred for an in-person excipient skin testing appointment at either MGH or BWH. Approximately 14% of patients with first-dose mRNA COVID-19 vaccine reactions were referred for in-person excipient skin testing using our algorithm.13
At MGH, PEG skin testing was performed using MiraLAX, a laxative containing PEG-3350, epicutaneous and methylprednisolone acetate (containing PEG-3350 only13), intradermally (ID) (see Table E1 in this article’s Online Repository at www.jaci-inpractice.org).13 If methylprednisolone acetate was positive, methylprednisolone sodium succinate was used as a control to exclude methylprednisolone as the cause of allergy. At MGH, for individuals with first-dose mRNA COVID-19 vaccine reactions, skin testing to polysorbate 80 (using triamcinolone acetonide14) was performed if PEG skin testing was positive, to guide future COVID-19 vaccination or if the patient reported a history of a reaction to another vaccine containing polysorbate 80. At BWH, PEG (MiraLAX and methylprednisolone acetate containing PEG 3350 only) skin testing was similar, although polysorbate 80 testing used Refresh Tears (Allergan, Madison, NJ) and was performed regardless of the PEG skin testing result. Both sites required adequate positive (histamine) and negative (saline) controls for epicutaneous and ID tests. Patients with negative skin testing were advised to proceed to second-dose mRNA COVID-19 vaccination. Shared decision-making was used for patients with positive skin test to PEG and/or polysorbate 80.
We used excipient skin testing alone (without mRNA COVID-19 vaccine skin testing, which has been described elsewhere15 , 16) because we did not have access at the time to mRNA COVID-19 vaccine for skin testing purposes and the mRNA COVID-19 vaccines were the first vaccines of their kind. It was not apparent that prior vaccine guidance17 could be accurately applied to these vaccines and there was initial concern regarding stability for its use as a skin testing product or as a graded challenge.18
Owing to concerns that arose during this study regarding irritant skin testing reactions to Refresh Tears, control skin tests were performed on 25 nonallergic subjects who reported clinical tolerance to polysorbate 80.
Data collection and definitions
Clinical data were gathered from manual electronic health record review by trained research assistants (A.E.M. and A.S.C.) with allergist oversight (A.R.W., L.B.R., and L.L.). Structured MGB allergist note templates used during clinic visits facilitated accurate demographic, medical, and allergy history; skin test results; and outcomes. Study data were collected and managed using Research Electronic Data Capture tools.19 , 20
Immediate allergic reactions were defined as occurring within the first 4 hours; delayed allergic reactions occurred anytime thereafter. Reaction details were categorized by organ system of involvement. Cutaneous included itching, rash, hives, flushing, and swelling. Cardiovascular included hypotension and tachycardia. Lower respiratory included cough, wheezing, chest tightness, or shortness of breath. Upper airway included globus, hoarseness, and throat swelling. Gastrointestinal included abdominal pain, nausea, vomiting, and diarrhea.
For patients who received the second mRNA COVID-19 vaccination, we assessed tolerance of the vaccine using 3 days of electronically disseminated symptoms surveys. If patients did not respond to all 3 days of the survey, or if they reported allergic symptoms on the survey, they were contacted by phone or e-mail message by an allergist/immunologist (A.R.W.) to assess tolerance of the second dose of mRNA COVID-19 vaccine. Individuals who received the Janssen vaccine in lieu of second mRNA COVID-19 vaccines were considered not to have received the second mRNA COVID-19 vaccine dose but to have been completely vaccinated.
The index allergic reaction to the first mRNA COVID-19 vaccine and, when relevant, the allergic reaction to the second mRNA COVID-19 vaccine, were graded using Ring and Messmer criteria21 (see Table E2 in this article’s Online Repository at www.jaci-inpractice.org) by 2 allergists independently. In the case of discordant grading, a third allergist was used to determine the final grade (A.R.W., L.B.R., and L.L.). We defined second-dose tolerance as either no allergic reaction or a grade 0 reaction with mild subjective symptoms that did not require treatment.
This study was approved by the MGB Partners Institutional Review Board, Protocol No. 2020P004068.
Data analysis
Data are presented as counts with frequencies for categorical data and means with SDs or medians with interquartile ranges for continuous variables, as indicated. All analyses were performed in SAS software (version 9.4, Cary, NC). We calculated the NPV for PEG skin testing result and having a reaction with mRNA COVID-19 vaccine dose 2 for the entire sample and considering only patients with immediate reactions.
Results
Eighty patients underwent excipient skin testing at MGB after a reported allergic reaction to the first dose of mRNA COVID-19 vaccine (Table I ). Of those, 62 (77%) reported a reaction to the Moderna and 18 (23%) to the Pfizer-BioNTech COVID-19 mRNA vaccine. In total, 38,933 Moderna vaccines (60%) and 25,906 Pfizer-BioNTech vaccines (40%) were administered at MGB during the study period and 576 allergy/immunology telehealth visits were performed for COVID-19 vaccine reactions.
Table I.
Characteristics of patients who completed skin testing
| Characteristic | All (n = 80) | Immediate allergic reaction∗ (n = 65) | Delayed allergic reaction† (n = 15) |
|---|---|---|---|
| Demographics | |||
| Age, y (mean [SD]) | 40.9 (±13.6) | 42.4 (±12.4) | 34.5 (±16.6) |
| Female sex, n (%) | 71 (89) | 57 (88) | 14 (93) |
| Race, n (%) | |||
| White | 62 (78) | 51 (79) | 11 (73) |
| Asian | 7 (9) | 5 (8) | 2 (13) |
| Black | 4 (5) | 3 (5) | 1 (7) |
| Other | 7 (9) | 6 (9) | 1 (7) |
| History of atopy, n (%) | 56 (70) | 45 (69) | 11 (73) |
| Food allergy | 29 (36) | 24 (37) | 5 (33) |
| Asthma | 25 (31) | 19 (29) | 6 (40) |
| History of anaphylaxis | 22 (28) | 17 (26)‡ | 5 (33)§ |
| Allergic rhinitis | 20 (25) | 15 (23) | 5 (33) |
| History of polysorbate allergy | 5 (6) | 3 (5) | 2 (13) |
| Atopic dermatitis | 4 (5) | 3 (5) | 1 (7) |
| History of polyethylene glycol allergy | 2 (3) | 2 (3) | 0 |
| Reaction history, n (%)‖ | |||
| Cutaneous symptoms¶ | 65 (81) | 51 (78) | 14 (93) |
| Lower respiratory symptoms# | 18 (23) | 14 (22) | 4 (27) |
| Cardiovascular symptoms∗∗ | 11 (14) | 10 (15) | 1 (7) |
| Gastrointestinal symptoms†† | 9 (11) | 7 (11) | 2 (13) |
| Upper airway symptoms‡‡ | 6 (8) | 6 (9) | 0 |
| Other symptoms | 51 (64) | 21 (32)§§ | 1 (7)‖‖ |
| Management of allergic reaction, n (%) | |||
| Antihistamines | 54 (68) | 47 (72) | 7 (47) |
| Corticosteroids | 12 (15) | 11 (17) | 1 (7) |
| Intramuscular epinephrine | 6 (8) | 6 (9) | 0 |
| Emergency room visit | 19 (24) | 18 (28) | 1 (7) |
| Allergic reaction to (n [%]): | |||
| Dose 1 (Moderna) | 62 (78) | 48 (74) | 14 (93) |
| Dose 1 (Pfizer) | 18 (23) | 17 (26) | 1 (7) |
| Ring and Messmer17 criteria, n (%) | |||
| Grade 0 | 22 (28) | 21 (32) | 1 (7) |
| Grade 1 | 46 (58) | 33 (51) | 13 (87) |
| Grade 2 | 12 (15) | 11 (17) | 1 (7) |
| Positive skin test results, n (%) | 14 (18) | 8 (12) | 6 (40) |
| MiraLAX 1:100 (SPT) | 1 (1) | 1 (2) | 0 |
| MiraLAX 1:10 (SPT) | 0 | 0 | 0 |
| MiraLAX 1:1 (SPT) | 0 | 0 | 0 |
| Depo-Medrol 0.4 mg/mL (IDT) | 3 (4) | 2 (3) | 1 (7) |
| Depo-Medrol 4 mg/mL (IDT) | 1 (1) | 1 (2) | 0 |
| Refresh eye drops 1:1 (SPT) | 0 | 0 | 0 |
| Refresh eye drops 1:100 (IDT) | 10 (13) | 5 (8) | 5 (33) |
| Refresh eye drops 1:10 (IDT) | 2 (3) | 1 (2) | 1 (7) |
IDT, intradermal test; SPT, skin prick test.
All patients were referred for skin testing owing to symptoms suggestive of an IgE reaction.
Immediate was defined as 4 or fewer hours; 47 reactions (72%) were within 1 h (median, 10 min; interquartile range, 5-15 minutes).
Delayed was defined as more than 4 h; 11 reactions (73%) were within 24 h.
Culprit was a medication: 9 (14%); food: 6 (9%); and venom: 2 (3%).
Culprit was a medication: 3 (20%); and food: 2 (13%).
Patients may have more than 1 symptom.
Hives, rash, itching, swelling (not throat), and flushing.
Wheezing, chest tightness, shortness of breath, and cough.
Tachycardia and hypotension.
Gastrointestinal upset, diarrhea, vomiting, and nausea.
Throat swelling, hoarseness, and globus.
Tingling (n = 14; 22%), lightheadedness (n = 4; 6%), chest pain (n = 1; 2%), feeling hot (n = 1; 2%), syncope (n = 1; 2%), and red eye (n = 1; 2%). Patients may have more than 1 symptom.
Sneezing (n = 1; 7%) and tingling (n = 1; 7%).
Patient characteristics
Patients were predominantly female (89%) and White (78%), mean age 40.9 ± 13.6 years. Medical history included a history of nonanaphylactic IgE-mediated food allergy (n = 29; 36%); asthma (n = 25; 31%); anaphylaxis to medications, foods, venom, or latex (n = 22; 28%); allergic rhinitis (n = 20; 25%); polysorbate allergy (n = 5; 6%); atopic dermatitis (n = 4; 5%); and PEG allergy (n = 2; 3%) (Table I).
Reaction characteristics
A total of 65 patients reported an immediate reaction (81%); 40 occurred within the first 30 minutes (62%). Median onset of symptoms was within 10 minutes (interquartile range, 5-15 minutes). Reported symptoms were cutaneous (n = 51; 78%), lower respiratory (n = 14; 22%), cardiovascular (n = 10; 15%), and subjective, including lightheadedness and tingling (n = 21; 32%). Moreover, 47 patients received antihistamine treatment (72%) and 6 received epinephrine (9%); 18 were treated in the emergency department (28%). Of the 65 immediate allergic reactions, 21 were grade 0 (32%), 33 were grade 1 (51%), and 11 were grade 2 (17%). All individuals recovered from the reaction without long-term sequalae.
There were 15 patients who experienced delayed allergic reactions (19%): 14 patients received Moderna (93%) and 1 patient received Pfizer (7%) (Table I). Of these, 11 had symptoms within the first 24 hours after receiving the vaccine (73%) and 4 had symptoms starting 24 hours or more after the vaccine was administered (27%). Delayed symptoms were most frequently cutaneous (n = 14; 93%); other symptoms are listed in Table I. One patient was treated in the emergency department.
Skin testing results
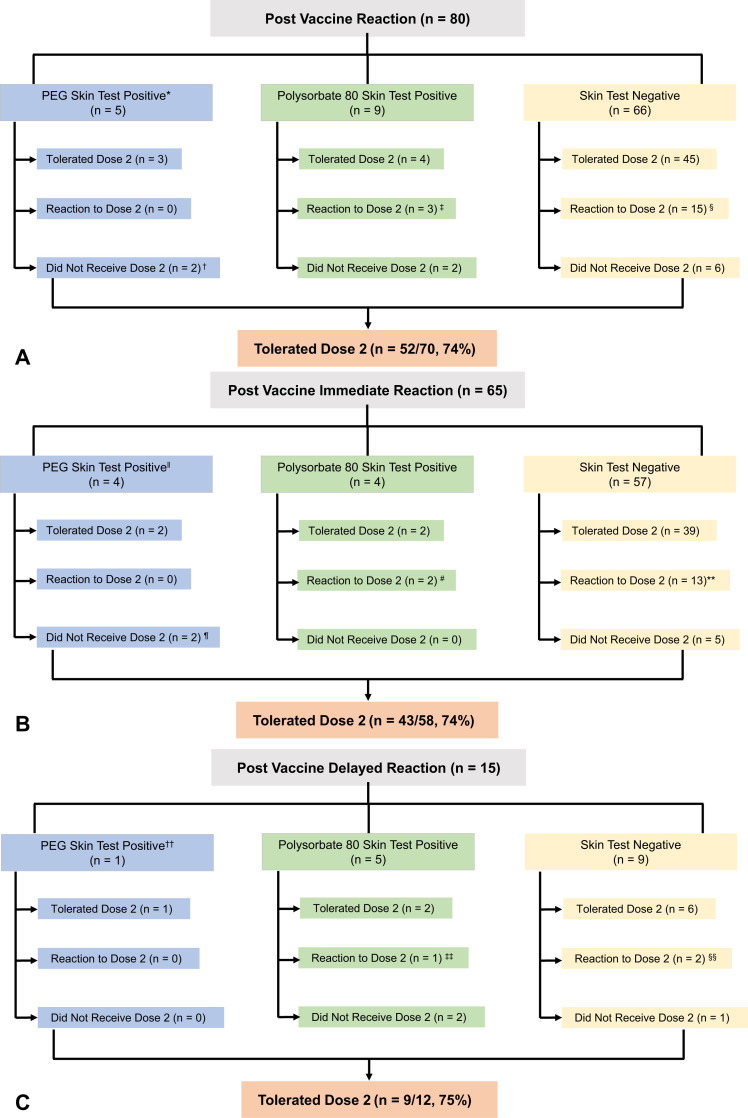
Of the 80 patients who underwent excipient skin testing, 33 had testing to PEG only; 47 had testing to PEG and polysorbate 80 (n = 41 to Refresh Tears and n = 6 to triamcinolone acetonide). In all, 14 had a positive skin test result (18%) (Table II ): PEG only (n = 2), PEG and polysorbate 80 (n = 3), and polysorbate 80 only (n = 9) (Figure 1 , A). All polysorbate 80-positive individuals were positive on skin testing to Refresh Tears (n = 12), which was found to be an irritant in 13 of 25 (52%) nonallergic controls (see Table E3 in this article’s Online Repository at www.jaci-inpractice.org). Among the 65 patients presenting with an immediate allergic reaction, 8 had positive skin tests (12%): PEG (n = 2), PEG and polysorbate 80 (n = 2), and polysorbate 80 (n = 4) (Figure 1, B). Patients with PEG-positive skin tests were positive on ID testing to methylprednisolone acetate 4 mg/mL (n = 1), methylprednisolone acetate 0.4 mg/mL (n = 2), and MiraLAX 1:100 epicutaneous (n = 1). Among the 15 patients with a delayed allergic reaction, 6 had positive skin testing (40%): 5 to polysorbate 80 (33%) and 1 to both PEG (ID testing to methylprednisolone acetate 0.4 mg/mL) and polysorbate 80 (Figure 1, C) (7%). No allergic or adverse events occurred during any skin testing procedures.
Table II.
Patients with positive skin testing results
| Timing of reaction | Age, y | Sex | History | Dose 1 Reaction | Skin test results | Dose 2 outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergies or allergic reaction | History of anaphylaxis | Vaccine | Onset of symptoms (h) | Signs and symptoms | Treatment | Treatment setting/disposition | Ring and Messmer grade | |||||
| Immediate (<4-h) reaction | 46 | F | Hymenoptera venom | Yes (Hymenoptera venom) | Moderna | 0.16 | Dizziness, nausea, difficulty swallowing, swelling of eyes and tongue, hives | Diphenhydramine | Home | 1 | Methylprednisolone acetate IDT 4 mg/mL (10 ×14 mm) Refresh IDT 1:10 (9 × 21 mm) |
Tolerated Janssen |
| 40 | F | None | No | Pfizer | 0.16 | Flushing, tachycardia, tongue itching, swelling of fingers, vomiting, diarrhea | Diphenhydramine, intravenous fluids, intramuscular epinephrine, methylprednisolone succinate, ondansetron. | Emergency department | 1 | MiraLAX skin prick test 1:100 (6 × 25 mm) | Recommended not to receive dose 2 | |
| 26 | F | Ginger | No | Moderna | 0.25 | Wheezing | No treatment needed | Home | 2 | Methylprednisolone acetate IDT 0.4 mg/mL (16 × 44 mm) | Tolerated Moderna | |
| 56 | F | Omalizumab, cefaclor, sulfonamide antibiotics, tree nuts, shellfish, latex | No | Moderna | 0.3 | Stinging in eyes, tingling of tongue and lips, mild itching | Cetirizine, famotidine | Home | 0 | Refresh IDT 1:100 (8 × 11 mm) | Pretreated with cetirizine 10 mg and prednisone 20 mg. At 5 min after receiving dose 2 (Moderna), patient experienced pruritus. Treated with cetirizine 20 mg | |
| 49 | F | None | No | Moderna | 1 | Flushing, hives, itching | Fexofenadine | Home | 1 | Refresh IDT (5 × 15 mm) | Tolerated Moderna | |
| 26 | F | Environmental | No | Moderna | 3 | Wheezing, chest tightness, dry cough | Albuterol | Home | 2 | Methylprednisolone acetate IDT 0.4 mg/mL (10 × 20 mm) Refresh IDT 1:100 (8 × 18 mm) |
Tolerated Moderna | |
| 54 | M | None | No | Moderna | 3 | Itching, lip swelling | No treatment needed | Home | 1 | Refresh IDT 1:100 (9 × 21 mm) | 4-6 h after receiving dose 2 (Moderna), patient experienced lip swelling, which self-resolved | |
| 42 | F | Penicillin, hymenoptera venom | No | Moderna | 4 | Hives | No treatment needed | Home | 1 | Refresh IDT 1:100 (6 × 24 mm) | Tolerated Moderna | |
| Delayed (>4-h) reaction | 28 | F | None | No | Moderna | 10-12 | Itching, hives | No treatment needed | Home | 1 | Refresh IDT 1:100 (4 × 12 mm) | Awaiting vaccination |
| 29 | F | Penicillin | No | Moderna | 12-16 | Palpitations, diarrhea, delayed hives | Loperamide | Home | 0 | Refresh IDT 1:100 (7 × 18 mm) | Tolerated Moderna | |
| 33 | F | None | No | Moderna | 12-24 | Diffuse hives | Diphenhydramine | Home | 1 | Refresh IDT 1:100 (10 × 40 mm) | Tolerated Moderna | |
| 24 | F | Environmental | No | Moderna | 18-24 | Wheezing, chest tightness, hives, itching | Diphenhydramine, topical hydrocortisone | Home | 1 | Methylprednisolone acetate IDT 0.4 mg/mL (9 × 22 mm) Refresh IDT 1:100 (13 × 32 mm) |
Tolerated Moderna | |
| 49 | F | None | No | Pfizer | 20 | Tongue swelling, sneezing, nasal congestion, itchy eyes, chest congestion, cough, swelling of fingers | Diphenhydramine, Acetaminophen, pseudoephedrine | Home | 1 | Refresh IDT 1:100 (12 × 20 mm) | Patient preference not to receive dose 2 | |
| 19 | F | Peanut, tree nuts | Yes (peanut, tree nuts) | Moderna | 48 | Hives, throat closing | Diphenhydramine | Home | 1 | Refresh IDT 1:10 (11 × 17 mm) | Pretreated with cetirizine 10 mg and prednisone 20 mg. At 20 min after receiving dose 2 (Moderna), patient experienced dizziness, numbness in legs and fingers, and warm feeling in throat. Treated with cetirizine 10 mg | |
IDT, intradermal test.
Clinical characteristics, details of the first dose reaction, skin testing result, and details of the second dose for patients with positive skin testing results are provided.
Figure 1.
Patients who were skin tested owing to symptoms after mRNA COVID-19 vaccination that were concerning for an allergic reaction. (A) All patients. (B) Patients with immediate allergic reactions. (C) Patients with delayed allergic reactions. ∗Three patients who had positive skin testing to both polyethylene glycol (PEG) and polysorbate are represented in the PEG category only for the purpose of illustration. †One patient did not receive the next dose in the series and instead received Janssen. ‡Two patients had grade 0 reactions and 1 had a grade 1 reaction. §Three patients had a grade 0 reaction, 9 had a grade 1 reaction, and 3 had a grade 2 reaction. ‖Two patients who had positive skin testing to both PEG and polysorbate are represented in the PEG category only for the purpose of illustration. ¶One patient did not receive the next dose in the series and instead received Janssen. #One patient had a grade 0 reaction and 1 had a grade 1 reaction. ∗∗Two patients had a grade 0 reaction, 8 patients had a grade 1 reaction, and 3 had a grade 2 reaction. ††One patient who had positive skin testing to both PEG and polysorbate is represented in the PEG category only for the purpose of illustration. ‡‡One patient had a grade 0 reaction. §§One patient had a grade 0 reaction and 1 had a grade 1 reaction.
Overall tolerance of second dose of vaccine
Of the 80 patients who underwent excipient skin testing, 70 (88%) received a second dose of the same mRNA COVID-19 vaccine and 52 (74%) tolerated the second dose vaccine without a reaction (Figure 1, A). The NPV of a negative skin test to PEG predicted a 75% likelihood of tolerating the second dose of the vaccine.
Among the 65 patients who reported an immediate allergic reaction to the first dose of mRNA COVID-19 vaccine, 58 (89%) received the second dose of the vaccine and 43 (74%) tolerated the second dose without a reaction (Figure 1, B); 2 (3%) received epinephrine treatment for the second-dose reaction. Among the 65 patients who reported an immediate reaction to the first dose of mRNA COVID-19 vaccine, the NPV of a negative skin test to PEG was 75%.
Tolerance of second dose of vaccine among skin test-negative patients
Overall, 66 patients (83%) were skin test-negative and 60 (91%) of those patients went on to receive the second dose of the vaccine; 45 (75%) ultimately had the second dose of the vaccine without recurrent symptoms. Among the 65 patients who reported an immediate allergic reaction to the first dose of mRNA COVID vaccine, 57 (88%) were skin test-negative; 52 (80%) received their second dose of the vaccine and 39 (75%) had no symptoms with the second dose. Thirteen patients (25%) were skin test-negative but reacted to the second dose of the vaccine; the most common symptoms were cutaneous (n = 12), but 4 (31%) patients were seen in the emergency department and 2 (15%) received epinephrine (Table III ).
Table III.
Patients with negative skin testing results but reporting reaction to second dose
| Timing of reaction | Age, y | Sex | Allergies or allergic reaction | Vaccine | Dose 1 reaction | Dose 2 reaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset of symptoms (h) | Signs and symptoms | Treatment and treatment setting | Grade∗ | Onset of symptoms (h) | Signs and symptoms | Treatment and treatment setting | Grade∗ | |||||
| Immediate (<4-h) reaction | 29 | F | Cefuroxime, sulfonamide antibiotics, clindamycin, iodinated contrast media, ketoconazole, moxifloxacin, nystatin | Moderna | 0 | Dizziness, fatigue, hives | Levocetirizine and diphenhydramine at home | 1 | 0.2 | Hives | Diphenhydramine at vaccine clinic and cetirizine, famotidine, and prednisone at home | 1 |
| 62 | F | Sulfonamide antibiotics, latex, loratadine, morphine | Moderna | 0.1 | Lip tingling, dizziness, eye and facial swelling | No treatment was sought | 1 | 0.5 | Itching of ear, eye swelling | Diphenhydramine at vaccine clinic and prednisone at home | 1 | |
| 56 | M | None | Pfizer | 0.1 | Tongue tingling, lower lip swelling, mild throat clearing | Epinephrine, diphenhydramine, solumedrol at emergency department | 1 | 0.1 | Lower lip swelling | Cetirizine at home | 1 | |
| 47 | F | Latex, tramadol, amoxicillin, sulfamethoxazole-trimethoprim, fluconazole | Moderna | 0.1 | Itching, lip tingling, subjective lip and tongue swelling, hive-like rash | Diphenhydramine at vaccine clinic and loratadine and cetirizine at home | 1 | 12 | Eye swelling and itching | Loteprednol and cetirizine at home | 1 | |
| 56 | F | Shrimp, nitrofurantoin, ibuprofen, sulfamethoxazole trimethoprim | Moderna | 0.1 | Generalized itching, throat itchiness, fatigue | Diphenhydramine at emergency department and albuterol at home | 0 | 0.2 | Itching, headache, throat tightness and itching, warmth, flushing | Diphenhydramine at emergency department | 0 | |
| 57 | F | None | Moderna | 0.1 | Headache, throat tightness, fatigue, hypertension, redness on legs, joint and muscle aches, shakes | Ibuprofen and unspecified antihistamine at home | 0 | 12-24 | Itching, rash, diarrhea, vomiting, headache, fatigue, fever, cough, muscle aches, and joint pain | Acetaminophen and diphenhydramine at home | 1 | |
| 46 | F | Pepper, procaine | Moderna | 0.2 | Tingling of left arm, roof of mouth, and tongue, numbness. | Cetirizine at emergency department | 0 | 0 | Numbness, chest tightness, dry heaving, chills, hot sensation in lips | Diphenhydramine, ondansetron, and methylprednisolone at emergency department | 0 | |
| 24 | F | Amoxicillin, clindamycin, pineapple, benzonatate | Moderna | 0.25 | Globus and throat itching, cough, erythematous oropharynx, hoarse voice | Epinephrine, methylprednisolone sodium succinate, and diphenhydramine at emergency department | 2 | 0.6 | Throat tightness and itching, cough, erythematous posterior pharynx, watery eyes | Cetirizine, albuterol, and epinephrine at vaccine clinic and methylprednisolone sodium succinate, diphenhydramine, and famotidine at emergency department | 2 | |
| 29 | F | Measles, mumps, and rubella vaccine, penicillin | Pfizer | 0.3 | Hives, cough, hoarseness | Epinephrine, prednisone, and cetirizine at emergency department | 2 | 0.3 | Itching, rash, hives, throat tightness, cough | Epinephrine, cetirizine, and albuterol at emergency department | 2 | |
| 58 | F | Ciprofloxacin, cephalexin, macrolide antibiotics, nonsteroidal anti-inflammatory drugs, omeprazole, penicillin, theophylline, codeine | Moderna | 0.5 | Lip tingling, eye, lip, facial, and leg swelling, throat tightness | No treatment was sought | 1 | 12 | Lip tingling, lip swelling, sensation of facial swelling | Unspecified antihistamine at home | 1 | |
| 59 | F | Succinylcholine, midazolam, promethazine, gabapentin, fentanyl, prochlorperazine, cefazolin | Pfizer | 1.25 | Sensation of throat closing, dysphagia, shortness of breath, itching of palms, soles, and torso | Diphenhydramine at vaccine clinic | 0 | 0.3 | Face, neck, and chest flushing, itching over palms and feet, lip fullness and tingling | Diphenhydramine at vaccine clinic | 1 | |
| 43 | F | Ibuprofen, penicillin | Pfizer | 2 | Lip swelling, full body itching, facial swelling, headache | Acetaminophen at home | 1 | 0.75 | Tongue numbness, hive-like rash, wheezing | No treatment was sought | 2 | |
| 43 | F | None | Moderna | 2-3 | Itchy rash on right arm, red and itchy right eye | No treatment was sought | 1 | 0.1 | Chest flushing | No treatment was sought | 1 | |
| Delayed (>4-h) reaction | 37 | F | Cephalosporins, penicillin, shellfish, latex, hymenoptera venom, influenza vaccine | Moderna | 8.5 | Hives, eye and lip swelling | Diphenhydramine and loratadine at home | 1 | 0.25 | Arm and neck itching, arm erythema | Cetirizine in vaccine clinic | 0 |
| 62 | F | Iodine, shellfish | Moderna | 10 | Hives, nausea, diarrhea | Unspecified anti-itch cream at home | 1 | Unknown | Throat, tongue, and eye swelling, itching of chest and face, nausea, headache, lethargy | No treatment was sought | 1 | |
Clinical characteristics and details of the first and second dose reactions for patients with negative skin testing results, who developed a reaction to dose 2, are provided.
Ring and Messmer grade.13
Among the 15 individuals who reported a delayed reaction after the first dose of mRNA COVID-19 vaccine, 9 (60%) were skin test negative and 8 (89%) received the second vaccine dose; 6 of 8 (75%) tolerated the second dose without a reaction. Two patients were skin test-negative but reacted to the second dose of the vaccine: 1 had mild swelling of the throat, tongue, and eyes, and pruritus without rash on the face and chest. No treatment was sought. The other patient had pruritus and erythema of the arms, with pruritus of the lips and neck, treated with antihistamines.
Tolerance of second dose of vaccine among skin test-positive patients
Among the 8 patients (12%) who reported a history of an immediate allergic reaction and a positive skin test, 4 (67%) tolerated the vaccine (skin test-positive to polysorbate 80/Refresh Tears [n = 2], ID PEG/methylprednisolone acetate [n = 1], ID PEG/methylprednisolone acetate and polysorbate 80/Refresh Tears [n = 1]), 2 (33%) had mild allergic reactions, and 2 (25%) opted to not receive the second mRNA COVID-19 vaccine dose after shared decision-making (both were skin test-positive to PEG; 1 patient was skin prick-positive to MiraLAX and 1 opted instead to receive the Janssen vaccine) (Table II). The 2 patients with reactions to the second dose were positive to Refresh Tears, and both had mild symptoms. The 2 patients who were PEG skin test-positive but tolerated the vaccine were positive to methylprednisolone acetate on intradermal testing. The only patient with a positive reaction to MiraLAX on epicutaneous testing had experienced anaphylaxis requiring epinephrine after the first dose of the vaccine and was not rechallenged.
Among the 15 patients who reported a delayed allergic reaction to the first dose of mRNA COVID-19 vaccine, 6 had a positive skin test, 3 tolerated the vaccine (the skin test was positive to polysorbate 80/Refresh Tears [n = 2], and ID PEG/methylprednisolone acetate and polysorbate 80/Refresh Tears [n = 1]), 1 had a mild allergic reaction, and 2 did not receive the second dose (the skin test was positive to polysorbate 80/Refresh Tears). The patient who had a reaction to the second dose was positive to polysorbate 80/Refresh Tears and experienced subjective symptoms (Table II). The patient with positive skin testing to PEG was positive only on ID testing to methylprednisolone acetate.
Discussion
Among 80 individuals referred for excipient skin testing after a reported allergic reaction to the first dose of mRNA COVID-19 vaccine, the vast majority of patients received the second mRNA COVID-19 vaccine dose safely irrespective of skin test results. Two patients who were treated with epinephrine after the second dose had been treated with epinephrine after the first dose. However, 3 patients who received epinephrine after the first dose tolerated the second vaccine dose. Therefore, although there are no clear risk factors, this is an important issue for physicians to discuss with patients who were treated with epinephrine after the first dose of the mRNA COVID-19 vaccine. Notably, it is possible that epinephrine was not necessary in these cases. Overall, excipient skin testing did not add value to the clinical risk assessment in those reporting first-dose mRNA COVID-19 vaccine reactions, even when applied to this highly selected group that represented just 14% of mRNA COVID-19 vaccine reactions evaluated by MGB allergy/immunology overall in this period. However, PEG skin prick testing may be useful in guiding second-dose vaccination in individuals with anaphylaxis to the first dose of the mRNA COVID-19 vaccine, although its positive predictive value remains unknown.
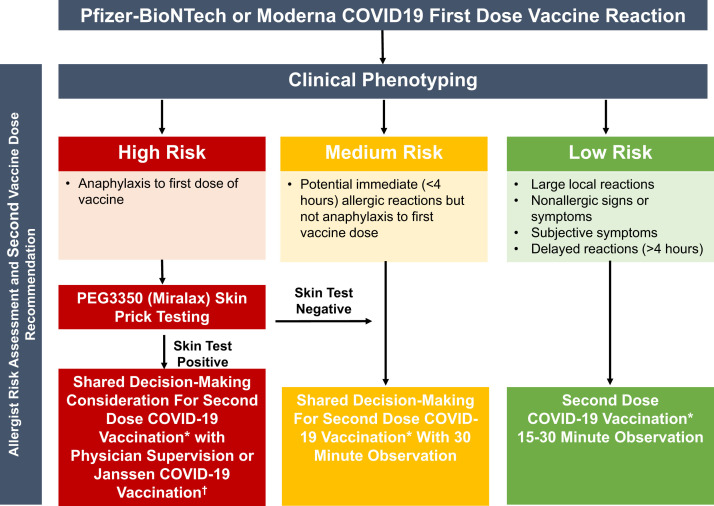
Based on our data and experience to date, we propose an updated risk stratification algorithm after reported first-dose mRNA COVID-19 vaccine allergic reactions (Figure 2 ). First, the clinical history remains the most important aspect in the allergist evaluation. Using shared decision-making, most individuals with reactions to the first dose of an mRNA COVID-19 vaccine can proceed safely with the second dose.22, 23, 24 Second, excipient skin testing is favored over vaccine skin testing,15 , 16 because the vaccine is largely inaccessible to most allergists and the mRNA vaccines are still under EUA. Third, in terms of premedication, although some patients in the cohort were pretreated with antihistamines or steroids, this study was not designed to determine an optimal premedication regimen. The authors have used premedication with nonsedating antihistamines for patients with mild symptoms such as delayed urticaria but have tried to restrict the use of glucocorticoids that may have an unfavorable immunologic effect.25 Fourth, in our experience to date, PEG skin testing was of limited utility (n = 5 with positive skin tests) and should be considered only in specific cases (eg, patients with a convincing history of anaphylaxis to mRNA COVID-19 vaccine or recently to oral PEG-containing medications). Finally, methylprednisolone acetate or PEG ID skin testing did not provide additional information to guide the safety of second-dose mRNA COVID-19 vaccination, because in our experience, patients with positive skin testing to this tolerated the vaccine (n = 3). We thus suggest that when PEG excipient testing is warranted, epicutaneous testing to MiraLAX (PEG3350) alone is adequate.10, 11, 12 In our experience, consistent with rare true cases of anaphylaxis to the COVID-19 mRNA vaccines, MiraLAX skin testing was positive in 1 patient who had symptoms consistent with anaphylaxis. This patient did not receive a second dose of COVID-19 vaccine, so the positive predictive value of MiraLAX skin testing remains unknown10 , 26 (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org).
Figure 2.
Management of patients who present with symptoms concerning for an allergic reaction to the first dose of mRNA COVID-19 vaccine. Use of Janssen vaccine (if available) may be appropriate after allergic reaction to the first dose of mRNA COVID-19 vaccine if allergy evaluation is not feasible or owing to shared decision-making between the patient and physician. PEG, polyethylene glycol. ∗Same COVID-19 vaccine manufactured as first dose. †Limited supply of Janssen COVID vaccine; only use when clinically necessary.
Figure E1.
Skin testing protocol for polyethylene glycol (PEG) using MiraLAX for epicutaneous testing without PEG intradermal testing and PEG and polysorbate 80.
The cause of mRNA COVID-19 vaccine allergic reactions remains unclear and may be multifactorial, but 1 case report linked PEG as a possible allergen.10 An improved understanding of the role of excipient skin testing to PEG in individuals with a history of anaphylaxis to mRNA COVID-19 vaccines is still needed. Although we were able to calculate the NPV for PEG skin testing and tolerance with the second dose, we were unable to calculate sensitivity, specificity, and/or positive predictive value. Two patients reported histories of being allergic to PEG (reported after the first-dose mRNA COVID-19 vaccine reaction). Interestingly, both were PEG skin test-negative and 1 patient tolerated the second dose without symptoms; the other patient developed subjective globus sensation after the second dose, which was treated with antihistamines only. Three patients positive on intradermal PEG (methylprednisolone acetate) skin testing tolerated the second mRNA COVID-19 vaccine dose with no reactions. In addition, in our experience, a portion of skin test-negative patients had reactions to the mRNA COVID-19 vaccine second dose. Although most had easily treatable reactions and are now fully vaccinated, importantly, 2 were treated with epinephrine and 4 were treated in the emergency department, which emphasizes the important role of an allergist in the care of these patients. Thus, because skin test-positive patients tolerated the second dose of the vaccine and skin test-negative patients had reactions, skin testing did not add information regarding the tolerance of the second mRNA COVID-19 vaccine dose.
Owing to the high rate of skin test positivity to Refresh Tears, which was used as a source of polysorbate 80 in the BWH study population, we tested a new control group (Table E3). Approximately half of the nonallergic controls had positive skin test results, which suggests that Refresh Tears likely causes an irritant, or false-positive, skin test reaction. These findings prompted us to modify our initial algorithmic approach and to contact all MGB patients with positive skin tests to Refresh Tears to modify our initial advice and encourage mRNA COVID-19 vaccination. No patients with positive skin tests to Refresh Tears who received the second dose of the mRNA COVID-19 vaccine developed an allergic reaction, but 3 patients had subjective symptoms treated with antihistamines only.
Vaccine hesitancy is an important issue that may be exacerbated by adverse and/or allergic symptoms after the first dose of the mRNA COVID-19 vaccine.27 We are eager to reassure patients regarding the vaccine’s safety to encourage vaccination.28 In this cohort of 80 high-risk individuals referred for skin testing, 10 of 80 have not received the second dose of mRNA COVID-19 vaccine, although only 2 were advised by allergists not to receive mRNA COVID-19 vaccine (a third patient received Janssen vaccine). The reasons for incomplete vaccination are unclear but may include vaccine administration logistics, unclear benefit of second-dose vaccination, and/or concern about having a recurrent allergic or adverse reaction.27 , 29 The allergist may not be able to address all of these barriers, but the allergist can acknowledge them30 and, based on these data, provide reassurance regarding receiving the second dose if the clinical history is low-risk.12 , 13 , 16 , 31 The allergist also has an important role in helping to recognize and treat allergic reactions correctly, so allergist-observed second-dose vaccinations may reassure patients.
Recently, the CDC added guidance allowing Janssen COVID-19 vaccine at least 28 days after an allergic reaction to a first dose of mRNA COVID vaccine, broadening patients’ options.5 The Janssen COVID-19 vaccine contains the excipient polysorbate 80 but not PEG; therefore, it could be administered without skin testing to PEG in patients concerned about PEG allergy. Janssen COVID-19 vaccine could also be considered in an individual with a history of PEG allergy or PEG skin test positivity but a negative polysorbate 80 skin test or tolerance of vaccine with polysorbate 80 (Figure 2). Reassuringly, to date, the rate of allergic reactions to the Janssen vaccine appears exceedingly low.32 One patient in this cohort who had reported an immediate allergic reaction to Moderna, and then had positive skin testing to methylprednisolone acetate and Refresh Tears, subsequently tolerated the Janssen vaccine. This is an important option for high-risk patients with possible PEG allergy.
Limitations of our study include generalizability because our study population was a referred population consisting of MGB patients evaluated by a specialist. Most of these patients were White females, which was likely a reflection of the overrepresentation of White females in the health care worker population in the Boston area. The demographics may limit generalizability across demographics, although they closely match those reported nationally.3 , 6 , 7 These data are subject to information bias because we do not know why some individuals chose not to receive the second dose of the vaccine, even when the allergist advised that it was safe to proceed. Because proceeding to a second mRNA COVID-19 vaccine dose was not done at random, we acknowledge that there is a bias in calculating a NPV. However, although NPV could be calculated, given that only 9% of skin test-negative patients did not receive the second dose, we were unable to calculate PPV because 40% of skin test-positive patients did not receive the second dose and the sample size was just 5. Another limitation is that symptoms were self-reported. Although we intended to use excipient skin testing only in high-risk individuals, some individuals reported minor and subjective symptoms (eg, itch, globus, dizziness). Including these symptoms in our analysis might have made it more difficult to ascertain the association of excipient skin testing with true mRNA COVID-19 vaccine allergy. However, the inclusion of these patients was due to patient preference and shared decision-making.
Overall, our initially published risk stratification protocol13 allowed safe second-dose mRNA COVID-19 vaccination for the vast majority of individuals despite a reported first-dose allergic reaction. We have since learned that excipient skin testing may be of little utility in assessing these reactions, particularly in individuals without anaphylaxis. More data are needed to identify whether PEG skin prick testing after a reaction clinically consistent with severe anaphylaxis to mRNA COVID vaccine is predictive of a reaction with subsequent mRNA COVID-19 vaccine. Additional studies focusing on specific reaction phenotypes, notably anaphylaxis, may enable us to better risk stratify and optimize recommendations for subsequent COVID-19 vaccination after immediate allergic reactions. As experiences accumulate, similar to the revised algorithm included in this study, we will update our clinical approach to provide up-to-date guidance for individuals with a presumed reaction to the first dose of the mRNA COVID-19 vaccine.
Footnotes
This work was supported by National Institutes of Health Grant K01AI125631 and the Massachusetts General Hospital Department of Medicine Transformative Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Massachusetts General Hospital.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
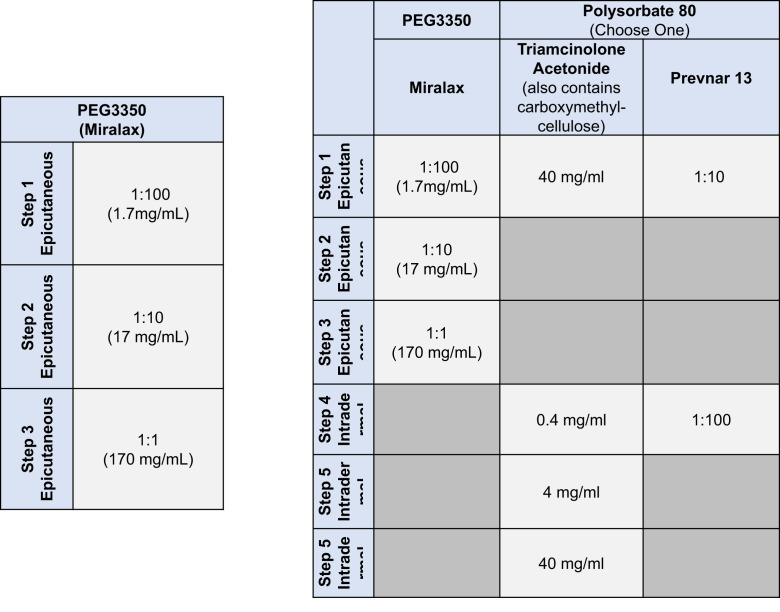
Table E1.
Skin testing protocols used to test for PEG and polysorbate 80 allergyE1
| Drug | Skin prick testing (mg/mL) | Intradermal (mg/mL) |
|---|---|---|
| PEG3350 | ||
| MiraLAX | 1.7 | |
| 17 | ||
| 170 | ||
| Methylprednisolone acetate∗ | 40 | 0.4 4 |
| Control | ||
| Methylprednisolone sodium succinate | 40 | 0.4 4 |
| Polysorbate 80 | ||
| Triamcinolone acetonide | 40 | 0.4 4 40 |
| Refresh Tears | 1:1 | 1:10 |
PEG, polyethylene glycol.
Refresh Tears, which contains polysorbate 80 0.5%, was subsequently found to be irritating. Triamcinolone acetonide contains polysorbate 80 0.04%.
Some brands of methylprednisolone acetate contain polysorbate and PEG3350 whereas others have only PEG3350; use methylprednisolone acetate containing PEG3350 only.
Table E2.
Ring and Messmer grading for allergic reactionsE2
| Grade 1: Cutaneous signs |
| Urticaria |
| Angioedema |
| Generalized erythema |
| Grade 2: Measurable but not life-threatening symptoms |
| Cutaneous signs |
| Hypotension (defined as a decrease of more than 30% in blood pressure with tachycardia) |
| Cough |
| Difficulty with mechanical ventilation |
| Grade 3: Life-threatening symptoms |
| Cardiovascular collapse |
| Tachycardia or bradycardia (due not include mild sinus tachycardia) |
| Arrhythmias |
| Severe bronchospasm |
| Grade 4: Death |
| Cardiac arrest |
| Respiratory arrest |
Table E3.
Refresh Tears skin testing results on nonallergic, clinically tolerant controls, reflecting a high frequency of false-positive, irritant reactions
| Patient | Refresh Tears 1:100 (intradermal), mm (wheal flare) | Refresh Tears 1:10 (intradermal), mm (wheal ×flare) |
|---|---|---|
| 1 | 10 × 20 | — |
| 2 | 0 | 7 × 15 |
| 3 | 0 | 8 × 18 |
| 4 | 0 | 0 |
| 5 | 8 × 18 | — |
| 6 | 9 × 14 | — |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 8 × 13 | — |
| 12 | 0 | 0 |
| 13 | 9 × 20 | — |
| 14 | 0 | 7 × 18 |
| 15 | 0 | 0 |
| 16 | 0 | — |
| 17 | 0 | — |
| 18 | 0 | 6 × 24 |
| 19 | 0 | 0 |
| 20 | 0 | 0 |
| 21 | 0 | 0 |
| 22 | 8 × 18 | — |
| 23 | 7 × 8 | — |
| 24 | 0 | 4 × 8 |
| 25 | 5 × 10 | — |
| Total | 8 (32%) | 5 (20%) |
Among 25 nonallergic controls, there were 13 positive reactions (52%) to Refresh Tears on intradermal testing, with 8 positive at the 1:100 concentration (32%) and 5 positive at the 1:10 concentration (20%).
References
- 1.Johns Hopkins Coronavirus Resource Center COVID-19 map. https://coronavirus.jhu.edu/map.html Available from:
- 2.US Food and Drug Administration Pfizer-BioNTech COVID-19 vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine Available from:
- 3.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention COVID-19 vaccines for people with allergies. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html Available from: [PubMed]
- 5.Centers for Disease Control and Prevention What to do if you have an allergic reaction after getting a COVID-19 vaccine. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html Available from:
- 6.Centers for Disease Control and Prevention (CDC) COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellaturay P., Nasser S.M., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitlick M.M., Sitek A.N., Kinate S.A., Joshi A.Y., Park M.A. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021;126:735–738. doi: 10.1016/j.anai.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1427. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broyles A.D., Banerji A., Barmettler S., Biggs C.M., Blumenthal K., Brennan P.J. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8(9 suppl):S16–S116. doi: 10.1016/j.jaip.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Marcelino J., Farinha S., Silva R., Didenko I., Proença M., Tomás E. Non-irritant concentrations for skin testing with SARS-CoV-2 mRNA Vaccine. J Allergy Clin Immunol Pract. 2021;9:2476–2477. doi: 10.1016/j.jaip.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi L., Biondi F., Hansel K., Murgia N., Tramontana M., Stingeni L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: limits of intradermal testing. Allergy. 2021;76:2605–2607. doi: 10.1111/all.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa S.S., Ramsey A., Staicu M.L. Administration of a second dose of the Moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174:1177–1178. doi: 10.7326/L21-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 22.Iammatteo M., Ferastraoaru D., Koransky R., Alvarez-Arango S., Thota N., Akenroye A. Identifying allergic drug reactions through placebo-controlled graded challenges. J Allergy Clin Immunol Pract. 2017;5:711–717.e2. doi: 10.1016/j.jaip.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Sampath V., Rabinowitz G., Shah M., Jain S., Diamant Z., Jesenak M. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76:1640–1660. doi: 10.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelso J.M. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127:133–134. doi: 10.1016/j.anai.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deepak P., Kim W., Paley M.A., Yang M., Carvidi A.B., El-Qunni A.A. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. [preprint published online April 9, 2021]. medRxiv. [DOI]
- 26.Turk V.E. Anaphylaxis associated with the mRNA COVID-19 vaccines: approach to allergy investigation. Clin Immunol. 2021;227:108748. doi: 10.1016/j.clim.2021.108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson L.B., Landman A.B., Shenoy E.S., Hashimoto D., Fu X., Camargo C.A., Jr Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2019;9:3200–3202. doi: 10.1016/j.jaip.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenhawt M., Abrams E.M., Oppenheimer J., Vander Leek T.K., Mack D.P., Singer A.G. The COVID-19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021;9:1438–1441. doi: 10.1016/j.jaip.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hause A.M., Gee J., Johnson T., Jazwa A., Marquez P., Miller E. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination - five U.S. mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:685–688. doi: 10.15585/mmwr.mm7018e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenthal K.G., Li Y., Acker W.W., Chang Y., Banerji A., Ghaznavi S. Multiple drug intolerance syndrome and multiple drug allergy syndrome: epidemiology and associations with anxiety and depression. Allergy. 2018;73:2012–2023. doi: 10.1111/all.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaker M., Phillips E., Blumenthal K.G., Abrams E.M., Banerji A., Oppenheimer J. The importance of a timely second dose of the 2021 COVID-19 mRNA vaccine depends on the protection afforded by a first dose and subsequent risk of anaphylaxis. J Allergy Clin Immunol Pract. 2021;9:2556–2561. doi: 10.1016/j.jaip.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen Global Services Fact sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) https://www.janssenlabels.com/emergency-use-authorization/Janssen+COVID-19+Vaccine-Recipient-fact-sheet.pdf Available from:
References
- Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1427. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]