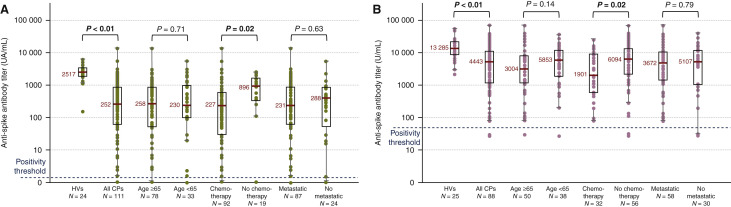
Recent data, including ours, show that patients treated for solid cancers (SCs) had low anti-spike antibody responses after a first dose of messenger RNA (mRNA) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine, with seroconversion rates ranging from 38% to 55%, in comparison with healthy controls having seroconversion rates ranging from 94% to 100%.1, 2, 3 This humoral response was more impaired in elderly and chemotherapy-treated patients. In this study we aimed to compare the proportion and the level of antibody response 3-4 weeks after the second injection of the BNT162b2 (Pfizer-BioNTech) vaccine in patients with SCs using healthy volunteers (HVs) as a control population.
Patients with SCs on active treatment or who received treatment in the past 2 years and HVs who underwent SARS-CoV-2 vaccination between 5 January 2021 and 2 April 2021 at the Pitié-Salpêtrière and Tenon hospitals, Paris, France, and at the Saint Jean Polyclinic, Nice, France, were selected for analysis. The titration of anti-SARS-CoV-2 antibodies was proposed 3-4 weeks after the second injection of BNT162b2 (Pfizer-BioNTech) vaccine. Anti-spike antibodies were detected using different assays (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.06.018). For quantitative analysis, only titrations using the Abbott Alinity SARS-CoV-2 immunoglobulin (Ig) G chemiluminescent microparticle immunoassay (detection threshold: 50 UA/ml), and the Roche Elecsys SARS-CoV-2 total Ig electrochemiluminescent immunoassay (detection threshold: 0.8 U/ml) were analyzed. Median titers of anti-spike antibodies were compared between patients with SCs and HVs, and between different subpopulations of patients using Kruskal-Wallis tests. This study was approved by the ‘Commission Nationale de l'Informatique et des Libertés’ (MR004, registration number: 2221945).
No patients had prior exposure to COVID-19 as none of them had IgG anti-nucleoprotein before vaccination. SARS-CoV-2 antibodies were measured in 223 patients and 49 HVs (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.06.018). The median age of patients was 67 years [interquartile range (IQR) 60-75 years], with 142 women (64%) and 81 men (36%). 129 (58%) patients were treated with chemotherapy at the time of vaccination. The seroconversion rate was 94% in patients and 100% in HVs. The 13/223 (6%) non-seroconverter patients were 8 women and 5 men, with ages ranging from 45 to 90 years, mostly metastatic (n = 8), including 10/13 treated with chemotherapy. Titers of anti-spike antibodies were significantly lower in patients with SCs in comparison with HVs, and significantly lower in those receiving chemotherapy (in combination or not with other treatments as targeted therapies), regardless of the assay used (Figure 1 ). Titers of anti-spike antibodies did not differ depending on age, sex, cancer location and metastatic status. Only one mild case of COVID-19 occurred after the first injection of vaccine, in a patient with colon cancer.
Figure 1.
Anti-spike antibody titers in HVs and cancer patients (CPs), using Roche Elecsys assay (A) and Abbott Alinity assay (B).
HVs, healthy volunteers.
In summary, the mRNA vaccine boost led to a high seroconversion rate, reinforcing the need not to delay the second dose. However, anti-spike antibody titers were 3-10 times lower in patients with SCs than in healthy controls, raising concerns about impaired humoral immunity, especially in patients treated with chemotherapy. At the same time, the seroconversion data are rather reassuring among patients on anti-Human Epidermal Growth Factor Receptor 2, anti-Programmed cell death protein 1/Programmed death-ligand 1, antiangiogenic treatment or hormone therapy without associated chemotherapy. Nevertheless, correlates of immunity to SARS-CoV-2 are still not defined, and further studies are required to determine the SARS-CoV-2 correlates of vaccine-induced protection based on neutralizing antibodies and cellular immunity.4 We still lack the insight required to determine when a third dose (second booster) should be offered to patients with SCs. Pending additional data, we would highly recommend vaccination for family and friendship circles, to provide an indirect protection against COVID-19 to this population.
Acknowledgments
Funding
None declared.
Disclosure
JPS declares he has received advisory fees and meeting invitations from Roche, BMS, MSD, Pfizer, Lilly, PFO, Leo Pharma, Myriads, Biogaran, AZ and Gilead. JG declares he has received advisory fees and meeting invitations from AZ, Exact Science, Lilly, Novartis, Pierre Fabre, Pfizer, Roche and Seagen. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palich R., Veyri M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.04.020. 32(8):1051-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.04.019. 32(8):1053-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.