Abstract
A 66-year-old Caucasian man was initially admitted with a metastatic small cell lung carcinoma, hyponatraemia and obstructive pneumonia. His transthoracic echocardiogram (TTE) was normal. Ten days after admission, he was diagnosed with a non-ST segment elevation myocardial infarction (MI). Both a repeated TTE and a transoesophageal echocardiogram identified thickened, myxomatous mitral valve leaflet tips with small, mobile masses identified as vegetations, and new, eccentric, severe mitral regurgitation. Subsequent cardiac catheterisation recorded thrombotic occlusion of the right coronary artery. Successful coronary thrombectomy was carried out, but the patient died. A diagnosis of non-bacterial thrombotic endocarditis leading to coronary embolisation and MI was made. The clinical course and treatment choices are discussed.
Keywords: cardiovascular medicine, cancer - see oncology, interventional cardiology, valvar diseases, cardiovascular system
Background
Non-bacterial thrombotic endocarditis (NBTE) is characterised by fibrin and platelet aggregate vegetations on a cardiac valve in the absence of bacteria or inflammation.1 The incidence of NBTE obtained from postmortem studies in the general population ranges from 0.3% to 9.3%2–4 and is initiated by the immune complexes, hypoxia and hypercoagulability associated with malignancy.1 NBTE has been associated with mucin-producing adenocarcinomata of the pancreas and the lungs.5 Our patient is unusual, having a non-mucin producing carcinoma and a subsequent myocardial infarction (MI). NBTE has a higher incidence of systemic embolisation and may be misdiagnosed as infective endocarditis.6 There are only seven reported cases of NBTE complicated by MI in the last decade (table 1).
Table 1.
Case reports of acute myocardial infarction and non-bacterial thrombotic endocarditis in cancer patients in the past 10 years
| Publication | Date published | Age/sex | Underlying pathology | Pathology treatment | Valve involved | MI type | AC/DAPT Treatment | Surgical intervention | Outcome |
| Bathina et al16 | 2010 | 76/M | Non-SCLC | N/A | MV | NSTEMI | Aspirin | None | Death (multiorgan failure) |
| 53/M | Oesophageal cancer | N/A | MV | STEMI | Aspirin, UFH | None | Death (multiorgan failure) | ||
| 55/M | Non-SCLC | N/A | MV | STEMI | Aspirin | None | Death (multiorgan failure) | ||
| 44/F | Non-SCLC | N/A | AV | NSTEMI | Aspirin, clopidogrel | None | Discharge, death 2 months later (cancer progression) | ||
| Scalia et al9 | 2012 | 68/M | Adenocarcinoma (unspecified) | N/A | AV | ACS | N/A | AV replacement | Death (multiorgan failure) |
| Tiong et al7 | 2013 | 57/F | Cervical adenocarcinoma | Carboplatin, paclitaxel (palliative) | AV | STEMI | Aspirin, clopidogrel, enoxaparin | Aspiration thrombectomy (Second obtuse MA) | Discharged, death 6 weeks later (cancer progression) |
| Sia et al18 | 2016 | 54/F | Pancreatic adenocarcinoma | None | AV | Unspecified | Unspecified | None | Death (multiorgan failure) |
AC, anticoagulation; ACS, acute coronary syndrome; AV, aortic valve; DAPT, dual-antiplatelet therapy; F, female; M, male; MA, marginal artery; MI, myocardial infarction; MV, mitral valve; N/A, not available; NSTEMI, non-ST segment elevation myocardial infarction; SCLC, small cell lung carcinoma; STEMI, ST-elevation myocardial infarction; UFH, unfractionated heparin.
Case presentation
A 66-year-old Caucasian man with small cell lung carcinoma, chronic hepatitis C, chronic obstructive lung disease and hypertension presented with nausea, vomiting and a sodium level of 118 mmol/dL due to the paraneoplastic syndrome of inappropriate antidiuretic syndrome.
Investigations
Thoracic and abdominal CT revealed a right hilar mass with lymphangitic spread to the middle and lower lobes of the right lung, obstructive lobar pneumonia and hepatic metastases.
On day 5, the patient was placed on bilevel positive airway pressure due to the development of acute hypoxic respiratory failure without evidence of pulmonary embolism on CT angiogram. An ECG recorded new-onset atrial fibrillation with a rapid ventricular response. Administration of diltiazem and metoprolol resulted in conversion to normal sinus rhythm. Intravenous amiodarone and heparin were commenced in the setting of borderline blood pressure. Serum troponin levels from day 1 to 5 ranged from 0.009 to 0.018 ng/mL (normal range <0.04 ng/mL). Initial transthoracic echocardiogram (TTE) showed left ventricular diastolic dysfunction and very mild mitral regurgitation.
On day 6, the patient was commenced on carboplatin and etoposide. On day 9, he developed acute pulmonary oedema and required intubation. A repeat TTE showed eccentric, severe mitral regurgitation (regurgitant fraction: 65%). On day 10, serum troponin levels increased to 45 ng/mL. An ECG showed recurrence of atrial fibrillation without ST segment elevation. A transoesophageal echocardiogram (TOE) recorded thickening of the tips of the mitral valve leaflets with multiple, small valvular vegetations (figure 1 and video 1) and confirmed severe mitral regurgitation (regurgitant fraction: >60%). Cardiac catheterisation identified a 100% occlusion of the right coronary artery; the anterior descending and circumflex left coronary vessels were patent. An aspiration thrombectomy was performed. Of note, blood cultures taken on days 1, 6 and 10 were negative.
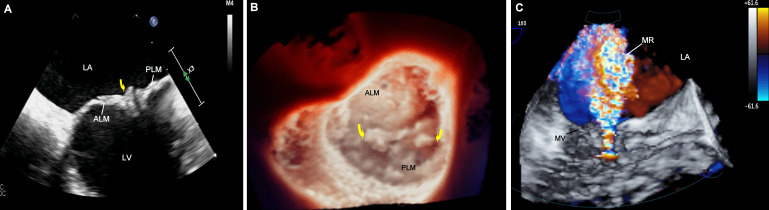
Figure 1.
A- 2D TOE showing mitral valve vegetations (arrow), B- 3D TOE surgical true view of the mitral valve showing vegetations (arrows), C-3D color flow Doppler showing mitral regurgitation jet (arrow). ALM, anterior leaflet of the mitral valve; LA, left atrium; LV, left ventricle; MR, mitral regurgitation; PLM, posterior leaflet of the mitral valve; TOE, transesophageal echocardiogram.
Video 1.
Differential diagnosis
Infective endocarditis, suggested by the valvular vegetations seen on the echocardiograms, was ruled out by the serial negative blood cultures. Sterile vegetations associated with an underlying malignancy suggested NBTE. This diagnosis was further supported by the location of the vegetations on the mitral valve; NBTE is more frequently associated with left-sided and/or bilateral valvular vegetations.6 MI due to an embolic event associated with non-atherosclerotic coronary vessels together with gross examination of the aspirated material strongly suggested the diagnosis of thrombotic embolism due to NBTE. The possibility of an atrial thrombus was less likely with transient atrial fibrillation and the absence of atrial or atrial appendage thrombus on TOE. The mobile masses seen on the mitral valve leaflets were the more likely source of the thrombotic embolus.
Treatment
Aspiration thrombectomy was performed and thrombolysis in myocardial infarction (TIMI) 3 coronary blood flow was restored (figure 2). A 72-hour infusion of heparin was commenced. Dual-antiplatelet therapy (DAPT) was considered; only clopidogrel was given due to an aspirin allergy. The allergy-immunology team did not recommend aspirin desensitisation while the patient was medically unstable. Cardiothoracic surgery did not consider the patient to be a suitable candidate for mitral valve replacement.
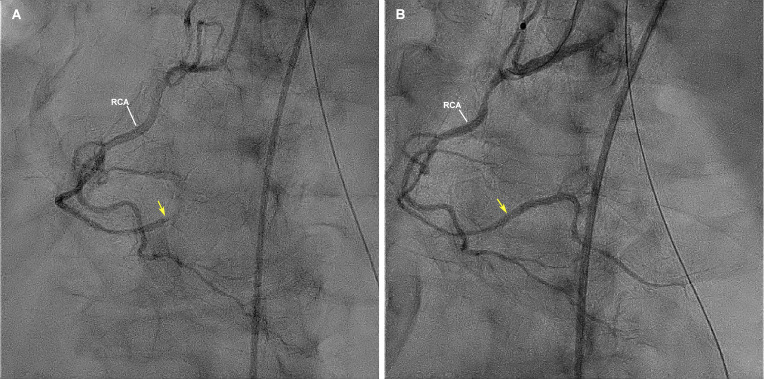
Figure 2.
Coronary angiogram before (A) and after (B) thrombectomy, showing thrombotic occlusion of the distal right coronary artery (arrow) and a patent distal right coronary artery after aspiration thrombectomy (arrow). RCA, right coronary artery.
Outcome and follow-up
The patient’s status failed to improve, remaining dependent on ventilator respiratory support and vasopressors. On day 14, he was transitioned to comfort measures. The patient died from multiorgan failure after extubation.
Discussion
Hypercoagulability with malignancy is multifactorial and associated with the aberrant behaviour of the coagulation cascade.4 This prothrombotic state may present as migratory superficial thrombophlebitis, venous and arterial thrombosis, thrombotic microangiopathy, disseminated intravascular coagulation and NBTE.7 The pathology of NBTE, formerly referenced as marantic endocarditis, is not well understood. Clotting abnormalities, together with endothelial damage, hypoxia and immune complex deposition may contribute to its pathogenesis.1 A postmortem analysis of NBTE valvular vegetations has revealed primarily an amorphous mixture of fibrin and platelets.8
The greatest risk of NBTE is thromboembolism. Unlike infective endocarditis, there is minimal inflammation associated with NBTE vegetations, which predisposes them to detachment more easily from the valves, resulting in multiorgan embolism.6 A small prospective study by Edoute et al discusses the rare complication of MI, estimated at 6.7-9%, in NBTE.5
In a case study by Scalia et al, a patient with resulting stroke and MI from underlying NBTE underwent extensive treatment, including valve replacement, with later findings of underlying colonic adenocarcinoma. In retrospect, the authors discussed the option of prioritising comfort care, in view of a poor prognosis, and potentially foregoing the invasive procedure.9 Our patient underwent invasive treatment followed by a rapidly fatal decline. Considering the responsiveness of small cell lung carcinoma to chemotherapy, though not curative, the patient’s life could have been prolonged in comfort if his hypercoagulability had been better managed to prevent NBTE and fatal thrombosis.
Treatments vary, but heparin is typically the first drug of choice as an anticoagulant. A study by Lee et al showed Coumadin (warfarin) to be less effective at preventing recurrent venous thromboembolism in patients with cancer compared with heparin.10 The use of warfarin alone in malignancy-associated NBTE is also relatively contraindicated due to recurrent thromboembolic events.4 The treatment of NBTE with unfractionated heparin was successful in a patient with pancreatic cancer, who developed the condition while already on prophylactic direct oral anticoagulant (rivaroxaban).11 Other studies show an adequate response to treatment with continuous intravenous heparin alone, which was superior to the subcutaneous form.2 12–14
A patient with cervical adenocarcinoma was treated with warfarin.7 Despite a supratherapeutic international normalized ratio (INR) of 4.4, thrombotic complications continued. Enoxaparin was started, but within a week, the patient developed NBTE of the aortic valve and a STEMI. She underwent coronary thrombectomy and, in addition to the enoxaparin, started DAPT with aspirin and clopidogrel. Findings of NBTE were absent on subsequent TTE.7 This suggests that using warfarin or low-molecular-weight heparin alone for cancer-related hypercoagulability, as suggested by the American College of Chest Physician guidelines,15 may not be sufficient prophylaxis unless combined with DAPT.7
Prior to the advent of DAPT or the absence of NBTE, there was significant evidence of reduced thrombotic and embolic events using only heparin.2 10 Two other cases showed better outcomes following the administration of DAPT, with one patient already taking enoxaparin when recurrent emboli occurred.7 16 Another case had resolution and recurrence of NBTE with enoxaparin, followed by resolved symptoms after treatment with anticoagulation (rivaroxaban) and antithrombotic therapy (clopidogrel).17
The treatment results in the cases mentioned suggest a platelet-driven process, rather than only a coagulation factor-based phenomenon. This pathogenesis is supported by the composition of vegetations being mainly fibrin and platelets.8
In the case of MI, thrombolytics are controversial considering the risk of fatal intracerebral and gastrointestinal bleeding,16 whereas percutaneous coronary intervention has proven effective for immediate relief, as shown in our patient and others.7 16 This treatment strategy does not address the problem of recurring embolism where heparin alone may not be sufficient and neurologicalcomplications may be fatal.18 Further studies are needed to evaluate the combined role of DAPT and heparin at the first signs of hypercoagulability in cancer patients to reach a consensus on optimal management.
Patient’s perspective.
Although our patient died, there was an opportunity months later to interview his brother. As next of kin, he made the decision to extubate. He states ‘I still wonder if I made the right decision’ in regards to ending life support. He expresses, ‘I think it was the best choice with what the doctors were saying about how long he had left to live with his cancer and also they said he had a heart attack. He wasn’t in good shape. I just don’t understand how they didn’t find the cancer at earlier doctor’s appointments. That doesn’t make sense to me. Then again, he never took very good care of himself. He’d always ignore the way he was feeling and never even got a colonoscopy. He would often say that he ‘felt invincible’. It’s really weird to me that he would say that and I guess he wouldn’t have if he knew all this was going to happen. It still doesn’t feel real. When I come home, I imagine he’s going to walk out of his room like he always did. I’m the only one left of my family and I don’t really understand why.’
Learning points.
Consider non-bacterial thrombotic endocarditis when a patient with a history of cancer presents with left-sided vegetations on echocardiogram.
Treatment of culture negative endocarditis may include a combination of heparin and dual-antiplatelet therapy to prevent fatal thromboembolic events.
Hypercoagulability in patients with cancer cannot be under looked as a potential cause of thrombotic events and may provide even more reason for prompt oncological treatment.
The stability and overall condition of a patient needs to be considered prior to invasive procedures as symptomatic treatment may be best with poor prognostic status, such as with advanced underlying carcinoma.
Acknowledgments
We are grateful to Dr J.V. Nixon, Professor Emeritus, Internal Medicine, Virginia Commonwealth University School of Medicine, Richmond VA, for his assistance in revising the manuscript. Also to Jerome Escano, PhD, for his help in editing and revising the tables within the manuscript.
Footnotes
Contributors: MA and BN oversaw all patient care, while KMG and LB help tend to the patient in the hospital setting. MA conceptualised the case report. BN and KMG reviewed the patient chart for details included in the case report. KMG was responsible for the original draft of the manuscript, acquisition and interpretation of data. MA and BN were responsible for interpretation of data and revision of both intellectual content and writing style. LB helped with writing and revision of the manuscript. MA captured images from echocardiograms and created figures with corresponding descriptions.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007;32:696–701. 10.1016/j.ejcts.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 2.Lopez JA, Ross RS, Fishbein MC, et al. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773–84. 10.1016/0002-8703(87)90719-8 [DOI] [PubMed] [Google Scholar]
- 3.Kuramoto K, Matsushita S, Yamanouchi H. Nonbacterial thrombotic endocarditis as a cause of cerebral and myocardial infarction. Jpn Circ J 1984;48:1000–6. 10.1253/jcj.48.1000 [DOI] [PubMed] [Google Scholar]
- 4.Mazokopakis EE, Syros PK, Starakis IK. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets 2010;10:84–6. 10.2174/187152910791292484 [DOI] [PubMed] [Google Scholar]
- 5.Edoute Y, Haim N, Rinkevich D, et al. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med 1997;102:252–8. 10.1016/S0002-9343(96)00457-3 [DOI] [PubMed] [Google Scholar]
- 6.Lee V, Gilbert JD, Byard RW. Marantic endocarditis - A not so benign entity. J Forensic Leg Med 2012;19:312–5. 10.1016/j.jflm.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Tiong IS, Williams MJA, Perez DJ. Nonbacterial thrombotic endocarditis with ST-elevation myocardial infarction treated with percutaneous coronary aspiration thrombectomy. Heart Lung Circ 2013;22:386–9. 10.1016/j.hlc.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 8.Chino F, Kodama A, Otake M, et al. Nonbacterial thrombotic endocarditis in a Japanese autopsy sample. A review of eighty cases. Am Heart J 1975;90:190–8. 10.1016/0002-8703(75)90119-2 [DOI] [PubMed] [Google Scholar]
- 9.Scalia GM, Tandon AK, Robertson JA, Stroke RJA. Stroke, aortic vegetations and disseminated adenocarcinoma--a case of marantic endocarditis. Heart Lung Circ 2012;21:234–6. 10.1016/j.hlc.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Lee AYY, Levine MN, Baker RI, et al. Low-Molecular-Weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53. 10.1056/NEJMoa025313 [DOI] [PubMed] [Google Scholar]
- 11.Mantovani F, Navazio A, Barbieri A, et al. A first described case of cancer-associated non-bacterial thrombotic endocarditis in the era of direct oral anticoagulants. Thromb Res 2017;149:45–7. 10.1016/j.thromres.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 12.Rogers LR, Cho ES, Kempin S, et al. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746–56. 10.1016/0002-9343(87)90908-9 [DOI] [PubMed] [Google Scholar]
- 13.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease--native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:457S–82. 10.1378/chest.126.3_suppl.457S [DOI] [PubMed] [Google Scholar]
- 14.Sack GH, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine 1977;56:1–37. [PubMed] [Google Scholar]
- 15.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTe disease: chest guideline and expert panel report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 16.Bathina JD, Daher IN, Plana JC, et al. Acute myocardial infarction associated with nonbacterial thrombotic endocarditis. Tex Heart Inst J 2010;37:208–12. [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann CC, Wessely E, Huber K. Non-bacterial thrombotic endocarditis in the context of pulmonary adenocarcinoma: a case report. Eur Heart J Case Rep 2020;4:1–5. 10.1093/ehjcr/ytaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia C-H, Lim JSJ, Poh KK, et al. A classical case of non-bacterial thrombotic endocarditis from pancreatic adenocarcinoma presenting as multiple strokes, myocardial infarction and acute limb ischaemia. Oxf Med Case Reports 2016;2016:omw084. 10.1093/omcr/omw084 [DOI] [PMC free article] [PubMed] [Google Scholar]