Abstract
Background
Stress from obstructive sleep apnoea (OSA) stimulates catecholamine release and consequently can exacerbate hypertension, even in the absence of a catecholamine-producing tumour (phaeochromocytoma). As such, a positive screening test for suspected phaeochromocytoma may be misleading. There exists only a handful case reports, and no controlled trials, how continuous positive airway pressure (CPAP) to treat OSA influences catecholamine levels. We examined changes to levels of urinary catecholamine and blood pressure in response to CPAP treatment.
Methods
We conducted a meta-analysis of data aggregated from published case reports of individual patient data up to April 2020. The quality of the reports was evaluated using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool.
Results
A total of 13 cases (seven men and six women) from seven reports met our search criteria. Patients had mean age of 49.1 years (range = 36–62) and body mass index of 37.4 kg/m2 (range = 27–56). Most had moderate to severe OSA with CPAP treatment. Nine cases had 24-hour urinary noradrenaline assessment before and after CPAP treatment. CPAP treatment led to a 21% reduction (104 nmol/24-hours, 95% credible interval =59 to 148) in 24-hour urinary noradrenaline to within reference ranges, and 25% reduction (from 131 to 100 mmHg) in mean arterial pressure. The risk of overall bias evaluated by the ROBINS-I tool was found to be low in the majority of reports.
Conclusions
Investigations of patients suspected of phaeochromocytoma, particularly obese individuals, should exclude OSA and treat this condition if present before performing screening tests to assess for catecholamine levels.
Keywords: Hypertension, obesity, stress hormones, sympathetic activity
Introduction
In normal physiological states, catecholamines are rapidly released as an acute response to stress through activation of the sympathetic system and they return to baseline levels once the stress is withdrawn. 1 In contrast, patients with phaeochromocytoma, whereby excessive catecholamines are episodically released into the circulation by a tumour, can present with paroxysmal or persistent hypertension and a number of symptoms such as headaches, palpitations and sweating. 2 In clinical practice, when phaeochromocytoma is suspected, the initial endocrine investigation frequently involves screening tests such as 24-hour urinary free catecholamines: dopamine, adrenaline and noradrenaline, or their products metadrenalines (metanephrines) 3-methoxytyramine, normetadrenaline (normetanephrine) and metadrenaline (metanephrine).2,3
Obstructive sleep apnoea (OSA) commonly occurs among individuals with essential hypertension, ranging between 30 and 50% of cases,4–6 but many remain undiagnosed.7,8 OSA elicits significant stress to the patient, stimulating the release of catecholamines and as a consequence can exacerbate hypertension, even in the absence of a phaechromocytoma.9,10 In such patients, a positive screening test may therefore falsely indicate the presence of a phaeochromocytoma. Hitherto, only a handful of case reports have been published showing reductions in the levels of catecholamines or metanephrines by treatment with continuous positive airway pressure (CPAP). Therefore, due to a lack of randomised controlled trials, results from existing case reports are not well publicised or included in clinical guidelines for investigation of phaeochromocytoma. 11 Consequently, many patients are subjected to unnecessary investigations including radiological procedures and complex endocrine dynamic function tests. These investigations require hospital visits and admissions which may cause anxiety to the patient and impose a substantial cost to healthcare services.
This study was undertaken to determine whether OSA should be considered in cases where there is some indication that a phaeochromocytoma is present, before further evaluation, by conducting a meta-analysis of data aggregated from published clinical case reports of pseudophaeochromocytoma.
Methods
Search criteria
Two investigators performed independently a literature search of MEDLINE and Google Scholar up to April 2020 using the key terms (British or US usage and abbreviations, e.g. CPAP and OSA): obstructive sleep apnoea, continuous positive airway pressure, pseudophaeochromocytoma, phaeochromocytoma, catecholamines, adrenaline (epinephrine), noradrenaline (norepinephrine), 3-methoxytyramine, metanephrines, normetanephrine, metanephrine and dopamine, and hypertension. No language or data filters were applied. The Boolean operators “AND” and “OR” were used to combine search terms. Relevant studies were hand-searched within these references.
Selection criteria
Studies reported catecholamines or metanephrines as primary outcome from CPAP treatment in patients with OSA in the absence of phaeochromocytoma, as confirmed by radiological investigations including computerised tomography or magnetic resonance imaging scans and metaiodobenzylguanidine or octreotide scans, as well as endocrine procedures such as a pentolinium suppression test and adrenal venous sampling.
Risk of bias
The quality of the reports was evaluated using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool that covers seven distinct domains: Two pre-intervention domains (bias due to confounding and selection of participants into the study), one domain at intervention (classification of interventions), and four post-intervention domains (deviations from intended interventions, missing data, measurement of outcomes and selection of the reported results. 12 The risk of bias for each report was rated independently from low, moderate, serious to critical level by two authors and any discrepancies were resolved by reciprocal discussion.
Statistical analysis
Meta-analysis of individual patient data was conducted using Bayesian approach to synthesise the evidence and estimate relative treatment effects for all procedures within the case studies. This technique was used for its ability to handle studies with small sample size, allowing the incorporation of prior information on model parameters and derivation of effect size from the posterior distribution for all studies based on Markov chain Monte Carlo simulations. 13 When an unexplained heterogeneity occurred, a random-effects model with a common heterogeneity parameter was preferred to a fixed-effects model. Deviance information criteria were used to assess goodness-of-fit of the models. The analysis included non-informative priors for model parameters, and ran Markov chain Monte Carlo sampling for four chains, where first 1,00,000 posterior samples burn-in period were discarded and then another 1,00,000 posterior samples were saved in an interval of 10 in each chain. 14 Convergence was attained based on visual inspection of time-series plots and using the Brooks-Gelman-Rubin test. 13 The treatment effects were obtained from the posterior distributions of the Bayesian analysis and reported as a mean difference with 95% associated credible interval, which is a Bayesian analogy of the 95% confidence interval from traditional meta-analyses. 15 The results were presented as forest plots, which display the mean difference for both the individual trials and also the pooled results. Because the reference ranges for the levels of catecholamines were variably reported between studies, percentage changes in post-CPAP treatment levels relative to pre-CPAP treatment levels were also presented. The analysis and graphics were performed and produced using the software R v3.6.2 and Just Another Gibbs Sampler (JAGS) which generates inferences.13,16
Results
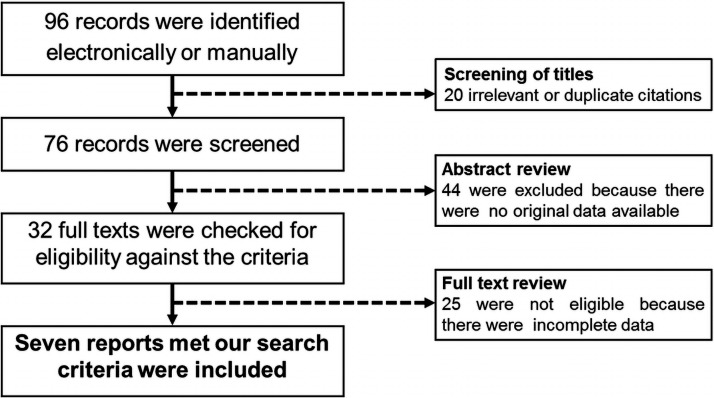
The initial search yielded 96 potentially relevant records. Screening of titles identified 20 irrelevant or duplicate citations. After a review of abstracts, 44 articles were excluded because the original data were unavailable. Further detailed evaluation of full text found seven studies met our search criteria (Figure 1), comprising a total of 13 cases (seven men and six women).17–23 Nine cases had 24-hour urinary noradrenaline assessment before and after CPAP treatment (cases 1–8)17–20 or before and after a weight loss of 15 kg (12%) body weight in one patient (case 9). 20 Of the remaining four cases, one had noradrenaline measured at baseline and normetanephrine/creatinine ratio after CPAP treatment (case 10), 20 two had 24-hour urinary normetadrenaline measured (case 11 and case 12),21,22 and one had catecholamines measured but no details of the levels were available (case 13). 23 The patients had mean age of 49.1 years (range: 36–62 years) and body mass index of 37.4 kg/m2 (range: 27–56 kg/m2). All patients were in the obese category (body mass index ≥30 kg/m2), except one patient who was overweight (case 3; body mass index of 27 kg/m2). Most patients had moderate to severe OSA, with CPAP treatment from a few weeks to several months (Table 1). Radiological investigations and endocrine dynamic function tests confirmed none of the patients had evidence of phaeochromocytoma (Table 2).
Figure 1.
QUORUM flow chart of literature search.
Table 1.
Clinical characteristics and changes in blood pressure with CPAP treatment.
|
Baseline characteristics |
Blood pressure (mmHg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (years) | BMI(kg/m2) | BP drugs (n) | AHI (events/hr) | OSA severity | Pre-CPAP | Post- CPAP | CPAP duration | |
|
1. Hoy et al., 2004 17 |
F | 62 | 56.0 | 1 | >30 | Severe | 204/100 | 135/75 | Not stated |
|
2. Hoy et al., 2004 17 |
F | 52 | 41.0 | 3 | 15–30 | Moderate | 200/110 | 120/80 | Not stated |
|
3. Hoy et al., 2004 17 |
M | 42 | 27.0 | 3 | >30 | Severe | 160/110 | 135/84 | Not stated |
|
4. Hoy et al., 2004 17 |
M | 48 | 35.0 | 3 | 15–30 | Moderate | 170/100 | 140/85 | Not stated |
|
5. Hoy et al., 2004 17 |
F | 38 | 36.0 | 3 | 15–30 | Moderate | 160/100 | 130/75 | Not stated |
|
6. Makino et al., 2006 18 |
F | 55 | 35.4 | 4 | >30 | Severe | 247/140 | 160/100 | 2 weeks |
|
7. Cheezum et al., 2010 19 |
M | 39 | 30.0 | 3 | 112 | Severe | 156/89 | 116/76 | 4 weeks |
|
8. Kahal et al., 2013 20 |
M | 39 | 35.0 | 2 | 52 | Severe | 180/120 | 146/95 | 6 months |
|
9. Kahal et al., 2013 20 |
M | 68 | 36.0 | 0 | 10 | Mild‡ | 134/77* | -- | -- |
|
10. Kahal et al., 2013 20 |
M | 51 | 42.0 | 4 | 40 | Severe | 174/133 | -- | 2 years |
|
11. Brainard et al., 2014 21 |
M | 36 | -- | 2 | -- | Positive | 154/104 | 118/82 | 7 weeks |
|
12. Weeks et al., 2015 22 |
F | 56 | 43.8 | 0 | -- | Positive | 166/100 | -- | 7 weeks |
|
13. Marmouch et al., 2021 23 |
F | 52 | 31.0 | 4 | >30 | Severe | 200/120 | 130/80 | 4 weeks |
| All cases | 7M: 6 F | 49.1 | 37.4 | 177/109 | 133/83 | ||||
BMI, body mass index; BP, blood pressure; AHI, apnoea hypoapnoea index; OSA, obstructive sleep apnoea; CPAP, continuous positive airway pressure.
*This patient had mild OSA after weight loss of 15 kg (12% of body weight).
Table 2.
Urinary screening test and radiological investigations.
|
Investigations |
|||
|---|---|---|---|
| 24-hour urinary screening test | Adrenal CT | MIBG | |
|
1. Hoy et al., 2004 17 |
Noradrenaline | Adenoma | Normal |
|
2. Hoy et al., 2004 17 |
Noradrenaline | Normal | Normal* |
|
3. Hoy et al., 2004 17 |
Noradrenaline | Normal | Normal* |
|
4. Hoy et al., 2004 17 |
Noradrenaline | Normal | Normal |
|
5. Hoy et al., 2004 17 |
Noradrenaline | Normal | Normal |
|
6. Makino et al., 2006 18 |
Noradrenaline | Normal | Normal |
|
7. Cheezum et al., 2010 19 |
Noradrenaline | Normal | Normal† |
|
8. Kahal et al., 2013 20 |
Noradrenaline | Normal | Normal |
|
9. Kahal et al., 2013 20 |
Noradrenaline | Normal | Normal |
|
10. Kahal et al., 2013 20 |
Noradrenaline§ | -- | -- |
|
11. Brainard et al., 2014 21 |
Normetadrenaline | -- | -- |
|
12. Weeks et al., 2015 22 |
Normetadrenaline | Adenoma¶ | -- |
|
13. Marmouch et al., 2021 23 |
Noradrenaline | Normal | Normal |
CT, computerised tomography; MIBG, metaiodobenzylguanidine.
*Both MIBG and octreotide tests were done; †Only octreotide test was done. §Only done at baseline; ¶Adrenalectomy showed adrenal cortical adenoma (no evidence of phaeochromocytoma)..
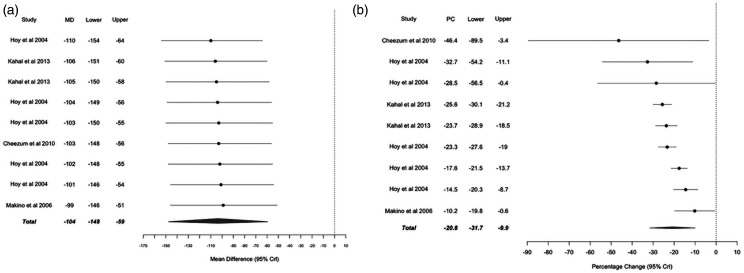
Of the thirteen cases studied, CPAP treatment reduced the levels of catecholamines or metanephrines to within local reference ranges in each case except one, case 12 (92.3% of all cases). 22 Analysis of individual cases and pooled data showed clear differences in noradrenaline levels before and after CPAP treatment (Figure 2). The absolute reduction of urinary noradrenaline levels after CPAP treatment was very similar among individual cases, regardless of the initial value. The overall mean (±SD) urinary noradrenaline before CPAP treatment was 499 ± 46 nmol/24-hours and after CPAP treatment was 395 ± 40 nmol/24-hours, i.e. a reduction of 104 nmol/24-hours (95% credible interval = 59 to 148 nmol/24-hours) (Figure 3(a)). Calculation of the percentage reduction of noradrenaline before and after CPAP treatment levels yielded an overall reduction of 21% (95% credible interval = 10 to 32%) (Figure 3(b)). For case 10, noradrenaline levels were raised by 30% above the upper reference limit at presentation (689 nmol/24 hours) which were normalised after CPAP treatment; although specific values for post-CPAP noradrenaline levels were not reported by the authors, post-CPAP normetanephrine/creatinine ratio for this patient was reported to be normal at 0.21 µmol/mmol (reference range <0.35 µmol/mmol). 20 For the two patients (case 11 and case 12) where urinary normetadrenaline levels were reported, there was a reduction of 32.5% after CPAP treatment.21,22 Case 13 had noradrenaline levels at presentation of 3.5 times the upper limit of reference and were reduced to within reference limits after CPAP treatment. 23
Figure 2.
Noradrenaline levels before (○) and after CPAP treatment (●) for individual cases and for pooled results (□open square = before CPAP treatment, ▪ solid square = after CPAP treatment). Noradrenaline levels were reduced to within local reference ranges for all cases after CPAP treatment.
Figure 3.
Mean difference (MD) in noradrenaline levels calculated as post-CPAP treatment adrenaline levels minus pre-CPAP treatment adrenaline levels (A), and percentage change (PC) in noradrenaline levels calculated as (post-CPAP treatment adrenaline levels minus pre-CPAP treatment adrenaline levels)/pre-CPAP treatment adrenaline levels (B) for individual cases (●) and for pooled results (♦).
Mean systolic/diastolic blood pressure fell from 177/109 mmHg before CPAP to 133/83 mmHg after CPAP treatment, equating to a reduction of 31 mmHg (25.4%) in mean arterial pressure, from 131 mmHg to 100 mmHg, respectively. Because of the improvement in blood pressure, the number of antihypertensive medications that patients were taking before CPAP treatment was either unchanged or reduced in all cases after CPAP treatment (Table 1).
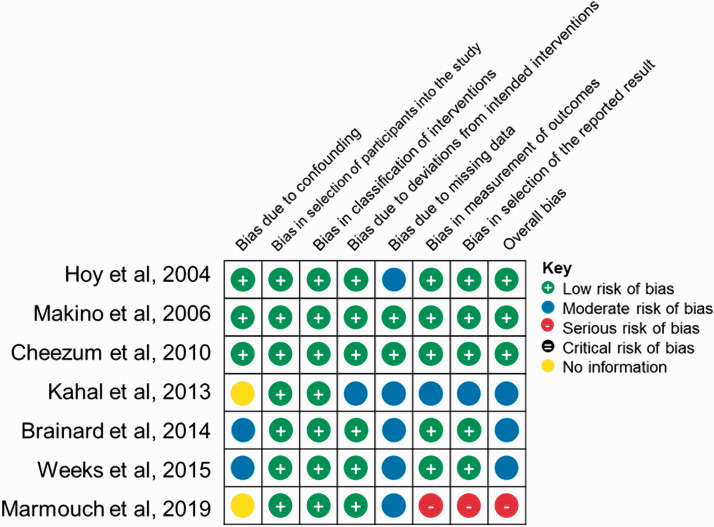
The risk of bias evaluated by the ROBINS-I tool was found to be low in the majority of reports (Figure 4). The few issues identified were related to bias due to pre-intervention domains (lack of information on confounding factors such as co-morbidities in two studies) and post-intervention domains (incomplete data including the actual values of catecholamines) in one report.
Figure 4.
Risk of bias summary for included reports evaluated by the ROBINS-I tool.
Discussion
The present study showed that the levels of catecholamines and metanephrines are reduced by CPAP treatment in those with OSA without evidence of phaeochromocytoma. These findings support the need to perform sleep studies for patients with hypertension who display features of OSA (snoring, witnessed apnoea, irregular breathing during sleep, restless sleeping, and chronic morning fatigue), 24 before embarking on investigations for phaeochromocytoma.
In parallel to the reduction of catecholamines or metadrenalines, both systolic and diastolic blood pressure also fell to an acceptable target treatment range while the number of antihypertensive medications at presentation were either reduced or unchanged after CPAP treatment. These observations suggest that the removal of stress due to OSA, significantly diminished sympathetic overdrive25,26 in patients without evidence of phaeochromocytoma. Our findings are consistent with those observed in clinical studies of the effects of CPAP treatment on catecholamines and blood pressure in patients with OSA, but the reduction in blood pressure is greater in this study (31 mmHg) compared with that in clinical studies (<10 mmHg).27,28 There are a number factors that may contribute to this difference. In this study, phaeochromocytoma was excluded in all patients, therefore a good response to CPAP treatment would be expected. On the other hand, phaeochromocytoma may be present in clinical studies since this condition is not uncommon – estimated to be between one to six in a thousand individuals with hypertension29–31; such individuals would not respond so well to CPAP treatment due to autonomous catecholamine release. Other reasons may be due to the small numbers of subjects in case reports who may have been preferentially selected; the baseline blood pressure which appears to be high in reported cases; the duration of CPAP treatment and antihypertensive therapy; and compliance with CPAP may lead to a lack of treatment response, which may occur more frequently in clinical studies.
The levels of the other major stress hormone cortisol have also been shown to be elevated in patients with OSA. This is thought to be due to activation of the hypothalamic-pituitary-adrenal axis or the sympathetic nervous system by stress or disruptive sleep patterns in these individuals. 32 Similar to the reduction in the levels of catecholamines demonstrated in this study of pseudophaeochromocytoma, abnormal screening tests such as 24-hour urinary free cortisol and the low-dose dexamethasone suppression test observed in untreated patients are also normalised by CPAP treatment in patients with pseudo-Cushing’s syndrome with coexisting OSA. 33 Therefore, indiscriminate screening for phaeochromocytoma or Cushing’s syndrome without first excluding OSA would lead to a misdiagnosis, subjecting the patient to more invasive procedures and causing them unnecessary anxiety, as well as imposing additional costs to the healthcare system.
Strengths and limitations
Although findings from case reports are often considered to provide weak scientific evidence, aggregation of results from case reports for systematic review and meta-analysis has been increasingly used.34,35 The advantage in the study of case reports is that a wealth of detailed individual data is available which can be synthesised and analysed.36,37 Studies have also shown that meta-analysis of case reports provides similar findings to those of clinical studies that comprise multiple participants. 38 In addition, meta-analysis of individual patient data has also been shown to provide less bias and more reliable than meta-analysis of clinical trials. 39 In the present study, we applied the ROBINS-I tool for assessing risk of bias 12 and found the majority of the included reports to be at low risk of overall bias and with consistency between reports. These findings suggest good quality of the published data which enhances the overall certainty of evidence from individual reports on the reduction of catecholamine levels and blood pressure by CPAP. As with most meta-analyses, this study may suffer from potential bias from unpublished reports, especially those with negative results, leading to an over-estimation of the effect of CPAP treatment on blood pressure. The 44 articles excluded in this study were judged on the basis of their lack of data necessary for the purpose of our analysis (not due to negative results), therefore they would be unlikely to introduce bias to the findings of this study.
In conclusion, investigations of patients with suspected phaeochromocytoma, particularly obese individuals, should exclude OSA and treat this condition if present before performing screening tests to assess for catecholamine levels.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: None.
Guarantor: TSH is the guarantor of this study.
Contributorship: TSH created the study concept and design. TSH and GK-D performed data collection. GK-D analysed the data. TSH wrote the first draft. MW, PS, CHF and TSH further edited the draft. All authors checked, interpreted the results and approved the final manuscript.
Provenance: Invited contribution.
ORCID iD: Thang S Han https://orcid.org/0000-0003-2570-0938
References
- 1.Widmaier EP, Raff H, Strang KT. Vander's human physiology. New York, NY: McGraw-Hill; 2006. [Google Scholar]
- 2.Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005; 366: 665–675. [DOI] [PubMed] [Google Scholar]
- 3.Kudva YC, Sawka AM, Young WF., Jr. The laboratory diagnosis of adrenal pheochromocytoma: the Mayo clinic experience. J Clin Endocrinol Metab 2003; 88: 4533–4539. [DOI] [PubMed] [Google Scholar]
- 4.Kales A, Cadieux R, Shaw IIL, et al. Sleep apnoea in a hypertensive population. Lancet 1984; 324: 1005–1008. [DOI] [PubMed] [Google Scholar]
- 5.Williams AJ, Houston D, Finberg S, et al. Sleep apnea syndrome and essential hypertension. Am J Cardiol 1985; 55: 1019–1022. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg DF, Iaina A, Oksenberg A. Treating obstructive sleep apnea improves essential hypertension and quality of life. Am Fam Physician 2002; 65: 229–236. [PubMed] [Google Scholar]
- 7.Fletcher EC, DeBehnke RD, Lovoi MS, et al. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med 1985; 103: 190–195. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg DS, Oksenberg A, Iaina A. Sleep related breathing disorders are common contributing factors to the production of essential hypertension but are neglected, underdiagnosed, and undertreated. Am J Hypertens 1997; 10: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 9.Elmasry A, Lindberg E, Hedner J, et al. Obstructive sleep apnoea and urine catecholamines in hypertensive males: a population-based study. Eur Respir J 2002; 19: 511–517. [DOI] [PubMed] [Google Scholar]
- 10.Trakada G, Chrousos GP, Pejovic S, et al. Sleep apnea and its association with the stress system, inflammation, insulin resistance and visceral obesity. Sleep Med Clin 2007; 2: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenders JW, Duh QY, Eisenhofer G, et al.; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014; 99: 1915–1942. [DOI] [PubMed] [Google Scholar]
- 12.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelman A, Carlin JB, Stern HS, et al. Bayesian data analysis. London: CRC Press, 2013. [Google Scholar]
- 14.Welton NJ, Sutton AJ, Cooper N, et al. Evidence synthesis for decision making in healthcare. New York: John Wiley & Sons, 2012. [Google Scholar]
- 15.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 16.Lunn D, Jackson C, Best N, et al. The BUGS book. A practical introduction to Bayesian analysis. London: CRC Press, 2012. [Google Scholar]
- 17.Hoy LJ, Emery M, Wedzicha JA, et al. Obstructive sleep apnea presenting as pseudopheochromocytoma: a case report. J Clin Endocrinol Metab 2004; 89: 2033–2038. [DOI] [PubMed] [Google Scholar]
- 18.Makino S, Iwata M, Fujiwara M, et al. A case of sleep apnea syndrome manifesting severe hypertension with high plasma norepinephrine levels. Endocr J 2006; 53: 363–369. [DOI] [PubMed] [Google Scholar]
- 19.Cheezum MK, Lettieri CJ. Obstructive sleep apnea presenting as pseudopheochromocytoma. J Clin Sleep Med 2010; 6: 190–191. [PMC free article] [PubMed] [Google Scholar]
- 20.Kahal H, Tahrani AA, George JT, et al. Obstructive sleep apnoea; a rare cause of pseudophaeochromocytoma. QJM 2013; 106: 1133–1136. [DOI] [PubMed] [Google Scholar]
- 21.Brainard T. Obstructive sleep apnea and tricyclic antidepressant use presenting as a pseudopheochromocytoma in an active duty sailor: a case report. Mil Med 2014; 179: e120–e123. [DOI] [PubMed] [Google Scholar]
- 22.Weeks AC, Kimple ME, Davis DB. The importance of exclusion of obstructive sleep apnea during screening for adrenal adenoma and diagnosis of pheochromocytoma. J Investig Med High Impact Case Rep 2015; 3: 2324709615607062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmouch H, Jenzri H, Mrabet H, et al. Two cases of pseudopheochromocytoma due to obstructive sleep apnea: which mechanisms? EJEA 2019; 63: GP4. [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, the National High Blood Pressure Education Program Coordinating Committee; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003; 42: 1206–1252. [DOI] [PubMed] [Google Scholar]
- 25.Hedner J, Darpo B, Ejnell H, et al. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 1995; 8: 222–229. [DOI] [PubMed] [Google Scholar]
- 26.Marrone O, Salvaggio A, Bue AL, et al. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: relationship with nocturnal sympathetic activity. Clin Exp Hypertens 2011; 33: 373–380. [DOI] [PubMed] [Google Scholar]
- 27.Green M, Ken-Dror G, Fluck D, et al. Meta-analysis of changes in the levels of catecholamines and blood pressure with continuous positive airway pressure therapy in obstructive sleep apnea. J Clin Hypertens 2021; 23: 12–20. DOI: 10.1111/jch.14061. [DOI] [PMC free article] [PubMed]
- 28.Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J 2008; 32: 1488–1496. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair AM, Isles CG, Brown I, et al. Secondary hypertension in a blood pressure clinic. Arch Intern Med 1987; 147: 1289–1293. [PubMed] [Google Scholar]
- 30.Anderson GH, Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens 1994; 12: 609–615. [DOI] [PubMed] [Google Scholar]
- 31.Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 2004; 27: 193–202. [DOI] [PubMed] [Google Scholar]
- 32.Kritikou I, Basta M, Vgontzas AN, et al. Sleep apnoea and the hypothalamic–pituitary–adrenal axis in men and women: effects of continuous positive airway pressure. Eur Respir J 2016; 47: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bravis V, Todd J, Dhillo W, et al. Severe obstructive sleep apnoea causing a pseudo-Cushing. In: Society for Endocrinology BES 2009 March 1 (Vol. 19). Bristol: BioScientifica, 2009.
- 34.Groen RJ, Middel B, Meilof JF, de Vos-van de Biezenbos JM, et al. Operative treatment of anterior thoracic spinal cord herniation: three new cases and an individual patient data meta-analysis of 126 case reports. Oper Neurosurg 2009; 64: ONS145–ONS160. [DOI] [PubMed] [Google Scholar]
- 35.Gupta N, Asi N, Farah W, et al. Clinical features and management of non-HIV–related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab 2017; 102: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson D, Daly J, Saltman DC. Aggregating case reports: a way for the future of evidence-based health care? Clin Case Rep 2014; 2: 23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampayo-Cordero M, Miguel-Huguet B, Pardo-Mateos A, et al. Agreement between the results of Meta-analyses from case reports and from clinical studies regarding the efficacy of laronidase therapy in patients with mucopolysaccharidosis type I who initiated enzyme replacement therapy in adult age: an example of case reports meta-analyses as an useful tool for evidence-based medicine in rare diseases. Mol Genet Metab 2018; 123: 69–75. [DOI] [PubMed] [Google Scholar]
- 39.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993; 341: 418–422. [DOI] [PubMed] [Google Scholar]