Abstract
Background
Neck pain is frequent in patients with migraine. Likewise, evidence for inflammatory processes in the trapezius muscles is accumulating. However, non-invasive and objectively assessable correlates are missing in vivo.
Methods
Twenty-one subjects with episodic migraine (mean age: 24.6 ± 3.1 years, 18 females) and 22 controls (mean age: 23.0 ± 2.2 years, 17 females) without any history of headache prospectively underwent physical examination and quantitative magnetic resonance imaging of the trapezius muscles. A T2‐prepared turbo spin-echo sequence was acquired for manual segmentation of the trapezius muscles and extraction of mean T2 values.
Results
There were no statistically significant differences regarding age, sex, body mass index, or number of myofascial trigger points (mTrPs) between groups. All patients with migraine presented with mTrPs in the trapezius muscles. T2 of the entire trapezius muscles was significantly higher in the migraine group when compared to controls (31.1 ± 0.8 ms vs. 30.1 ± 1.1 ms; p = 0.002).
Conclusions
Elevated T2 values of the trapezius muscles may indicate subtle inflammatory processes within musculature among patients with migraine because T2 increase is likely to stem from edematous changes. Future work may validate this finding in larger cohorts, but muscle T2 might have potential to develop into a viable in vivo biomarker for muscular affection in migraine.
Keywords: Magnetic resonance imaging, migraine, myofascial trigger points, trapezius muscle, T2 mapping, trigemino-cervical complex
Abbreviations
3D: Three-dimensional
BMI: Body mass index
CGRP: Calcitonin gene-related peptide
FOV: Field of view
ICHD: International Classification of Headache Disorders
MIDAS: Migraine Disability Assessment
MRI: Magnetic resonance imaging
mTrP: Myofascial trigger point
RMS: Root-mean-square
RMSCV: Root-mean-square coefficient of variation
rPMS: Repetitive peripheral magnetic stimulation
ROI: Region of interest
SD: Standard deviation
TCC: Trigemino-cervical complex
TSE: Turbo spin-echo
Introduction
Migraine is ranked among the top ten of the most disabling disorders worldwide (1). Regarding migraine pathophysiology, research has focused on its central mechanisms. Beyond central mechanisms, evidence accumulates that headache disorders, and particularly migraine, may be closely linked to structures of the musculoskeletal system. Nociceptive input stemming from the pericranial and the neck and shoulder region could be transmitted to the brainstem and meninges, thus leading to the experience of headache within the context of the trigemino-cervical complex (TCC) (2,3).
Muscular pain in the neck and shoulder region is a common finding in subjects suffering from migraine (4–7). Neck pain has been identified as a more frequent accompaniment of migraine than nausea, and the prevalence of neck pain shows associations to headache chronicity (7). However, objectively assessable correlates of such involvement of the musculoskeletal system have not yet been identified in patients with migraine. Yet, a sparse amount of previous work using in vivo imaging has investigated the role of myofascial trigger points (mTrPs) for migraine. Ultrasound studies were incoherent in differentiating mTrPs from surrounding musculature, pointing at a lack of correlation between clinically identified active mTrPs and ultrasound findings (8), or, conversely, revealed mTrPs as focal, hypoechoic regions or as regions with reduced vibration amplitude, indicating increased stiffness (9,10). On a similar note, studies using magnetic resonance imaging (MRI) pointed at low agreement between physicians and imaging raters for mTrPs using elastography for evaluating myofascial pain-associated taut bands (11), while a small study in three patients suffering from migraine pointed at focal, partly T2-hyperintense signal alterations in overlap with clinically defined mTrPs of the trapezius muscles (12). Using T2 mapping in ten patients with migraine, suspected mTrPs were characterised as T2 elevations in relation to surrounding muscle tissue of the trapezius (13). Such T2 evaluations might be the correlate of focal edematous changes within the trapezius muscles (13); yet, direct correlations between spots of T2 increase, potentially attributable to mTrPs, and tissue probes has not been achieved. However, whether or not the neck and shoulder musculature beyond mTrPs would present signal alterations in patients with migraine has not been investigated. Specifically, it is not evident why potential alterations of the musculoskeletal system in the course of migraine should be restricted to mTrPs only.
Inflammatory processes represent one of the pathophysiological mechanisms that may contribute to migraine development and maintenance (14). Biopsy samples of the calvarial periosteum identified increased expression of proinflammatory genes and decreased expression of genes that suppress inflammation and immune cell differentiation in patients with chronic migraine with muscle tenderness, providing support for a localised extracranial pathophysiology of migraine (15). On the muscular level, microdialysis of probes of upper trapezius muscles has also revealed elevated concentrations of inflammation-related substances among subjects with neck pain and mTrPs compared to subjects without neck pain with or without mTrPs (16). However, potential evidence of such muscular inflammatory changes in the course of migraine by means of in vivo imaging is lacking to date.
Against this background, this study aims at evaluating the trapezius muscles in patients with migraine applying quantitative high-resolution MRI with T2 mapping. We hypothesise that migraineurs with mTrPs in the trapezius muscles may show increases in muscle T2 as a potential correlate of ongoing inflammation causing edematous changes.
Methods
Participants and design
This prospective, monocentric study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
The following inclusion criteria were defined for this study: i) written informed consent, ii) age between 18 and 30 years, and iii) diagnosis of migraine (migraine group; according to the German version of the headache questionnaire modified according to the International Classification of Headache Disorders, 3rd edition, beta version [ICHD-3 beta] (17)), or absence of any history of migraine or other types of headache (control group). Exclusion criteria were a) any history of muscular or neurological disorders (except for migraine), b) diagnosis of tension-type headache as an additional diagnosis (migraine group) or isolated diagnosis (control group), c) pregnancy, d) general contraindications for MRI, e) previous injury, surgery, or implants at the neck and shoulder region, f) a body mass index (BMI) indicating underweight or obesity (BMI < 18.5 or BMI > 30.0 kg/m2), and g) reported participation in competitive sports, extensive physical activity, or weight lifting/body building.
Overall, 21 subjects with diagnosed episodic migraine and 22 healthy control subjects were enrolled. Study participation included two visits, 14 days apart, to assess eligibility and clinical and headache characteristics including physical examination with screening for mTrPs of the neck and shoulder area, specifically of the trapezius muscles (first study visit) and imaging of the neck and shoulder area by MRI (second study visit). All subjects considered eligible during the first study visit were advised to not perform any kind of sports activity and to not undergo physiotherapy or massage during the upcoming two weeks until the appointment for the second study visit. The visits were scheduled between July 2019 and August 2020.
Clinical and physical examination
During the first study visit, standard clinical examinations, including assessment of headache characteristics, were performed by a board-certified medical doctor, including completion of the Migraine Disability Assessment (MIDAS) questionnaire in subjects diagnosed with migraine (with reference to the 30 days prior to the first study visit) (18).
Manual palpation of the trapezius muscles was performed by a certified physiotherapist, with the aim to detect mTrPs using the approach as described by Simons et al. (19). During manual palpation, the physiotherapist let the trapezius muscle slide through between thumb and index finger (pincer grip) under medium pressure. Specifically, to diagnose a latent mTrP, the following criteria had to be fulfilled: i) presence of a palpable taut band with a local hypersensitive spot, or ii) presence of a local hypersensitive spot with occurrence of a referred sensation during palpation of this spot, or iii) presence of a palpable taut band with a local hypersensitive spot and occurrence of a referred sensation during palpation of this spot (20–23). To qualify as an active mTrP, the referred sensation caused by palpation of a hypersensitive spot had to be in the same location with similar characteristics and intensity as the typical headache of the respective study participant (20–23). No specific location was considered to be a particular mTrP location, thus examining the entire anatomical course of the trapezius muscles of both sides.
The number of active and latent mTrPs and their distinct locations within the trapezius muscles with respect to proportional distance between anatomical landmarks were documented (os occiput to cervical vertebra 7, cervical vertebra 7 to acromion).
Magnetic resonance imaging
Image acquisition
Imaging of the neck and shoulder region was performed with a 3-Tesla MRI scanner (Ingenia Elition, Philips Healthcare, Best, The Netherlands) in supine position during the second study visit. A 16-channel anterior coil, 12-channel built-in posterior coil, and 16-channel head coil were used during scanning, applying a T2‐prepared three-dimensional (3D) turbo spin-echo (TSE) sequence for T2 mapping with the following sequence parameters: repetition time/echo time = 1500/16 ms, field of view (FOV) = 480 × 200 × 84 mm3, acquisition voxel = 1.75 × 1.75 × 2.0 mm3, reconstruction voxel = 1.5 × 1.5 × 2.0 mm3, echo train length = 55, echo spacing = 2.3 ms, compressed SENSE (reduction factor R = 5.5), no partial Fourier, and fat suppression using spectral inversion recovery. The acquisition time of this sequence amounted to 7 min 53 sec per study participant.
A T2 preparation duration of 15 – 30 – 45 ms was applied and an additional saturation preparation scan was acquired to mitigate quantification errors due to B0 inhomogeneities. The flip angle train was determined according to the vendor’s routines, leading to a constant signal over the entire shot for the relaxation properties of skeletal musculature (13,24). The FOV of the sequence covered the entire course of the trapezius muscle in the vertical axis and the entire body width at the shoulder level in the horizontal axis.
Processing of images for T2 extraction
The T2-weighted raw images of the T2‐prepared 3D TSE sequence were processed by in-house developed scripts coded in MATLAB. A voxel-by-voxel fitting for the T2 value with additional accounting for B0 field inhomogeneities was applied as described previously (13,24,25). The obtained datasets were transferred to the open-source software Medical Imaging Interaction Toolkit (MITK; developed by the Division of Medical and Biological Informatics, German Cancer Research Center, Heidelberg, Germany; www.mitk.org), followed by visual quality assessment. None of the study participants had to be excluded due to image quality issues.
Image segmentation
Manual segmentations of image data were performed by placement of regions of interest (ROIs) using tools implemented in MITK. One reader (PS), supervised by a board-certified radiologist with ten years of experience (TB), performed the segmentations in all enrolled subjects. Furthermore, this reader performed segmentations in five randomly selected subjects a second time to assess reproducibility (8 weeks after the initial segmentations), supplemented by evaluation of these five subjects by a second independent reader with 8 years of experience (NS) to evaluate inter-reader reliability. The readers were strictly blinded to the results of physical examination as well as the group assignments (migraine group vs. control group).
The entire trapezius muscles were segmented in the axial slices of the images with the shortest T2 preparation duration, with polygonal ROIs being manually placed on each slice in consecutive order to separately enclose the right and left trapezius muscles. A margin of approximately 5 mm to the outer contour of the trapezius muscles was considered to avoid accidental inclusion of muscular fascia or intermuscular fat, and the segmentations were stopped in the vertical direction when no more muscle tissue was identified at the transition zone to the muscle tendons of the trapezius muscles bilaterally (Figure 1). Additionally, a multi-planar reconstruction of the images was performed in coronal and sagittal orientation, which was considered to avoid unwilling inclusion of vessel structures as far as possible. Mean T2 values >100 ms were removed from the datasets prior to extraction of T2 values using our MATLAB-coded scripts because such high T2 values most likely stem from measurements in areas with very high fluid components, such as vasculature (25).
Figure 1.

Segmentation of the trapezius muscle. T2-weighted raw data (with the weakest T2-weighting, in arbitrary units [a.u.]) for an exemplary case. On the raw data, the masks obtained from segmentation of the trapezius muscle on the left side are indicated by an overlay in a representative axial slice.
The mean T2 values (in ms) of the entire left and right trapezius muscles were extracted and averaged for each subject. Additionally, T2 values were extracted and averaged between sides for the single slice that presented the largest axial cross-sectional area of the trapezius muscles.
Statistical analysis
Statistics and generation of graphs were performed with GraphPad Prism (version 6.0; GraphPad Software Inc., San Diego, CA, USA). A p-value < 0.05 was defined as statistically significant (two-sided).
Descriptive statistics including mean, standard deviation (SD), median, ranges, or absolute frequencies were calculated. Shapiro-Wilk normality tests were performed and indicated Gaussian distribution for age, BMI, and the extracted T2 values. Unpaired t-tests were conducted for age and BMI between the subjects assigned to the migraine and control group, χ2 tests were performed to assess sex distribution and number of mTrPs per side between groups. The results of the MIDAS questionnaire were used to generate an overall MIDAS score (0–5 points: None to minimal impairment, 6–10 points: Mild impairment, 11–20 points: Moderate impairment, and > 20 points: Severe impairment) (18,26,27).
To evaluate differences in T2 values between groups, unpaired t-tests were performed for the segmentations of the entire trapezius muscles as well as for the segmentations derived from the slice with the largest cross-sectional area, respectively. To evaluate the reproducibility of T2 values of the segmentations of the trapezius muscles, we evaluated the measurements performed two times by the first reader in five subjects by calculating the root-mean-square (RMS) error (absolute units [ms]) and root-mean-square coefficient of variation (RMSCV; relative units [%]) for repeated measurements (28,29). Analogously, we used the RMS and RMSCV to assess inter-reader reliability based on the measurements among these five subjects as obtained from the two readers.
Results
Cohort characteristics
The results of the MIDAS questionnaire and derived MIDAS scores are depicted in Table 1. There were no statistically significant differences between the migraine and control group regarding age, sex distribution or BMI (p > 0.05 each; Table 2). Fourteen of the included 21 migraineurs had a diagnosis of migraine with aura. None of the migraineurs presented with an acute migraine attack on the days of the study visits.
Table 1.
Migraine Disability Assessment (MIDAS) questionnaire. This table shows the results of the MIDAS questionnaire and derived MIDAS score as mean ± standard deviation (SD) and ranges.
| MIDAS Questionnaire | |
|---|---|
| Item | Mean ± SD(range) |
| Missing school/work (days) | 1.7 ± 2.0 (0–8) |
| Productivity at school/work reduced by half (days) | 6.2 ± 4.9 (0–20) |
| Could not do household work (days) | 2.7 ± 3.1 (0–12) |
| Household work productivity reduced by half (days) | 4.1 ± 5.0 (0–18) |
| Missing family, social, or leisure activities (days) | 3.0 ± 2.5 (0–10) |
| Frequency of headache (days) | 21.5 ± 10.2 (9–45) |
| Intensity (scale of 0–10) | 5.8 ± 1.5 (3–8) |
| MIDAS score | 17.8 ± 13.6 (3–59) |
Table 2.
Cohort characteristics and myofascial trigger points (mTrPs). This table shows age, body mass index (BMI), and number of latent and active mTrPs for migraineurs and control subjects. Given p-values are derived from unpaired t-tests (age, BMI) or χ2 tests (number of mTrPs).
| Cohort characteristics | ||||
|---|---|---|---|---|
| Mean ± SD (range) | ||||
| Item | Migraine group (n = 21) | Control group (n = 22) | p-value | |
| Age (years) | 24.6 ± 3.1 (20.0–31.7) | 23.0 ± 2.2 (19.7–28.1) | 0.060 | |
| BMI (kg/m2) | 21.6 ± 1.9 (18.6–25.4) | 22.1 ± 2.3 (19.1–28.7) | 0.434 | |
| All mTrPs | ||||
| Right trapezius muscle | 2.1 ± 1.9 (0–8) | 2.0 ± 2.0 (0–6) | 0.880 | |
| Left trapezius muscle | 2.2 ± 1.7 (0–6) | 2.0 ± 2.2 (0–7) | ||
| Active mTrPs | ||||
| Right trapezius muscle | 0.3 ± 1.1 (0–4) | 0 | – | |
| Left trapezius muscle | 0.3 ± 1.1 (0–5) | 0 | ||
According to physical examination, all subjects of the migraine cohort showed at least one mTrP in the trapezius muscles. Two subjects of the migraine group showed active mTrPs bilaterally, while the remaining 19 subjects of the migraine group only presented with latent mTrPs (Table 2). Subjects of the control group did not show any active mTrPs (Table 2).
T2 values of the trapezius muscles
The T2 values of the trapezius muscles were significantly higher in the migraine group when compared to the control group for the entire trapezius muscles (mean ± SD and range: 31.1 ± 0.8 ms, 30.0–32.6 ms vs. 30.1 ± 1.1 ms, 28.4–32.0 ms; p = 0.002; Figure 2) as well as for the single slice with the largest cross-sectional area of the trapezius muscles (mean ± SD and range: 31.1 ± 0.8 ms, 30.0–32.8 ms vs. 30.2 ± 1.1 ms, 28.0–31.9 ms; p = 0.002).
Figure 2.
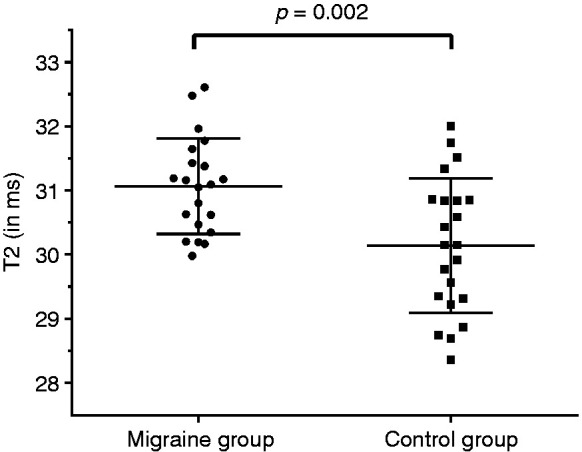
T2 values of the trapezius muscles. This graph illustrates the T2 values derived from segmentations of the entire trapezius muscles of both sides in subjects with migraine as well as controls. The circles and squares indicate individual T2 values (in ms), horizontal lines represent the mean and standard deviation (SD). The difference in T2 values between groups was statistically significant (p = 0.002).
Reproducibility and inter-reader reliability
The reproducibility of T2 measurements for the trapezius muscles, as assessed by the RMS error, was 0.14 ms (RMSCV: mean ± SD and range: 0.12 ± 0.07%, 0.01–0.23%). Similarly, inter-reader reliability showed an RMS error amounting to 1.57 ms (RMSCV: mean ± SD and range: 1.43 ± 0.64%, 0.90–2.50%).
Discussion
This study applied quantitative MRI by means of T2 mapping in patients with migraine and controls to evaluate T2 values of the trapezius muscles bilaterally. We found that the muscle T2 is significantly higher in subjects with episodic migraine when compared to controls without a history of migraine or other types of headache. Therefore, our hypothesis of potentially ongoing inflammation causing edematous changes, most probably the cause of such increased T2 values in the trapezius muscles of migraineurs, could be confirmed. Prior research has repeatedly described that muscular pain of the neck and shoulder region is frequent in subjects suffering from migraine (4–7). Triggering of headache by muscular pain of the neck and shoulder region in migraine seems likely within the concept of the TCC, which includes a convergence of nociceptive inputs originating from the neck and shoulder muscles and the trigeminal nerve in the caudal trigeminal nuclei (2,3). Accordingly, hyperalgesia at the level of the neck and shoulder muscles could influence the development and/or maintenance of migraine or vice versa. In this regard, the finding of increased muscle T2 for the trapezius muscles in migraine may point at edematous changes, which may most likely stem from inflammatory mechanisms. Increases on T2-weighted MRI sequences or, in a quantifiable manner, increases in measurable T2 values are almost specific for edema (30,31).
Our findings align with evidence for peripheral inflammatory mechanisms in migraine from previous work, demonstrating enhanced expression of proinflammatory genes (e.g. CCL8, TLR2) and decreased expression of genes suppressing inflammation and immune cell differentiation (e.g. IL10RA, CSF1R) in biopsy samples of the calvarial periosteum derived from patients with chronic migraine and muscle tenderness (15). On a similar note, subjects with trapezius myalgia, not necessarily related to migraine, showed higher interstitial serotonin and higher glutamate than controls according to microdialysis evaluation of trapezius muscle probes (32). Moreover, microdialysis of samples of the upper trapezius muscles has revealed elevated concentrations of substances including bradykinin, calcitonin gene-related peptide (CGRP), substance P, tumour necrosis factor-α, interleukin-1β, serotonin, and norepinephrine in subjects with neck pain and mTrPs when compared to subjects without neck pain and with or without mTrPs (16). In particular, CGRP has played a major role in migraine research during the last decade and is reported as one of the most important substances in inter-axonal signaling in the trigeminal loops and the trigeminal ganglion itself (33–35). Specifically, CGRP is a key neurotransmitter in the neuro-immune axis, with unmyelinated nerve fibres (c-fibres) containing CGRP (36). In this regard, T2 mapping of the trapezius muscles in patients with migraine and a muscular component may represent a novel, objective outcome measure to assess the treatment response to acute or preventive therapies. Further, elevated T2 values may serve as future response predictors for such therapies on the long run. Vice versa, CGRP levels may serve as markers for prediction or assessment of treatment responses of approaches focusing on the neck and shoulder muscles, such as repetitive peripheral magnetic stimulation (rPMS) (37–39). Yet, another interesting perspective may be to assess the effects of CGRP-targeting medications applied subcutaneously in the neck and shoulder region. However, such integrative approaches combining objective diagnostics by MRI using T2 mapping with medication and/or interventions such as rPMS are beyond this study’s scope, but should be designed as the next steps.
The finding of elevated T2 values of the trapezius muscles was obtained in a cohort that did not show significant differences to controls for age, BMI, sex distribution, or the total number of mTrPs of the trapezius muscles. A previous study evaluating signal alterations by means of T2 mapping related to mTrPs reported on lower mean T2 values for the right and left trapezius muscles, which were only derived from measurements in a single slice at the level of the individually marked mTrP location in ten subjects (13). Discrepancies in absolute values may be related to differences in sequence details when compared to the present study or could be linked to the respective slice evaluated. The very good values for reproducibility and inter-reader reliability, however, nearly exclude variability in values due to potentially divergent segmentation approaches. In this regard, the application of T2 mapping becomes possible by a high-resolution T2-prepared 3D TSE sequence that offers an accurate and fast T2 quantification with sufficient robustness to B1 and B0 errors even at a challenging region such as the neck and shoulder region where large B0 variations are frequently observed (13,24). In contrast, previous conventional T2 mapping approaches, applied for various other purposes than evaluations of trapezius muscles in migraine, were primarily based on two-dimensional, multi‐slice multi-echo spin echo sequences that were error-prone due to the dependence of the T2 quantification on B1 and B0 errors (40,41).
The following limitations have to be acknowledged when interpreting the results of this study. First, we only included young adults and the predominantly female study cohort has a rather narrow age range, which limits the generalisability of the presented findings. Hence, future studies may include larger samples of different ages and may additionally investigate potential sex differences for muscle T2 values. Second, we did not specifically assess whether neck pain was closely linked to the presence of a migraine attack, but upcoming studies may evaluate potential differences in mTrP characteristics and, importantly, T2 values considering interictal intervals and ictal phases with concomitant neck pain. Third, related to the period of 14 days between the two study visits, it is currently unclear to what extent the CGRP status or the number of mTrPs may change within a subject’s individual migraine cycle. Yet, given the likelihood of manual palpation of mTrP assessments causing microtrauma and edema, synchronising manual palpation and MRI acquisition on the same day or within only few days apart may reflect a source of bias, with the risk of arbitrarily increased T2 values captured by too-early MRI acquisitions after physical examination. Yet, upcoming studies may more specifically assess muscular symptoms during the course of a migraine cycle, including standardised measures of muscular hyperalgesia (e.g. pressure pain thresholds), to be able to potentially provide surrogate markers of migraine-related neck pain on the day of MRI acquisition. Fourth, the contribution of latent or active mTrPs of the trapezius muscles to the overall muscle T2 values is unclear and not evaluated in this study. Previous work in a small cohort of ten subjects has pointed at increased T2 values for suspected mTrPs when compared to surrounding musculature (13), yet this previous work has not evaluated T2 values of the entire trapezius muscles. However, the size of mTrPs is rather small, and we do not expect considerable impact of these points on overall muscle T2 values. In this regard, upcoming analyses will have to validate increased T2 values related to mTrPs of the trapezius muscles and their role for signal quantifications in MRI of the neck and shoulder area. Fifth, the absolute difference in muscle T2 values between subjects with migraine and controls was in the range of only few ms. While increased T2 values may be interpreted as evidence for inflammatory changes related to migraine, reference values for edematous changes of musculature are not available and would be subject to stark differences between sequences and MRI systems. Yet, the difference between groups was observed using the same scanner and sequence protocol in all subjects, but confirmation in larger samples and also with other scanning environments seems mandatory to draw more final conclusions.
Conclusion
By means of in vivo, quantitative high-resolution MRI of the neck and shoulder region, this study revealed a significant difference in muscle T2 values between patients with migraine with mTrPs and controls for the trapezius muscles. The higher T2 values for the migraine group may be attributed to subtle edematous changes within the musculature, potentially related to peripheral inflammatory mechanisms of migraine. Related to this finding, migraine could not only be seen as an isolated “brain state”, but rather as a comprehensive pathophysiological concept integrating a “brain and muscle state”, which seems at least applicable to cohorts of migraineurs with mTrPs. Yet, confirmation of results in larger samples is needed prior to determining the distinct value of muscle T2 as a potential muscular biomarker in migraine.
Clinical implications
This study investigated the trapezius muscles by means of magnetic resonance imaging with T2 mapping.
Subjects with episodic migraine show increased T2 values of the trapezius muscles compared to controls.
This finding may relate to edematous changes of the trapezius muscles in migraine, likely stemming from subtle inflammatory processes.
Muscle T2 could translate into a viable biomarker for peripheral components of migraine pathophysiology.
Acknowledgements
We thank Helene König and Matthias Lechner for their contribution during manual palpation and assessment of myofascial trigger points.
Footnotes
Ethic approval and patient consent: This study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The work of MVB in the field of migraine research is supported by a scholarship of the Bavarian Gender Equality Grant. Furthermore, the work of NS in the field of musculoskeletal imaging and/or migraine research is supported by the Dr.-Ing. Leonhard Lorenz Foundation, the German Society of Musculoskeletal Radiology (DGMSR), and the Joachim Herz Foundation.
ORCID iDs: Nico Sollmann https://orcid.org/0000-0002-8120-2223
Michaela V Bonfert https://orcid.org/0000-0003-0995-9050
References
- 1.Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: Current concepts and synthesis. Curr Pain Headache Rep 2003; 7: 371–376. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-de-Las-Penas C. Myofascial head pain. Curr Pain Headache Rep 2015; 19: 28. [DOI] [PubMed] [Google Scholar]
- 4.Blaschek A, Milde-Busch A, Straube A, et al. Self-reported muscle pain in adolescents with migraine and tension-type headache. Cephalalgia 2012; 32: 241–249. [DOI] [PubMed] [Google Scholar]
- 5.De Marinis M, Accornero N. Recurrent neck pain as a variant of migraine: Description of four cases. J Neurol Neurosurg Psych 1997; 62: 669–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashina S, Bendtsen L, Lyngberg AC, et al. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015; 35: 211–219. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun AH, Ford S, Millen C, et al. The prevalence of neck pain in migraine. Headache 2010; 50: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 8.Lewis J, Tehan P. A blinded pilot study investigating the use of diagnostic ultrasound for detecting active myofascial trigger points. Pain 1999; 79: 39–44. [DOI] [PubMed] [Google Scholar]
- 9.Sikdar S, Shah JP, Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil 2009; 90: 1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikdar S, Shah JP, Gilliams E, et al. Assessment of myofascial trigger points (MTrPs): A new application of ultrasound imaging and vibration sonoelastography. In: Proceedings of IEEE Engineering in Medicine & Biology Society Conference, Vancouver, Canada, 20–25 August 2008, pp.5585–5588. [DOI] [PubMed]
- 11.Chen Q, Wang HJ, Gay RE, et al. Quantification of myofascial taut bands. Arch Phys Med Rehabil 2016; 97: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landgraf MN, Ertl-Wagner B, Koerte IK, et al. Alterations in the trapezius muscle in young patients with migraine – a pilot case series with MRI. Eur J Paediatr Neurol 2015; 19: 372–376. [DOI] [PubMed] [Google Scholar]
- 13.Sollmann N, Mathonia N, Weidlich D, et al. Quantitative magnetic resonance imaging of the upper trapezius muscles – assessment of myofascial trigger points in patients with migraine. J Headache Pain 2019; 20: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol 2019; 15: 483–490. [DOI] [PubMed] [Google Scholar]
- 15.Perry CJ, Blake P, Buettner C, et al. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: Implications for extracranial origin of headache. Ann Neurol 2016; 79: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah JP, Phillips TM, Danoff JV, et al. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985) 2005; 99: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 17.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 18.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001; 56: S20–S28. [DOI] [PubMed] [Google Scholar]
- 19.Simons DG, Travell J, Simons LS. Myofascial pain and dysfunction: The trigger point manual, 2nd edn. Baltimore: Williams & Wilkins, 1999. [Google Scholar]
- 20.Fernandez-de-Las-Penas C, Simons D, Cuadrado ML, et al. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep 2007; 11: 365–372. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-de-Las-Penas C, Dommerholt J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A Delphi study. Pain Med 2018; 19: 142–150. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez DJ, Rockwell PG. Trigger points: Diagnosis and management. Am Fam Physician 2002; 65: 653–660. [PubMed] [Google Scholar]
- 23.Davidoff RA. Trigger points and myofascial pain: Toward understanding how they affect headaches. Cephalalgia 1998; 18: 436–448. [DOI] [PubMed] [Google Scholar]
- 24.Weidlich D, Schlaeger S, Kooijman H, et al. T2 mapping with magnetization-prepared 3D TSE based on a modified BIR-4 T2 preparation. NMR Biomed 2017; 30: e3773. [DOI] [PubMed] [Google Scholar]
- 25.Sollmann N, Weidlich D, Cervantes B, et al. High isotropic resolution T2 mapping of the lumbosacral plexus with T2-prepared 3D turbo spin echo. Clin Neuroradiol 2019; 29: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton RB, Stewart WF, Sawyer J, et al. Clinical utility of an instrument assessing migraine disability: The Migraine Disability Assessment (MIDAS) questionnaire. Headache 2001; 41: 854–861. [PubMed] [Google Scholar]
- 27.Sajobi TT, Amoozegar F, Wang M, et al. Global assessment of migraine severity measure: Preliminary evidence of construct validity. BMC Neurol 2019; 19: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barchard K. Examining the reliability of interval level data using root mean square differences and concordance correlation coefficients. Psychol Meth 2011; 17: 294–308. [DOI] [PubMed] [Google Scholar]
- 29.Gluer CC, Blake G, Lu Y, et al. Accurate assessment of precision errors: How to measure the reproducibility of bone densitometry techniques. Osteoporos Int 1995; 5: 262–270. [DOI] [PubMed] [Google Scholar]
- 30.McMahon CJ, Wu JS, Eisenberg RL. Muscle edema. AJR Am J Roentgenol 2010; 194: W284–W292. [DOI] [PubMed] [Google Scholar]
- 31.Kumar Y, Wadhwa V, Phillips L, et al. MR imaging of skeletal muscle signal alterations: Systematic approach to evaluation. Euro J Radiol 2016; 85: 922–935. [DOI] [PubMed] [Google Scholar]
- 32.Rosendal L, Larsson B, Kristiansen J, et al. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: Microdialysis in rest and during exercise. Pain 2004; 112: 324–334. [DOI] [PubMed] [Google Scholar]
- 33.Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019; 394: 1765–1774. [DOI] [PubMed] [Google Scholar]
- 34.Edvinsson L, Goadsby PJ. Discovery of CGRP in relation to migraine. Cephalalgia 2019; 39: 331–332. [DOI] [PubMed] [Google Scholar]
- 35.Eftekhari S, Salvatore CA, Calamari A, et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010; 169: 683–696. [DOI] [PubMed] [Google Scholar]
- 36.Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci 2014; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renner T, Sollmann N, Trepte-Freisleder F, et al. Repetitive peripheral magnetic stimulation (rPMS) in subjects with migraine-setup presentation and effects on skeletal musculature. Front Neurol 2019; 10: 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sollmann N, Trepte-Freisleder F, Albers L, et al. Magnetic stimulation of the upper trapezius muscles in patients with migraine – a pilot study. Eur J Paediatr Neurol 2016; 20: 888–897. [DOI] [PubMed] [Google Scholar]
- 39.Renner T, Sollmann N, Heinen F, et al. Alleviation of migraine symptoms by application of repetitive peripheral magnetic stimulation to myofascial trigger points of neck and shoulder muscles – a randomized trial. Sci Rep 2020; 10: 5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majumdar S, Orphanoudakis SC, Gmitro A, et al. Errors in the measurements of T2 using multiple-echo MRI techniques. II. Effects of static field inhomogeneity. Mag Reson Med 1986; 3: 562–574. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar S, Orphanoudakis SC, Gmitro A, et al. Errors in the measurements of T2 using multiple-echo MRI techniques. I. Effects of radiofrequency pulse imperfections. Mag Reson Med 1986; 3: 397–417. [DOI] [PubMed] [Google Scholar]


