Abstract
Objectives
The aim of this study was to compare the effects of acupuncture and medical training therapy alone and in combination with those of usual care on the pain sensation of patients with frequent episodic and chronic tension-type headache.
Design
This was a prospective single-centre randomised controlled trial with four balanced treatment arms. The allocation was carried out by pre-generated randomisation lists in the ratio 1:1:1:1 with different permutation block sizes.
Setting
The study was undertaken in the outpatient clinic of Rehabilitation Medicine of the Hannover Medical School.
Participants and interventions: Ninety-six adult patients with tension-type headache were included and randomised into usual care (n = 24), acupuncture (n = 24), medical training (n = 24), and combination of acupuncture and medical training (n = 24). One patient was excluded from analysis because of withdrawing her/his consent, leaving 95 patients for intention to treat analysis. Each therapy arm consisted of 6 weeks of treatment with 12 interventions. Follow-up was at 3 and 6 months.
Main outcome measures
Pain intensity (average, maximum and minimum), frequency of headache, responder rate (50% frequency reduction), duration of headache and use of headache medication.
Clinical results: The combination of acupuncture and medical training therapy significantly reduced mean pain intensity compared to usual care (mean = −38%, standard deviation = 25%, p = 0.012). Comparable reductions were observed for maximal pain intensity (−25%, standard deviation = 20%, 0.014) and for minimal pain intensity (−35%, standard deviation = 31%, 0.03). In contrast, neither acupuncture nor medical training therapy differed significantly from usual care. No between-group differences were found in headache frequency, mean duration of headache episodes, and pain medication intake. At 3 months, the majority of all patients showed a reduction of at least 50% in headache frequency. At 6 months, significantly higher responder rates were found in all intervention groups compared to usual care.
Conclusions
In contrast to monotherapy, only the combination of acupuncture and medical training therapy was significantly superior in reduction of pain intensity compared to usual care.
Trial registration: Registered on 11 February 2019. German Clinical Trials Register, DRKS00016723.
Keywords: Tension type headache, acupuncture, medical training, exercise therapy, traditional Chinese medicine
Introduction
Non-drug treatments in addition to drug strategies for the treatment of tension type headache (TTH) are laid down in the national and international guidelines (1,2). Patients with high headache frequency are particularly at risk of excessive misuse of headache medication, resulting in medication overuse headache. Consequently, non-drug treatment strategies are of high therapeutic importance (3–5). More than 80% of patients with chronic TTH previously had episodic TTH, which is associated with significantly higher limitations in quality of life (6). In order to counteract this chronification, the focus is on patient-centred, well accepted and well tolerated strategies such as relaxation techniques, biofeedback and prophylactic medication with few side effects. Some studies showed that multimodal therapy strategies are likely to be superior to unimodal treatment (7,8). For both episodic and chronic TTH, acupuncture (AP) and medical training therapy (MTT) may be effective treatment options with high patient acceptance and low side effects (9–12). MTT is a physician-prescribed active form of physiotherapy in small groups focusing on muscle strength, endurance, and cardiopulmonary capability (13). The main components of training were initial ergometer training, strength training on medical training machines, postural-proprioceptive training with flexible oscillating rods and unstable oscillating surfaces, and flexibility and coordinative training on the soft floor mat (see Table 1). As AP may help to relieve pain mainly by activation of different pain inhibitory control systems, MTT may additionally regulate the autonomic nervous system in a long-lasting way and may be associated with beneficial neuroplastic changes (14–16). Therefore, the combination therapy of both AP and MTT has the potential to show supra-additive effects on chronic pain states. So far, only one small study comparing AP plus aerobic exercise against AP alone in myofascial pain syndrome of the neck could not demonstrate any additional benefit on pain scores and quality of life by the adjunct of aerobic exercise (17). However, since combination therapy of AP with high-end MTT has not yet been sufficiently investigated in clinical randomised comparative four-arm studies, we designed a trial addressing this issue in frequent episodic and chronic TTH (18).
Table 1.
Detailed description of medical training therapy for clinical reproducibility. (Equipment: GENIUS ECO, FREI medical GmbH; Propriomed and Posturomed, HAIDER BIOSWING GmbH).
| Method | Intervention | Intensity | Frequency | Duration |
|---|---|---|---|---|
| Cardiovascular training | Ergometry | 75% HR max | 1 | 15 min |
| Rowing machine | ||||
| Cross-trainer | ||||
| Treadmill | ||||
| Strength-endurance training | Pull-down | 40% of max strength | 2 * 25 | 10 min |
| Butterfly reverse | ||||
| Row | ||||
| Coordinative training | Postural training | Individual level | 1 | 3 min |
| Proprioceptive training | Segmental stabilisation of the spine in flection and extension | 2.5–4.8 Hz | 5 * 15 sec | |
| Segmental stabilisation of the spine in lateral flexion | ||||
| Reducing muscle tension | ||||
| Training of mobility and flexibility | Stretching of the erector spinae muscles | Individual level | 1 | 30 sec |
| Stretching of iliopsoas muscle and extension of flank | 4 * 1 | 4 * 20 sec | ||
| Spinal mobilisation from the quadruped stand | 4 * 1 | 4 * 20 sec | ||
| Supine – extension and abdominal respiration, shoulder bridge and “candle” | 2 | 30 sec | ||
| Forearm crutch for stabilisation of the trunk | 2 * 1 | 30 sec | ||
| Rotational strain of the spine with breathing recess | 5 | 5 * 10sec | ||
| Stretching of the trapezius and pectoralis muscles | 2 | 30 sec |
HR: heart rate.
Methods
Ethical approval was provided by the Ethics Committee of the Hannover Medical School, all participants subscribed informed consent prior to study inclusion. The study protocol was previously published in 2019 under the reference number doi: 10.1186/s13063-019-3700-1 in the journal Trials (18). The main objective criteria of the study are presented in this publication.
Study design and sample size estimation
The study is a randomised, unblinded controlled prospective clinical trial with four balanced treatment arms. It was carried out in the outpatient department of Rehabilitation Medicine of the Hannover Medical School.
The sample size estimation is based on the results of Endres et al. to test interaction effect (time × intervention), the differences between the repeated measurements of the four study groups were analysed (19). The study was conducted to detect significant differences for the primary endpoint (α = 0.05) with a power of 90% using a generalised estimation equation (GEE). Assuming a 20% drop-out rate during the course of the study, 96 (24 per arm) participants had to be recruited.
Screening and recruitment
Between July 2018 and May 2019, 212 test persons were screened. Recruitment was previously carried out via local and regional digital and print media, attracting patients from the pain clinic and primary care centre of the Hannover Medical School as well as from pain and primary care practices in the Hannover area. Subsequently, the initial personal medical contact was made in order to clarify whether the headache symptoms of the participants match the criteria of TTH of the International Headache Society International Classification of Headache Disorders, third edition (ICHD-3), and to provide comprehensive oral and written information about the content, possible risks and side effects of the study. Initially, all study participants were reminded about the risks of frequently taking abortive headache medication, recalling the use of headache medication for no more than 10 days/month. In addition, the use of preventive medication and non-medication options was communicated in accordance with guidelines for the treatment of TTH (1). Any abortive (e.g. ibuprofen) and prophylactic (e.g. amitriptyline) drug treatment existing according to these guidelines had to be continued and documented as usual. Preventive medication was on a stable level for a period of at least 3 months prior to inclusion into the study and no adjustments during the course of the study were allowed.
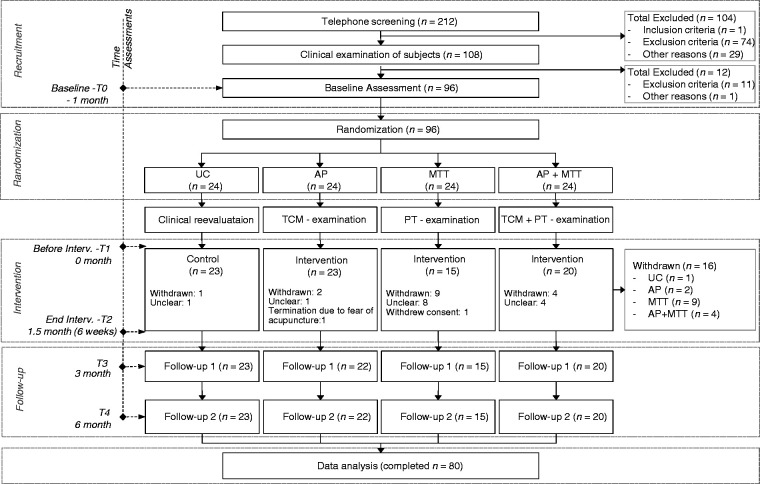
The total study period was 7 months for each participant. After an initial 1-month pre-intervention period after T0, an intervention period of 1.5 months (6 weeks) followed. Two follow-up appointments were made 3 (T3) and 6 (T4) months after the start of the intervention (see Figure 1). Measurements were undertaken prior to and following all these periods.
Figure 1.
CONSORT flowchart of the study.
AP: acupuncture; MTT: medical training therapy; n: number; PT: physiotherapeutic; TCM: traditional Chinese medicine; UC: usual care.
Inclusion and exclusion criteria
Participants between 18 and 65 years who suffered from frequent episodic (headache on 1–14 days/month) or chronic TTH (headache on > 14 days/month) for more than 1 year were included in the study (20). Participants were excluded based on the following criteria: Infrequent episodic TTH (headache <1day/month) inability to speak/write in German, treatment of TTH by AP or MTT within the last 6 months, severe somatic or psychiatric illness, drug abuse or use of headache medication on more than 10 days/month, >1 × migraine/year, pregnant and breastfeeding women, onset of headache after 50 years of age and other medical causes of headache (21).
After written consent, 96 participants were included.
Randomisation
By means of pre-generated randomisation lists (i.e. group ratio of 1:1:1) with varying permutation block sizes by an independent statistician, all participants were assigned to one of the four intervention groups. After the lists were prepared, the written assignment sheets were handed over to the treating study physicians in opaque sealed envelopes. The assignment was made by the study participant randomly drawing one of the opaque randomisation envelopes after inclusion in the study.
Interventions
During the intervention phase, the participants in the intervention groups received a total of 12 treatment units in decreasing frequency (3 × week in weeks 1 + 2, 2 × week in weeks 3 + 4 and 1 × week in weeks 5 + 6). To ensure the therapy compliance of the study participants, the usual care group was offered free therapy of their choice after the last follow-up. The participants of the MTT group were also offered to receive AP free of charge after completion of the last follow-up.
From the participants of the usual care group (UC), a comprehensive medical history and physical exam were obtained. The participants of the UC had contact with the study physicians and the study nurse at all five examination times but had no additional appointments to compensate the 12 therapeutic measures in the intervention groups. They were informed about the disease and their treatment options and did not receive any additional intervention; the treatment of their headaches was to be continued as usual as outlined in “screening and recruitment”.
AP was performed as a semi-standardised treatment concept based on the principles of traditional Chinese medicine (TCM), the scientific literature and the opinion of international experts (22). A total of seven standardised AP points, as well as 3–5 individual points identified according to pain localisation (meridian localisation), were used (Table 2). This concept fulfils both the requirement of an individual TCM basic diagnosis and a standardised AP treatment for patients with comparable symptoms. In addition, 1–4 needles could be placed in so-called “Ashi” points (the most painful points). All AP points were manually stimulated after insertion and after 30 min before removing the needle to induce a Deqi sensation. Sterile disposable AP needles were used (25–40 mm × 0.25–0.3 mm; SuzhouTianxie). The AP was applied without any additional technique enhancing methods and was performed by an experienced acupuncturist with more than 5 years of clinical experience. The exercise treatment was generally based on the principles of MTT and includes a combination of strength, endurance, flexibility and coordination training (Table 1) (23,24). After initial physiotherapeutic anamnesis and diagnostic evaluation, a cardiovascular stress test, a test of strength, endurance, posture and flexibility followed to create an individual treatment plan. MTT equipment, flexible oscillating rods and sensorimotor therapy equipment with unstable oscillating therapy surfaces were used. In addition, all participants in the MTT and combination groups were given a compact written 15-min self-exercise program to independently exercise three times a week. Each of the 60-min treatment sessions took place in the clinic’s gym in small groups of four participants, was adapted in intensity to the previously prepared treatment plan and personally supervised by an experienced qualified physiotherapist. The combination of initial MTT, a short 5–10 min break and subsequent AP represented the complex intersectoral health intervention in this study. Both procedures were performed as described above with the same frequency, but the time required was approximately 100 min, correspondingly 40–60 min longer than in the monotherapies.
Table 2.
Detailed description of acupuncture treatment.
| Point selection | Pain localisation | Acupuncture points | Depth (cun) |
|---|---|---|---|
| Standard | Baihui (GV20) | 0.5–0.8 | |
| Taiyang (EX-HN5) | 0.3–0.5 | ||
| Fengchi (GB20) | 0.8–1.2 | ||
| Hegu (LI4) | 0.5–1.0 | ||
| Yangming meridian | Front of the head, forehead, brow edge | Neiting (ST44) | 0.5–0.8 |
| Yintang (GV29) | 0.3–0.5 | ||
| Shaoyang meridian | Side of the head, temporal | Zulinqi (GB41) | 0.3–0.5 |
| Waiguan (SJ5) | 0.5–1.0 | ||
| Taiyang meridian | Back of the head, occipital | Kunlun (BL60) | 0.5–1.0 |
| Houxi (SI3) | 0.5–1.0 | ||
| Jueyin meridian | Top of the head | Taichong (LR3) | 0.5–0.8 |
| Neiguan (PC6) | 0.5–1.0 | ||
| Sishencong (EX-HN1) | 0.5–0.8 |
Outcome parameter
The primary outcome was the average intensity of pain, assessed using a verbal rating scale (VRS). The valid and reliable one-dimensional 11-point scaled metric scales for subjective assessment of average pain intensity on a scale of 0–10 (0 = no pain to 10 = worst imaginable pain) was used (25,26). Pain intensity was assessed verbally at T0 (baseline), T3 (3 months after intervention start) and T4 (6 months after intervention start) during the consultation with the study physician. Patients were asked to verbally evaluate not only the average but also the maximum and minimum intensity of headache for the previous 4 weeks.
Secondary outcomes were the frequency of days with TTH per month, the mean duration of pain attacks in hours/month and the frequency of days/month on which headache medication was taken. These results were obtained using continuous headache diaries (www.dmkg.de) (27). Study participants with more than 50% reduction of headache days per month compared between T0 and T3/T4 were considered responders.
Adverse events/side-effects were continuously documented in the headache diary and classified by one of the study doctors. The treatment methods were presented as equally effective in order to objectify their credibility and the expectations of the participants. All study participants in the intervention groups were asked about their expectations of the therapy assigned to them, the fulfilment of those expectations and the recommendation of the assigned therapy to a close friend or family member.
Statistical analysis
Data entry was carried out by transferring the written data into electronic databases by academic employees of the clinic who were not involved in the treatment and evaluation process. Subsequently, the digitised data were evaluated by employees of the Goethe University Frankfurt who were not involved in the data collection and therapy process.
Potential T0 differences between the four groups were revealed using (ordinal and nominal scaled data) cross-tables and χ2 tests and, for the interval, proportion, and pseudo-interval scaled data, using one-way analyses of variances (ANOVAs). Main analyses were performed for change scores; differences between T0 and T3 for the main effects analyses and between T0 and T4 for the sustainability analyses. All analyses were performed as confirmatory, where possible on an intention-to-treat-basis, and no between-hypotheses alpha-error adjustment was performed. Associated data were displayed as mean and standard deviations and as box plots. By means of standardised mean difference calculations, effect sizes (Cohen’s d) were calculated. On group level, change scores were checked for variance homogeneity using Levene’s test. Data distribution was visually examined. The omnibus test was selected based on variances: In case of variance homogeneity, repeated measures analyses of variances (rmANOVAs, main and interaction effects) were calculated, in case of variance heterogeneity, Kruskal-Wallis-test (two times, once for Tt0-Tt3 changes and once for Tt0–Tt4 changes) were performed. For the responder-rates, Pearson- χ2 tests were calculated. Significant omnibus tests were followed by Bonferroni-Holm-adjusted post-hoc comparisons (t-test in case of normal distribution, Man-Whitney U test in case of non-parametric distributions, Fisher’s exact tests for the responder rates) of the intervention groups and the usual care group. To prevent a loss in power, only many-one comparisons with the usual care group as fixed control were performed. The box plots were created using jamovi (version 1.0.7.0, the jamovi project (2019, USA). All other analyses were performed using SPSS (Version 24, IBM SPSS, Armonk, NY, USA). For all significance testing, an alpha-error of 5% was considered as a valid cut-off, all p-values below are interpreted as statistically significant.
Results
Figure 1 shows the process and follow-up of the study participants and the drop outs. Two hundred and twelve persons were screened, 96 were randomised to one of the four groups. Overall, 16 participants were excluded or withdrew consent during the course of the study. Most withdrawal reasons were not specified.
Baseline values
Table 3 shows the sociodemographic, anthropometric, and baseline characteristics of the study population. More than two third were females, frequent episodic tension-type headache associated with pericranial tenderness was more frequent than frequent episodic tension-type headache not associated with pericranial tenderness.
Table 3.
Sociodemographic, anthropometric, disease- and intervention-related characteristics. Values are displayed for the total sample and separated for each group. Interval and pseudo-interval values are displayed as mean and standard deviations (SD), ordinal and nominal values as numbers/frequencies. Corresponding statistical values (one-way ANOVAs and χ2 values).
| Parameter |
Total |
ANOVAχ2 |
UC |
AP |
MTT |
AP + MTT |
|---|---|---|---|---|---|---|
| Mean (SD) bzw. % | F/χ2 value (p-value) | Mean (SD)/numbers | Mean (SD)/numbers | Mean (SD)/numbers | Mean (SD)/numbers | |
| Sociodemographics | ||||||
| Age (years) | 38.7 (13.3) | .18 (.9) | 38.7 (14.6) | 39.8 (12.2) | 37.0 (15.3) | 39.0 (11.6) |
| Sex: Female/male (n) | 75/20 | 3.5 (.3) | 17/7 | 18/6 | 18/5 | 22/2 |
| IHS classification | ||||||
| ETTH associated with pericranial tenderness | 54 | 5.1 (.2) | 13 | 14 | 17 | 10 |
| ETTH not associated with pericranial tenderness | 12 | 3.0 (.4) | 3 | 1 | 3 | 5 |
| CTTH associated with pericranial tenderness | 24 | 10.7 (.01)* | 7 | 9 | 0 | 8 |
| CTTH not associated with pericranial tenderness | 5 | NA | 1 | 0 | 3 | 1 |
| Pain | ||||||
| Mean intensity (VRS, points) | 5.5 (1.2) | .18 (.9) | 5.5 (1.1) | 5.6 (1.3) | 5.4 (1.4) | 5.5 (1.0) |
| Maximal intensity (VRS, points) | 8.1 (1.1) | .89 (.5) | 8.2 (0.8) | 8.0 (1.0) | 7.8 (1.3) | 8.3 (1.1) |
| Minmal intensity (VRS, points) | 4.7 (1.5) | .28 (.8) | 4.5 (1.8) | 4.9 (1.6) | 4.8 (1.4) | 4.8 (1.2) |
| Symptoms duration (years) | 14.8 (12.6) | .87 (.5) | 17.3 (13.4) | 12.4 (13.3) | 13.1 (10.9) | 16.2 (12.6) |
| Number of painful days last 3 months (days) | 32.0 (27.5) | .45 (7) | 36.7 (32.6) | 29.6 (24.1) | 28.2 (24.5) | 33.4 (28.3) |
| Sleeping quality | ||||||
| Sleeping disorder (n) | 41 | 5.9 (.7) | 9 | 13 | 8 | 11 |
| Analgesics | ||||||
| General (n) | 88 | 1.6 (0.7) | 22 | 23 | 22 | 21 |
| Training expectation and recommendation | ||||||
| Training effect expectation (points) | 6.2 (2.7) | 1.3 (.3) | N/A | 6.8 (2.4) | 5.2 (2.7) | 6.3 (3.1) |
| Training recommendation yes/no (n) | 53/3 | 1.6 (.4) | N/A | 22/1 | 13/0 | 18/2 |
| Side effects Occurrence yes/no (n) | ||||||
| Mild | 25/54 | 24.2 (.001) | 0/21 | 13/10 | 2/13 | 10/10 |
| Moderate | 0/0 | N/A | 0 | 0 | 0 | 0 |
| Severe | 0/0 | N/A | 0 | 0 | 0 | 0 |
| Mild side effect, if yes: Type | ||||||
| Acute symptom worsening | 12 | 18.7 (.02) | 0 | 5 | 1 | 6 |
| Hematome | 7 | 0 | 6 | 0 | 1 | |
| Fatigue | 2 | 0 | 0 | 0 | 2 | |
| Local pain | 3 | 0 | 2 | 0 | 1 | |
| Dizziness | 1 | 0 | 0 | 1 | 0 |
UC: usual care; AP: acupuncture; MTT: medical training therapy; ETTH: frequent episodic tension-type headache; CTTH: chronic tension-type headache; VRS: verbal rating scale.
With the exception of IHS diagnosis of chronic TTH with pericranial tenderness and mild side effects, all data were homogeneously distributed with regard to group differences and did not show significant differences (p > 0.05). In the MTT group, only three patients with CTTH were included, in these patients no association with pericranial tenderness was found. The proportion of patients with CTTH in the other groups ranged from eight to nine patients. In our opinion, the influence on the outcome is small due to the small number of cases, but should be considered. In the explorative analysis (Table 4), no influence of the IHS classification between episodic and chronic on the outcome could be calculated (p > 0.05).
Table 4.
Explorative analyses for the change score (dependent variable: primary outcome mean pain intensity change score (T0–T3)). 1: Pearson product-moment correlations; 2: binary logistic.
| Independent variable | UCr-value (p-value) | APr-value (p-value) | MTTr-value (p-value) | AP + MTT r-value (p-value) |
|---|---|---|---|---|
| Baseline pain intensity (VRS)1 | −.56 (.01)* | −.55 (.01)* | −.54 (.04)* | −.42 (.06) |
| Episodic versus chronic type2 | .40 (.06) | .08 (.7) | .37 (.2) | .18 (.5) |
| Pericranial tenderness: Yes versus no2 | −.19 (.4) | −.25 (.3) | .54 (.04)** N-statistics: 2.32, p < .01 | −.40 (.08) |
| Symptoms duration (years)1 | .045 (.8) | .08 (7) | .06 (.8) | −002 (.9) |
| Sleeping duration (hours/night)1 | .07 (.7) | .13 (.6) | −.08 (.8) | .01 (.9) |
| Sleeping disorder yes/no (n)2 | −.49 (.02)* | .08 (.7) | .28 (.3) | −.43 (.05) |
| Effect expectation (points) | N.A. | −.27 (.2) | −.28 (.4) | .07 (.5) |
*Significant against 5%; ** significant when compared to the control group.
UC: usual care; AP: acupuncture; MTT: medical training therapy; VRS: verbal rating scale.
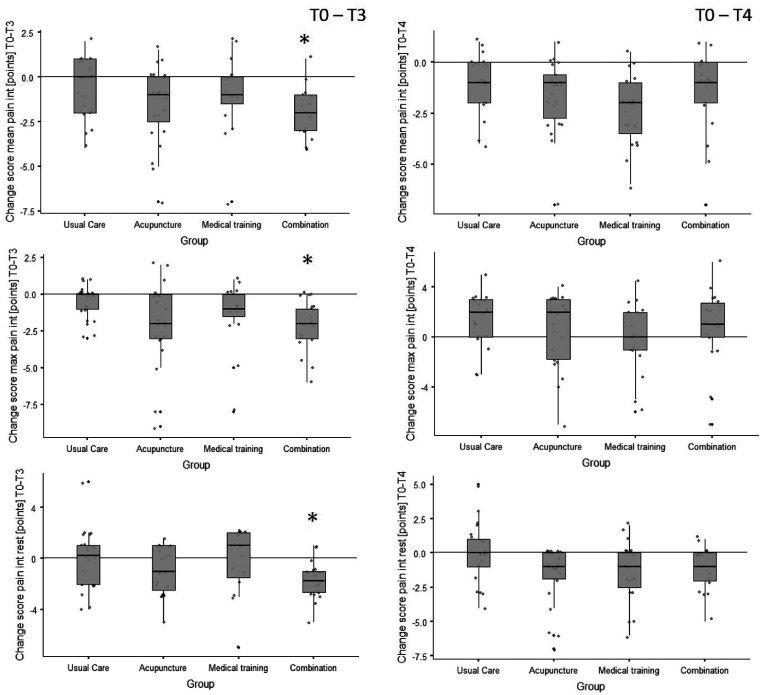
Primary outcome: The changes in mean, maximum and minimum pain intensity are shown in Figure 2 for all groups at 3- (T3) and 6-month (T4) follow-up. Changes in mean values, effect sizes (Cohen’s d) and p-values are shown in Table 5.
Figure 2.
Box plots of the pain intensity change scores (differences) from baseline to 3-month follow-up (T0–T3) and to 6-month follow-up (T0–T4, sustainability). The plots include individual data (dots), medians (horizontal lines), interquartile ranges and whisker bars (90 % intervals, vertical lines). The asterisks (*) indicate a significant group difference to the usual care group (refer to Table 5).
int: intensity; max: maximal; n: number.
Table 5.
Descriptive and inference statistical outcomes for the pain intensity variables. For each outcome, omnibus test characteristics, in case of significance post-hoc test characteristics, as well as means (and standard deviations), and effect sizes are displayed.
| Change score (difference) for outcome | Statistical parameter | Control group | Acupuncture group | Medical training group | Combination group |
|---|---|---|---|---|---|
| Mean pain intensity (points) T0–T3 | Mean (SD); % (SD) | −0.6 (1.6); −11 (29) % | −1.6 (2.1); −28 (38) % | −1 (2.2); −19 (40) % | −2.1 (1.4); −38 (25) % |
| Kruskal-Wallis χ2 (p-value) | 9.4 (.024) | ||||
| Post-hoc Mann-Whitney-U t-value (p-value adjusted)* | Comparator | 1.5 (.24) | .43 (.67) | 3.0 (.012) | |
| Cohen’s d * | Comparator | −0.83 | −0.33 | −1.25 | |
| Mean pain intensity (points) T0–T4 | Mean (SD); % (SD) | −.98 (1.4); −18 (25) % | −1.7 (1.8); −30 (32) % | −2.4 (1.8); −44 (33) % | −1.5 (2.1); −27 (38) % |
| Kruskal-Wallis χ2 (p-value) | 2.5 (.48), | ||||
| Maximal pain intensity (points) T0–T3 | Mean (SD); % (SD) | −0.6 (1.2); −7 (14) % | −1.9 (2.8); −23 (35) % | −1.3 (2.4); −17 (31) % | −2.1 (1.7); −25 (20) % |
| Kruskal-Wallis χ2 (p-value) | 8.5 (.036) | ||||
| Post-hoc Mann-Whitney-U t-value (p-value adjusted)* | Comparator | 1.9 (.11) | .92 (.35) | 2.9 (.014) | |
| Cohen’s d * | Comparator | −0.87 | −0.49 | −0.99 | |
| Maximal pain intensity (points) T0–T4 | Mean (SD); % (SD) | 1.4 (2.0); 25 (36) % | .57 (2.9); 10 (52) % | .05 (2.7); 1 (50) % | .9 (3); 16 (55) % |
| Kruskal-Wallis χ2 (p-value) | 4.3 (.23) | ||||
| Minimal pain intensity [points] T0–T3 | Mean (SD); % (SD) | −0.023 (2.3); 0 (50) % | −0.91 (1.8); −18 (37) % | −0.27 (2.6); − 6 (54) % | −1.7 (1.5); −35 (31) % |
| Kruskal-Wallis χ2 (p-value) | 8.7 (.033) | ||||
| Post-hoc Mann-Whitney U t-value (p-value adjusted)* | Comparator | 1.3 (.36) | 0.21 (83) | 2.6 (.03) | |
| Cohen’s d * | Comparator | −0.81 | −0.22 | −1.5 | |
| Minimal pain intensity (points) T0–T4 | Mean (SD); % (SD) | −.05 (2.2); −1 (40) % | −1.7 (2.2); −30 (39) % | −1.5 (2.2); −28 (41) % | −1.1 (1.6); −20 (29) % |
| Kruskal-Wallis χ2 (p-value) | 5.7 (.13) | ||||
*Many-one comparison; always contrasted to the control group (standardised mean difference or pairwise comparison). Significant comparisons are highlighted in bold.
For the mean pain intensity, a 38% (standard deviation of 25%) reduction between T0 and first follow-up was shown with strong and clinically relevant effect size (d = −1.25) for the combination of AP and MTT (28). Only the combination showed a significant difference in the post-hoc test compared to UC (p = 0.012). The maximum and minimum pain intensity measured between T0 and T3 showed only significant changes for the combination compared to UC (pmax = 0.014; pmin = 0.03) with strong effect sizes (dmax= −0.99; dmin = −1.5). Large effects could also be shown for AP for all pain intensity parameters between T0 and T3 (dmean = −0.83; dmax = −0.87; dmin = −0.81). Also significant differences between UC and the monotherapies as well as lasting differences (T0–T4) could not be observed (p > 0.05).
Secondary outcomes
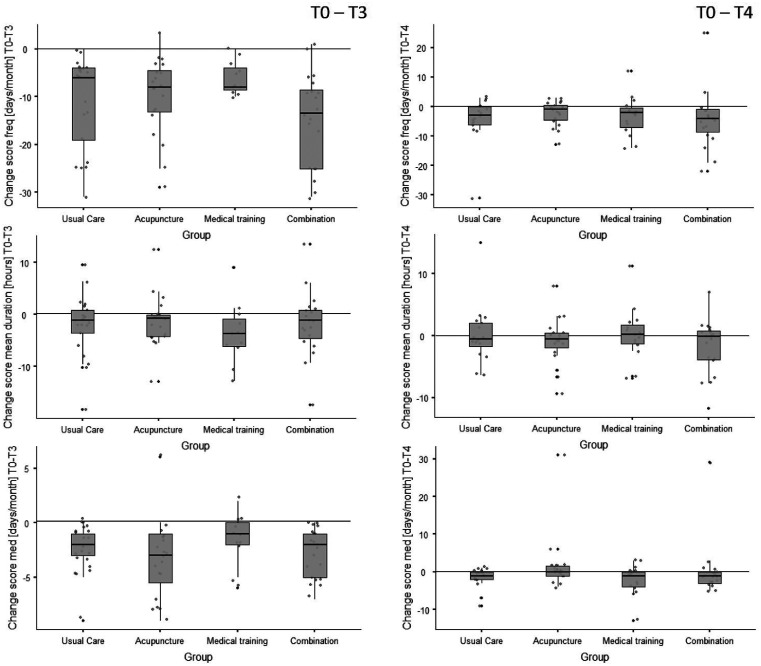
In Figure 3, the changes in headache frequency, mean duration of headache episodes and intake of headache medication between T0 and T3/4 are shown for all groups. Both the main effects (T0 to T3) and the sustainability change scores (T0 to T4) are displayed. The corresponding mean (standard deviation) and percentage change values for headache days/month and duration of pain episodes are, accompanied by the corresponding effect sizes, displayed in Table 6.
Figure 3.
Box plots of the headache frequency, duration, and medication intake change scores (differences) from baseline to 3-month follow-up (T0–T3) and to 6-month follow-up (T0–T4, sustainability). The plots include individual data (dots), medians (horizontal lines), interquartile ranges and whisker bars (90 % intervals, vertical lines).
freq: frequency; med: medication.
Table 6.
Descriptive and inference statistical outcomes for the headache frequency (with responder rates (equals at least 50% reduction in day/month)), duration, and medication intake variables. For each outcome, omnibus test characteristics, in case of significance post hoc test characteristics, as well as means (and standard deviations), percentage changes, and effect sizes are displayed. Significant comparisons are highlighted in bold.
| Change score for outcome | Statistical parameter | UC | AP | MTT | AP + MTT |
|---|---|---|---|---|---|
| Headache frequency (days/month) T0–T3 | Mean (SD);% | −11.1 (9.7); −80% | −9.8 (8.2); −82% | −6.1 (3.5); −88% | −14.7 (9.7); −84% |
| Kruskal-Wallis χ2 (p-value) | 6.9 (.076) | ||||
| Responder rate (>50% frequency reduction) (n); (%) | 19; 91% | 19; 95% | 9; 90% | 18; 95% | |
| Pearson χ2 (p-value) | .52 (.9) | ||||
| Headache frequency (days/month) T0–T4 | Mean (SD);% | −4.3 (7.7); −31% | −2.2 (4.2); −18% | −3.3 (6.7); −48% | −4.6 (9.8); −26% |
| Kruskal-Wallis χ2 (p-value) | 7.3 (.062) | ||||
| Responder rate (>50% frequency reduction) (n); (%) | 2; 10% | 10; 53% | 6; 60% | 7; 39% | |
| Pearson χ2 (p-value) | 11.1 (.01) | ||||
| Post-hoc Fisher’s exact test (p-value adjusted)* | Comparator | .01 | .01 | .04 | |
| Mean duration of headache episodes (hours) T0–T3 | Mean (SD);% | −2.1 (6.0); −29% | −1.3 (4.9); −19% | −3.6 (5.9); −54% | −1.8 (6.3); −28% |
| Kruskal-Wallis χ2 (p-value) | 1.9 (.59) | ||||
| Mean duration of headache episodes (hours) T0–T4 | Mean (SD);% | 0.2 (4.9); +2% | −0.83 (3.8); −12% | 0.23 (4.3); +3% | −1.8 (4.4); −28% |
| Kruskal-Wallis χ2 (p-value) | 3.6 (.31) | ||||
| Headache medication intake [days/month] T0–T3 | Mean (SD);% | −2.3 (2.3); −70% | −3.5 (3.6); −78% | −1.6 (2.3); −70% | −2.8 (2.3); −85% |
| Kruskal-Wallis χ2 (p-value) | 4.9 (.18) | ||||
| Headache medication intake [days/month] T0–T4 | Mean (SD);% | −1.5 (2.7); −46% | 1.7 (7.4); +38% | −2.1 (4.1); −91% | 0.05 (7.3); +2% |
| Kruskal-Wallis χ2 (p-value) | 6.3 (.10) | ||||
UC: usual care; AP: acupuncture; MTT: medical training therapy.
In all groups, the frequency of headache days/month was, from baseline (T0) to the first follow-up (T3), significantly reduced by more than 80% (Table 6). At T4, the reduction of headache days was between 18% and 48% compared with baseline. Although large effect sizes were found, no differences between the groups were found at T3 (p = 0.76) and T4 (p = 0.62).
In all groups, the duration of pain episodes (19–54%) and the use of headache medication (70–85%) was substantially reduced between T0 and T3. No differences between UC, AP, MTT and AP + MTT could be shown (pduration = 0.59; pmedication = 0.18). At T3, all groups had a very high responder rate of > 90% (see Table 5, no group differences). At T4, the responder rate was significantly higher in the intervention groups than in the UC group (Table 6). All intervention groups differed from the UC’s responder rate (p < 0.04).
Explorative analyses
Explorative analyses for the change score (primary outcome pain) are displayed in Table 4. Exploratively (when controlled for the control group associations), participants with pericranial sensitivity seem to profit more from MTT (p = 0.04) whereas patients without pericranial sensitivity profit more from AP and combination treatments.
Safety endpoint
The most frequent mild adverse events throughout the intervention were minor hematomas around the insertion point (n = 12) when AP was applied, and a temporary initial worsening of headache symptoms (n = 7). No moderate or serious unexpected events occurred during the study (Table 3).
Discussion
In contrast to monotherapy, only the combination of AP and MTT was significantly superior in reduction of pain intensity compared to usual care. However, significant group differences compared to the other intervention groups could not be shown. Furthermore, no between-group-differences were found in headache frequency, mean duration of headache episodes, and frequency of pain medication use. While in all groups responder rates were remarkably high, only the intervention groups showed a significant long-lasting effect. The effect sizes for MTT, AP, and the combination reached a level of up to −0.5, and above −0.9 and −1.5, respectively.
These results indicate that both AP and MTT seem to contribute to reducing chronic pain in frequent episodic and chronic TTH by different mechanisms. In fact, the major pathophysiological concept of TTH includes local tenderness of pericranial myofascial tissue and, especially in frequent episodic and chronic TTH, substantial central sensitisation caused by reduced endogenous pain control systems (29,30). It is well known that physical exercise generally improves pain sensitivity by influencing neuroplasticity and reducing tenderness (16,31). In contrast, due to its local and segmental effects, AP seems to reduce muscle tenderness; that, however, may not result in a specific general benefit in chronic TTH (29). Reasons for this may be found in the fact that in frequent episodic and chronic TTH, central sensitisation is triggered by psychological stress and poor coping mechanisms (32). These stress-induced sensitisation mechanisms seem to weaken the AP effects on frequent episodic and chronic TTH (33). In line with this, exploratory analysis in our study showed that patients with pericranial tenderness apparently benefited more from MTT compared to AP in regard to pain intensity (Table 4).
In contrast to our results, Cochrane analyses on AP for TTH from two large studies comparing AP versus routine care showed, for all parameters (pain intensity, number of headache days, response rate), significant superiority of AP (10). Similar results have been reported in studies in which AP was compared to sham (seven trials). However, when comparators were physiotherapy, massage or exercise as analysed in four trials, no significant superiority of AP was found (10).
AP, as a biomedical information therapy, addresses the allostatic (information) systems of the body (14). As other complex approaches may act in a similar holistic way, differences may fade away (15,34,35).
It is more difficult to explain positive changes of the control group (UC), which exceed the data from the literature and accordingly produce missing group differences compared to the intervention groups (10,36). This can be explained by three aspects that fall under the generic term of contextual healing (37). The reasons for this are firstly the participation in a medical study (Hawthorne effect), secondly the ritual of medical education on the current guideline-based therapy, the physical and technical examination and confirmation of diagnosis, and thirdly the positive interaction between physician and patient (38,39). The desired adherence of the study participant, despite being assigned to an inactive study group, was established through friendly and empathetic interaction. It is to be assumed that this addressed binding interaction, and in addition to the comprehensible adherence, this has had a particularly strong effect on the level of complaint reduction (40,41).
Despite the firm offer to receive AP free of charge after the study, eight of the 24 patients discontinued the MTT without giving any reason. We attribute this high dropout rate on the one hand to the randomised allocation mode and an associated increased preference of participants for AP, which could not be fulfilled promptly due to the long study period of 7 months (42). Furthermore, this form of therapy is physically strenuous and less individual than AP. It is known from other studies that dropout rates can reach up to 25% in active exercise interventions (43). However, it is interesting to note that in comparable multi-arm studies comparing both active physiotherapy and AP, no group differences in drop-out rates were reported (44).
Limitations
The guidelines for controlled trials of drugs in TTH outlined that days with TTH per 4 weeks can be the primary efficacy measure (21). However, as we used non-pharmacological interventions addressing directly and mainly pain intensity and pain thresholds, we decided to use the measure of pain intensity as the primary outcome and days with TTH per 4 weeks as the secondary outcome. In addition, the period of our trial was rather short while the guidelines assumed that the counting of headache days per month would be most useful in large-scale long-term pragmatic trials (21).
The recording of the pain intensities of the past 4 weeks during consultation with the study staff could lead to bias in reporting due to fulfilling expectations of the investigators. However, this bias would affect all participants of the study, including the UC group. Recording of the pain intensities during consultation might have resulted in the loss of data related to the onset of pain reduction and its fluctuation over time. However, we decided to follow this approach to collect data from all patients, especially from those with low frequency headache. While choosing the narrow time span of the last 4 weeks, we assumed a low risk of recall bias.
Contextual therapy effects due to differences in provider interaction, in particular empathetic, friendly and compliance-maintaining behaviour of the contact persons in the study, cannot be estimated exactly and could be a source of bias. In the future, these effects could be made more transparent by minimising personal contact with the usual care group (e.g. through digital contact), but on the other hand they will probably also lead to reduced adherence. The implementation of the self-exercise programs could only be examined to a limited extent. Continuous feedback on the fulfilment of self-exercise; for example, through a digital “training app”, would be a useful addition. Due to the low number of cases and the high discontinuation rate in the MTT group, the results are limited in their informative value. In future studies, we recommend optimising the adherence and number of cases according to our results by methodical procedures.
Conclusion
The combination of two non-drug therapeutic strategies effectively reduces the average, maximum and minimal pain intensity of patients with frequent episodic and chronic TTH and could be implemented excellently in the daily clinical routine of a university outpatient clinic. The additive effect based on the combination of Western and traditional Chinese medical strategies could be demonstrated. These results scientifically support the clinical application of this complex health intervention with high patient acceptance. In all participants, the frequency of headaches, the duration of headache episodes and the frequency of taking headache medication were reduced. From these results of a limited group of patients, studies with higher case numbers can be planned due to the exploratory approach in order to test the therapeutic effects on larger samples.
Article highlights
Overall, a strong and meaningful reduction of the average, maximum and minimal pain intensity in patients with frequent episodic and chronic tension-type headache can be reached when combining acupuncture and medical training therapy.
Patients with pericranial sensitivity may profit more from medical training therapy.
At 6 months, responder-rates are larger in all interventions when compared to the usual care group.
The implementation of this complex intervention at a university outpatient clinic into everyday clinical practice is feasible and is characterised by high patient acceptance.
Acknowledgements
We would like to thank K Hoepner and M Lemke for their excellent physiotherapy and T Weiberlenn and L Jiang for their advice on TCM issues. Furthermore, we would like to thank V Bono-Contioso and A Ostermann for the personal support of the study participants and the organisation of the dates. We would like to thank J Briest and X Liu for their statistical cooperation. Last but not least, we would like to thank all study participants for their motivated participation in this project. All authors met the ICMJE authorship criteria. No fees or payments were made for authorship.
Footnotes
Ethics approval: The final revised version of the study protocol and the declaration of consent to the study were reviewed and approved by the Ethics Committee of the Hannover Medical School (No. 7751_BO_S_2018). The written declaration of consent of each study participant had to be obtained prior to registration.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was performed as part of the development of a research ambulance for integrative Chinese medicine. The project was sponsored by Tasly Healthcare Deutschland GmbH (Bahnhofstr. 8, 30159 Hannover). Tasly has and will not influence the unbiased and scientific character of the publication.
ORCID iD: Joerg Schiller https://orcid.org/0000-0002-1775-1092
References
- 1.Bendtsen L, Evers S, Linde M, et al. EFNS guideline on the treatment of tension-type headache – Report of an EFNS task force. Eur J Neurol 2010; 17: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 2.Kommission Leitlinien der Deutschen Gesellschaft für Neurologie. Therapie des episodischen und chronischen Kopfschmerzes vom Spannungstyp und anderer chronischer täglicher Kopfschmerzen, 2015. https://dgn.org/wp-content/uploads/2012/11/030077_LL_Therapie_chronischer_Kopfschmerzen_final.pdf (accessed 20 January 2021). [Google Scholar]
- 3.Scher AI, Stewart WF, Ricci JA, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003; 106: 81–89. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson P, Hedenrud T, Linde M. Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia 2011; 31: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 5.Katsarava Z, Jensen R. Medication-overuse headache: Where are we now? Curr Opin Neurol 2007; 20: 326–330. [DOI] [PubMed] [Google Scholar]
- 6.Spierings ELH, Ranke AH, Schroevers M, et al. Chronic daily headache: A time perspective. Headache 2000; 40: 306–310. [DOI] [PubMed] [Google Scholar]
- 7.Wallasch TM, Kropp P. Multidisciplinary integrated headache care: A prospective 12-month follow-up observational study. J Headache Pain 2012; 13: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaul C, Van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: An observational study. J Headache Pain 2011; 12: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammill JM, Cook TM, Rosecrance JC. Effectiveness of a physical therapy regimen in the treatment of tension-type headache. Headache 1996; 36: 149–153. [DOI] [PubMed] [Google Scholar]
- 10.Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of tension‐type headache. Cochrane Database Syst Rev 2016; 4: CD007587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil Martinez A, Kindelan P, Agudo-Carmona D, et al. Therapeutic exercise as treatment for migraine and tension-type headaches: A systematic review of randomised clinical trials. Revista De Neurologia 2013; 57: 433–443. [PubMed] [Google Scholar]
- 12.Torelli P, Jensen R, Olesen J. Physiotherapy for tension-type headache: A controlled study. Cephalalgia 2004; 24: 29–36. [DOI] [PubMed] [Google Scholar]
- 13.Scharrer M, Ebenbichler G, Pieber K, et al. A systematic review on the effectiveness of medical training therapy for subacute and chronic low back pain. Eur J Phys Rehabil Med 2012; 48: 361–371. [PubMed] [Google Scholar]
- 14.Karst M, Fink M. Acupuncture – a biomedical information therapy: A translational analysis. Med Acupunct 2016; 28: 308–315. [Google Scholar]
- 15.Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia pain-free and chronic pain populations: State of the art and future directions. J Pain 2019; 20: 1249–1266. [DOI] [PubMed] [Google Scholar]
- 16.Nugraha B, Karst M, Engeli S, et al. Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: A mini review. Rheumatol Int 2011; 32: 2593–2599. [DOI] [PubMed] [Google Scholar]
- 17.Eftekharsadat B, Porjafar E, Eslamian F, et al. Combination of exercise and acupuncture versus acupuncture alone for treatment of myofascial pain syndrome: A randomized clinical trial. J Acupunct Meridian Stud 2018; 11: 315–322. [DOI] [PubMed] [Google Scholar]
- 18.Schiller J, Kellner T, Briest J, et al. The best from East and West? Acupuncture and medical training therapy as monotherapies or in combination for adult patients with episodic and chronic tension-type headache: Study protocol for a randomized controlled trial. Trials 2019; 20: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endres HG, Böwing G, Diener HC, et al. Acupuncture for tension-type headache: A multicentre, sham-controlled, patient-and observer-blinded, randomised trial. J Headache Pain 2007; 8: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 21.Bendtsen L, Bigal ME, Cerbo R, et al. Guidelines for controlled trials of drugs in tension‐type headache: Guidelines for controlled trials of drugs in tension‐type headache, second edition. Cephalalgia 2010; 30: 1–16. [DOI] [PubMed] [Google Scholar]
- 22.MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): Extending the CONSORT statement. Acupunct Rel Ther 2015; 3: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheibe J. Sport als Therapie. Berlin/Wiesbaden: Ullstein Mosby, 1994. [Google Scholar]
- 24.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994; 58: 387–392. [DOI] [PubMed] [Google Scholar]
- 26.Hawker GA, Mian S, Kendzerska T, et al. Measures of adult pain: Visual Analog Scale for Pain (VAS pain), Numeric Rating Scale for Pain (NRS pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011; 63: 240–252. [DOI] [PubMed] [Google Scholar]
- 27.Russell MB, Rasmussen BK, Brennum J, et al. Presentation of a new instrument: The diagnostic headache diary. Cephalalgia 1992; 12: 369–374. [DOI] [PubMed] [Google Scholar]
- 28.Fritz CO, Morris PE, Richler JJ. Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen 2012; 141: 2–18. [DOI] [PubMed] [Google Scholar]
- 29.Karst M, Rollnik JD, Fink M, et al. Pressure pain threshold and needle acupuncture in chronic tension-type headache – a double-blind placebo-controlled study. Pain 2000; 88: 199–203. [DOI] [PubMed] [Google Scholar]
- 30.Söderberg E, Carlsson J, Stener-Victorin E. Chronic tension-type headache treated with acupuncture, physical training and relaxation training. Between-group differences. Cephalalgia 2006; 26: 1320–1329. [DOI] [PubMed] [Google Scholar]
- 31.Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 2013; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y. Advances in the pathophysiology of tension-type headache: From stress to central sensitization. Curr Pain Headache Rep 2009; 13: 484–494. [DOI] [PubMed] [Google Scholar]
- 33.Karst M, Reinhard M, Thum P, et al. Needle acupuncture in tension-type headache: A randomized , placebo-controlled study. Cephalalgia 2001; 21: 637–642. [DOI] [PubMed] [Google Scholar]
- 34.Jena S, Witt CM, Brinkhaus B, et al. Acupuncture in patients with headache. Cephalalgia 2008; 28: 969–979. [DOI] [PubMed] [Google Scholar]
- 35.Melchart D, Streng A, Hoppe A, et al. Acupuncture in patients with tension-type headache: Randomised controlled trial. BMJ 2005; 331: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Groot FM, Voogt-Bode A, Passchier J, et al. Headache: The placebo effects in the control groups in randomized clinical trials; an analysis of systematic reviews. J Manip Physiol Ther 2011; 34: 297–305. [DOI] [PubMed] [Google Scholar]
- 37.Miller FG, Kaptchuk TJ. The power of context: Reconceptualizing the placebo effect. J R Soc Med 2008; 101: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franke RH, Kaul JD. The Hawthorne experiments: First statistical interpretation. Am Sociol Rev 1978; 43: 623–643. [Google Scholar]
- 39.Autret A, Valade D, Debiais S. Placebo and other psychological interactions in headache treatment. J Headache Pain 2012; 13: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008; 336: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med 2015; 373: 8–9. [DOI] [PubMed] [Google Scholar]
- 42.Härtel U, Volger E. Use and acceptance of classical natural and alternative medicine in Germany – findings of a representative population-based survey. Forsch Komplementarmed Klass Naturheilkd (Research in complementary and natural classical medicine) 2004; 11: 327–334. [DOI] [PubMed] [Google Scholar]
- 43.Schuch FB, Stubbs B. The role of exercise in preventing and treating depression. Curr Sports Med Rep 2019; 18: 299–304. [DOI] [PubMed] [Google Scholar]
- 44.Williamson L, Wyatt MR, Yein K, et al. Severe knee osteoarthritis: A randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology 2007; 46: 1445–1449. [DOI] [PubMed] [Google Scholar]